Adipose-Derived Stem Cell Therapy in Spinal Cord Injury
Abstract
1. Introduction
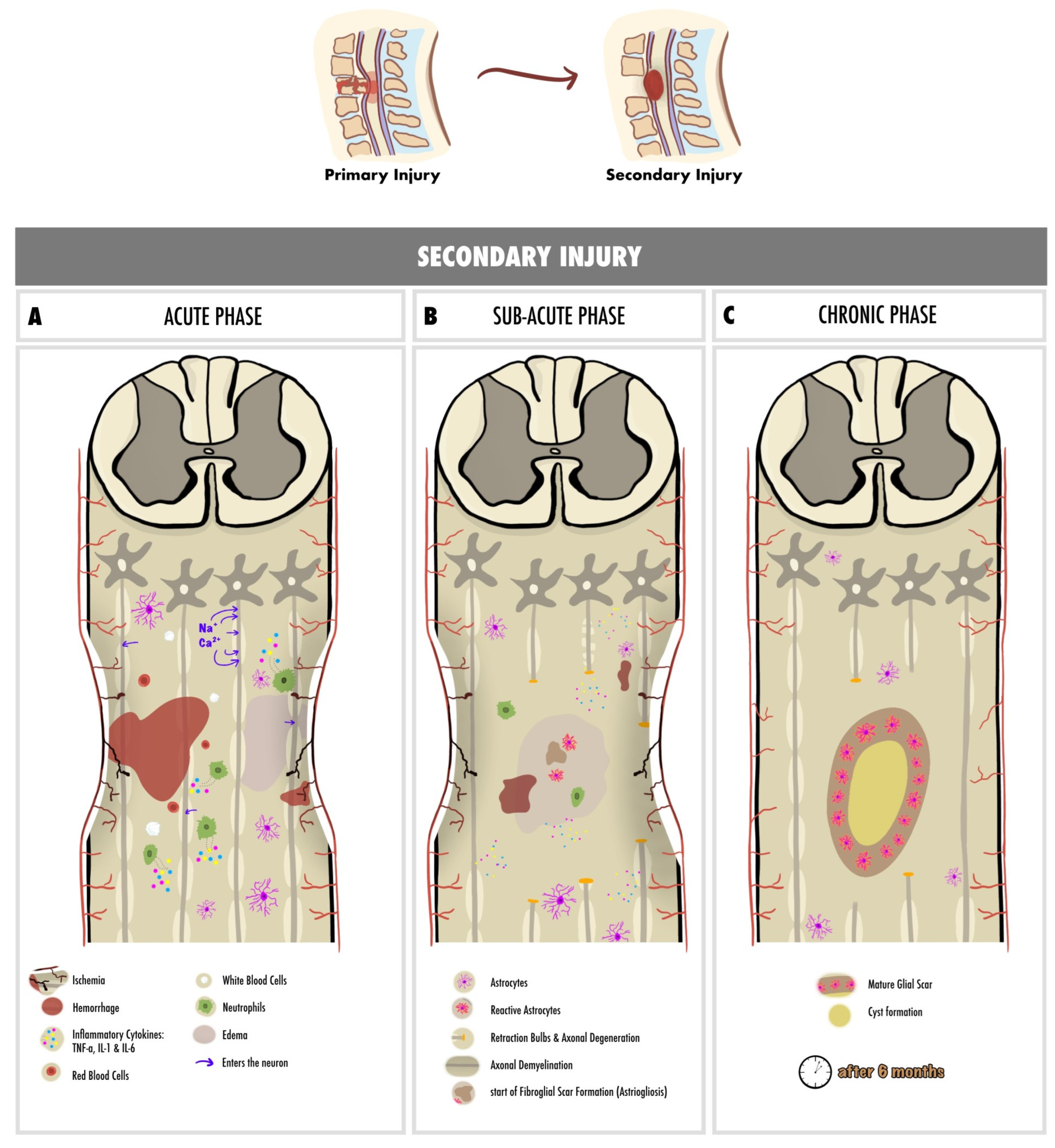
2. Pathophysiology of Spinal Cord Injury
2.1. Primary Injury
2.1.1. Non-Traumatic SCI
2.1.2. Traumatic SCI
2.2. Secondary Injury
3. Adipose-Derived Stem Cells
4. Animal Models for ADSC in SCI
5. Application Methods of ADSC in SCI
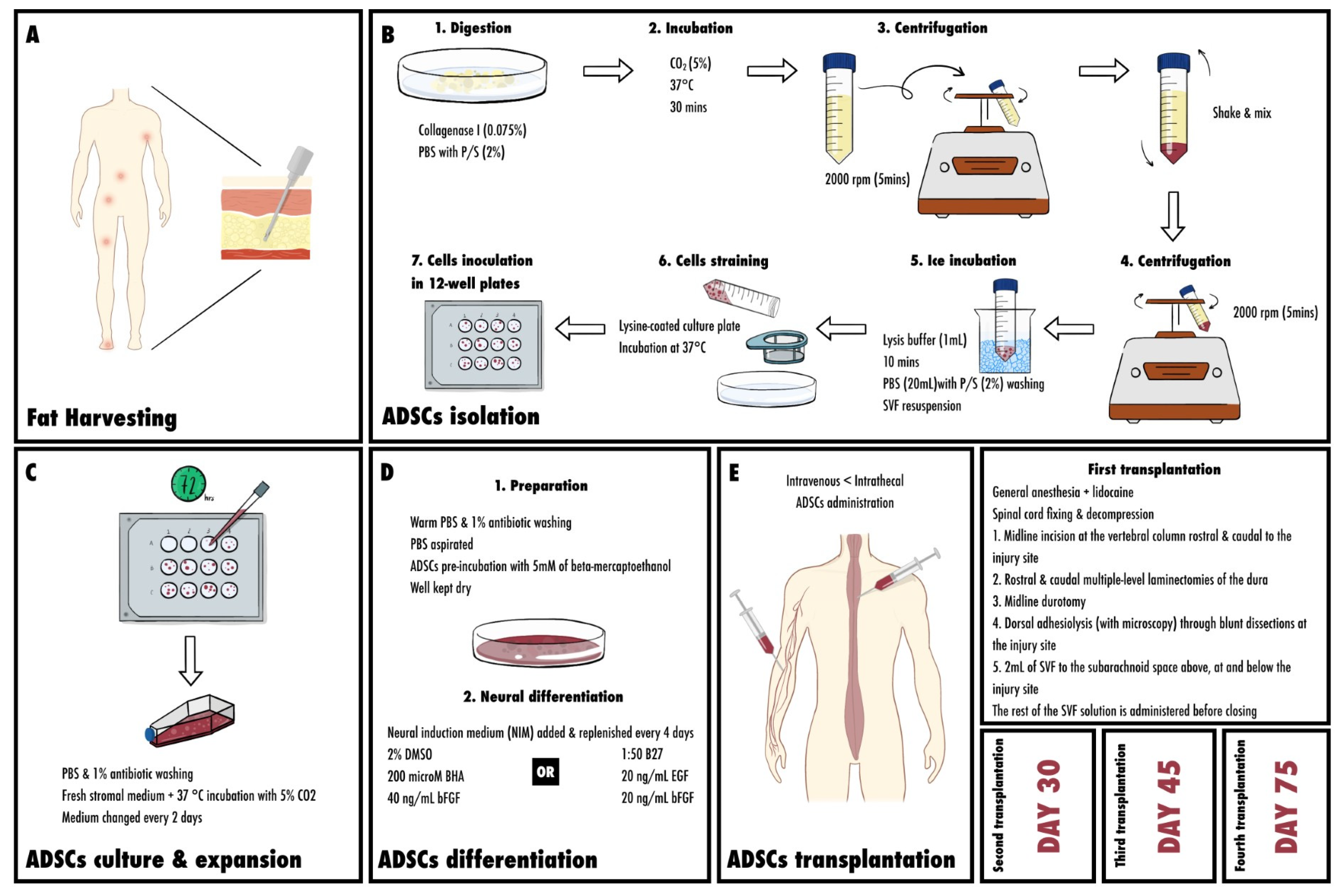
5.1. Fat Harvesting Sites
5.2. Isolation of Mesenchymal Stem Cells from Adipose Tissue
5.3. Culture and Expansion of ASCs
5.4. Differentiation
5.5. Neural Differentiation of ASCs
5.6. Transplanting ADSC (Injection; Surgical Procedures; Intrathecal vs. Intravenous; Amounts)
5.6.1. First Transplantation
5.6.2. Second and Third ADSC Transplantation
5.6.3. Fourth ADSCs Transplantation
6. Inflammation, Nociception and Neuroprotection
7. Neurogenesis, Neuronal Regeneration and Axonal Regrowth
8. Angiogenesis and Vascularization
9. Outcomes (Studied Factors with the Outcomes Reached)
9.1. Locomotion and Functional Recovery
9.2. Neural Relay Restoration, Sensory Rehabilitation and Pain Reduction
9.3. Neurogenic Bladder Recovery
10. Clinical Application
11. Challenges and Future Perspectives
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Spinal Cord Injury. Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury (accessed on 29 December 2023).
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global Prevalence and Incidence of Traumatic Spinal Cord Injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar] [CrossRef] [PubMed]
- Spinal Cord Injury—Symptoms and Causes. Available online: https://www.mayoclinic.org/diseases-conditions/spinal-cord-injury/symptoms-causes/syc-20377890 (accessed on 29 December 2023).
- Müller-Jensen, L.; Ploner, C.J.; Kroneberg, D.; Schmidt, W.U. Clinical Presentation and Causes of Non-Traumatic Spinal Cord Injury: An Observational Study in Emergency Patients. Front. Neurol. 2021, 12, 701927. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, Regional, and National Burden of Traumatic Brain Injury and Spinal Cord Injury, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Spinal Cord Injury | National Institute of Neurological Disorders and Stroke. Available online: https://www.ninds.nih.gov/health-information/disorders/spinal-cord-injury (accessed on 29 December 2023).
- Acute Spinal Cord Injury. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/acute-spinal-cord-injury (accessed on 29 December 2023).
- Spinal Cord Injury Facts and Figures at a Glance. J. Spinal Cord. Med. 2014, 37, 117–118. [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic Spinal Cord Injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Vawda, R.; Fehlings, M.G. Mesenchymal Cells in the Treatment of Spinal Cord Injury: Current & Future Perspectives. Curr. Stem Cell Res. Ther. 2013, 8, 25–38. [Google Scholar] [CrossRef]
- Dasari, V.R.; Veeravalli, K.K.; Dinh, D.H. Mesenchymal Stem Cells in the Treatment of Spinal Cord Injuries: A Review. World J. Stem Cells 2014, 6, 120–133. [Google Scholar] [CrossRef]
- Pang, Q.-M.; Chen, S.-Y.; Fu, S.-P.; Zhou, H.; Zhang, Q.; Ao, J.; Luo, X.-P.; Zhang, T. Regulatory Role of Mesenchymal Stem Cells on Secondary Inflammation in Spinal Cord Injury. J. Inflamm. Res. 2022, 15, 573–593. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem Cells: Their Source, Potency and Use in Regenerative Therapies with Focus on Adipose-Derived Stem Cells—A Review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Vismara, I.; Papa, S.; Rossi, F.; Forloni, G.; Veglianese, P. Current Options for Cell Therapy in Spinal Cord Injury. Trends Mol. Med. 2017, 23, 831–849. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-Assisted Lipotransfer for Cosmetic Breast Augmentation: Supportive Use of Adipose-Derived Stem/Stromal Cells. Aesthetic Plast. Surg. 2008, 32, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Moraci, M.; Armenia, E.; Orabona, C.; Sergio, R.; De Sena, G.; Capuozzo, V.; Barbarisi, M.; Rosso, F.; Giordano, G.; et al. Therapy with Autologous Adipose-Derived Regenerative Cells for the Care of Chronic Ulcer of Lower Limbs in Patients with Peripheral Arterial Disease. J. Surg. Res. 2013, 185, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Khalifian, S.; Sarhane, K.A.; Tammia, M.; Ibrahim, Z.; Mao, H.-Q.; Cooney, D.S.; Shores, J.T.; Lee, W.P.A.; Brandacher, G. Stem Cell-Based Approaches to Improve Nerve Regeneration: Potential Implications for Reconstructive Transplantation? Arch. Immunol. Ther. Exp. 2015, 63, 15–30. [Google Scholar] [CrossRef]
- New, P.W.; Eriks-Hoogland, I.; Scivoletto, G.; Reeves, R.K.; Townson, A.; Marshall, R.; Rathore, F.A. Important Clinical Rehabilitation Principles Unique to People with Non-Traumatic Spinal Cord Dysfunction. Top. Spinal Cord. Inj. Rehabil. 2017, 23, 299–312. [Google Scholar] [CrossRef]
- Guest, J.; Datta, N.; Jimsheleishvili, G.; Gater, D.R. Pathophysiology, Classification and Comorbidities after Traumatic Spinal Cord Injury. J. Pers. Med. 2022, 12, 1126. [Google Scholar] [CrossRef]
- Molinares, D.M.; Gater, D.R.; Daniel, S.; Pontee, N.L. Nontraumatic Spinal Cord Injury: Epidemiology, Etiology and Management. J. Pers. Med. 2022, 12, 1872. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke: From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J. Vasc. Interv. Radiol. 2018, 29, 441–453. [Google Scholar] [CrossRef]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Eli, I.; Lerner, D.P.; Ghogawala, Z. Acute Traumatic Spinal Cord Injury. Neurol. Clin. 2021, 39, 471–488. [Google Scholar] [CrossRef]
- Rouanet, C.; Reges, D.; Rocha, E.; Gagliardi, V.; Silva, G.S. Traumatic Spinal Cord Injury: Current Concepts and Treatment Update. Arq. Neuropsiquiatr. 2017, 75, 387–393. [Google Scholar] [CrossRef]
- Sekhon, L.H.; Fehlings, M.G. Epidemiology, Demographics, and Pathophysiology of Acute Spinal Cord Injury. Spine (Phila. Pa. 1976) 2001, 26, S2–S12. [Google Scholar] [CrossRef]
- Dimitrijevic, M.R.; Danner, S.M.; Mayr, W. Neurocontrol of Movement in Humans With Spinal Cord Injury. Artif. Organs 2015, 39, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after Spinal Cord Injury: A Review of the Critical Timeline of Signaling Cues and Cellular Infiltration. J. Neuro Inflamm. 2021, 18, 284. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Bieler, L.; Vogl, M. Pathophysiology of Traumatic Spinal Cord Injury. In Neurological Aspects of Spinal Cord Injury; Weidner, N., Rupp, R., Tansey, K.E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 503–528. ISBN 978-3-319-46293-6. [Google Scholar]
- Vanzulli, I.; Butt, A.M. MGluR5 Protect Astrocytes from Ischemic Damage in Postnatal CNS White Matter. Cell Calcium 2015, 58, 423–430. [Google Scholar] [CrossRef]
- Ginet, V.; Spiehlmann, A.; Rummel, C.; Rudinskiy, N.; Grishchuk, Y.; Luthi-Carter, R.; Clarke, P.G.; Truttmann, A.C.; Puyal, J. Involvement of Autophagy in Hypoxic-Excitotoxic Neuronal Death. Autophagy 2014, 10, 846. [Google Scholar] [CrossRef]
- Williams, P.R.; Marincu, B.-N.; Sorbara, C.D.; Mahler, C.F.; Schumacher, A.-M.; Griesbeck, O.; Kerschensteiner, M.; Misgeld, T. A Recoverable State of Axon Injury Persists for Hours after Spinal Cord Contusion in Vivo. Nat. Commun. 2014, 5, 5683. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, M.; Almazan, G.; Tabrizian, M. Oligodendrocyte-Protection and Remyelination Post-Spinal Cord Injuries: A Review. Prog. Neurobiol. 2012, 96, 322–339. [Google Scholar] [CrossRef]
- Karimi-Abdolrezaee, S.; Billakanti, R. Reactive Astrogliosis after Spinal Cord Injury-Beneficial and Detrimental Effects. Mol. Neurobiol. 2012, 46, 251–264. [Google Scholar] [CrossRef]
- Göritz, C.; Dias, D.O.; Tomilin, N.; Barbacid, M.; Shupliakov, O.; Frisén, J. A Pericyte Origin of Spinal Cord Scar Tissue. Science 2011, 333, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.S.; Loeffler, M. Stem Cells: Attributes, Cycles, Spirals, Pitfalls and Uncertainties. Lessons for and from the Crypt. Development 1990, 110, 1001–1020. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z.; Li, H. The Role of Endothelial Progenitor Cells in Ischemic Cerebral and Heart Diseases. Cell Transplant. 2007, 16, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Zhu, M.; Wheeler, E.S. Differences in Stem and Progenitor Cell Yield in Different Subcutaneous Adipose Tissue Depots. Cytotherapy 2007, 9, 459–467. [Google Scholar] [CrossRef]
- Liu, W.; Song, F.; Ren, L.; Guo, W.; Wang, T.; Feng, Y.; Tang, L.; Li, K. The Multiple Functional Roles of Mesenchymal Stem Cells in Participating in Treating Liver Diseases. J. Cell Mol. Med. 2015, 19, 511–520. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Brown, J.C.; Shang, H.; Li, Y.; Yang, N.; Patel, N.; Katz, A.J. Isolation of Adipose-Derived Stromal Vascular Fraction Cells Using a Novel Point-of-Care Device: Cell Characterization and Review of the Literature. Tissue Eng. Part C Methods 2017, 23, 125–135. [Google Scholar] [CrossRef]
- Salgado, A.J.B.O.G.; Reis, R.L.G.; Sousa, N.J.C.; Gimble, J.M. Adipose Tissue Derived Stem Cells Secretome: Soluble Factors and Their Roles in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef]
- Kaewsuwan, S.; Song, S.Y.; Kim, J.H.; Sung, J.-H. Mimicking the Functional Niche of Adipose-Derived Stem Cells for Regenerative Medicine. Expert. Opin. Biol. Ther. 2012, 12, 1575–1588. [Google Scholar] [CrossRef]
- Yousefifard, M.; Shamseddin, J.; Babahajian, A.; Sarveazad, A. Efficacy of Adipose Derived Stem Cells on Functional and Neurological Improvement Following Ischemic Stroke: A Systematic Review and Meta-Analysis. BMC Neurol. 2020, 20, 294. [Google Scholar] [CrossRef]
- Chen, S.-H.; Wu, C.-C.; Tseng, W.-L.; Lu, F.-I.; Liu, Y.-H.; Lin, S.-P.; Lin, S.-C.; Hsueh, Y.-Y. Adipose-Derived Stem Cells Modulate Neuroinflammation and Improve Functional Recovery in Chronic Constriction Injury of the Rat Sciatic Nerve. Front. Neurosci. 2023, 17, 1172740. [Google Scholar] [CrossRef]
- Chang, K.-A.; Lee, J.-H.; Suh, Y.-H. Therapeutic Potential of Human Adipose-Derived Stem Cells in Neurological Disorders. J. Pharmacol. Sci. 2014, 126, 293–301. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Wang, D.; Liu, J.; Pan, J. Adipose Stem Cell-Based Clinical Strategy for Neural Regeneration: A Review of Current Opinion. Stem Cells Int. 2019, 2019, 8502370. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, L.; Zhong, J.; Gu, H.; Feng, D.; Johnstone, B.H.; March, K.L.; Farlow, M.R.; Du, Y. Adipose Stromal Cells-Secreted Neuroprotective Media against Neuronal Apoptosis. Neurosci. Lett. 2009, 462, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.Y.; Clavijo-Alvarez, J.; Brayfield, C.; Rubin, J.P.; Marra, K.G. Delivery of Adipose-Derived Precursor Cells for Peripheral Nerve Repair. Cell Transplant. 2009, 18, 145–158. [Google Scholar] [CrossRef]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel Autologous Cell Therapy in Ischemic Limb Disease through Growth Factor Secretion by Cultured Adipose Tissue-Derived Stromal Cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.A.; Pentecost, M.; Lenin, R.; Klaic, L.; Elshaer, S.L.; Gentry, J.; Russell, J.M.; Beland, A.; Reiner, A.; Jotterand, V.; et al. Concentrated Conditioned Media from Adipose Tissue Derived Mesenchymal Stem Cells Mitigates Visual Deficits and Retinal Inflammation Following Mild Traumatic Brain Injury. Int. J. Mol. Sci. 2018, 19, 2016. [Google Scholar] [CrossRef]
- Sánchez-Castillo, A.I.; Sepúlveda, M.R.; Marín-Teva, J.L.; Cuadros, M.A.; Martín-Oliva, D.; González-Rey, E.; Delgado, M.; Neubrand, V.E. Switching Roles: Beneficial Effects of Adipose Tissue-Derived Mesenchymal Stem Cells on Microglia and Their Implication in Neurodegenerative Diseases. Biomolecules 2022, 12, 219. [Google Scholar] [CrossRef]
- Duijvestein, M.; Wildenberg, M.E.; Welling, M.M.; Hennink, S.; Molendijk, I.; van Zuylen, V.L.; Bosse, T.; Vos, A.C.W.; de Jonge-Muller, E.S.M.; Roelofs, H.; et al. Pretreatment with Interferon-γ Enhances the Therapeutic Activity of Mesenchymal Stromal Cells in Animal Models of Colitis. Stem Cells 2011, 29, 1549–1558. [Google Scholar] [CrossRef]
- Chidlow, J.H.; Shukla, D.; Grisham, M.B.; Kevil, C.G. Pathogenic Angiogenesis in IBD and Experimental Colitis: New Ideas and Therapeutic Avenues. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G5–G18. [Google Scholar] [CrossRef]
- Marycz, K.; Michalak, I.; Kocherova, I.; Marędziak, M.; Weiss, C. The Cladophora Glomerata Enriched by Biosorption Process in Cr(III) Improves Viability, and Reduces Oxidative Stress and Apoptosis in Equine Metabolic Syndrome Derived Adipose Mesenchymal Stromal Stem Cells (ASCs) and Their Extracellular Vesicles (MV’s). Mar. Drugs 2017, 15, 385. [Google Scholar] [CrossRef]
- Xie, J.; Broxmeyer, H.E.; Feng, D.; Schweitzer, K.S.; Yi, R.; Cook, T.G.; Chitteti, B.R.; Barwinska, D.; Traktuev, D.O.; Van Demark, M.J.; et al. Human Adipose-Derived Stem Cells Ameliorate Cigarette Smoke-Induced Murine Myelosuppression via Secretion of TSG-6. Stem Cells 2015, 33, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Park, J.-S.; Jeong, H.-S. Neural Differentiation of Human Adipose Tissue-Derived Stem Cells Involves Activation of the Wnt5a/JNK Signalling. Stem Cells Int. 2015, 2015, 178618. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, J.; Kim, M.Y.; Bae, Y.-S.; Ryu, S.H.; Lee, T.G.; Kim, J.H. Proteomic Analysis of Tumor Necrosis Factor-Alpha-Induced Secretome of Human Adipose Tissue-Derived Mesenchymal Stem Cells. J. Proteome Res. 2010, 9, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Shang, X.; Xu, H.; Lü, S.; Dong, T.; Liang, C.; Yuan, Y. Rho-Associated Coiled Kinase Inhibitor Y-27632 Promotes Neuronal-like Differentiation of Adult Human Adipose Tissue-Derived Stem Cells. Chin. Med. J. 2012, 125, 3332–3335. [Google Scholar]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The Current State of Animal Models in Research: A Review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef]
- Patrick, C.W.; Uthamanthil, R.; Beahm, E.; Frye, C. Animal Models for Adipose Tissue Engineering. Tissue Eng. Part. B Rev. 2008, 14, 167–178. [Google Scholar] [CrossRef]
- Bukowska, J.; Szóstek-Mioduchowska, A.Z.; Kopcewicz, M.; Walendzik, K.; Machcińska, S.; Gawrońska-Kozak, B. Adipose-Derived Stromal/Stem Cells from Large Animal Models: From Basic to Applied Science. Stem Cell Rev. Rep. 2021, 17, 719–738. [Google Scholar] [CrossRef]
- Sharif-Alhoseini, M.; Khormali, M.; Rezaei, M.; Safdarian, M.; Hajighadery, A.; Khalatbari, M.M.; Safdarian, M.; Meknatkhah, S.; Rezvan, M.; Chalangari, M.; et al. Animal Models of Spinal Cord Injury: A Systematic Review. Spinal Cord. 2017, 55, 714–721. [Google Scholar] [CrossRef]
- Cizkova, D.; Murgoci, A.-N.; Cubinkova, V.; Humenik, F.; Mojzisova, Z.; Maloveska, M.; Cizek, M.; Fournier, I.; Salzet, M. Spinal Cord Injury: Animal Models, Imaging Tools and the Treatment Strategies. Neurochem. Res. 2020, 45, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, J.Y.; Cates, L.N.; Hyde, J.E.; Hofstetter, C.P.; Yang, C.C.; Khaing, Z.Z. Early Detrusor Application of Botulinum Toxin A Results in Reduced Bladder Hypertrophy and Fibrosis after Spinal Cord Injury in a Rodent Model. Toxins 2022, 14, 777. [Google Scholar] [CrossRef] [PubMed]
- Cheriyan, T.; Ryan, D.J.; Weinreb, J.H.; Cheriyan, J.; Paul, J.C.; Lafage, V.; Kirsch, T.; Errico, T.J. Spinal Cord Injury Models: A Review. Spinal Cord. 2014, 52, 588–595. [Google Scholar] [CrossRef]
- Mattucci, S.; Speidel, J.; Liu, J.; Kwon, B.K.; Tetzlaff, W.; Oxland, T.R. Basic Biomechanics of Spinal Cord Injury—How Injuries Happen in People and How Animal Models Have Informed Our Understanding. Clin. Biomech. 2019, 64, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Ridlen, R.; McGrath, K.; Gorrie, C.A. Animal Models of Compression Spinal Cord Injury. J. Neurosci. Res. 2022, 100, 2201–2212. [Google Scholar] [CrossRef]
- Han, B.; Liang, W.; Hai, Y.; Sun, D.; Ding, H.; Yang, Y.; Yin, P. Neurophysiological, Histological, and Behavioral Characterization of Animal Models of Distraction Spinal Cord Injury: A Systematic Review. Neural Regen. Res. 2023, 19, 563–570. [Google Scholar] [CrossRef]
- Fiford, R.J.; Bilston, L.E.; Waite, P.; Lu, J. A Vertebral Dislocation Model of Spinal Cord Injury in Rats. J. Neurotrauma 2004, 21, 451–458. [Google Scholar] [CrossRef]
- Choo, A.M.-T.; Liu, J.; Liu, Z.; Dvorak, M.; Tetzlaff, W.; Oxland, T.R. Modeling Spinal Cord Contusion, Dislocation, and Distraction: Characterization of Vertebral Clamps, Injury Severities, and Node of Ranvier Deformations. J. Neurosci. Methods 2009, 181, 6–17. [Google Scholar] [CrossRef]
- Jurgens, W.J.F.M.; Oedayrajsingh-Varma, M.J.; Helder, M.N.; Zandiehdoulabi, B.; Schouten, T.E.; Kuik, D.J.; Ritt, M.J.P.F.; van Milligen, F.J. Effect of Tissue-Harvesting Site on Yield of Stem Cells Derived from Adipose Tissue: Implications for Cell-Based Therapies. Cell Tissue Res. 2008, 332, 415–426. [Google Scholar] [CrossRef]
- Wickham, M.Q.; Erickson, G.R.; Gimble, J.M.; Vail, T.P.; Guilak, F. Multipotent Stromal Cells Derived from the Infrapatellar Fat Pad of the Knee. Clin. Orthop. Relat. Res. 2003, 412, 196–212. [Google Scholar] [CrossRef]
- Iyyanki, T.; Hubenak, J.; Liu, J.; Chang, E.I.; Beahm, E.K.; Zhang, Q. Harvesting Technique Affects Adipose-Derived Stem Cell Yield. Aesthet. Surg. J. 2015, 35, 467–476. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Kaneda, M.; Mady, E.A.; Yoshida, T.; Doghish, A.S.; Tanaka, R. Impact of Adipose Tissue Depot Harvesting Site on the Multilineage Induction Capacity of Male Rat Adipose-Derived Mesenchymal Stem Cells: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 7513. [Google Scholar] [CrossRef] [PubMed]
- Miana, V.V.; González, E.A.P. Adipose Tissue Stem Cells in Regenerative Medicine. Ecancermedicalscience 2018, 12, 822. [Google Scholar] [CrossRef] [PubMed]
- Egro, F.M.; Roy, E.; Rubin, J.P.; Coleman, S.R. Evolution of the Coleman Technique. Plast. Reconstr. Surg. 2022, 150, 329e. [Google Scholar] [CrossRef]
- Rodbell, M. The Metabolism of Isolated Fat Cells. IV. Regulation of Release of Protein by Lipolytic Hormones and Insulin. J. Biol. Chem. 1966, 241, 3909–3917. [Google Scholar] [CrossRef]
- Deslex, S.; Negrel, R.; Vannier, C.; Etienne, J.; Ailhaud, G. Differentiation of Human Adipocyte Precursors in a Chemically Defined Serum-Free Medium. Int. J. Obes. 1987, 11, 19–27. [Google Scholar] [PubMed]
- Van, R.L.; Bayliss, C.E.; Roncari, D.A. Cytological and Enzymological Characterization of Adult Human Adipocyte Precursors in Culture. J. Clin. Investig. 1976, 58, 699–704. [Google Scholar] [CrossRef]
- Illouz, Y.G. Body Contouring by Lipolysis: A 5-Year Experience with over 3000 Cases. Plast. Reconstr. Surg. 1983, 72, 591–597. [Google Scholar] [CrossRef]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Nagase, T.; Koshima, I.; et al. Characterization of Freshly Isolated and Cultured Cells Derived from the Fatty and Fluid Portions of Liposuction Aspirates. J. Cell Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-Derived Stem Cells: Isolation, Expansion and Differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Trentz, O.A.; Reddy, M.S.; Rela, M.; Kandasamy, M.; Sellathamby, S. In Vitro Transdifferentiation of Human Adipose Tissue-Derived Stem Cells to Neural Lineage Cells—A Stage-Specific Incidence. Adipocyte 2019, 8, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.B.; Trentz, O.A.; Arikketh, D.; Senthinathan, V.; Rosario, B.; Mohandas, P.V.A. Detection of Embryonic Stem Cell Markers in Adult Human Adipose Tissue-Derived Stem Cells. Indian J. Pathol. Microbiol. 2011, 54, 501–508. [Google Scholar] [CrossRef]
- Schreml, S.; Babilas, P.; Fruth, S.; Orsó, E.; Schmitz, G.; Mueller, M.B.; Nerlich, M.; Prantl, L. Harvesting Human Adipose Tissue-Derived Adult Stem Cells: Resection versus Liposuction. Cytotherapy 2009, 11, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, D.; Schwarz, E.J.; Prockop, D.J.; Black, I.B. Adult Rat and Human Bone Marrow Stromal Cells Differentiate into Neurons. J. Neurosci. Res. 2000, 61, 364–370. [Google Scholar] [CrossRef]
- Intrathecal Delivery of Stem Cells in the Subarachnoid Space. Available online: https://stemcellthailand.org/intrathecal-administration-stem-cells/ (accessed on 22 December 2023).
- Zhou, Z.; Tian, X.; Mo, B.; Xu, H.; Zhang, L.; Huang, L.; Yao, S.; Huang, Z.; Wang, Y.; Xie, H.; et al. Adipose Mesenchymal Stem Cell Transplantation Alleviates Spinal Cord Injury-Induced Neuroinflammation Partly by Suppressing the Jagged1/Notch Pathway. Stem Cell Res. Ther. 2020, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.L.B.; Hoa, N.D.; Thanh, V.V.; Thach, N.V.; Ngoc, V.T.N.; Dinh, T.C.; Phuong, T.N.T.; Toi, P.L.; Chu, D.T. Autologous Transplantation of Adipose-Derived Stem Cells to Treat Acute Spinal Cord Injury: Evaluation of Clinical Signs, Mental Signs, and Quality of Life. Open Access Maced. J. Med. Sci. 2019, 7, 4399–4405. [Google Scholar] [CrossRef]
- Féron, F.; Perry, C.; Cochrane, J.; Licina, P.; Nowitzke, A.; Urquhart, S.; Geraghty, T.; Mackay-Sim, A. Autologous Olfactory Ensheathing Cell Transplantation in Human Spinal Cord Injury. Brain 2005, 128, 2951–2960. [Google Scholar] [CrossRef]
- Ohta, Y.; Hamaguchi, A.; Ootaki, M.; Watanabe, M.; Takeba, Y.; Iiri, T.; Matsumoto, N.; Takenaga, M. Intravenous Infusion of Adipose-Derived Stem/Stromal Cells Improves Functional Recovery of Rats with Spinal Cord Injury. Cytotherapy 2017, 19, 839–848. [Google Scholar] [CrossRef]
- Yin, T.-C.; Shao, P.-L.; Chen, K.-H.; Lin, K.-C.; Chiang, J.Y.; Sung, P.-H.; Wu, S.-C.; Li, Y.-C.; Yip, H.-K.; Lee, M.S. Synergic Effect of Combined Therapy of Hyperbaric Oxygen and Adipose-Derived Mesenchymal Stem Cells on Improving Locomotor Recovery After Acute Traumatic Spinal Cord Injury in Rat Mainly Through Downregulating Inflammatory and Cell-Stress Signalings. Cell Transplant. 2022, 31, 9636897221133820. [Google Scholar] [CrossRef]
- Carelli, S.; Colli, M.; Vinci, V.; Caviggioli, F.; Klinger, M.; Gorio, A. Mechanical Activation of Adipose Tissue and Derived Mesenchymal Stem Cells: Novel Anti-Inflammatory Properties. Int. J. Mol. Sci. 2018, 19, 267. [Google Scholar] [CrossRef]
- Sun, L.; Li, M.; Ma, X.; Feng, H.; Song, J.; Lv, C.; He, Y. Inhibition of HMGB1 Reduces Rat Spinal Cord Astrocytic Swelling and AQP4 Expression after Oxygen-Glucose Deprivation and Reoxygenation via TLR4 and NF-ΚB Signaling in an IL-6-Dependent Manner. J. Neuro Inflamm. 2017, 14, 231. [Google Scholar] [CrossRef]
- Sarveazad, A.; Janzadeh, A.; Taheripak, G.; Dameni, S.; Yousefifard, M.; Nasirinezhad, F. Co-Administration of Human Adipose-Derived Stem Cells and Low-Level Laser to Alleviate Neuropathic Pain after Experimental Spinal Cord Injury. Stem Cell Res. Ther. 2019, 10, 183. [Google Scholar] [CrossRef]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but Elusive Drug Targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Perrelet, D.; Ferri, A.; Liston, P.; Muzzin, P.; Korneluk, R.G.; Kato, A.C. IAPs Are Essential for GDNF-Mediated Neuroprotective Effects in Injured Motor Neurons in Vivo. Nat. Cell Biol. 2002, 4, 175–179. [Google Scholar] [CrossRef]
- Macias, M.Y.; Syring, M.B.; Pizzi, M.A.; Crowe, M.J.; Alexanian, A.R.; Kurpad, S.N. Pain with No Gain: Allodynia Following Neural Stem Cell Transplantation in Spinal Cord Injury. Exp. Neurol. 2006, 201, 335–348. [Google Scholar] [CrossRef]
- Zanjani, T.M.; Sabetkasaei, M.; Mosaffa, N.; Manaheji, H.; Labibi, F.; Farokhi, B. Suppression of Interleukin-6 by Minocycline in a Rat Model of Neuropathic Pain. Eur. J. Pharmacol. 2006, 538, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Castonguay, A.; Cottet, M.; Little, J.W.; Chen, Z.; Symons-Liguori, A.M.; Doyle, T.; Egan, T.M.; Vanderah, T.W.; De Koninck, Y.; et al. Engagement of the GABA to KCC2 Signaling Pathway Contributes to the Analgesic Effects of A3AR Agonists in Neuropathic Pain. J. Neurosci. 2015, 35, 6057–6067. [Google Scholar] [CrossRef]
- Moon, H.C.; Lee, Y.J.; Cho, C.B.; Park, Y.S. Suppressed GABAergic Signaling in the Zona Incerta Causes Neuropathic Pain in a Thoracic Hemisection Spinal Cord Injury Rat Model. Neurosci. Lett. 2016, 632, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Menezes, K.; Nascimento, M.A.; Gonçalves, J.P.; Cruz, A.S.; Lopes, D.V.; Curzio, B.; Bonamino, M.; de Menezes, J.R.L.; Borojevic, R.; Rossi, M.I.D.; et al. Human Mesenchymal Cells from Adipose Tissue Deposit Laminin and Promote Regeneration of Injured Spinal Cord in Rats. PLoS ONE 2014, 9, e96020. [Google Scholar] [CrossRef]
- Menezes, K.; de Menezes, J.R.L.; Nascimento, M.A.; Santos, R.d.S.; Coelho-Sampaio, T. Polylaminin, a Polymeric Form of Laminin, Promotes Regeneration after Spinal Cord Injury. FASEB J. 2010, 24, 4513–4522. [Google Scholar] [CrossRef]
- Aras, Y.; Sabanci, P.A.; Kabatas, S.; Duruksu, G.; Subasi, C.; Erguven, M.; Karaoz, E. The Effects of Adipose Tissue-Derived Mesenchymal Stem Cell Transplantation During the Acute and Subacute Phases Following Spinal Cord Injury. Turk. Neurosurg. 2016, 26, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Kim, I.-S.; Han, N.; Yun, S.; Park, K.I.; Yoo, K.-H. Real-Time Discrimination between Proliferation and Neuronal and Astroglial Differentiation of Human Neural Stem Cells. Sci. Rep. 2014, 4, 6319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, W.; Reiser, G. Activation of Protease-Activated Receptors in Astrocytes Evokes a Novel Neuroprotective Pathway through Release of Chemokines of the Growth-Regulated Oncogene/Cytokine-Induced Neutrophil Chemoattractant Family. Eur. J. Neurosci. 2007, 26, 3159–3168. [Google Scholar] [CrossRef] [PubMed]
- Kazanis, I.; Lathia, J.D.; Vadakkan, T.J.; Raborn, E.; Wan, R.; Mughal, M.R.; Eckley, D.M.; Sasaki, T.; Patton, B.; Mattson, M.P.; et al. Quiescence and Activation of Stem and Precursor Cell Populations in the Subependymal Zone of the Mammalian Brain Are Associated with Distinct Cellular and Extracellular Matrix Signals. J. Neurosci. 2010, 30, 9771–9781. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Wang, Y.; Kokovay, E.; Lin, G.; Chuang, S.-M.; Goderie, S.K.; Roysam, B.; Temple, S. Adult SVZ Stem Cells Lie in a Vascular Niche: A Quantitative Analysis of Niche Cell-Cell Interactions. Cell Stem Cell 2008, 3, 289–300. [Google Scholar] [CrossRef]
- Lathia, J.D.; Patton, B.; Eckley, D.M.; Magnus, T.; Mughal, M.R.; Sasaki, T.; Caldwell, M.A.; Rao, M.S.; Mattson, M.P.; ffrench-Constant, C. Patterns of Laminins and Integrins in the Embryonic Ventricular Zone of the CNS. J. Comp. Neurol. 2007, 505, 630–643. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Liu, M.; Gao, G.; Zhao, W.; Fu, Q.; Wang, Y. Implantation of Adipose-Derived Mesenchymal Stem Cell Sheets Promotes Axonal Regeneration and Restores Bladder Function after Spinal Cord Injury. Stem Cell Res. Ther. 2022, 13, 503. [Google Scholar] [CrossRef]
- Safford, K.M.; Hicok, K.C.; Safford, S.D.; Halvorsen, Y.-D.C.; Wilkison, W.O.; Gimble, J.M.; Rice, H.E. Neurogenic Differentiation of Murine and Human Adipose-Derived Stromal Cells. Biochem. Biophys. Res. Commun. 2002, 294, 371–379. [Google Scholar] [CrossRef]
- Deng, J.; Petersen, B.E.; Steindler, D.A.; Jorgensen, M.L.; Laywell, E.D. Mesenchymal Stem Cells Spontaneously Express Neural Proteins in Culture and Are Neurogenic after Transplantation. Stem Cells 2006, 24, 1054–1064. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Yuan, X.; Yuan, W.; Ding, L.; Shi, M.; Luo, L.; Wan, Y.; Oh, J.; Zhou, Y.; Bian, L.; Deng, D.Y.B. Cell-Adaptable Dynamic Hydrogel Reinforced with Stem Cells Improves the Functional Repair of Spinal Cord Injury by Alleviating Neuroinflammation. Biomaterials 2021, 279, 121190. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ji, Z.; Wang, Y.; Li, T.; Luo, J.; Li, J.; Shi, X.; Li, L.; He, L.; Wu, W. Human Adipose-Derived Stem Cells Combined with Nano-Hydrogel Promote Functional Recovery after Spinal Cord Injury in Rats. Biology 2022, 11, 781. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, X.; Ji, L.; Wang, K.; Qiu, Y. Effects of Brain-derived Neurotrophic Factor and Neurotrophin-3 on the Neuronal Differentiation of Rat Adipose-derived Stem Cells. Mol. Med. Rep. 2015, 12, 4981–4988. [Google Scholar] [CrossRef]
- Takahashi, A.; Nakajima, H.; Kubota, A.; Watanabe, S.; Matsumine, A. Adipose-Derived Mesenchymal Stromal Cell Transplantation for Severe Spinal Cord Injury: Functional Improvement Supported by Angiogenesis and Neuroprotection. Cells 2023, 12, 1470. [Google Scholar] [CrossRef] [PubMed]
- Hausman, G.J.; Richardson, R.L. Adipose Tissue Angiogenesis. J. Anim. Sci. 2004, 82, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Hamano, K.; Li, T.S.; Katoh, T.; Kobayashi, S.; Matsuzaki, M.; Esato, K. Enhancement of Angiogenesis by the Implantation of Self Bone Marrow Cells in a Rat Ischemic Heart Model. J. Surg. Res. 2000, 89, 189–195. [Google Scholar] [CrossRef]
- Bergers, G.; Song, S. The Role of Pericytes in Blood-Vessel Formation and Maintenance. Neuro Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef]
- Menezes, K.; Rosa, B.G.; Freitas, C.; da Cruz, A.S.; de Siqueira Santos, R.; Nascimento, M.A.; Alves, D.V.L.; Bonamino, M.; Rossi, M.I.; Borojevic, R.; et al. Human Mesenchymal Stromal/Stem Cells Recruit Resident Pericytes and Induce Blood Vessels Maturation to Repair Experimental Spinal Cord Injury in Rats. Sci. Rep. 2020, 10, 19604. [Google Scholar] [CrossRef]
- Rafiei Alavi, S.N.; Madani Neishaboori, A.; Hossein, H.; Sarveazad, A.; Yousefifard, M. Efficacy of Adipose Tissue-Derived Stem Cells in Locomotion Recovery after Spinal Cord Injury: A Systematic Review and Meta-Analysis on Animal Studies. Syst. Rev. 2021, 10, 213. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yousefifard, M.; Sarveazad, A.; Janzadeh, A.; Behroozi, Z.; Nasirinezhad, F. Pain Alleviating Effect of Adipose-Derived Stem Cells Transplantation on the Injured Spinal Cord: A Behavioral and Electrophysiological Evaluation. J. Stem Cells Regen. Med. 2022, 18, 53–63. [Google Scholar] [CrossRef]
- Levin, E.D.; Cerutti, D.T. Behavioral Neuroscience of Zebrafish. In Methods of Behavior Analysis in Neuroscience; Buccafusco, J.J., Ed.; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009; ISBN 978-1-4200-5234-3. [Google Scholar]
- Ryu, H.H.; Lim, J.H.; Byeon, Y.E.; Park, J.R.; Seo, M.S.; Lee, Y.W.; Kim, W.H.; Kang, K.S.; Kweon, O.K. Functional Recovery and Neural Differentiation after Transplantation of Allogenic Adipose-Derived Stem Cells in a Canine Model of Acute Spinal Cord Injury. J. Vet. Sci. 2009, 10, 273–284. [Google Scholar] [CrossRef]
- Lim, J.H.; Byeon, Y.E.; Ryu, H.H.; Jeong, Y.H.; Lee, Y.W.; Kim, W.H.; Kang, K.S.; Kweon, O.K. Transplantation of Canine Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Experimentally Induced Spinal Cord Injured Dogs. J. Vet. Sci. 2007, 8, 275–282. [Google Scholar] [CrossRef]
- Oh, J.S.; Park, I.S.; Kim, K.N.; Yoon, D.H.; Kim, S.-H.; Ha, Y. Transplantation of an Adipose Stem Cell Cluster in a Spinal Cord Injury. Neuroreport 2012, 23, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Dalghi, M.G.; Montalbetti, N.; Carattino, M.D.; Apodaca, G. The Urothelium: Life in a Liquid Environment. Physiol. Rev. 2020, 100, 1621–1705. [Google Scholar] [CrossRef] [PubMed]
- Home—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ (accessed on 16 December 2023).
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of Intravenous Infusion of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Animals and Humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef]
- Hur, J.W.; Cho, T.-H.; Park, D.-H.; Lee, J.-B.; Park, J.-Y.; Chung, Y.-G. Intrathecal Transplantation of Autologous Adipose-Derived Mesenchymal Stem Cells for Treating Spinal Cord Injury: A Human Trial. J. Spinal Cord. Med. 2016, 39, 655–664. [Google Scholar] [CrossRef]
- Bydon, M.; Dietz, A.B.; Goncalves, S.; Moinuddin, F.M.; Alvi, M.A.; Goyal, A.; Yolcu, Y.; Hunt, C.L.; Garlanger, K.L.; Del Fabro, A.S.; et al. CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury. Mayo Clin. Proc. 2020, 95, 406–414. [Google Scholar] [CrossRef]
- Lai, A.; Iliff, D.; Zaheer, K.; Wang, D.; Gansau, J.; Laudier, D.M.; Zachariou, V.; Iatridis, J.C. Spinal Cord Sensitization and Spinal Inflammation from an In Vivo Rat Endplate Injury Associated with Painful Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2023, 24, 3425. [Google Scholar] [CrossRef]
- Vilalta, M.; Dégano, I.R.; Bagó, J.; Gould, D.; Santos, M.; García-Arranz, M.; Ayats, R.; Fuster, C.; Chernajovsky, Y.; García-Olmo, D.; et al. Biodistribution, Long-Term Survival, and Safety of Human Adipose Tissue-Derived Mesenchymal Stem Cells Transplanted in Nude Mice by High Sensitivity Non-Invasive Bioluminescence Imaging. Stem Cells Dev. 2008, 17, 993–1003. [Google Scholar] [CrossRef]
- Baranovskii, D.S.; Klabukov, I.D.; Arguchinskaya, N.V.; Yakimova, A.O.; Kisel, A.A.; Yatsenko, E.M.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Adverse Events, Side Effects and Complications in Mesenchymal Stromal Cell-Based Therapies. Stem Cell Investig. 2022, 9, 7. [Google Scholar] [CrossRef]
- Chang, S.-H.; Kim, H.J.; Park, C.-G. Allogeneic ADSCs Induce the Production of Alloreactive Memory-CD8 T Cells through HLA-ABC Antigens. Cells 2020, 9, 1246. [Google Scholar] [CrossRef]
- Prigozhina, T.B.; Khitrin, S.; Elkin, G.; Eizik, O.; Morecki, S.; Slavin, S. Mesenchymal Stromal Cells Lose Their Immunosuppressive Potential after Allotransplantation. Exp. Hematol. 2008, 36, 1370–1376. [Google Scholar] [CrossRef]
- Bajek, A.; Gurtowska, N.; Olkowska, J.; Kazmierski, L.; Maj, M.; Drewa, T. Adipose-Derived Stem Cells as a Tool in Cell-Based Therapies. Arch. Immunol. Ther. Exp. 2016, 64, 443–454. [Google Scholar] [CrossRef]
- Mazini, L.; Ezzoubi, M.; Malka, G. Overview of Current Adipose-Derived Stem Cell (ADSCs) Processing Involved in Therapeutic Advancements: Flow Chart and Regulation Updates before and after COVID-19. Stem Cell Res. Ther. 2021, 12, 1. [Google Scholar] [CrossRef]
- Wei, H.-J.; Zeng, R.; Lu, J.-H.; Lai, W.-F.T.; Chen, W.-H.; Liu, H.-Y.; Chang, Y.-T.; Deng, W.-P. Adipose-Derived Stem Cells Promote Tumor Initiation and Accelerate Tumor Growth by Interleukin-6 Production. Oncotarget 2015, 6, 7713–7726. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Liu, C.; Han, X.; Gu, X.; Zhou, S. Deciphering Glial Scar after Spinal Cord Injury. Burns Trauma. 2021, 9, tkab035. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Shen, P.-P.; Wang, B. Gene-Modified Stem Cells for Spinal Cord Injury: A Promising Better Alternative Therapy. Stem Cell Rev. Rep. 2022, 18, 2662–2682. [Google Scholar] [CrossRef]
- Clifford, T.; Finkel, Z.; Rodriguez, B.; Joseph, A.; Cai, L. Current Advancements in Spinal Cord Injury Research—Glial Scar Formation and Neural Regeneration. Cells 2023, 12, 853. [Google Scholar] [CrossRef]

| NCT Number | Study Title | Study Status | Results | Sex | Enrollment | Phase | Start Date | Duration | Location |
|---|---|---|---|---|---|---|---|---|---|
| NCT02981576 | Safety and Effectiveness of BM-MSC vs. AT-MSC in the Treatment of SCI Patients. | Completed | No | All | 14 | I/II | November 2016 | 3 years | Jordan |
| NCT02917291 | Safety and Preliminary Efficacy of FAB117-HC in Patients With Acute Traumatic Spinal Cord Injury. | Unknown | No | All | 48 | I/II | December 2016 | 7 years | Spain |
| NCT02034669 | Transplantation of Autologous Adipose Derived Stem Cells (ADSCs) in Spinal Cord Injury Treatment. | Unknown | No | All | 48 | I/II | February 2013 | 2 years | Vietnam |
| NCT01769872 | Safety and Effect of Adipose Tissue Derived Mesenchymal Stem Cell Implantation in Patients With Spinal Cord Injury. | Completed | No | All | 15 | I/II | January 2013 | 3 years | Republic of Korea |
| NCT01624779 | Intrathecal Transplantation Of Autologous Adipose Tissue Derived MSC in the Patients With Spinal Cord Injury. | Completed | Yes | All | 15 | I | April 2012 | 2 years | Republic of Korea |
| NCT01274975 | Autologous Adipose Derived MSCs Transplantation in Patient With Spinal Cord Injury. | Completed | Yes | Male | 8 | I | July 2009 | 1 year | Republic of Korea |
| NCT05018793 | Safety of Cultured Autologous Adult Adipose Derived Mesenchymal Stem Cell Intrathecal Injection for SCI. | Suspended | No | All | 15 | I | December 2012 | 4 years | Greece |
| NCT04520373 | Autologous Adipose Derived Mesenchymal Stem Cells for Spinal Cord Injury Patients. | Recruiting | No | All | 40 | II | June 2020 | 4 years | United States |
| NCT04064957 | Individual Patient Expanded Access IND of Hope Biosciences Autologous Adipose-derived Mesenchymal Stem Cells for Spinal Cord Injury. | No longer available | No | All | N/A | N/A | N/A | N/A | United States |
| NCT03925649 | Individual Patient Expanded Access IND of Hope Biosciences Autologous Adipose-derived Mesenchymal Stem Cells for Treatment of SCI. | No longer available | No | Male | N/A | N/A | N/A | N/A | United States |
| NCT03308565 | Adipose Stem Cells for Traumatic Spinal Cord Injury. | Completed | Yes | All | 10 | I | December 2017 | 4 years | United States |
| Title | Study Design | Primary Objectives | Inclusion Criteria | Intervention |
|---|---|---|---|---|
| Autologous Adipose Derived MSCs Transplantation in Patient with Spinal Cord Injury | Randomized Open Label Single group Assignment | Assess the safety of intravenous autologous AD-MSC transplant in SCI patients. | Males between 19 and 60 years. AIS grade A, B or C. Duration of injury: >2 months | Group 1: IV injection of AD-MSC (400 million cells) (Astrostem®) |
| Intrathecal Transplantation of Autologous Adipose Tissue Derived MSC in the Patients With Spinal Cord Injury | Open Label Factorial Assignment | Assess the effect of intrathecal transplantation of autologous AD-MSC in the patients with SCI. | Male or female aging between 19 and 70 years. No chance of improving neurological function despite performed the optimal treatment after SCI. No change in neurological function for 4-week intervals by at least 2 clinical medical specialists | Group 1: Intrathecal injections of AD-MSC (90 million cells) at day 1, after 1 month and after 2 months. |
| Safety and Effect of Adipose Tissue Derived Mesenchymal Stem Cell Implantation in Patients With Spinal Cord Injury | Open Label Single group assignment | Investigate the efficacy and safety of autologous transplantation of AD-MSC in patient with SCI | Male or female aging between 19 and 70 years. AIS grade A, B or C. Duration of injury > 3 months. | Group 1: IV and Intrathecal injections of AS-MSC. |
| Transplantation of Autologous Adipose Derived Stem Cells (ADSCs) in Spinal Cord Injury Treatment | Randomized Open Label Parallel Assignment | Assess the safety and effect of AD-MSC transplantation in acute SCI patients. | Male or female aging between 19 and 60 years. AIS grade A. Patients with complete spinal cord < 2 weeks in acute category. | Group 1: AD-MSC transplantation 4 Interventions: laminectomy, intradural space at damage site, intrathecal at lumbar puncture, and IV. Group 2: only laminectomy. |
| Safety and Preliminary Efficacy of FAB117-HC in Patients with Acute Traumatic Spinal Cord Injury | Randomized Double Masked Parallel Assignment | Evaluate the safety and tolerability of FAB117-HC (a medicinal product containing human AD-MSC expanded and pulsed with H2O2, HC016 cells) administered at a single-time point to patients with acute thoracic SCI. | Male or female between 16 and 70 years. AIS grade A and B. Single traumatic spinal cord injury as defined by MRI. Either a level of injury between D1–D12 both inclusive. Injury occurred between 72 and 120 h before undergoing DSS and treatment. Clinically and hemodynamically stable, under medical criteria, enough to undergo DSS. | Phase 1: (only grade A) Intramedullary injection of Drug: FAB117-HC (3 patients with 20 million cells and 5 patients with 40 million cells) Phase 2: (grade A or B) Group 1: Intramedullary injection of FAB117-HC (20 or 40 million cells) Group 2: No treatment |
| Safety and Effectiveness of BM-MSC vs. AT-MSC in the Treatment of SCI Patients. | Randomized Open Label Parallel Assignment | Assess and compare the safety and effectiveness of autologous BM-MSC vs. autologous AD-MSC in SCI patients. | Male or Female between 18 and 70 years. Complete SCI grade AIS A or B, or incomplete C. At least 2 weeks since time of injury. | Group 1: Intrathecal injection of AD-MSC 3 times. Group 2: Intrathecal injection of BM-MSC 3 times. |
| Adipose Stem Cells for Traumatic Spinal Cord Injury | Open Label Single group assignment | Determine if AD-MSC can be safely administered into the cerebrospinal fluid of patients with SCI. | Male or female older than 18 years. AIS grade A or B. SCI must be traumatic, blunt/non-penetrating in nature and not degenerative. SCI must be within two weeks and up to 1 year after the event | Group 1: Intrathecal injection of AD-MSC (100 million cells). |
| Autologous Adipose Derived Mesenchymal Stem Cells for Spinal Cord Injury Patients | Randomized Open Label Crossover Assignment | Investigate the safety and potential therapeutic effects of autologous, culture-expanded, AD-MSC intrathecal injections in the treatment of SCI. | Male or female older than 18 years. AIS grade A or B. SCI must be traumatic, blunt/non-penetrating in nature and not degenerative. | Group 1: Intrathecal injection of AD-MSC. Group 2: 6 months attending physical and occupational therapy. Then, intrathecal injection of AD-MSC. |
| Safety of Cultured Autologous Adult Adipose Derived Mesenchymal Stem Cell Intrathecal Injection for SCI | Open Label Single group assignment | Study the safety and efficacy of intrathecal injection of cultured autologous AD-MSC for the treatment of SCI. | Male or female. Presence of a diagnosis of SCI. | Group 1: Intrathecal injection of AD-MSC (100 million cells). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Masri, J.; Fadlallah, H.; Al Sabsabi, R.; Afyouni, A.; Al-Sayegh, M.; Abou-Kheir, W. Adipose-Derived Stem Cell Therapy in Spinal Cord Injury. Cells 2024, 13, 1505. https://doi.org/10.3390/cells13171505
El Masri J, Fadlallah H, Al Sabsabi R, Afyouni A, Al-Sayegh M, Abou-Kheir W. Adipose-Derived Stem Cell Therapy in Spinal Cord Injury. Cells. 2024; 13(17):1505. https://doi.org/10.3390/cells13171505
Chicago/Turabian StyleEl Masri, Jad, Hiba Fadlallah, Rahaf Al Sabsabi, Ahmad Afyouni, Mohamed Al-Sayegh, and Wassim Abou-Kheir. 2024. "Adipose-Derived Stem Cell Therapy in Spinal Cord Injury" Cells 13, no. 17: 1505. https://doi.org/10.3390/cells13171505
APA StyleEl Masri, J., Fadlallah, H., Al Sabsabi, R., Afyouni, A., Al-Sayegh, M., & Abou-Kheir, W. (2024). Adipose-Derived Stem Cell Therapy in Spinal Cord Injury. Cells, 13(17), 1505. https://doi.org/10.3390/cells13171505









