The Role of αvβ3 Integrin in Lamina Cribrosa Cell Mechanotransduction in Glaucoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and LC Cells Characterization
2.2. LC Cells of Normal Donors Cultured on Stiffened Matrices
2.3. Cilengitide Treatment
2.4. β3 Integrin siRNA Transfection
2.5. Total RNA Extraction and Quantitative RT-PCR
2.6. Cell Lysate Preparation and Western Blot Analysis
2.7. Cell Viability Assay (MTT)
2.8. Cell Proliferation Assay
2.9. Statistical Analyses
3. Results
3.1. Expression of αVβ3 and αVβ5 Integrins in LC Cells and in Human ONH
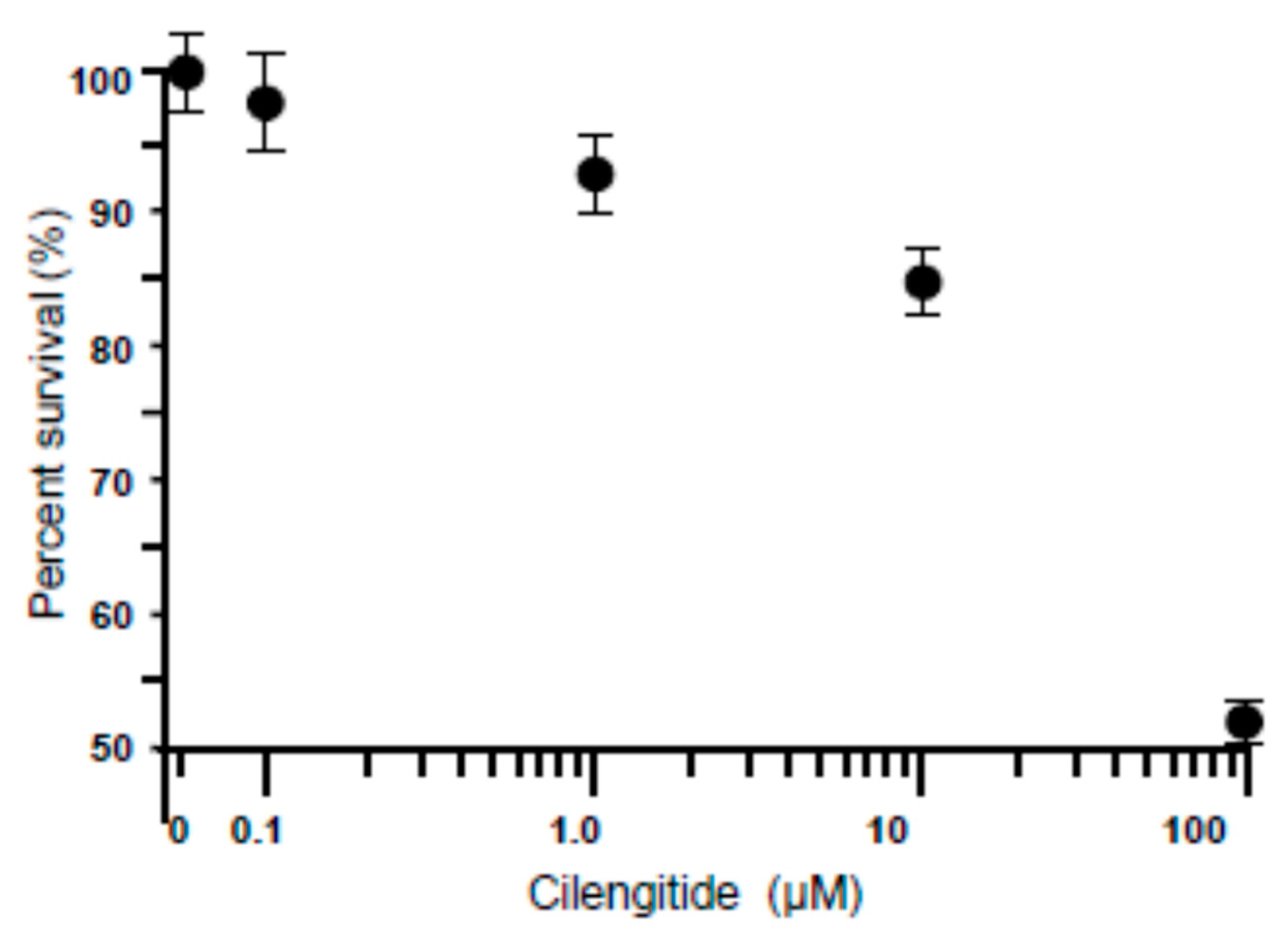
3.2. Dose-Dependent Effect of Cilengitide on LC Cell Viability
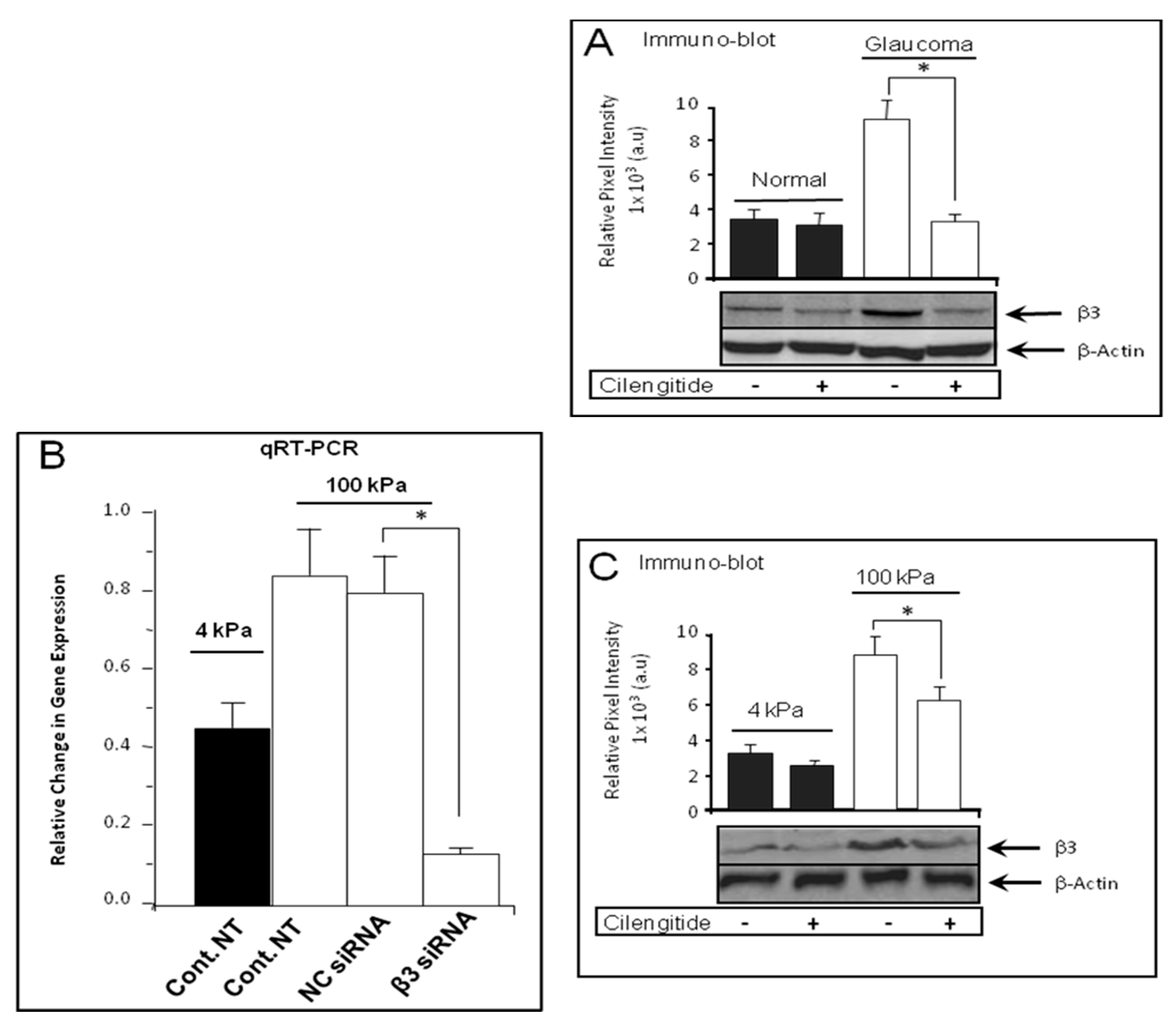
3.3. Cilengitide Treatment Down-Regulated the Expression of β3 Integrin in Glaucoma LC Cells
3.4. Cilengitide Treatment Down-Regulated the Stiffness-Induced Expression of β3 Integrin in Normal LC Cells
3.5. SiRNA Knockdown Treatment Down-Regulated the Expression of β3 Integrin in Glaucoma LC Cells
3.6. siRNA Knockdown Treatment Inhibited the Stiffness-Induced Expression of β3 Integrin in Normal LC Cells
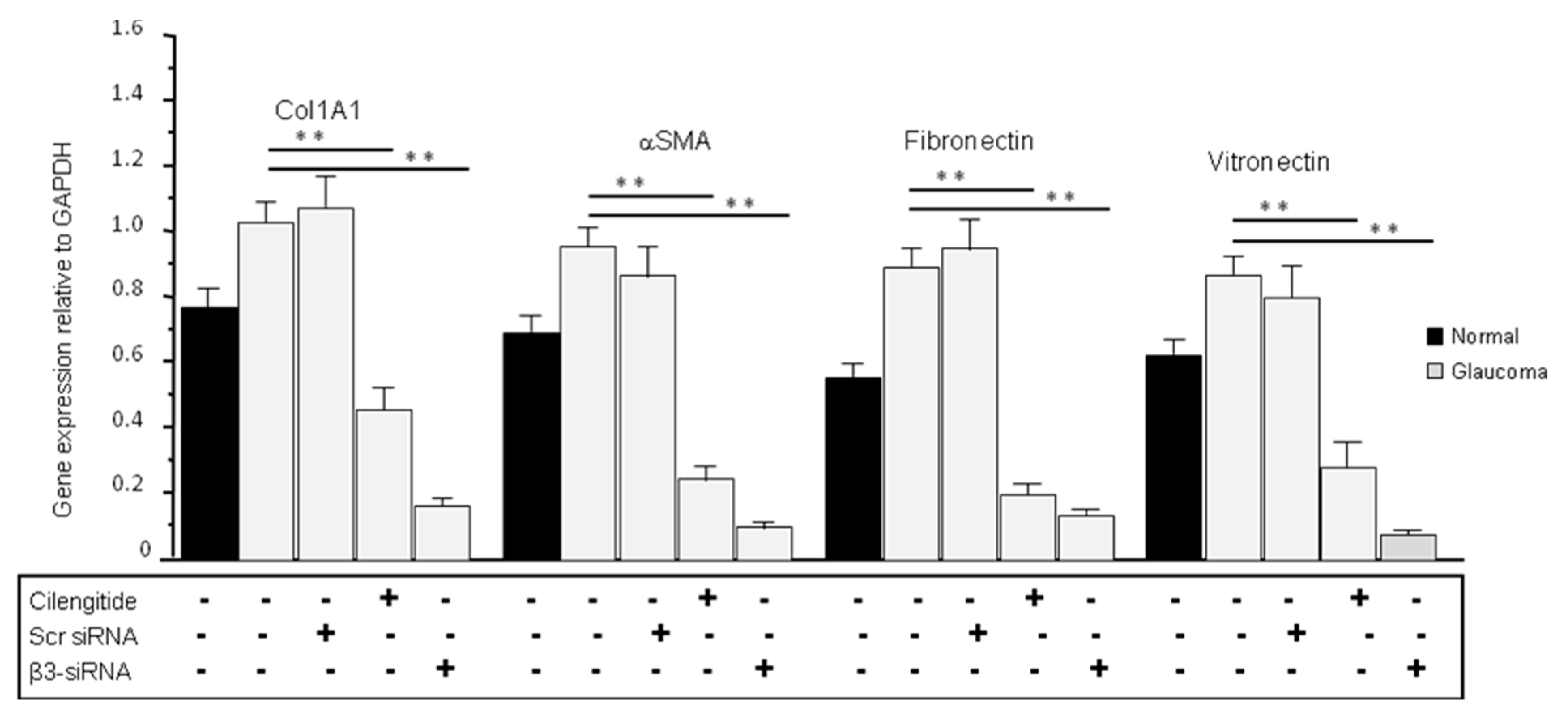
3.7. Cilengitide and siRNA Treatments Reduced the Expression of ECM Production in Glaucoma LC Cells
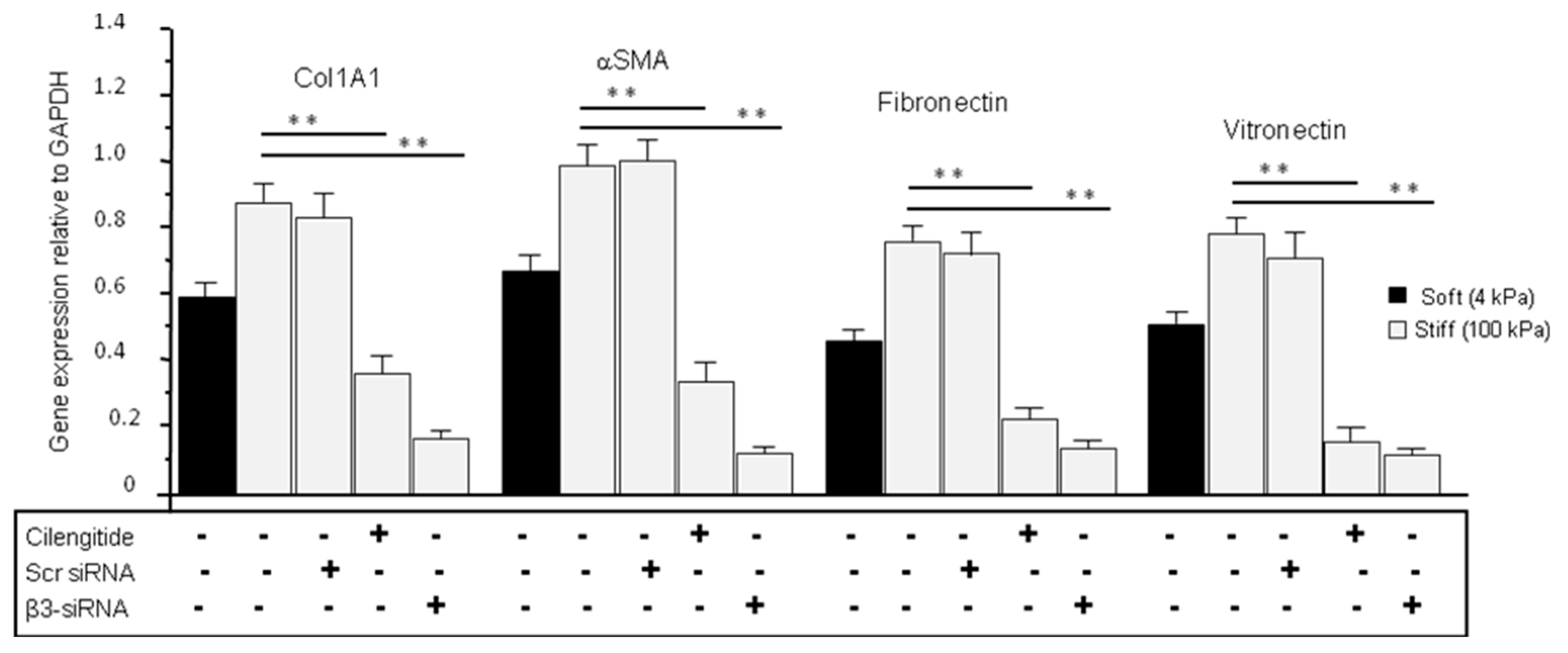
3.8. Cilengitide and siRNA Treatments Reduced the Stiffness-Induced Expression of ECM in Normal LC Cells Grown on Stiff (100 kPa) Substrate
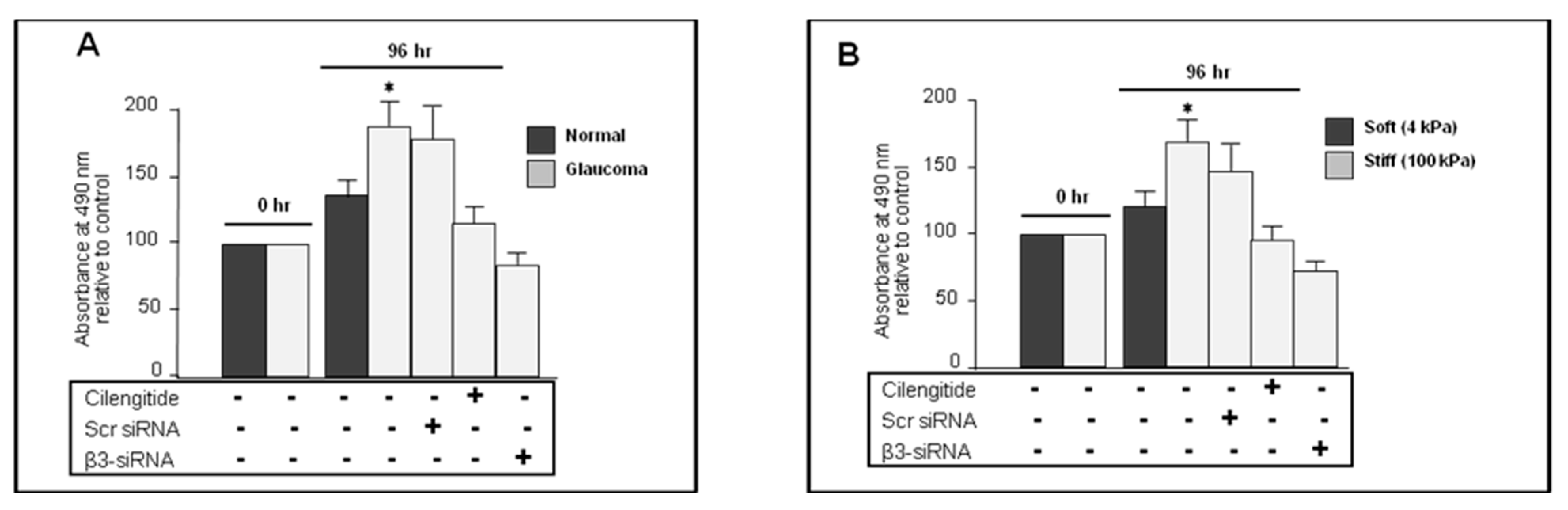
3.9. Cilengitide and siRNA Knockdown of αVβ3 Integrin Inhibited Proliferation in Glaucoma LC Cells
3.10. Cilengitide and siRNA Anti-β3 Integrin Inhibited the Stiffness-Induced Proliferation in Normal LC Cells
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Quigley, H.A.; Enger, C.; Katz, J.; Sommer, A.; Scott, R.; Gilbert, D. Risk factors for the development of glaucomatous visual field loss in ocular hypertension. Arch. Ophthalmol. 1994, 112, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Crawley, L.; Zamir, S.M.; Cordeiro, M.F.; Guo, L. Clinical Options for the Reduction of Elevated Intraocular Pressure. Ophthalmol. Eye Dis. 2012, 4, 43–64. [Google Scholar] [CrossRef]
- Peters, D.; Bengtsson, B.; Heijl, A. Lifetime Risk of Blindness in Open-Angle Glaucoma. Am. J. Ophthalmol. 2013, 156, 724–730. [Google Scholar] [CrossRef]
- Quigley, H.A.; Hohman, R.M.; Addicks, E.M.; Massof, R.W.; Green, W.R. Morphologic Changes in the Lamina Cribrosa Correlated with Neural Loss in Open-Angle Glaucoma. Am. J. Ophthalmol. 1983, 95, 673–691. [Google Scholar] [CrossRef]
- Quigley, H.A.; Addicks, E.M. Chronic experimental glaucoma in primates. I. Production of elevated intraocular pressure by anterior chamber injection of autologous ghost red blood cells. Investig. Ophthalmol. Vis. Sci. 1980, 19, 126–136. [Google Scholar]
- Downs, J.C.; Roberts, M.D.; Burgoyne, C.F. Mechanical environment of the optic nerve head in glaucoma. Optom. Vis. Sci. 2008, 85, 425–435. [Google Scholar] [CrossRef]
- Albon, J.; Purslow, P.P.; Karwatowski, W.S.S.; Easty, D.L. Age related compliance of the lamina cribrosa in human eyes. Br. J. Ophthalmol. 2000, 84, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Albon, J.; Karwatowski, W.S.; Avery, N.; Easty, D.L.; Duance, V.C. Changes in the collagenous matrix of the aging human lamina cribrosa. Br. J. Ophthalmol. 1995, 79, 368–375. [Google Scholar] [CrossRef]
- Burgoyne, C.F.; Crawford Downs, J.; Bellezza, A.J.; Francis Suh, J.-K.; Hart, R.T. The optic nerve head as a biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res. 2005, 24, 39–73. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.A.; Murphy, R.; Irnaten, M.; Wallace, D.M.; Quill, B.; O’Brien, C. The role of lamina cribrosa tissue stiffness and fibrosis as fundamental biomechanical drivers of pathological glaucoma cupping. Am. J. Physiol. Physiol. 2020, 319, C611–C623. [Google Scholar] [CrossRef] [PubMed]
- Zeimer, R.C.; Ogura, Y. The Relation Between Glaucomatous Damage and Optic Nerve Head Mechanical Compliance. Arch. Ophthalmol. 1989, 107, 1232–1234. [Google Scholar] [CrossRef]
- Burgoyne, C.F.; Varma, R.; Quigley, H.A.; Vitale, S.; Pease, M.E.; Lenane, P.L. Global and regional detection of induced optic disc change by digitised image analysis. Arch. Ophthalmol. 1994, 112, 1261–1268. [Google Scholar] [CrossRef]
- Burgoyne, C.F.; Quigley, H.A.; Thompson, H.W.; Vitale, S.; Varma, R. Measurement of optic disc compliance by digitised image analysis in the normal monkey eye. Ophthalmology 1995, 102, 1790–1799. [Google Scholar] [CrossRef]
- Burgoyne, C.F.; Quigley, H.A.; Thompson, H.W.; Vitale, S.; Varma, R. Early changes in optic disc compliance and surface position in experimental glaucoma. Ophthalmology 1995, 102, 1800–1809. [Google Scholar] [CrossRef]
- Coleman, A.L.; Quigley, H.A.; Vitale, S.; Dunkelberger, G. Displacement of the optic nerve head by acute changes in intraocular pressure in monkey eyes. Ophthalmology 1991, 98, 35–40. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Andrzejewska, W.M.; Neufeld, A.H. Changes in the extracellular matrix of the human optic nerve head in primary open-angle glaucoma. Am. J. Ophthalmol. 1990, 109, 180–188. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Igoe, F.; Neufeld, A.H. Cell culture of the human lamina cribrosa. Investig. Ophthalmol. Vis. Sci. 1988, 29, 78–89. [Google Scholar]
- Last, J.A.; Pan, T.; Ding, Y.; Reilly, C.M.; Keller, K.; Acott, T.S.; Fautsch, M.P.; Murphy, C.J.; Russell, P. Elastic Modulus Determination of Normal and Glaucomatous Human Trabecular Meshwork. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2147–2152. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Johnstone, M.A.; Xin, C.; Song, S.; Padilla, S.; Vranka, J.A.; Acott, T.S.; Zhou, K.; Schwaner, S.A.; Wang, R.K.; et al. Estimating Human Trabecular Meshwork Stiffness by Numerical Modeling and Advanced OCT Imaging. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4809–4817. [Google Scholar] [CrossRef]
- Wang, K.; Read, A.T.; Sulchek, T.; Ethier, C.R. Trabecular meshwork stiffness in glaucoma. Exp. Eye Res. 2017, 158, 3–12. [Google Scholar] [CrossRef]
- Wang, A.G.; Yen, M.Y.; Hsu, W.M.; Fann, M.J. Induction of vitronectin and integrin αv in the retina after optic nerve injury. Mol. Vis. 2006, 12, 76–84. [Google Scholar]
- Vahabikashi, A.; Gelman, A.; Dong, B.; Gong, L.; Cha, E.D.K.; Schimmel, M.; Tamm, E.R.; Perkumas, K.; Stamer, W.D.; Sun, C.; et al. Increased stiffness and flow resistance of the inner wall of Schlemm’s canal in glaucomatous human eyes. Proc. Natl. Acad. Sci. USA 2019, 116, 26555–26563. [Google Scholar] [CrossRef]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119 Pt 19, 3901–3903. [Google Scholar] [CrossRef] [PubMed]
- Malgorzata, B.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef]
- Seguin, L.; Desgrosellier, J.S.; Weisand, S.M.; Cheresh, D.A. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015, 25, 234–240. [Google Scholar] [CrossRef]
- Gerthoffer, W.T.; Gunst, S.J. Invited Review: Focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J. Appl. Physiol. 2001, 91, 963–972. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Conroy, K.P.; Kitto, L.J.; Henderson, N.C. αv integrins: Key regulators of tissue fibrosis. Cell Tissue Res. 2016, 365, 511–519. [Google Scholar] [CrossRef]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef]
- Sheppard, D. The role of integrins in pulmonary fibrosis. Eur. Respir. Rev. 2008, 17, 157–162. [Google Scholar] [CrossRef]
- Henderson, N.C.; Sheppard, D. Integrin-mediated regulation of TGFβ in fibrosis. Biochim. Biophys. Acta 2013, 1832, 891–896. [Google Scholar] [CrossRef]
- Patsenker, E.; Stickel, F. Role of integrins in fibrosing liver diseases. Am. J. Physiol. Liver Physiol. 2011, 301, G425–G434. [Google Scholar] [CrossRef]
- Fiore, V.F.; Wong, S.S.; Tran, C.; Tan, C.; Xu, W.; Sulchek, T.; White, E.S.; Hagood, J.S.; Barker, T.H. αvβ3 Integrin drives fibroblast contraction and strain stiffening of soft provisional matrix during progressive fibrosis. JCI Insight 2018, 3, e97597. [Google Scholar] [CrossRef]
- Koivisto, L.; Heino, J.; Hakkinen, L.; Larjava, H. Integrins in wound healing. Adv. Wound Care 2014, 3, 762–783. [Google Scholar] [CrossRef]
- Filla, M.S.; Meyer, K.K.; Faralli, J.A.; Peters, D.M. Overexpression and Activation of αvβ3 Integrin Differentially Affects TGFβ2 Signaling in Human Trabecular Meshwork Cells. Cells 2021, 10, 1923. [Google Scholar] [CrossRef]
- Morrison, J.C. Integrins in the optic nerve head: Potential roles in glaucomatous optic neuropathy (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 453–477. [Google Scholar]
- Bermudez, J.Y.; Montecchi-Palmer, M.; Mao, W.; Clark, A.F. Cross-linked actin networks (CLANs) in glaucoma. Exp. Eye Res. 2017, 159, 16–22. [Google Scholar] [CrossRef]
- Filla, M.S.; Woods, A.; Kaufman, P.L.; Peters, D.M. β1 and β3 integrins cooperate to induce syndecan-4-containing cross-linked actin networks in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1956–1967. [Google Scholar] [CrossRef]
- Filla, M.S.; Faralli, J.A.; Peotter, J.L.; Peters, D.M. The role of integrins in glaucoma. Exp. Eye Res. 2017, 158, 124–136. [Google Scholar] [CrossRef]
- Sheppard, D.; Cohen, D.S.; Wang, A.; Busk, M. Transforming growth factor beta differentially regulates expression of integrin subunits in guinea pig airway epithelial cells. J. Biol. Chem. 1992, 267, 17409–17414. [Google Scholar] [CrossRef]
- Gagen, D.; Faralli, J.A.; Filla, M.S.; Peters, D.M. The Role of Integrins in the Trabecular Meshwork. J. Ocul. Pharmacol. Ther. 2013, 30, 110–120. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, W.; Lu, X.; Qian, C.; Zhang, J.; Li, P.; Shi, L.; Zhao, P.; Fu, Z.; Pu, P.; et al. Overexpression of osteopontin induces angiogenesis of endothelial progenitor cells via the avβ3/PI3K/AKT/eNOS/NO signaling pathway in glioma cells. Eur. J. Cell Biol. 2011, 90, 642–648. [Google Scholar] [CrossRef]
- Chang, J.Y.H.; Stamer, W.D.; Bertrand, J.; Read, A.T.; Marando, C.M.; Ethier, C.R.; Overby, D.R. Role of nitric oxide in murine conventional outflow physiology. Am. J. Physiol. Physiol. 2015, 309, C205–C214. [Google Scholar] [CrossRef]
- Faralli, J.A.; Gagen, D.; Filla, M.S.; Crotti, T.N.; Peters, D.M. Dexamethasone increases αvβ3 integrin expression and affinity through a calcineurin/NFAT pathway. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 3306–3313. [Google Scholar] [CrossRef]
- Tzima, E.; del Pozo, M.A.; Shattil, S.J.; Chien, S.; Schwartz, M.A. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001, 20, 4639–4647. [Google Scholar] [CrossRef]
- Schwarzbauer, J.E.; DeSimone, D.W. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 2011, 3, a005041. [Google Scholar] [CrossRef]
- Kirwan, R.P.; Leonard, M.O.; Murphy, M.; Clark, A.F.; O’Brien, C.J. Transforming Growth factor-β-regulated gene transcription and protein expression in human GFAP-negative lamina cribrosa cells. Glia 2005, 52, 309–324. [Google Scholar] [CrossRef] [PubMed]
- McElnea, E.M.; Quill, B.; Docherty, N.G.; Irnaten, M.; Siah, W.; Clark, A.; O’brien, C.; Wallace, D. Oxidative stress, mitochondrial dysfunction and calcium overload in human lamina cribrosa cells from glaucoma donors. Mol. Vis. 2011, 17, 1182–1191. [Google Scholar]
- Kirwan, R.P.; Wordinger, R.J.; Clark, A.F.; O’Brien, C.J. Differential global and extra-cellular matrix focused gene expression patterns between normal and glaucomatous human lamina cribrosa cells. Mol. Vis. 2009, 15, 76–88. [Google Scholar] [PubMed]
- Liu, B.; Kilpatrick, J.I.; Lukasz, B.; Jarvis, S.P.; McDonnell, F.; Wallace, D.M.; Clark, A.F.; O’Brien, C.J. Increased substrate stiffness elicits a myofibroblastic phenotype in human lamina cribrosa cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.N.; Clark, A.F.; Tovar-Vidales, T. Isolation and characterization of human optic nerve head astrocytes and lamina cribrosa cells. Exp. Eye Res. 2020, 197, 108103. [Google Scholar] [CrossRef] [PubMed]
- Lambert, W.S.; Clark, A.F.; Wordinger, R.J. Neurotrophin and Trk expression by cells of the human lamina cribrosa following oxygen-glucose deprivation. BMC Neurosci. 2004, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Irnaten, M.; Zhdanov, A.; Brennan, D.; Crotty, T.; Clark, A.; Papkovsky, D.; O’Brien, C. Activation of the NFAT-calcium signaling pathway in human lamina cribrosa cells in glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 831–842. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Goodman, S.L.; Holzemann, G.; Sulyok, G.A.; Kessler, H. Nanomolar small molecule inhibitors for αvβ6, αvβ5, and αvβ3 integrins. J. Med. Chem. 2002, 45, 1045–1051. [Google Scholar] [CrossRef]
- Wang, J.; Harris, A.; Prendes, M.A.; Alshawa, L.; Gross, J.C.; Wentz, S.M.; Rao, A.B.; Kim, N.J.; Synder, A.; Siesky, B. Targeting Transforming Growth Factor-β Signaling in Primary Open-Angle Glaucoma. J. Glaucoma 2017, 26, 390–395. [Google Scholar] [CrossRef]
- Damsky, C.H.; Werb, Z. Signal transduction by integrin receptors for extracellular matrix: Cooperative processing of extracellular information. Curr. Opin. Cell Biol. 1992, 4, 772–781. [Google Scholar] [CrossRef]
- Filla, M.S.; Faralli, J.A.; Desikan, H.; Peotter, J.L.; Wannow, A.C.; Peters, D.M. Activation of αvβ3 integrin alters fibronectin fibril formation in human trabecular meshwork cells in a ROCK-independent manner. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3897–3913. [Google Scholar] [CrossRef]
- Pena, J.D.; Taylor, A.W.; Ricard, C.S.; Vidal, I.; Hernandez, M.R. Transforming growth factor beta isoforms in human optic nerve heads. Br. J. Ophthalmol. 1999, 83, 209–218. [Google Scholar] [CrossRef]
- Wallace, D.M.; Murphy-Ullrich, J.E.; Downs, J.C.; O’Brien, C.J. The role of matricellular proteins in glaucoma. Matrix Biol. 2014, 37, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.L. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am. J. Pathol. 2009, 175, 1362–1370. [Google Scholar] [CrossRef]
- Brown, N.F.; Marshall, J.F. Integrin-Mediated TGFβ Activation Modulates the Tumour Microenvironment. Cancers 2019, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Faralli, J.A.; Filla, M.S.; Peters, D.M. Effect of αvβ3 integrin expression and activity on intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1776–1788. [Google Scholar] [CrossRef]
- Ellison, J.A.; Barone, F.C.; Feuerstein, G.Z. Matrix remodeling after stroke. De novo expression of matrix proteins and integrin receptors. Ann. N. Y. Acad. Sci. 1999, 890, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Leyton, L.; Schneider, P.; Labra, C.V.; Rüegg, C.; Hetz, C.A.; Quest, A.F.; Bron, C. Thy-1 binds to integrin β3 on astrocytes and triggers formation of focal contact sites. Curr. Biol. 2001, 11, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.E.; Ramirez, G.; Hagood, J.S. Roles and regulation of Thy-1, a context dependent modulator of cell phenotype. Biofactors 2009, 35, 258–265. [Google Scholar] [CrossRef]
- Schlamp, C.L.; Johnson, E.C.; Li, Y.; Morrison, J.C.; Nickells, R.W. Changes in Thy1 gene expression associated with damaged retinal ganglion cells. Mol. Vis. 2001, 7, 192–201. [Google Scholar]
- Hermosilla, T.; Muñoz, D.; Herrera-Molina, R.; Valdivia, A.; Muñoz, N.; Nham, S.U.; Schneider, P.; Burridge, K.; Quest, A.F.; Leyton, L. Direct Thy-1/αVβ3 integrin interaction mediates neuron to astrocyte communication. Biochim. Biophys. Acta 2008, 1783, 1111–1120. [Google Scholar] [CrossRef]
- Henríquez, M.; Herrera-Molina, R.; Valdivia, A.; Alvarez, A.; Kong, M.; Muñoz, N.; Eisner, V.; Jaimovich, E.; Schneider, P.; Quest, A.F.; et al. ATP release due to Thy-1-integrin binding induces P2X7-mediated calcium entry required for focal adhesion formation. J. Cell Sci. 2011, 124, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Molina, R.; Frischknecht, R.; Maldonado, H.; Seidenbecher, C.I.; Gundelfinger, E.D.; Hetz, C.; Aylwin Mde, L.; Schneider, P.; Quest, A.F.; Leyton, L. Astrocytic αVβ3 integrin inhibits neurite outgrowth and promotes retraction of neuronal processes by clustering Thy-1. PLoS ONE 2012, 7, e34295. [Google Scholar] [CrossRef]
- Lagos-Cabré, R.; Brenet, M.; Díaz, J.; Pérez, R.D.; Pérez, L.A.; Herrera-Molina, R.; Quest, A.F.G.; Leyton, L. Intracellular Ca2+ increases and connexin 43 hemichannel opening are necessary but not sufficient for thy-1-induced astrocyte migration. Int. J. Mol. Sci. 2018, 19, 2179. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Schlötzer-Schrehardt, U.; Pal-Ghosh, S.; Stepp, M.A. Integrin: Basement membrane adhesion by corneal epithelial and endothelial cells. Exp. Eye Res. 2020, 198, 108138. [Google Scholar] [CrossRef]
- Małgorzata, M.; Bryl, A.; Falkowski, M.; Zorena, K. Integrins: An Important Link between Angiogenesis, Inflammation and Eye Diseases. Cells 2021, 10, 1703. [Google Scholar] [CrossRef]
- Wu, W.; Hutcheon, A.E.K.; Sriram, S.; Tran, J.A.; Zieske, J.D. Initiation of fibrosis in the integrin Avβ6 knockout mice. Exp. Eye Res. 2019, 180, 23–28. [Google Scholar] [CrossRef]
- Weller, J.M.; Zenkel, M.; Schlotzer-Schrehardt, U.; Bachmann, B.O.; Tourtas, T.; Kruse, F.E. Extracellular matrix alterations in late-onset Fuchs’ corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3700–3708. [Google Scholar] [CrossRef]
- Storm, R.J.; Persson, B.D.; Skalman, L.N.; Frangsmyr, L.; Lindstrom, M.; Rankin, G.; Lundmark, R.; Domellof, F.P.; Arnberg, N. Human Adenovirus Type 37 Uses αVβ1 and α3β1 Integrins for Infection of Human Corneal Cells. J. Virol. 2017, 91, e02019-16. [Google Scholar] [CrossRef]
- Perrucci, G.L.; Barbagallo, V.A.; Corlianò, M.; Tosi, D.; Santoro, R.; Nigro, P.; Poggio, P.; Bulfamante, G.; Lombardi, F.; Pompilio, G. Integrin ανβ5 in vitro inhibition limits pro-fibrotic response in cardiac fibroblasts of spontaneously hypertensive rats. J. Transl. Med. 2018, 16, 352. [Google Scholar] [CrossRef]
- Bouvet, M.; Claude, O.; Roux, M.; Skelly, D.; Masurkar, N.; Mougenot, N.; Nadaud, S.; Blanc, C.; Delacroix, C.; Chardonnet, S.; et al. Anti-integrin αv therapy improves cardiac fibrosis after myocardial infarction by blunting cardiac PW1+ stromal cells. Sci. Rep. 2020, 10, 11404. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Quinones, L.; Kasiganesan, H.; Zhang, Y.; Pleasant, D.L.; Sundararaj, K.P.; Zile, M.R.; Bradshaw, A.D.; Kuppuswamy, D. β3integrinin cardiac fibroblast is critical for extracellular matrix accumulation during pressure overload hypertrophy in mouse. PLoS ONE 2012, 7, e45076. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Gustafson, D.; Holden, S.; McConkey, D.; Davis, D.; Morrow, M.; Basche, M.; Gore, L.; Zang, C.; O’Bryant, C.L.; et al. Assessment of the biological and pharmacological effects of the ανβ3 and ανβ5 integrin receptor antagonist, cilengitide (EMD 121974), in patients with advanced solid tumors. Ann. Oncol. 2007, 18, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Nabors, L.B.; Stupp, R.; Mikkelsen, T. Cilengitide: An integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin. Investig. Drugs 2008, 17, 1225–1235. [Google Scholar] [CrossRef]
- Patsenker, E.; Popov, Y.; Wiesner, M.; Goodman, S.L.; Schuppan, D. Pharmacological inhibition of the vitronectin receptor abrogates PDGF-BB-induced hepatic stellate cell migration and activation in vitro. J. Hepatol. 2007, 46, 878–887. [Google Scholar] [CrossRef]


| Donor ID | Age | Gender | Disease State | Eye Bank |
|---|---|---|---|---|
| 41-02 | 88 | F | Non-glaucoma | Central Florida (Tampa) |
| 82-02 | 88 | F | Non-glaucoma | Central Florida (Tampa) |
| 439-02 | 79 | M | Non-glaucoma | Central Florida (Tampa) |
| 444-02 | 87 | M | Non-glaucoma | Central Florida (Tampa) |
| 553-02 | 87 | F | Non-glaucoma | Central Florida (Tampa) |
| 58-02 | 84 | F | Glaucoma | Central Florida (Tampa) |
| 350-02 | 84 | F | Glaucoma | Central Florida (Tampa) |
| 412-02 | 85 | M | Glaucoma | Central Florida (Tampa) |
| 600-02 | 86 | M | Glaucoma | Central Florida (Tampa) |
| 652-02 | 79 | M | Glaucoma | Central Florida (Tampa) |
| Human Genes | Forward | Reverse |
|---|---|---|
| Integrin αv | AATCTTATTGAGGATATCAC | AAAACAAGTAGCAACAAT |
| Integrin β5 | GGAGCCAGAGTGTGGAAACA | GAAACTTTGCAAACTCCCTC |
| Integrin β3 | GTCACCTGAAGGAGAATCTGC | TTCTTCGAATCATCTGGCC |
| αSMA | CCGACCGAATGCAGAAGGA | ACAGAGTATTTGCGCTCCGAA |
| Col1A1 | ACGAAGACATCCCACCAATC | ATGGTACCTGAGGCCGTTC |
| Fibronectin | ACAACACCGAGGTGACTGAGAC | GGACACAACGATGCTTCCTGAG |
| Vitronectin | ATGGGTTGCTCTGGCTGAC | CTGCTGGGGGCTGAGGTCT |
| 18S | CTGGGACGACATGGAGAAAA | AAGGAAGGCTGGAAGAGTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irnaten, M.; Gaynor, E.; O’Brien, C. The Role of αvβ3 Integrin in Lamina Cribrosa Cell Mechanotransduction in Glaucoma. Cells 2024, 13, 1487. https://doi.org/10.3390/cells13171487
Irnaten M, Gaynor E, O’Brien C. The Role of αvβ3 Integrin in Lamina Cribrosa Cell Mechanotransduction in Glaucoma. Cells. 2024; 13(17):1487. https://doi.org/10.3390/cells13171487
Chicago/Turabian StyleIrnaten, Mustapha, Ellen Gaynor, and Colm O’Brien. 2024. "The Role of αvβ3 Integrin in Lamina Cribrosa Cell Mechanotransduction in Glaucoma" Cells 13, no. 17: 1487. https://doi.org/10.3390/cells13171487
APA StyleIrnaten, M., Gaynor, E., & O’Brien, C. (2024). The Role of αvβ3 Integrin in Lamina Cribrosa Cell Mechanotransduction in Glaucoma. Cells, 13(17), 1487. https://doi.org/10.3390/cells13171487







