Immunopathology of Corneal Allograft Rejection and Donor-Specific Antibodies (DSAs) as Immunological Predictors of Corneal Transplant Failure
Abstract
1. Introduction
2. MHC-HLA Antigens in Transplantation
3. Immune Privilege of the Cornea
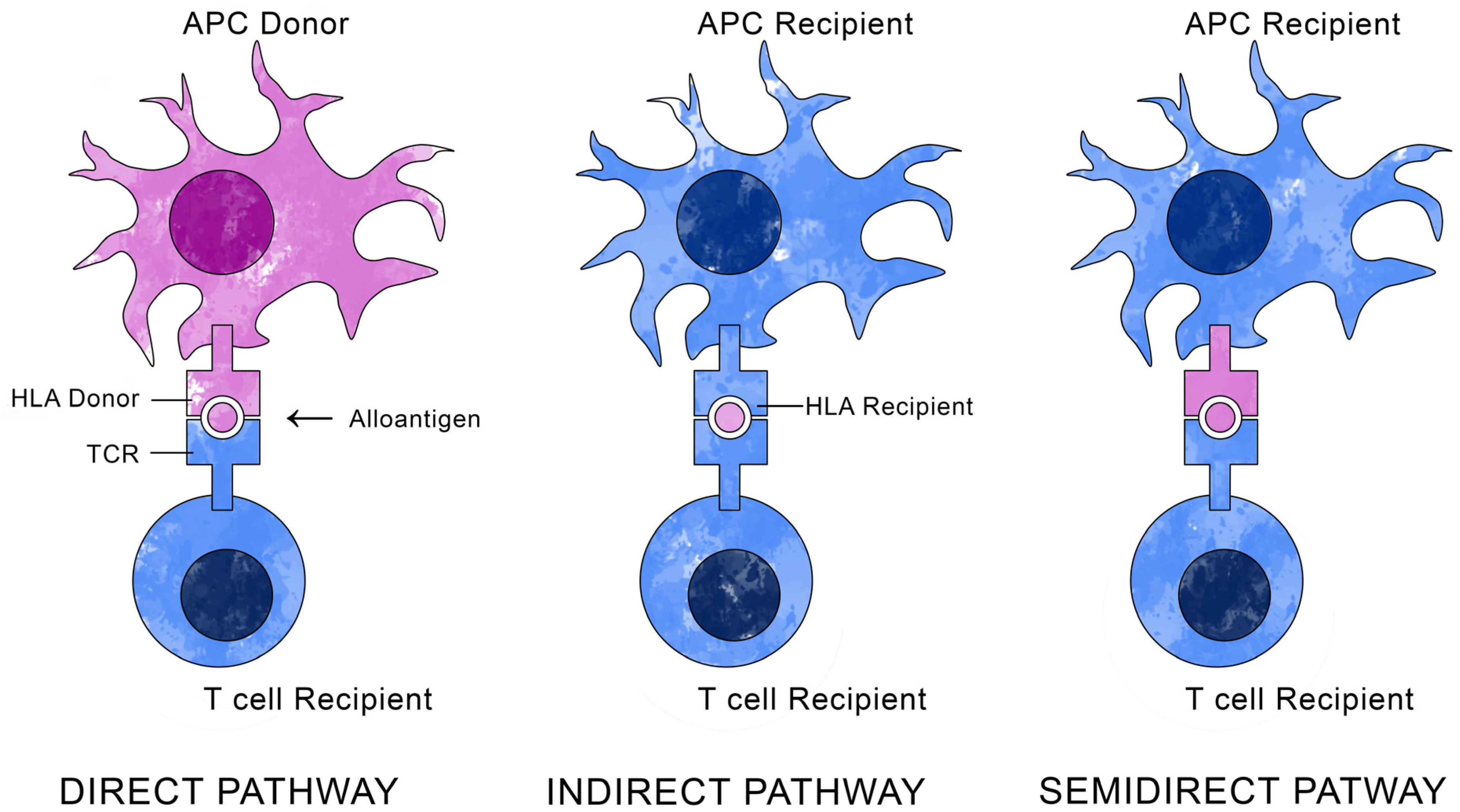
4. Immunopathology of Allogeneic Corneal Transplant Rejection
5. Role of Antibodies in Corneal Transplant Rejection
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mishra, S.K.; Joshi, A.; Ginu, P.M.; Sati, A.; Kumar, S.V. Corneal transplantation: A walk to vision. Med. J. Armed Forces India 2023, 79, 645–650. [Google Scholar] [CrossRef]
- Crawford, A.Z.; Patel, D.V.; McGhee, C.N. A brief history of corneal transplantation: From ancient to modern. Oman J. Ophthalmol. 2013, 6 (Suppl. S1), S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudlou, P.; Sood, G.; Gurnani, B.; Akhondi, H. Cornea Transplantation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK539690/ (accessed on 14 August 2024).
- Rao, S.K.; Fogla, R.; Sitalakshmi, G.; Padmanabhan, P. Corneal autografting: A systematic approach. Ophthalmic Surg. Lasers 2000, 31, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Major, J.; Foroncewicz, B.; Szaflik, J.P.; Mucha, K. Immunology and Donor-Specific Antibodies in Corneal Transplantation. Arch. Immunol. Ther. Exp. 2021, 69, 32. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gupta, N.; Vanathi, M.; Tandon, R. Corneal transplantation in the modern era. Indian J. Med. Res. 2019, 150, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Flockerzi, E.; Maier, P.; Böhringer, D.; Reinshagen, H.; Kruse, F.; Cursiefen, C.; Reinhard, T.; Geerling, G.; Torun, N.; Seitz, B. Trends in Corneal Transplantation from 2001 to 2016 in Germany: A Report of the DOG-Section Cornea and its Keratoplasty Registry. Am. J. Ophthalmol. 2018, 188, 91–98. [Google Scholar] [CrossRef]
- Le, R.; Yucel, N.; Khattak, S.; Yucel, Y.H.; Prud’homme, G.J.; Gupta, N. Current indications and surgical approaches to corneal transplants at the University of Toronto: A clinical-pathological study. Can. J. Ophthalmol. 2017, 52, 74–79. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Rubenstein, D.; Price, A.J.; Côté, E.; Levitt, M.; Sharpen, L.; Slomovic, A. Evolving surgical techniques of and indications for corneal transplantation in Ontario: 2000–2012. Can. J. Ophthalmol. 2013, 48, 153–159. [Google Scholar] [CrossRef]
- Liu, S.; Wong, Y.L.; Walkden, A. Current Perspectives on Corneal Transplantation. Clin. Ophthalmol. 2022, 16, 631–646. [Google Scholar] [CrossRef]
- Sakowska, J.; Glasner, P.; Zieliński, M.; Trzonkowski, P.; Glasner, L. Corneal Allografts: Factors for and against Acceptance. J. Immunol. Res. 2021, 2021, 5372090. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Armitage, W.J.; Winton, H.L.; Jones, M.N.A.; Crewe, J.M.; Rogers, C.A.; Tole, D.M.; Dick, A.D. Corneal transplant follow-up study II (CTFS II): A prospective clinical trial to determine the influence of HLA class II matching on corneal transplant rejection: Baseline donor and recipient characteristics. Br. J. Ophthalmol. 2019, 103, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Musch, D.C.; Sutphin, J.E.; Farjo, A.A. Extended long-term outcomes of penetrating keratoplasty for keratoconus. Ophthalmology 2006, 113, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.W.; Price, M.O.; Bowers, P.J.; Price, F.W. Long-term graft survival after penetrating keratoplasty. Ophthalmology 2003, 110, 1396–1402. [Google Scholar] [CrossRef]
- Amouzegar, A.; Chauhan, S.K. Effector and Regulatory T Cell Trafficking in Corneal Allograft Rejection. Mediat. Inflamm. 2017, 2017, 8670280. [Google Scholar] [CrossRef]
- Hori, J.; Yamaguchi, T.; Keino, H.; Hamrah, P.; Maruyama, K. Immune privilege in corneal transplantation. Prog. Retin. Eye Res. 2019, 72, 100758. [Google Scholar] [CrossRef]
- Alio, J.L. Corneal transplantation surgery: Where we are and where are we going? Taiwan J. Ophthalmol. 2024, 14, 1–2. [Google Scholar] [CrossRef]
- McCaughan, J.; Xu, Q.; Tinckam, K. Detecting donor-specific antibodies: The importance of sorting the wheat from the chaff. Hepatobiliary Surg. Nutr. 2019, 8, 37–52. [Google Scholar] [CrossRef]
- Wood-Trageser, M.A.; Xu, Q.; Zeevi, A.; Randhawa, P.; Lesniak, D.; Demetris, A.J. Precision transplant pathology. Curr. Opin. Organ Transplant. 2020, 25, 412–419. [Google Scholar] [CrossRef]
- Rock, K.L.; Reits, E.; Neefjes, J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016, 37, 724–737. [Google Scholar] [CrossRef]
- Shiina, T.; Hosomichi, K.; Inoko, H.; Kulski, J.K. The HLA genomic loci map: Expression, interaction, diversity and disease. J. Hum. Genet. 2009, 54, 15–39. [Google Scholar] [CrossRef]
- Trivedi, V.B.; Dave, A.P.; Dave, J.M.; Patel, B.C. Human leukocyte antigen and its role in transplantation biology. Transplant. Proc. 2007, 39, 688–693. [Google Scholar] [CrossRef] [PubMed]
- van Essen, T.H.; Roelen, D.L.; Williams, K.A.; Jager, M.J. Matching for Human Leukocyte Antigens (HLA) in corneal transplantation—To do or not to do. Prog. Retin. Eye Res. 2015, 46, 84–110. [Google Scholar] [CrossRef] [PubMed]
- DeWolf, S.; Sykes, M. Alloimmune T cells in transplantation. J. Clin. Investig. 2017, 127, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Carnel, N.; Lancia, H.H.; Guinier, C.; Benichou, G. Pathways of Antigen Recognition by T Cells in Allograft Rejection. Transplantation 2023, 107, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Sato, A. The HLA System. N. Engl. J. Med. 2000, 343, 702–709. [Google Scholar] [CrossRef]
- Li, X.C.; Raghavan, M. Structure and function of major histocompatibility complex class I antigens. Curr. Opin. Organ Transplant. 2010, 15, 499–504. [Google Scholar] [CrossRef]
- Roche, P.A.; Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef]
- Böhringer, D.; Grotejohann, B.; Ihorst, G.; Reinshagen, H.; Spierings, E.; Reinhard, T.; FANCY Study Group. Rejection Prophylaxis in Corneal Transplant. Dtsch. Arztebl. Int. 2018, 115, 259–265. [Google Scholar] [CrossRef]
- Klein, J.; Sato, A. The HLA system. Second of two parts. N. Engl. J. Med. 2000, 343, 782–786. [Google Scholar] [CrossRef]
- van den Broek, D.A.J.; Meziyerh, S.; Budde, K.; Lefaucheur, C.; Cozzi, E.; Bertrand, D.; López del Moral, C.; Dorling, A.; Emonds, M.P.; Naesens, M.; et al. The Clinical Utility of Post-Transplant Monitoring of Donor-Specific Antibodies in Stable Renal Transplant Recipients: A Consensus Report With Guideline Statements for Clinical Practice. Transpl. Int. 2023, 36, 11321. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Terasaki, P.I. Significance of the positive crossmatch test in kidney transplantation. N. Engl. J. Med. 1969, 280, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, W. A historical perspective on HLA. Immunother. Adv. 2023, 3, ltad014. [Google Scholar] [CrossRef]
- Hori, J.; Kunishige, T.; Nakano, Y. Immune Checkpoints Contribute Corneal Immune Privilege: Implications for Dry Eye Associated with Checkpoint Inhibitors. Int. J. Mol. Sci. 2020, 21, 3962. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Rummelt, C.; Jünemann, A.; Vorwerk, C.; Neuhuber, W.; Kruse, F.E.; Schroedl, F. Absence of blood and lymphatic vessels in the developing human cornea. Cornea 2006, 25, 722–726. [Google Scholar] [CrossRef]
- Leong, Y.Y.; Tong, L. Barrier Function in the Ocular Surface: From Conventional Paradigms to New Opportunities. Ocul. Surf. 2015, 13, 103–109. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011, 21 (Suppl. 6), S3–S9. [Google Scholar] [CrossRef]
- Knickelbein, J.E.; Watkins, S.C.; McMenamin, P.G.; Hendricks, R.L. Stratification of Antigen-presenting Cells within the Normal Cornea. Ophthalmol. Eye Dis. 2009, 1, 45–54. [Google Scholar] [CrossRef]
- Ferguson, T.A.; Griffith, T.S. A vision of cell death: Fas ligand and immune privilege 10 years later. Immunol. Rev. 2006, 213, 228–238. [Google Scholar] [CrossRef]
- Taylor, A.W. Ocular immune privilege. Eye 2009, 23, 1885–1889. [Google Scholar] [CrossRef]
- Ratajczak, W.; Tokarz-Deptuła, B.; Deptuła, W. Immunology of the eye. Postepy Hig. Med. Dosw 2018, 72, 318–326. [Google Scholar] [CrossRef]
- Bauer, J.; Bahmer, F.A.; Wörl, J.; Neuhuber, W.; Schuler, G.; Fartasch, M. A strikingly constant ratio exists between Langerhans cells and other epidermal cells in human skin. A stereologic study using the optical disector method and the confocal laser scanning microscope. J. Investig. Dermatol. 2001, 116, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Grzywnowicz, M.; Giannopoulos, K. The role of receptor programmed death-1 and its ligands in immune system and tumors. Acta Haematol. Pol. 2012, 43, 132–145. [Google Scholar] [CrossRef]
- Wang, S.; Gottlieb, J.L.; Sorenson, C.M.; Sheibani, N. Modulation of Thrombospondin-1 and Pigmented Epithelium Derived Factor Levels in Vitreous Fluid of Patients with Diabetes. Arch. Ophthalmol. 2009, 127, 507. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.; Onderka, J.; Braun, G.; Schneider, A.C.; Hos, D.; Bi, Y.; Bachmann, B.O.; Cursiefen, C. Identification of Novel Endogenous Anti(lymph)angiogenic Factors in the Aqueous Humor. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6554–6560. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Iwata, C.; Suzuki, H.I.; Kiyono, K.; Morishita, Y.; Watabe, T.; Komuro, A.; Kano, M.R.; Miyazono, K. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood 2008, 111, 4571–4579. [Google Scholar] [CrossRef] [PubMed]
- Ambati, B.K.; Nozaki, M.; Singh, N.; Takeda, A.; Jani, P.D.; Suthar, T.; Albuquerque, R.J.; Richter, E.; Sakurai, E.; Newcomb, M.T.; et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature 2006, 443, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.; Maruyama, K.; Regenfuss, B.; Hos, D.; Steven, P.; Heindl, L.M.; Cursiefen, C. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog. Retin. Eye Res. 2013, 34, 89–124. [Google Scholar] [CrossRef] [PubMed]
- Sekiyama, E.; Nakamura, T.; Kawasaki, S.; Sogabe, H.; Kinoshita, S. Different expression of angiogenesis-related factors between human cultivated corneal and oral epithelial sheets. Exp. Eye Res. 2006, 83, 741–746. [Google Scholar] [CrossRef]
- Tan, Y.; Cruz-Guilloty, F.; Medina-Mendez, C.A.; Cutrufello, N.J.; Martinez, R.E.; Urbieta, M.; Wilson, D.; Li, Y.; Perez, V.L. Immunological disruption of antiangiogenic signals by recruited allospecific T cells leads to corneal allograft rejection. J. Immunol. 2012, 188, 5962–5969. [Google Scholar] [CrossRef]
- Barabino, S.; Chen, Y.; Chauhan, S.; Dana, R. Ocular Surface Immunity: Homeostatic Mechanisms and Their Disruption in Dry Eye Disease. Prog. Retin. Eye Res. 2012, 31, 271–285. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, F.A.; Fiorot, S.H.S.; Benchimol, E.I.; Provenzano, J.; Martins, V.J.; Levy, R.A. The autoimmune diseases of the eyes. Autoimmun. Rev. 2016, 15, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.W. Ocular immunosuppressive microenvironment. Chem. Immunol. Allergy 2007, 92, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.B.; Kaiser-Marko, C.; Spurr-Michaud, S.; Tisdale, A.S.; Gipson, I.K. Suppression of Toll-like Receptor-Mediated Innate Immune Responses at the Ocular Surface by the Membrane-associated Mucins MUC1 and MUC16. Mucosal Immunol. 2015, 8, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Mantelli, F.; Mauris, J.; Argüeso, P. The ocular surface epithelial barrier and other mechanisms of mucosal protection: From allergy to infectious diseases. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Le Discorde, M.; Moreau, P.; Sabatier, P.; Legeais, J.M.; Carosella, E.D. Expression of HLA-G in human cornea, an immune-privileged tissue. Hum. Immunol. 2003, 64, 1039–1044. [Google Scholar] [CrossRef]
- Riteau, B.; Rouas-Freiss, N.; Menier, C.; Paul, P.; Dausset, J.; Carosella, E. HLA-G2, -G3, and -G4 Isoforms Expressed as Nonmature Cell Surface Glycoproteins Inhibit NK and Antigen-Specific CTL Cytolysis. J. Immunol. 2001, 166, 5018–5026. [Google Scholar] [CrossRef]
- Treacy, O.; Fahy, G.; Ritter, T.; O’Flynn, L. Corneal Immunosuppressive Mechanisms, Anterior Chamber-Associated Immune Deviation (ACAID) and Their Role in Allograft Rejection. Methods Mol. Biol. 2016, 1371, 205–214. [Google Scholar] [CrossRef]
- Taylor, A.W. Ocular Immune Privilege and Transplantation. Front. Immunol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Panda, A.; Vanathi, M.; Kumar, A.; Dash, Y.; Priya, S. Corneal Graft Rejection. Surv. Ophthalmol. 2007, 52, 375–396. [Google Scholar] [CrossRef]
- Nuzzi, R.; Rossi, A. Pediatric Keratoplasty: The Success of a Tailor-Made Surgical Management. Case Rep. Ophthalmol. 2020, 11, 639–646. [Google Scholar] [CrossRef]
- Bachmann, B.; Taylor, R.S.; Cursiefen, C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: An evidence-based meta-analysis. Ophthalmology 2010, 117, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, S.; Dana, M.R. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1293–1298. [Google Scholar] [CrossRef]
- Tan, Y.; Abdulreda, M.H.; Cruz-Guilloty, F.; Cutrufello, N.; Shishido, A.; Martinez, R.E.; Duffort, S.; Xia, X.; Echegaray-Mendez, J.; Levy, R.B.; et al. Role of T cell recruitment and chemokine-regulated intra-graft T cell motility patterns in corneal allograft rejection. Am. J. Transplant. 2013, 13, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Coster, D.J.; Williams, K.A. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am. J. Ophthalmol. 2005, 140, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, C.F.; Stulting, R.D. The distribution of HLA antigens on human corneal tissue. Investig. Ophthalmol. Vis. Sci. 1984, 25, 519–524. [Google Scholar]
- Maier, P.; Heizmann, U.; Böhringer, D.; Kern, Y.; Reinhard, T. Predicting the risk for corneal graft rejection by aqueous humor analysis. Mol. Vis. 2011, 17, 1016–1023. [Google Scholar]
- Stuart, P.M.; Griffith, T.S.; Usui, N.; Pepose, J.; Yu, X.; Ferguson, T.A. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J. Clin. Investig. 1997, 99, 396–402. [Google Scholar] [CrossRef]
- Janyst, M.; Kaleta, B.; Janyst, K.; Zagożdżon, R.; Kozlowska, E.; Lasek, W. Comparative Study of Immunomodulatory Agents to Induce Human T Regulatory (Treg) Cells: Preferential Treg-Stimulatory Effect of Prednisolone and Rapamycin. Arch. Immunol. Ther. Exp. 2020, 68, 20. [Google Scholar] [CrossRef]
- Schönberg, A.; Hamdorf, M.; Bock, F. Immunomodulatory Strategies Targeting Dendritic Cells to Improve Corneal Graft Survival. J. Clin. Med. 2020, 9, 1280. [Google Scholar] [CrossRef]
- Delbosc, B.; Fellmann, D.; Piquot, X.; Montard, M.; Royer, J. HLA antigenicity of normal and pathological corneas. J. Fr. Ophtalmol. 1990, 13, 535–541. [Google Scholar] [PubMed]
- Zhu, S.N.; Dana, M.R. Expression of cell adhesion molecules on limbal and neovascular endothelium in corneal inflammatory neovascularization. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1427–1434. [Google Scholar]
- Zhu, S.; Dekaris, I.; Duncker, G.; Dana, M.R. Early expression of proinflammatory cytokines interleukin-1 and tumor necrosis factor-alpha after corneal transplantation. J. Interferon Cytokine Res. 1999, 19, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Liu, J.J.; Lee, J.S.; Mohan, R.R.; Mohan, R.R.; Woods, D.J.; He, Y.G.; Wilson, S.E. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2795–2803. [Google Scholar]
- Flynn, T.H.; Mitchison, N.A.; Ono, S.J.; Larkin, D.F.P. Aqueous humor alloreactive cell phenotypes, cytokines and chemokines in corneal allograft rejection. Am. J. Transplant. 2008, 8, 1537–1543. [Google Scholar] [CrossRef]
- Bachmann, B.O.; Bock, F.; Wiegand, S.J.; Maruyama, K.; Dana, M.R.; Kruse, F.E.; Luetjen-Drecoll, E.; Cursiefen, C. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch. Ophthalmol. 2008, 126, 71–77. [Google Scholar] [CrossRef]
- Koenig, Y.; Bock, F.; Horn, F.; Kruse, F.; Straub, K.; Cursiefen, C. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 1375–1382. [Google Scholar] [CrossRef]
- Bhatti, N.; Qidwai, U.; Hussain, M.; Kazi, A. Efficacy of sub-conjunctival and topical bevacizumab in high-risk corneal transplant survival. J. Pak. Med. Assoc. 2013, 63, 1256–1259. [Google Scholar]
- January, S.E.; Fester, K.A.; Halverson, L.P.; Witt, C.A.; Byers, D.E.; Vazquez-Guillamet, R.; Alexander-Brett, J.; Tague, L.K.; Kreisel, D.; Gelman, A.; et al. Tocilizumab for antibody-mediated rejection treatment in lung transplantation. J. Heart Lung Transplant. 2023, 42, 1353–1357. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Saban, D.R.; Lee, H.K.; Dana, R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J. Immunol. 2009, 182, 148–153. [Google Scholar] [CrossRef]
- Tahvildari, M.; Omoto, M.; Chen, Y.; Emami-Naeini, P.; Inomata, T.; Dohlman, T.H.; Kaye, A.E.; Chauhan, S.K.; Dana, R. In vivo expansion of regulatory T cells by low-dose interleukin-2 treatment increases allograft survival in corneal transplantation. Transplantation 2016, 100, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Plitas, G.; Rudensky, A.Y. Regulatory T Cells: Differentiation and Function. Cancer Immunol. Res. 2016, 4, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.; Zhang, E.P.; Pohl, T.; Kunzendorf, U.; Wachtlin, J.; Bulfone-Paus, S. Inhibition of corneal allograft reaction by CTLA4-Ig. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, F.; Hori, J.; Kamiya, K.; Kaji, Y.; Oshika, T.; Amano, S.; Yamagami, S.; Tsuru, T.; Tanaka, S.; Matsuda, H.; et al. Inhibition of murine corneal allograft rejection by treatment with antibodies to CD80 and CD86. Exp. Eye Res. 2002, 74, 131–139. [Google Scholar] [CrossRef]
- Roy, R.; Boisjoly, H.M.; Wagner, E.; Langlois, A.; Bernard, P.M.; Bazin, R.; Laughrea, P.A.; Dubé, I. Pretransplant and posttransplant antibodies in human corneal transplantation. Transplantation 1992, 54, 463–467. [Google Scholar] [CrossRef]
- Hahn, A.B.; Foulks, G.N.; Enger, C.; Fink, N.; Stark, W.J.; Hopkins, K.A.; Sanfilippo, F.; Collaborative Corneal Transplantation Studies Research Group. The association of lymphocytotoxic antibodies with corneal allograft rejection in high risk patients. The Collaborative Corneal Transplantation Studies Research Group. Transplantation 1995, 59, 21–27. [Google Scholar] [CrossRef]
- Jager, M.J.; Vos, A.; Pasmans, S.; Hoekzema, R.; Broersma, L.; van der Gaag, R. Circulating cornea-specific antibodies in corneal disease and cornea transplantation. Graefes Arch. Clin. Exp. Ophthalmol. 1994, 232, 82–86. [Google Scholar] [CrossRef]
- Hargrave, S.L.; Mayhew, E.; Hegde, S.; Niederkorn, J. Are corneal cells susceptible to antibody-mediated killing in corneal allograft rejection? Transpl. Immunol. 2003, 11, 79–89. [Google Scholar] [CrossRef]
- Sel, S.; Schlaf, G.; Schurat, O.; Altermann, W.W. A novel ELISA-based crossmatch procedure to detect donor-specific anti-HLA antibodies responsible for corneal allograft rejections. J. Immunol. Methods 2012, 381, 23–31. [Google Scholar] [CrossRef]
- Worthington, J.E.; Martin, S.; Al-Husseini, D.M.; Dyer, P.A.; Johnson, R.W.G. Posttransplantation production of donor HLA-specific antibodies as a predictor of renal transplant outcome. Transplantation 2003, 75, 1034–1040. [Google Scholar] [CrossRef]
- Terasaki, P.I. Humoral theory of transplantation. Am. J. Transplant. 2003, 3, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Yabu, J.M.; Higgins, J.P.; Chen, G.; Sequeira, F.; Busque, S.; Tyan, D.B. C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation 2011, 91, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Yell, M.; Muth, B.L.; Kaufman, D.B.; Djamali, A.; Ellis, T.M. C1q Binding Activity of De Novo Donor-specific HLA Antibodies in Renal Transplant Recipients With and Without Antibody-mediated Rejection. Transplantation 2015, 99, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Goslings, W.R.; Yamada, J.; Dana, M.R.; Streilein, J.W.; van Beelen, E.; Prodeus, A.P.; Carroll, M.C.; Jager, M.J. Corneal transplantation in antibody-deficient hosts. Investig. Ophthalmol. Vis. Sci. 1999, 40, 250–253. [Google Scholar]
- Hug, M.N.; Keller, S.; Marty, T.; Gygax, D.; Meinel, D.; Spies, P.; Handschin, J.; Kleiser, M.; Vazquez, N.; Linnik, J.; et al. HLA antibody affinity determination: From HLA-specific monoclonal antibodies to donor HLA specific antibodies (DSA) in patient serum. HLA 2023, 102, 278–300. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejkowska, N.; Gorczyca, I.; Rękas, M.; Garley, M. Immunopathology of Corneal Allograft Rejection and Donor-Specific Antibodies (DSAs) as Immunological Predictors of Corneal Transplant Failure. Cells 2024, 13, 1532. https://doi.org/10.3390/cells13181532
Olejkowska N, Gorczyca I, Rękas M, Garley M. Immunopathology of Corneal Allograft Rejection and Donor-Specific Antibodies (DSAs) as Immunological Predictors of Corneal Transplant Failure. Cells. 2024; 13(18):1532. https://doi.org/10.3390/cells13181532
Chicago/Turabian StyleOlejkowska, Natalia, Iwona Gorczyca, Marek Rękas, and Marzena Garley. 2024. "Immunopathology of Corneal Allograft Rejection and Donor-Specific Antibodies (DSAs) as Immunological Predictors of Corneal Transplant Failure" Cells 13, no. 18: 1532. https://doi.org/10.3390/cells13181532
APA StyleOlejkowska, N., Gorczyca, I., Rękas, M., & Garley, M. (2024). Immunopathology of Corneal Allograft Rejection and Donor-Specific Antibodies (DSAs) as Immunological Predictors of Corneal Transplant Failure. Cells, 13(18), 1532. https://doi.org/10.3390/cells13181532





