Dysregulation of miR-223, miR-146a, and miR-193a Expression Profile in Acute and Chronic Phases of Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of EAE
2.3. Histological Analysis
2.4. Cell Culture and Nucleic Acid Preparation
2.5. Real-Time PCR
2.6. Statistical Analysis
3. Results
3.1. EAE Development Exacerbated the Clinical Manifestations
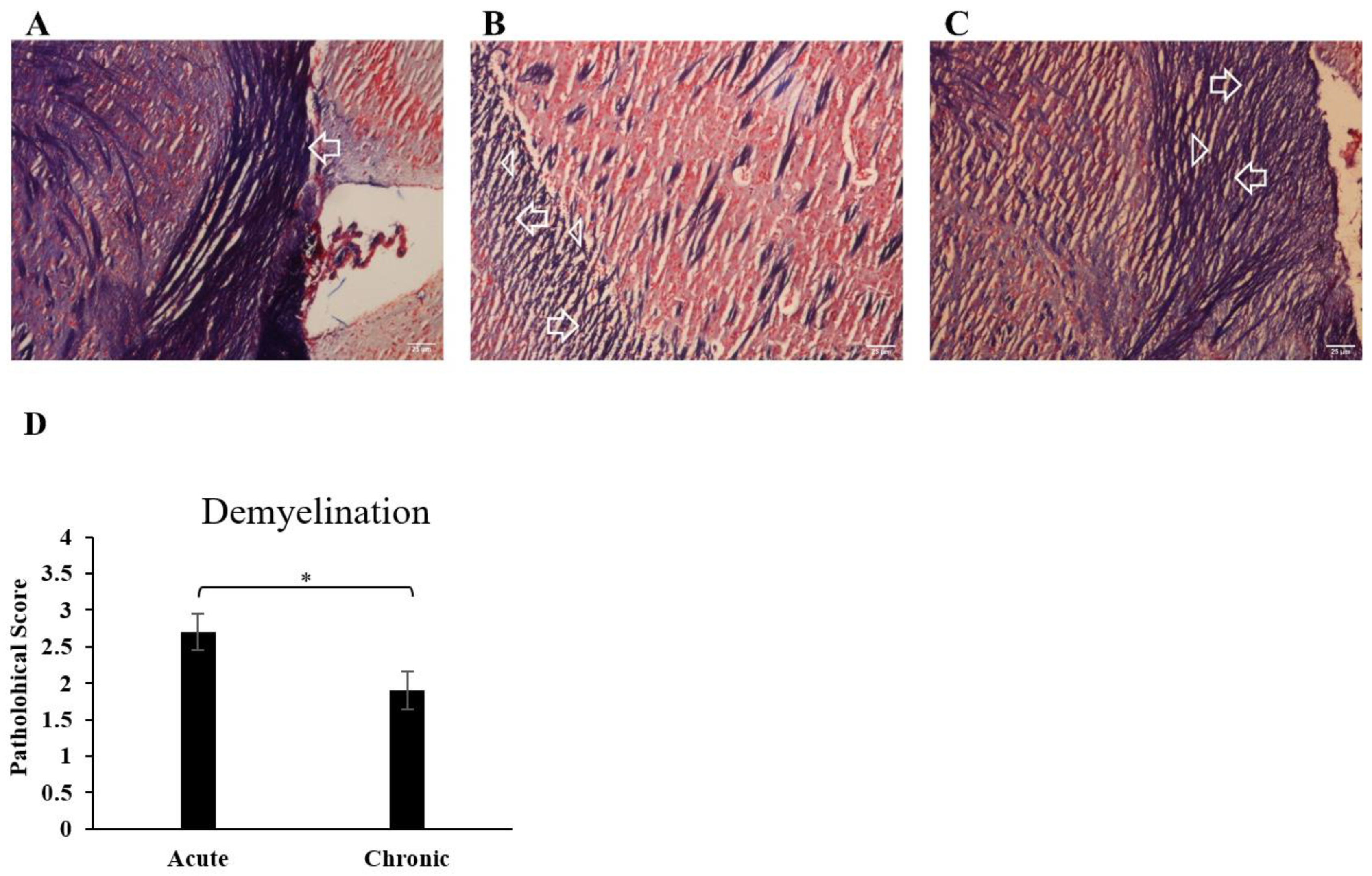
3.2. Immune Cell Infiltration into the CNS and Demyelination of the Brain Increased during EAE
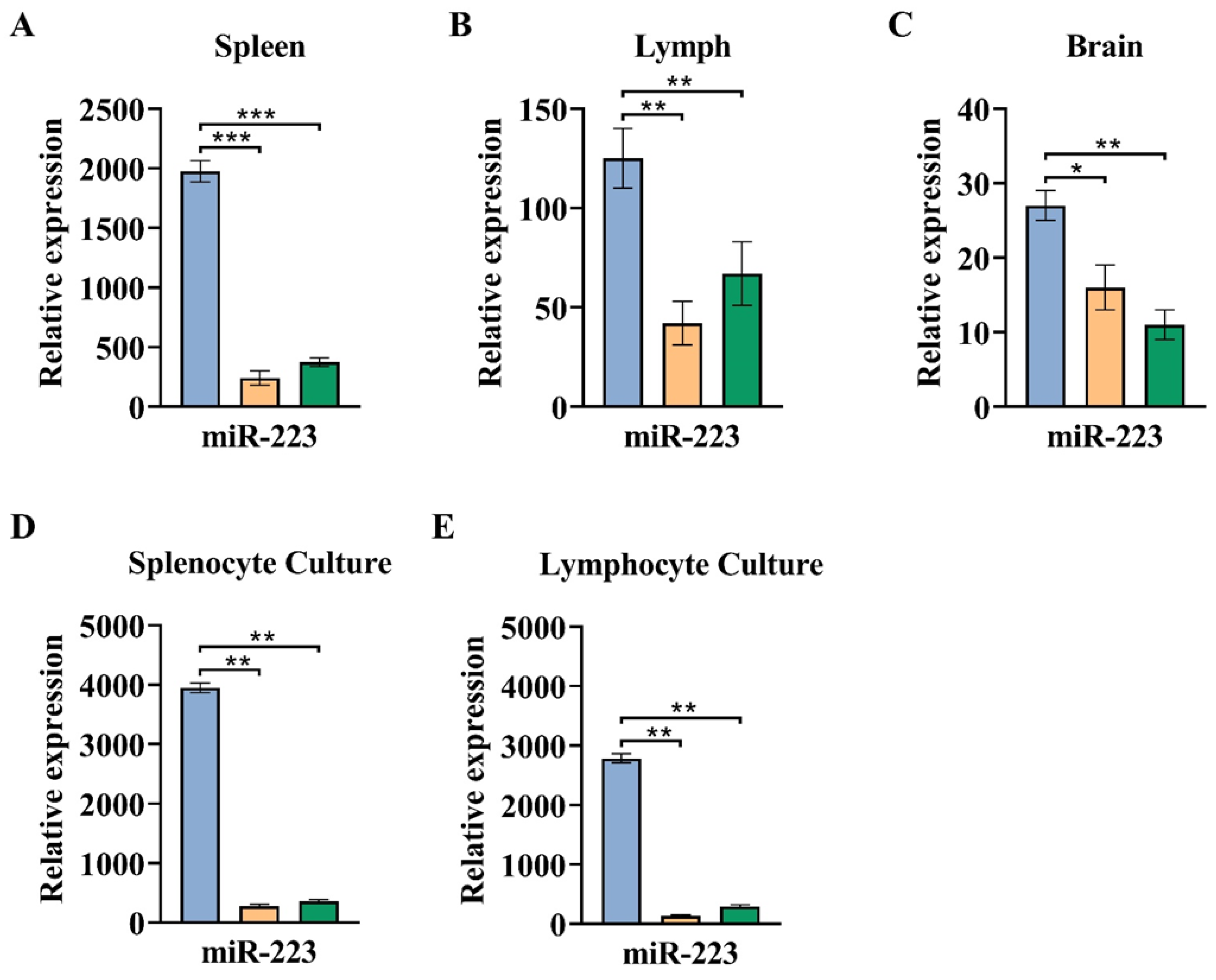
3.3. The Greater Expression of miR-223 Observed in the Acute Phase of EAE Compared to a Chronic Phase
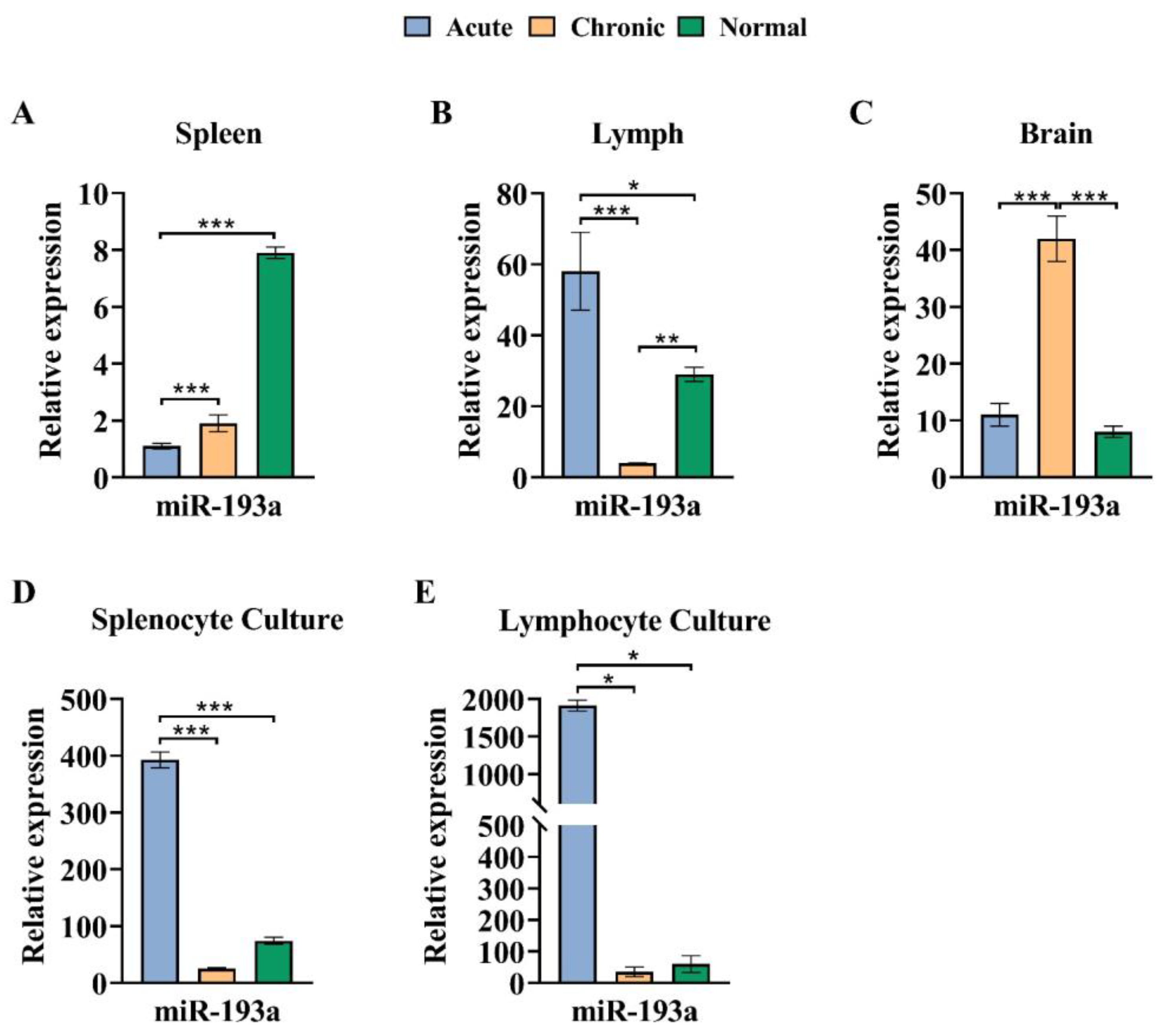
3.4. The miR-193a Expression Pattern in the Brain Decreased in the Chronic Phase of EAE
3.5. The Expression Pattern of miR-146a Declined in the Chronic Phase of EAE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandi, D.; Fricska-Nagy, Z.; Bencsik, K.; Vécsei, L. Neurodegeneration in multiple sclerosis: Symptoms of silent progression, biomarkers and neuroprotective therapy—Kynurenines are important players. Molecules 2021, 26, 3423. [Google Scholar] [CrossRef]
- Bjelobaba, I.; Begovic-Kupresanin, V.; Pekovic, S.; Lavrnja, I. Animal models of multiple sclerosis: Focus on experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2018, 96, 1021–1042. [Google Scholar] [CrossRef] [PubMed]
- Kipp, M.; van der Star, B.; Vogel, D.Y.; Puentes, F.; van der Valk, P.; Baker, D.; Amor, S. Experimental in vivo and in vitro models of multiple sclerosis: EAE and beyond. Mult. Scler. Relat. Disord. 2012, 1, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Bolton, C.; Smith, P. Defining and regulating acute inflammatory lesion formation during the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. CNS Neurol. Disord. Drug Targets 2015, 14, 915–935. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, B.; Song, J.-H.; Zhen, T.; Wang, B.-Y.; Li, X.; Liu, P.; Yang, X.; Zhang, Q.-L.; Xi, X.-D.; et al. Eriocalyxin B ameliorates experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells. Proc. Natl. Acad. Sci. USA 2013, 110, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Piket, E.; Zheleznyakova, G.Y.; Kular, L.; Jagodic, M. Small non-coding RNAs as important players, biomarkers and therapeutic targets in multiple sclerosis: A comprehensive overview. J. Autoimmun. 2019, 101, 17–25. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, J.; Zhong, Y.; Jiang, L.; Mu, P.; Li, Y.; Singh, N.; Nagarkatti, M.; Nagarkatti, P. Expression, regulation and function of microRNAs in multiple sclerosis. Int. J. Med. Sci. 2014, 11, 810–818. [Google Scholar] [CrossRef]
- Iborra, M.; Bernuzzi, F.; Invernizzi, P.; Danese, S. MicroRNAs in autoimmunity and inflammatory bowel disease: Crucial regulators in immune response. Autoimmun. Rev. 2012, 11, 305–314. [Google Scholar] [CrossRef]
- Chandan, K.; Gupta, M.; Sarwat, M. Role of host and pathogen-derived microRNAs in immune regulation during infectious and inflammatory diseases. Front. Immunol. 2020, 10, 3081. [Google Scholar] [CrossRef]
- Amoruso, A.; Blonda, M.; Gironi, M.; Grasso, R.; Di Francescantonio, V.; Scaroni, F.; Furlan, R.; Verderio, C.; Avolio, C. Immune and central nervous system-related miRNAs expression profiling in monocytes of multiple sclerosis patients. Sci. Rep. 2020, 10, 6125. [Google Scholar] [CrossRef]
- Saba, R.; Sorensen, D.L.; Booth, S.A. MicroRNA-146a: A dominant, negative regulator of the innate immune response. Front. Immunol. 2014, 5, 578. [Google Scholar] [CrossRef]
- Marino, M.; Mele, E.; Pastorino, G.M.; Meccariello, R.; Operto, F.F.; Santoro, A.; Viggiano, A. Neuroinflammation: Molecular mechanisms and therapeutic perspectives. Cent. Nerv. Syst. Agents Med. Chem. 2022, 22, 160–174. [Google Scholar]
- Aslani, M.; Mortazavi-Jahromi, S.S.; Mirshafiey, A. Efficient roles of miR-146a in cellular and molecular mechanisms of neuroinflammatory disorders: An effectual review in neuroimmunology. Immunol. Lett. 2021, 238, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.V.S.; Monteiro, V.V.S.; Navegantes-Lima, K.C.; de Brito Oliveira, A.L.; de França Gaspar, S.L.; Quadros, L.B.G.; Monteiro, M.C. MicroRNAs in cell cycle progression and proliferation: Molecular mechanisms and pathways. Non-Coding RNA Investig. 2018, 2, 28. [Google Scholar] [CrossRef]
- Khordadmehr, M.; Shahbazi, R.; Sadreddini, S.; Baradaran, B. miR-193: A new weapon against cancer. J. Cell. Physiol. 2019, 234, 16861–16872. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambantham, K.; Islam, V.I.H.; Saravanan, S.; Kumar, V.S.; Ashok, G.; Chellappandian, M. Role of miRNA in Multiple Sclerosis. In MicroRNA: Perspectives in Health and Diseases; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Huehnchen, P.; Prozorovski, T.; Klaissle, P.; Lesemann, A.; Ingwersen, J.; Wolf, S.A.; Kupsch, A.; Aktas, O.; Steiner, B. Modulation of adult hippocampal neurogenesis during myelin-directed autoimmune neuroinflammation. Glia 2011, 59, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Mosayebi, G.; Haghmorad, D.; Namaki, S.; Ghazavi, A.; Ekhtiari, P.; Mirshafiey, A. Therapeutic effect of EDTA in experimental model of multiple sclerosis. Immunopharmacol. Immunotoxicol. 2010, 32, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Haghmorad, D.; Mahmoudi, M.B.; Mahmoudi, M.; Rab, S.Z.; Rastin, M.; Shegarfi, H.; Azizi, G.; Mirshafiey, A. Calcium intervention ameliorates experimental model of multiple sclerosis. Oman Med. J. 2014, 29, 185–189. [Google Scholar] [CrossRef]
- Soltanmohammadi, A.; Tavaf, M.J.; Zargarani, S.; Yazdanpanah, E.; Sadighi-Moghaddam, B.; Yousefi, B.; Sameni, H.R.; Haghmorad, D. Daphnetin alleviates experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating Th2 and regulatory T cells. Acta Neurobiol. Exp. 2022, 82, 273–283. [Google Scholar] [CrossRef]
- Horstmann, L.; Schmid, H.; Heinen, A.P.; Kurschus, F.C.; Dick, H.B.; Joachim, S.C. Inflammatory demyelination Induces glia alterations and ganglion cell loss in the retina of an experimental autoimmune encephalomyelitis model. J. Neuroinflamm. 2013, 10, 120. [Google Scholar] [CrossRef]
- Sloka, S.; Metz, L.M.; Hader, W.; Starreveld, Y.; Yong, V.W. Reduction of microglial activity in a model of multiple sclerosis by dipyridamole. J. Neuroinflamm. 2013, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Niino, M. Epigenetics in multiple sclerosis. Clin. Exp. Neuroimmunol. 2015, 6, 49–58. [Google Scholar] [CrossRef]
- Gao, F.-B. Context-dependent functions of specific microRNAs in neuronal development. Neural Dev. 2010, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Scott, H. Extracellular microRNAs as messengers in the central and peripheral nervous system. Neuronal Signal. 2017, 1, NS20170112. [Google Scholar] [CrossRef] [PubMed]
- Harraz, M.M.; Eacker, S.M.; Wang, X.; Dawson, T.M.; Dawson, V.L. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 18962–18967. [Google Scholar] [CrossRef]
- Satoorian, T.; Li, B.; Tang, X.; Xiao, J.; Xing, W.; Shi, W.; Lau, K.H.W.; Baylink, D.J.; Qin, X. MicroRNA223 promotes pathogenic T-cell development and autoimmune inflammation in central nervous system in mice. Immunology 2016, 148, 326–338. [Google Scholar] [CrossRef]
- Hosseini, A.; Ghaedi, K.; Tanhaei, S.; Ganjalikhani-Hakemi, M.; Teimuri, S.; Etemadifar, M.; Nasr Esfahani, M.H. Upregulation of CD4+T-Cell Derived MiR-223 in The Relapsing Phase of Multiple Sclerosis Patients. Cell J. 2016, 18, 371–380. [Google Scholar]
- Ifergan, I.; Chen, S.; Zhang, B.; Miller, S.D. Cutting Edge: MicroRNA-223 Regulates Myeloid Dendritic Cell-Driven Th17 Responses in Experimental Autoimmune Encephalomyelitis. J. Immunol. 2016, 196, 1455–1459. [Google Scholar] [CrossRef]
- Ridolfi, E.; Fenoglio, C.; Cantoni, C.; Calvi, A.; De Riz, M.; Pietroboni, A.; Villa, C.; Serpente, M.; Bonsi, R.; Vercellino, M.; et al. Expression and Genetic Analysis of MicroRNAs Involved in Multiple Sclerosis. Int. J. Mol. Sci. 2013, 14, 4375–4384. [Google Scholar] [CrossRef]
- Cantoni, C.; Cignarella, F.; Ghezzi, L.; Mikesell, B.; Bollman, B.; Berrien-Elliott, M.M.; Ireland, A.R.; Fehniger, T.A.; Wu, G.F.; Piccio, L. Mir-223 regulates the number and function of myeloid-derived suppressor cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 2017, 133, 61–77. [Google Scholar] [CrossRef]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zheng, L.Y.; Zhang, P.; Yu, C.Q. miR-146a and miR-155 expression in PBMCs from patients with Sjogren’s syndrome. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2014, 43, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, X.; Cui, H.; Ni, X.; Yuan, M.; Guo, Y.; Huang, X.; Zhou, H.; de Vries, N.; Tak, P.P.; et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009, 60, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wan, Y.; Guo, Q.; Zou, L.; Zhang, J.; Fang, Y.; Zhang, J.; Zhang, J.; Fu, X.; Liu, H.; et al. Altered microRNA expression profile with miR-146a upregulation in CD4(+) T cells from patients with rheumatoid arthritis. Arthritis Res. Ther. 2010, 12, R81. [Google Scholar] [CrossRef] [PubMed]
- Lescher, J.; Paap, F.; Schultz, V.; Redenbach, L.; Scheidt, U.; Rosewich, H.; Nessler, S.; Fuchs, E.; Gartner, J.; Bruck, W.; et al. MicroRNA regulation in experimental autoimmune encephalomyelitis in mice and marmosets resembles regulation in human multiple sclerosis lesions. J. Neuroimmunol. 2012, 246, 27–33. [Google Scholar] [CrossRef]
- Li, Y.; Du, C.; Wang, W.; Ma, G.; Cui, L.; Zhou, H.; Tao, H.; Yao, L.; Zhao, B.; Li, K. Genetic Association of MiR-146a with Multiple Sclerosis Susceptibility in the Chinese Population. Cell. Physiol. Biochem. 2015, 35, 281–291. [Google Scholar] [CrossRef]
- Pauley, K.M.; Satoh, M.; Chan, A.L.; Bubb, M.R.; Reeves, W.H.; Chan, E.K.L. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res. Ther. 2008, 10, R101. [Google Scholar] [CrossRef]
- Niimoto, T.; Nakasa, T.; Ishikawa, M.; Okuhara, A.; Izumi, B.; Deie, M.; Suzuki, O.; Adachi, N.; Ochi, M. MicroRNA-146a expresses in interleukin-17 producing T cells in rheumatoid arthritis patients. BMC Musculoskelet. Disord. 2010, 11, 209. [Google Scholar] [CrossRef]
- Grossi, I.; Salvi, A.; Abeni, E.; Marchina, E.; De Petro, G. Biological Function of MicroRNA193a-3p in Health and Disease. Int. J. Genom. 2017, 2017, 5913195. [Google Scholar] [CrossRef]
- Otaegui, D.; Baranzini, S.E.; Armañanzas, R.; Calvo, B.; Muñoz-Culla, M.; Khankhanian, P.; Inza, I.; Lozano, J.A.; Castillo-Triviño, T.; Asensio, A. Differential micro RNA expression in PBMC from multiple sclerosis patients. PLoS ONE 2009, 4, e6309. [Google Scholar] [CrossRef]



| Group | Maximum Score | p-Value | Mean Score (Last Day) | p-Value * |
|---|---|---|---|---|
| Acute | 4.8 ± 0.83 (n = 7) | p < 0.05 | 4.8 ± 0.83 (n = 7) | p < 0.05 |
| Chronic | 4.8 ± 0.52 (n = 8) | 2.4 ± 1.3 (n = 8) |
| Tissue/Cell | Spleen | Lymph Nodes | Brain | Cells Cultured from Spleen | Cells Cultured from Lymph Nodes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| miRNA | Disease phase | Acute | Chronic | Normal | Acute | Chronic | Normal | Acute | Chronic | Normal | Acute | Chronic | Normal | Acute | Chronic | Normal |
| miR-223 | Acute | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | |||||
| Chronic | ⬇ | ----- | ⬇ | ----- | ⬇ | ----- | ⬇ | ----- | ⬇ | ----- | ||||||
| miR-146a | Acute | ⬆ | ----- | ----- | ----- | ----- | ----- | ⬆ | ----- | ⬆ | ⬆ | |||||
| Chronic | ⬇ | ----- | ----- | ----- | ----- | ----- | ⬇ | ⬇ | ⬇ | ----- | ||||||
| miR-193a | Acute | ----- | ⬇ | ⬆ | ⬆ | ⬇ | ----- | ⬆ | ⬆ | ⬆ | ⬆ | |||||
| Chronic | ----- | ⬇ | ⬇ | ⬇ | ⬆ | ⬆ | ⬇ | ----- | ⬇ | ----- | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharibi, S.; Moghimi, B.; Mahmoudi, M.B.; Shahvazian, E.; Yazd, E.F.; Yadegari, M.; Tahoori, M.T.; Yazdanpanah, E.; Haghmorad, D.; Oksenych, V. Dysregulation of miR-223, miR-146a, and miR-193a Expression Profile in Acute and Chronic Phases of Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice. Cells 2024, 13, 1499. https://doi.org/10.3390/cells13171499
Gharibi S, Moghimi B, Mahmoudi MB, Shahvazian E, Yazd EF, Yadegari M, Tahoori MT, Yazdanpanah E, Haghmorad D, Oksenych V. Dysregulation of miR-223, miR-146a, and miR-193a Expression Profile in Acute and Chronic Phases of Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice. Cells. 2024; 13(17):1499. https://doi.org/10.3390/cells13171499
Chicago/Turabian StyleGharibi, Saba, Bahram Moghimi, Mohammad Bagher Mahmoudi, Ensieh Shahvazian, Ehsan Farashahi Yazd, Maryam Yadegari, Mohammad Taher Tahoori, Esmaeil Yazdanpanah, Dariush Haghmorad, and Valentyn Oksenych. 2024. "Dysregulation of miR-223, miR-146a, and miR-193a Expression Profile in Acute and Chronic Phases of Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice" Cells 13, no. 17: 1499. https://doi.org/10.3390/cells13171499
APA StyleGharibi, S., Moghimi, B., Mahmoudi, M. B., Shahvazian, E., Yazd, E. F., Yadegari, M., Tahoori, M. T., Yazdanpanah, E., Haghmorad, D., & Oksenych, V. (2024). Dysregulation of miR-223, miR-146a, and miR-193a Expression Profile in Acute and Chronic Phases of Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice. Cells, 13(17), 1499. https://doi.org/10.3390/cells13171499







