Bleomycin-Induced Pulmonary Fibrosis in Transgenic Mice Carrying the Human MUC5B rs35705950 Variant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Generation of Human MUC5B [rs35705950] Transgenic Mice
2.3. Pulmonary Fibrosis Induction
2.4. Collection of BALF and Peripheral Blood
2.5. Lung Tissue Collection
2.6. Biochemical Analysis
2.7. Immunohistochemical Staining
2.8. Gene Expression Analysis
2.9. Statistical Analysis
3. Results
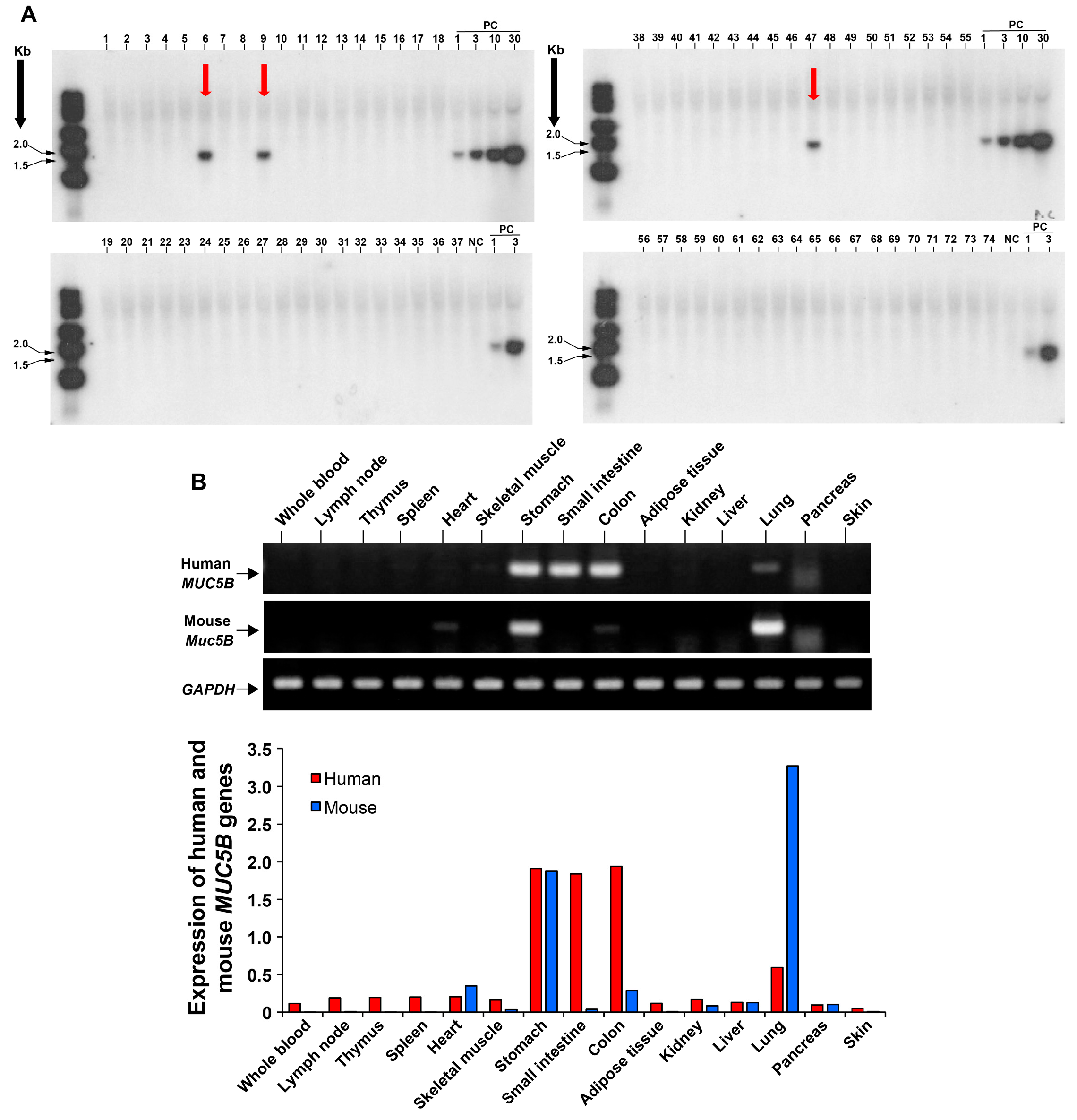
3.1. Development of a Transgenic Mouse Model Expressing the Human MUC5B rs35705950 with the Mutant T Allele
3.2. Significant Expression of MUC5B in the Proximal Bronchial Segments in Human MUC5B rs35705950 Transgenic Mice with Lung Fibrosis
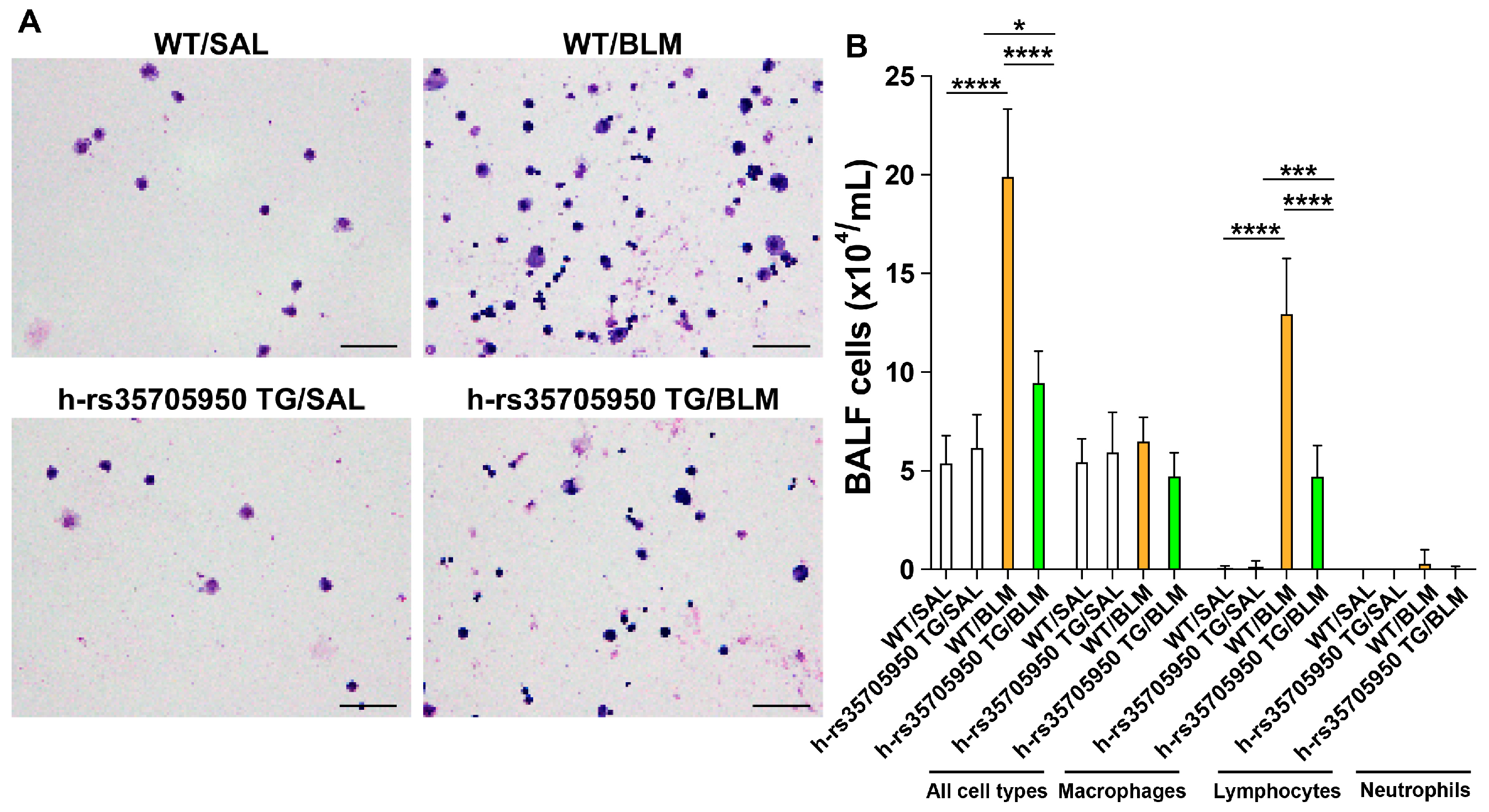
3.3. Reduced Infiltration of Inflammatory Cells in Human MUC5B rs35705950 Transgenic Mice with Lung Fibrosis
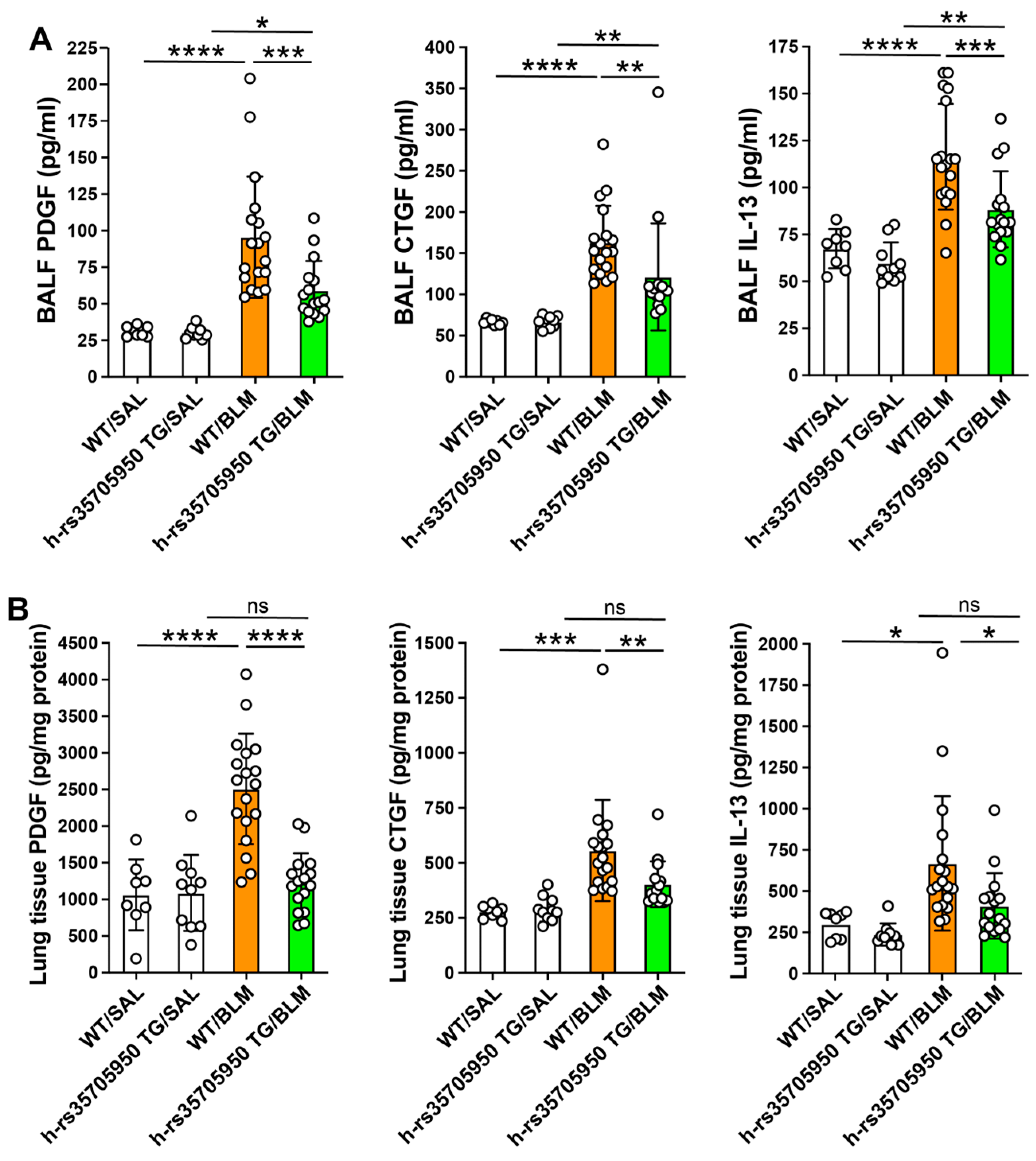
3.4. Reduced Expression of Inflammatory Cytokines in Human MUC5B rs35705950 Transgenic Mice with Lung Fibrosis
3.5. Decreased Expression of Growth Factors in Human MUC5B rs35705950 Transgenic Mice with Lung Fibrosis
3.6. Reduced Expression of Extracellular Matrix Markers in Human MUC5B rs35705950 Transgenic Mice with Lung Fibrosis
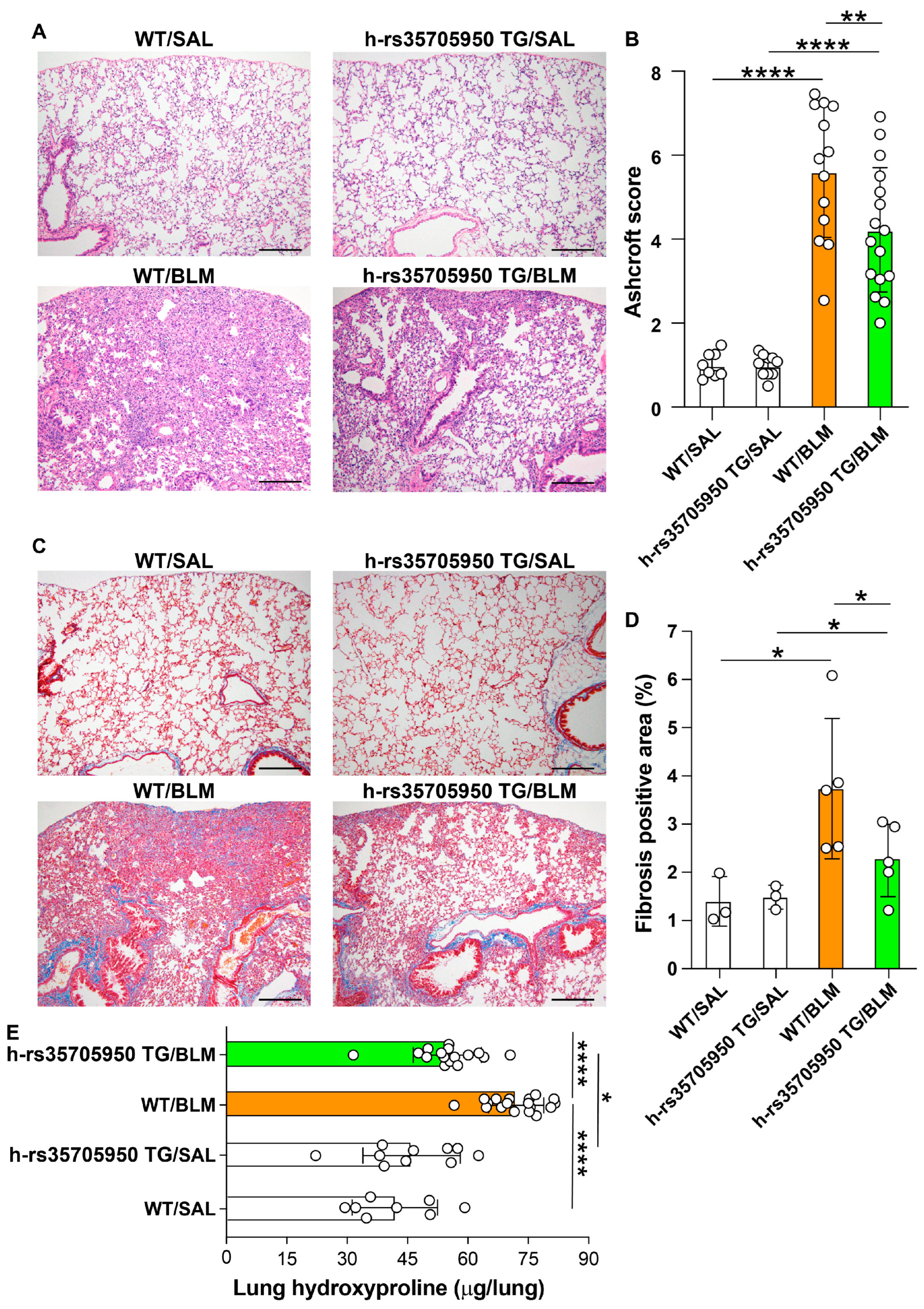
3.7. Decreased Lung Fibrosis in Human MUC5B rs35705950 Transgenic Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lettieri, S.; Bertuccio, F.R.; Del Frate, L.; Perrotta, F.; Corsico, A.G.; Stella, G.M. The Plastic Interplay between Lung Regeneration Phenomena and Fibrotic Evolution: Current Challenges and Novel Therapeutic Perspectives. Int. J. Mol. Sci 2023, 25, 547. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M. Interstitial Lung Disease: A Review. JAMA 2024, 331, 1655. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.J.; Ha, M.; Shin, D.H.; Lee, H.R.; Kim, Y.H.; Cho, W.H. Development of a Novel Biomarker for the Progression of Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2024, 25, 599. [Google Scholar] [CrossRef] [PubMed]
- Baratella, E.; Ruaro, B.; Giudici, F.; Wade, B.; Santagiuliana, M.; Salton, F.; Confalonieri, P.; Simbolo, M.; Scarpa, A.; Tollot, S.; et al. Evaluation of Correlations between Genetic Variants and High-Resolution Computed Tomography Patterns in Idiopathic Pulmonary Fibrosis. Diagnostics 2021, 11, 762. [Google Scholar] [CrossRef]
- Zaman, T.; Lee, J.S. Risk Factors for the Development of Idiopathic Pulmonary Fibrosis: A Review. Curr. Pulmonol. Rep. 2018, 7, 118–125. [Google Scholar] [CrossRef]
- Fingerlin, T.E.; Murphy, E.; Zhang, W.; Peljto, A.L.; Brown, K.K.; Steele, M.P.; Loyd, J.E.; Cosgrove, G.P.; Lynch, D.; Groshong, S.; et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat. Genet. 2013, 45, 613–620. [Google Scholar] [CrossRef]
- Newton, C.A.; Oldham, J.M.; Ley, B.; Anand, V.; Adegunsoye, A.; Liu, G.; Batra, K.; Torrealba, J.; Kozlitina, J.; Glazer, C.; et al. Telomere length and genetic variant associations with interstitial lung disease progression and survival. Eur. Respir. J. 2019, 53, 1801641. [Google Scholar] [CrossRef]
- Seibold, M.A.; Wise, A.L.; Speer, M.C.; Steele, M.P.; Brown, K.K.; Loyd, J.E.; Fingerlin, T.E.; Zhang, W.; Gudmundsson, G.; Groshong, S.D.; et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N. Engl. J. Med. 2011, 364, 1503–1512. [Google Scholar] [CrossRef]
- Wu, X.; Li, W.; Luo, Z.; Chen, Y. The minor T allele of the MUC5B promoter rs35705950 associated with susceptibility to idiopathic pulmonary fibrosis: A meta-analysis. Sci. Rep. 2021, 11, 24007. [Google Scholar] [CrossRef]
- Zhang, D.; Newton, C.A.; Wang, B.; Povysil, G.; Noth, I.; Martinez, F.J.; Raghu, G.; Goldstein, D.; Garcia, C.K. Utility of whole genome sequencing in assessing risk and clinically relevant outcomes for pulmonary fibrosis. Eur. Respir. J. 2022, 60, 2200577. [Google Scholar] [CrossRef]
- Hancock, L.A.; Hennessy, C.E.; Solomon, G.M.; Dobrinskikh, E.; Estrella, A.; Hara, N.; Hill, D.B.; Kissner, W.J.; Markovetz, M.R.; Grove Villalon, D.E.; et al. Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat. Commun. 2018, 9, 5363. [Google Scholar] [CrossRef] [PubMed]
- Kurche, J.S.; Dobrinskikh, E.; Hennessy, C.E.; Huber, J.; Estrella, A.; Hancock, L.A.; Schwarz, M.I.; Okamoto, T.; Cool, C.D.; Yang, I.V.; et al. Muc5b Enhances Murine Honeycomb-like Cyst Formation. Am. J. Respir. Cell Mol. Biol. 2019, 61, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Stancil, I.T.; Michalski, J.E.; Davis-Hall, D.; Chu, H.W.; Park, J.A.; Magin, C.M.; Yang, I.V.; Smith, B.J.; Dobrinskikh, E.; Schwartz, D.A. Pulmonary fibrosis distal airway epithelia are dynamically and structurally dysfunctional. Nat. Commun. 2021, 12, 4566. [Google Scholar] [CrossRef] [PubMed]
- Biondini, D.; Cocconcelli, E.; Bernardinello, N.; Lorenzoni, G.; Rigobello, C.; Lococo, S.; Castelli, G.; Baraldo, S.; Cosio, M.G.; Gregori, D.; et al. Prognostic role of MUC5B rs35705950 genotype in patients with idiopathic pulmonary fibrosis (IPF) on antifibrotic treatment. Respir. Res. 2021, 22, 98. [Google Scholar] [CrossRef]
- Molyneaux, P.L.; Cox, M.J.; Willis-Owen, S.A.; Mallia, P.; Russell, K.E.; Russell, A.M.; Murphy, E.; Johnston, S.L.; Schwartz, D.A.; Wells, A.U.; et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2014, 190, 906–913. [Google Scholar] [CrossRef]
- Peljto, A.L.; Zhang, Y.; Fingerlin, T.E.; Ma, S.F.; Garcia, J.G.; Richards, T.J.; Silveira, L.J.; Lindell, K.O.; Steele, M.P.; Loyd, J.E.; et al. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA 2013, 309, 2232–2239. [Google Scholar] [CrossRef]
- van der Vis, J.J.; Prasse, A.; Renzoni, E.A.; Stock, C.J.W.; Caliskan, C.; Maher, T.M.; Bonella, F.; Borie, R.; Crestani, B.; Petrek, M.; et al. MUC5B rs35705950 minor allele associates with older age and better survival in idiopathic pulmonary fibrosis. Respirology 2023, 28, 455–464. [Google Scholar] [CrossRef]
- Cosgrove, G.P.; Groshong, S.D.; Peljto, A.L.; Talbert, J.; McKean, D.; Markin, C.; Kidd, R.; Cool, C.D.; Seibold, M.; Murphy, E.; et al. The MUC5B promoter polymorphism is associated with a less severe pathological form of familial interstitial pneumonia. Am. J. Respir. Crit. Care Med. 2012, 185, A6865. [Google Scholar]
- Stock, C.J.; Sato, H.; Fonseca, C.; Banya, W.A.; Molyneaux, P.L.; Adamali, H.; Russell, A.M.; Denton, C.P.; Abraham, D.J.; Hansell, D.M.; et al. Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax 2013, 68, 436–441. [Google Scholar] [CrossRef]
- Schwartz, D.A. Idiopathic Pulmonary Fibrosis Is a Genetic Disease Involving Mucus and the Peripheral Airways. Ann. Am. Thorac. Soc. 2018, 15 (Suppl. S3), 192–197. [Google Scholar] [CrossRef]
- Thornton, D.J.; Rousseau, K.; McGuckin, M.A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2008, 70, 459–486. [Google Scholar] [CrossRef] [PubMed]
- Fadista, J.; Kraven, L.M.; Karjalainen, J.; Andrews, S.J.; Geller, F.; Initiative, C.-H.G.; Baillie, J.K.; Wain, L.V.; Jenkins, R.G.; Feenstra, B. Shared genetic etiology between idiopathic pulmonary fibrosis and COVID-19 severity. EBioMedicine 2021, 65, 103277. [Google Scholar] [CrossRef] [PubMed]
- van Moorsel, C.H.M.; van der Vis, J.J.; Duckworth, A.; Scotton, C.J.; Benschop, C.; Ellinghaus, D.; Ruven, H.J.T.; Quanjel, M.J.R.; Grutters, J.C. The MUC5B Promoter Polymorphism Associates With Severe COVID-19 in the European Population. Front. Med. 2021, 8, 668024. [Google Scholar] [CrossRef] [PubMed]
- Muyrers, J.P.; Zhang, Y.; Benes, V.; Testa, G.; Ansorge, W.; Stewart, A.F. Point mutation of bacterial artificial chromosomes by ET recombination. EMBO Rep. 2000, 1, 239–243. [Google Scholar] [CrossRef]
- Abe, K.; Hazama, M.; Katoh, H.; Yamamura, K.; Suzuki, M. Establishment of an efficient BAC transgenesis protocol and its application to functional characterization of the mouse Brachyury locus. Exp. Anim. 2004, 53, 311–320. [Google Scholar] [CrossRef]
- D’Alessandro-Gabazza, C.N.; Kobayashi, T.; Yasuma, T.; Toda, M.; Kim, H.; Fujimoto, H.; Hataji, O.; Takeshita, A.; Nishihama, K.; Okano, T.; et al. A Staphylococcus pro-apoptotic peptide induces acute exacerbation of pulmonary fibrosis. Nat. Commun. 2020, 11, 1539. [Google Scholar] [CrossRef]
- D’Alessandro-Gabazza, C.N.; Yasuma, T.; Kobayashi, T.; Toda, M.; Abdel-Hamid, A.M.; Fujimoto, H.; Hataji, O.; Nakahara, H.; Takeshita, A.; Nishihama, K.; et al. Inhibition of lung microbiota-derived proapoptotic peptides ameliorates acute exacerbation of pulmonary fibrosis. Nat. Commun. 2022, 13, 1558. [Google Scholar] [CrossRef]
- Inoue, R.; Yasuma, T.; Fridman D’Alessandro, V.; Toda, M.; Ito, T.; Tomaru, A.; D’Alessandro-Gabazza, C.N.; Tsuruga, T.; Okano, T.; Takeshita, A.; et al. Amelioration of Pulmonary Fibrosis by Matrix Metalloproteinase-2 Overexpression. Int. J. Mol. Sci. 2023, 24, 6695. [Google Scholar] [CrossRef]
- Ma, J.; Rubin, B.K.; Voynow, J.A. Mucins, Mucus, and Goblet Cells. Chest 2018, 154, 169–176. [Google Scholar] [CrossRef]
- Williams, O.W.; Sharafkhaneh, A.; Kim, V.; Dickey, B.F.; Evans, C.M. Airway mucus: From production to secretion. Am. J. Respir. Cell Mol. Biol. 2006, 34, 527–536. [Google Scholar] [CrossRef]
- Abrami, M.; Biasin, A.; Tescione, F.; Tierno, D.; Dapas, B.; Carbone, A.; Grassi, G.; Conese, M.; Di Gioia, S.; Larobina, D.; et al. Mucus Structure, Viscoelastic Properties, and Composition in Chronic Respiratory Diseases. Int. J. Mol. Sci. 2024, 25, 1933. [Google Scholar] [CrossRef] [PubMed]
- Button, B.M.; Button, B. Structure and function of the mucus clearance system of the lung. Cold Spring Harb. Perspect. Med. 2013, 3, a009720. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, M.C.; Brown, R.; Ryan, S.; Mall, M.A.; Weldon, S.; Taggart, C.C. Proteases, Mucus, and Mucosal Immunity in Chronic Lung Disease. Int. J. Mol. Sci. 2021, 22, 5018. [Google Scholar] [CrossRef] [PubMed]
- Hauber, H.P.; Foley, S.C.; Hamid, Q. Mucin overproduction in chronic inflammatory lung disease. Can. Respir. J. 2006, 13, 327–335. [Google Scholar] [CrossRef]
- Meldrum, O.W.; Chotirmall, S.H. Mucus, Microbiomes and Pulmonary Disease. Biomedicines 2021, 9, 675. [Google Scholar] [CrossRef]
- Shah, B.K.; Singh, B.; Wang, Y.; Xie, S.; Wang, C. Mucus Hypersecretion in Chronic Obstructive Pulmonary Disease and Its Treatment. Mediat. Inflamm. 2023, 2023, 8840594. [Google Scholar] [CrossRef]
- Tian, P.W.; Wen, F.Q. Clinical significance of airway mucus hypersecretion in chronic obstructive pulmonary disease. J. Transl. Int. Med. 2015, 3, 89–92. [Google Scholar] [CrossRef]
- Bonser, L.R.; Erle, D.J. Airway Mucus and Asthma: The Role of MUC5AC and MUC5B. J. Clin. Med. 2017, 6, 112. [Google Scholar] [CrossRef]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef]
- Jaramillo, A.M.; Vladar, E.K.; Holguin, F.; Dickey, B.F.; Evans, C.M. Emerging cell and molecular targets for treating mucus hypersecretion in asthma. Allergol. Int. 2024, 73, 375–381. [Google Scholar] [CrossRef]
- Valque, H.; Gouyer, V.; Duez, C.; Leboeuf, C.; Marquillies, P.; Le Bert, M.; Plet, S.; Ryffel, B.; Janin, A.; Gottrand, F.; et al. Muc5b-deficient mice develop early histological lung abnormalities. Biol. Open 2019, 8, bio046359. [Google Scholar] [CrossRef] [PubMed]
- Ballester, B.; Milara, J.; Cortijo, J. The role of mucin 1 in respiratory diseases. Eur. Respir. Rev. 2021, 30, 200149. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Boucher, R.C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 2002, 109, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.G.; Livraghi-Butrico, A.; Fletcher, A.A.; McElwee, M.M.; Evans, S.E.; Boerner, R.M.; Alexander, S.N.; Bellinghausen, L.K.; Song, A.S.; Petrova, Y.M.; et al. Muc5b is required for airway defence. Nature 2014, 505, 412–416. [Google Scholar] [CrossRef]




| Gene & Direction | Sequence (5′ to 3′) | Length (nt) | Tm (°C) | Accession Number | Position | Product |
|---|---|---|---|---|---|---|
| hMUC5B | ||||||
| Forward | GCCCACATCTCCACCTATGAT | 21 | 61.1 | NM_002458.3 | 1349-1369 | 141 bp |
| Reverse | GCAGTTCTCGTTGTCCGTCA | 20 | 62.4 | 1489-1470 | ||
| mMuc5B | ||||||
| Forward | TCCCTAGCATGAGCGCCTTA | 20 | 62.6 | NM_028801.2 | 197-216 | 178 bp |
| Reverse | CCACGACGCAGTTGGATGTT | 20 | 63 | 374-355 | ||
| mPeriostin | ||||||
| Forward | CACGGCATGGTTATTCCTTCA | 21 | 60.4 | NM_001198766.1 | 547-567 | 151 bp |
| Reverse | TCAGGACACGGTCAATGACAT | 21 | 61.1 | 697-677 | ||
| mFibronectin | ||||||
| Forward | TTCAAGTGTGATCCCCATGAAG | 22 | 60 | NM_010233.2 | 7126-7147 | 154 bp |
| Reverse | CAGGTCTACGGCAGTTGTCA | 20 | 61.5 | 7279-7260 | ||
| mCollagen I | ||||||
| Forward | TAAGGGTCCCCAATGGTGAGA | 21 | 63.8 | NM007742.4 | 107-127 | 203 bp |
| Reverse | GGGTCCCTCGACTCCTACAT | 20 | 60.3 | 309-290 | ||
| mα-SMA | ||||||
| Forward | CAGGATGCAGAAGGAGATCAC | 21 | 60.7 | NM007392.2 | 1009-1029 | 364 bp |
| Reverse | TGTTGCTAGGCCAGGGCTAC | 20 | 62.6 | 1372-1353 | ||
| mGapdh | ||||||
| Forward | TGGCCTTCCGTGTTCCTAC | 19 | 61.3 | NM_008084.4 | 686-704 | 178 bp |
| Reverse | GAGTTGCTGTTGAAGTCGCA | 20 | 60.9 | 863-844 | ||
| Gapdh: glyceraldehyde 3-phosphate dehydrogenase; αSMA, αsmooth muscle actin; h, human; m, mouse. | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tharavecharak, S.; Fujimoto, H.; Yasuma, T.; D’Alessandro-Gabazza, C.N.; Toda, M.; Tomaru, A.; Saiki, H.; Uemura, M.; Kogue, Y.; Ito, T.; et al. Bleomycin-Induced Pulmonary Fibrosis in Transgenic Mice Carrying the Human MUC5B rs35705950 Variant. Cells 2024, 13, 1523. https://doi.org/10.3390/cells13181523
Tharavecharak S, Fujimoto H, Yasuma T, D’Alessandro-Gabazza CN, Toda M, Tomaru A, Saiki H, Uemura M, Kogue Y, Ito T, et al. Bleomycin-Induced Pulmonary Fibrosis in Transgenic Mice Carrying the Human MUC5B rs35705950 Variant. Cells. 2024; 13(18):1523. https://doi.org/10.3390/cells13181523
Chicago/Turabian StyleTharavecharak, Suphachai, Hajime Fujimoto, Taro Yasuma, Corina N. D’Alessandro-Gabazza, Masaaki Toda, Atsushi Tomaru, Haruko Saiki, Mei Uemura, Yurie Kogue, Toshiyuki Ito, and et al. 2024. "Bleomycin-Induced Pulmonary Fibrosis in Transgenic Mice Carrying the Human MUC5B rs35705950 Variant" Cells 13, no. 18: 1523. https://doi.org/10.3390/cells13181523







