Application of Single Cell Type-Derived Spheroids Generated by Using a Hanging Drop Culture Technique in Various In Vitro Disease Models: A Narrow Review
Abstract
:1. Introduction
1.1. Various Methods for Single-Cell 3D Spheroid Generation
1.1.1. Scaffold-Assisted Methods
Hydrogel-Assisted Cultures
Biofilm EPS-Assisted Cultures
Gelatin Microparticle-Assisted Cultures
Magnetic Particle-Assisted Cultures
1.1.2. Biomaterial Non-Assisted Methods
Static Suspension Cultures
Floating Cultures
Hanging Drop Cultures
1.1.3. Comparison of the Various Influencing Factors among the 3D Spheroid Culture Methods
2. Preparation and Characterization of 3D Spheroids Formed by Using a 384-Hanging Drop Array Culture Plate
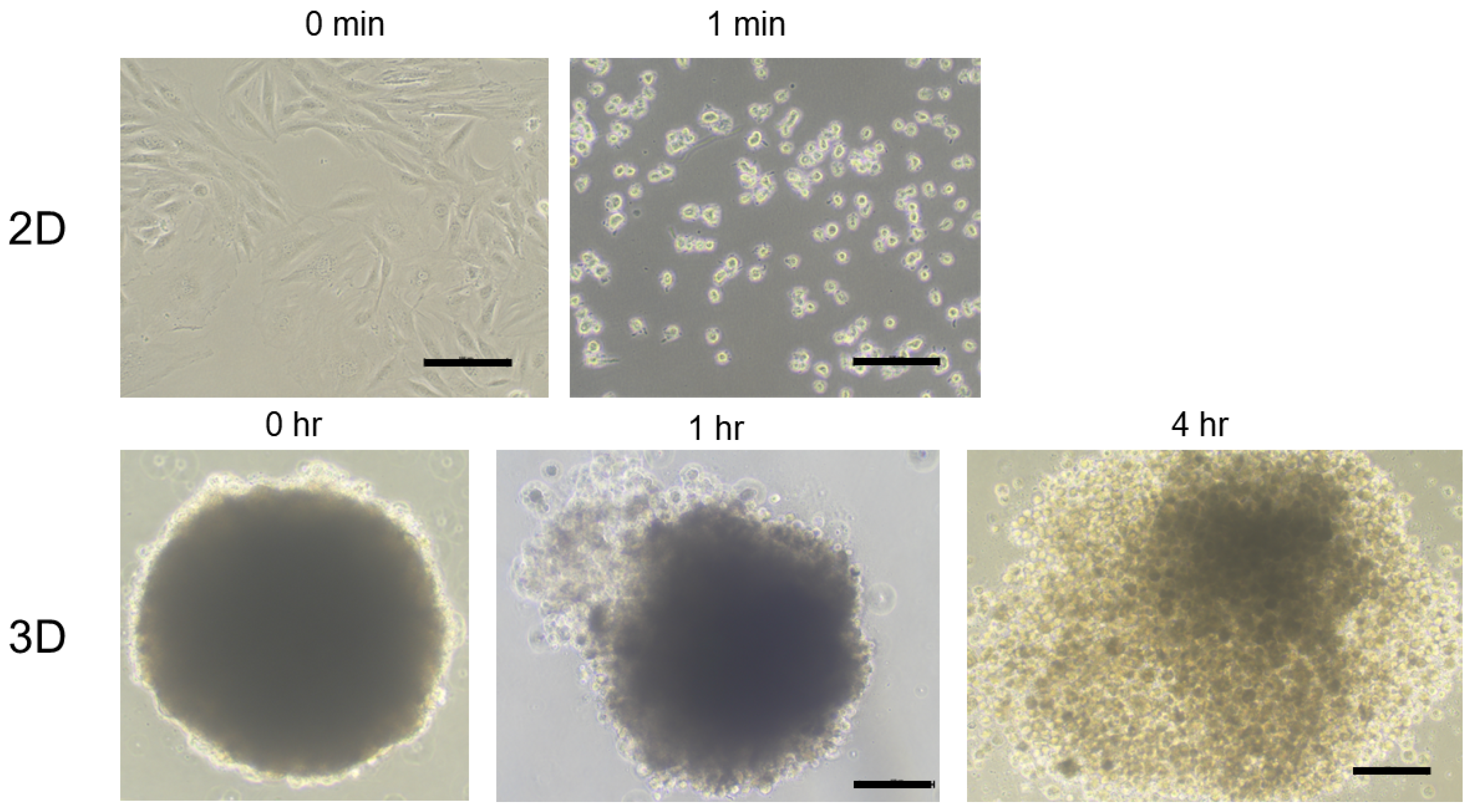
2.1. Representative Protocol of Preparation (Figure 1)
- Step 1. Cells to be used for spheroid generation are prepared by conventional 2D planar culture in 100 mm or 150 mm dishes until the cells reach approximately 90% confluence at 37 °C using a CO2 cell incubator, as described elsewhere.
- Step 2. The cell pellet is collected from the 2D cell culture plate using 0.25% Tryp sin/EDTA and centrifugation after washing with phosphate-buffered saline (PBS) on a clean bench.
- Step 3. The cells are resuspended in the 2D culture medium supplemented with methylcellulose (Methocel A4M, Sigma-Aldrich Co., St. Louis, MO, USA) to facilitate stable morphology at a concentration of 20,000 cells in 28 μL of the culture medium on a clean bench.
- Step 4. A 28 μL aliquot of the cell suspension is placed in each well of a hanging drop culture plate (# HDP1385, Sigma-Aldrich Co., St. Louis, MO, USA) on a clean bench, and then the culture plate is placed in a CO2 cell incubator.
- Step 5. Half of the medium (14 μL) in each well is replaced daily by 14 μL of a fresh culture medium on a clean bench, and then the culture plate is placed in a CO2 cell incubator.

2.2. Analyses of the Physical Properties, Including Size and Stiffness, and the Morphology of 3D Spheroids
2.2.1. Measurement of the Sizes of 3D Spheroids
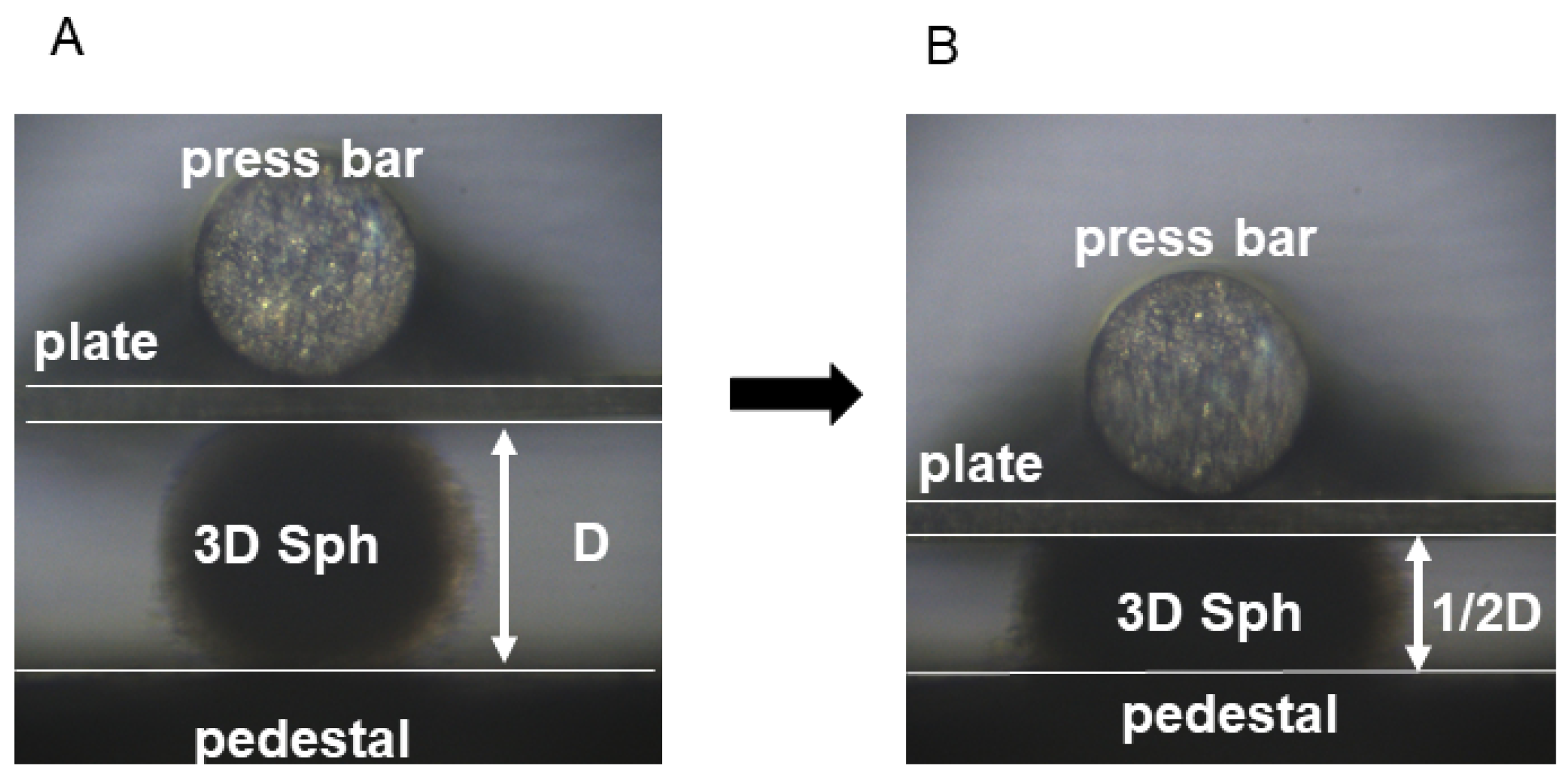
2.2.2. Measurement of the Stiffness of 3D Spheroids
2.2.3. Analysis of Morphology by Scanning Electron Microscopy and Immunocytochemistry
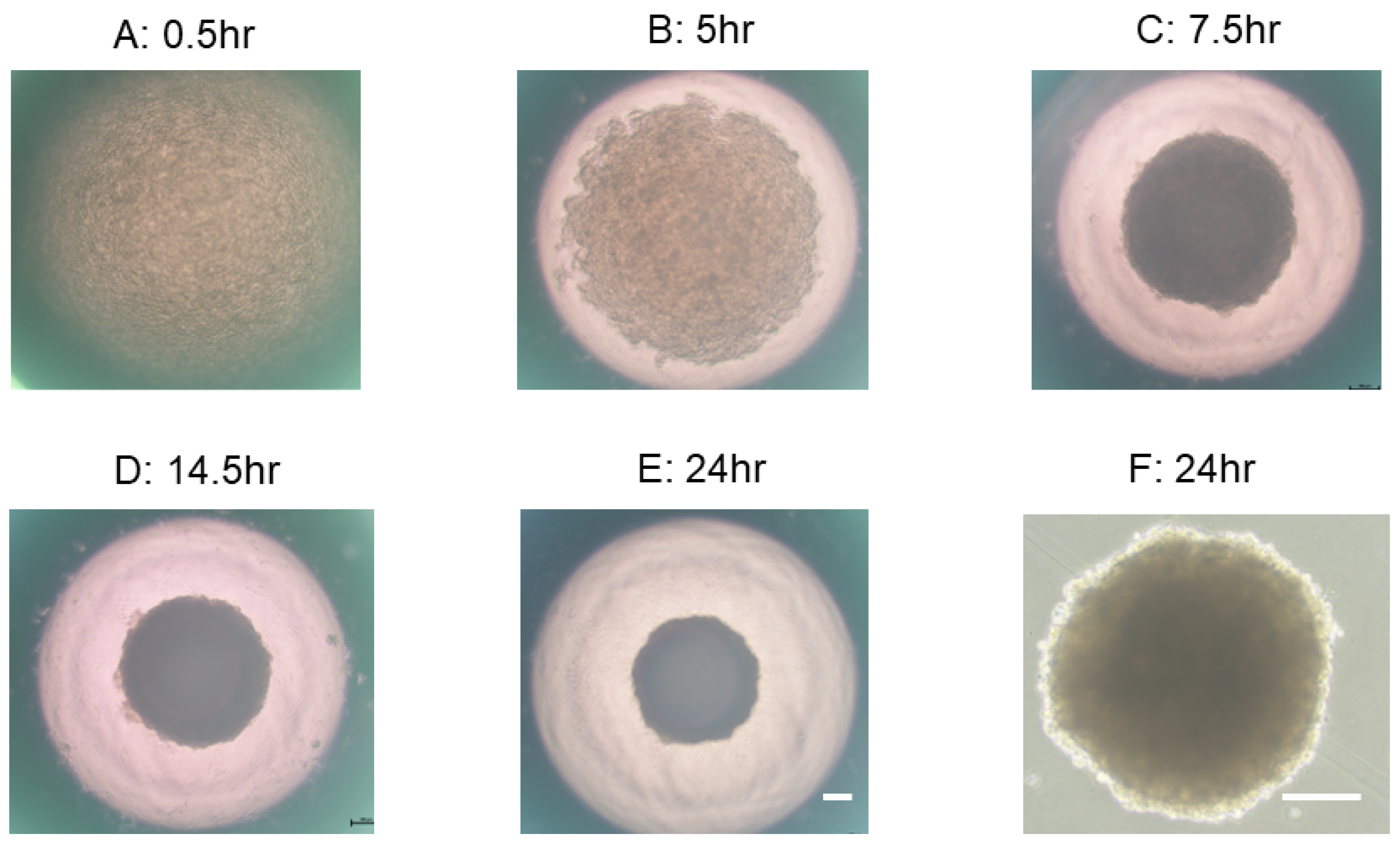
2.3. Representative Process of 3D Spheroid Maturation
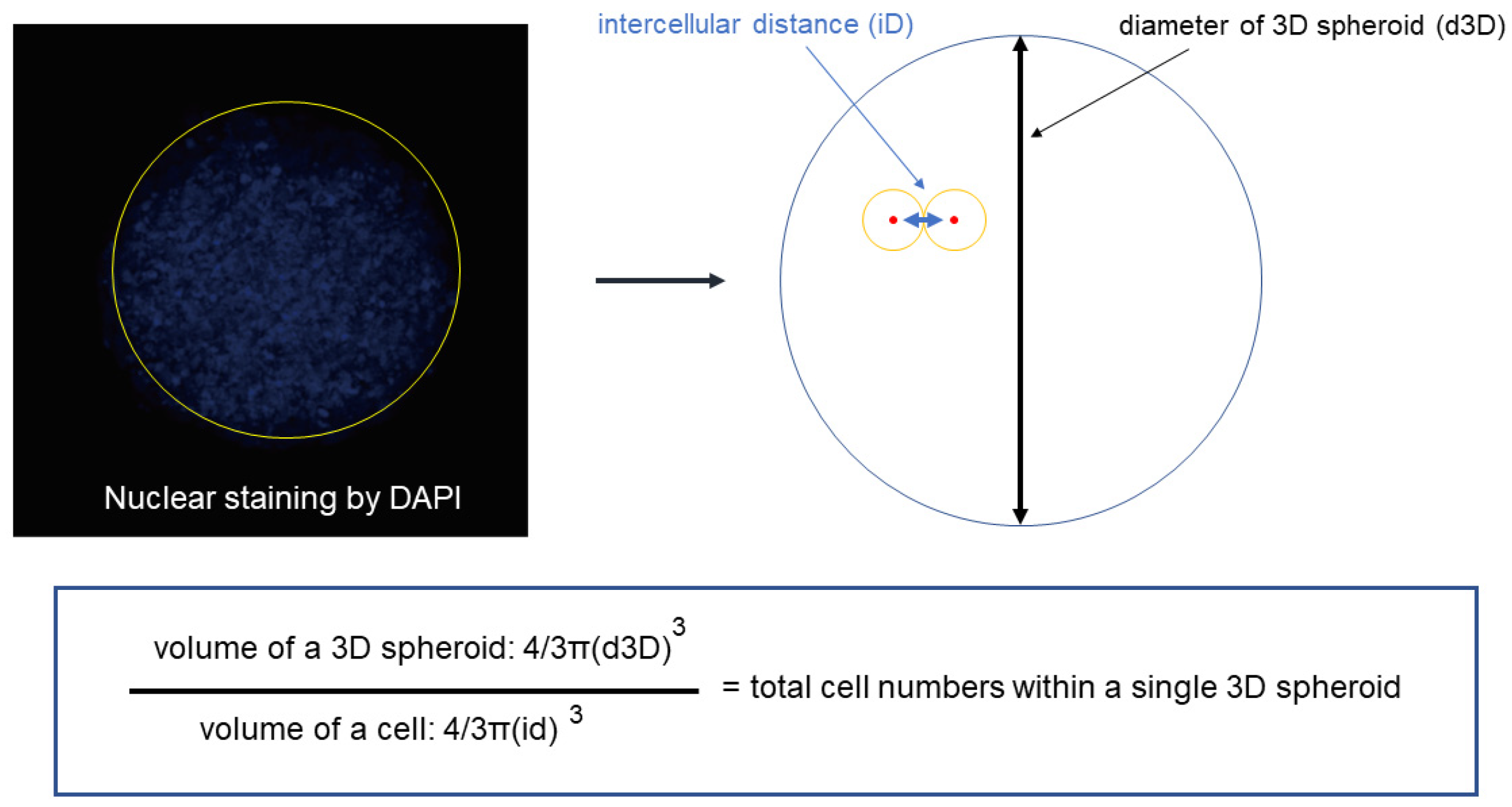
2.4. Estimation of the Total Number of Cells in a Single Spheroid
2.5. Cellular Metabolic Analysis of 3D Spheroid
3. Application of 3D Spheroids for Various Fields in Biological Science
3.1. Study of Adipogenesis Using 3T3-L1-1 Cells (Figure 6)
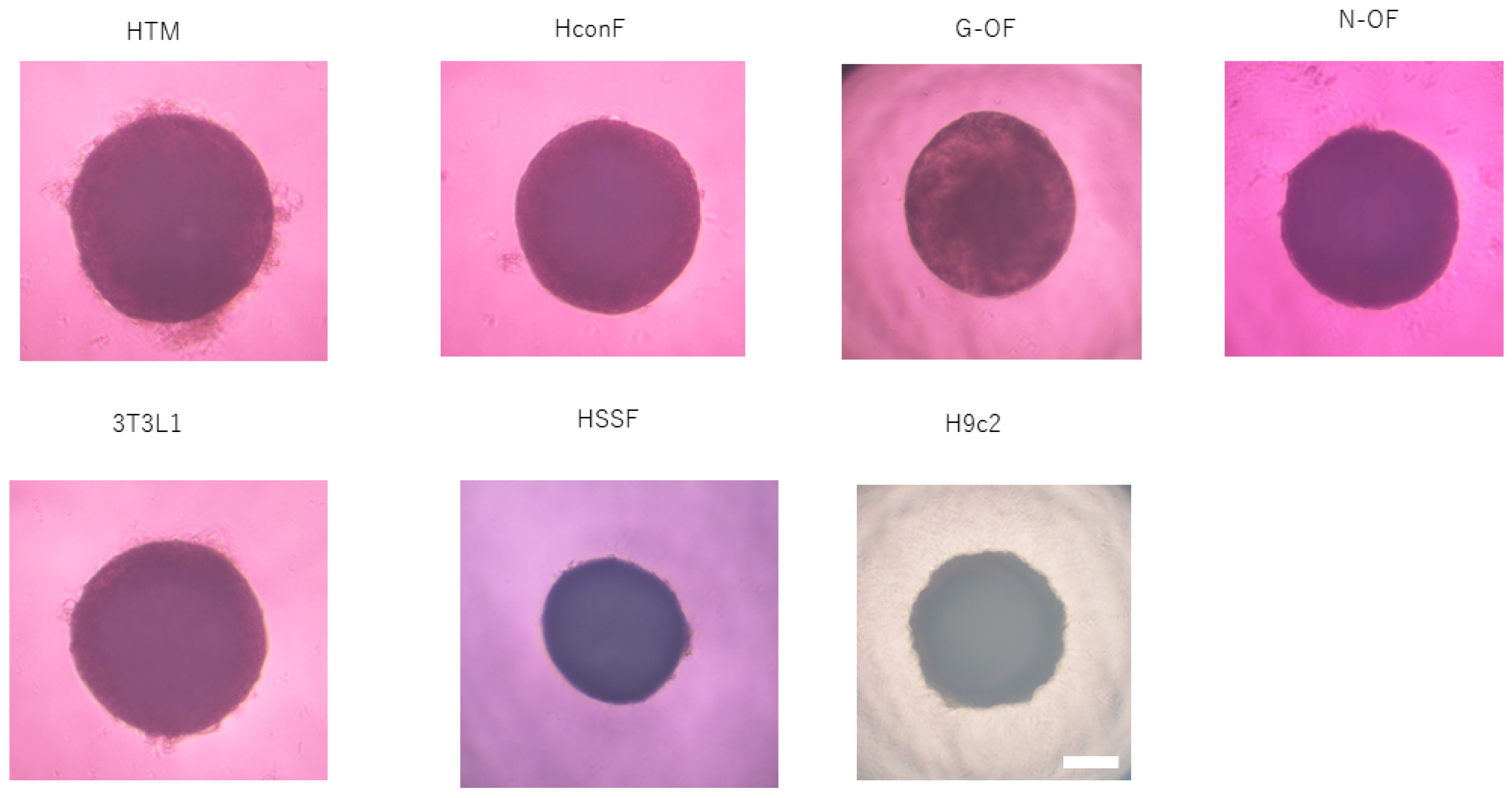
3.2. Study for Cardiology Using H9c2 Cells (Figure 6)
4. Studies for Ocular Pathophysiology
4.1. Ocular Surface and Sclera
4.2. Intraocular Segments
4.3. Orbit
4.3.1. Lacrimal Gland (LG)
4.3.2. Orbital Fatty Tissue
5. Three-Dimensional Spheroids Obtained from Various Cancerous Cells
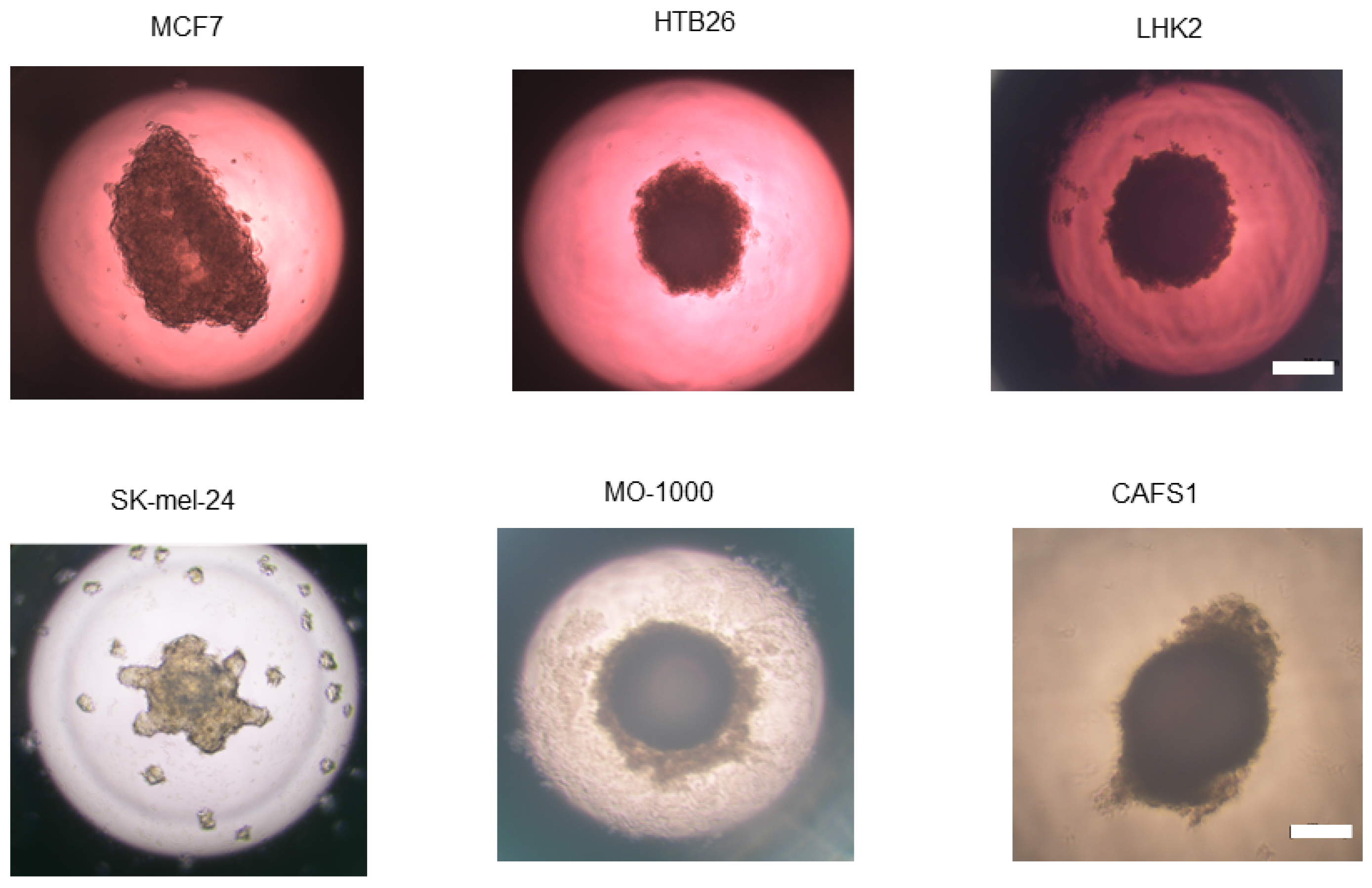
5.1. Malignant Melanoma (MM)
5.2. Oral Squamous Cell Carcinoma (OSCC)
5.3. Cancer-Associated Fibroblasts (CAFs)
6. Summary of Current Concepts of the Biological Significance of Hanging Drop Cultures of Single Cell Type-Derived Spheroids and Their Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sumbalova Koledova, Z. 3D Cell Culture: Techniques For and Beyond Organoid Applications. Methods Mol. Biol. 2024, 2764, 1–12. [Google Scholar] [PubMed]
- Boucherit, N.; Gorvel, L.; Olive, D. 3D Tumor Models and Their Use for the Testing of Immunotherapies. Front. Immunol. 2020, 11, 603640. [Google Scholar] [CrossRef] [PubMed]
- Ryu, N.E.; Lee, S.H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Koo, I.S.; Hwang, H.J.; Lee, D.W. In Vitro three-dimensional (3D) cell culture tools for spheroid and organoid models. SLAS Discov. Adv. Life Sci. R. D 2023, 28, 119–137. [Google Scholar] [CrossRef]
- Hikage, F.; Atkins, S.; Kahana, A.; Smith, T.J.; Chun, T.H. HIF2A-LOX Pathway Promotes Fibrotic Tissue Remodeling in Thyroid-Associated Orbitopathy. Endocrinology 2019, 160, 20–35. [Google Scholar] [CrossRef]
- Ohguro, H.; Ida, Y.; Hikage, F.; Umetsu, A.; Ichioka, H.; Watanabe, M.; Furuhashi, M. STAT3 Is the Master Regulator for the Forming of 3D Spheroids of 3T3-L1 Preadipocytes. Cells 2022, 11, 300. [Google Scholar] [CrossRef]
- Ohguro, H.; Watanabe, M.; Sato, T.; Hikage, F.; Furuhashi, M.; Okura, M.; Hida, T.; Uhara, H. 3D Spheroid Configurations Are Possible Indictors for Evaluating the Pathophysiology of Melanoma Cell Lines. Cells 2023, 12, 759. [Google Scholar] [CrossRef]
- Watanabe, M.; Ida, Y.; Ohguro, H.; Ota, C.; Hikage, F. Establishment of appropriate glaucoma models using dexamethasone or TGFβ2 treated three-dimension (3D) cultured human trabecular meshwork (HTM) cells. Sci. Rep. 2021, 11, 19369. [Google Scholar] [CrossRef]
- Nishikiori, N.; Takada, K.; Sato, T.; Miyamoto, S.; Watanabe, M.; Hirakawa, Y.; Sekiguchi, S.; Furuhashi, M.; Yorozu, A.; Takano, K.; et al. Physical Properties and Cellular Metabolic Characteristics of 3D Spheroids Are Possible Definitive Indices for the Biological Nature of Cancer-Associated Fibroblasts. Cells 2023, 12, 2160. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Choi, Y.S.; Seo, Y.J.; Lee, M.Y.; Jeon, S.Y.; Ku, B.; Kim, S.; Yi, S.H.; Nam, D.H. High-throughput screening (HTS) of anticancer drug efficacy on a micropillar/microwell chip platform. Anal. Chem. 2014, 86, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Keown, A.T.; Addison, B.; Leach, J.K. Cell Migration and Bone Formation from Mesenchymal Stem Cell Spheroids in Alginate Hydrogels Are Regulated by Adhesive Ligand Density. Biomacromolecules 2017, 18, 4331–4340. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.C.; Whitehead, J.; Zhou, D.; Ho, S.S.; Leach, J.K. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomater. 2017, 64, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S.; Lewis, E.E.; Mullin, M.; Wheadon, H.; Dalby, M.J.; Berry, C.C. Magnetically levitated mesenchymal stem cell spheroids cultured with a collagen gel maintain phenotype and quiescence. J. Tissue Eng. 2017, 8, 2041731417704428. [Google Scholar] [CrossRef]
- Gwon, K.; Kim, E.; Tae, G. Heparin-hyaluronic acid hydrogel in support of cellular activities of 3D encapsulated adipose derived stem cells. Acta Biomater. 2017, 49, 284–295. [Google Scholar] [CrossRef]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From discovery and ECM mimicry to assays and models for cancer research. Adv. Drug Deliv. Rev. 2014, 79, 3–18. [Google Scholar] [CrossRef]
- Benton, G.; Kleinman, H.K.; George, J.; Arnaoutova, I. Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Int. J. Cancer 2011, 128, 1751–1757. [Google Scholar] [CrossRef]
- Dolega, M.E.; Abeille, F.; Picollet-D’hahan, N.; Gidrol, X. Controlled 3D culture in Matrigel microbeads to analyze clonal acinar development. Biomaterials 2015, 52, 347–357. [Google Scholar] [CrossRef]
- Chen, Y.H.; Ku, Y.H.; Wang, K.C.; Chiang, H.C.; Hsu, Y.P.; Cheng, M.T.; Wang, C.S.; Wee, Y. Bioinspired Sandcastle Worm-Derived Peptide-Based Hybrid Hydrogel for Promoting the Formation of Liver Spheroids. Gels 2022, 8, 149. [Google Scholar] [CrossRef]
- Li, Y.; Kumacheva, E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018, 4, eaas8998. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef]
- Cometta, S.; Hutmacher, D.W.; Chai, L. In vitro models for studying implant-associated biofilms—A review from the perspective of bioengineering 3D microenvironments. Biomaterials 2024, 309, 122578. [Google Scholar] [CrossRef]
- Huang, G.S.; Dai, L.G.; Yen, B.L.; Hsu, S.H. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 2011, 32, 6929–6945. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.Y.; Liu, B.H.; Hsu, S.H. The calcium-dependent regulation of spheroid formation and cardiomyogenic differentiation for MSCs on chitosan membranes. Biomaterials 2012, 33, 8943–8954. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Baipaywad, P.; Jeong, Y.; Park, H. Incorporation of gelatin microparticles on the formation of adipose-derived stem cell spheroids. Int. J. Biol. Macromol. 2018, 110, 472–478. [Google Scholar] [CrossRef]
- Hayashi, K.; Tabata, Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011, 7, 2797–2803. [Google Scholar] [CrossRef]
- Baraniak, P.R.; Cooke, M.T.; Saeed, R.; Kinney, M.A.; Fridley, K.M.; McDevitt, T.C. Stiffening of human mesenchymal stem cell spheroid microenvironments induced by incorporation of gelatin microparticles. J. Mech. Behav. Biomed. Mater. 2012, 11, 63–71. [Google Scholar] [CrossRef]
- Bratt-Leal, A.M.; Nguyen, A.H.; Hammersmith, K.A.; Singh, A.; McDevitt, T.C. A microparticle approach to morphogen delivery within pluripotent stem cell aggregates. Biomaterials 2013, 34, 7227–7235. [Google Scholar] [CrossRef]
- Anil-Inevi, M.; Yaman, S.; Yildiz, A.A.; Mese, G.; Yalcin-Ozuysal, O.; Tekin, H.C.; Ozcivici, E. Biofabrication of in situ Self Assembled 3D Cell Cultures in a Weightlessness Environment Generated using Magnetic Levitation. Sci. Rep. 2018, 8, 7239. [Google Scholar] [CrossRef]
- Meng, R.; Xu, H.Y.; Di, S.M.; Shi, D.Y.; Qian, A.R.; Wang, J.F.; Shang, P. Human mesenchymal stem cells are sensitive to abnormal gravity and exhibit classic apoptotic features. Acta Biochim. Biophys. Sin. 2011, 43, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Sytkowski, A.J.; Davis, K.L. Erythroid cell growth and differentiation in vitro in the simulated microgravity environment of the NASA rotating wall vessel bioreactor. Vitr. Cell. Dev. Biol. Anim. 2001, 37, 79–83. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Zitzmann, F.D.; Schmidt, S.; Frank, R.; Weigel, W.; Meier, M.; Jahnke, H.G. Microcavity well-plate for automated parallel bioelectronic analysis of 3D cell cultures. Biosens. Bioelectron. 2024, 250, 116042. [Google Scholar] [CrossRef]
- Kukla, D.A.; Belair, D.G.; Stresser, D.M. Evaluation and Optimization of a Microcavity Plate-Based Human Hepatocyte Spheroid Model for Predicting Clearance of Slowly Metabolized Drug Candidates. Drug Metab. Dispos. Biol. Fate Chem. 2024, 52, 797–812. [Google Scholar] [CrossRef]
- Kim, J.B. Three-dimensional tissue culture models in cancer biology. Semin. Cancer Biol. 2005, 15, 365–377. [Google Scholar] [CrossRef]
- Achilli, T.M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert. Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylostalo, J.H. Preparation of anti-inflammatory mesenchymal stem/precursor cells (MSCs) through sphere formation using hanging-drop culture technique. Curr. Protoc. Stem Cell Biol. 2014, 28, 2b.6.1–2b.6.23. [Google Scholar] [CrossRef]
- Kelm, J.M.; Timmins, N.E.; Brown, C.J.; Fussenegger, M.; Nielsen, L.K. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol. Bioeng. 2003, 83, 173–180. [Google Scholar] [CrossRef]
- Tung, Y.C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.S.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Ida, Y.; Hikage, F.; Ohguro, H. ROCK inhibitors enhance the production of large lipid-enriched 3D organoids of 3T3-L1 cells. Sci. Rep. 2021, 11, 5479. [Google Scholar] [CrossRef]
- Duraj, T.; Carrión-Navarro, J.; Seyfried, T.N.; García-Romero, N.; Ayuso-Sacido, A. Metabolic therapy and bioenergetic analysis: The missing piece of the puzzle. Mol. Metab. 2021, 54, 101389. [Google Scholar] [CrossRef]
- Rodríguez-Enríquez, S.; Gallardo-Pérez, J.C.; Avilés-Salas, A.; Marín-Hernández, A.; Carreño-Fuentes, L.; Maldonado-Lagunas, V.; Moreno-Sánchez, R. Energy metabolism transition in multi-cellular human tumor spheroids. J. Cell. Physiol. 2008, 216, 189–197. [Google Scholar] [CrossRef]
- Mandujano-Tinoco, E.A.; Gallardo-Pérez, J.C.; Marín-Hernández, A.; Moreno-Sánchez, R.; Rodríguez-Enríquez, S. Anti-mitochondrial therapy in human breast cancer multi-cellular spheroids. Biochim. Biophys. Acta 2013, 1833, 541–551. [Google Scholar] [CrossRef]
- Marín-Hernández, Á.; Gallardo-Pérez, J.C.; Hernández-Reséndiz, I.; Del Mazo-Monsalvo, I.; Robledo-Cadena, D.X.; Moreno-Sánchez, R.; Rodríguez-Enríquez, S. Hypoglycemia Enhances Epithelial-Mesenchymal Transition and Invasiveness, and Restrains the Warburg Phenotype, in Hypoxic HeLa Cell Cultures and Microspheroids. J. Cell. Physiol. 2017, 232, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K.; Smith, H.; van Hamel Parsons, V.; Gavaghan, D.; Kelly, C.; Fletcher, A.; Maini, P.; Callaghan, R. Metabolic alterations during the growth of tumour spheroids. Cell Biochem. Biophys. 2014, 68, 615–628. [Google Scholar] [CrossRef]
- Javed, Z.; Worley, B.L.; Stump, C.; Shimko, S.S.; Crawford, L.C.; Mythreye, K.; Hempel, N. Optimization of Extracellular Flux Assay to Measure Respiration of Anchorage-independent Tumor Cell Spheroids. Bio-Protoc. 2022, 12, e4321. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Z.; Xu, E.; Shen, X.; Wang, X.; Li, Z.; Yu, H.; Chen, K.; Hu, Q.; Xia, X.; et al. Apolipoprotein C-II induces EMT to promote gastric cancer peritoneal metastasis via PI3K/AKT/mTOR pathway. Clin. Transl. Med. 2021, 11, e522. [Google Scholar] [CrossRef]
- Bonuccelli, G.; Sotgia, F.; Lisanti, M.P. Identification of natural products and FDA-approved drugs for targeting cancer stem cell (CSC) propagation. Aging 2022, 14, 9466–9483. [Google Scholar] [CrossRef]
- Campioni, G.; Pasquale, V.; Busti, S.; Ducci, G.; Sacco, E.; Vanoni, M. An Optimized Workflow for the Analysis of Metabolic Fluxes in Cancer Spheroids Using Seahorse Technology. Cells 2022, 11, 866. [Google Scholar] [CrossRef] [PubMed]
- Ichioka, H.; Hirohashi, Y.; Sato, T.; Furuhashi, M.; Watanabe, M.; Ida, Y.; Hikage, F.; Torigoe, T.; Ohguro, H. G-Protein-Coupled Receptors Mediate Modulations of Cell Viability and Drug Sensitivity by Aberrantly Expressed Recoverin 3 within A549 Cells. Int. J. Mol. Sci. 2023, 24, 771. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Watanabe, M.; Takada, K.; Sato, T.; Hikage, F.; Umetsu, A.; Muramatsu, J.; Furuhashi, M.; Ohguro, H. Modulation of Epithelial-Mesenchymal Transition Is a Possible Underlying Mechanism for Inducing Chemoresistance in MIA PaCa-2 Cells against Gemcitabine and Paclitaxel. Biomedicines 2024, 12, 1011. [Google Scholar] [CrossRef]
- Nishikiori, N.; Watanabe, M.; Sato, T.; Furuhashi, M.; Okura, M.; Hida, T.; Uhara, H.; Ohguro, H. Significant and Various Effects of ML329-Induced MITF Suppression in the Melanoma Cell Line. Cancers 2024, 16, 263. [Google Scholar] [CrossRef]
- Miyamoto, S.; Nishikiori, N.; Sato, T.; Watanabe, M.; Umetsu, A.; Tsugeno, Y.; Hikage, F.; Sasaya, T.; Kato, H.; Ogi, K.; et al. Three-Dimensional Spheroid Configurations and Cellular Metabolic Properties of Oral Squamous Carcinomas Are Possible Pharmacological and Pathological Indicators. Cancers 2023, 15, 2793. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Sato, T.; Umetsu, A.; Watanabe, M.; Hikage, F.; Ida, Y.; Ohguro, H.; Furuhashi, M. 3D culture induction of adipogenic differentiation in 3T3-L1 preadipocytes exhibits adipocyte-specific molecular expression patterns and metabolic functions. Heliyon 2023, 9, e20713. [Google Scholar] [CrossRef]
- Watanabe, M.; Sato, T.; Tsugeno, Y.; Umetsu, A.; Suzuki, S.; Furuhashi, M.; Ida, Y.; Hikage, F.; Ohguro, H. Human Trabecular Meshwork (HTM) Cells Treated with TGF-β2 or Dexamethasone Respond to Compression Stress in Different Manners. Biomedicines 2022, 10, 1338. [Google Scholar] [CrossRef]
- Watanabe, M.; Sato, T.; Tsugeno, Y.; Higashide, M.; Furuhashi, M.; Umetsu, A.; Suzuki, S.; Ida, Y.; Hikage, F.; Ohguro, H. All-trans Retinoic Acids Synergistically and Beneficially Affect In Vitro Glaucomatous Trabecular Meshwork (TM) Models Using 2D and 3D Cell Cultures of Human TM Cells. Int. J. Mol. Sci. 2022, 23, 9912. [Google Scholar] [CrossRef]
- Tsugeno, Y.; Sato, T.; Watanabe, M.; Furuhashi, M.; Umetsu, A.; Ida, Y.; Hikage, F.; Ohguro, H. Benzalkonium Chloride, Even at Low Concentrations, Deteriorates Intracellular Metabolic Capacity in Human Conjunctival Fibroblasts. Biomedicines 2022, 10, 2315. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dessels, C.; Durandt, C.; Pepper, M.S. Genome-wide analysis of gene expression during adipogenesis in human adipose-derived stromal cells reveals novel patterns of gene expression during adipocyte differentiation. Stem Cell Res. 2016, 16, 725–734. [Google Scholar] [CrossRef]
- Sadowski, H.B.; Wheeler, T.T.; Young, D.A. Gene expression during 3T3-L1 adipocyte differentiation. Characterization of initial responses to the inducing agents and changes during commitment to differentiation. J. Biol. Chem. 1992, 267, 4722–4731. [Google Scholar] [CrossRef] [PubMed]
- Aulthouse, A.L.; Freeh, E.; Newstead, S.; Stockert, A.L. Part 1: A Novel Model for Three-Dimensional Culture of 3T3-L1 Preadipocytes Stimulates Spontaneous Cell Differentiation Independent of Chemical Induction Typically Required in Monolayer. Nutr. Metab. Insights 2019, 12, 1178638819841399. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.H.; Inoue, M. 3-D adipocyte differentiation and peri-adipocyte collagen turnover. Methods Enzymol. 2014, 538, 15–34. [Google Scholar] [PubMed]
- Davidenko, N.; Campbell, J.J.; Thian, E.S.; Watson, C.J.; Cameron, R.E. Collagen-hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010, 6, 3957–3968. [Google Scholar] [CrossRef]
- Fischbach, C.; Seufert, J.; Staiger, H.; Hacker, M.; Neubauer, M.; Göpferich, A.; Blunk, T. Three-dimensional in vitro model of adipogenesis: Comparison of culture conditions. Tissue Eng. 2004, 10, 215–229. [Google Scholar] [CrossRef]
- Fischbach, C.; Spruss, T.; Weiser, B.; Neubauer, M.; Becker, C.; Hacker, M.; Göpferich, A.; Blunk, T. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp. Cell Res. 2004, 300, 54–64. [Google Scholar] [CrossRef]
- Josan, C.; Kakar, S.; Raha, S. Matrigel® enhances 3T3-L1 cell differentiation. Adipocyte 2021, 10, 361–377. [Google Scholar] [CrossRef]
- Daquinag, A.C.; Souza, G.R.; Kolonin, M.G. Adipose tissue engineering in three-dimensional levitation tissue culture system based on magnetic nanoparticles. Tissue Eng. Part. C Methods 2013, 19, 336–344. [Google Scholar] [CrossRef]
- Tseng, H.; Daquinag, A.C.; Souza, G.R.; Kolonin, M.G. Three-Dimensional Magnetic Levitation Culture System Simulating White Adipose Tissue. Methods Mol. Biol. 2018, 1773, 147–154. [Google Scholar]
- Turner, P.A.; Garrett, M.R.; Didion, S.P.; Janorkar, A.V. Spheroid Culture System Confers Differentiated Transcriptome Profile and Functional Advantage to 3T3-L1 Adipocytes. Ann. Biomed. Eng. 2018, 46, 772–787. [Google Scholar] [CrossRef]
- Turner, P.A.; Gurumurthy, B.; Bailey, J.L.; Elks, C.M.; Janorkar, A.V. Adipogenic Differentiation of Human Adipose-Derived Stem Cells Grown as Spheroids. Process Biochem. 2017, 59, 312–320. [Google Scholar] [CrossRef]
- Ida, Y.; Hikage, F.; Itoh, K.; Ida, H.; Ohguro, H. Prostaglandin F2α agonist-induced suppression of 3T3-L1 cell adipogenesis affects spatial formation of extra-cellular matrix. Sci. Rep. 2020, 10, 7958. [Google Scholar] [CrossRef]
- Ida, Y.; Hikage, F.; Umetsu, A.; Ida, H.; Ohguro, H. Omidenepag, a non-prostanoid EP2 receptor agonist, induces enlargement of the 3D organoid of 3T3-L1 cells. Sci. Rep. 2020, 10, 16018. [Google Scholar] [CrossRef]
- Ida, Y.; Watanabe, M.; Ohguro, H.; Hikage, F. Simultaneous Use of ROCK Inhibitors and EP2 Agonists Induces Unexpected Effects on Adipogenesis and the Physical Properties of 3T3-L1 Preadipocytes. Int. J. Mol. Sci. 2021, 22, 4648. [Google Scholar] [CrossRef]
- Ida, Y.; Watanabe, M.; Umetsu, A.; Ohguro, H.; Hikage, F. Addition of EP2 agonists to an FP agonist additively and synergistically modulates adipogenesis and the physical properties of 3D 3T3-L1 sphenoids. Prostaglandins Leukot. Essent. Fat. Acids 2021, 171, 102315. [Google Scholar] [CrossRef]
- Mery, B.; Vallard, A.; Rowinski, E.; Magne, N. High-throughput sequencing in clinical oncology: From past to present. Swiss Med. Wkly. 2019, 149, w20057. [Google Scholar] [CrossRef]
- Albi, E.; Curcio, F.; Lazzarini, A.; Floridi, A.; Cataldi, S.; Lazzarini, R.; Loreti, E.; Ferri, I.; Ambesi-Impiombato, F.S. A firmer understanding of the effect of hypergravity on thyroid tissue: Cholesterol and thyrotropin receptor. PLoS ONE 2014, 9, e98250. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, C.G.; Wu, H.M.; Oh, C.S.; Chung, S.W.; Kim, S.G. A load of mice to hypergravity causes AMPKα repression with liver injury, which is overcome by preconditioning loads via Nrf2. Sci. Rep. 2015, 5, 15643. [Google Scholar] [CrossRef]
- Cavey, T.; Pierre, N.; Nay, K.; Allain, C.; Ropert, M.; Loréal, O.; Derbré, F. Simulated microgravity decreases circulating iron in rats: Role of inflammation-induced hepcidin upregulation. Exp. Physiol. 2017, 102, 291–298. [Google Scholar] [CrossRef]
- Picca, A.; Mankowski, R.T.; Burman, J.L.; Donisi, L.; Kim, J.S.; Marzetti, E.; Leeuwenburgh, C. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat. Rev. Cardiol. 2018, 15, 543–554. [Google Scholar] [CrossRef]
- Kolwicz, S.C., Jr.; Purohit, S.; Tian, R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ. Res. 2013, 113, 603–616. [Google Scholar] [CrossRef]
- Chen, X.F.; Chen, X.; Tang, X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin. Sci. 2020, 134, 657–676. [Google Scholar] [CrossRef]
- Heusch, G.; Libby, P.; Gersh, B.; Yellon, D.; Böhm, M.; Lopaschuk, G.; Opie, L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014, 383, 1933–1943. [Google Scholar] [CrossRef]
- Patel, K.V.; Pandey, A.; de Lemos, J.A. Conceptual Framework for Addressing Residual Atherosclerotic Cardiovascular Disease Risk in the Era of Precision Medicine. Circulation 2018, 137, 2551–2553. [Google Scholar] [CrossRef]
- Poole, D.C.; Hirai, D.M.; Copp, S.W.; Musch, T.I. Muscle oxygen transport and utilization in heart failure: Implications for exercise (in)tolerance. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1050–H1063. [Google Scholar] [CrossRef]
- Zuppinger, C. 3D Cardiac Cell Culture: A Critical Review of Current Technologies and Applications. Front. Cardiovasc. Med. 2019, 6, 87. [Google Scholar] [CrossRef]
- Arai, K.; Kitsuka, T.; Nakayama, K. Scaffold-based and scaffold-free cardiac constructs for drug testing. Biofabrication 2021, 13, 042001. [Google Scholar] [CrossRef]
- Navaee, F.; Renaud, P.; Kleger, A.; Braschler, T. Highly Efficient Cardiac Differentiation and Maintenance by Thrombin-Coagulated Fibrin Hydrogels Enriched with Decellularized Porcine Heart Extracellular Matrix. Int. J. Mol. Sci. 2023, 24, 2842. [Google Scholar] [CrossRef]
- Navaee, F.; Khornian, N.; Longet, D.; Heub, S.; Boder-Pasche, S.; Weder, G.; Kleger, A.; Renaud, P.; Braschler, T. A Three-Dimensional Engineered Cardiac In Vitro Model: Controlled Alignment of Cardiomyocytes in 3D Microphysiological Systems. Cells 2023, 12, 576. [Google Scholar] [CrossRef]
- Finosh, G.T.; Jayabalan, M.; Vandana, S.; Raghu, K.G. Hybrid alginate-polyester bimodal network hydrogel for tissue engineering—Influence of structured water on long-term cellular growth. Colloids Surf. B Biointerfaces 2015, 135, 855–864. [Google Scholar] [CrossRef]
- Chin, I.L.; Amos, S.E.; Jeong, J.H.; Hool, L.; Hwang, Y.; Choi, Y.S. Mechanosensation mediates volume adaptation of cardiac cells and spheroids in 3D. Mater. Today. Bio 2022, 16, 100391. [Google Scholar] [CrossRef]
- Hu, Y.; Jia, Y.; Wang, H.; Cao, Q.; Yang, Y.; Zhou, Y.; Tan, T.; Huang, X.; Zhou, Q. Low-intensity pulsed ultrasound promotes cell viability and inhibits apoptosis of H9C2 cardiomyocytes in 3D bioprinting scaffolds via PI3K-Akt and ERK1/2 pathways. J. Biomater. Appl. 2022, 37, 402–414. [Google Scholar] [CrossRef]
- Mei, C.; Chao, C.W.; Lin, C.W.; Li, S.T.; Wu, K.H.; Yang, K.C.; Yu, J. Three-dimensional spherical gelatin bubble-based scaffold improves the myotube formation of H9c2 myoblasts. Biotechnol. Bioeng. 2019, 116, 1190–1200. [Google Scholar] [CrossRef]
- Punia, K.; Bucaro, M.; Mancuso, A.; Cuttitta, C.; Marsillo, A.; Bykov, A.; L’Amoreaux, W.; Raja, K.S. Rediscovering Chemical Gardens: Self-Assembling Cytocompatible Protein-Intercalated Silicate-Phosphate Sponge-Mimetic Tubules. Langmuir ACS J. Surf. Colloids 2016, 32, 8748–8758. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Wang, Y.; Su, Y.; Chen, M. 3D myotube guidance on hierarchically organized anisotropic and conductive fibers for skeletal muscle tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111070. [Google Scholar] [CrossRef]
- Li, D.; Tao, L.; Shen, Y.; Sun, B.; Xie, X.; Ke, Q.; Mo, X.; Deng, B. Fabrication of Multilayered Nanofiber Scaffolds with a Highly Aligned Nanofiber Yarn for Anisotropic Tissue Regeneration. ACS Omega 2020, 5, 24340–24350. [Google Scholar] [CrossRef]
- Zhang, Y.; Le Friec, A.; Chen, M. 3D anisotropic conductive fibers electrically stimulated myogenesis. Int. J. Pharm. 2021, 606, 120841. [Google Scholar] [CrossRef]
- Rogers, A.J.; Miller, J.M.; Kannappan, R.; Sethu, P. Cardiac Tissue Chips (CTCs) for Modeling Cardiovascular Disease. IEEE Trans. Bio-Med. Eng. 2019, 66, 3436–3443. [Google Scholar] [CrossRef]
- Ciofani, G.; Ricotti, L.; Mattoli, V. Preparation, characterization and in vitro testing of poly(lactic-co-glycolic) acid/barium titanate nanoparticle composites for enhanced cellular proliferation. Biomed. Microdevices 2011, 13, 255–266. [Google Scholar] [CrossRef]
- Onbas, R.; Arslan Yildiz, A. Biopatterning of 3D Cellular Model by Contactless Magnetic Manipulation for Cardiotoxicity Screening. Tissue Eng. Part. A 2023, 30, 367–376. [Google Scholar] [CrossRef]
- Di, Y.; Zhao, S.; Fan, H.; Li, W.; Jiang, G.; Wang, Y.; Li, C.; Wang, W.; Wang, J. Mass Production of Rg1-Loaded Small Extracellular Vesicles Using a 3D Bioreactor System for Enhanced Cardioprotective Efficacy of Doxorubicin-Induced Cardiotoxicity. Pharmaceutics 2024, 16, 593. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yano, T.; Sato, T.; Umetsu, A.; Higashide, M.; Furuhashi, M.; Ohguro, H. mTOR Inhibitors Modulate the Physical Properties of 3D Spheroids Derived from H9c2 Cells. Int. J. Mol. Sci. 2023, 24, 11459. [Google Scholar] [CrossRef] [PubMed]
- Layer, P.G. In a century from agitated cells to human organoids. J. Neurosci. Methods 2024, 405, 110083. [Google Scholar] [CrossRef]
- Lu, Q.; Yin, H.; Grant, M.P.; Elisseeff, J.H. An In Vitro Model for the Ocular Surface and Tear Film System. Sci. Rep. 2017, 7, 6163. [Google Scholar] [CrossRef]
- Gleixner, S.; Zahn, I.; Dietrich, J.; Singh, S.; Drobny, A.; Schneider, Y.; Schwendner, R.; Socher, E.; Blavet, N.; Bräuer, L.; et al. A New Immortalized Human Lacrimal Gland Cell Line. Cells 2024, 13, 622. [Google Scholar] [CrossRef] [PubMed]
- Rodboon, T.; Yodmuang, S.; Chaisuparat, R.; Ferreira, J.N. Development of high-throughput lacrimal gland organoid platforms for drug discovery in dry eye disease. SLAS Discov. Adv. Life Sci. R. D 2022, 27, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Tsugeno, Y.; Sato, T.; Umetsu, A.; Nishikiori, N.; Furuhashi, M.; Ohguro, H. TGF-β Isoforms Affect the Planar and Subepithelial Fibrogenesis of Human Conjunctival Fibroblasts in Different Manners. Biomedicines 2023, 11, 2005. [Google Scholar] [CrossRef]
- Tsugeno, Y.; Furuhashi, M.; Sato, T.; Watanabe, M.; Umetsu, A.; Suzuki, S.; Ida, Y.; Hikage, F.; Ohguro, H. FGF-2 enhances fibrogenetic changes in TGF-β2 treated human conjunctival fibroblasts. Sci. Rep. 2022, 12, 16006. [Google Scholar] [CrossRef]
- Oouchi, Y.; Watanabe, M.; Ida, Y.; Ohguro, H.; Hikage, F. Rosiglitasone and ROCK Inhibitors Modulate Fibrogenetic Changes in TGF-β2 Treated Human Conjunctival Fibroblasts (HconF) in Different Manners. Int. J. Mol. Sci. 2021, 22, 7335. [Google Scholar] [CrossRef]
- Tsugeno, Y.; Sato, T.; Watanabe, M.; Higashide, M.; Furuhashi, M.; Umetsu, A.; Suzuki, S.; Ida, Y.; Hikage, F.; Ohguro, H. All Trans-Retinoic Acids Facilitate the Remodeling of 2D and 3D Cultured Human Conjunctival Fibroblasts. Bioengineering 2022, 9, 463. [Google Scholar] [CrossRef]
- Tsugeno, Y.; Sato, T.; Watanabe, M.; Furuhashi, M.; Ohguro, H. Prostanoid FP and EP2 Receptor Agonists Induce Epithelial and Subepithelial Fibrogenetic Changes in Human Conjunctival Fibroblasts in Different Manners. J. Ocul. Pharmacol. Ther. 2023, 39, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Q.; Yang, Y.; Guo, X.; Lian, R.; Li, S.; Wang, C.; Zhang, S.; Chen, J. The effects of ROCK inhibitor Y-27632 on injectable spheroids of bovine corneal endothelial cells. Cell. Reprogram. 2015, 17, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, Y.; Lei, H.; Zeng, Y.; Cui, Z.; Zeng, Q.; Zhu, D.; Lian, R.; Zhang, J.; Chen, Z.; et al. In vitro biomimetic platforms featuring a perfusion system and 3D spheroid culture promote the construction of tissue-engineered corneal endothelial layers. Sci. Rep. 2017, 7, 777. [Google Scholar] [CrossRef]
- Ida, Y.; Umetsu, A.; Furuhashi, M.; Watanabe, M.; Hikage, F.; Ohguro, H. The EP2 agonist, omidenepag, alters the physical stiffness of 3D spheroids prepared from human corneal stroma fibroblasts differently depending on the osmotic pressure. Faseb J. 2022, 36, e22067. [Google Scholar] [CrossRef]
- Ida, Y.; Umetsu, A.; Furuhashi, M.; Watanabe, M.; Tsugeno, Y.; Suzuki, S.; Hikage, F.; Ohguro, H. ROCK 1 and 2 affect the spatial architecture of 3D spheroids derived from human corneal stromal fibroblasts in different manners. Sci. Rep. 2022, 12, 7419. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Furuhashi, M.; Umetsu, A.; Hikage, F.; Watanabe, M.; Ohguro, H.; Ida, Y. Modulation of the Physical Properties of 3D Spheroids Derived from Human Scleral Stroma Fibroblasts (HSSFs) with Different Axial Lengths Obtained from Surgical Patients. Curr. Issues Mol. Biol. 2021, 43, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Wang, D.; Geng, A.; Seo, R.; Gong, X. Growth of hollow cell spheroids in microbead templated chambers. Biomaterials 2017, 143, 57–64. [Google Scholar] [CrossRef]
- Tirendi, S.; Saccà, S.C.; Vernazza, S.; Traverso, C.; Bassi, A.M.; Izzotti, A. A 3D Model of Human Trabecular Meshwork for the Research Study of Glaucoma. Front. Neurol. 2020, 11, 591776. [Google Scholar] [CrossRef]
- Buffault, J.; Brignole-Baudouin, F.; Labbé, A.; Baudouin, C. An Overview of Current Glaucomatous Trabecular Meshwork Models. Curr. Eye Res. 2023, 48, 1089–1099. [Google Scholar] [CrossRef]
- Torrejon, K.Y.; Papke, E.L.; Halman, J.R.; Bergkvist, M.; Danias, J.; Sharfstein, S.T.; Xie, Y. TGFβ2-induced outflow alterations in a bioengineered trabecular meshwork are offset by a rho-associated kinase inhibitor. Sci. Rep. 2016, 6, 38319. [Google Scholar] [CrossRef]
- Adhikari, B.; Osmond, M.J.; Pantcheva, M.B.; Krebs, M.D. Glycosaminoglycans Influence Extracellular Matrix of Human Trabecular Meshwork Cells Cultured on 3D Scaffolds. ACS Biomater. Sci. Eng. 2022, 8, 5221–5232. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Stinson, B.S.; Osmond, M.J.; Pantcheva, M.B.; Krebs, M.D. Photoinduced Gelatin-Methacrylate Scaffolds to Examine the Impact of Extracellular Environment on Trabecular Meshwork Cells. Ind. Eng. Chem. Res. 2021, 60, 17417–17428. [Google Scholar] [CrossRef] [PubMed]
- Vernazza, S.; Tirendi, S.; Scarfì, S.; Passalacqua, M.; Oddone, F.; Traverso, C.E.; Rizzato, I.; Bassi, A.M.; Saccà, S.C. 2D- and 3D-cultures of human trabecular meshwork cells: A preliminary assessment of an in vitro model for glaucoma study. PLoS ONE 2019, 14, e0221942. [Google Scholar] [CrossRef]
- Kumon, M.; Fuwa, M.; Shimazaki, A.; Odani-Kawabata, N.; Iwamura, R.; Yoneda, K.; Kato, M. Downregulation of COL12A1 and COL13A1 by a selective EP2 receptor agonist, omidenepag, in human trabecular meshwork cells. PLoS ONE 2023, 18, e0280331. [Google Scholar] [CrossRef]
- Watanabe, M.; Ida, Y.; Furuhashi, M.; Tsugeno, Y.; Ohguro, H.; Hikage, F. Screening of the Drug-Induced Effects of Prostaglandin EP2 and FP Agonists on 3D Cultures of Dexamethasone-Treated Human Trabecular Meshwork Cells. Biomedicines 2021, 9, 930. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Furuhashi, M.; Tsugeno, Y.; Umetsu, A.; Ida, Y.; Hikage, F.; Ohguro, H.; Watanabe, M. Comparison of the Drug-Induced Efficacies between Omidenepag Isopropyl, an EP2 Agonist and PGF2α toward TGF-β2-Modulated Human Trabecular Meshwork (HTM) Cells. J. Clin. Med. 2022, 11, 1652. [Google Scholar] [CrossRef]
- Kalouche, G.; Beguier, F.; Bakria, M.; Melik-Parsadaniantz, S.; Leriche, C.; Debeir, T.; Rostène, W.; Baudouin, C.; Vigé, X. Activation of Prostaglandin FP and EP2 Receptors Differently Modulates Myofibroblast Transition in a Model of Adult Primary Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1816–1825. [Google Scholar] [CrossRef]
- Watanabe, M.; Furuhashi, M.; Tsugeno, Y.; Ida, Y.; Hikage, F.; Ohguro, H. Autotaxin May Have Lysophosphatidic Acid-Unrelated Effects on Three-Dimension (3D) Cultured Human Trabecular Meshwork (HTM) Cells. Int. J. Mol. Sci. 2021, 22, 12039. [Google Scholar] [CrossRef]
- Bouchemi, M.; Roubeix, C.; Kessal, K.; Riancho, L.; Raveu, A.L.; Soualmia, H.; Baudouin, C.; Brignole-Baudouin, F. Effect of benzalkonium chloride on trabecular meshwork cells in a new in vitro 3D trabecular meshwork model for glaucoma. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2017, 41, 21–29. [Google Scholar] [CrossRef]
- Ota, C.; Ida, Y.; Ohguro, H.; Hikage, F. ROCK inhibitors beneficially alter the spatial configuration of TGFβ2-treated 3D organoids from a human trabecular meshwork (HTM). Sci. Rep. 2020, 10, 20292. [Google Scholar] [CrossRef]
- Watanabe, M.; Ida, Y.; Ohguro, H.; Ota, C.; Hikage, F. Diverse effects of pan-ROCK and ROCK2 inhibitors on 2 D and 3D cultured human trabecular meshwork (HTM) cells treated with TGFβ2. Sci. Rep. 2021, 11, 15286. [Google Scholar] [CrossRef]
- Buffault, J.; Brignole-Baudouin, F.; Reboussin, É.; Kessal, K.; Labbé, A.; Mélik Parsadaniantz, S.; Baudouin, C. The Dual Effect of Rho-Kinase Inhibition on Trabecular Meshwork Cells Cytoskeleton and Extracellular Matrix in an In Vitro Model of Glaucoma. J. Clin. Med. 2022, 11, 1001. [Google Scholar] [CrossRef]
- Watanabe, M.; Ida, Y.; Furuhashi, M.; Tsugeno, Y.; Hikage, F.; Ohguro, H. Pan-ROCK and ROCK2 Inhibitors Affect Dexamethasone-Treated 2D- and 3D-Cultured Human Trabecular Meshwork (HTM) Cells in Opposite Manners. Molecules 2021, 26, 6382. [Google Scholar] [CrossRef]
- Watanabe, M.; Sato, T.; Tsugeno, Y.; Higashide, M.; Furuhashi, M.; Umetsu, A.; Suzuki, S.; Ida, Y.; Hikage, F.; Ohguro, H. An α2-Adrenergic Agonist, Brimonidine, Beneficially Affects the TGF-β2-Treated Cellular Properties in an In Vitro Culture Model. Bioengineering 2022, 9, 310. [Google Scholar] [CrossRef]
- Zhang, Y.; Tseng, S.C.G.; Zhu, Y.T. Suppression of TGF-β1 signaling by Matrigel via FAK signaling in cultured human trabecular meshwork cells. Sci. Rep. 2021, 11, 7319. [Google Scholar] [CrossRef]
- Han, H.; Kampik, D.; Grehn, F.; Schlunck, G. TGF-β2-induced invadosomes in human trabecular meshwork cells. PLoS ONE 2013, 8, e70595. [Google Scholar] [CrossRef]
- Watanabe, M.; Sato, T.; Tsugeno, Y.; Higashide, M.; Furuhashi, M.; Ohguro, H. TGF-β-3 Induces Different Effects from TGF-β-1 and -2 on Cellular Metabolism and the Spatial Properties of the Human Trabecular Meshwork Cells. Int. J. Mol. Sci. 2023, 24, 4181. [Google Scholar] [CrossRef]
- Zhou, M.; Geathers, J.S.; Grillo, S.L.; Weber, S.R.; Wang, W.; Zhao, Y.; Sundstrom, J.M. Role of Epithelial-Mesenchymal Transition in Retinal Pigment Epithelium Dysfunction. Front. Cell Dev. Biol. 2020, 8, 501. [Google Scholar] [CrossRef]
- Raimondi, R.; Zollet, P.; De Rosa, F.P.; Tsoutsanis, P.; Stravalaci, M.; Paulis, M.; Inforzato, A.; Romano, M.R. Where Are We with RPE Replacement Therapy? A Translational Review from the Ophthalmologist Perspective. Int. J. Mol. Sci. 2022, 23, 682. [Google Scholar] [CrossRef]
- Blenkinsop, T.A.; Saini, J.S.; Maminishkis, A.; Bharti, K.; Wan, Q.; Banzon, T.; Lotfi, M.; Davis, J.; Singh, D.; Rizzolo, L.J.; et al. Human Adult Retinal Pigment Epithelial Stem Cell-Derived RPE Monolayers Exhibit Key Physiological Characteristics of Native Tissue. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7085–7099. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med. 2018, 10, eaao4097. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef]
- Hunt, N.C.; Hallam, D.; Karimi, A.; Mellough, C.B.; Chen, J.; Steel, D.H.W.; Lako, M. 3D culture of human pluripotent stem cells in RGD-alginate hydrogel improves retinal tissue development. Acta Biomater. 2017, 49, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Xie, M.; Gademann, F.; Cao, J.; Wang, P.; Guo, Y.; Zhang, L.; Su, T.; Zhang, J.; Chen, J. Protective effects of human iPS-derived retinal pigmented epithelial cells on retinal degenerative disease. Stem Cell Res. Ther. 2020, 11, 98. [Google Scholar] [CrossRef]
- Kuwahara, A.; Nakano, T.; Eiraku, M. Generation of a Three-Dimensional Retinal Tissue from Self-Organizing Human ESC Culture. Methods Mol. Biol. 2017, 1597, 17–29. [Google Scholar]
- Shokoohmand, A.; Jeon, J.E.; Theodoropoulos, C.; Baldwin, J.G.; Hutmacher, D.W.; Feigl, B. A Novel 3D Cultured Model for Studying Early Changes in Age-Related Macular Degeneration. Macromol. Biosci. 2017, 17, 1700221. [Google Scholar] [CrossRef]
- Tian, Y.; Zonca, M.R., Jr.; Imbrogno, J.; Unser, A.M.; Sfakis, L.; Temple, S.; Belfort, G.; Xie, Y. Polarized, Cobblestone, Human Retinal Pigment Epithelial Cell Maturation on a Synthetic PEG Matrix. ACS Biomater. Sci. Eng. 2017, 3, 890–902. [Google Scholar] [CrossRef]
- Isla-Magrané, H.; Veiga, A.; García-Arumí, J.; Duarri, A. Multiocular organoids from human induced pluripotent stem cells displayed retinal, corneal, and retinal pigment epithelium lineages. Stem Cell Res. Ther. 2021, 12, 581. [Google Scholar] [CrossRef]
- Stahl, A.; Paschek, L.; Martin, G.; Feltgen, N.; Hansen, L.L.; Agostini, H.T. Combinatory inhibition of VEGF and FGF2 is superior to solitary VEGF inhibition in an in vitro model of RPE-induced angiogenesis. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von. Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2009, 247, 767–773. [Google Scholar] [CrossRef]
- Suzuki, S.; Sato, T.; Watanabe, M.; Higashide, M.; Tsugeno, Y.; Umetsu, A.; Furuhashi, M.; Ida, Y.; Hikage, F.; Ohguro, H. Hypoxia Differently Affects TGF-β2-Induced Epithelial Mesenchymal Transitions in the 2D and 3D Culture of the Human Retinal Pigment Epithelium Cells. Int. J. Mol. Sci. 2022, 23, 5473. [Google Scholar] [CrossRef]
- Ida, Y.; Sato, T.; Watanabe, M.; Umetsu, A.; Tsugeno, Y.; Furuhashi, M.; Hikage, F.; Ohguro, H. The Selective α1 Antagonist Tamsulosin Alters ECM Distributions and Cellular Metabolic Functions of ARPE 19 Cells in a Concentration-Dependent Manner. Bioengineering 2022, 9, 556. [Google Scholar] [CrossRef]
- Veernala, I.; Jaffet, J.; Fried, J.; Mertsch, S.; Schrader, S.; Basu, S.; Vemuganti, G.K.; Singh, V. Lacrimal gland regeneration: The unmet challenges and promise for dry eye therapy. Ocul. Surf. 2022, 25, 129–141. [Google Scholar] [CrossRef]
- Bannier-Hélaouët, M.; Post, Y.; Korving, J.; Trani Bustos, M.; Gehart, H.; Begthel, H.; Bar-Ephraim, Y.E.; van der Vaart, J.; Kalmann, R.; Imhoff, S.M.; et al. Exploring the human lacrimal gland using organoids and single-cell sequencing. Cell Stem Cell 2021, 28, 1221–1232.e7. [Google Scholar] [CrossRef]
- Hayashi, R.; Okubo, T.; Kudo, Y.; Ishikawa, Y.; Imaizumi, T.; Suzuki, K.; Shibata, S.; Katayama, T.; Park, S.J.; Young, R.D.; et al. Generation of 3D lacrimal gland organoids from human pluripotent stem cells. Nature 2022, 605, 126–131. [Google Scholar] [CrossRef]
- Chen, H.; Huang, P.; Zhang, Y. Three-Dimensional, Serum-Free Culture System for Lacrimal Gland Stem Cells. J. Vis. Exp. 2022, 184, e63585. [Google Scholar]
- Jaffet, J.; Mohanty, A.; Veernala, I.; Singh, S.; Ali, M.J.; Basu, S.; Vemuganti, G.K.; Singh, V. Human Lacrimal Gland Derived Mesenchymal Stem Cells—Isolation, Propagation, and Characterization. Invest. Ophthalmol. Vis. Sci. 2023, 64, 12. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Y. Establishment of long-term serum-free culture for lacrimal gland stem cells aiming at lacrimal gland repair. Stem Cell Res. Ther. 2020, 11, 20. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Choi, W.H.; Jeon, S.G.; Lee, S.; Park, J.M.; Park, M.; Lee, H.; Lew, H.; Yoo, J. Establishment of functional epithelial organoids from human lacrimal glands. Stem Cell Res. Ther. 2021, 12, 247. [Google Scholar] [CrossRef]
- Massie, I.; Spaniol, K.; Barbian, A.; Geerling, G.; Metzger, M.; Schrader, S. Development of lacrimal gland spheroids for lacrimal gland tissue regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e2001–e2009. [Google Scholar] [CrossRef]
- Li, H.; Fitchett, C.; Kozdon, K.; Jayaram, H.; Rose, G.E.; Bailly, M.; Ezra, D.G. Independent adipogenic and contractile properties of fibroblasts in Graves’ orbitopathy: An in vitro model for the evaluation of treatments. PLoS ONE 2014, 9, e95586. [Google Scholar] [CrossRef]
- Hikage, F.; Ichioka, H.; Watanabe, M.; Umetsu, A.; Ohguro, H.; Ida, Y. Addition of ROCK inhibitors to prostaglandin derivative (PG) synergistically affects adipogenesis of the 3D spheroids of human orbital fibroblasts (HOFs). Hum. Cell 2022, 35, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ichioka, H.; Ida, Y.; Watanabe, M.; Ohguro, H.; Hikage, F. Prostaglandin F2α and EP2 agonists, and a ROCK inhibitor modulate the formation of 3D organoids of Grave’s orbitopathy related human orbital fibroblasts. Exp. Eye Res. 2021, 205, 108489. [Google Scholar] [CrossRef] [PubMed]
- Ida, Y.; Ichioka, H.; Furuhashi, M.; Hikage, F.; Watanabe, M.; Umetsu, A.; Ohguro, H. Reactivities of a Prostanoid EP2 Agonist, Omidenepag, Are Useful for Distinguishing between 3D Spheroids of Human Orbital Fibroblasts without or with Graves’ Orbitopathy. Cells 2021, 10, 3196. [Google Scholar] [CrossRef]
- Hikage, F.; Ichioka, H.; Watanabe, M.; Umetsu, A.; Ohguro, H.; Ida, Y. ROCK inhibitors modulate the physical properties and adipogenesis of 3D spheroids of human orbital fibroblasts in different manners. FASEB Bioadv. 2021, 3, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Ida, Y.; Ohguro, H.; Hikage, F. Prostaglandin F2α agonists induced enhancement in collagen1 expression is involved in the pathogenesis of the deepening of upper eyelid sulcus. Sci. Rep. 2021, 11, 9002. [Google Scholar] [CrossRef] [PubMed]
- Hikage, F.; Watanabe, M.; Sato, T.; Umetsu, A.; Tsugeno, Y.; Furuhashi, M.; Ohguro, H. Simultaneous Effects of a Selective EP2 Agonist, Omidenepag, and a Rho-Associated Coiled-Coil Containing Protein Kinase Inhibitor, Ripasudil, on Human Orbital Fibroblasts. J. Ocul. Pharmacol. Ther. 2023, 39, 439–448. [Google Scholar] [CrossRef]
- Hikage, F.; Ida, Y.; Ouchi, Y.; Watanabe, M.; Ohguro, H. Omidenepag, a Selective, Prostanoid EP2 Agonist, Does Not Suppress Adipogenesis in 3D Organoids of Human Orbital Fibroblasts. Transl. Vis. Sci. Technol. 2021, 10, 6. [Google Scholar] [CrossRef]
- Krieger, C.C.; Perry, J.D.; Morgan, S.J.; Kahaly, G.J.; Gershengorn, M.C. TSH/IGF-1 Receptor Cross-Talk Rapidly Activates Extracellular Signal-Regulated Kinases in Multiple Cell Types. Endocrinology 2017, 158, 3676–3683. [Google Scholar] [CrossRef]
- Dik, W.A.; Virakul, S.; van Steensel, L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Exp. Eye Res. 2016, 142, 83–91. [Google Scholar] [CrossRef]
- Turcu, A.F.; Kumar, S.; Neumann, S.; Coenen, M.; Iyer, S.; Chiriboga, P.; Gershengorn, M.C.; Bahn, R.S. A small molecule antagonist inhibits thyrotropin receptor antibody-induced orbital fibroblast functions involved in the pathogenesis of Graves ophthalmopathy. J. Clin. Endocrinol. Metab. 2013, 98, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J. TSH-receptor-expressing fibrocytes and thyroid-associated ophthalmopathy. Nat. Rev. Endocrinol. 2015, 11, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Ida, Y.; Sato, T.; Umetsu, A.; Watanabe, M.; Furuhashi, M.; Hikage, F.; Ohguro, H. Addition of ROCK Inhibitors Alleviates Prostaglandin-Induced Inhibition of Adipogenesis in 3T3L-1 Spheroids. Bioengineering 2022, 9, 702. [Google Scholar] [CrossRef]
- Ida, Y.; Furuhashi, M.; Watanabe, M.; Umetsu, A.; Hikage, F.; Ohguro, H. Prostaglandin F2 and EP2 Agonists Exert Different Effects on 3D 3T3-L1 Spheroids during Their Culture Phase. Biomedicines 2021, 9, 1821. [Google Scholar] [CrossRef]
- Umetsu, A.; Ida, Y.; Sato, T.; Watanabe, M.; Tsugeno, Y.; Furuhashi, M.; Hikage, F.; Ohguro, H. Brimonidine Modulates the ROCK1 Signaling Effects on Adipogenic Differentiation in 2D and 3D 3T3-L1 Cells. Biomedicines 2022, 9, 327. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef]
- Butelmann, T.; Gu, Y.; Li, A.; Tribukait-Riemenschneider, F.; Hoffmann, J.; Molazem, A.; Jaeger, E.; Pellegrini, D.; Forget, A.; Shastri, V.P. 3D Printed Solutions for Spheroid Engineering and Cancer Research. Int. J. Mol. Sci. 2022, 23, 8188. [Google Scholar] [CrossRef]
- Zhao, L.; Xiu, J.; Liu, Y.; Zhang, T.; Pan, W.; Zheng, X.; Zhang, X. A 3D Printed Hanging Drop Dripper for Tumor Spheroids Analysis Without Recovery. Sci. Rep. 2019, 9, 19717. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.L.F.; Miranda, M.; Marcato, P.D.; Swiech, K. Comparative Analysis of 3D Bladder Tumor Spheroids Obtained by Forced Floating and Hanging Drop Methods for Drug Screening. Front. Physiol. 2017, 8, 605. [Google Scholar] [CrossRef]
- Shi, H.; Rath, E.M.; Lin, R.C.Y.; Sarun, K.H.; Clarke, C.J.; McCaughan, B.C.; Ke, H.; Linton, A.; Lee, K.; Klebe, S.; et al. 3-Dimensional mesothelioma spheroids provide closer to natural pathophysiological tumor microenvironment for drug response studies. Front. Oncol. 2022, 12, 973576. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, A.; Mostafa, A.; Saavedra, C.; Kim, Y.; Le, P.; Faramarzi, V.; Feathers, R.W.; Berger, J.; Ramos-Cruz, K.P.; Adeniba, O.; et al. Three-dimensional microscale hanging drop arrays with geometric control for drug screening and live tissue imaging. Sci. Adv. 2021, 7, eabc1323. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.A.; Fisher, D.E. The melanoma revolution: From UV carcinogenesis to a new era in therapeutics. Science 2014, 346, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef]
- Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Antonescu, C.R.; Wolchok, J.D.; Chapman, P.B.; Roman, R.A.; Teitcher, J.; Panageas, K.S.; Busam, K.J.; Chmielowski, B.; Lutzky, J.; et al. KIT as a therapeutic target in metastatic melanoma. Jama 2011, 305, 2327–2334. [Google Scholar] [CrossRef]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef]
- Wang, F.; Weaver, V.M.; Petersen, O.W.; Larabell, C.A.; Dedhar, S.; Briand, P.; Lupu, R.; Bissell, M.J. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: A different perspective in epithelial biology. Proc. Natl. Acad. Sci. USA 1998, 95, 14821–14826. [Google Scholar] [CrossRef]
- Beaumont, K.A.; Mohana-Kumaran, N.; Haass, N.K. Modeling Melanoma In Vitro and In Vivo. Healthcare 2013, 2, 27–46. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.; Busam, K.J. Spitz melanocytic tumours—A review. Histopathology 2022, 80, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Haass, N.K. Microenvironment-Driven Dynamic Heterogeneity and Phenotypic Plasticity as a Mechanism of Melanoma Therapy Resistance. Front. Oncol. 2018, 8, 173. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA A Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.J.; Prasad, G.; Farah, C.S. Oral mucosal malignancy and potentially malignant lesions: An update on the epidemiology, risk factors, diagnosis and management. Aust. Dent. J. 2010, 55 (Suppl. S1), 61–65. [Google Scholar] [CrossRef]
- Farah, C.S.; Woo, S.B.; Zain, R.B.; Sklavounou, A.; McCullough, M.J.; Lingen, M. Oral cancer and oral potentially malignant disorders. Int. J. Dent. 2014, 2014, 853479. [Google Scholar] [CrossRef]
- Abrahão, R.; Anantharaman, D.; Gaborieau, V.; Abedi-Ardekani, B.; Lagiou, P.; Lagiou, A.; Ahrens, W.; Holcatova, I.; Betka, J.; Merletti, F.; et al. The influence of smoking, age and stage at diagnosis on the survival after larynx, hypopharynx and oral cavity cancers in Europe: The ARCAGE study. Int. J. Cancer 2018, 143, 32–44. [Google Scholar] [CrossRef]
- Chitturi Suryaprakash, R.T.; Kujan, O.; Shearston, K.; Farah, C.S. Three-Dimensional Cell Culture Models to Investigate Oral Carcinogenesis: A Scoping Review. Int. J. Mol. Sci. 2020, 21, 9520. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Miki, Y.; Ono, K.; Hata, S.; Suzuki, T.; Kumamoto, H.; Sasano, H. The advantages of co-culture over mono cell culture in simulating in vivo environment. J. Steroid Biochem. Mol. Biol. 2012, 131, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Soon, R.H.; Lim, C.T.; Khoo, B.L.; Han, J. Microfluidic modelling of the tumor microenvironment for anti-cancer drug development. Lab. A Chip 2019, 19, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef]
- Zanoni, M.; Pignatta, S.; Arienti, C.; Bonafè, M.; Tesei, A. Anticancer drug discovery using multicellular tumor spheroid models. Expert Opin. Drug Discov. 2019, 14, 289–301. [Google Scholar] [CrossRef]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kang, S.H.; Oh, S.Y.; Lee, K.Y.; Lee, H.J.; Gum, S.; Kwon, T.G.; Kim, J.W.; Lee, S.T.; Hong, Y.J.; et al. Differential Angiogenic Potential of 3-Dimension Spheroid of HNSCC Cells in Mouse Xenograft. Int. J. Mol. Sci. 2021, 22, 8245. [Google Scholar] [CrossRef]
- Di Modugno, F.; Colosi, C.; Trono, P.; Antonacci, G.; Ruocco, G.; Nisticò, P. 3D models in the new era of immune oncology: Focus on T cells, CAF and ECM. J. Exp. Clin. Cancer Res. CR 2019, 38, 117. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Parsonage, G.; Filer, A.D.; Haworth, O.; Nash, G.B.; Rainger, G.E.; Salmon, M.; Buckley, C.D. A stromal address code defined by fibroblasts. Trends Immunol. 2005, 26, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M.; Fujiwara, H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 2011, 3, a005124. [Google Scholar] [CrossRef]
- Lynch, M.D.; Watt, F.M. Fibroblast heterogeneity: Implications for human disease. J. Clin. Investig. 2018, 128, 26–35. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Lavie, D.; Ben-Shmuel, A.; Erez, N.; Scherz-Shouval, R. Cancer-associated fibroblasts in the single-cell era. Nat. Cancer 2022, 3, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kasashima, H.; Fukui, Y.; Tsujio, G.; Yashiro, M.; Maeda, K. The heterogeneity of cancer-associated fibroblast subpopulations: Their origins, biomarkers, and roles in the tumor microenvironment. Cancer Sci. 2023, 114, 16–24. [Google Scholar] [CrossRef]
- Kanzaki, R.; Pietras, K. Heterogeneity of cancer-associated fibroblasts: Opportunities for precision medicine. Cancer Sci. 2020, 111, 2708–2717. [Google Scholar] [CrossRef]
- Kehrberg, R.J.; Bhyravbhatla, N.; Batra, S.K.; Kumar, S. Epigenetic regulation of cancer-associated fibroblast heterogeneity. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188901. [Google Scholar] [CrossRef]
- Nurmik, M.; Ullmann, P.; Rodriguez, F.; Haan, S.; Letellier, E. In search of definitions: Cancer-associated fibroblasts and their markers. Int. J. Cancer 2020, 146, 895–905. [Google Scholar] [CrossRef]
| Methods | Place to Generate 3D Spheroid | Effects of Gravity Force | Effects of Buoyant Force | Contribution of Additive Factors | |
|---|---|---|---|---|---|
| scaffold-assisted | |||||
| hydrogel-assisted culture | on the ground | less | low | hydrogels | |
| biofilm-assisted culture | on the ground | less | low | biofilms | |
| particle-assisted culture | on the ground | less | low | particles | |
| magnet particle-assisted culture | above the ground | moderate | strong | magnetic particles, magnetic force | |
| scaffold non-assisted | |||||
| static suspension culture | on the ground | less | low | none | |
| floating culture | above the ground | moderate | strong | centrifugal force | |
| hanging drop culture | under the ground | strong | moderate | none |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohguro, H.; Watanabe, M.; Sato, T.; Nishikiori, N.; Umetsu, A.; Higashide, M.; Yano, T.; Suzuki, H.; Miyazaki, A.; Takada, K.; et al. Application of Single Cell Type-Derived Spheroids Generated by Using a Hanging Drop Culture Technique in Various In Vitro Disease Models: A Narrow Review. Cells 2024, 13, 1549. https://doi.org/10.3390/cells13181549
Ohguro H, Watanabe M, Sato T, Nishikiori N, Umetsu A, Higashide M, Yano T, Suzuki H, Miyazaki A, Takada K, et al. Application of Single Cell Type-Derived Spheroids Generated by Using a Hanging Drop Culture Technique in Various In Vitro Disease Models: A Narrow Review. Cells. 2024; 13(18):1549. https://doi.org/10.3390/cells13181549
Chicago/Turabian StyleOhguro, Hiroshi, Megumi Watanabe, Tatsuya Sato, Nami Nishikiori, Araya Umetsu, Megumi Higashide, Toshiyuki Yano, Hiromu Suzuki, Akihiro Miyazaki, Kohichi Takada, and et al. 2024. "Application of Single Cell Type-Derived Spheroids Generated by Using a Hanging Drop Culture Technique in Various In Vitro Disease Models: A Narrow Review" Cells 13, no. 18: 1549. https://doi.org/10.3390/cells13181549








