The Relationship between HERV, Interleukin, and Transcription Factor Expression in ZIKV Infected versus Uninfected Trophoblastic Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Infections
2.2. Nucleic Acid Extraction
2.3. Molecular Detection
2.4. Detection of ERV Expression in Placenta Cell Line
2.5. Expression of Interleukins and Transcription Factors Related to the Immune Response
2.6. Data Analysis
3. Results
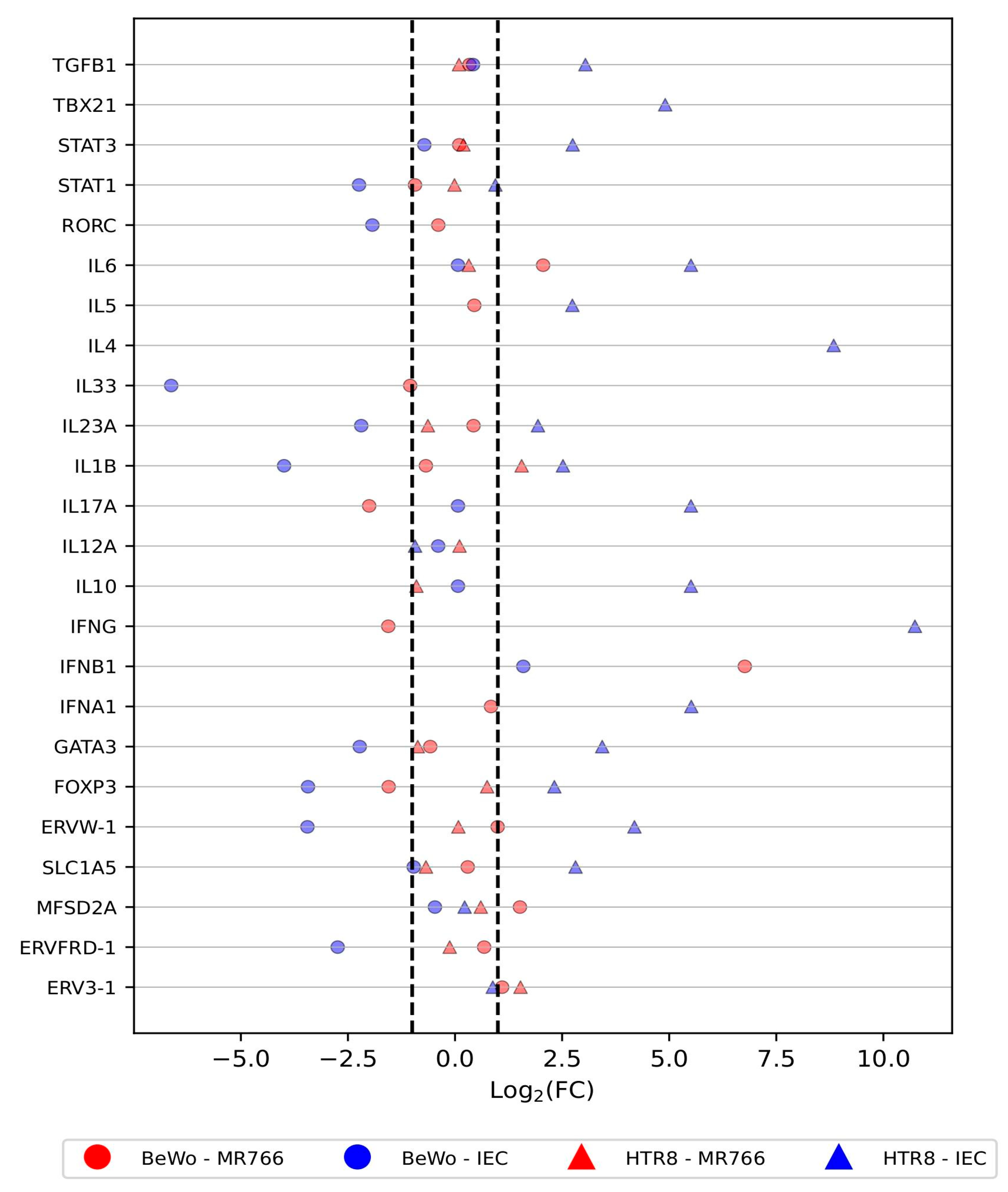
3.1. Differential Expression of Host Defense Genes in Trophoblastic Cells in Response to ZIKV Infection
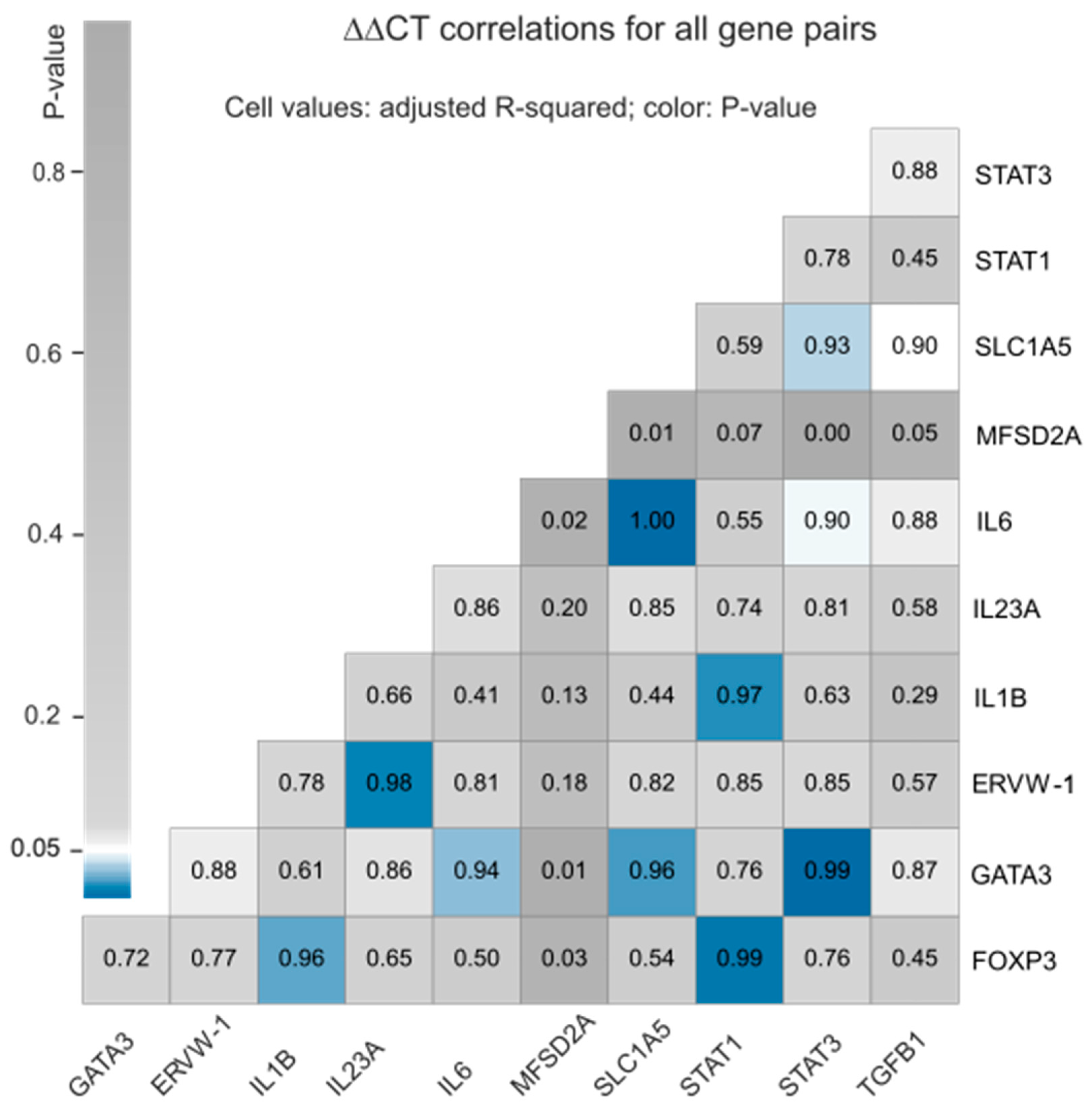
3.2. Correlation between the Expression Profiles of Selected Host Defense Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Davison, A.J.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; et al. Changes to virus taxonomy and to the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses. Arch. Virol. 2021, 166, 2633–2648. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses Executive Committee. The new scope of virus taxonomy: Partitioning the virosphere into 15 hierarchical ranks. Nat. Microbiol. 2020, 5, 668–674. [Google Scholar] [CrossRef]
- Postler, T.S.; Beer, M.; Blitvich, B.J.; Bukh, J.; de Lamballerie, X.; Drexler, J.F.; Imrie, A.; Kapoor, A.; Karganova, G.G.; Lemey, P.; et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch.Virol. 2023, 168, 224. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Gubler, D.J. 2016. Zika virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef]
- Faye, O.; Freire, C.C.M.; Iamarino, A.; Faye, O.; de Oliveira, J.V.C.; Diallo, M.; Zanotto, P.M.A.; Sall, A.A. Molecular evolution of Zika virus during its emergence in the 20th century. PLoS Neglected Trop. Dis. 2014, 8, e2636. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, D.; Kuhn, R.J. Zika virus structure, maturation, and receptors. J. Infect. Dis. 2017, 8, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.; Ksiazek, T.G. Zika virus, a cause of fever in Central Java, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 389–393. [Google Scholar] [CrossRef]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika virus outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885. [Google Scholar] [CrossRef]
- Zanluca, C.; Melo, V.C.; Mosimann, A.L.; Santos, G.I.; Santos, C.N.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz. 2015, 110, 569–572. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.D.; Costa, A.; Prieto-Oliveira, P.; Andreata-Santos, R.; Peter, C.M.; Zanotto, P.M.; Janini, L.M.R. Proposal of Model for Evaluation of Viral Kinetics of African/Asian/Brazilian—Zika virus Strains (Step Growth Curve) in Trophoblastic Cell Lines. Viruses 2023, 15, 1446. [Google Scholar] [CrossRef] [PubMed]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.; Guimarães, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Blaise, S.; de Parseval, N.; Heidmann, T. Functional characterization of two newly identified Human Endogenous Retrovirus coding envelope genes. Retrovirology 2005, 2, 19. [Google Scholar] [CrossRef]
- International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Hayward, A.; Cornwallis, C.K.; Jern, P. Pan-vertebrate comparative genomics unmasks retrovirus macroevolution. Proc. Natl. Acad. Sci. USA 2015, 112, 464–469. [Google Scholar] [CrossRef]
- Johnson, W.E.; Coffin, J.M. Constructing primate phylogenies from ancient retrovirus sequences. Proc. Natl. Acad. Sci. USA 1999, 96, 10254–10260. [Google Scholar] [CrossRef]
- Tristem, M. Identification and characterization of novel human endogenous retrovirus families by phylogenetic screening of the human genome mapping project database. J. Virol. 2000, 74, 3715–3730. [Google Scholar] [CrossRef]
- de Parseval, N.; Lazar, V.; Casella, J.F.; Benit, L.; Heidmann, T. Survey of human genes of retroviral origin: Identification and transcriptome of the genes with coding capacity for complete envelope proteins. J. Virol. 2003, 77, 10414–10422. [Google Scholar] [CrossRef]
- Szpakowski, S.; Sun, X.; Lage, J.M.; Dyer, A.; Rubinstein, J.; Kowalski, D.; Sasaki, C.; Costa, J.; Lizardi, P.M. Loss of epigenetic silencing in tumors preferentially affects primate-specific retroelements. Gene 2009, 448, 151–167. [Google Scholar] [CrossRef]
- Lavie, L.; Kitova, M.; Maldener, E.; Meese, E.; Mayer, J. CpG methylation directly regulates transcriptional activity of the human endogenous retrovirus family HERV-K(HML-2). J. Virol. 2005, 79, 876–883. [Google Scholar] [CrossRef]
- Blond, J.L.; Lavillette, D.; Cheynet, V.; Bouton, O.; Oriol, G.; Chapel-Fernandes, S.; Mandrand, B.; Mallet, F.; Cosset, F.L. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 2000, 74, 3321–3329. [Google Scholar] [CrossRef]
- Chen, C.P.; Chen, L.F.; Yang, S.R.; Chen, C.Y.; Ko, C.C.; Chang, G.D.; Chen, H. Functional characterization of the human placental fusogenic membrane protein syncytin 2. Biol. Reprod. 2008, 79, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Esnault, C.; Priet, S.; Ribet, D.; Vernochet, C.; Bruls, T.; Lavialle, C.; Weissenbach, J.; Heidmann, T. A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc. Natl. Acad. Sci. 2008, 105, 17532–17537. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Mosmann, T.R.; Guilbert, L.; Tuntipopipat, S.; Wegmann, T.G. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 1993, 151, 4562–4573. [Google Scholar] [CrossRef] [PubMed]
- Marzi, M.; Vigano, A.; Trabattoni, D.; Villa, M.L.; Salvaggio, A.; Clerici, E.; Cleirici, M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin. Exp. Immunol. 1996, 106, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar] [CrossRef]
- Del Prete, G.F.; De Carli, M.; Mastromauro, C.; Biagiotti, R.; Macchia, D.; Falagiani, P.; Ricci, M.; Romagnani, S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J. Clin. Investig. 1991, 88, 346–350. [Google Scholar] [CrossRef]
- Tolosa, J.M.; Schjenken, J.E.; Clifton, V.L.; Vargas, A.; Barbeau, B.; Lowry, P.; Maiti, K.; Smith, R. The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta 2012, 33, 933–941. [Google Scholar] [CrossRef]
- Lokossou, A.G.; Toudic, C.; Nguyen, P.T.; Elisseeff, X.; Vargas, A.; Rassart, É.; Lafond, J.; Leduc, L.; Bourgault, S.; Gilbert, C.; et al. Endogenous retrovirus-encoded Syncytin-2 contributes to exosome-mediated immunosuppression of T cells†. Biol. Reprod. 2020, 102, 185–198. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Joyce, J.; Fitch, F.W. Antiproliferative effect of IFN-γ in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-γ. J. Immunol. 1989, 143, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Seder, R.A.; Paul, W.E.; Davis, M.M.; Fazekas de St Groth, B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J. Exp. Med. 1992, 176, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, S.; Mandrekar, P. Cellular stress response and innate immune signaling: Integrating pathways in host defense and inflammation. J. Leukoc. Biol. 2013, 94, 1167–1184. [Google Scholar] [CrossRef]
- Schust, D.J.; Bonney, E.A.; Sugimoto, J.; Ezashi, T.; Roberts, R.M.; Choi, S.; Zhou, J. The immunology of syncytialized trophoblast. Int. J. Mol. Sci. 2021, 22, 4–1767. [Google Scholar] [CrossRef] [PubMed]
- Girsch, J.H.; Mejia Plazas, M.C.; Olivier, A.; Farah, M.; Littlefield, D.; Behl, S.; Punia, S.; Sakemura, R.; Hemsath, J.R.; Norgan, A.; et al. Host-viral interactions at the maternal-fetal interface. What we know and what we need to know. Frontiers 2022, 2, 833106. [Google Scholar] [CrossRef]
- Ponferrada, V.G.; Mauck, B.S.; Wooley, D.P. The envelope glycoprotein of human endogenous retrovirus HERV-W induces cellular resistance to spleen necrosis virus. Arch. Virol. 2003, 48, 659–675. [Google Scholar] [CrossRef]
- Rabelo, K.; de Souza, L.J.; Salomão, N.G.; Machado, L.N.; Pereira, P.G.; Portari, E.A.; Basílio-de-Oliveira, R.; Dos Santos, F.B.; Neves, L.D.; Morgade, L.F.; et al. Zika Induces Human Placental Damage and Inflammation. Front. Immunol. 2020, 11, 2146. [Google Scholar] [CrossRef]
- Pattillo, R.A.; Gey, G.O. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968, 7, 1231–1236. [Google Scholar]
- Graham, C.H.; Hawley, T.S.; Hawley, R.C.; MacDougall, J.R.; Kerbel, R.S.; Khoo, N.; Lala, P.K. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 1993, 206, 204–211. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Geddes, V.E.V.; Brustolini, O.J.B.; Cavalcante, L.T.D.F.; Moreira, F.R.R.; De Castro, F.L.; Guimaraes, A.P.D.C.; Gerber, A.L.; Figueiredo, C.M.; Diniz, L.P.; Neto, E.D.A.; et al. Common Dysregulation of Innate Immunity Pathways in Human Primary Astrocytes Infected With Chikungunya, Mayaro, Oropouche, and Zika Viruses. Front. Cell. Infect. Microbiol. 2021, 11, 641261. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.L.D.; Brustolini, O.J.B.; Geddes, V.E.V.; Souza, J.P.B.M.D.; Alves-Leon, S.V.; Aguiar, R.S.; Vasconcelos, A.T.R. Modulation of HERV Expression by Four Different Encephalitic Arboviruses during Infection of Human Primary Astrocytes. Viruses 2022, 14, 2505. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.H.M.; Moises, R.N.C.; Alves, B.E.B.; Pereira, H.W.B.; de Paiva, A.A.P.; Morais, I.C.; Nascimento, Y.M.; Monteiro, J.D.; de Souto, J.T.; Nascimento, M.S.L.; et al. Innate immune response in patients with acute Zika virus infection. Med. Microbiol. Immunol. 2019, 208, 703–714. [Google Scholar] [CrossRef]
- Chang, C.; Yen, M.; Chen, Y.; Chien, C.; Huang, H.; Bai, C.; Yen, B.L. Placenta-Derived Multipotent Cells Exhibit Immunosuppressive Properties That Are Enhanced in the Presence of Interferon-γ. Stem Cells 2006, 24, 2466–2477. [Google Scholar] [CrossRef]
- Culouscou, J.M.; Remacle-Bonnet, M.M.; Pommier, G.; Rance, R.J.; Depieds, R.C. Immunosuppressive properties of human placenta: Study of supernatants from short-term syncytiotrophoblast cultures. J. Reprod. Immunol. 1986, 9, 33–47. [Google Scholar] [CrossRef]
- Menu, E.; Kaplan, L.; Andreu, G.; Denver, L.; Chaouat, G. Immunoactive products of human placenta. I. An immunoregulatory factor obtained from explant cultures of human placenta inhibits CTL generation and cytotoxic effector activity. Cell. Immunol. 1989, 119, 341–352. [Google Scholar] [CrossRef]
- Tolosa, J.M.; Parsons, K.S.; Hansbro, P.M.; Smith, R.; Wark, P.A.B. The Placental Protein Syncytin-1 Impairs Antiviral Responses and Exaggerates Inflammatory Responses to Influenza. PLoS ONE 2015, 10, e0118629. [Google Scholar] [CrossRef]
- Piccinni, M.P.; Beloni, L.; Livi, C.; Maggi, E.; Scarselli, G.; Romagnani, S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat. Med. 1998, 4, 1020–1024. [Google Scholar] [CrossRef]
- Raghupathy, R.; Makhseed, M.; Azizieh, F.; Omu, A.; Gupta, M.; Farhat, R. Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum. Reprod. 2000, 15, 713–718. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, P.L. Increased CD56+ NK cells and enhanced Th1 responses in human unexplained recurrent spontaneous abortion. Genet. Mol. Res. 2015, 14, 18103–18109. [Google Scholar] [CrossRef]
- Sakakibara, M.; Maeda, Y.; Nakamura, K. Fetal loss due to Th1-skewed Th1/Th2 balance with increase (not decrease) of regulatory T cells in abortion-prone mouse model. J. Toxicol. Sci. 2022, 47, 327–336. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Aste-Amezaga, M.; Valiante, N.M.; Ma, X.; Kubin, M.; Trinchieri, G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993, 178, 1041–1048. [Google Scholar] [CrossRef]
- Aste-Amezaga, M.; Ma, X.; Sartori, A.; Trinchieri, G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J. Immunol. 1998, 160, 5936–5944. [Google Scholar] [CrossRef] [PubMed]
- Aste-Amezaga, M.; D’Andrea, A.; Kubin, M.; Trinchieri, G. Cooperation of Natural Killer Cell Stimulatory Factor/Interleukin-12 with Other Stimuli in the Induction of Cytokines and Cytotoxic Cell-Associated Molecules in Human T and NK Cells. Cell. Immunol. 1994, 156, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, L.; Aguet, M.; Trinchieri, G.; Garotta, G. The in vivo antiviral activity of interleukin-12 is mediated by gamma interferon. J. Virol. 1995, 69, 8147–8150. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.M.; Harvey, C.; Trinchieri, G. Role of Interleukin-12 in Primary Influenza Virus Infection. J. Virol. 1998, 72, 4825–4831. [Google Scholar] [CrossRef]
- Lu, J.; Su, Z.; Li, W.; Ling, Z.; Cheng, B.; Yang, X.; Tao, X. ASCT2-mediated glutamine uptake of epithelial cells facilitates CCL5-induced T cell infiltration via ROS-STAT3 pathway in oral lichen planus. Int. Immunopharmacol. 2023, 119, 110216. [Google Scholar] [CrossRef]
- Mangeney, M.; Renard, M.; Schlecht-Louf, G.; Bouallaga, I.; Heidmann, O.; Letzelter, C.; Richaud, A.; Ducos, B.; Heidmann, T. Placental syncytins: Genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc. Natl. Acad. Sci. USA 2007, 104, 20534–20539. [Google Scholar] [CrossRef]
- Gottfried-Blackmore, A.; Kaunzner, U.W.; Idoyaga, J.; Felger, J.C.; McEwen, B.S.; Bulloch, K. Acute in vivo exposure to interferon-γ enables resident brain dendritic cells to become effective antigen presenting cells. Proc. Natl. Acad. Sci. USA 2009, 106, 20918–20923. [Google Scholar] [CrossRef]
- Bendriss-Vermare, N.; Burg, S.; Kanzler, H.; Chaperot, L.; Duhen, T.; de Bouteiller, O.; D’Agostini, M.; Bridon, J.-M.; Durand, I.; Sederstrom, J.M.; et al. Virus overrides the propensity of human CD40L-activated plasmacytoid dendritic cells to produce Th2 mediators through synergistic induction of IFN-γ and Th1 chemokine production. J. Leukoc. Biol. 2005, 78, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Brown, H.M.; Hwang, S. Direct Antiviral Mechanisms of Interferon-Gamma. Immune Netw. 2018, 18, e33. [Google Scholar] [CrossRef] [PubMed]
- Lucinda, N.; Figueiredo, M.M.; Pessoa, N.L.; Santos, B.S.d.S.; Lima, G.K.; Freitas, A.M.; Machado, A.M.V.; Kroon, E.G.; Antonelli, L.R.D.V.; Campos, M.A. Dendritic cells, macrophages, NK and CD8+ T lymphocytes play pivotal roles in controlling HSV-1 in the trigeminal ganglia by producing IL1-beta, iNOS and granzyme B. Virol. J. 2017, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sergerie, Y.; Rivest, S.; Boivin, G. Tumor Necrosis Factor–α and Interleukin-1β Play a Critical Role in the Resistance against Lethal Herpes Simplex Virus Encephalitis. J. Infect. Dis. 2007, 196, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rodriguez, E.V.; Napolitani, G.; Lanzavecchia, A.; Sallusto, F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17–producing human T helper cells. Nat. Immunol. 2007, 8, 942–949. [Google Scholar] [CrossRef]
- Tsugawa, Y.; Kato, H.; Fujita, T.; Shimotohno, K.; Hijikata, M. Critical Role of Interferon-α Constitutively Produced in Human Hepatocytes in Response to RNA Virus Infection. PLOS ONE 2014, 9, e89869. [Google Scholar] [CrossRef]
- Sakuragi, S.; Liao, H.; Yajima, K.; Fujiwara, S.; Nakamura, H. Rubella Virus Triggers Type I Interferon Antiviral Response in Cultured Human Neural Cells: Involvement in the Control of Viral Gene Expression and Infectious Progeny Production. Int. J. Mol. Sci. 2022, 23, 9799. [Google Scholar] [CrossRef]
- A Meraz, M.; White, J.; Sheehan, K.C.; A Bach, E.; Rodig, S.J.; Dighe, A.S.; Kaplan, D.H.; Riley, J.K.; Greenlund, A.C.; Campbell, D.; et al. Targeted Disruption of the Stat1 Gene in Mice Reveals Unexpected Physiologic Specificity in the JAK–STAT Signaling Pathway. Cell 1996, 84, 431–442. [Google Scholar] [CrossRef]
- E Durbin, J.; Hackenmiller, R.; Simon, M.; E Levy, D. Targeted Disruption of the Mouse Stat1 Gene Results in Compromised Innate Immunity to Viral Disease. Cell 1996, 84, 443–450. [Google Scholar] [CrossRef]
- Lugo-Villarino, G.; Maldonado-López, R.; Possemato, R.; Peñaranda, C.; Glimcher, L.H. T-bet is required for optimal production of IFN-γ and antigen-specific T cell activation by dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 7749–7754. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Z.; Zhang, Y.; Hu, J.; Li, D.; Zang, G.; Yu, Y. Lentivirally overexpressed T-bet regulates T-helper cell lineage commitment in chronic hepatitis B patients. Mol. Med. Rep. 2012, 6, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.N.; Chang, H.-C.; Zisoulis, D.G.; Kapur, R.; Belladonna, M.L.; Kansas, G.S.; Kaplan, M.H. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood 2006, 108, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, A.M.; Banerjee, A.; Takemoto, N.; Gordon, S.M.; DeJong, C.S.; Shin, H.; Hunter, C.A.; Wherry, E.J.; Lindsten, T.; Reiner, S.L. Anomalous Type 17 Response to Viral Infection by CD8 + T Cells Lacking T-bet and Eomesodermin. Science 2008, 321, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.M.; Chaix, J.; Rupp, L.J.; Wu, J.; Madera, S.; Sun, J.C.; Lindsten, T.; Reiner, S.L. The Transcription Factors T-bet and Eomes Control Key Checkpoints of Natural Killer Cell Maturation. Immunity 2012, 36, 55–67. [Google Scholar] [CrossRef]
- Furusawa, J.-I.; Moro, K.; Motomura, Y.; Okamoto, K.; Zhu, J.; Takayanagi, H.; Kubo, M.; Koyasu, S. Critical Role of p38 and GATA3 in Natural Helper Cell Function. J. Immunol. 2013, 191, 1818–1826. [Google Scholar] [CrossRef]
- Zhu, J.; Min, B.; Hu-Li, J.; Watson, C.J.; Grinberg, A.; Wang, Q.; Killeen, N.; Urban, J.F.; Guo, L.; E Paul, W. Conditional deletion of Gata3 shows its essential function in TH1-TH2 responses. Nat. Immunol. 2004, 5, 1157–1165. [Google Scholar] [CrossRef]
- Pai, S.-Y.; Truitt, M.L.; Ting, C.-N.; Leiden, J.M.; Glimcher, L.H.; Ho, I.-C. Critical Roles for Transcription Factor GATA-3 in Thymocyte Development. Immunity 2003, 19, 863–875. [Google Scholar] [CrossRef]
- Wang, Y.; Su, M.A.; Wan, Y.Y. An Essential Role of the Transcription Factor GATA-3 for the Function of Regulatory T Cells. Immunity 2011, 35, 337–348. [Google Scholar] [CrossRef]
- Bending, D.; Ono, M. From stability to dynamics: Understanding molecular mechanisms of regulatory T cells through Foxp3 transcriptional dynamics. Clin. Exp. Immunol. 2018, 197, 14–23. [Google Scholar] [CrossRef]
- Kawakami, R.; Kitagawa, Y.; Chen, K.Y.; Arai, M.; Ohara, D.; Nakamura, Y.; Yasuda, K.; Osaki, M.; Mikami, N.; Lareau, C.A.; et al. Distinct Foxp3 enhancer elements coordinate development, maintenance, and function of regulatory T cells. Immunity 2021, 54, 947–961.e8. [Google Scholar] [CrossRef]
- Yang, X.O.; Pappu, B.P.; Nurieva, R.; Akimzhanov, A.; Kang, H.S.; Chung, Y.; Ma, L.; Shah, B.; Panopoulos, A.D.; Schluns, K.S.; et al. T Helper 17 Lineage Differentiation Is Programmed by Orphan Nuclear Receptors RORα and RORγ. Immunity 2008, 28, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Croft, C.A.; Thaller, A.; Marie, S.; Doisne, J.-M.; Surace, L.; Yang, R.; Puel, A.; Bustamante, J.; Casanova, J.-L.; Di Santo, J.P. Notch, RORC and IL-23 signals cooperate to promote multi-lineage human innate lymphoid cell differentiation. Nat. Commun. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- A Seder, R.; Gazzinelli, R.; Sher, A.; E Paul, W. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc. Natl. Acad. Sci. USA 1993, 90, 10188–10192. [Google Scholar] [CrossRef]
- Yang, Q.; Li, G.; Zhu, Y.; Liu, L.; Chen, E.; Turnquist, H.; Zhang, X.; Finn, O.J.; Chen, X.; Lu, B. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells. Eur. J. Immunol. 2011, 41, 3351–3360. [Google Scholar] [CrossRef] [PubMed]
- Meyaard, L.; Hovenkamp, E.; A Otto, S.; Miedema, F. IL-12-induced IL-10 production by human T cells as a negative feedback for IL-12-induced immune responses. J. Immunol. 1996, 156, 2776–2782. [Google Scholar] [CrossRef]
- Han, X.; Chai, R.; Qi, F.; Bai, S.; Cui, Y.; Teng, Y.; Liu, B. Natural Helper Cells Mediate Respiratory Syncytial Virus-Induced Airway Inflammation by Producing Type 2 Cytokines in an IL-33-Dependent Manner. Immunotherapy 2017, 9, 715–722. [Google Scholar] [CrossRef]
- Gao, X.; Chi, X.; Wang, X.; Wu, R.; Xu, H.; Zhan, M.; Li, D.; Ding, Y.; Xu, D.; Niu, J. IL-33 Inhibits Hepatitis B Virus through Its Receptor ST2 in Hydrodynamic HBV Mouse Model. Mediat. Inflamm. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Faas, M.; Ipseiz, N.; Ackermann, J.; Culemann, S.; Grüneboom, A.; Schröder, F.; Rothe, T.; Scholtysek, C.; Eberhardt, M.; Böttcher, M.; et al. IL-33-induced metabolic reprogramming controls the differentiation of alternatively activated macrophages and the resolution of inflammation. Immunity 2021, 54, 2531–2546.e5. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Flavell, R.A. T Cell-Produced Transforming Growth Factor-β1 Controls T Cell Tolerance and Regulates Th1- and Th17-Cell Differentiation. Immunity 2007, 26, 579–591. [Google Scholar] [CrossRef]
- Gorelik, L.; A Flavell, R. Abrogation of TGFβ Signaling in T Cells Leads to Spontaneous T Cell Differentiation and Autoimmune Disease. Immunity 2000, 12, 171–181. [Google Scholar] [CrossRef]
- Robinson, R.T.; Gorham, J.D. TGF-β1 Regulates Antigen-Specific CD4+ T Cell Responses in the Periphery. J. Immunol. 2007, 179, 71–79. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Vodovotz, Y.; Nathan, C. Macrophage deactivation by interleukin 10. J. Exp. Med. 1991, 174, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Rabin, E.M.; Ohara, J.; E Paul, W. B-cell stimulatory factor 1 activates resting B cells. Proc. Natl. Acad. Sci. USA 1985, 82, 2935–2939. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Sad, S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 1996, 17, 138–146. [Google Scholar] [CrossRef]
- Hober, D.; Benyoucef, S.; Chehadeh, W.; Chieux, V.; La Tribonniere, D.; Mouton, Y.; Bocket, L.; Wattre, P. Production of Interleukin-4, Interferon (IFN)-γ and IFN-α in Human Immunodeficiency Virus-1 Infection: An Imbalance of Type 1 and Type 2 Cytokines may Reduce the Synthesis of IFN-α. Scand. J. Immunol. 1998, 48, 436–442. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.L.d.; Prieto-Oliveira, P.; Duarte-Barbosa, M.; Andreata-Santos, R.; Peter, C.M.; Prolo de Brito, T.; Antoneli, F.; Durães-Carvalho, R.; Briones, M.R.S.; Maricato, J.T.; et al. The Relationship between HERV, Interleukin, and Transcription Factor Expression in ZIKV Infected versus Uninfected Trophoblastic Cells. Cells 2024, 13, 1491. https://doi.org/10.3390/cells13171491
Costa ALd, Prieto-Oliveira P, Duarte-Barbosa M, Andreata-Santos R, Peter CM, Prolo de Brito T, Antoneli F, Durães-Carvalho R, Briones MRS, Maricato JT, et al. The Relationship between HERV, Interleukin, and Transcription Factor Expression in ZIKV Infected versus Uninfected Trophoblastic Cells. Cells. 2024; 13(17):1491. https://doi.org/10.3390/cells13171491
Chicago/Turabian StyleCosta, Anderson Luís da, Paula Prieto-Oliveira, Márcia Duarte-Barbosa, Robert Andreata-Santos, Cristina M. Peter, Thamires Prolo de Brito, Fernando Antoneli, Ricardo Durães-Carvalho, Marcelo R. S. Briones, Juliana T. Maricato, and et al. 2024. "The Relationship between HERV, Interleukin, and Transcription Factor Expression in ZIKV Infected versus Uninfected Trophoblastic Cells" Cells 13, no. 17: 1491. https://doi.org/10.3390/cells13171491







