The Role of circHIPK3 in Tumorigenesis and Its Potential as a Biomarker in Lung Cancer
Abstract
:1. Introduction
2. Structure and Biogenesis
3. Role of CircHIPK3 in Other Cancers
| Disease | Role of CircHIPK3 | References |
|---|---|---|
| Osteoarthritis | Upregulated in osteoarthritis cartilage tissues; silencing promotes chondrocyte apoptosis via miR-124/SOX8. | [40] |
| Acute Pancreatitis | Promotes pyroptosis via miR-193a-5p/GSDMD axis, aggravating acute pancreatitis; potentially plays a role in disease severity. Potential to be a biomarker for different severe acute pancreatitis stages; important for management of complications like systemic inflammatory response syndrome and multiple organ failure. | [45,46] |
| Pulmonary Fibrosis | High expression in idiopathic pulmonary fibrosis; induces FMT via miR-338-3p, a potential target for treatment. | [47,48] |
| Cardiac Fibrosis | Increased expression post-Ang II treatment; associated with cardiac fibrosis; reduced by silencing Ang II. | [49] |
| Atherosclerosis | Decreased in atherosclerotic mice; suppresses autophagy. Overexpression reverses the inhibition of autophagy by ox-LDL and improves symptoms via the miR-190b/ATG7 pathway. | [50,51,52,53,54,55,56,57] |
| Asthma | Upregulated in airway smooth muscle cells; promotes proliferation and migration, inhibits apoptosis. Inhibits miR-326, activating STIM1; silences miR-375, upregulating MMP-16. | [58,59] |
| Age-related Cataract | Downregulated in age-related cataract; inhibits apoptosis and promotes proliferation by targeting miR-221-3p/PI3K/AKT pathways. | [60] |
| Diabetes Mellitus (DM) and Its Complications | Elevated in Type 2 diabetes mellitus and associated with HbA1c, fasting blood glucose; involved in diabetic nephropathy, cardiomyopathy, neuropathic pain, retinal damage, and aortic endothelial cell proliferation. Sponges various miRNAs (e.g., miR-185, miR-29b-3p, miR-192-5p, miR-124, miR-30a-3p, miR-106a-5p) affecting genes like cyclin D1, PCNA, TGF-β1, Col1a1, Col3a1, FOXO1, VEGF-C, FZD4, WNT2 | [61,62,63,64,65] |
4. The Function of CircHIPK3 in Lung Cancer Tumorigenesis
| miRNA | Onco-Suppressive Role | Effect of Sponging by CircHIPK3 | References |
|---|---|---|---|
| miR-124 | Significant suppression of metastasis, active inhibition of NSCLC cell invasion and progression, reduction in cell proliferation, regulation of reversing resistance to gefitinib treatment | Enhancing the survival and proliferation of cancer cells | [87,89,90,91,99] |
| miR-381-3p | Inhibition of NSCLC cell proliferation, migration, glycolysis, and promotion of apoptosis of lung cancer cells, reduction in resistance to anti-programmed cell death 1-based therapy | Promotion of NSCLC cell proliferation, migration, glycolysis, and reduction in lung cancer cell apoptosis | [94,105] |
| miR-149 | Inhibition of NSCLC cell proliferation and metastasis, promotion of cell autophagy and apoptosis | Reduction in NSCLC cell proliferation, migration and invasion. Reduction in apoptosis | [95,96,106] |
| miR-107 | Inhibition of NSCLC cell proliferation and migration | Promotion of NSCLC cell proliferation and migration | [86] |
5. Biomarker
6. Conclusions
7. Methods
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kuno, H.; Hiyama, T.; Oda, S.; Masuoka, S.; Miyasaka, Y.; Taki, T.; Nagasaki, Y.; Ohtani-Kim, S.J.; Ishii, G.; et al. 2021 WHO Classification of Lung Cancer: Molecular Biology Research and Radiologic-Pathologic Correlation. Radiographics 2024, 44, e230136. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Kuhn, E.; Morbini, P.; Cancellieri, A.; Damiani, S.; Cavazza, A.; Comin, C.E. Adenocarcinoma classification: Patterns and prognosis. Pathologica 2018, 110, 5–11. [Google Scholar] [PubMed]
- Olivares-Hernández, A.; González Del Portillo, E.; Tamayo-Velasco, Á.; Figuero-Pérez, L.; Zhilina-Zhilina, S.; Fonseca-Sánchez, E.; Miramontes-González, J.P. Immune checkpoint inhibitors in non-small cell lung cancer: From current perspectives to future treatments-a systematic review. Ann. Transl. Med. 2023, 11, 354. [Google Scholar] [CrossRef]
- Meijer, J.J.; Leonetti, A.; Airò, G.; Tiseo, M.; Rolfo, C.; Giovannetti, E.; Vahabi, M. Small cell lung cancer: Novel treatments beyond immunotherapy. Semin. Cancer Biol. 2022, 86, 376–385. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Prim. 2021, 7, 3. [Google Scholar] [CrossRef]
- Alberg, A.J.; Brock, M.V.; Ford, J.G.; Samet, J.M.; Spivack, S.D. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e1S–e29S. [Google Scholar] [CrossRef]
- Spaulding, A.C.; Eldridge, G.D.; Chico, C.E.; Morisseau, N.; Drobeniuc, A.; Fils-Aime, R.; Day, C.; Hopkins, R.; Jin, X.; Chen, J.; et al. Smoking in Correctional Settings Worldwide: Prevalence, Bans, and Interventions. Epidemiol. Rev. 2018, 40, 82–95. [Google Scholar] [CrossRef]
- Huang, R.; Wei, Y.; Hung, R.J.; Liu, G.; Su, L.; Zhang, R.; Zong, X.; Zhang, Z.F.; Morgenstern, H.; Brüske, I.; et al. Associated Links Among Smoking, Chronic Obstructive Pulmonary Disease, and Small Cell Lung Cancer: A Pooled Analysis in the International Lung Cancer Consortium. EBioMedicine 2015, 2, 1677–1685. [Google Scholar] [CrossRef]
- Hristova, V.A.; Chan, D.W. Cancer biomarker discovery and translation: Proteomics and beyond. Expert Rev. Proteom. 2019, 16, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Zhand, S.; Liao, J.; Castorina, A.; Yuen, M.-L.; Ebrahimi Warkiani, M.; Cheng, Y.-Y. Small Extracellular Vesicle-Derived Circular RNA hsa_circ_0007386 as a Biomarker for the Diagnosis of Pleural Mesothelioma. Cells 2024, 13, 1037. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Zimta, A.A.; Harangus, A.; Iurca, I.; Irimie, A.; Coza, O.; Berindan-Neagoe, I. The Function of Non-Coding RNAs in Lung Cancer Tumorigenesis. Cancers 2019, 11, 605. [Google Scholar] [CrossRef]
- Wei, Z.; Shi, Y.; Xue, C.; Li, M.; Wei, J.; Li, G.; Xiong, W.; Zhou, M. Understanding the Dual Roles of CircHIPK3 in Tumorigenesis and Tumor Progression. J. Cancer 2022, 13, 3674–3686. [Google Scholar] [CrossRef]
- Zhu, Y.; Shen, L.; Xia, Q.; Tao, H.; Liu, Z.; Wang, M.; Zhang, X.; Zhang, J.; Lv, J. Extracellular vesicle-derived circHIPK3: Novel diagnostic biomarker for lung cancer. Adv. Med. Sci. 2023, 68, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, R.; Su, W.; Yang, X.; Geng, Q.; Guo, C.; Wang, Z.; Wang, J.; Kresty, L.A.; Beer, D.G.; et al. Circular RNA circHIPK3 modulates autophagy via MIR124-3p-STAT3-PRKAA/AMPKα signaling in STK11 mutant lung cancer. Autophagy 2020, 16, 659–671. [Google Scholar] [CrossRef]
- Chen, L.L.; Yang, L. Regulation of circRNA biogenesis. RNA Biol. 2015, 12, 381–388. [Google Scholar] [CrossRef]
- Rybak-Wolf, A.; Stottmeister, C.; Glažar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R.; et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell 2015, 58, 870–885. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Jakobsen, T.; Hager, H.; Kjems, J. The emerging roles of circRNAs in cancer and oncology. Nat. Rev. Clin. Oncol. 2022, 19, 188–206. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Ashwal-Fluss, R.; Meyer, M.; Pamudurti, N.R.; Ivanov, A.; Bartok, O.; Hanan, M.; Evantal, N.; Memczak, S.; Rajewsky, N.; Kadener, S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 2014, 56, 55–66. [Google Scholar] [CrossRef]
- Ragan, C.; Goodall, G.J.; Shirokikh, N.E.; Preiss, T. Insights into the biogenesis and potential functions of exonic circular RNA. Sci. Rep. 2019, 9, 2048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, L.; Niu, Y.; Wang, Z.; Xu, X.; Li, Y.; Yu, Y. Circular RNA in Lung Cancer Research: Biogenesis, Functions, and Roles. Int. J. Biol. Sci. 2020, 16, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Chen, L.L. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol. Cell 2018, 71, 428–442. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Du, W.W.; Zhang, C.; Yang, W.; Yong, T.; Awan, F.M.; Yang, B.B. Identifying and Characterizing circRNA-Protein Interaction. Theranostics 2017, 7, 4183–4191. [Google Scholar] [CrossRef]
- Wang, L.; Luo, T.; Bao, Z.; Li, Y.; Bu, W. Intrathecal circHIPK3 shRNA alleviates neuropathic pain in diabetic rats. Biochem. Biophys. Res. Commun. 2018, 505, 644–650. [Google Scholar] [CrossRef]
- Teng, F.; Xu, J.; Zhang, M.; Liu, S.; Gu, Y.; Zhang, M.; Wang, X.; Ni, J.; Qian, B.; Shen, R.; et al. Comprehensive circular RNA expression profiles and the tumor-suppressive function of circHIPK3 in ovarian cancer. Int. J. Biochem. Cell Biol. 2019, 112, 8–17. [Google Scholar] [CrossRef]
- Altesha, M.A.; Ni, T.; Khan, A.; Liu, K.; Zheng, X. Circular RNA in cardiovascular disease. J. Cell Physiol. 2019, 234, 5588–5600. [Google Scholar] [CrossRef]
- Yang, L.; Han, B.; Zhang, Z.; Wang, S.; Bai, Y.; Zhang, Y.; Tang, Y.; Du, L.; Xu, L.; Wu, F.; et al. Extracellular Vesicle–Mediated Delivery of Circular RNA SCMH1 Promotes Functional Recovery in Rodent and Nonhuman Primate Ischemic Stroke Models. Circulation 2020, 142, 556–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Zhu, W.K.; Qi, F.Y.; Che, F.Y. CircHIPK3 promotes neuroinflammation through regulation of the miR-124-3p/STAT3/NLRP3 signaling pathway in Parkinson’s disease. Adv. Clin. Exp. Med. 2023, 32, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shan, G. CircRNA in cancer: Fundamental mechanism and clinical potential. Cancer Lett. 2021, 505, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Liao, J.; Liang, J.; Chen, X.P.; Zhang, B.; Chu, L. Circular RNA HIPK3: A Key Circular RNA in a Variety of Human Cancers. Front. Oncol. 2020, 10, 773. [Google Scholar] [CrossRef]
- Panda, A.C.; Grammatikakis, I.; Munk, R.; Gorospe, M.; Abdelmohsen, K. Emerging roles and context of circular RNAs. Wiley Interdiscip. Rev. RNA 2017, 8, e1386. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, J. CircHIPK3 regulates melanoma cell behaviors by binding with miR-215-5p to upregulate YY1. Mol. Cell Probes 2020, 53, 101644. [Google Scholar] [CrossRef]
- Zhang, H.D.; Jiang, L.H.; Sun, D.W.; Hou, J.C.; Ji, Z.L. CircRNA: A novel type of biomarker for cancer. Breast Cancer 2018, 25, 1–7. [Google Scholar] [CrossRef]
- Legnini, I.; Di Timoteo, G.; Rossi, F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M.; et al. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol. Cell 2017, 66, 22–37.e29. [Google Scholar] [CrossRef]
- Zheng, Q.; Bao, C.; Guo, W.; Li, S.; Chen, J.; Chen, B.; Luo, Y.; Lyu, D.; Li, Y.; Shi, G.; et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016, 7, 11215. [Google Scholar] [CrossRef]
- Wu, Q.; Yuan, Z.H.; Ma, X.B.; Tang, X.H. Low expression of CircRNA HIPK3 promotes osteoarthritis chondrocyte apoptosis by serving as a sponge of miR-124 to regulate SOX8. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7937–7945. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, B.; Bin, X.; Xie, C.; Li, B.; Liu, O.; Tang, Z. CircHIPK3: Key Player in Pathophysiology and Potential Diagnostic and Therapeutic Tool. Front. Med. 2021, 8, 615417. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhuo, H.; Xu, M.; Wang, L.; Xu, H.; Peng, J.; Hou, J.; Lin, L.; Cai, J. Regulatory network of circRNA-miRNA-mRNA contributes to the histological classification and disease progression in gastric cancer. J. Transl. Med. 2018, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-G.; Zhao, H.-J.; Lin, L.; Liu, J.-B.; Bai, J.-Z.; Wang, G.-S. Circular RNA CirCHIPK3 promotes cell proliferation and invasion of breast cancer by sponging miR-193a/HMGB1/PI3K/AKT axis. Thorac. Cancer 2020, 11, 2660–2671. [Google Scholar] [CrossRef] [PubMed]
- Zaiou, M. The Emerging Role and Promise of Circular RNAs in Obesity and Related Metabolic Disorders. Cells 2020, 9, 1473. [Google Scholar] [CrossRef]
- Lankisch, P.G.; Apte, M.; Banks, P.A. Acute pancreatitis. Lancet 2015, 386, 85–96. [Google Scholar] [CrossRef]
- Zerem, E. Treatment of severe acute pancreatitis and its complications. World J. Gastroenterol. 2014, 20, 13879–13892. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Toews, G.B.; White, E.S.; Lynch, J.P., 3rd; Martinez, F.J. Mechanisms of pulmonary fibrosis. Annu. Rev. Med. 2004, 55, 395–417. [Google Scholar] [CrossRef]
- Meyer, K.C. Pulmonary fibrosis, part I: Epidemiology, pathogenesis, and diagnosis. Expert Rev. Respir. Med. 2017, 11, 343–359. [Google Scholar] [CrossRef]
- Ni, H.; Li, W.; Zhuge, Y.; Xu, S.; Wang, Y.; Chen, Y.; Shen, G.; Wang, F. Inhibition of circHIPK3 prevents angiotensin II-induced cardiac fibrosis by sponging miR-29b-3p. Int. J. Cardiol. 2019, 292, 188–196. [Google Scholar] [CrossRef]
- Triska, J.; Mathew, C.; Zhao, Y.; Chen, Y.E.; Birnbaum, Y. Circular RNA as Therapeutic Targets in Atherosclerosis: Are We Running in Circles? J. Clin. Med. 2023, 12, 4446. [Google Scholar] [CrossRef]
- Kong, P.; Yu, Y.; Wang, L.; Dou, Y.Q.; Zhang, X.H.; Cui, Y.; Wang, H.Y.; Yong, Y.T.; Liu, Y.B.; Hu, H.J.; et al. circ-Sirt1 controls NF-κB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res. 2019, 47, 3580–3593. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, Q.; Hu, N.; Zheng, F.; Zhang, X.; Ni, Y.; Liu, J. Downregulation of hsa_circ_0068087 ameliorates TLR4/NF-κB/NLRP3 inflammasome-mediated inflammation and endothelial cell dysfunction in high glucose conditioned by sponging miR-197. Gene 2019, 709, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Hu, Y.; Lou, J.; Yin, S.; Wang, W.; Wang, Y.; Xia, Y.; Wu, W. CircRNA-0044073 is upregulated in atherosclerosis and increases the proliferation and invasion of cells by targeting miR-107. Mol. Med. Rep. 2019, 19, 3923–3932. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Yue, M.; Li, Y.; Bi, J.; Liu, H. Angiotensin II inhibits apoptosis of mouse aortic smooth muscle cells through regulating the circNRG-1/miR-193b-5p/NRG-1 axis. Cell Death Dis. 2019, 10, 362. [Google Scholar] [CrossRef]
- Pan, L.; Lian, W.; Zhang, X.; Han, S.; Cao, C.; Li, X.; Li, M. Human circular RNA-0054633 regulates high glucose-induced vascular endothelial cell dysfunction through the microRNA-218/roundabout 1 and microRNA-218/heme oxygenase-1 axes. Int. J. Mol. Med. 2018, 42, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Wang, X.; Bai, M. CircTM7SF3 contributes to oxidized low-density lipoprotein-induced apoptosis, inflammation and oxidative stress through targeting miR-206/ASPH axis in atherosclerosis cell model in vitro. BMC Cardiovasc. Disord. 2021, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Feng, X.; Zhang, J. Circular RNA circHIPK3 modulates the proliferation of airway smooth muscle cells by miR-326/STIM1 axis. Life Sci. 2020, 255, 117835. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Guo, X.; Qin, J. Silencing of circHIPK3 hampers platelet-derived growth factor-induced proliferation and migration in airway smooth muscle cells through the miR-375/MMP-16 axis. Cytotechnology 2021, 73, 629–642. [Google Scholar] [CrossRef]
- Cui, G.; Wang, L.; Huang, W. Circular RNA HIPK3 regulates human lens epithelial cell dysfunction by targeting the miR-221-3p/PI3K/AKT pathway in age-related cataract. Exp. Eye Res. 2020, 198, 108128. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, M.; Ge, Y. Circular RNA HIPK3 exacerbates diabetic nephropathy and promotes proliferation by sponging miR-185. Gene 2021, 765, 145065. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Jiang, Z.; Yang, X.; Lin, J.; Cai, Q.; Li, X. Circular RNA HIPK3 contributes to hyperglycemia and insulin homeostasis by sponging miR-192-5p and upregulating transcription factor forkhead box O1. Endocr. J. 2020, 67, 397–408. [Google Scholar] [CrossRef]
- Wang, S.; Shi, M.; Li, J.; Zhang, Y.; Wang, W.; Xu, P.; Li, Y. Endothelial cell-derived exosomal circHIPK3 promotes the proliferation of vascular smooth muscle cells induced by high glucose via the miR-106a-5p/Foxo1/Vcam1 pathway. Aging 2021, 13, 25241–25255. [Google Scholar] [CrossRef]
- Tremblay, J.; Hamet, P. Environmental and genetic contributions to diabetes. Metabolism 2019, 100s, 153952. [Google Scholar] [CrossRef]
- Rezaeinejad, F.; Mirzaei, A.; Khalvati, B.; Sabz, G.; Alipoor, B. Circulating expression levels of CircHIPK3 and CDR1as circular-RNAs in type 2 diabetes patients. Mol. Biol. Rep. 2022, 49, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.G.; Xu, Q. Upregulation of circHIPK3 promotes the progression of gastric cancer via Wnt/β-catenin pathway and indicates a poor prognosis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7905–7912. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Xu, H.; Wei, W.; Wang, Z.; Zhang, Q.; De, W.; Shu, Y. circHIPK3 Promotes Cell Proliferation and Migration of Gastric Cancer by Sponging miR-107 and Regulating BDNF Expression. OncoTargets Ther. 2020, 13, 1613–1624. [Google Scholar] [CrossRef]
- Jablonski, E.M.; Mattocks, M.A.; Sokolov, E.; Koniaris, L.G.; Hughes, F.M., Jr.; Fausto, N.; Pierce, R.H.; McKillop, I.H. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett. 2007, 250, 36–46. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, L.; Dong, L.; Wang, J.; Xiao, Q.; Yu, X.; Zhu, H. CircHIPK3 Promotes Gemcitabine (GEM) Resistance in Pancreatic Cancer Cells by Sponging miR-330-5p and Targets RASSF1. Cancer Manag. Res. 2020, 12, 921–929. [Google Scholar] [CrossRef]
- Ilson, D.H. Advances in the treatment of gastric cancer. Curr. Opin. Gastroenterol. 2018, 34, 465–468. [Google Scholar] [CrossRef]
- Yan, Y.; Su, M.; Qin, B. CircHIPK3 promotes colorectal cancer cells proliferation and metastasis via modulating of miR-1207-5p/FMNL2 signal. Biochem. Biophys. Res. Commun. 2020, 524, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, A.; de Bono, J.S. Prostate Cancer 2020: "The Times They Are a “Changing”. Cancer Cell 2020, 38, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhi, Y.; Wang, K.; Zhang, P.; Ji, Z.; Xie, C.; Sun, F. CircHIPK3 overexpression accelerates the proliferation and invasion of prostate cancer cells through regulating miRNA-338-3p. OncoTargets Ther. 2019, 12, 3363–3372. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shi, Y.; Liu, M.; Sun, J. circHIPK3 regulates cell proliferation and migration by sponging miR-124 and regulating AQP3 expression in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 175. [Google Scholar] [CrossRef]
- Gomez, E.W.; De Paula, L.B.; Weimer, R.D.; Hellwig, A.; Rodrigues, G.M.; Alegretti, A.P.; de Oliveira, J.R. The potential of circHIPK3 as a biomarker in chronic myeloid leukemia. Front. Oncol. 2024, 14, 1330592. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, K.L.; Gupta, A.; Yadav, A.; Kumar, A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J. Gastroenterol. 2017, 23, 3978–3998. [Google Scholar] [CrossRef]
- Xiao-Long, M.; Kun-Peng, Z.; Chun-Lin, Z. Circular RNA circ_HIPK3 is down-regulated and suppresses cell proliferation, migration and invasion in osteosarcoma. J. Cancer 2018, 9, 1856–1862. [Google Scholar] [CrossRef]
- Van Meir, E.G.; Hadjipanayis, C.G.; Norden, A.D.; Shu, H.K.; Wen, P.Y.; Olson, J.J. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. CA Cancer J. Clin. 2010, 60, 166–193. [Google Scholar] [CrossRef]
- Jin, P.; Huang, Y.; Zhu, P.; Zou, Y.; Shao, T.; Wang, O. CircRNA circHIPK3 serves as a prognostic marker to promote glioma progression by regulating miR-654/IGF2BP3 signaling. Biochem. Biophys. Res. Commun. 2018, 503, 1570–1574. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, S.; Wang, P.; Yang, C.; Shang, C.; Yang, J.; Wang, J. LncRNA, TUG1 regulates the oral squamous cell carcinoma progression possibly via interacting with Wnt/β-catenin signaling. Gene 2017, 608, 49–57. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.Y.; Ouyang, S.S.; Huang, Z.K.; Luo, Q.; Liao, L. Circular RNA circHIPK3 acts as the sponge of microRNA-124 to promote human oral squamous cell carcinoma cells proliferation. Zhonghua Kou Qiang Yi Xue Za Zhi 2018, 53, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Xie, F.; Zheng, C.; Chen, D. CircHIPK3 promotes proliferation and invasion in nasopharyngeal carcinoma by abrogating miR-4288-induced ELF3 inhibition. J. Cell Physiol. 2019, 234, 1699–1706. [Google Scholar] [CrossRef]
- Qian, W.; Huang, T.; Feng, W. Circular RNA HIPK3 Promotes EMT of Cervical Cancer Through Sponging miR-338-3p to Up-Regulate HIF-1α. Cancer Manag. Res. 2020, 12, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Vera, R.; Dotor, E.; Feliu, J.; González, E.; Laquente, B.; Macarulla, T.; Martínez, E.; Maurel, J.; Salgado, M.; Manzano, J.L. SEOM Clinical Guideline for the treatment of pancreatic cancer (2016). Clin. Transl. Oncol. 2016, 18, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wu, J.; Han, P.; Zhao, Z.; Song, X. Circular RNA expression profiles and features in human tissues: A study using RNA-seq data. BMC Genom. 2017, 18, 680. [Google Scholar] [CrossRef]
- Hong, W.; Zhang, Y.; Ding, J.; Yang, Q.; Xie, H.; Gao, X. circHIPK3 Acts as Competing Endogenous RNA and Promotes Non-Small-Cell Lung Cancer Progression through the miR-107/BDNF Signaling Pathway. Biomed. Res. Int. 2020, 2020, 6075902. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.; Jiang, P. Circular RNA HIPK3 exerts oncogenic properties through suppression of miR-124 in lung cancer. Biochem. Biophys. Res. Commun. 2018, 506, 455–462. [Google Scholar] [CrossRef]
- Volinia, S.; Galasso, M.; Costinean, S.; Tagliavini, L.; Gamberoni, G.; Drusco, A.; Marchesini, J.; Mascellani, N.; Sana, M.E.; Abu Jarour, R.; et al. Reprogramming of miRNA networks in cancer and leukemia. Genome Res. 2010, 20, 589–599. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, Y.; Li, M.; Zhang, Y.; Zhang, H.; Chen, J.; Liu, Z.; Yuan, P.; Yang, Z.; Wang, X. MiR-124-3p impedes the metastasis of non-small cell lung cancer via extracellular exosome transport and intracellular PI3K/AKT signaling. Biomark. Res. 2023, 11, 1. [Google Scholar] [CrossRef]
- Li, H.; Guo, X.; Li, Q.; Ran, P.; Xiang, X.; Yuan, Y.; Dong, T.; Zhu, B.; Wang, L.; Li, F.; et al. Long non-coding RNA 1308 promotes cell invasion by regulating the miR-124/ADAM 15 axis in non-small-cell lung cancer cells. Cancer Manag. Res. 2018, 10, 6599–6609. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, Y.; Wei, K.; Yao, G.; Pan, C.; Liu, B.; Xia, Y.; He, Z.; Qi, X.; Li, Z.; et al. MicroRNA-124 Functions as a Tumor Suppressor by Regulating CDH2 and Epithelial-Mesenchymal Transition in Non-Small Cell Lung Cancer. Cell Physiol. Biochem. 2016, 38, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Van Der Steen, N.; Lyu, Y.; Hitzler, A.K.; Becker, A.C.; Seiler, J.; Diederichs, S. The Circular RNA Landscape of Non-Small Cell Lung Cancer Cells. Cancers 2020, 12, 1091. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Q.; Lei, Z.; Feng, M.; Zhao, Z.; Wang, Y.; Wei, G. DTL promotes cancer progression by PDCD4 ubiquitin-dependent degradation. J. Exp. Clin. Cancer Res. 2019, 38, 350. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Zhang, J.; Yan, L.; Li, D. CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway. Open Life Sci. 2020, 15, 683–695. [Google Scholar] [CrossRef]
- Wei, H.; Li, L.; Zhang, H.; Xu, F.; Chen, L.; Che, G.; Wang, Y. Circ-FOXM1 knockdown suppresses non-small cell lung cancer development by regulating the miR-149-5p/ATG5 axis. Cell Cycle 2021, 20, 166–178. [Google Scholar] [CrossRef]
- Lu, H.; Han, X.; Ren, J.; Ren, K.; Li, Z.; Sun, Z. Circular RNA HIPK3 induces cell proliferation and inhibits apoptosis in non-small cell lung cancer through sponging miR-149. Cancer Biol. Ther. 2020, 21, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Musa, S.; Amara, N.; Selawi, A.; Wang, J.; Marchini, C.; Agbarya, A.; Mahajna, J. Overcoming Chemoresistance in Cancer: The Promise of Crizotinib. Cancers 2024, 16, 2479. [Google Scholar] [CrossRef]
- Katopodi, T.; Petanidis, S.; Domvri, K.; Zarogoulidis, P.; Anestakis, D.; Charalampidis, C.; Tsavlis, D.; Bai, C.; Huang, H.; Freitag, L.; et al. Kras-driven intratumoral heterogeneity triggers infiltration of M2 polarized macrophages via the circHIPK3/PTK2 immunosuppressive circuit. Sci. Rep. 2021, 11, 15455. [Google Scholar] [CrossRef]
- Hu, F.-y.; Cao, X.-n.; Xu, Q.-z.; Deng, Y.; Lai, S.-y.; Ma, J.; Hu, J.-b. miR-124 modulates gefitinib resistance through SNAI2 and STAT3 in non-small cell lung cancer. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 839–845. [Google Scholar] [CrossRef]
- Zhang, P.-F.; Pei, X.; Li, K.-S.; Jin, L.-N.; Wang, F.; Wu, J.; Zhang, X.-M. Circular RNA circFGFR1 promotes progression and anti-PD-1 resistance by sponging miR-381-3p in non-small cell lung cancer cells. Mol. Cancer 2019, 18, 179. [Google Scholar] [CrossRef]
- Minneci, P.C.; Deans, K.J. Clinical trials. Semin. Pediatr. Surg. 2018, 27, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, J.; Sun, T.; Zheng, X.; Li, J.; Sun, H.; Zhou, X.; Zhou, C.; Zhang, H.; Cheng, Z.; et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci. Rep. 2017, 7, 1339. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Wang, X.; Ma, L.; Li, J.; Cao, G.; Zhang, S.; Lin, W. CircVAPA promotes small cell lung cancer progression by modulating the miR-377-3p and miR-494-3p/IGF1R/AKT axis. Mol. Cancer 2022, 21, 123. [Google Scholar] [CrossRef] [PubMed]
- Chao, F.; Zhang, Y.; Lv, L.; Wei, Y.; Dou, X.; Chang, N.; Yi, Q.; Li, M. Extracellular Vesicles Derived circSH3PXD2A Inhibits Chemoresistance of Small Cell Lung Cancer by miR-375-3p/YAP1. Int. J. Nanomed. 2023, 18, 2989–3006. [Google Scholar] [CrossRef]
- Zhan, R.; Yu, H.; Zhang, G.; Ding, Q.; Li, H.; Li, X.; Tang, X. Exosomal EGFR and miR-381-3P Mediate HPV-16 E7 Oncoprotein-Induced Angiogenesis of Non-Small Cell Lung Cancer. Front. Biosci. 2024, 29, 189. [Google Scholar] [CrossRef]
- Du, W.; Yin, F.; Zhong, Y.; Luo, M.; Wang, Z.; Lin, P.; Liu, Q.; Yang, H. CircUCP2 promotes the tumor progression of non-small cell lung cancer through the miR-149/UCP2 pathway. Oncol. Res. 2023, 31, 929–936. [Google Scholar] [CrossRef]
- Mayeux, R. Biomarkers: Potential uses and limitations. NeuroRx 2004, 1, 182–188. [Google Scholar] [CrossRef]
- Cortes, J.; Perez-García, J.M.; Llombart-Cussac, A.; Curigliano, G.; El Saghir, N.S.; Cardoso, F.; Barrios, C.H.; Wagle, S.; Roman, J.; Harbeck, N.; et al. Enhancing global access to cancer medicines. CA Cancer J. Clin. 2020, 70, 105–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Liao, Q. CircHIPK3: A promising cancer-related circular RNA. Am. J. Transl. Res. 2020, 12, 6694–6704. [Google Scholar]
- Yan, B.; Zhang, Y.; Liang, C.; Liu, B.; Ding, F.; Wang, Y.; Zhu, B.; Zhao, R.; Yu, X.Y.; Li, Y. Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/ FOXO3a pathway. Theranostics 2020, 10, 6728–6742. [Google Scholar] [CrossRef]
- Carter, B.W.; Lichtenberger, J.P., 3rd; Benveniste, M.K.; de Groot, P.M.; Wu, C.C.; Erasmus, J.J.; Truong, M.T. Revisions to the TNM Staging of Lung Cancer: Rationale, Significance, and Clinical Application. Radiographics 2018, 38, 374–391. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Guo, C.; Zheng, H.; Zhao, K.; Luo, Y.; An, M.; Lin, Y.; Chen, J.; Li, Y.; Li, Y.; et al. SUMOylation-triggered ALIX activation modulates extracellular vesicles circTLCD4-RWDD3 to promote lymphatic metastasis of non-small cell lung cancer. Signal Transduct. Target. Ther. 2023, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Jordan-Alejandre, E.; Campos-Parra, A.D.; Castro-López, D.L.; Silva-Cázares, M.B. Potential miRNA Use as a Biomarker: From Breast Cancer Diagnosis to Metastasis. Cells 2023, 12, 525. [Google Scholar] [CrossRef]
- Liu, B.N.; Chen, J.; Piao, Y. Global research and emerging trends in autophagy in lung cancer: A bibliometric and visualized study from 2013 to 2022. Front. Pharmacol. 2024, 15, 1352422. [Google Scholar] [CrossRef] [PubMed]
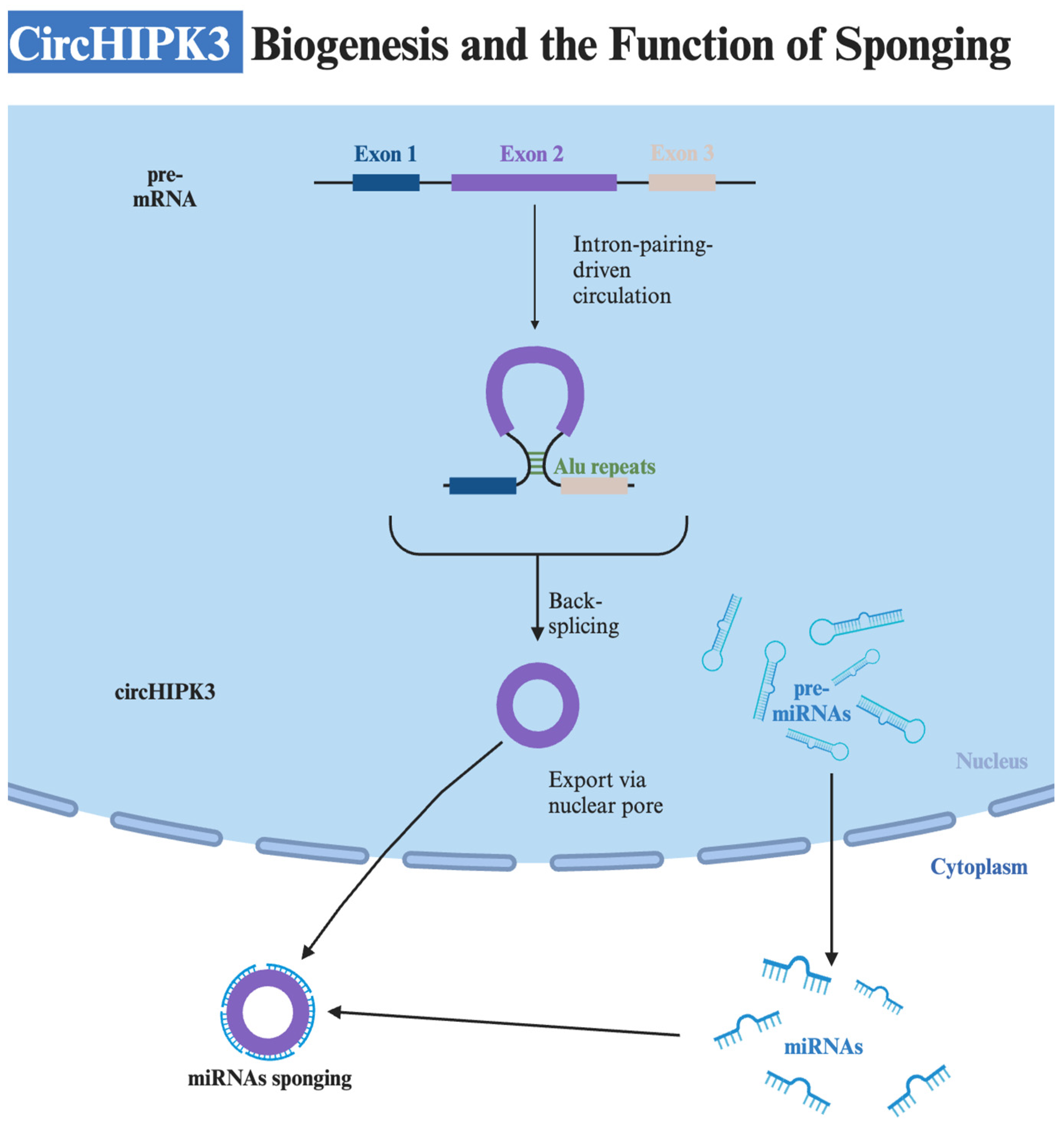
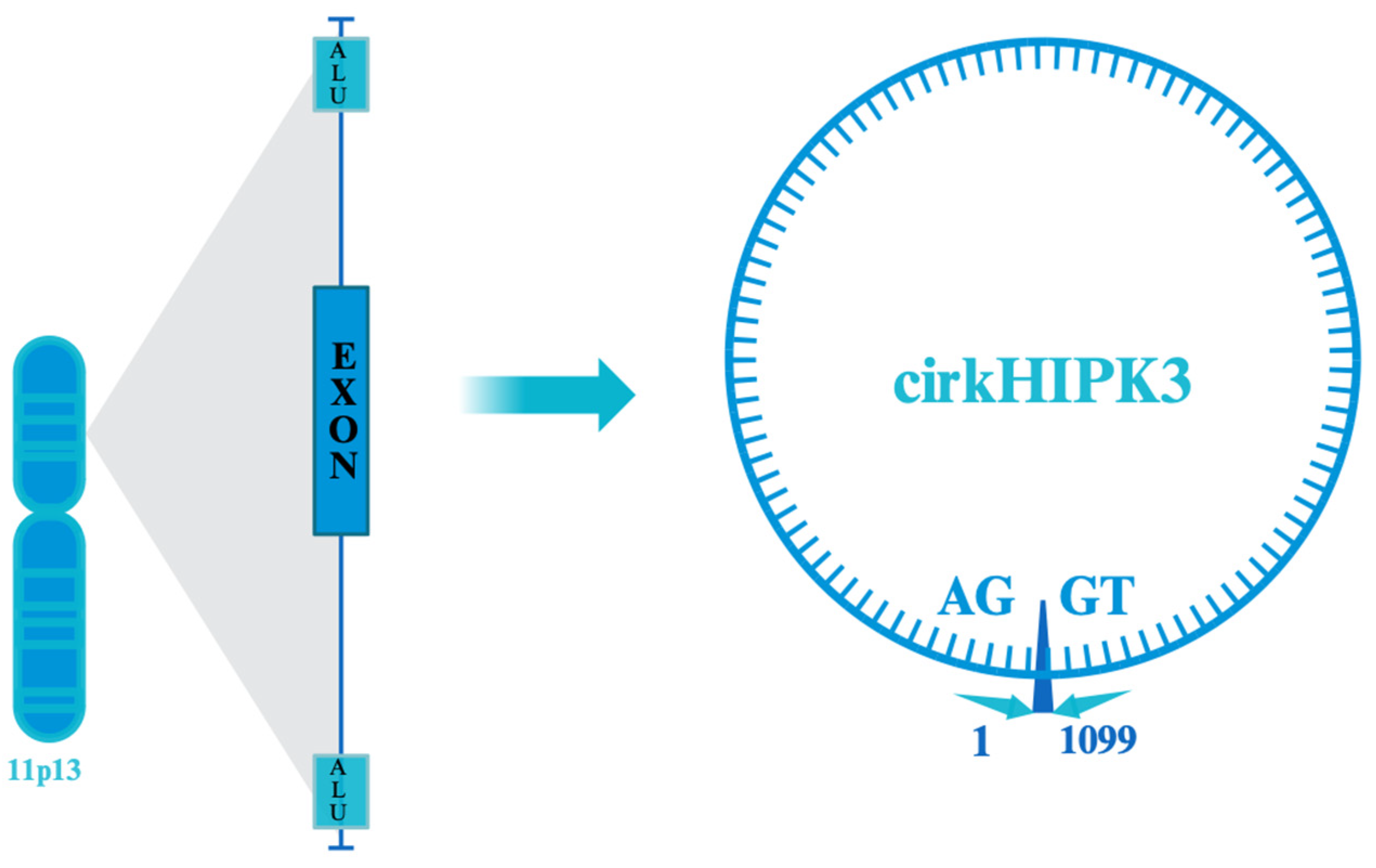
| Mechanism | Description | miRNAs Involved | References |
|---|---|---|---|
| Regulation of cell growth | It influences cell cycle regulators and enhances cell proliferation and growth. | miR-124, miR-193a, miR-29b, | [66,67] |
| Modulation of apoptosis | Controls apoptosis-related proteins, impacting cell survival. | miR-193a | [68] |
| Influence on metastasis | Affects genes involved in cell migration and invasion, modulating metastatic behaviour. | miR-193a | [43] |
| Drug resistance | Modulates autophagy (negatively in bladder cancer) and survival pathways, contributing to chemoresistance. | miR-330-5p | [69] |
| Cancer Type | Role of CircHIPK3 | Mechanism of Action | References |
|---|---|---|---|
| Gastric Cancer | Promotes gastric cancer progression; associated with poor prognosis | Sponges miR-124 and miR-29b to regulate COL1A1, COL4A1, and CDK6 | [42,67,70] |
| Colorectal Cancer | Functions as an oncogene; promotes proliferation, migration, and invasion; decreases apoptosis | Sponges miR-7 and miR-1207-5p to regulate FMNL2 expression | [71] |
| Prostate Cancer | Upregulated in prostate cancer tissues and cells; promotes proliferation and invasiveness | Sponges miR-338-3p to regulate ADAM17 expression | [72,73] |
| Hepatocellular Carcinoma | Significantly upregulated; promotes proliferation and migration | Sponges miR-124 to regulate AQP3 expression | [74] |
| Chronic Myeloid Leukaemia | Upregulated in peripheral blood mononuclear cells and serum; promotes progression. | Not specified | [75] |
| Gallbladder Cancer | Higher expression in cancer cells; inhibits survival and proliferation | Sponges miR-124 to regulate ROCK1 and CDK6 | [76] |
| Osteosarcoma | Downregulated; associated with poor prognosis; suppresses proliferation, migration, and invasion | Not specified | [77] |
| Glioma | Functions as an oncogene; promotes tumour growth | Sponges miR-124-3p and miR-654, regulating STAT3 and IGF2BP3 expression | [78,79] |
| Oral Squamous Cell Carcinoma | Upregulated; promotes proliferation | Regulates miR-124 | [80,81] |
| Epithelial Ovarian Cancer | Downregulated; promotes proliferation, migration, and invasion | Not specified | [29] |
| Nasopharyngeal Carcinoma | Upregulated; promotes proliferation, migration, and invasion | Sponges miR-4288 to regulate ELF3 expression | [82] |
| Cervical Cancer | Upregulated; promotes cell proliferation and EMT, resulting in tumorigenesis | Sponges miR-338-3p, resulting in upregulation of HIF-1α expression | [83] |
| Melanoma | Overexpressed in melanoma cells; promotes cell growth and mitigates cell death | Sponges miR-215-5p, upregulating YY1 expression | [36] |
| Pancreatic Cancer | Upregulated in cancer cells; associated with gemcitabine resistance | Sponges miR-330-5p, upregulating RASSF1, regulating proliferation, invasion, migration, EMT, and apoptosis | [69,84,85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siedlecki, E.; Remiszewski, P.; Stec, R. The Role of circHIPK3 in Tumorigenesis and Its Potential as a Biomarker in Lung Cancer. Cells 2024, 13, 1483. https://doi.org/10.3390/cells13171483
Siedlecki E, Remiszewski P, Stec R. The Role of circHIPK3 in Tumorigenesis and Its Potential as a Biomarker in Lung Cancer. Cells. 2024; 13(17):1483. https://doi.org/10.3390/cells13171483
Chicago/Turabian StyleSiedlecki, Eryk, Piotr Remiszewski, and Rafał Stec. 2024. "The Role of circHIPK3 in Tumorigenesis and Its Potential as a Biomarker in Lung Cancer" Cells 13, no. 17: 1483. https://doi.org/10.3390/cells13171483






