The Effect of Synthetic Polyamine BPA-C8 on the Fertilization Process of Intact and Denuded Sea Urchin Eggs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gamete Preparation and Fertilization
2.2. Visualization of the Egg-Incorporated Sperm
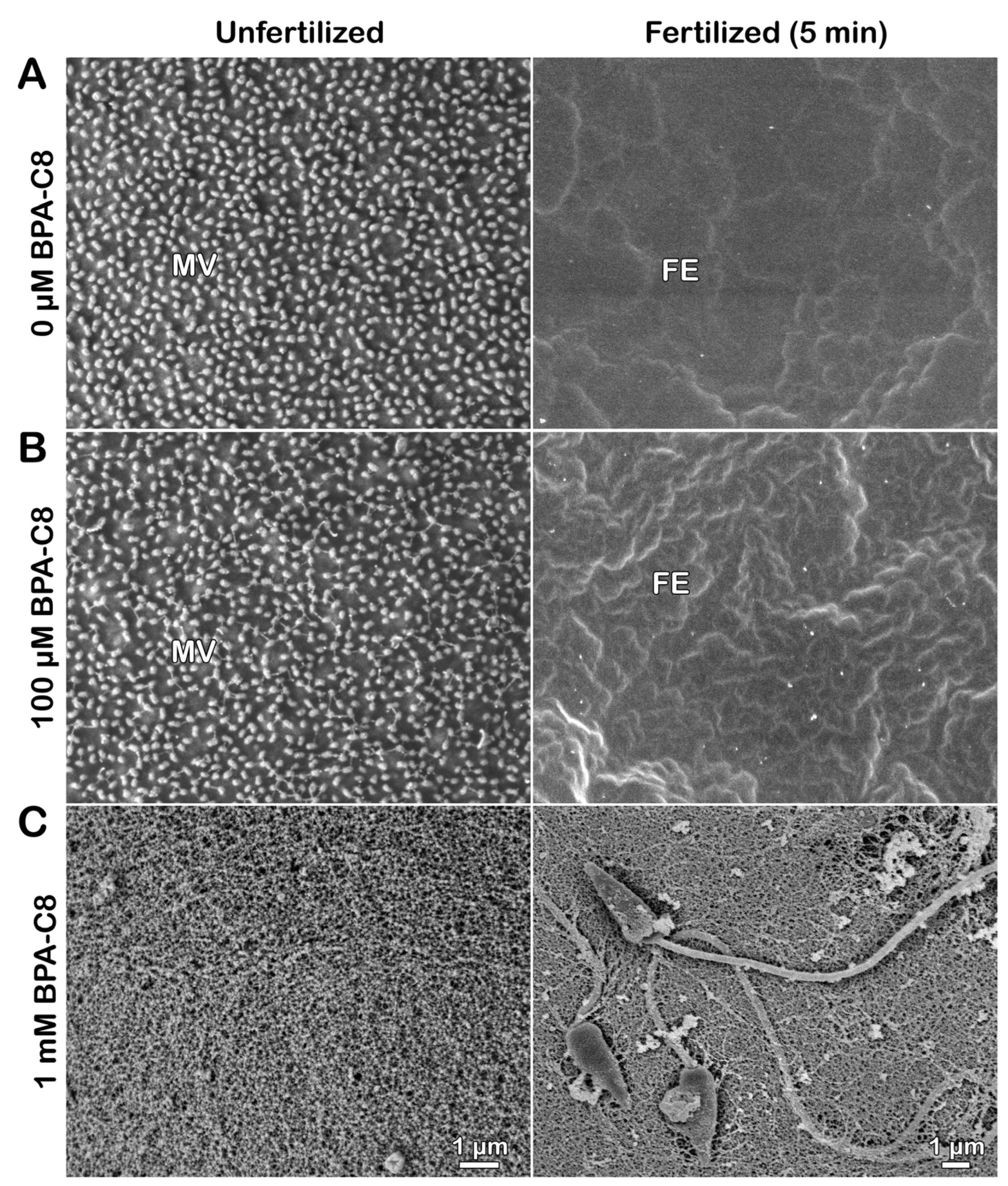
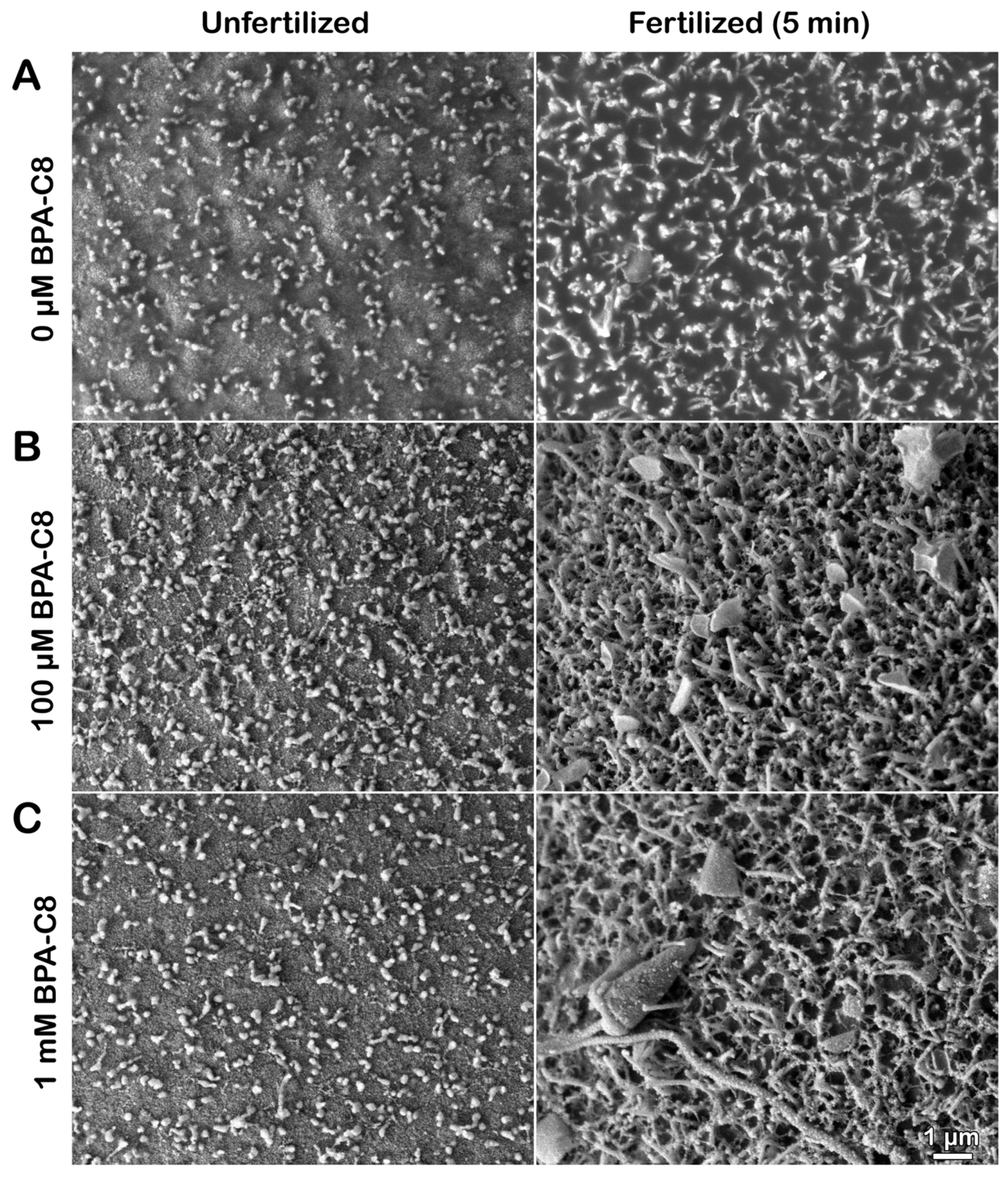
2.3. Scanning Electron Microscopy (SEM)
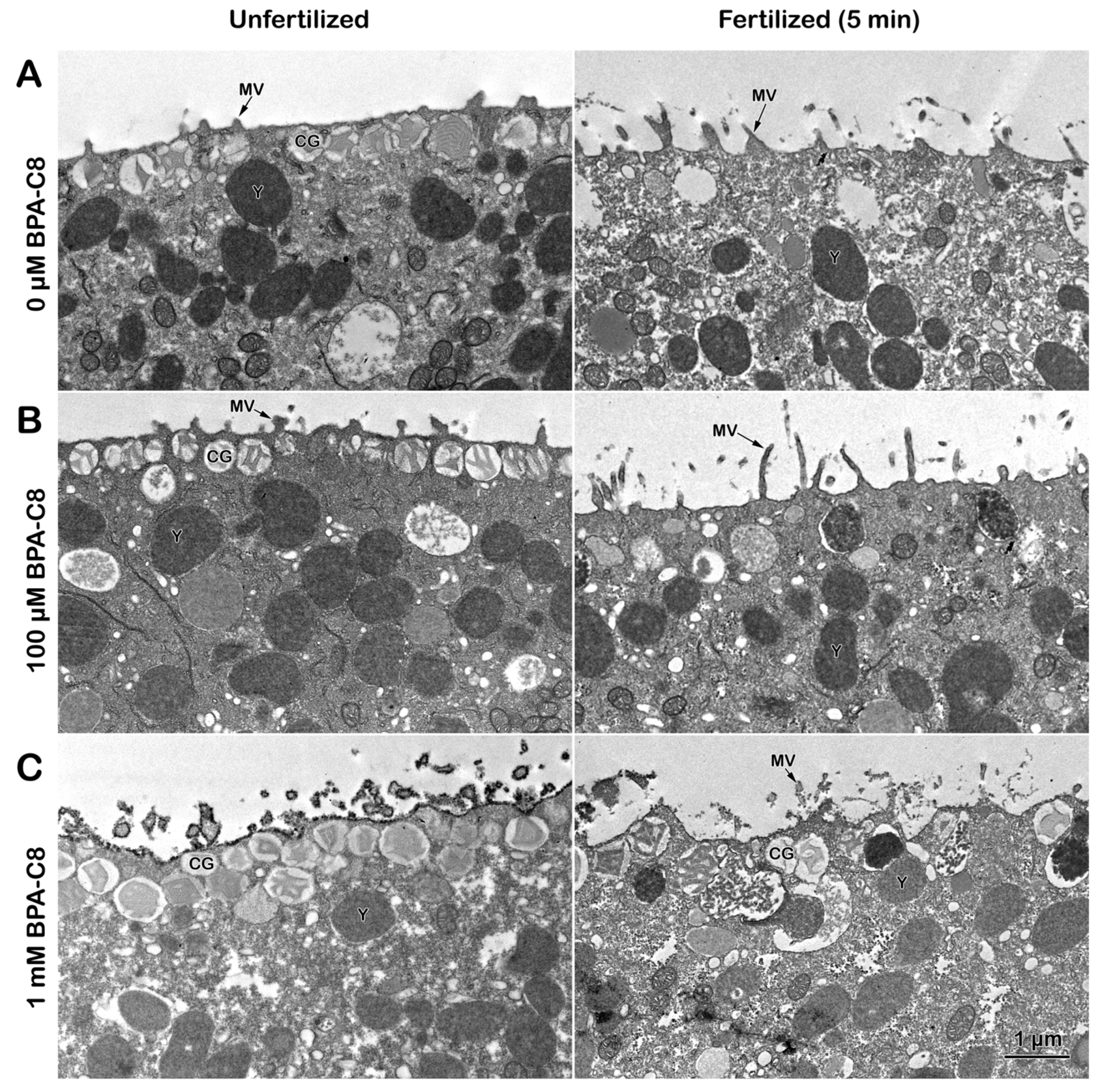
2.4. Transmission Electron Microscopy (TEM)
2.5. Chemicals, Reagents, and Recombinant Proteins
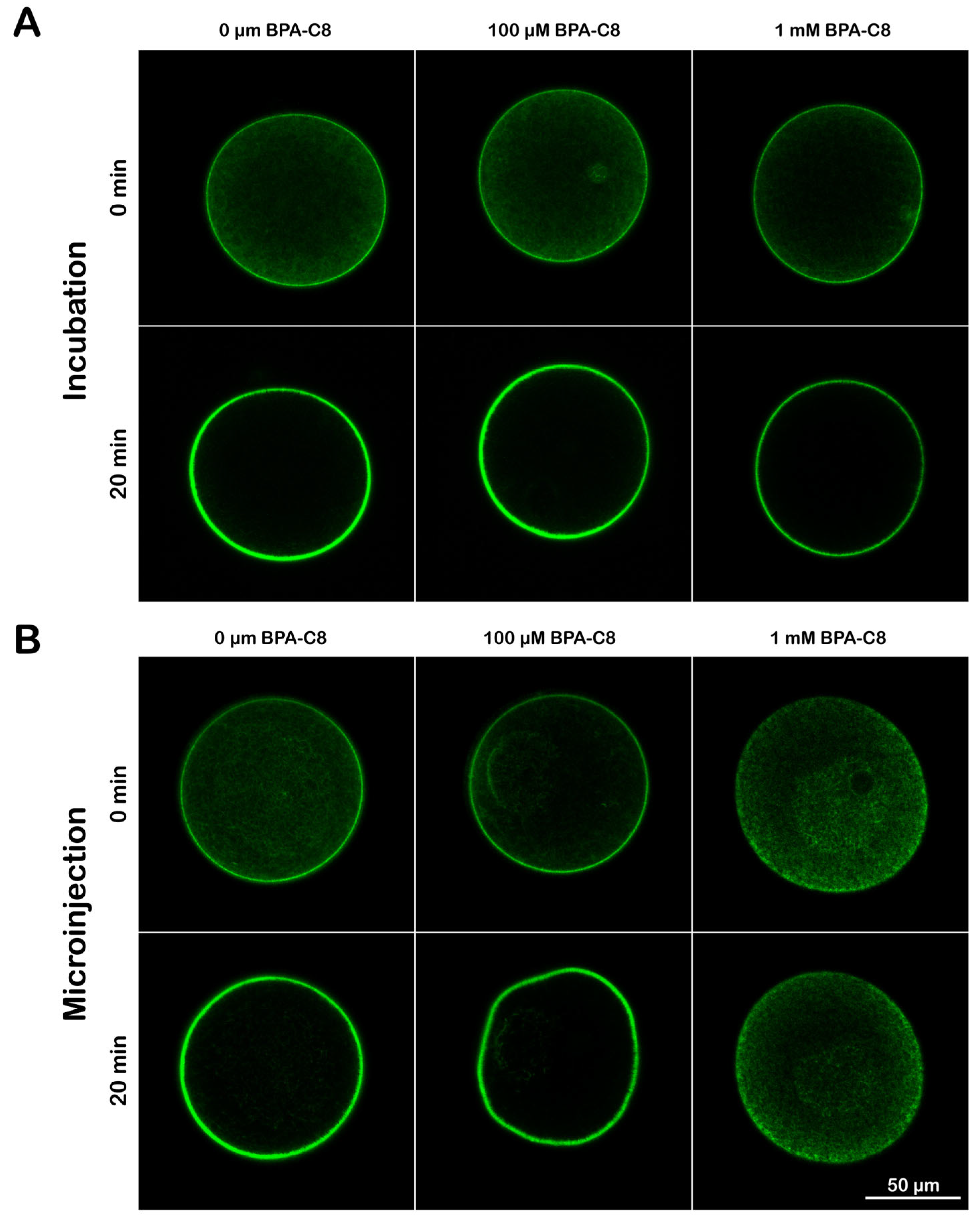
2.6. Microinjection, Ca2+ Imaging, and Confocal Microscopy
2.7. Visualization of Actin Filaments
2.8. Statistical Analysis
3. Results
3.1. Effect of BPA-C8 on the Surface of Unfertilized and Fertilized Sea Urchin Eggs Visualized with Electron Microscopy
3.2. Effect of BPA-C8 on the Denuded Eggs of P. lividus Visualized with Electron Microscopy
3.3. Structure of the Actin Cytoskeleton in Sea Urchin Eggs Treated with BPA-C8
3.4. Effect of BPA-C8 on Actin Dynamics in Sea Urchin Eggs
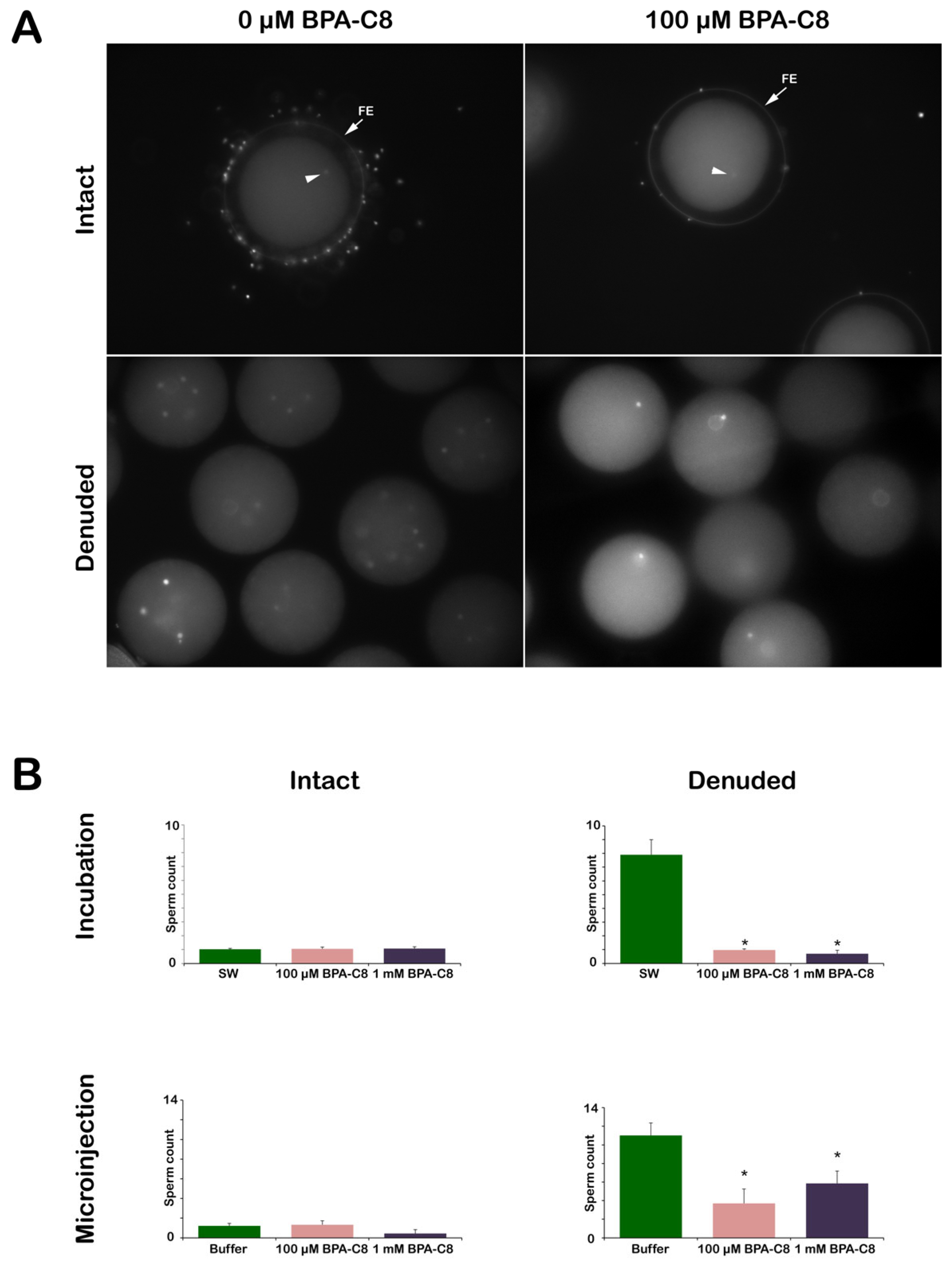
3.5. Effect of BPA-C8 on Sperm Entry into the Intact and Denuded Eggs
3.6. Effect of BPA-C8 on Ca2+ Signals in Intact and Denuded Eggs at Fertilization
3.7. Sperm Entry during the Fertilization Envelope Elevation at the Early Fertilization Stage in Sea Urchin Eggs
4. Discussion
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishihara, K. Chemical analysis of glycoproteins in the egg surface of the sea urchin, Arbacia punctulata. Biol. Bull. 1968, 134, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Hagström, B.E. Further experiments on jelly-free sea urchin eggs. Exp. Cell Res. 1959, 17, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Byrd, E.W.; Collins, F.D. Absence of fast block to polyspermy in eggs of sea urchin Strongylocentrotus purpuratus. Nature 1975, 257, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.; Monroy, A. How is polyspermy prevented? Gamete Res. 1981, 4, 151–169. [Google Scholar] [CrossRef]
- Farley, G.S.; Levitan, D.R. The role of jelly coats in sperm-egg encounters, fertilization success, and selection on egg size in broadcast spawners. Am. Nat. 2001, 157, 626–636. [Google Scholar] [CrossRef]
- Nakano, E.; Ohashi, S. On the carbohydrate component of the jelly coat and related substances of eggs from Japanese sea urchins. Embryologia 1954, 2, 81–85. [Google Scholar] [CrossRef]
- SeGall, G.K.; Lennarz, W.J. Chemical characterization of the component of the jelly coat from sea urchin eggs responsible for induction of the acrosome reaction. Dev. Biol. 1979, 71, 33–48. [Google Scholar] [CrossRef]
- Bonnell, B.S.; Keller, S.H.; Vacquier, V.D.; Chandler, D.E. The sea urchin egg jelly coat consists of globular glycoproteins bound to a fibrous fucan superstructure. Dev. Biol. 1994, 162, 313–324. [Google Scholar] [CrossRef]
- Ward, G.E.; Brokaw, C.J.; Garbers, D.L.; Vacquier, V.D. Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J. Cell Biol. 1985, 101, 2324–2329. [Google Scholar] [CrossRef]
- Ramarao, C.S.; Garbers, D.L. Receptor-mediated regulation of guanylate cyclase activity in spermatozoa. J. Biol. Chem. 1985, 260, 8390–8396. [Google Scholar] [CrossRef]
- Metz, E.C.; Kane, R.E.; Yanagimachi, H.; Palumbi, S.R. Fertilization Between Closely Related Sea Urchins Is Blocked by Incompatibilities During Sperm-Egg Attachment and Early Stages of Fusion. Biol. Bull. 1994, 187, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Ohlendieck, K.; Lennarz, W.J. Molecular mechanisms of gamete recognition in sea urchin fertilization. Curr. Top. Dev. Biol. 1996, 32, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Mah, S.A.; Swanson, W.J.; Vacquier, V.D. Positive selection in the carbohydrate recognition domains of sea urchin sperm receptor for egg jelly (suREJ) proteins. Mol. Biol. Evol. 2005, 22, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Vilela-Silva, A.C.; Hirohashi, N.; Mourão, P.A. The structure of sulfated polysaccharides ensures a carbohydrate-based mechanism for species recognition during sea urchin fertilization. Int. J. Dev. Biol. 2008, 52, 551–559. [Google Scholar] [CrossRef]
- Jessen, H.; Behnke, O.; Wingstrand, K.G.; Rostgaard, J. Actin-like filaments in the acrosomal apparatus of spermatozoa of a sea urchin. Exp. Cell Res. 1972, 80, 47–54. [Google Scholar] [CrossRef]
- Vacquier, V.D.; Moy, G.W. Isolation of bindin: The protein responsible for adhesion of sperm to sea urchin eggs. Proc. Natl. Acad. Sci. USA 1977, 74, 2456–2460. [Google Scholar] [CrossRef]
- Glabe, C.G.; Grabel, L.B.; Vacquier, V.D.; Rosen, S.D. Carbohydrate specificity of sea urchin sperm bindin: A cell surface lectin mediating sperm-egg adhesion. J. Cell Biol. 1982, 94, 123–128. [Google Scholar] [CrossRef]
- Longo, F.J.; Georgiou, C.; Cook, S. Membrane specializations associated with the acrosomal complex of sea urchin sperm as revealed by immunocytochemistry and freeze fracture replication. Gamete Res. 1989, 23, 429–440. [Google Scholar] [CrossRef]
- Glabe, C.G.; Lennarz, W.J. Species-specific sperm adhesion in sea urchins. A quantitative investigation of bindin-mediated egg agglutination. J. Cell Biol. 1979, 83, 595–604. [Google Scholar] [CrossRef]
- DeAngelis, P.L.; Glabe, C.G. Polysaccharide structural features that are critical for the binding of sulfated fucans to bindin, the adhesive protein from sea urchin sperm. J. Biol. Chem. 1987, 262, 13946–13952. [Google Scholar] [CrossRef]
- Longo, F.J.; Cook, S.; McCulloh, D.H.; Ivonnet, P.I.; Chambers, E.L. Stages leading to and following fusion of sperm and egg plasma membranes. Zygote 1994, 2, 317–331. [Google Scholar] [CrossRef]
- Aketa, K. On the sperm-egg bonding as the initial step of fertilization in the sea urchin. Embryologia 1967, 9, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Aketa, K.; Tsuzuki, H. Sperm-binding capacity of the S-S reduced protein of the vitelline membrane of the sea urchin egg. Exp. Cell Res. 1968, 50, 675–676. [Google Scholar] [CrossRef] [PubMed]
- Aketa, K.; Onitake, K.; Tsuzuki, H. Tryptic disruption of sperm-binding site of sea urchin egg surface. Exp. Cell Res. 1972, 71, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Vacquier, V.D.; Tegner, M.J.; Epel, D. Protease released from sea urchin eggs at fertilization alters the vitelline layer and aids in preventing polyspermy. Exp. Cell Res. 1973, 80, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Glabe, C.G.; Vacquier, V.D. Isolation and characterization of the vitelline layer of sea urchin eggs. J. Cell Biol. 1977, 75, 410–421. [Google Scholar] [CrossRef]
- Veron, M.; Shapiro, B.M. Binding of concanavalin A to the surface of sea urchin eggs and its alteration upon fertilization. J. Biol. Chem. 1977, 252, 1286–1292. [Google Scholar] [CrossRef]
- Gache, C.; Niman, H.L.; Vacquier, V.D. Monoclonal antibodies to the sea urchin egg vitelline layer inhibit fertilization by blocking sperm adhesion. Exp. Cell Res. 1983, 147, 75–84. [Google Scholar] [CrossRef]
- Chandler, D.E.; Heuser, J. The vitelline layer of the sea urchin egg and its modification during fertilization. A freeze-fracture study using quick-freezing and deep-etching. J. Cell Biol. 1980, 84, 618–632. [Google Scholar] [CrossRef]
- Chandler, D.E.; Kazilek, C.J. Extracellular coats on the surface of Strongylocentrotus purpuratus eggs: Stereo electron microscopy of quick-frozen and deep-etched specimens. Cell Tissue Res. 1986, 246, 153–161. [Google Scholar] [CrossRef]
- Glabe, C.G. Interaction of the sperm adhesive protein, bindin, with phospholipid vesicles. I. Specific association of bindin with gel-phase phospholipid vesicles. J. Cell Biol. 1985, 100, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Wessel, G.M.; Wada, Y.; Yajima, M.; Kiyomoto, M. Bindin is essential for fertilization in the sea urchin. Proc. Natl. Acad. Sci. USA 2021, 118, e2109636118. [Google Scholar] [CrossRef]
- Vacquier, V.D. The quest for the sea urchin egg receptor for sperm. Biochem. Biophys. Res. Commun. 2012, 425, 583–587. [Google Scholar] [CrossRef]
- Ohlendieck, K.; Partin, J.S.; Lennarz, W.J. The biologically active form of the sea urchin egg receptor for sperm is a disulfide-bonded homo-multimer. J. Cell Biol. 1994, 125, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Ohlendieck, K.; Partin, J.S.; Stears, R.L.; Lennarz, W.J. Developmental expression of the sea urchin egg receptor for sperm. Dev. Biol. 1994, 165, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Stears, R.L.; Lennarz, W.J. Mapping sperm binding domains on the sea urchin egg receptor for sperm. Dev. Biol. 1997, 187, 200–208. [Google Scholar] [CrossRef]
- Hirohashi, N.; Lennarz, W.J. Role of a vitelline layer-associated 350 kDa glycoprotein in controlling species-specific gamete interaction in the sea urchin. Dev. Growth Differ. 2001, 43, 247–255. [Google Scholar] [CrossRef]
- Kamei, N.; Glabe, C.G. The species-specific egg receptor for sea urchin sperm adhesion is EBR1,a novel ADAMTS protein. Genes. Dev. 2003, 17, 2502–2507. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Santella, L. Species-Specific Gamete Interaction during Sea Urchin Fertilization: Roles of the Egg Jelly and Vitelline Layer. Cells 2022, 11, 2984. [Google Scholar] [CrossRef]
- Kidd, P. The jelly and vitelline coats of the sea urchin egg: New ultrastructural features. J. Ultrastruct. Res. 1978, 64, 204–215. [Google Scholar] [CrossRef]
- Carron, C.P.; Longo, F.J. Relation of cytoplasmic alkalinization to microvillar elongation and microfilament formation in the sea urchin egg. Dev. Biol. 1982, 89, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Begg, D.A.; Rebhun, L.I.; Hyatt, H. Structural organization of actin in the sea urchin egg cortex: Microvillar elongation in the absence of actin filament bundle formation. J. Cell Biol. 1982, 93, 24–32. [Google Scholar] [CrossRef]
- Bonder, E.M.; Fishkind, D.J.; Cotran, N.M.; Begg, D.A. The cortical actin-membrane cytoskeleton of unfertilized sea urchin eggs: Analysis of the spatial organization and relationship of filamentous actin, nonfilamentous actin, and egg spectrin. Dev. Biol. 1989, 134, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.; Aplin, A.E.; Alahari, S.K.; Juliano, R.L. Integrin signaling and cell growth control. Curr. Opin. Cell Biol. 1998, 10, 220–231. [Google Scholar] [CrossRef]
- Chan, D.; Thomas, C.J.; Taylor, V.J.; Burke, R.D. Integrins on eggs: Focal adhesion kinase is activated at fertilization, forms a complex with integrins, and is necessary for cortex formation and cell cycle initiation. Mol. Biol. Cell. 2013, 24, 3472–3481. [Google Scholar] [CrossRef]
- Vasilev, F.; Ezhova, Y.; Chun, J.T. Signaling Enzymes and Ion Channels Being Modulated by the Actin Cytoskeleton at the Plasma Membrane. Int. J. Mol. Sci. 2021, 22, 10366. [Google Scholar] [CrossRef]
- Ziomek, C.A.; Epel, D. Polyspermy block of Spisula eggs is prevented by cytochalasin B. Science 1975, 189, 139–141. [Google Scholar] [CrossRef]
- Misamore, M.J.; Lynn, J.W. Role of the cytoskeleton in sperm entry during fertilization in the freshwater bivalve Dreissena polymorpha. Biol. Bull. 2000, 199, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.T.; Limatola, N.; Vasilev, F.; Santella, L. Early events of fertilization in sea urchin eggs are sensitive to actin-binding organic molecules. Biochem. Biophys. Res. Commun. 2014, 450, 1166–1174. [Google Scholar] [CrossRef]
- Santella, L.; Limatola, N.; Vasilev, F.; Chun, J.T. Maturation and fertilization of echinoderm eggs: Role of actin cytoskeleton dynamics. Biochem. Biophys. Res. Commun. 2018, 506, 361–371. [Google Scholar] [CrossRef]
- Limatola, N.; Vasilev, F.; Santella, L.; Chun, J.T. Nicotine Induces Polyspermy in Sea Urchin Eggs through a Non-Cholinergic Pathway Modulating Actin Dynamics. Cells 2020, 9, 63. [Google Scholar] [CrossRef]
- Limatola, N.; Vasilev, F.; Chun, J.T.; Santella, L. Altered actin cytoskeleton in ageing eggs of starfish affects fertilization process. Exp. Cell Res. 2019, 381, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Puppo, A.; Chun, J.T.; Gragnaniello, G.; Garante, E.; Santella, L. Alteration of the cortical actin cytoskeleton deregulates Ca2+ signaling, monospermic fertilization, and sperm entry. PLoS ONE 2008, 10, e3588. [Google Scholar] [CrossRef]
- Santella, L.; Limatola, N.; Chun, J.T. Cellular and molecular aspects of oocyte maturation and fertilization: A perspective from the actin cytoskeleton. Zool. Lett. 2020, 6, 5. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Cherraben, S.; Schmitt, J.L.; Lehn, J.M.; Santella, L. Effects of Dithiothreitol on Fertilization and Early Development in Sea Urchin. Cells 2021, 10, 3573. [Google Scholar] [CrossRef] [PubMed]
- Limatola, N.; Chun, J.T.; Schneider, S.C.; Schmitt, J.L.; Lehn, J.M.; Santella, L. The Effect of Acidic and Alkaline Seawater on the F-Actin-Dependent Ca2+ Signals Following Insemination of Immature Starfish Oocytes and Mature Eggs. Cells 2023, 12, 740. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Chiba, K.; Santella, L. Dithiothreitol Affects the Fertilization Response in Immature and Maturing Starfish Oocytes. Biomolecules 2023, 13, 1659. [Google Scholar] [CrossRef] [PubMed]
- Mangini, M.; Limatola, N.; Ferrara, M.A.; Coppola, G.; Chun, J.T.; De Luca, A.C.; Santella, L. Application of Raman spectroscopy to the evaluation of F-actin changes in sea urchin eggs at fertilization. Zygote 2024, 32, 38–48. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Santella, L. Regulation of the Actin Cytoskeleton-Linked Ca2+ Signaling by Intracellular pH in Fertilized Eggs of Sea Urchin. Cells 2022, 11, 1496. [Google Scholar] [CrossRef]
- Chun, J.T.; Puppo, A.; Vasilev, F.; Gragnaniello, G.; Garante, E.; Santella, L. The biphasic increase of PIP2 in the fertilized eggs of starfish: New roles in actin polymerization and Ca2+ signaling. PLoS ONE 2010, 5, e14100. [Google Scholar] [CrossRef]
- Oriol-Audit, C. Polyamine-induced actin polymerization. Eur. J. Biochem. 1978, 87, 371–376. [Google Scholar] [CrossRef]
- Grant, N.J.; Oriol-Audit, C.; Dickens, M.J. Supramolecular forms of actin induced by polyamines; an electron microscopic study. Eur. J. Cell Biol. 1983, 30, 67–73. [Google Scholar] [PubMed]
- Oriol-Audit, C.; Hosseini, M.W.; Lehn, J.M. ‘Superpolyamines’. Macrocyclic polyamines induce highly efficient actin polymerization. Eur. J. Biochem. 1985, 151, 557–559. [Google Scholar] [CrossRef]
- Nedeva, I.; Koripelly, G.; Caballero, D.; Chièze, L.; Guichard, B.; Romain, B.; Pencreach, E.; Lehn, J.M.; Carlier, M.F.; Riveline, D. Synthetic polyamines promote rapid lamellipodial growth by regulating actin dynamics. Nat. Commun. 2013, 4, 2165. [Google Scholar] [CrossRef]
- Mifková, A.; Kodet, O.; Szabo, P.; Kučera, J.; Dvořánková, B.; André, S.; Koripelly, G.; Gabius, H.J.; Lehn, J.M.; Smetana, K., Jr. Synthetic polyamine BPA-C8 inhibits TGF-β1-mediated conversion of human dermal fibroblast to myofibroblasts and establishment of galectin-1-rich extracellular matrix in vitro. Chembiochem 2014, 15, 1465–1470. [Google Scholar] [CrossRef]
- Vuento, M.; Vartio, T.; Saraste, M.; von Bonsdorff, C.H.; Vaheri, A. Spontaneous and polyamine-induced formation of filamentous polymers from soluble fibronectin. Eur. J. Biochem. 1980, 105, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Bratton, D.L.; Fadok, V.A.; Richter, D.A.; Kailey, J.M.; Frasch, S.C.; Nakamura, T.; Henson, P.M. Polyamine regulation of plasma membrane phospholipid flip-flop during apoptosis. J. Biol. Chem. 1999, 274, 28113–28120. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.A.; Johnson, L.R. Polyamines and cell migration. J. Physiol. Pharmacol. 2001, 52, 327–349. [Google Scholar]
- Wang, J.Y.; Casero, R.A. Polyamine Cell Signaling. In Physiology, Pharmacology, and Cancer Research; Humana Press, Inc.: Totowa, NJ, USA, 2006; pp. 1–137. [Google Scholar] [CrossRef]
- Coburn, R.F. Polyamine effects on cell function: Possible central role of plasma membrane PI(4,5)P2. J. Cell Physiol. 2009, 221, 544–551. [Google Scholar] [CrossRef]
- Seo, J.B.; Jung, S.R.; Huang, W.; Zhang, Q.; Koh, D.S. Charge Shielding of PIP2 by Cations Regulates Enzyme Activity of Phospholipase C. PLoS ONE 2015, 10, e0144432. [Google Scholar] [CrossRef]
- Molnar, M.M.; Liddell, S.C.; Wadkins, R.M. Effects of Polyamine Binding on the Stability of DNA i-Motif Structures. ACS Omega 2019, 4, 8967–8973. [Google Scholar] [CrossRef] [PubMed]
- Missiaen, L.; Wuytack, F.; Raeymaekers, L.; De Smedt, H.; Casteels, R. Polyamines and neomycin inhibit the purified plasma-membrane Ca2+ pump by interacting with associated polyphosphoinositides. Biochem. J. 1989, 261, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- McGurk, J.F.; Bennett, M.V.; Zukin, R.S. Polyamines potentiate responses of N-methyl-D-aspartate receptors expressed in xenopus oocytes. Proc. Natl. Acad. Sci. USA 1990, 87, 9971–9974. [Google Scholar] [CrossRef] [PubMed]
- Chini, E.N.; Beers, K.W.; Chini, C.C.; Dousa, T.P. Specific modulation of cyclic ADP-ribose-induced Ca2+ release by polyamines. Am. J. Physiol. 1995, 269, 1042–1047. [Google Scholar] [CrossRef]
- Raucher, D.; Stauffer, T.; Chen, W.; Shen, K.; Guo, S.; York, J.D.; Sheetz, M.P.; Meyer, T. Phosphatidylinositol 4,5-Bisphosphate Functions as a Second Messenger that Regulates Cytoskeleton–Plasma Membrane Adhesion. Cell 2000, 100, 221–228. [Google Scholar] [CrossRef]
- Schroeder, T.E. The jelly canal marker of polarity for sea urchin oocytes, eggs and embryos. Exp. Cell Res. 1980, 128, 490–494. [Google Scholar] [CrossRef]
- Vacquier, V.D. Laboratory on sea urchin fertilization. Mol. Reprod. Dev. 2011, 78, 553–564. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Santella, L. Fertilization and development of Arbacia lixula eggs are affected by osmolality conditions. Biosystems 2021, 206, 104448. [Google Scholar] [CrossRef]
- Epel, D.; Weaver, A.M.; Mazia, D. Methods for removal of the vitelline membrane of sea urchin eggs: I. Use of dithiothreitol (Cleland Reagent). Exp. Cell Res. 1970, 61, 64–68. [Google Scholar] [CrossRef]
- Riedl, J.; Crevenna, A.H.; Kessenbrock, K.; Yu, J.H.; Neukirchen, D.; Bista, M.; Bradke, F.; Jenne, D.; Holak, T.A.; Werb, Z.; et al. Lifeact: A versatile marker to visualize F-actin. Nat. Methods 2008, 5, 605–607. [Google Scholar] [CrossRef]
- Vasilev, F.; Limatola, N.; Chun, J.T.; Santella, L. Contributions of suboolemmal acidic vesicles and microvilli to the intracellular Ca2+ increase in the sea urchin eggs at fertilization. Int. J. Biol. Sci. 2019, 15, 757. [Google Scholar] [CrossRef] [PubMed]
- Tilney, L.G.; Jaffe, L.A. Actin, microvilli, and the fertilization cone of sea urchin eggs. J. Cell Biol. 1980, 87, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Shatten, G.; Mazia, D. The penetration of the spermatozoon through the sea urchin egg surface at fertilization. Observations from the outside on whole eggs and from the inside on isolated surfaces. Exp. Cell Res. 1976, 98, 325–337. [Google Scholar] [CrossRef]
- Barresi, M.J.; Gilbert, S.F. Fertilization. Beginning a new organism. In Developmental Biology, 13th ed.; Oxford University Press: New York, NY, USA, 2024; pp. 211–246, Chapter 7. [Google Scholar]
- Green, J.D.; Summers, R.G. Formation of the cortical concavity at fertilization in the sea urchin egg. Dev. Growth Differ. 1980, 22, 821–829. [Google Scholar] [CrossRef]
- Limatola, N.; Vasilev, F.; Chun, J.T.; Santella, L. Sodium-mediated fast electrical depolarization does not prevent polyspermic fertilization in Paracentrotus lividus eggs. Zygote 2019, 27, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Tabor, C.W.; Tabor, H. Polyamines. Annu. Rev. Biochem. 1984, 53, 749–790. [Google Scholar] [CrossRef]
- Smith, C.D.; Snyderman, R. Modulation of inositol phospholipid metabolism by polyamines. Biochem. J. 1988, 256, 125–130. [Google Scholar] [CrossRef]
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef]
- Manen, C.A.; Russell, D.H. Spermine is major polyamine in sea urchins: Studies of polyamines and their synthesis in developing sea urchins. J. Embryol. Exp. Morphol. 1973, 29, 331–345. [Google Scholar] [CrossRef]
- Manen, C.A.; Russell, D.H. Early cyclical changes in polyamine synthesis during sea-urchin development. J. Embryol. Exp. Morphol. 1973, 30, 243–256. [Google Scholar] [CrossRef]
- Kusunoki, S.; Yasumasu, I. Cyclic change in polyamine concentrations in sea urchin eggs related with cleavage cycle. Biochem. Biophys. Res. Commun. 1976, 68, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Canellakis, Z.N.; Bondy, P.K.; Infante, A.A. Spermidine is bound to a unique protein in early sea urchin embryos. Proc. Natl. Acad. Sci. USA 1985, 82, 7613–7615. [Google Scholar] [CrossRef] [PubMed]
- Vrijsen, S.; Houdou, M.; Cascalho, A.; Eggermont, J.; Vangheluwe, P. Polyamines in Parkinson’s Disease: Balancing Between Neurotoxicity and Neuroprotection. Annu. Rev. Biochem. 2023, 92, 435–464. [Google Scholar] [CrossRef]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef]
- Ohlendieck, K.; Dhume, S.T.; Partin, J.S.; Lennarz, W.J. The sea urchin egg receptor for sperm: Isolation and characterization of the intact, biologically active receptor. J. Cell Biol. 1993, 122, 887–895. [Google Scholar] [CrossRef]
- Schatten, G.; Mazia, D. The surface events of fertilization: The movements of the spermatozoon through the sea urchin egg surface and the roles of the surface layers. J. Supramol. Struct. 1976, 5, 343–369. [Google Scholar] [CrossRef]
- Janmey, P.A. Physical properties of actin. Curr. Opin. Cell Biol. 1991, 2, 11–17. [Google Scholar]
- Dominguez, R.; Holmes, K.C. Actin structure and function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef]
- Blanchoin, L.; Boujemaa-Paterski, R.; Sykes, C.; Plastino, J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 2014, 94, 235–263. [Google Scholar] [CrossRef]
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef]
- Lappalainen, P. Actin-binding Proteins: The Long Road to Understanding the Dynamic Landscape of Cellular Actin Networks. MBoC 2016, 27, 2519–2522. [Google Scholar] [CrossRef] [PubMed]
- Merino, F.; Pospich, S.; Raunser, S. Towards a structural understanding of the remodeling of the actin cytoskeleton. Semin. Cel. Dev. Biol. 2020, 102, 51–64. [Google Scholar] [CrossRef]
- Grant, N.J.; Oriol-Audit, C. Influence of the polyamine spermine on the organization of cortical filaments in isolated cortices of Xenopus laevis eggs. Eur. J. Cell Biol. 1985, 36, 239–246. [Google Scholar] [PubMed]
- Meijer, L.; Guerrier, P. Maturation and fertilization in starfish oocytes. Int. Rev. Cytol. 1984, 86, 129–196. [Google Scholar] [CrossRef] [PubMed]
- Santella, L.; Limatola, N.; Chun, J.T. Calcium and actin in the saga of awakening oocytes. Biochem. Biophys. Res. Commun. 2015, 460, 104–113. [Google Scholar] [CrossRef]
- Dan, J.C.; Kitahara, A.; Kohri, T. Studies on the acrosome. II. acrosome reaction in starfish spermatozoa. Biol. Bull. 1954, 107, 20–218. [Google Scholar] [CrossRef]
- Tilney, L.G.; Kiehart, D.P.; Sardet, C.; Tilney, M. Polymerization of actin. IV. Role of Ca++ and H+ in the assembly of actin and in membrane fusion in the acrosomal reaction of echinoderm sperm. J. Cell Biol. 1978, 77, 536–550. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kawase, O.; Islam, M.S.; Naruse, M.; Watanabe, S.N.; Ishikawa, R.; Hoshi, M. Regulation of the starfish sperm acrosome reaction by cGMP, pH, cAMP and Ca2+. Int. J. Dev. Biol. 2008, 52, 523–526. [Google Scholar] [CrossRef]
- Chun, J.T.; Vasilev, F.; Limatola, N.; Santella, L. Fertilization in Starfish and Sea Urchin: Roles of Actin. Results Probl. Cell Differ. 2018, 65, 33–47. [Google Scholar] [CrossRef]
- Su, Y.H.; Chen, S.H.; Zhou, H.; Vacquier, V.D. Tandem mass spectrometry identifies proteins phosphorylated by cyclic AMP-dependent protein kinase when sea urchin sperm undergo the acrosome reaction. Dev. Biol. 2005, 285, 116–125. [Google Scholar] [CrossRef]
- Hoshi, M.; Nishigaki, T.; Ushiyama, A.; Okinaga, T.; Chiba, K.; Matsumoto, M. Egg-jelly signal molecules for triggering the acrosome reaction in starfish spermatozoa. Int. J. Dev. Biol. 1994, 38, 167–174. [Google Scholar]
- Hoshi, M.; Moriyama, H.; Matsumoto, M. Structure of acrosome reaction-inducing substance in the jelly coat of starfish eggs: A mini review. Biochem. Biophys. Res. Commun. 2012, 425, 595–598. [Google Scholar] [CrossRef]
- Dale, B.; Dan-Sohkawa, M.; De Santis, A.; Hoshi, M. Fertilization of the starfish Astropecten aurantiacus. Exp. Cell Res. 1981, 132, 505–510. [Google Scholar] [CrossRef]
- Moy, G.W.; Vacquier, V.D. Immunoperoxidase localization of bindin during the adhesion of sperm to sea urchin eggs. Curr. Top. Dev. Biol. 1979, 13, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Monroy, A.; Rosati, F.; Dale, B. Sperm-egg interaction. Boll. Zool. 1984, 51, 103–119. [Google Scholar] [CrossRef]
- Yanagimachi, R.; Cherr, G.; Matsubara, T.; Andoh, T.; Harumi, T.; Vines, C.; Pillai, M.; Griffin, F.; Matsubara, H.; Weatherby, T.; et al. Sperm Attractant in the Micropyle Region of Fish and Insect Eggs. Biol. Reprod. 2013, 88, 47. [Google Scholar] [CrossRef]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: Its site and role in fertilization. Biol. Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Boveri, T. Die Polaritat von Ovocyte, Ei, und Larve des Strongylocentrotus lividus. Zool. Jahrb. Abt. Anat. Ontog. Tiere. 1901, 14, 630–653. [Google Scholar]
- Just, E.E. Biology of the Cell Surface; P. Blakiston’s Son & Co, Inc.: Philadelphia, PA, USA, 1939. [Google Scholar]
- Miyazaki, S.H.; Ohmori, H.; Sasaki, S. Action potential and non-linear current voltage relation in starfish oocytes. J. Physiol. 1975, 246, 37–54. [Google Scholar] [CrossRef]
- Lange, K. Fundamental role of microvilli in the main functions of differentiated cells: Outline of an universal regulating and signaling system at the cell periphery. J. Cell Physiol. 2011, 226, 896–927. [Google Scholar] [CrossRef]
- Lim, D.; Lange, K.; Santella, L. Activation of oocytes by latrunculin A. FASEB J. 2002, 16, 1050–1056. [Google Scholar] [CrossRef] [PubMed]




| BPA-C8 Concentration | |||||
|---|---|---|---|---|---|
| P. lividus Eggs | BPA-C8 Treatment | JAS Incubation | 0 μM | 100 μM | 1 mM |
| Intact | bath | 12 μM | 2.6 ± 0.21 | 2.4 ± 0.23 | 2.1 ± 0.07 |
| Intact | microinjection | 12 μM | 2.9 ± 0.22 | 3.6 ± 0.23 | 0.9 ± 0.28 * |
| BPA-C8 Concentration | ||||
|---|---|---|---|---|
| P. lividus Eggs | Treatment | 0 μM | 100 μM | 1 mM |
| Intact | bath | 1.02 ± 0.06 (80/80) | 1.05 ± 0.13 (100/100) | 1.07 ± 0.12 (80/80) |
| Denuded | bath | 7.9 ± 1.1 (40/40) | 0.97 ± 0.08 (39/40) * | 0.70 ± 0.25 (28/40) * |
| Intact | microinjection | 1.2 ± 0.25 (60/60) | 1.32 ± 0.41 (60/60) | 0.45 ± 0.39 (22/60) |
| Denuded | microinjection | 11 ± 1.35 (40/40) | 3.7 ± 1.55 (34/40) * | 5.85 ± 1.33 (38/40) * |
| BPA-C8 Concentration | |||||
|---|---|---|---|---|---|
| P. lividus Eggs | Treatment | Ca2+ Response | 0 μM | 100 μM | 1 mM |
| Intact | Bath | CF | 100 ± 16.1 (18) | 106.5 ± 17.5 (24) | 117.0 ± 28.4 (25) |
| Intact | Bath | CW | 100 ± 10.0 (24) | 81.7 ± 7.9 (28) * | 70.7 ± 13.1 (27) * |
| Denuded | Bath | CF | 100 ± 33.7 (13) | 101.1 ± 14.9 (13) | 61.8 ± 19.2 (12) § |
| Denuded | Bath | CW | 100 ± 22.4 (13) | 105.0 ± 22.8 (13) | 102.9 ± 11.6 (12) |
| Intact | microinjection | CF | 100 ± 15.9 (15) | 74.3 ± 23.0 (17) § | 25.6 ± 19.4 (15) * |
| Intact | microinjection | CW | 100 ± 8.7 (15) | 91.1 ± 8.9 (17) | 40.3 ± 18.8 (15) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limatola, N.; Chun, J.T.; Schmitt, J.-L.; Lehn, J.-M.; Santella, L. The Effect of Synthetic Polyamine BPA-C8 on the Fertilization Process of Intact and Denuded Sea Urchin Eggs. Cells 2024, 13, 1477. https://doi.org/10.3390/cells13171477
Limatola N, Chun JT, Schmitt J-L, Lehn J-M, Santella L. The Effect of Synthetic Polyamine BPA-C8 on the Fertilization Process of Intact and Denuded Sea Urchin Eggs. Cells. 2024; 13(17):1477. https://doi.org/10.3390/cells13171477
Chicago/Turabian StyleLimatola, Nunzia, Jong Tai Chun, Jean-Louis Schmitt, Jean-Marie Lehn, and Luigia Santella. 2024. "The Effect of Synthetic Polyamine BPA-C8 on the Fertilization Process of Intact and Denuded Sea Urchin Eggs" Cells 13, no. 17: 1477. https://doi.org/10.3390/cells13171477







