Keratin 8/18a.1 Expression Influences Embryonic Neural Crest Cell Dynamics and Contributes to Postnatal Corneal Regeneration in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Husbandry and Strain Description
2.2. Transcriptome Analysis
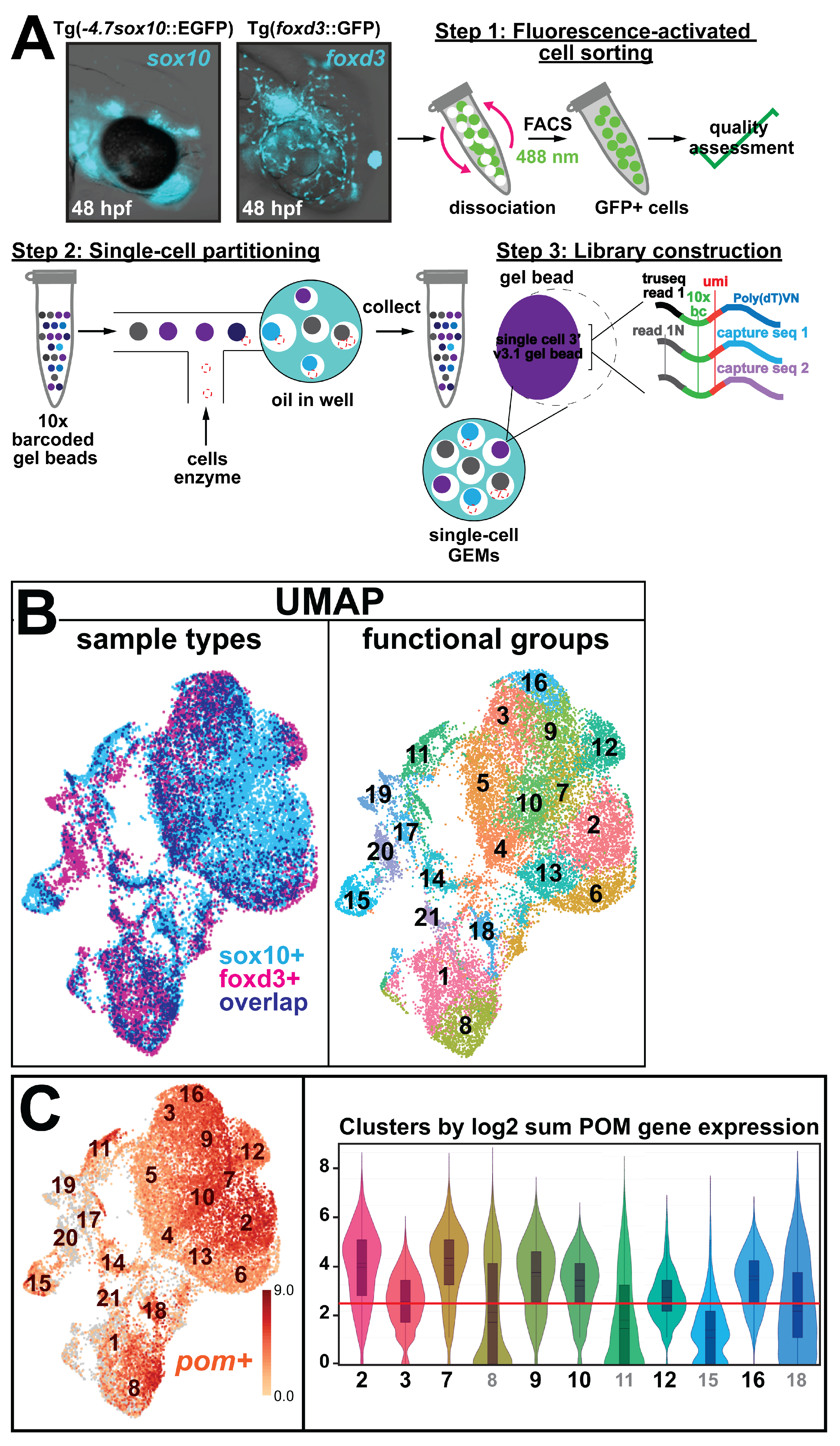
2.2.1. Fluorescence-Activated Cell Sorting
2.2.2. Library Preparation and Sequencing
2.2.3. Single-Cell RNA Sequencing Analysis
2.3. Imaging and Image Processing
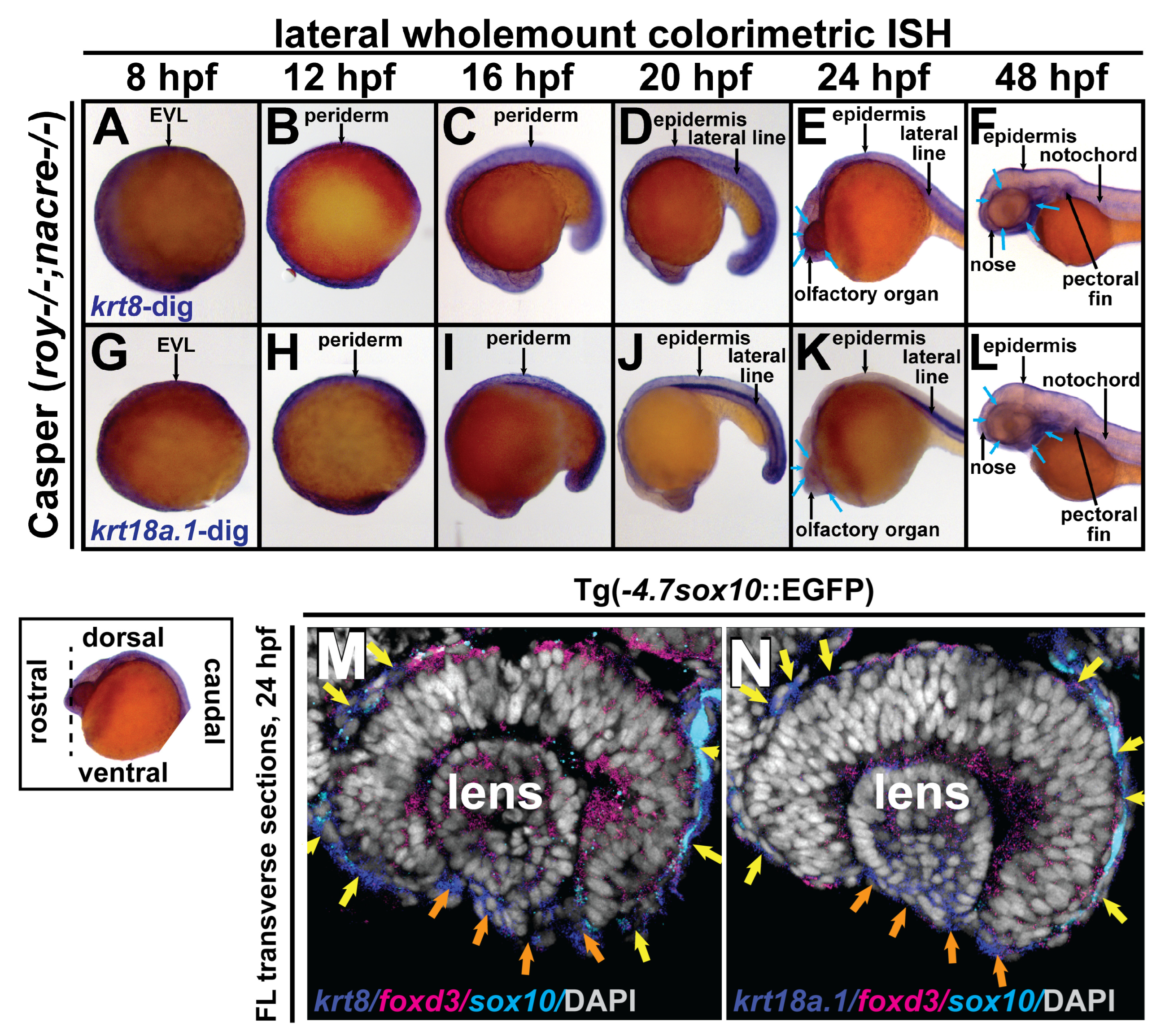
2.4. Wholemount In Situ Hybridization and Immunostaining
2.5. Pharmacological Treatments
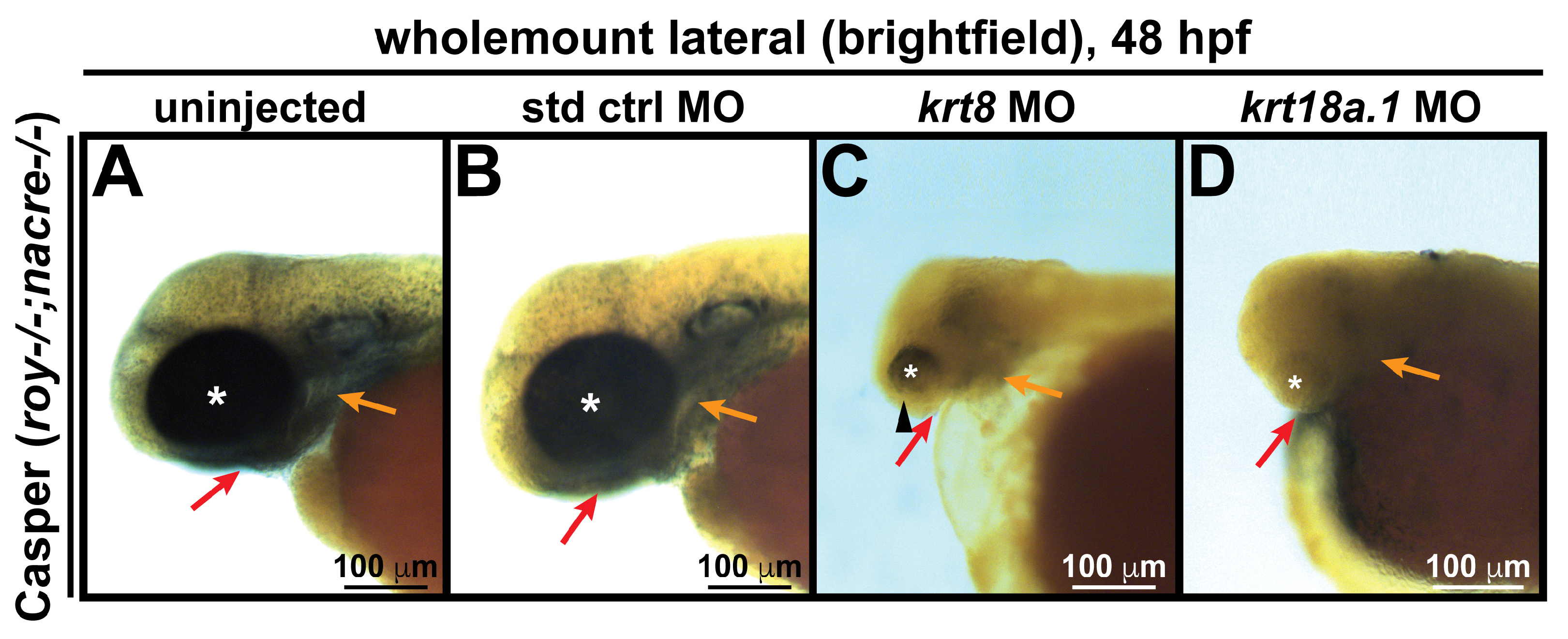
2.6. Morpholino Oligonucleotide Injections
2.7. Corneal Abrasion and Immunohistochemistry
3. Results
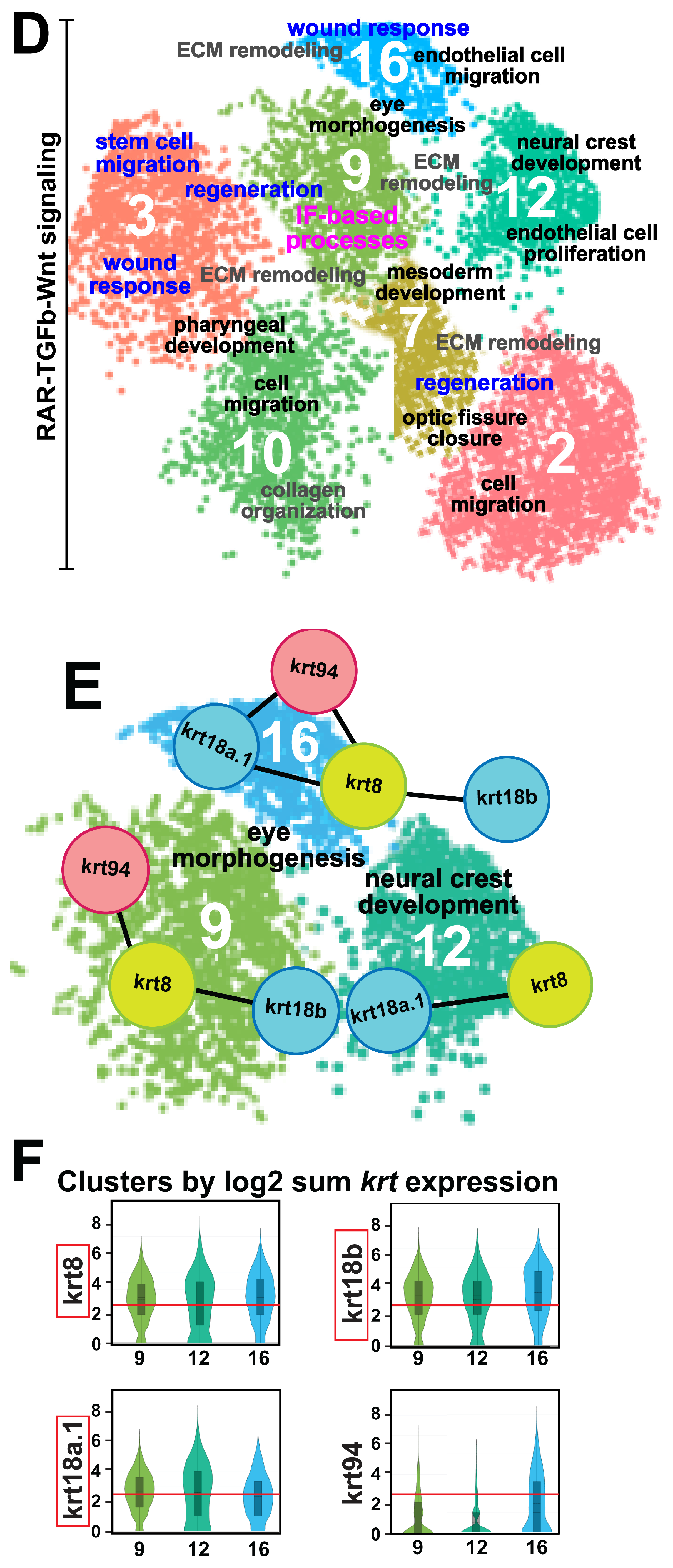
3.1. Single Cell Data Atlas Reveals the Differential Expression of Keratin Intermediate Filament Genes during Ocular Neural Crest Development
3.2. Krt8 and krt18a.1 Are Expressed at the Early Neural Plate Border and in Migrating Neural Crest Cells during Early Development in Zebrafish
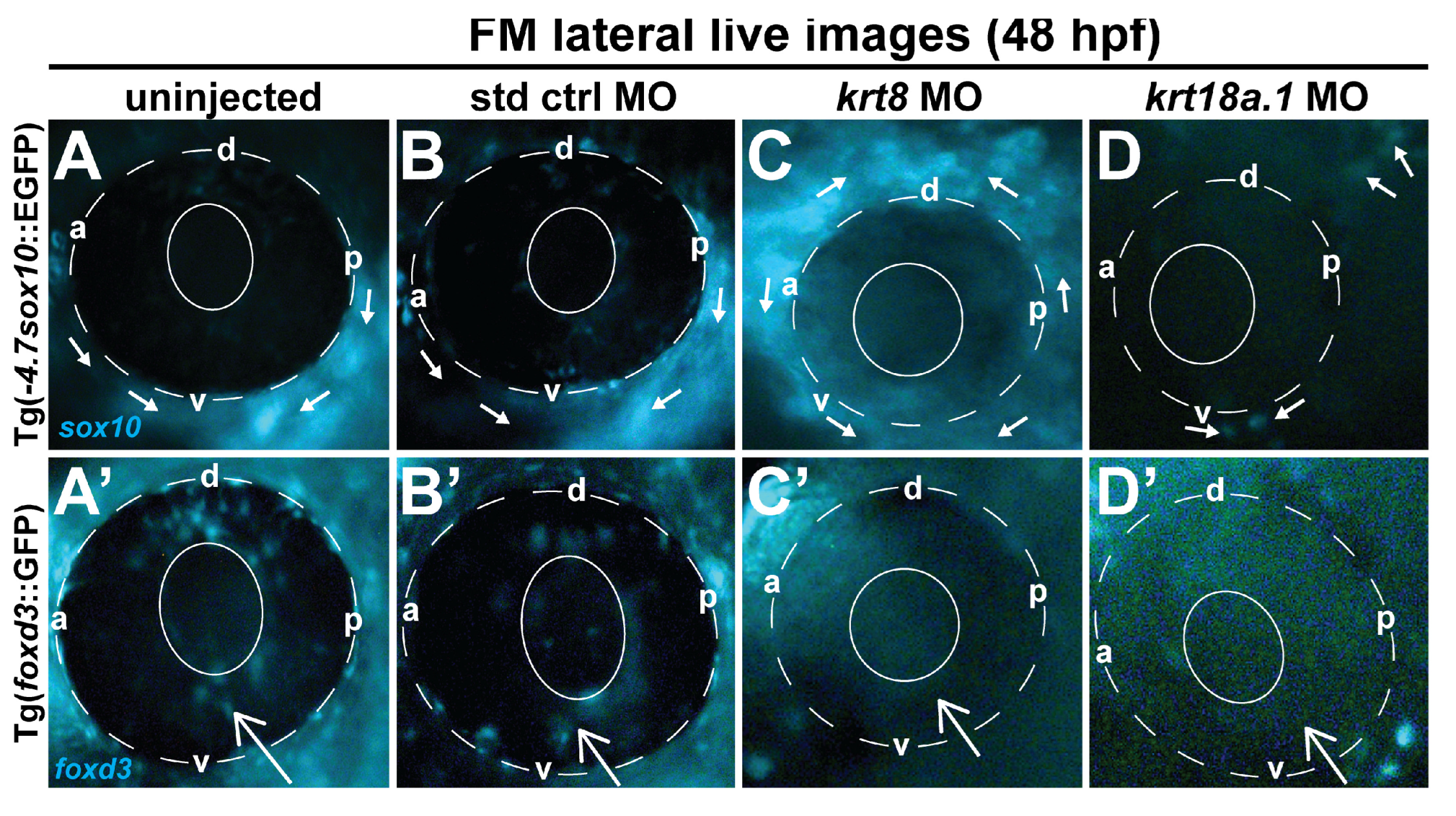
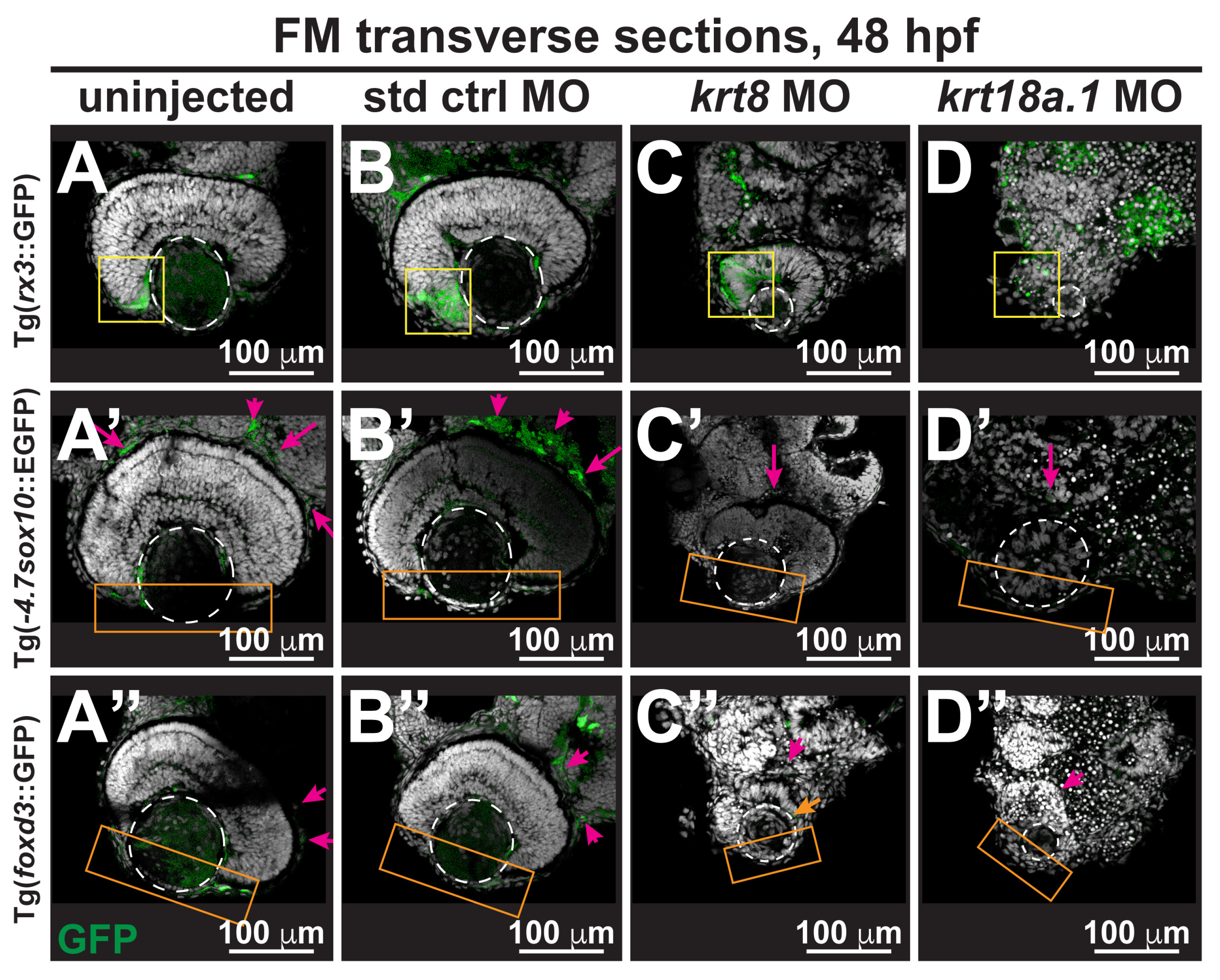
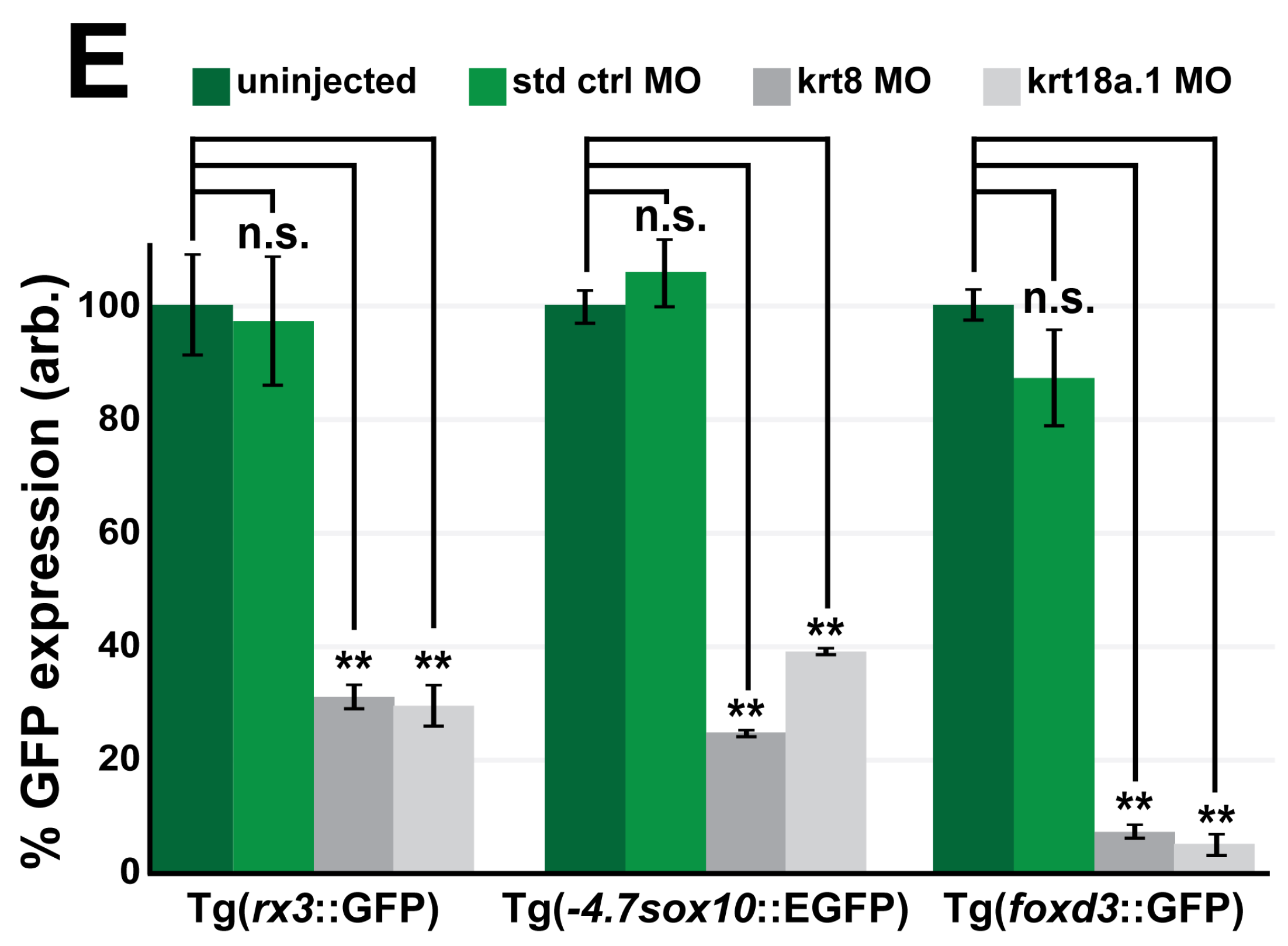
3.3. MO Knockdown of K8 and K8a.1 Disrupts Neural Crest Migration in the Ocular and Craniofacial Regions during Early Development
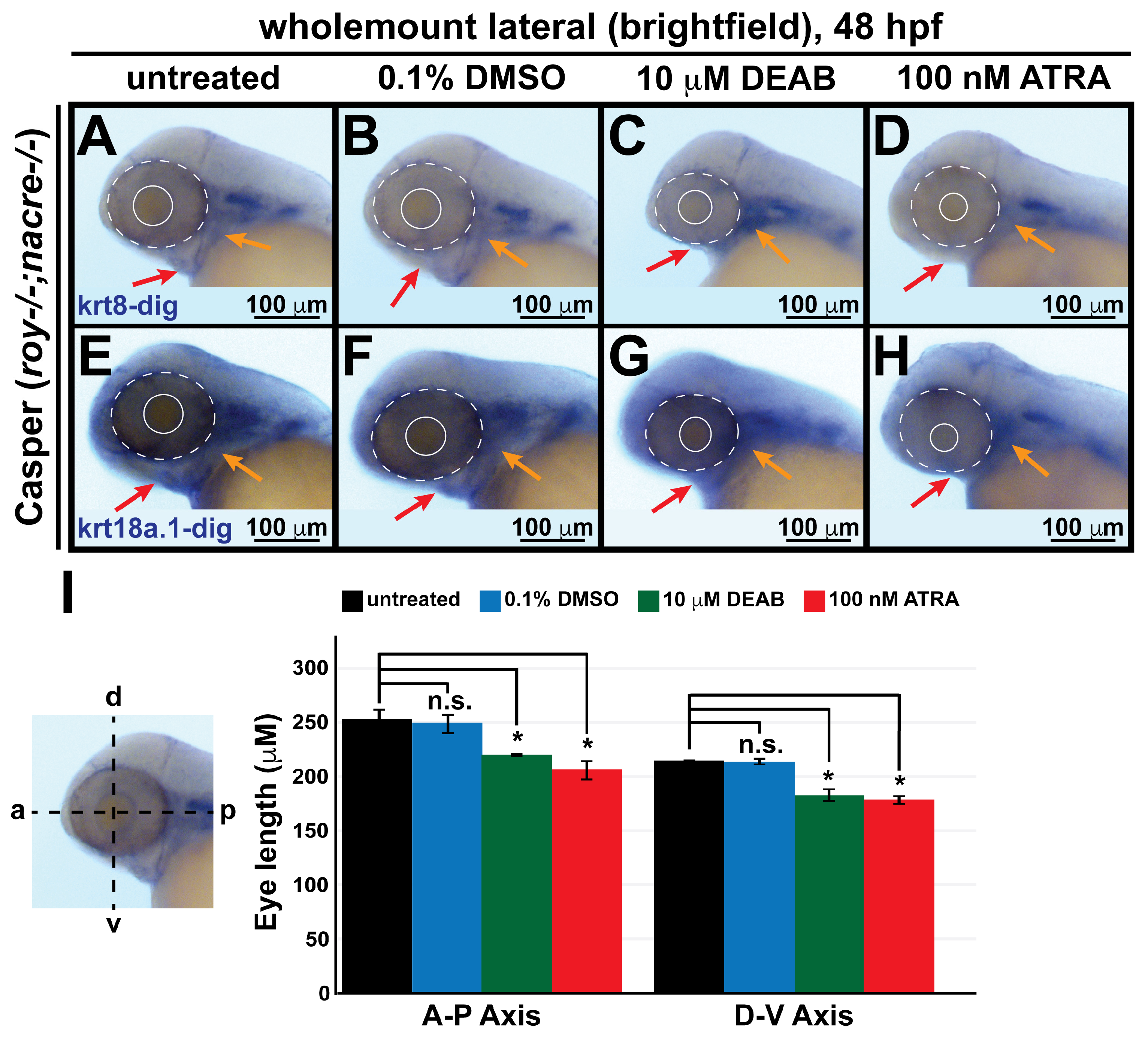
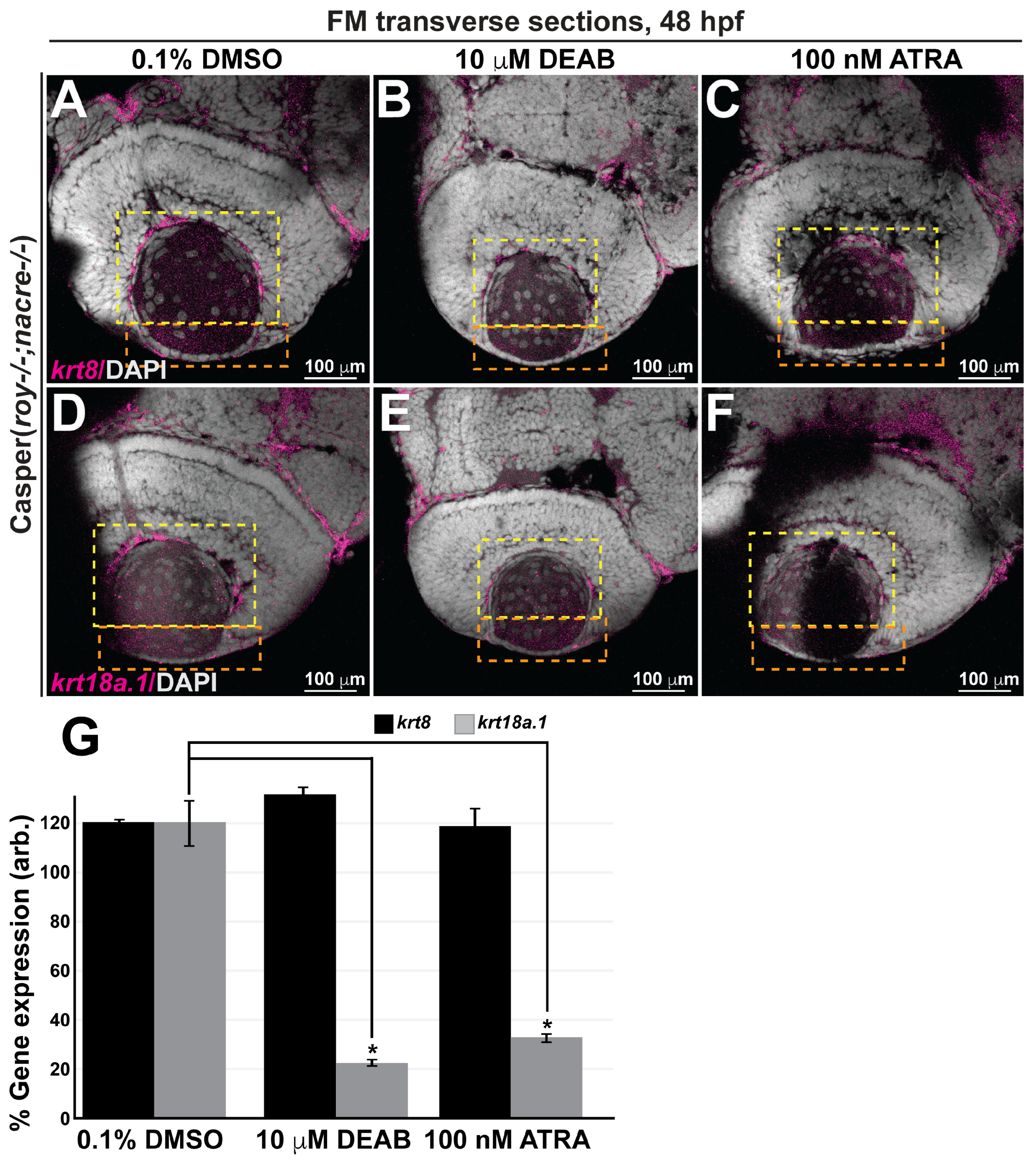
3.4. Retinoic Acid Regulates Keratin Intermediate Filament Gene Expression in the Ocular and Craniofacial Neural Crest during Embryonic Development
3.5. K8/18a.1 Is Expressed at Various Time Points during Postnatal Corneal Wound Healing
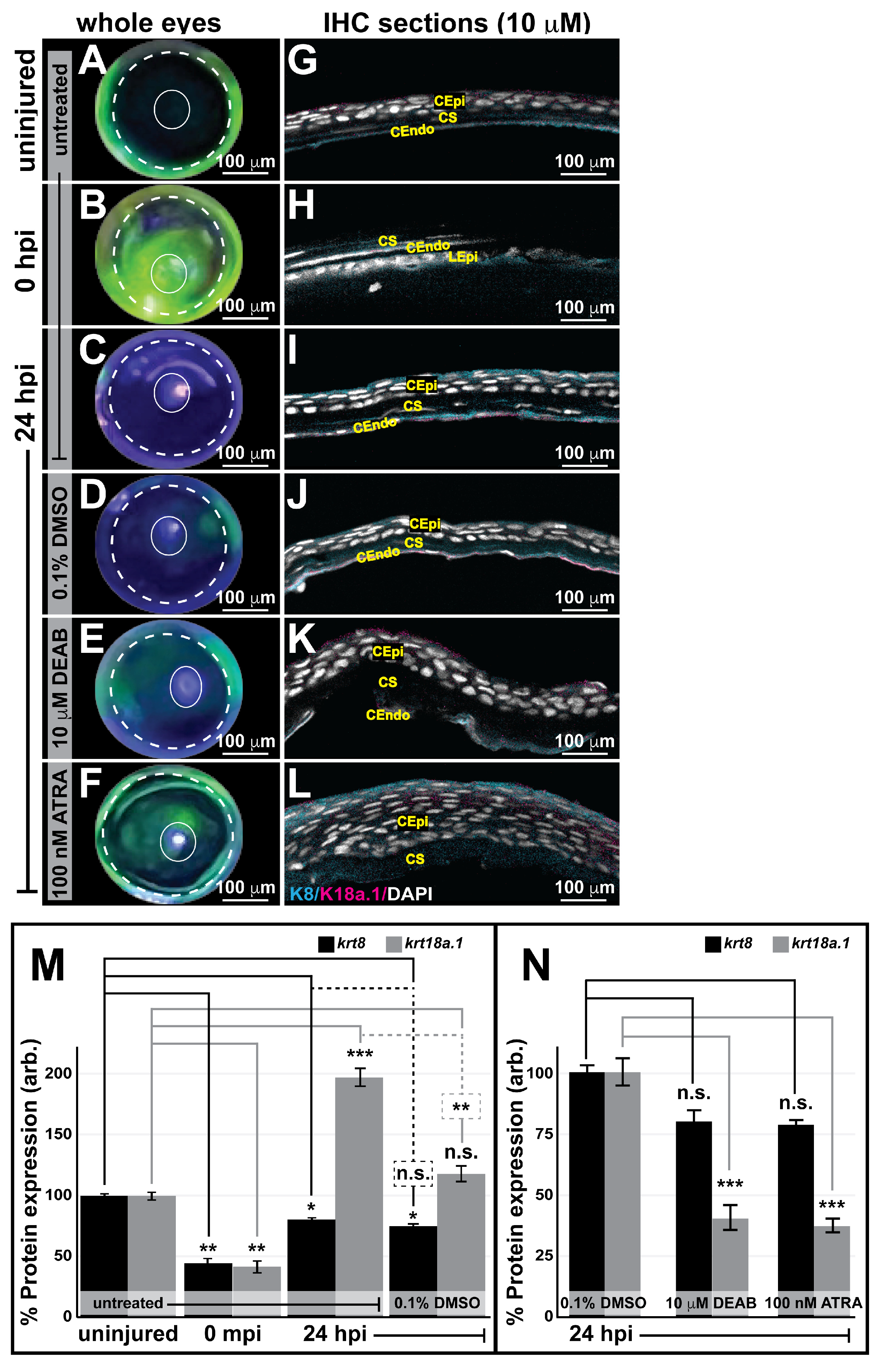
3.6. Retinoic Acid Regulates Corneal Regeneration and Alters K18a.1 Expression in the Adult Zebrafish Eye during Wound Healing
4. Discussion
4.1. Keratins as Hallmarks of Maturation in Neural Crest-Derived Ocular Tissues
4.2. Keratins as Cranial Neural Crest Cell Fate Signaling Molecules
4.3. Keratins as Downstream Targets of Retinoic Acid in the Ocular Anterior Segment
4.4. Keratins as Contributors to Corneal Wound Healing and Regeneration
4.4.1. Zebrafish Models of Corneal Regeneration and Injury Repair
4.4.2. Keratin Contributions to Corneal Wound Healing
4.4.3. Effects of Retinoic Acid on Keratin Expression during Wound Healing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menko, A.S.; Walker, J.L.; Stepp, M.A. Fibrosis: Shared Lessons From the Lens and Cornea. Anat. Rec. 2020, 303, 1689–1702. [Google Scholar] [CrossRef]
- Shu, D.Y.; Lovicu, F.J. Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog. Retin. Eye Res. 2017, 60, 44–65. [Google Scholar] [CrossRef]
- Stepp, M.A.; Menko, A.S. Immune responses to injury and their links to eye disease. Transl. Res. 2021, 236, 52–71. [Google Scholar] [CrossRef]
- Torricelli, A.A.; Santhanam, A.; Wu, J.; Singh, V.; Wilson, S.E. The corneal fibrosis response to epithelial-stromal injury. Exp. Eye Res. 2016, 142, 110–118. [Google Scholar] [CrossRef]
- Lahne, M.; Brecker, M.; Jones, S.E.; Hyde, D.R. The Regenerating Adult Zebrafish Retina Recapitulates Developmental Fate Specification Programs. Front. Cell Dev. Biol. 2020, 8, 617923. [Google Scholar] [CrossRef]
- Akula, M.; Park, J.W.; West-Mays, J.A. Relationship between neural crest cell specification and rare ocular diseases. J. Neurosci. Res. 2019, 97, 7–15. [Google Scholar] [CrossRef]
- Weigele, J.; Bohnsack, B.L. Genetics Underlying the Interactions between Neural Crest Cells and Eye Development. J. Dev. Biol. 2020, 8, 26. [Google Scholar] [CrossRef]
- Williams, A.L.; Bohnsack, B.L. Neural crest derivatives in ocular development: Discerning the eye of the storm. Birth Defects Res. Part C Embryo Today Rev. 2015, 105, 87–95. [Google Scholar] [CrossRef]
- Williams, A.L.; Bohnsack, B.L. The Ocular Neural Crest: Specification, Migration, and Then What? Front. Cell Dev. Biol. 2020, 8, 595896. [Google Scholar] [CrossRef]
- Liu, B.; Hunter, D.J.; Smith, A.A.; Chen, S.; Helms, J.A. The capacity of neural crest-derived stem cells for ocular repair. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 299–308. [Google Scholar] [CrossRef]
- Slack, J.M.W. Origin of Stem Cells in Organogenesis. Science 2008, 322, 1498–1501. [Google Scholar] [CrossRef]
- Cordero, D.R.; Brugmann, S.; Chu, Y.; Bajpai, R.; Jame, M.; Helms, J.A. Cranial neural crest cells on the move: Their roles in craniofacial development. Am. J. Med. Genet. Part A 2011, 155A, 270–279. [Google Scholar] [CrossRef]
- Kish, P.E.; Bohnsack, B.L.; Gallina, D.; Kasprick, D.S.; Kahana, A. The eye as an organizer of craniofacial development. Genesis 2011, 49, 222–230. [Google Scholar] [CrossRef]
- Parfejevs, V.; Antunes, A.T.; Sommer, L. Injury and stress responses of adult neural crest-derived cells. Dev. Biol. 2018, 444 (Suppl. S1), S356–S365. [Google Scholar] [CrossRef] [PubMed]
- West-Mays, J.A.; Dwivedi, D.J. The keratocyte: Corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol. 2006, 38, 1625–1631. [Google Scholar] [CrossRef]
- Baggiolini, A.; Varum, S.; Mateos, J.M.; Bettosini, D.; John, N.; Bonalli, M.; Ziegler, U.; Dimou, L.; Clevers, H.; Furrer, R.; et al. Premigratory and migratory neural crest cells are multipotent in vivo. Cell Stem Cell 2015, 16, 314–322. [Google Scholar] [CrossRef]
- Chawla, B.; Swain, W.; Williams, A.L.; Bohnsack, B.L. Retinoic Acid Maintains Function of Neural Crest-Derived Ocular and Craniofacial Structures in Adult Zebrafish. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1924–1935. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Bohnsack, B.L.; Gallina, D.; Kahana, A. Phenothiourea sensitizes zebrafish cranial neural crest and extraocular muscle development to changes in retinoic acid and IGF signaling. PLoS ONE 2011, 6, e22991. [Google Scholar] [CrossRef] [PubMed]
- Barthel, L.K.; Raymond, P.A. In situ hybridization studies of retinal neurons. Methods Enzymol. 2000, 316, 579–590. [Google Scholar] [PubMed]
- Kucenas, S.; Takada, N.; Park, H.-C.; Woodruff, E.; Broadie, K.; Appel, B. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat. Neurosci. 2008, 11, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.L.; Bohnsack, B.L. Zebrafish Model of Stickler Syndrome Suggests a Role for Col2a1a in the Neural Crest during Early Eye Development. J. Dev. Biol. 2022, 10, 42. [Google Scholar] [CrossRef]
- Ikkala, K.; Raatikainen, S.; Michon, F. Zebrafish Corneal Wound Healing: From Abrasion to Wound Closure Imaging Analysis. J. Vis. Exp. 2022, 181, e63605. [Google Scholar]
- Ikkala, K.; Stratoulias, V.; Michon, F. Unilateral zebrafish corneal injury induces bilateral cell plasticity supporting wound closure. Sci. Rep. 2022, 12, 161. [Google Scholar] [CrossRef]
- Creuzet, S.; Couly, G.; Le Douarin, N.M. Patterning the neural crest derivatives during development of the vertebrate head: Insights from avian studies. Am. J. Anat. 2005, 207, 447–459. [Google Scholar] [CrossRef]
- Drerup, C.M.; Wiora, H.M.; Topczewski, J.; Morris, J.A. Disc1 regulates foxd3 and sox10 expression, affecting neural crest migration and differentiation. Development 2009, 136, 2623–2632. [Google Scholar] [CrossRef]
- Dutton, K.A.; Pauliny, A.; Lopes, S.S.; Elworthy, S.; Carney, T.J.; Rauch, J.; Geisler, R.; Haffter, P.; Kelsh, R.N. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development 2001, 128, 4113–4125. [Google Scholar] [CrossRef]
- Kloss, B.A.V.; Reis, L.M.; Brémond-Gignac, D.; Glaser, T.; Semina, E.V. Analysis of FOXD3 sequence variation in human ocular disease. Mol. Vis. 2012, 18, 1740–1749. [Google Scholar]
- Langenberg, T.; Kahana, A.; Wszalek, J.A.; Halloran, M.C. The eye organizes neural crest cell migration. Dev. Dyn. 2008, 237, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Lister, J.A.; Cooper, C.; Nguyen, K.; Modrell, M.; Grant, K.; Raible, D.W. Zebrafish Foxd3 is required for development of a subset of neural crest derivatives. Dev. Biol. 2006, 290, 92–104. [Google Scholar] [CrossRef]
- Stewart, R.A.; Arduini, B.L.; Berghmans, S.; George, R.E.; Kanki, J.P.; Henion, P.D.; Look, A.T. Zebrafish foxd3 is selectively required for neural crest specification, migration and survival. Dev. Biol. 2006, 292, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meulen, K.L.; Vöcking, O.; Weaver, M.L.; Meshram, N.N.; Famulski, J.K. Spatiotemporal Characterization of Anterior Segment Mesenchyme Heterogeneity During Zebrafish Ocular Anterior Segment Development. Front. Cell Dev. Biol. 2020, 8, 379. [Google Scholar] [CrossRef]
- Wang, W.-D.; Melville, D.B.; Montero-Balaguer, M.; Hatzopoulos, A.K.; Knapik, E.W. Tfap2a and Foxd3 regulate early steps in the de-velopment of the neural crest progenitor population. Dev. Biol. 2011, 360, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Vöcking, O.; Van Der Meulen, K.; Patel, M.; Famulski, J. Zebrafish anterior segment mesenchyme progenitors are defined by function of tfap2a but not sox10. Differentiation 2023, 130, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Jacob, J.T.; Coulombe, P.A.; Kwan, R.; Omary, M.B. Types I and II Keratin Intermediate Filaments. Cold Spring Harb. Perspect. Biol. 2018, 10, a018275. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef]
- Ndiaye, A.-B.; Koenderink, G.H.; Shemesh, M. Intermediate Filaments in Cellular Mechanoresponsiveness: Mediating Cy-toskeletal Crosstalk From Membrane to Nucleus and Back. Front. Cell Dev. Biol. 2022, 10, 882037. [Google Scholar] [CrossRef]
- van Bodegraven, E.J.; Etienne-Manneville, S. Etienne-Manneville, Intermediate Filaments from Tissue Integrity to Single Molecule Mechanics. Cells 2021, 10, 1905. [Google Scholar] [CrossRef] [PubMed]
- García, D.M.; Bauer, H.; Dietz, T.; Schubert, T.; Markl, J.; Schaffeld, M. Identification of keratins and analysis of their expression in carp and goldfish: Comparison with the zebrafish and trout keratin catalog. Cell Tissue Res. 2005, 322, 245–256. [Google Scholar] [CrossRef]
- Pilon, N. Treatment and Prevention of Neurocristopathies. Trends Mol. Med. 2021, 27, 451–468. [Google Scholar] [CrossRef]
- Sato, T.S.; Handa, A.; Priya, S.; Watal, P.; Becker, R.M.; Sato, Y. Neurocristopathies: Enigmatic Appearances of Neural Crest Cell–derived Abnormalities. RadioGraphics 2019, 39, 2085–2102. [Google Scholar] [CrossRef]
- Watt, K.E.N.; Trainor, P.A. Neurocristopathies: The etiology and pathogenesis of disorders arising from defects in neural crest cell development. In Neural Crest Cells; Elsevier: Amsterdam, The Netherlands, 2014; pp. 361–394. [Google Scholar]
- Berenguer, M.; Duester, G. Retinoic acid, RARs and early development. J. Mol. Endocrinol. 2022, 69, T59–T67. [Google Scholar] [CrossRef] [PubMed]
- Chawla, B.; Schley, E.; Williams, A.L.; Bohnsack, B.L. Retinoic Acid and Pitx2 Regulate Early Neural Crest Survival and Migration in Craniofacial and Ocular Development. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2016, 107, 126–135. [Google Scholar] [CrossRef]
- Cvekl, A.; Wang, W.-L. Retinoic acid signaling in mammalian eye development. Exp. Eye Res. 2009, 89, 280–291. [Google Scholar] [CrossRef]
- Duester, G. Towards a Better Vision of Retinoic Acid Signaling during Eye Development. Cells 2022, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.L.; Bohnsack, B.L. What’s retinoic acid got to do with it? Retinoic acid regulation of the neural crest in crani-ofacial and ocular development. Genesis 2019, 57, e23308. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Garavaglia, S.; Ye, Z.; Moretti, A.; Belyaeva, O.V.; Beiser, A.; Ibrahim, M.; Wilk, A.; McClellan, S.; Klyuyeva, A.V.; et al. A specific inhibitor of ALDH1A3 regulates retinoic acid biosynthesis in glioma stem cells. Commun. Biol. 2021, 4, 1420. [Google Scholar] [CrossRef]
- Bohnsack, B.L.; Kasprick, D.S.; Kish, P.E.; Goldman, D.; Kahana, A. A zebrafish model of axenfeld-rieger syndrome reveals that pitx2 regulation by retinoic acid is essential for ocular and craniofacial development. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.E.; Dechat, T.; Grin, B.; Helfand, B.; Mendez, M.; Pallari, H.-M.; Goldman, R.D. Introducing intermediate filaments: From discovery to disease. J. Clin. Investig. 2009, 119, 1763–1771. [Google Scholar] [CrossRef]
- Herrmann, H.; Bär, H.; Kreplak, L.; Strelkov, S.V.; Aebi, U. Intermediate filaments: From cell architecture to nanomechanics. Nat. Rev. Mol. Cell Biol. 2007, 8, 562–573. [Google Scholar] [CrossRef]
- Infante, E.; Etienne-Manneville, S. Intermediate filaments: Integration of cell mechanical properties during migration. Front. Cell Dev. Biol. 2022, 10, 951816. [Google Scholar] [CrossRef]
- Omary, M.B.; Coulombe, P.A.; McLean, W.I. Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 2004, 351, 2087–2100. [Google Scholar] [CrossRef]
- Pollard, T.D.; Goldman, R.D. Overview of the Cytoskeleton from an Evolutionary Perspective. Cold Spring Harb. Perspect. Biol. 2018, 10, a030288. [Google Scholar] [CrossRef] [PubMed]
- Collomb, E.; Yang, Y.; Foriel, S.; Cadau, S.; Pearton, D.J.; Dhouailly, D. The corneal epithelium and lens develop independently from a common pool of precursors. Dev. Dyn. 2013, 242, 401–413. [Google Scholar] [CrossRef]
- Dhouailly, D.; Pearton, D.J.; Michon, F. The vertebrate corneal epithelium: From early specification to constant renewal. Dev. Dyn. 2014, 243, 1226–1241. [Google Scholar] [CrossRef]
- Kalha, S.; Kuony, A.; Michon, F. Corneal Epithelial Abrasion with Ocular Burr As a Model for Cornea Wound Healing. J. Vis. Exp. 2018, 137, 58071. [Google Scholar]
- Richardson, A.; Lobo, E.P.; Delic, N.C.; Myerscough, M.R.; Lyons, J.G.; Wakefield, D.; Di Girolamo, N. Keratin-14-Positive Precursor Cells Spawn a Population of Migratory Corneal Epithelia that Maintain Tissue Mass throughout Life. Stem Cell Rep. 2017, 9, 1081–1096. [Google Scholar] [CrossRef]
- Erickson, C.A.; Tucker, R.P.; Edwards, B.F. Changes in the distribution of intermediate-filament types in Japanese quail embryos during morphogenesis. Differentiation 1987, 34, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Kuburich, N.A.; Hollander, P.D.; Pietz, J.T.; Mani, S.A. Vimentin and cytokeratin: Good alone, bad together. Semin. Cancer Biol. 2022, 86 Pt 3, 816–826. [Google Scholar] [CrossRef]
- Sha, Y.; Haensel, D.; Gutierrez, G.; Du, H.; Dai, X.; Nie, Q. Intermediate cell states in epithelial-to-mesenchymal transition. Phys. Biol. 2019, 16, 021001. [Google Scholar] [CrossRef] [PubMed]
- Katagata, Y.; Aoki, T.; Kondo, S.; Kawa, Y.; Mizoguchi, M. Keratin subunit expression in human cultured melanocytes and mouse neural crest cells without formation of filamentous structures. J. Investig. Dermatol. Symp. Proc. 1999, 4, 110–115. [Google Scholar] [CrossRef]
- Katagata, Y.; Aoki, T.; Kondo, S. Detecting expression of keratins 8/18 in human HaCaT keratinocytes. J. Dermatol. Sci. 1999, 19, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Katagata, Y.; Kondo, S. The Correlation of the metastatic ability with keratin expression in cultured murine melanoma cell lines, B16-F1 and -F10. J. Dermatol. 1999, 26, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Cockerham, G.C.; Laver, N.V.; Hidayat, A.A.; McCoy, D.L. An immunohistochemical analysis and comparison of posterior polymorphous dystrophy with con-genital hereditary endothelial dystrophy. Cornea 2002, 21, 787–791. [Google Scholar] [CrossRef]
- Hidayat, A.A.; Cockerham, G.C. Epithelial metaplasia of the corneal endothelium in fuchs endothelial dystrophy. Cornea 2006, 25, 956–959. [Google Scholar] [CrossRef]
- Jirsova, K.; Merjava, S.; Martincova, R.; Gwilliam, R.; Ebenezer, N.D.; Liskova, P.; Filipec, M. Immunohistochemical characterization of cytokeratins in the abnormal corneal endothelium of posterior polymorphous corneal dystrophy patients. Exp. Eye Res. 2007, 84, 680–686. [Google Scholar] [CrossRef]
- Merjava, S.; Neuwirth, A.; Mandys, V.; Jirsova, K. Cytokeratins 8 and 18 in adult human corneal endothelium. Exp. Eye Res. 2009, 89, 426–431. [Google Scholar] [CrossRef]
- Creuzet, S.; Vincent, C.; Couly, G. Neural crest derivatives in ocular and periocular structures. Int. J. Dev. Biol. 2005, 49, 161–171. [Google Scholar] [CrossRef]
- Cvekl, A.; Tamm, E.R. Anterior eye development and ocular mesenchyme: New insights from mouse models and human diseases. BioEssays News Rev. Mol. Cell. Dev. Biol. 2004, 26, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, S. Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol. 2010, 93, 61–84. [Google Scholar]
- Gage, P.J.; Rhoades, W.; Prucka, S.K.; Hjalt, T. Fate maps of neural crest and mesoderm in the mammalian eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4200–4208. [Google Scholar] [CrossRef]
- Eason, J.; Williams, A.L.; Chawla, B.; Apsey, C.; Bohnsack, B.L. Differences in neural crest sensitivity to ethanol account for the infrequency of anterior segment defects in the eye compared with craniofacial anomalies in a zebrafish model of fetal alcohol syndrome. Birth Defects Res. 2017, 109, 1212–1227. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, M.; Stegmaier, J.; Kobitski, A.Y.; Schott, B.; Weger, B.D.; Margariti, D.; Cereceda Delgado, A.R.; Gourain, V.; Scherr, T.; Yang, L.; et al. Pax6 organizes the anterior eye segment by guiding two distinct neural crest waves. PLoS Genet. 2020, 16, e1008774. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.G.; Alvarez, Y.D.; Gasnier, M.; Wang, Y.; Tetlak, P.; Bissiere, S.; Wang, H.; Biro, M.; Plachta, N. Keratins are asymmetrically inherited fate determinants in the mammalian embryo. Nature 2020, 585, 404–409. [Google Scholar] [CrossRef]
- Discher, D.; Dong, C.; Fredberg, J.J.; Guilak, F.; Ingber, D.; Janmey, P.; Kamm, R.D.; Schmid-Schönbein, G.W.; Weinbaum, S. Biomechanics: Cell research and applications for the next decade. Ann. Biomed. Eng. 2009, 37, 847–859. [Google Scholar] [CrossRef]
- Bohnsack, B.L.; Kahana, A. Thyroid hormone and retinoic acid interact to regulate zebrafish craniofacial neural crest development. Dev. Biol. 2013, 373, 300–309. [Google Scholar] [CrossRef]
- Duester, G. Retinoic acid synthesis and Signaling during early organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef]
- Duester, G. Keeping an eye on retinoic acid signaling during eye development. Chem. Biol. Interact. 2009, 178, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, dev167502. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.N.; Walker, H.M.; Thompson, H.; Collinson, J.M.; Vargesson, N.; Erskine, L. Lens-regulated retinoic acid signalling controls expansion of the developing eye. Development 2018, 145, dev167171. [Google Scholar] [CrossRef]
- Balmer, J.E.; Blomhoff, R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002, 43, 1773–1808. [Google Scholar] [CrossRef]
- Törmä, H. Regulation of keratin expression by retinoids. Dermato-Endocrinol. 2011, 3, 136–140. [Google Scholar] [CrossRef]
- Aldehlawi, H.; Usman, S.; Lalli, A.; Ahmad, F.; Williams, G.; Teh, M.-T.; Waseem, A. Serum lipids, retinoic acid and phenol red differentially regulate expression of keratins K1, K10 and K2 in cultured keratinocytes. Sci. Rep. 2020, 10, 4829. [Google Scholar] [CrossRef] [PubMed]
- Giguère, V.; Evans, R.M. Chronicle of a discovery: The retinoic acid receptor. J. Mol. Endocrinol. 2022, 69, T1–T11. [Google Scholar] [CrossRef]
- Jiang, C.-K.; Epstein, H.; Tomic, M.; Freedberg, I.; Blumenberg, M. Epithelial-specific keratin gene expression: Identification of a 300 base-pair controlling segment. Nucleic Acids Res. 1990, 18, 247–253. [Google Scholar] [CrossRef]
- Lee, D.; Stojadinovic, O.; Krzyzanowska, A.; Vouthounis, C.; Blumenberg, M.; Tomic-Canic, M. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J. Cell. Physiol. 2009, 220, 427–439. [Google Scholar] [CrossRef]
- Ohtsuki, M.; Tomic-Canic, M.; Freedberg, I.M.; Blumenberg, M. Regulation of epidermal keratin expression by retinoic acid and thyroid hormone. J. Dermatol. 1992, 19, 774–780. [Google Scholar] [CrossRef]
- Ohtsuki, M.; Tomic-Canic, M.; Freedberg, I.M.; Blumenberg, M. Nuclear proteins involved in Transcription of the human K5 keratin gene. J. Investig. Dermatol. 1992, 99, 206–215. [Google Scholar] [CrossRef]
- Tomic, M.; Jiang, C.K.; Epstein, H.S.; Freedberg, I.M.; Samuels, H.H.; Blumenberg, M. Nuclear receptors for retinoic acid and thyroid hormone regulate transcription of keratin genes. Cell Regul. 1990, 1, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, S.L.; Nikapitiya, C.; Dananjaya, S.H.S.; Park, J.; Kim, D.; Choi, D.; De Zoysa, M. Effect of Polydeoxyribonucleotide (PDRN) Treatment on Corneal Wound Healing in Zebrafish (Danio rerio). Int. J. Mol. Sci. 2022, 23, 13525. [Google Scholar] [CrossRef] [PubMed]
- Heur, M.; Jiao, S.; Schindler, S.; Crump, J.G. Regenerative potential of the zebrafish corneal endothelium. Exp. Eye Res. 2013, 106, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Devi, S.; Lee, M.-S.; Jui, J.; Sahu, A.; Goldman, D. Vegf signaling between Müller glia and vascular endothelial cells is regulated by immune cells and stimulates retina regeneration. Proc. Natl. Acad. Sci. USA 2022, 119, e2211690119. [Google Scholar] [CrossRef]
- Poss, K.D.; Keating, M.T.; Nechiporuk, A. Tales of regeneration in zebrafish. Dev. Dyn. 2003, 226, 202–210. [Google Scholar] [CrossRef]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef]
- Vihtelic, T.S.; Hyde, D.R. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J. Neurobiol. 2000, 44, 289–307. [Google Scholar] [CrossRef]
- Wan, J.; Goldman, D. Retina regeneration in zebrafish. Curr. Opin. Genet. Dev. 2016, 40, 41–47. [Google Scholar] [CrossRef]
- Bukowiecki, A.; Hos, D.; Cursiefen, C.; Eming, S.A. Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities. Int. J. Mol. Sci. 2017, 18, 1257. [Google Scholar] [CrossRef]
- Kamil, S.; Mohan, R.R. Corneal stromal wound healing: Major regulators and therapeutic targets. Ocul. Surf. 2021, 19, 290–306. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, X.; Pan, B.; Zheng, C.; Hong, L.; Han, W. KRT8 Serves as a Novel Biomarker for LUAD and Promotes Metastasis and EMT via NF-kappaB Signaling. Front. Oncol. 2022, 12, 875146. [Google Scholar]
- Tan, H.-S.; Jiang, W.-H.; He, Y.; Wang, D.-S.; Wu, Z.-J.; Wu, D.-S.; Gao, L.; Bao, Y.; Shi, J.-Z.; Liu, B.; et al. KRT8 upregulation promotes tumor metastasis and is predictive of a poor prognosis in clear cell renal cell carcinoma. Oncotarget 2017, 8, 76189–76203. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Dang, Y.; Guo, J.; Sun, X.; Xie, T.; Zhang, L.; Yan, Z.; Amin, H.; Guo, X. High KRT8 Expression Independently Predicts Poor Prognosis for Lung Adenocarcinoma Patients. Genes 2019, 10, 36. [Google Scholar] [CrossRef]
- Chen, B.; Xu, X.; Lin, D.-D.; Chen, X.; Xu, Y.-T.; Liu, X.; Dong, W.-G. KRT18 Modulates Alternative Splicing of Genes Involved in Proliferation and Apoptosis Processes in Both Gastric Cancer Cells and Clinical Samples. Front. Genet. 2021, 12, 635429. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y.; Ding, G.; Du, H.; Fan, H. Keratin 18 induces proliferation, migration, and invasion in gastric cancer via the MAPK signalling pathway. Clin. Exp. Pharmacol. Physiol. 2020, 48, 147–156. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, S.; Li, Y. KRT18 is correlated with the malignant status and acts as an oncogene in colorectal cancer. Biosci. Rep. 2019, 39, BSR20190884. [Google Scholar] [CrossRef]
- Blum, N.; Begemann, G. Retinoic acid signaling controls the formation, proliferation and survival of the blastema during adult zebrafish fin regeneration. Development 2012, 139, 107–116. [Google Scholar] [CrossRef]
- De La Rosa-Reyes, V.; Duprey-Díaz, M.V.; Blagburn, J.M.; Blanco, R.E. Retinoic acid treatment recruits macrophages and increases axonal regeneration after optic nerve injury in the frog Rana pipiens. PLoS ONE 2021, 16, e0255196. [Google Scholar] [CrossRef]
- Kam, R.K.T.; Deng, Y.; Chen, Y.; Zhao, H. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci. 2012, 2, 11. [Google Scholar]
- Kartasheva-Ebertz, D.M.; Pol, S.; Lagaye, S. Retinoic Acid: A New Old Friend of IL-17A in the Immune Pathogeny of Liver Fi-brosis. Front. Immunol. 2021, 12, 691073. [Google Scholar] [CrossRef]
- Kikuchi, K.; Holdway, J.E.; Major, R.J.; Blum, N.; Dahn, R.D.; Begemann, G.; Poss, K.D. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 2011, 20, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Chen, R.; Sheu, M.; Kim, N.; Kim, S.; Islam, N.; Wier, E.M.; Wang, G.; Li, A.; Park, A.; et al. Noncoding dsRNA induces retinoic acid synthesis to stimulate hair follicle regeneration via TLR3. Nat. Commun. 2019, 10, 2811. [Google Scholar] [CrossRef]
- Maden, M. The role of retinoic acid in embryonic and post-embryonic development. Proc. Nutr. Soc. 2000, 59, 65–73. [Google Scholar] [CrossRef]
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Maden, M.; Hind, M. Retinoic acid, a regeneration-inducing molecule. Dev. Dyn. 2003, 226, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Mammadova, A.; Zhou, H.; Carels, C.E.; Hoff, J.W.V.D. Retinoic acid signalling in the development of the epidermis, the limbs and the secondary palate. Differentiation 2016, 92, 326–335. [Google Scholar] [CrossRef]
- McCaffery, P.J.; Adams, J.; Maden, M.; Rosa-Molinar, E. Too much of a good thing: Retinoic acid as an endogenous regulator of neural differentiation and ex-ogenous teratogen. Eur. J. Neurosci. 2003, 18, 457–472. [Google Scholar] [CrossRef]
- Puttagunta, R.; Di Giovanni, S. Retinoic acid signaling in axonal regeneration. Front. Mol. Neurosci. 2011, 4, 59. [Google Scholar] [CrossRef]
- Thompson, B.; Katsanis, N.; Apostolopoulos, N.; Thompson, D.C.; Nebert, D.W.; Vasiliou, V. Genetics and functions of the retinoic acid pathway, with special emphasis on the eye. Hum. Genom. 2019, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Azar, D.T. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 264–302. [Google Scholar] [PubMed]
- Saika, S.; Ikeda, K.; Yamanaka, O.; Flanders, K.C.; Okada, Y.; Miyamoto, T.; Kitano, A.; Ooshima, A.; Nakajima, Y.; Ohnishi, Y.; et al. Loss of tumor necrosis factor alpha potentiates transforming growth factor beta-mediated pathogenic tissue response during wound healing. Am. J. Pathol. 2006, 168, 1848–1860. [Google Scholar] [CrossRef]
- Saika, S.; Yamanaka, O.; Okada, Y.; Miyamoto, T.; Kitano, A.; Flanders, K.C.; Ohnishi, Y.; Nakajima, Y.; Kao, W.W.-Y.; Ikeda, K. Effect of overexpression of PPARgamma on the healing process of corneal alkali burn in mice. Am. J. Physiol.-Cell Physiol. 2007, 293, C75–C86. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, A.L.; Bohnsack, B.L. Keratin 8/18a.1 Expression Influences Embryonic Neural Crest Cell Dynamics and Contributes to Postnatal Corneal Regeneration in Zebrafish. Cells 2024, 13, 1473. https://doi.org/10.3390/cells13171473
Williams AL, Bohnsack BL. Keratin 8/18a.1 Expression Influences Embryonic Neural Crest Cell Dynamics and Contributes to Postnatal Corneal Regeneration in Zebrafish. Cells. 2024; 13(17):1473. https://doi.org/10.3390/cells13171473
Chicago/Turabian StyleWilliams, Antionette L., and Brenda L. Bohnsack. 2024. "Keratin 8/18a.1 Expression Influences Embryonic Neural Crest Cell Dynamics and Contributes to Postnatal Corneal Regeneration in Zebrafish" Cells 13, no. 17: 1473. https://doi.org/10.3390/cells13171473




