Functional Insights in PLS3-Mediated Osteogenic Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vivo Characterization of pls3 Knockdown Zebrafish Compensated with Actin-Bundling Proteins
2.2. Acquisition of Human Material
2.3. Cell Culture and Osteogenic Transdifferentiation
2.4. RNA Isolation, cDNA Synthesis, and qPCR
2.5. Western Blotting Analysis
2.6. Pls3 Knockdown in Murine MLO-Y4 Cell Line
2.7. RNA Extraction, cDNA Library Establishment, and Illumina Sequencing
2.8. RNA Sequence Data Analysis
2.9. Mineralization Assay
2.10. Statistical Analysis
3. Results
3.1. Actinin-1 and Actinin-4 Rescue Malformations of Craniofacial Bone Structure, Body Axis, and Tail in 5-Day-Old col1a1-eGFP Zebrafish with pls3-Mediated Morpholino Knockdown
3.2. PLS3 Expression in Fibroblasts of PLS3-Variant Osteoporosis Patients
3.3. Actin-Bundling Protein Expression in PLS3-Variant Patient Fibroblasts and Osteoblast-like Cells
3.4. Pls3 Knockdown in MLO-Y4 Cells
3.5. Differential Gene Expression in MLO-Y4 Cells Following Pls3 Knockdown
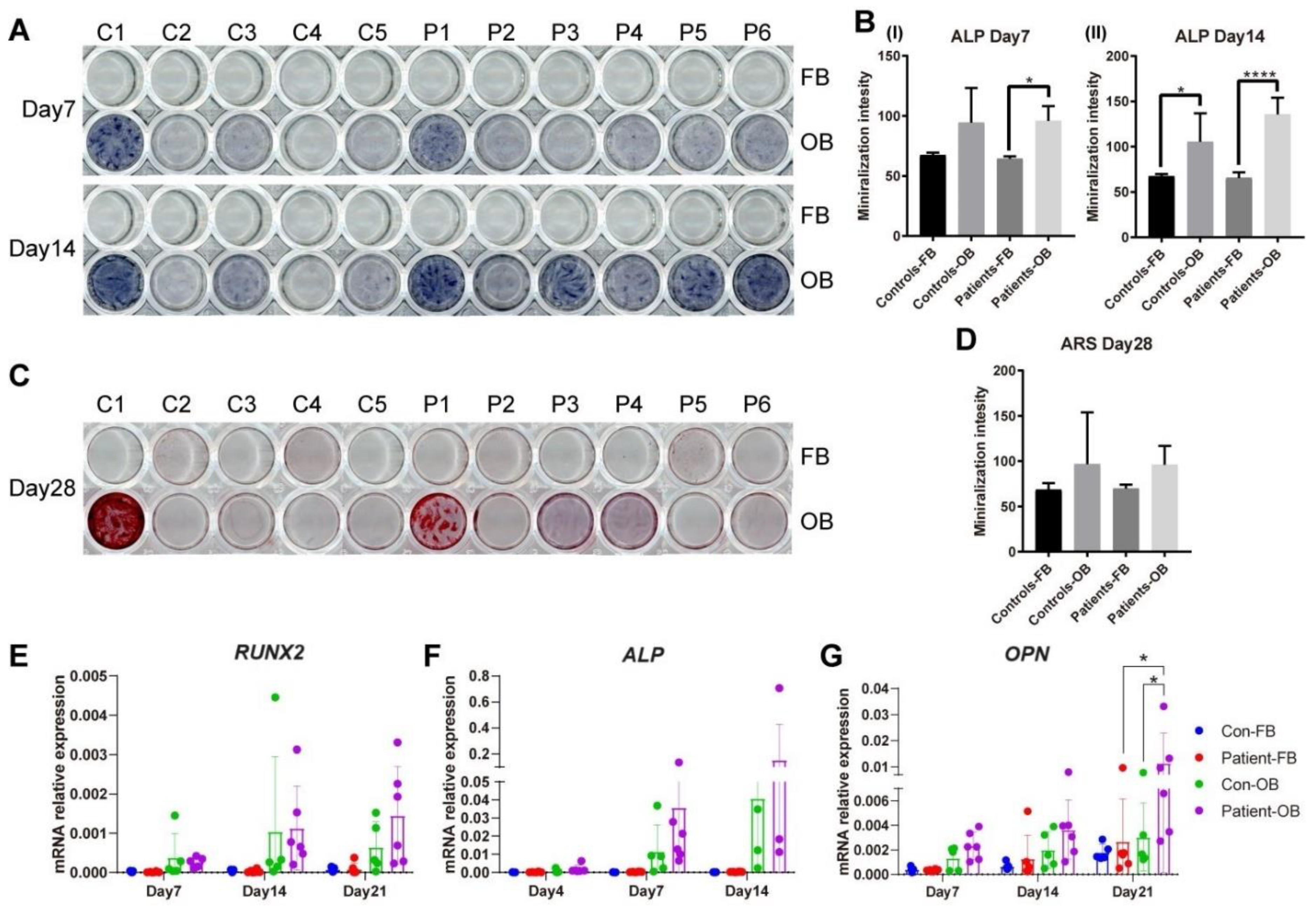
3.6. Osteogenic Transdifferentiation Potential of PLS3-Variant Patient Fibroblasts
3.7. Gene Expression of Proteins Involved in WNTSignaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Dijk, F.S.; Zillikens, M.C.; Micha, D.; Riessland, M.; Marcelis, C.L.; de Die-Smulders, C.E.; Milbradt, J.; Franken, A.A.; Harsevoort, A.J.; Lichtenbelt, K.D.; et al. PLS3 mutations in X-linked osteoporosis with fractures. N. Engl. J. Med. 2013, 369, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Apperley, L.J.; Albaba, S.; Dharmaraj, P.; Balasubramanian, M. PLS3 whole gene deletion as a cause of X-linked osteoporosis: Clinical report with review of published PLS3 literature. Clin. Dysmorphol. 2023, 32, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Wolff, L.; Strathmann, E.A.; Muller, I.; Mahlich, D.; Veltman, C.; Niehoff, A.; Wirth, B. Plastin 3 in health and disease: A matter of balance. Cell Mol. Life Sci. 2021, 78, 5275–5301. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Feng, Z.; Zhu, X.; Dai, Z.; Min, K.; Qiu, Y.; Yi, L.; Xu, L.; Zhu, Z. Identification of a novel splicing mutation and genotype-phenotype correlations in rare PLS3-related childhood-onset osteoporosis. Orphanet J. Rare Dis. 2022, 17, 247. [Google Scholar] [CrossRef] [PubMed]
- Besio, R.; Chow, C.W.; Tonelli, F.; Marini, J.C.; Forlino, A. Bone biology: Insights from osteogenesis imperfecta and related rare fragility syndromes. FEBS J. 2019, 286, 3033–3056. [Google Scholar] [CrossRef] [PubMed]
- Kampe, A.J.; Costantini, A.; Levy-Shraga, Y.; Zeitlin, L.; Roschger, P.; Taylan, F.; Lindstrand, A.; Paschalis, E.P.; Gamsjaeger, S.; Raas-Rothschild, A.; et al. PLS3 Deletions Lead to Severe Spinal Osteoporosis and Disturbed Bone Matrix Mineralization. J. Bone Miner. Res. 2017, 32, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Kampe, A.J.; Costantini, A.; Makitie, R.E.; Jantti, N.; Valta, H.; Mayranpaa, M.; Kroger, H.; Pekkinen, M.; Taylan, F.; Jiao, H.; et al. PLS3 sequencing in childhood-onset primary osteoporosis identifies two novel disease-causing variants. Osteoporos. Int. 2017, 28, 3023–3032. [Google Scholar] [CrossRef]
- Yorgan, T.A.; Sari, H.; Rolvien, T.; Windhorst, S.; Failla, A.V.; Kornak, U.; Oheim, R.; Amling, M.; Schinke, T. Mice lacking plastin-3 display a specific defect of cortical bone acquisition. Bone 2020, 130, 115062. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, J.; Heilig, J.; Hosseinibarkooie, S.; Ross, B.C.; Mendoza-Ferreira, N.; Nolte, F.; Peters, M.; Holker, I.; Hupperich, K.; Tschanz, T.; et al. Plastin 3 influences bone homeostasis through regulation of osteoclast activity. Hum. Mol. Genet. 2018, 27, 4249–4262. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, B.; Lin, X.; Zhang, Q.; Guan, F.; Sun, L.; Liu, J.; Wang, O.; Jiang, Y.; Xia, W.B.; et al. Impaired bone strength and bone microstructure in a novel early-onset osteoporotic rat model with a clinically relevant PLS3 mutation. Elife 2023, 12, e80365. [Google Scholar] [CrossRef]
- Maus, I.; Dreiner, M.; Zetzsche, S.; Metzen, F.; Ross, B.C.; Mählich, D.; Koch, M.; Niehoff, A.; Wirth, B. Osteoclast-specific Plastin 3 knockout in mice fail to develop osteoporosis despite dramatic increased osteoclast resorption activity. JBMR Plus 2024, 8, ziad009. [Google Scholar] [CrossRef] [PubMed]
- Schwebach, C.L.; Kudryashova, E.; Agrawal, R.; Zheng, W.; Egelman, E.H.; Kudryashov, D.S. Allosteric regulation controls actin-bundling properties of human plastins. Nat. Struct. Mol. Biol. 2022, 29, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book; ZFIN, University of Oregon Press: Eugene, OR, USA, 1995. [Google Scholar]
- Micha, D.; Voermans, E.; Eekhoff, M.E.W.; van Essen, H.W.; Zandieh-Doulabi, B.; Netelenbos, C.; Rustemeyer, T.; Sistermans, E.A.; Pals, G.; Bravenboer, N. Inhibition of TGFbeta signaling decreases osteogenic differentiation of fibrodysplasia ossificans progressiva fibroblasts in a novel in vitro model of the disease. Bone 2016, 84, 169–180. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq–A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Formosa, M.M.; Christou, M.A.; Makitie, O. Bone fragility and osteoporosis in children and young adults. J. Endocrinol. Investig. 2023, 47, 285–298. [Google Scholar] [CrossRef]
- Vignjevic, D.; Yarar, D.; Welch, M.D.; Peloquin, J.; Svitkina, T.; Borisy, G.G. Formation of filopodia-like bundles in vitro from a dendritic network. J. Cell Biol. 2003, 160, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, J.D.; Suarez, C.; Hocky, G.M.; Harker, A.J.; Morganthaler, A.N.; Christensen, J.R.; Voth, G.A.; Bartles, J.R.; Kovar, D.R. Fascin- and alpha-Actinin-Bundled Networks Contain Intrinsic Structural Features that Drive Protein Sorting. Curr. Biol. 2016, 26, 2697–2706. [Google Scholar] [CrossRef]
- Rajan, S.; Kudryashov, D.S.; Reisler, E. Actin Bundles Dynamics and Architecture. Biomolecules 2023, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.M.; Unnold-Cofre, C.; Naismith, T.; Jansen, S. The actin-bundling protein, PLS3, is part of the mechanoresponsive machinery that regulates osteoblast mineralization. Front. Cell Dev. Biol. 2023, 11, 1141738. [Google Scholar] [CrossRef] [PubMed]
- Laine, C.M.; Wessman, M.; Toiviainen-Salo, S.; Kaunisto, M.A.; Mayranpaa, M.K.; Laine, T.; Pekkinen, M.; Kroger, H.; Valimaki, V.V.; Valimaki, M.J.; et al. A novel splice mutation in PLS3 causes X-linked early onset low-turnover osteoporosis. J. Bone Miner. Res. 2015, 30, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Pathak, J.L.; Liang, Y.; Zhytnik, L.; Pals, G.; Eekhoff, E.M.W.; Bravenboer, N.; Micha, D. The intricate mechanism of PLS3 in bone homeostasis and disease. Front. Endocrinol. 2023, 14, 1168306. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Kamioka, K.; Kamioka, H.; Ris, H.; Lim, S.S. Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin-rich projections. J. Bone Miner. Res. 1998, 13, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Kamioka, H.; Sugawara, Y.; Honjo, T.; Yamashiro, T.; Takano-Yamamoto, T. Terminal differentiation of osteoblasts to osteocytes is accompanied by dramatic changes in the distribution of actin-binding proteins. J. Bone Miner. Res. 2004, 19, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Makitie, R.E.; Kampe, A.; Costantini, A.; Alm, J.J.; Magnusson, P.; Makitie, O. Biomarkers in WNT1 and PLS3 Osteoporosis: Altered Concentrations of DKK1 and FGF23. J. Bone Miner. Res. 2020, 35, 901–912. [Google Scholar] [CrossRef]
- Bafico, A.; Liu, G.; Yaniv, A.; Gazit, A.; Aaronson, S.A. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 2001, 3, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.B.; Robling, A.G. The Wnt pathway: An important control mechanism in bone’s response to mechanical loading. Bone 2021, 153, 116087. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, S.J.E.; Storoni, S.; Zhytnik, L.; Zhong, W.; Pals, G.; van Royen, B.J.; Elting, M.W.; Maugeri, A.; Eekhoff, E.M.W.; Micha, D. Medical Care Use Among Patients with Monogenic Osteoporosis Due to Rare Variants in LRP5, PLS3, or WNT1. Calcif. Tissue Int. 2023, 113, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 2022, 123, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Papagoras, C.; Chrysanthopoulou, A.; Mitsios, A.; Ntinopoulou, M.; Tsironidou, V.; Batsali, A.K.; Papadaki, H.A.; Skendros, P.; Ritis, K. IL-17A expressed on neutrophil extracellular traps promotes mesenchymal stem cell differentiation toward bone-forming cells in ankylosing spondylitis. Eur. J. Immunol. 2021, 51, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Jia, E.; Li, Z.; Geng, H.; Zhu, H.; Wang, Y.; Lin, F.; Jiang, Y.; Zhang, J. Neutrophil extracellular traps induce the bone erosion of gout. BMC Musculoskelet. Disord. 2022, 23, 1128. [Google Scholar] [CrossRef] [PubMed]
- Mallipattu, S.K.; Liu, R.; Zheng, F.; Narla, G.; Ma’ayan, A.; Dikman, S.; Jain, M.K.; Saleem, M.; D’Agati, V.; Klotman, P.; et al. Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation. J. Biol. Chem. 2012, 287, 19122–19135. [Google Scholar] [CrossRef] [PubMed]
- Fedou, C.; Camus, M.; Lescat, O.; Feuillet, G.; Mueller, I.; Ross, B.; Buleon, M.; Neau, E.; Alves, M.; Goudouneche, D.; et al. Mapping of the amniotic fluid proteome of fetuses with congenital anomalies of the kidney and urinary tract identifies plastin 3 as a protein involved in glomerular integrity. J. Pathol. 2021, 254, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.A.; Lanske, B.; Moe, O.W. Crosstalk between kidney and bone—Bench to bedside. Bone 2017, 100, 1–3. [Google Scholar] [CrossRef]
- Makitie, R.E.; Costantini, A.; Kampe, A.; Alm, J.J.; Makitie, O. New Insights Into Monogenic Causes of Osteoporosis. Front. Endocrinol. 2019, 10, 70. [Google Scholar] [CrossRef]
- Mak, W.; Shao, X.; Dunstan, C.R.; Seibel, M.J.; Zhou, H. Biphasic glucocorticoid-dependent regulation of Wnt expression and its inhibitors in mature osteoblastic cells. Calcif. Tissue Int. 2009, 85, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. Role of Dietary Polyphenols in the Activity and Expression of Nitric Oxide Synthases: A Review. Antioxidants 2023, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- van’t Hof, R.J.; Ralston, S.H. Nitric oxide and bone. Immunology 2001, 103, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Scala, M.; Accogli, A.; De Grandis, E.; Allegri, A.; Bagowski, C.P.; Shoukier, M.; Maghnie, M.; Capra, V. A novel pathogenic MYH3 mutation in a child with Sheldon-Hall syndrome and vertebral fusions. Am. J. Med. Genet. A 2018, 176, 663–667. [Google Scholar] [CrossRef]

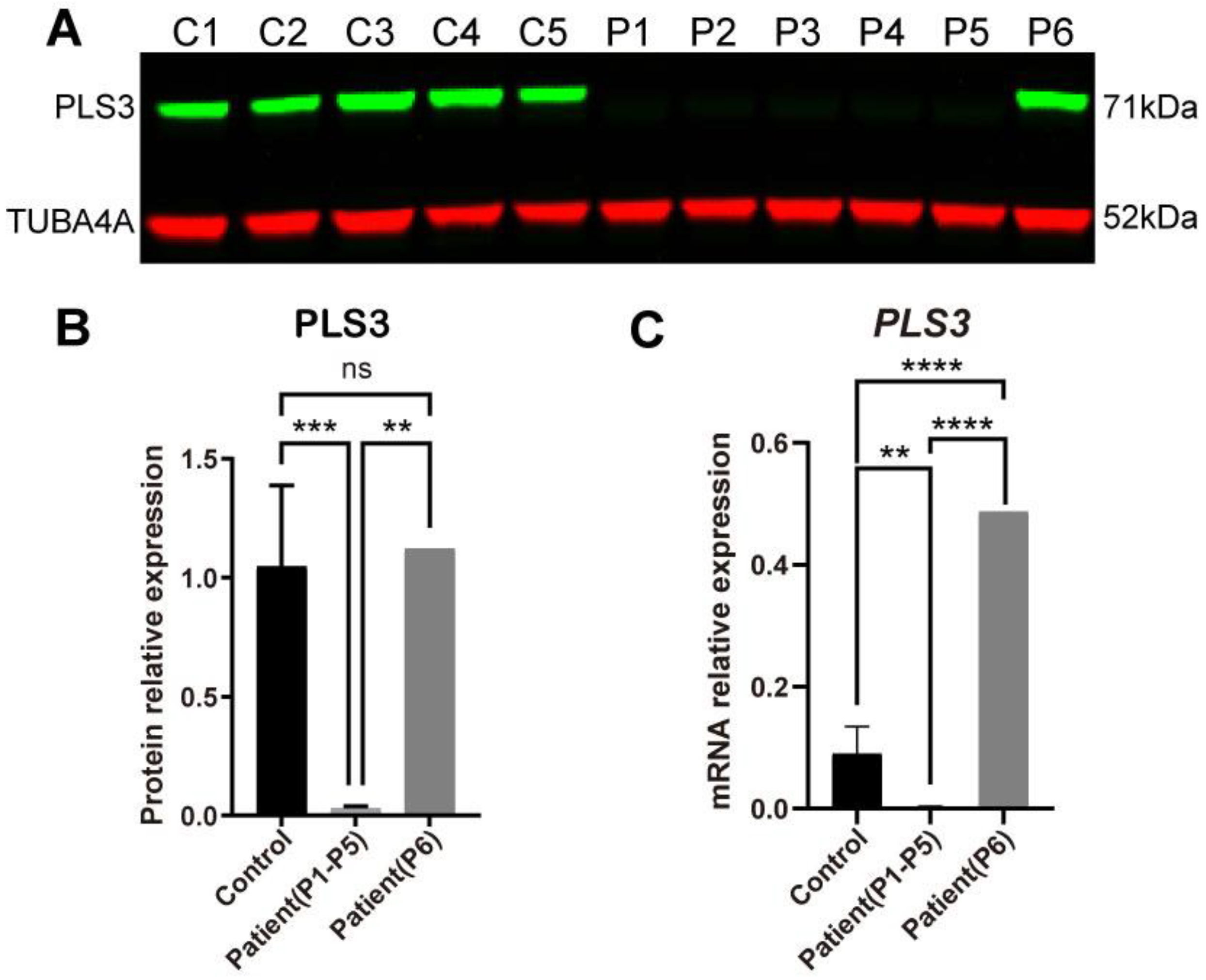
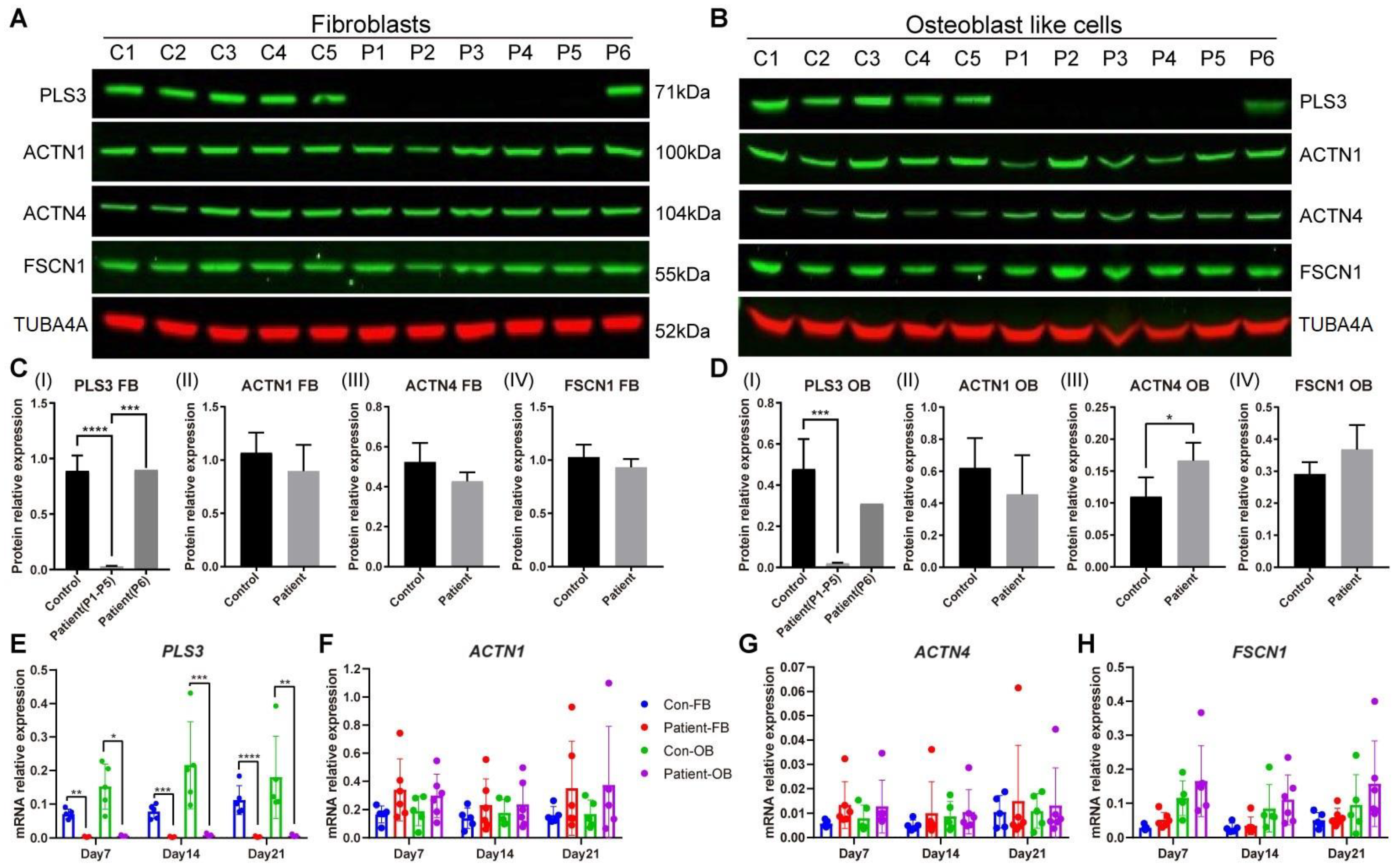
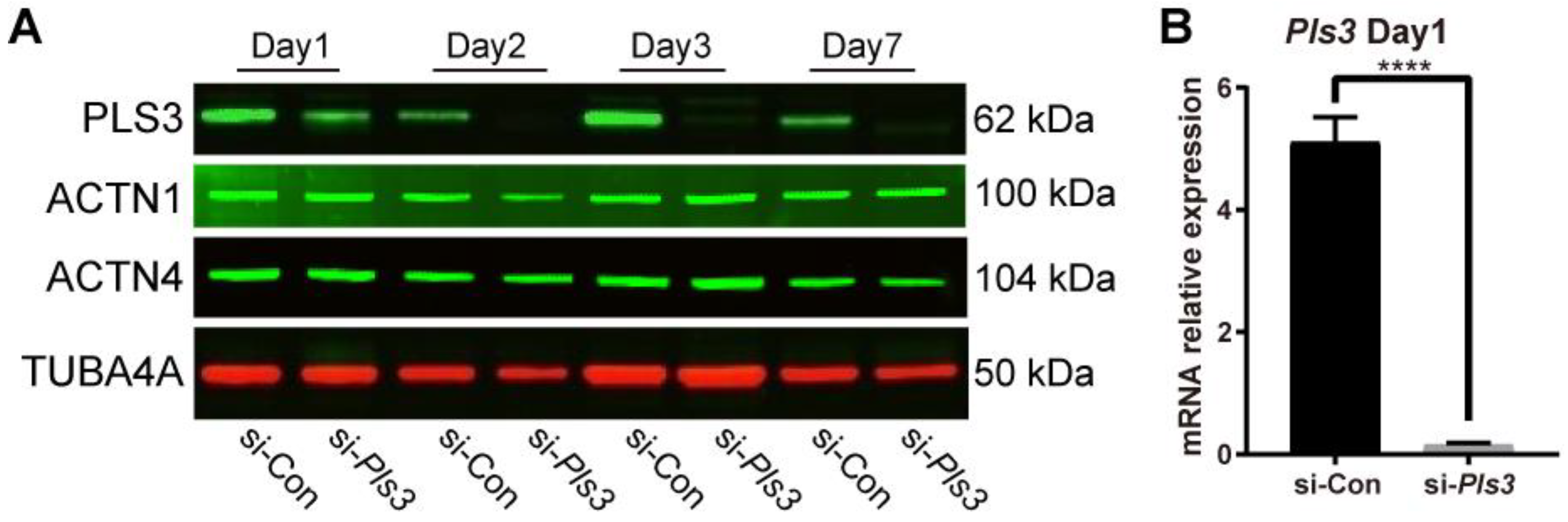
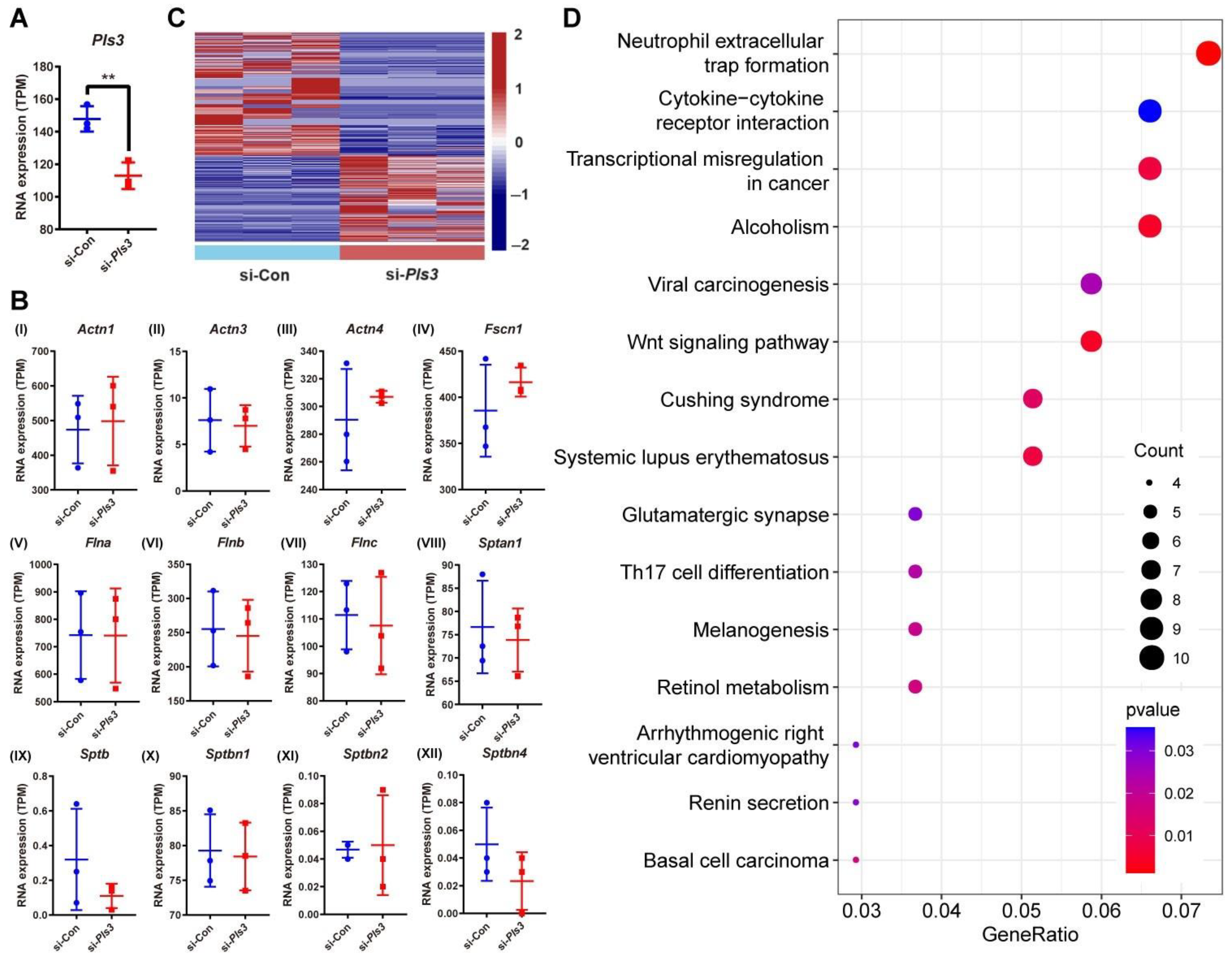

| Codes | Gender | Age at Biopsy | Mutation | Mutation Type | Low-Impact Peripheral Fractures | Multiple Vertebral Fractures | Other Clinical Findings |
|---|---|---|---|---|---|---|---|
| C1 | male | 4 | NA | NA | - | - | - |
| C2 | male | 45 | NA | NA | - | - | - |
| C3 | female | 54 | NA | NA | - | - | - |
| C4 | female | 45 | NA | NA | - | - | - |
| C5 | male | 47 | NA | NA | - | - | - |
| P1 | male | 10 | PLS3: c.235del p.(Tyr79fs) | Frameshift | 6 | No | Acute lymphatic leukemia |
| P2 | male | 32 | PLS3: c.235del p.(Tyr79fs) | Frameshift | 13 | Yes | None |
| P3 | male | 10 | PLS3: c.235del p.(Tyr79fs) | Frameshift | Multiple | No | Epilepsy and, in childhood, waddling gait |
| P4 | male | 6 | PLS3: c.235del p.(Tyr79fs) | Frameshift | 17 | No | Patent ductus arteriosus and, in childhood, waddling gait |
| P5 | male | 47 | PLS3: c.748+1G>A | Splice site variant | Multiple | Yes | Alcohol abuse and esophageal carcinoma |
| P6 | male | 54 | PLS3: c.759_760insAAT | Insertion | 1 | Yes | None |
| Gene | Accession Number | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|
| PLS3 | NM_005032.4 | CTCCCTGGTTGGCATTGGAG | GCCAAACTGGAGCTGATCGT |
| ACTN1 | NM_001130004.1 | CCCGAGCTGATTGACTACGG | ATGGCTTTCTCATCCGGTCG |
| ACTN4 | NM_004924.5 | GGCACAGACCAGAGCTGATT | AAGCGTGGTAGAAGCAGGAC |
| FSCN1 | NM_003088.3 | GTCGACTCGCTCATCACCCTC | GGAAGGTCTCCTGGTCGGTC |
| RUNX2 | NM_001024630.3 | ACTGCTTGCAGCCTTAAAT | ATGCTTCATTCGCCTCAC |
| ALP | NM_000478.5 | AGGGACATTGACGTGATCAT | CCTGGCTCGAAGAGACC |
| OPN | NM_001040058.1 | TTCCAAGTAAGTCCAACGAAAG | GTGACCAGTTCATCAGATTCAT |
| SFRP1 | NM_003013.2 | CGTGGGCTACAAGAAGATGG | AAGCCGAAGAACTGCATGAC |
| WNT1 | NM_005430.3 | GCCGATGGTGGGGTATTGTG | GATCCCCGGATTTTGGCGTA |
| CTNND2 | NM_001332.3 | CCGGGTGCCTAAGGAATGTT | CGCAGACTGGATCACGTACA |
| WNT2 | NM_003391.2 | CATCCAGATGTGATGCGTGC | GCAGATTCCCGACTACTTCG |
| NFATC2 | NM_012340.4 | CATGAGGGCAACCATCGACT | CCCCGTGAGGATCATTTGCT |
| NFATC1 | NM_006162.4 | AACCCCATCGAATGCTCCCAG | AGAAACTGACGTGAACGGGG |
| FZD3 | NM_017412.3 | CGCCGGGGTCTGAGATATT | GCTTGGTCTCACAAAAGGCG |
| GAPDH | NM_002046.6 | GCCCAATACGACCAAATCC | AGCCACATCGCTCAGACAC |
| Pls3 | NM_145629.1 | CAAGCACAATAACGCCAAGTACGC | ACTCTCTTCATCCCTCTGCCCATC |
| Tbp | NM_013684.2 | TGGCGGTTTGGCTAGGTTTC | CACCATGAAATAGTGATGCTGGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, W.; Neugebauer, J.; Pathak, J.L.; Li, X.; Pals, G.; Zillikens, M.C.; Eekhoff, E.M.W.; Bravenboer, N.; Zhang, Q.; Hammerschmidt, M.; et al. Functional Insights in PLS3-Mediated Osteogenic Regulation. Cells 2024, 13, 1507. https://doi.org/10.3390/cells13171507
Zhong W, Neugebauer J, Pathak JL, Li X, Pals G, Zillikens MC, Eekhoff EMW, Bravenboer N, Zhang Q, Hammerschmidt M, et al. Functional Insights in PLS3-Mediated Osteogenic Regulation. Cells. 2024; 13(17):1507. https://doi.org/10.3390/cells13171507
Chicago/Turabian StyleZhong, Wenchao, Janine Neugebauer, Janak L. Pathak, Xingyang Li, Gerard Pals, M. Carola Zillikens, Elisabeth M. W. Eekhoff, Nathalie Bravenboer, Qingbin Zhang, Matthias Hammerschmidt, and et al. 2024. "Functional Insights in PLS3-Mediated Osteogenic Regulation" Cells 13, no. 17: 1507. https://doi.org/10.3390/cells13171507








