Comparison of Tumor Microenvironments between Primary Tumors and Lymph Node Metastases in Head and Neck Squamous Cell Carcinoma and Their Predictive Role in Immune Checkpoint Inhibitor Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Procedures
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
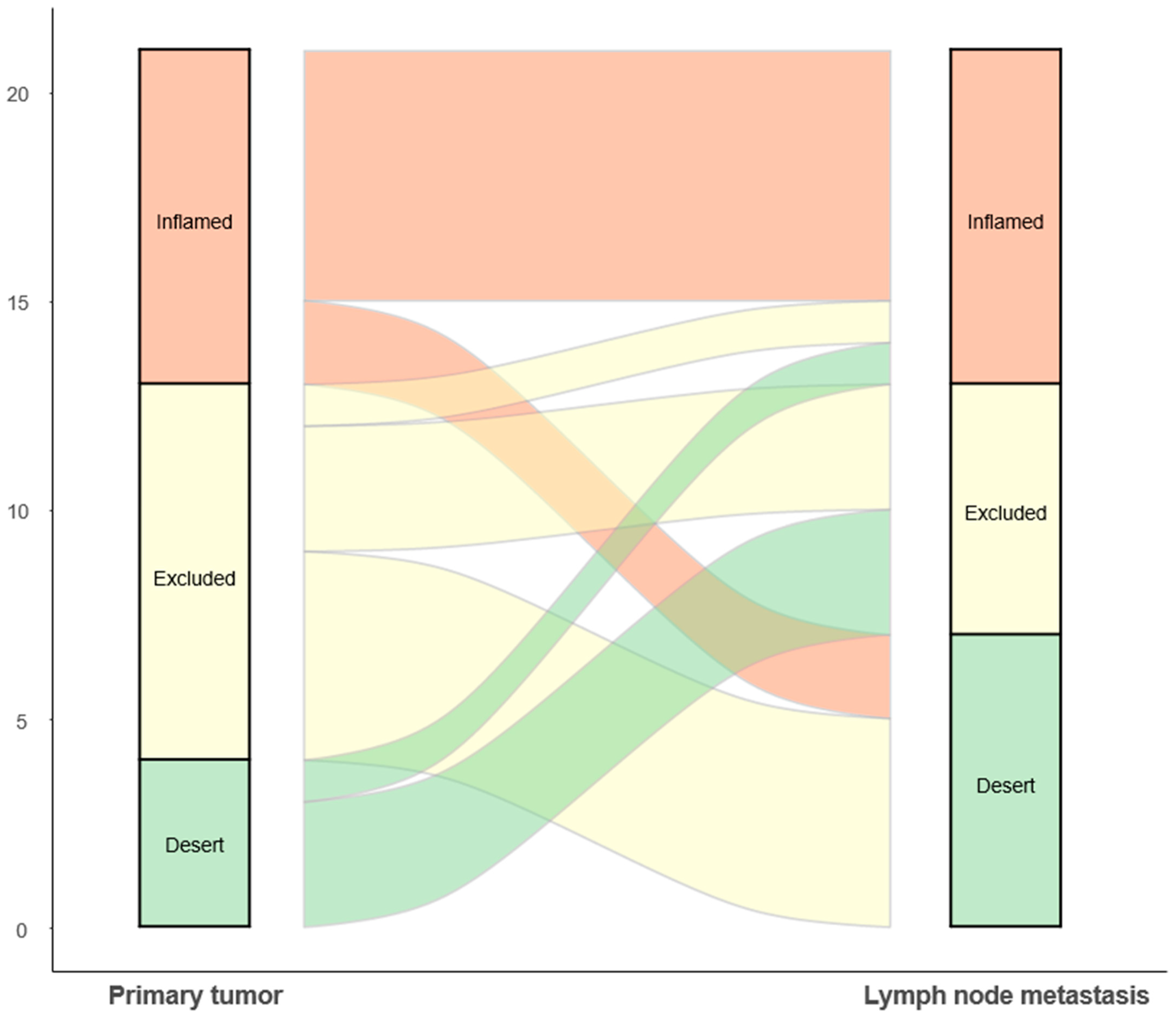
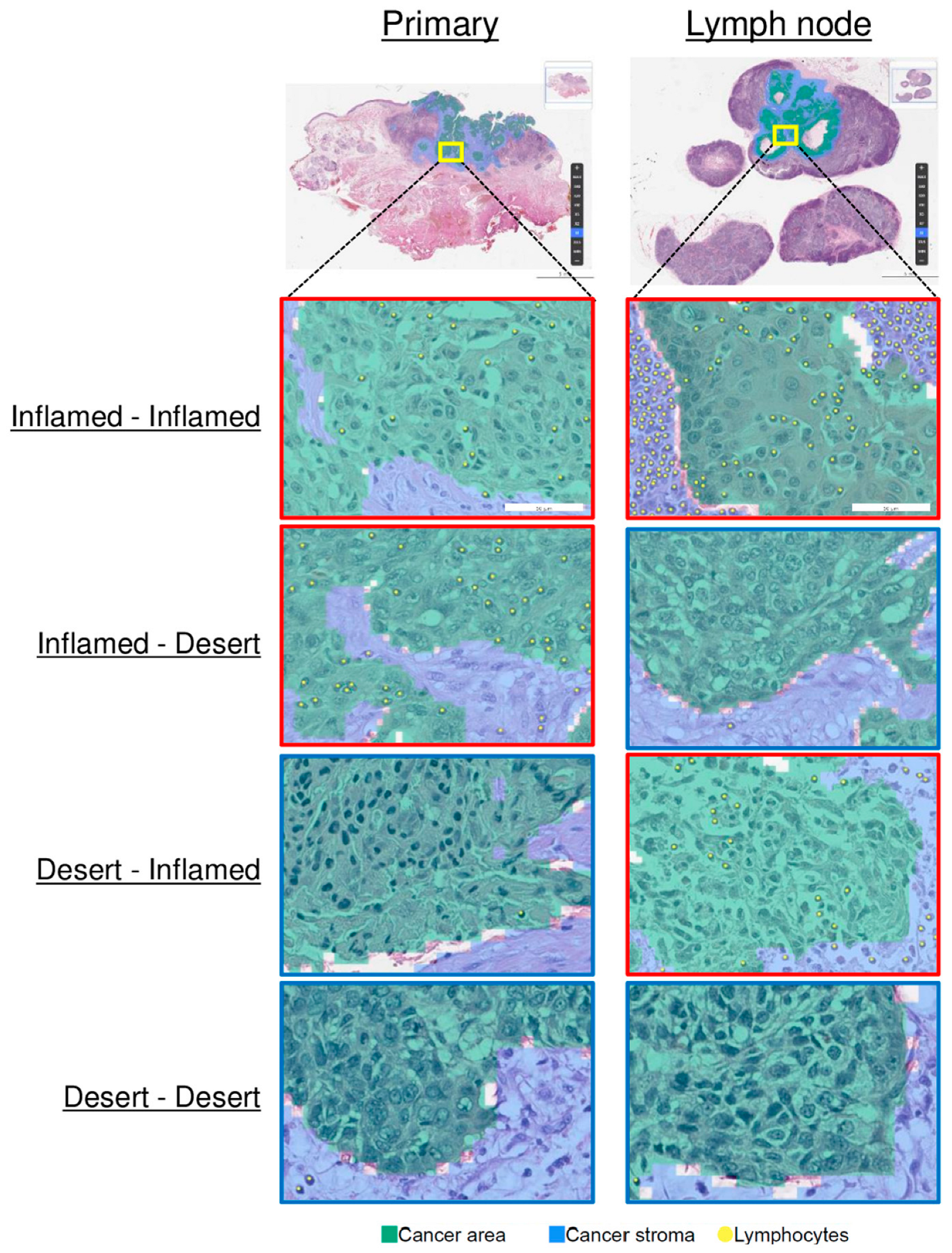
3.2. Comparison of the TMEs of Primary Tumors and LNMs
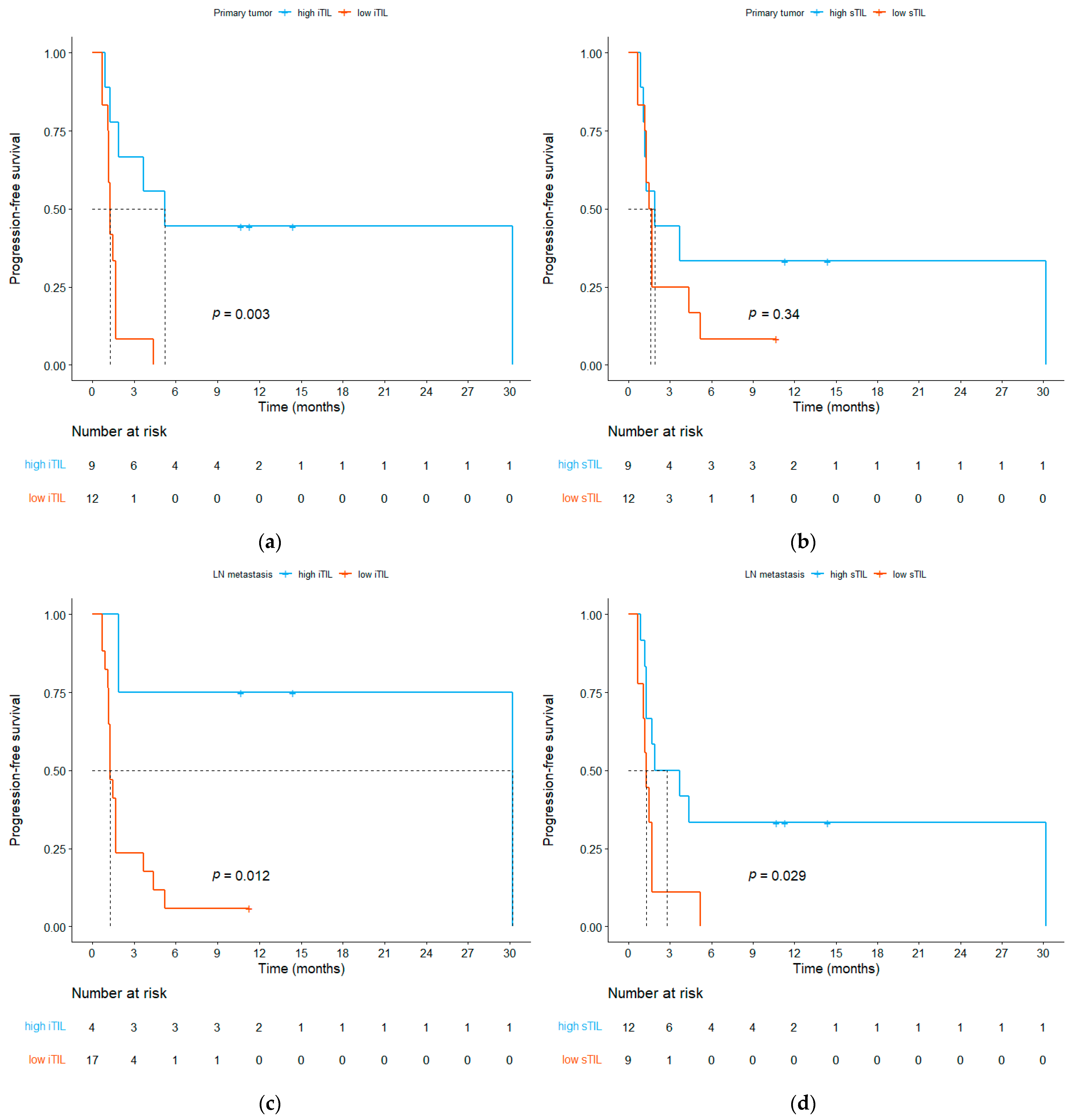
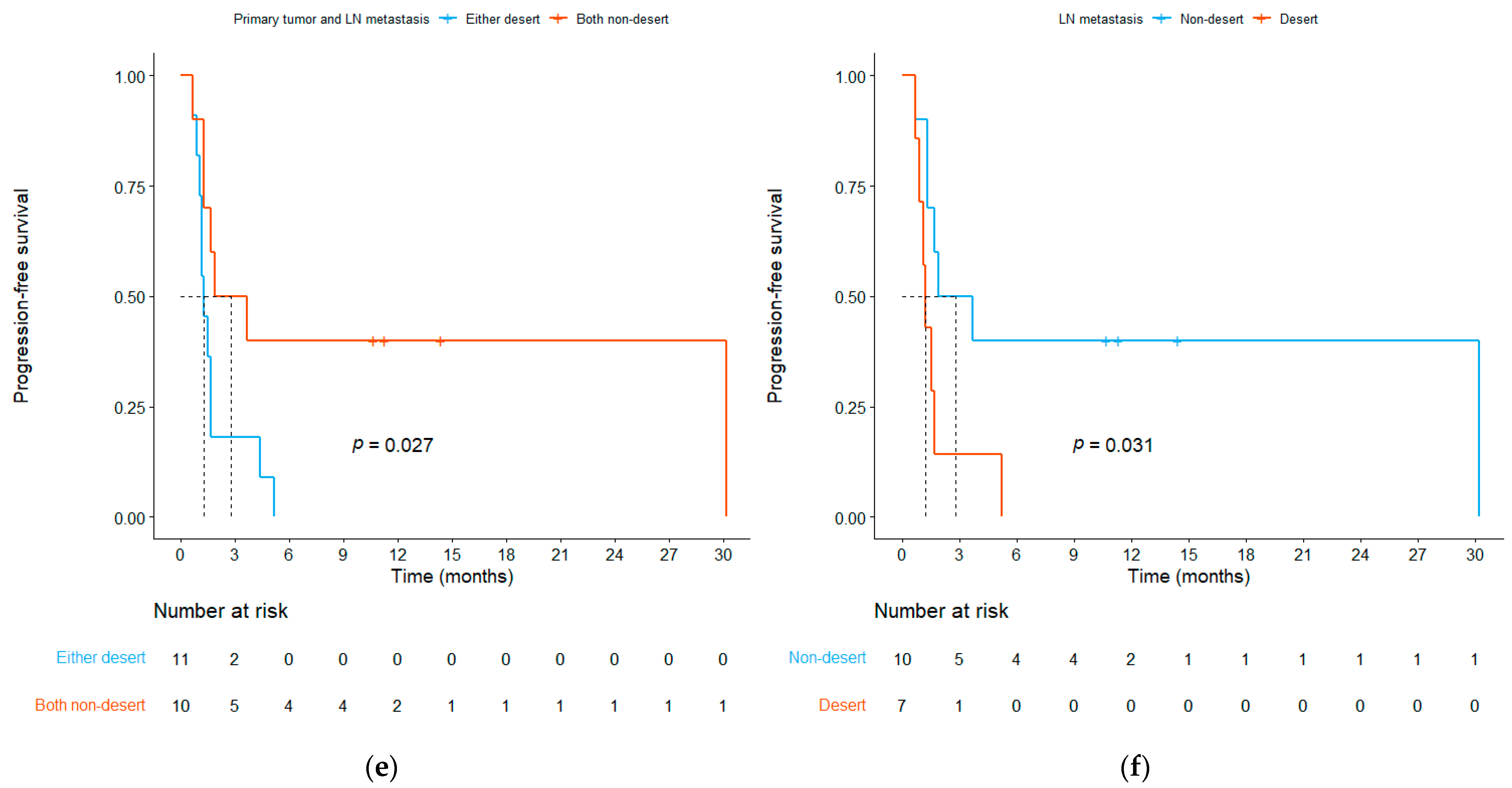
3.3. Relationship between ICI Efficacy and the TME
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marur, S.; Forastiere, A.A. Head and neck squamous cell carcinoma: Update on epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J.; Ferris, R.L.; Blumenschein, G.; Colevas, A.D.; Fayette, J.; Licitra, L.; Kasper, S.; Even, C.; Vokes, E.E.; Worden, F.; et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Bauml, J.; Seiwert, T.Y.; Pfister, D.G.; Worden, F.; Liu, S.V.; Gilbert, J.; Saba, N.F.; Weiss, J.; Wirth, L.; Sukari, A.; et al. Pembrolizumab for platinum-and cetuximab-refractory head and neck cancer: Results from a single-arm, phase II study. J. Clin. Oncol. 2017, 35, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.; Haddad, R.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.; Clement, P.M.; Mesia, R.; Kutukova, S.; Zholudeva, L.; et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann. Oncol. 2020, 31, 942–950. [Google Scholar] [CrossRef]
- Poulose, J.V.; Kainickal, C.T. Immune checkpoint inhibitors in head and neck squamous cell carcinoma: A systematic review of phase-3 clinical trials. World J. Clin. Oncol. 2022, 13, 388–411. [Google Scholar] [CrossRef]
- Park, J.H.; Chun, S.H.; Lee, Y.-G.; Chang, H.; Lee, K.-W.; Kim, H.R.; Shin, S.H.; An, H.J.; Lee, K.E.; Hwang, I.G.; et al. Hyperprogressive disease and its clinical impact in patients with recurrent and/or metastatic head and neck squamous cell carcinoma treated with immune-checkpoint inhibitors: Korean cancer study group HN 18–12. J. Cancer Res. Clin. Oncol. 2020, 146, 3359–3369. [Google Scholar] [CrossRef]
- Wang, H.-C.; Yeh, T.-J.; Chan, L.-P.; Hsu, C.-M.; Cho, S.-F. Exploration of feasible immune biomarkers for immune checkpoint inhibitors in head and neck squamous cell carcinoma treatment in real world clinical practice. Int. J. Mol. Sci. 2020, 21, 7621. [Google Scholar] [CrossRef]
- Rasmussen, J.H.; Lelkaitis, G.; Håkansson, K.; Vogelius, I.R.; Johannesen, H.H.; Fischer, B.M.; Bentzen, S.M.; Specht, L.; Kristensen, C.A.; von Buchwald, C.; et al. Intratumor heterogeneity of PD-L1 expression in head and neck squamous cell carcinoma. Br. J. Cancer 2019, 120, 1003–1006. [Google Scholar] [CrossRef]
- Oliva, M.; Spreafico, A.; Taberna, M.; Alemany, L.; Coburn, B.; Mesia, R.; Siu, L.L. Immune biomarkers of response to immune-checkpoint inhibitors in head and neck squamous cell carcinoma. Ann. Oncol. 2019, 30, 57–67. [Google Scholar] [CrossRef]
- Park, J.C.; Krishnakumar, H.N.; Saladi, S.V. Current and future biomarkers for immune checkpoint inhibitors in head and neck squamous cell carcinoma. Curr. Oncol. 2022, 29, 4185–4198. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Shen, J.; Lee, T.; Hwang, J.; Choi, Y.; Lee, S.; Kim, H.; Chung, J.; Bogdan, S.; Huang, M.; Raclin, T.; et al. Artificial intelligence-powered spatial analysis of tumor-infiltrating lymphocytes predicts survival after immune checkpoint inhibitor therapy across multiple cancer types. J. Clin. Oncol. 2021, 39, 2607. [Google Scholar] [CrossRef]
- Jung, H.A.; Park, K.-U.; Cho, S.; Lim, J.; Lee, K.-W.; Hong, M.H.; Yun, T.; An, H.J.; Park, W.-Y.; Pereira, S.; et al. A Phase II Study of Nivolumab plus Gemcitabine in Patients with Recurrent or Metastatic Nasopharyngeal Carcinoma (KCSG HN17–11). Clin. Cancer Res. 2022, 28, 4240–4247. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ock, C.-Y.; Kim, H.; Pereira, S.; Park, S.; Ma, M.; Choi, S.; Kim, S.; Shin, S.; Aum, B.J.; et al. Artificial intelligence–powered spatial analysis of tumor-infiltrating lymphocytes as complementary biomarker for immune checkpoint inhibition in non–small-cell lung cancer. J. Clin. Oncol. 2022, 40, 1916–1928. [Google Scholar] [CrossRef]
- Cho, J.-K.; Hyun, S.H.; Choi, N.; Kim, M.-J.; Padera, T.P.; Choi, J.Y.; Jeong, H.-S. Significance of lymph node metastasis in cancer dissemination of head and neck cancer. Transl. Oncol. 2015, 8, 119–125. [Google Scholar] [CrossRef]
- Paolino, G.; Pantanowitz, L.; Barresi, V.; Pagni, F.; Munari, E.; Moretta, L.; Brunelli, M.; Bariani, E.; Vigliar, E.; Pisapia, P.; et al. PD-L1 evaluation in head and neck squamous cell carcinoma: Insights regarding specimens, heterogeneity and therapy. Pathol.-Res. Pract. 2021, 226, 153605. [Google Scholar] [CrossRef]
- Ikemura, S.; Aramaki, N.; Fujii, S.; Kirita, K.; Umemura, S.; Matsumoto, S.; Yoh, K.; Niho, S.; Ohmatsu, H.; Kuwata, T.; et al. Changes in the tumor microenvironment during lymphatic metastasis of lung squamous cell carcinoma. Cancer Sci. 2017, 108, 136–142. [Google Scholar] [CrossRef]
- Paulsen, E.-E.; Kilvaer, T.K.; Rakaee, M.; Richardsen, E.; Hald, S.M.; Andersen, S.; Busund, L.-T.; Bremnes, R.M.; Donnem, T. CTLA-4 expression in the non-small cell lung cancer patient tumor microenvironment: Diverging prognostic impact in primary tumors and lymph node metastases. Cancer Immunol. Immunother. 2017, 66, 1449–1461. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lim, Y.; Kim, S.; Ock, C.Y.; Youk, J.; Kim, M.; Kim, T.M.; Kim, D.W.; Kim, H.J.; Koh, J.; et al. Artificial intelligence-powered spatial analysis of tumor-infiltrating lymphocytes as a biomarker in locally advanced unresectable thymic epithelial neoplasm: A single-center, retrospective, longitudinal cohort study. Thorac. Cancer 2023, 14, 3001–3011. [Google Scholar] [CrossRef] [PubMed]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-C.; Wu, C.-T.; Wang, C.-P.; Hsu, W.-L.; Yang, T.-L.; Lou, P.-J.; Ko, J.-Y.; Chang, Y.-L. Associations among pretreatment tumor necrosis and the expression of HIF-1α and PD-L1 in advanced oral squamous cell carcinoma and the prognostic impact thereof. Oral Oncol. 2015, 51, 1004–1010. [Google Scholar] [CrossRef]
- Scognamiglio, T.; Chen, Y.-T. Beyond the percentages of PD-L1-positive tumor cells: Induced versus constitutive PD-L1 expression in primary and metastatic head and neck squamous cell carcinoma. Head Neck Pathol. 2018, 12, 221–229. [Google Scholar] [CrossRef]
- Straub, M.; Drecoll, E.; Pfarr, N.; Weichert, W.; Langer, R.; Hapfelmeier, A.; Götz, C.; Wolff, K.-D.; Kolk, A.; Specht, K. CD274/PD-L1 gene amplification and PD-L1 protein expression are common events in squamous cell carcinoma of the oral cavity. Oncotarget 2016, 7, 12024–12034. [Google Scholar] [CrossRef]
- Brcic, I.; Gallob, M.; Schwantzer, G.; Zrnc, T.; Weiland, T.; Thurnher, D.; Wolf, A.; Brcic, L. Concordance of tumor infiltrating lymphocytes, PD-L1 and p16 expression in small biopsies, resection and lymph node metastases of oropharyngeal squamous cell carcinoma. Oral Oncol. 2020, 106, 104719. [Google Scholar] [CrossRef]
- Kaur, A.; Jamshidi, P.; Paintal, A. PD-L1 Combined Positive Score (CPS) Scoring in p16+ Oropharyngeal Squamous Cell Carcinoma (OPSCC): A Comparison of Scoring in Paired Primary Tumors and Lymph Node Metastases. Lab. Investig. 2020, 10, 1202–1203. [Google Scholar]
- Nathanson, S.D. Insights into the mechanisms of lymph node metastasis. Cancer 2003, 98, 413–423. [Google Scholar] [CrossRef]
- Yang, L.; Lin, P.C. Mechanisms that drive inflammatory tumor microenvironment, tumor heterogeneity, and metastatic progression. Semin. Cancer Biol. 2017, 47, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Bruno, A.; Gallo, C.; Pajardi, G.; Noonan, D.M.; Dallaglio, K. Cancer stem cells and the tumor microenvironment: Interplay in tumor heterogeneity. Connect. Tissue Res. 2015, 56, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Takes, R.P.; Baatenburg de Jong, R.J.; Wijffels, K.; Schuuring, E.; Litvinov, S.V.; Hermans, J.; Han JM van Krieken, J. Expression of genetic markers in lymph node metastases compared with their primary tumours in head and neck cancer. J. Pathol. 2001, 194, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Guervós, M.A.; Marcos, C.Á.; LLorente, J.L.; Nuño, A.S.; Suárez, C.; Hermsen, M. Genetic differences between primary larynx and pharynx carcinomas and their matched lymph node metastases by multiplex ligation-dependent probe amplification. Oral Oncol. 2009, 45, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, L.; Xu, Z.; Li, M.; Wang, W.; Bai, Y.; Xu, X.; Li, R.; Zhao, H. Lymph node metastasis-related gene signature shows good performance in predicting prognosis and immune infiltration in cervical cancer. Front. Oncol. 2023, 13, 1190251. [Google Scholar] [CrossRef]
- Roepman, P.; Wessels, L.F.; Kettelarij, N.; Kemmeren, P.; Miles, A.J.; Lijnzaad, P.; Tilanus, M.G.; Koole, R.; Hordijk, G.-J.; van der Vliet, P.C.; et al. An expression profile for diagnosis of lymph node metastases from primary head and neck squamous cell carcinomas. Nat. Genet. 2005, 37, 182–186. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Cai, X.; Xie, Z.; Zhou, L.; Cheng, B.; Zhong, R.; Xiong, S.; Li, J.; Chen, Z.; et al. The association between CD8+ tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: A systematic review and meta-analysis. EClinicalMedicine 2021, 41, 101134. [Google Scholar] [CrossRef]
- Kuba, K.; Inoue, H.; Matsumura, S.; Enoki, Y.; Kogashiwa, Y.; Ebihara, Y.; Nakahira, M.; Yamazaki, T.; Yasuda, M.; Kaira, K.; et al. A retrospective analysis of tumor infiltrating lymphocytes in head and neck squamous cell carcinoma patients treated with nivolumab. Sci. Rep. 2022, 12, 22557. [Google Scholar] [CrossRef]
- Ogiya, R.; Niikura, N.; Kumaki, N.; Bianchini, G.; Kitano, S.; Iwamoto, T.; Hayashi, N.; Yokoyama, K.; Oshitanai, R.; Terao, M.; et al. Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci. 2016, 107, 1730–1735. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, J.; Cai, W.; Lao, I.; Shi, Y.; Luo, X.; Yan, W. Changes in the tumor immune microenvironment in resected recurrent soft tissue sarcomas. Ann. Transl. Med. 2019, 7, 387. [Google Scholar] [CrossRef]
- So, Y.K.; Byeon, S.-J.; Ku, B.M.; Ko, Y.H.; Ahn, M.-J.; Son, Y.-I.; Chung, M.K. An increase of CD8+ T cell infiltration following recurrence is a good prognosticator in HNSCC. Sci. Rep. 2020, 10, 20059. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Tiwari, R.; Nauta, J.J.; Van der Waal, I.; Snow, G.B. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer 1993, 71, 452–456. [Google Scholar] [CrossRef] [PubMed]

| Variables | N = 21 |
|---|---|
| Median age, years (range) | 55 (36–72) |
| Age > 60, n (%) | 6 (28.6) |
| Sex, n (%) | |
| Male | 16 (76.2) |
| Female | 5 (23.8) |
| Current or former smokers, n (%) | 8 (38.1) |
| Primary tumor location, n (%) | |
| Oral cavity | 11 (52.4) |
| Oropharynx | 8 (38.1) |
| Hypopharynx | 2 (9.5) |
| HPV p16 status, n (%) | |
| Positive | 7 (33.3) |
| Negative | 5 (23.8) |
| Missing | 9 (42.9) |
| PD-L1 expression, n (%) | |
| CPS < 1 | 1 (4.8) |
| CPS ≥ 1 | 8 (38.1) |
| Not assessed | 12 (57.1) |
| Number of previous lines of chemotherapy before ICI, n (%) | |
| 0 | 6 (28.6) |
| 1 | 6 (28.6) |
| 2 or more | 9 (42.9) |
| Immune checkpoint inhibitor treatment, n (%) | |
| Pembrolizumab | 11 (52.4) |
| Nivolumab | 5 (23.8) |
| Nivolumab + Ipilimumab | 1 (4.8) |
| Durvalumab | 3 (14.3) |
| Durvalumab + Tremelimumab | 1 (4.8) |
| Primary Tumor N = 21 | LNM N = 21 | p-Value | |
|---|---|---|---|
| Median TIL density, /mm2 (range) | |||
| Intratumoral | 57.71 (3.32–753.19) | 105.29 (4.01–1559.75) | 0.424 |
| Stromal | 1153.2 (160.4–8725.8) | 1868.2 (78.1–14,728.4) | 0.237 |
| Immune score, %, median (range) | |||
| Inflamed score | 13.41 (0.47–73.95) | 20.48 (0–89.09) | 0.837 |
| Immune-excluded score | 29.86 (12.85–55.33) | 33.01 (4.49–68.25) | 0.781 |
| Immune-desert score | 41.64 (0.84–84.5) | 33.43 (2.91–95.06) | 0.628 |
| Immune phenotype, n (%) | 0.598 | ||
| Inflamed | 8 (38.1) | 8 (38.1) | |
| Immune-excluded | 9 (42.9) | 6 (28.6) | |
| Desert | 4 (19.0) | 7 (33.3) |
| Overall Response | p-Value | ||
|---|---|---|---|
| CR + PR (N = 4) | SD + PD (N = 17) | ||
| Median iTIL density, /mm2 (range) | |||
| Primary tumor | 297.9 (74.9–370.4) | 45.17 (3.32–753.19) | 0.031 |
| Lymph node metastasis | 377.4 (87.94–1559.8) | 60.53 (4.01–1490.14) | 0.031 |
| Median sTIL density, /mm2 (range) | |||
| Primary tumor | 1328.2 (428.9–5850.4) | 883.6 (160.4–8725.8) | 0.574 |
| Lymph node metastasis | 3495.6 (1397.6–14,728.4) | 1179.2 (78.1–7357.2) | 0.144 |
| Immune phenotype, n (%) | |||
| Primary tumor | 0.215 | ||
| Inflamed | 3 (75) | 5 (29.4) | |
| Immune-excluded | 1 (25) | 8 (47.1) | |
| Desert | 0 (0) | 4 (23.5) | |
| Lymph node metastasis | 0.179 | ||
| Inflamed | 3 (75) | 5 (29.4) | |
| Immune-excluded | 1 (25) | 5 (29.4) | |
| Desert | 0 (0) | 7 (41.2) | |
| Disease Control | p-Value | ||
| CR + PR + SD (N = 7) | PD (N = 14) | ||
| Median iTIL density, /mm2 (range) | |||
| Primary tumor | 275.95 (11.06–753.19) | 41.54 (3.32–322.17) | 0.038 |
| Lymph node metastasis | 270.4 (52.95–1559.75) | 55.86 (4.01–1490.14) | 0.020 |
| Median sTIL density, /mm2 (range) | |||
| Primary tumor | 1254.7 (160.4–8725.8) | 1018.4 (281.5–3773.1) | 0.971 |
| Lymph node metastasis | 3112.6 (93.9–14,728.4) | 1103.7 (78.1–7357.2) | 0.197 |
| Immune phenotype, n (%) | |||
| Primary tumor | 0.075 | ||
| Inflamed | 5 (71.4) | 3 (21.4) | |
| Immune-excluded | 1 (14.3) | 8 (57.1) | |
| Desert | 1 (14.3) | 3 (21.4) | |
| Lymph node metastasis | 0.084 | ||
| Inflamed | 5 (71.4) | 3 (21.4) | |
| Immune-excluded | 1 (14.3) | 5 (35.7) | |
| Desert | 1 (14.3) | 6 (42.9) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Ahn, J.S.; Kang, M.; Park, G.; Lim, Y.; Hwang, S.; Ock, C.-Y.; Koh, J.; Chung, E.-J.; Kwon, S.-K.; et al. Comparison of Tumor Microenvironments between Primary Tumors and Lymph Node Metastases in Head and Neck Squamous Cell Carcinoma and Their Predictive Role in Immune Checkpoint Inhibitor Treatment. Cells 2024, 13, 1557. https://doi.org/10.3390/cells13181557
Kim DH, Ahn JS, Kang M, Park G, Lim Y, Hwang S, Ock C-Y, Koh J, Chung E-J, Kwon S-K, et al. Comparison of Tumor Microenvironments between Primary Tumors and Lymph Node Metastases in Head and Neck Squamous Cell Carcinoma and Their Predictive Role in Immune Checkpoint Inhibitor Treatment. Cells. 2024; 13(18):1557. https://doi.org/10.3390/cells13181557
Chicago/Turabian StyleKim, Dong Hyun, Jong Seok Ahn, Mingu Kang, Gahee Park, Yoojoo Lim, Soohyun Hwang, Chan-Young Ock, Jiwon Koh, Eun-Jae Chung, Seong-Keun Kwon, and et al. 2024. "Comparison of Tumor Microenvironments between Primary Tumors and Lymph Node Metastases in Head and Neck Squamous Cell Carcinoma and Their Predictive Role in Immune Checkpoint Inhibitor Treatment" Cells 13, no. 18: 1557. https://doi.org/10.3390/cells13181557






