A Novel Recombinant Vitronectin Variant Supports the Expansion and Differentiation of Pluripotent Stem Cells in Defined Animal-Free Workflows
Abstract
1. Introduction
2. Materials and Methods
3. Results
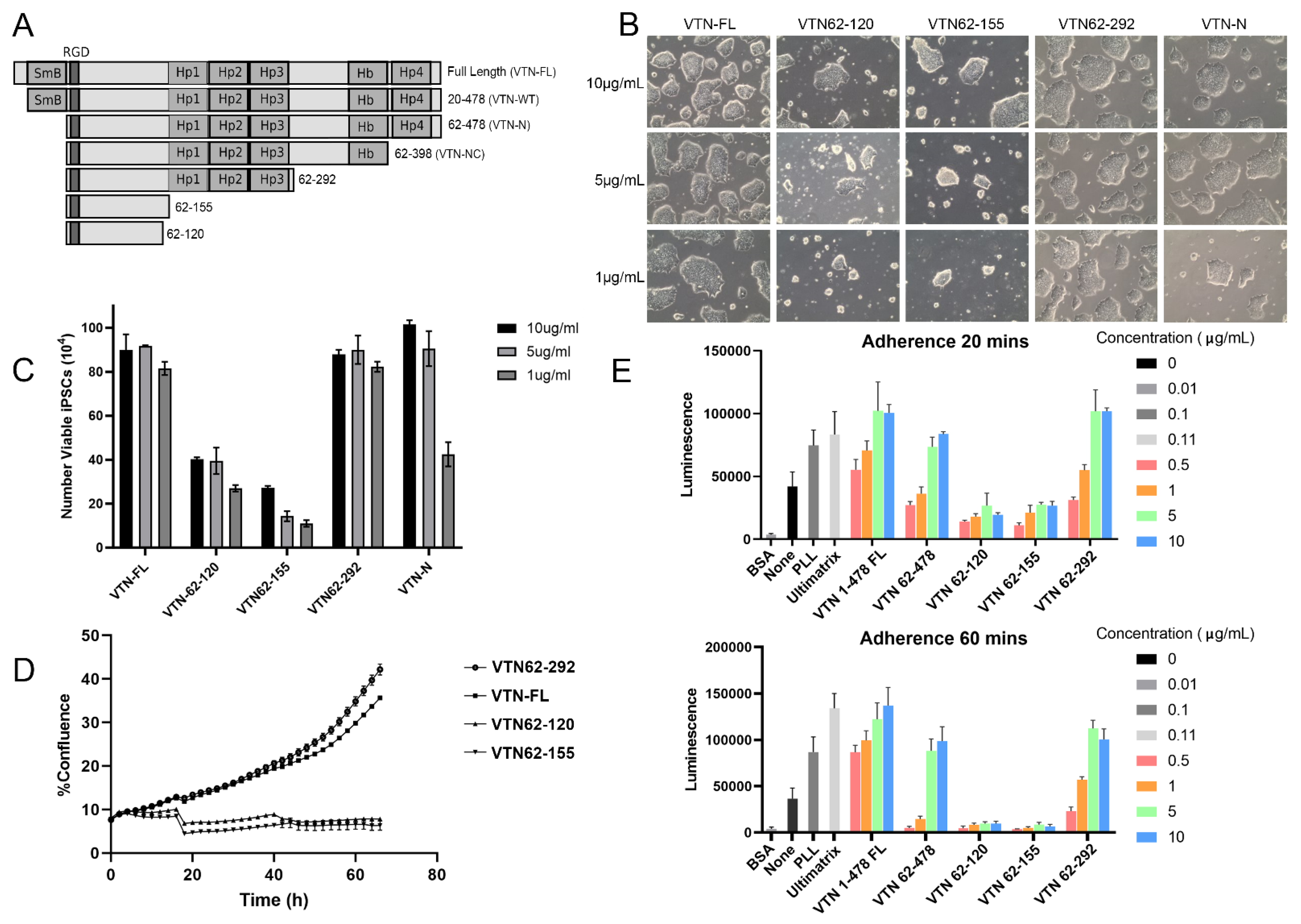
3.1. Design and Construction of Vitronectin Variants
3.2. Identification of Optimal Vitronectin Variant for Growth of iPSCs
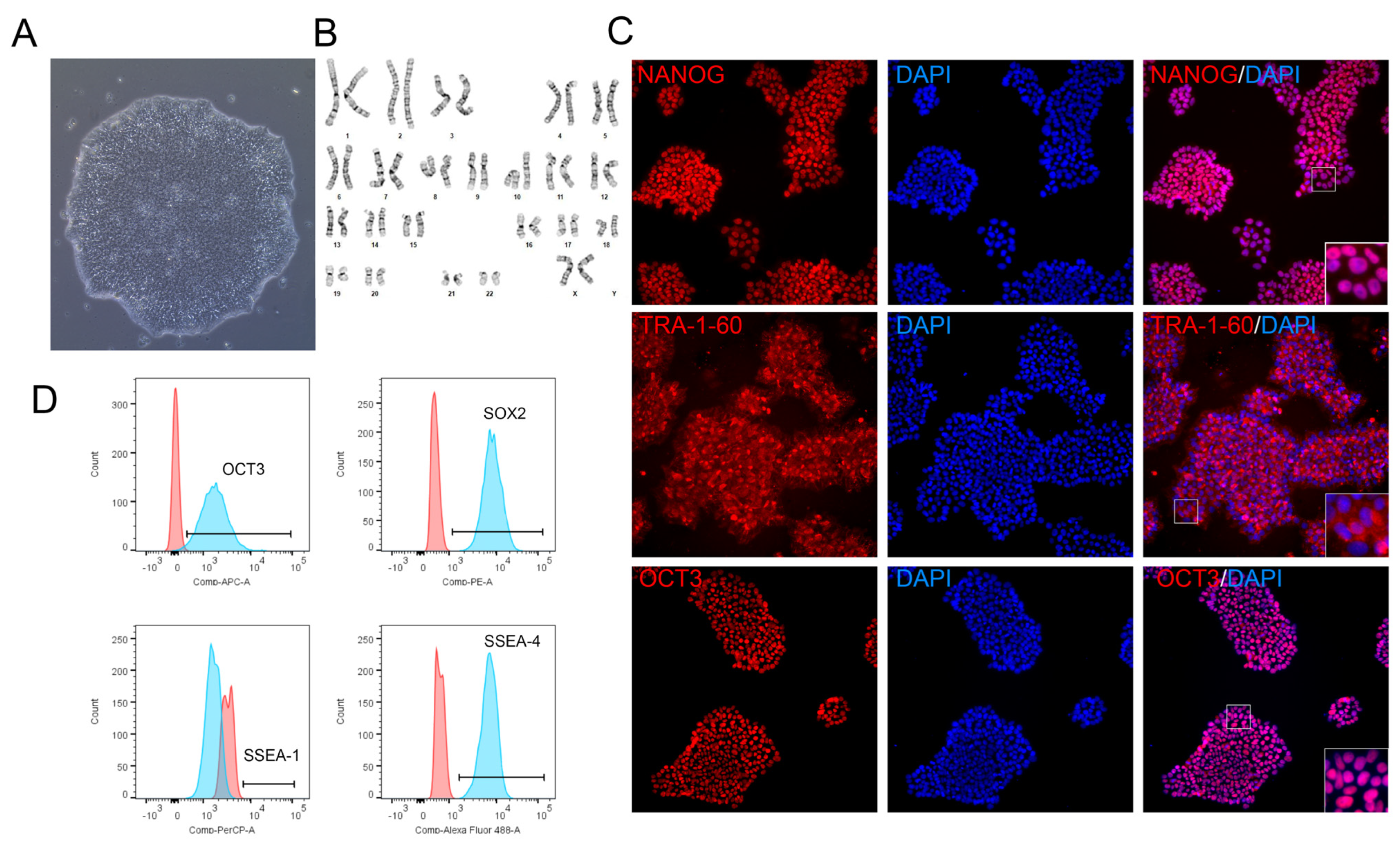
3.3. Long Term Growth of iPSCs on VTN62-292
3.4. Maintenance of Pluripotency on VTN62-292
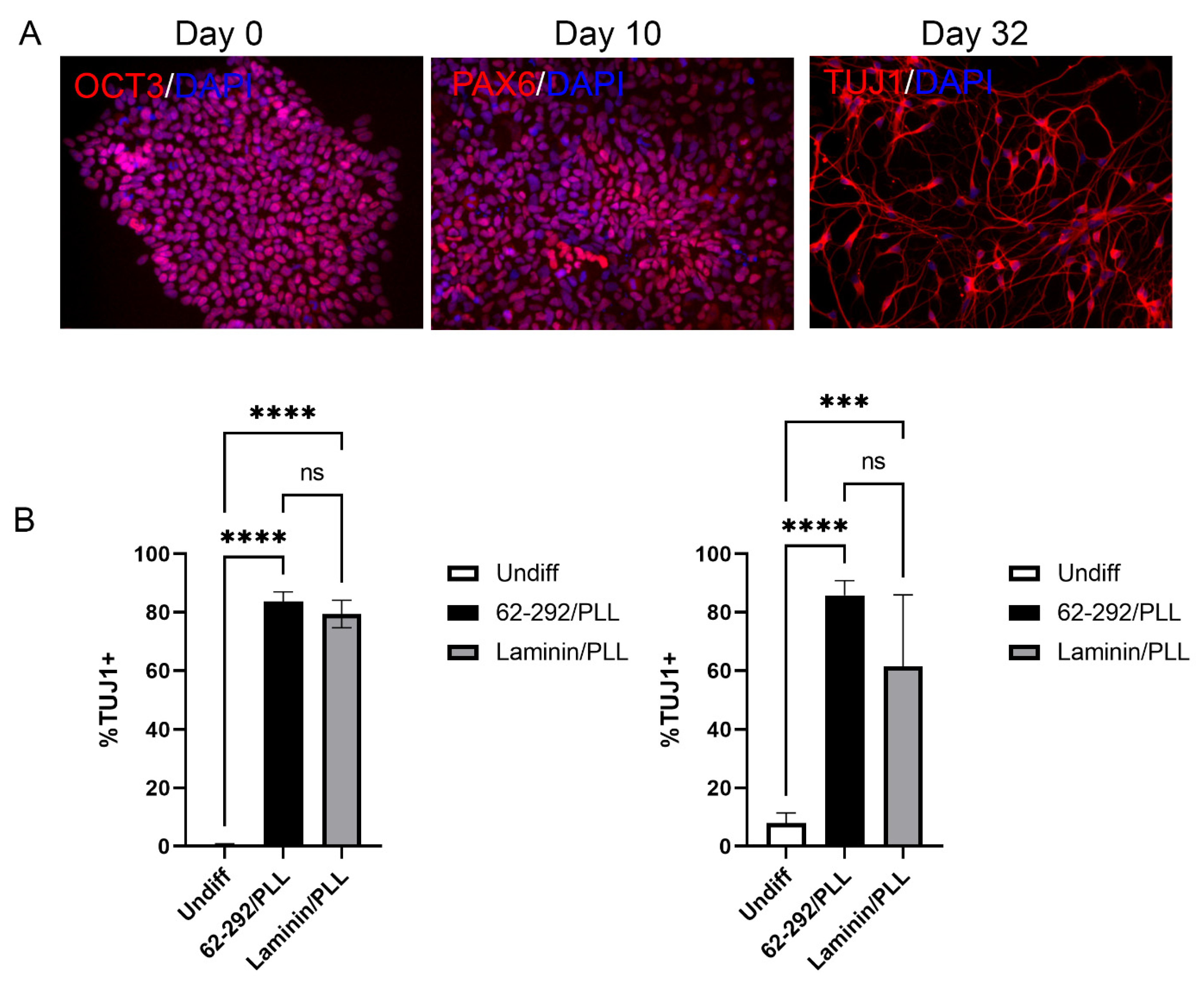
3.5. Animal-Free Differentiation of iPSCs into Neurons with VTN62-292
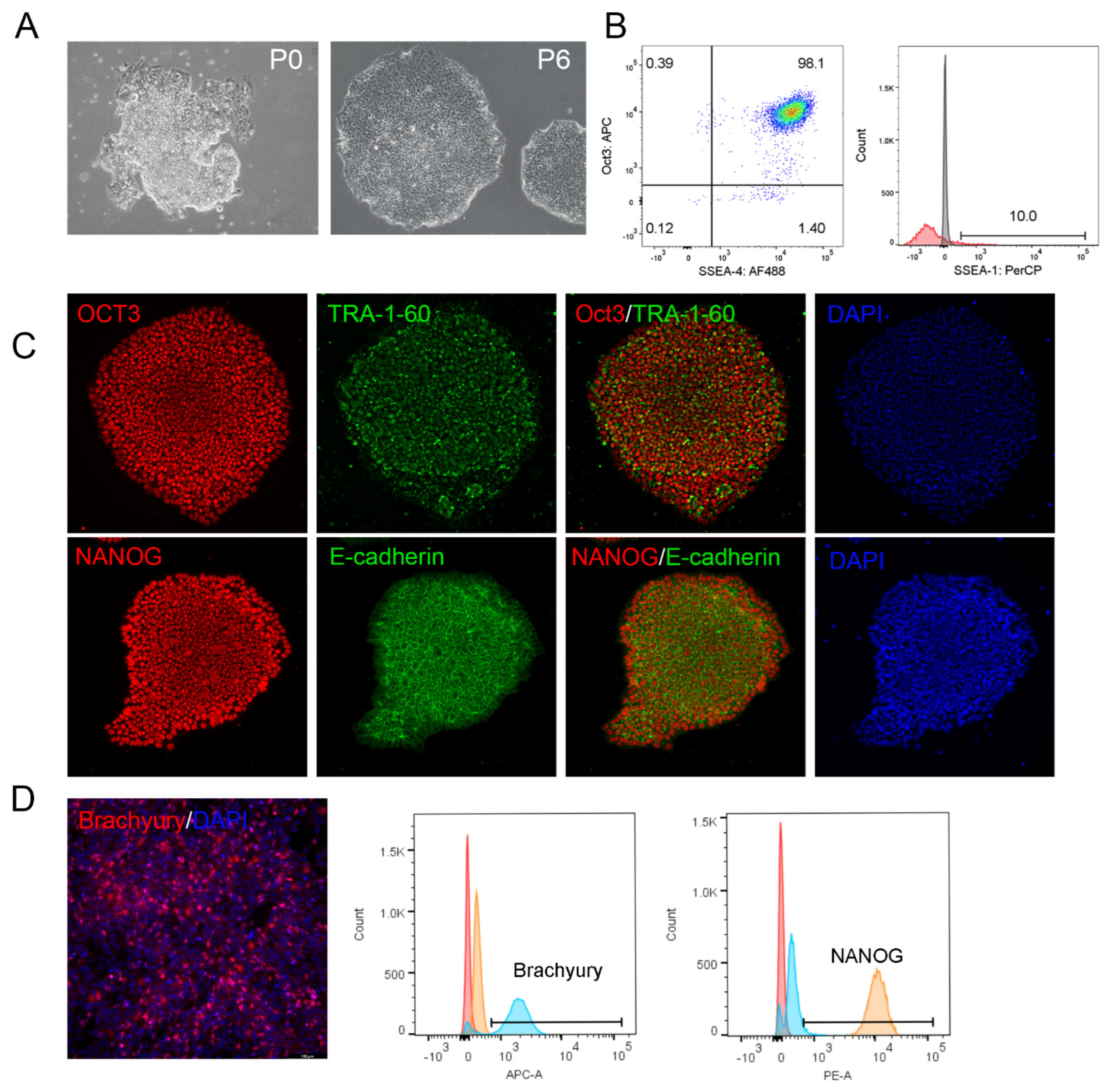
3.6. Reprogramming of PBMCs into iPSCs on VTN62-292
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishikawa, S.; Goldstein, R.A.; Nierras, C.R. The promise of human induced pluripotent stem cells for research and therapy. Nat. Rev. Mol. Cell Biol. 2008, 9, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Cruvinel, E.; Ogusuku, I.; Cerioni, R.; Rodrigues, S.; Goncalves, J.; Goes, M.E.; Alvim, J.M.; Silva, A.C.; Lino, V.S.; Boccardo, E.; et al. Long-term single-cell passaging of human iPSC fully supports pluripotency and high-efficient trilineage differentiation capacity. SAGE Open Med. 2020, 8, 2050312120966456. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Oikonomopoulos, A.; Sayed, N.; Wu, J.C. Modeling human diseases with induced pluripotent stem cells: From 2D to 3D and beyond. Development 2018, 145, dev156166. [Google Scholar] [CrossRef]
- Chen, K.G.; Mallon, B.S.; Park, K.; Robey, P.G.; McKay, R.D.G.; Gottesman, M.M.; Zheng, W. Pluripotent Stem Cell Platforms for Drug Discovery. Trends Mol. Med. 2018, 24, 805–820. [Google Scholar] [CrossRef]
- Young, J.S.; Morshed, R.A.; Kim, J.W.; Balyasnikova, I.V.; Ahmed, A.U.; Lesniak, M.S. Advances in stem cells, induced pluripotent stem cells, and engineered cells: Delivery vehicles for anti-glioma therapy. Expert Opin. Drug Deliv. 2014, 11, 1733–1746. [Google Scholar] [CrossRef]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef]
- Penney, J.; Ralvenius, W.T.; Tsai, L.H. Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol. Psychiatry 2020, 25, 148–167. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Yamazaki, Y.; Ren, Y.; Davis, M.D.; Liu, C.C.; Lu, W.; Wang, X.; Chen, K.; Cherukuri, Y.; et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat. Commun. 2020, 11, 5540. [Google Scholar] [CrossRef]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Hiller, B.M.; Marmion, D.J.; Thompson, C.A.; Elliott, N.A.; Federoff, H.; Brundin, P.; Mattis, V.B.; McMahon, C.W.; Kordower, J.H. Optimizing maturity and dose of iPSC-derived dopamine progenitor cell therapy for Parkinson’s disease. NPJ Regen. Med. 2022, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Israeli, Y.R.; Wasserman, A.H.; Gabalski, M.A.; Volmert, B.D.; Ming, Y.; Ball, K.A.; Yang, W.; Zou, J.; Ni, G.; Pajares, N.; et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 2021, 12, 5142. [Google Scholar] [CrossRef] [PubMed]
- Malan, D.; Friedrichs, S.; Fleischmann, B.K.; Sasse, P. Cardiomyocytes obtained from induced pluripotent stem cells with long-QT syndrome 3 recapitulate typical disease-specific features in vitro. Circ. Res. 2011, 109, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Bjordahl, R.; Gaidarova, S.; Mahmood, S.; Abujarour, R.; Wang, H.; Tuininga, K.; Felices, M.; Davis, Z.B.; Bendzick, L.; et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci. Transl. Med. 2020, 12, eaaz5618. [Google Scholar] [CrossRef]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018, 23, 181–192.e5. [Google Scholar] [CrossRef]
- Lee, G.; Studer, L. Modelling familial dysautonomia in human induced pluripotent stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2286–2296. [Google Scholar] [CrossRef][Green Version]
- Itzhaki, I.; Maizels, L.; Huber, I.; Zwi-Dantsis, L.; Caspi, O.; Winterstern, A.; Feldman, O.; Gepstein, A.; Arbel, G.; Hammerman, H.; et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 2011, 471, 225–229. [Google Scholar] [CrossRef]
- Hor, J.H.; Soh, E.S.; Tan, L.Y.; Lim, V.J.W.; Santosa, M.M.; Winanto; Ho, B.X.; Fan, Y.; Soh, B.S.; Ng, S.Y. Cell cycle inhibitors protect motor neurons in an organoid model of Spinal Muscular Atrophy. Cell Death Dis. 2018, 9, 1100. [Google Scholar] [CrossRef]
- Mithal, A.; Capilla, A.; Heinze, D.; Berical, A.; Villacorta-Martin, C.; Vedaie, M.; Jacob, A.; Abo, K.; Szymaniak, A.; Peasley, M.; et al. Generation of mesenchyme free intestinal organoids from human induced pluripotent stem cells. Nat. Commun. 2020, 11, 215. [Google Scholar] [CrossRef]
- Sekine, K.; Ogawa, S.; Tsuzuki, S.; Kobayashi, T.; Ikeda, K.; Nakanishi, N.; Takeuchi, K.; Kanai, E.; Otake, Y.; Okamoto, S.; et al. Generation of human induced pluripotent stem cell-derived liver buds with chemically defined and animal origin-free media. Sci. Rep. 2020, 10, 17937. [Google Scholar] [CrossRef] [PubMed]
- Papapetrou, E.P. Patient-derived induced pluripotent stem cells in cancer research and precision oncology. Nat. Med. 2016, 22, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Estrada, H.Q.; Patel, S.; Rabizadeh, S.; Casero, D.; Targan, S.R.; Barrett, R.J. Development of a Personalized Intestinal Fibrosis Model Using Human Intestinal Organoids Derived From Induced Pluripotent Stem Cells. Inflamm. Bowel Dis. 2022, 28, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Dannemann, M.; Gallego Romero, I. Harnessing pluripotent stem cells as models to decipher human evolution. FEBS J. 2022, 289, 2992–3010. [Google Scholar] [CrossRef] [PubMed]
- Santoso, M.R.; Ikeda, G.; Tada, Y.; Jung, J.H.; Vaskova, E.; Sierra, R.G.; Gati, C.; Goldstone, A.B.; von Bornstaedt, D.; Shukla, P.; et al. Exosomes From Induced Pluripotent Stem Cell-Derived Cardiomyocytes Promote Autophagy for Myocardial Repair. J. Am. Heart Assoc. 2020, 9, e014345. [Google Scholar] [CrossRef]
- Tang, Q.; Lu, B.; He, J.; Chen, X.; Fu, Q.; Han, H.; Luo, C.; Yin, H.; Qin, Z.; Lyu, D.; et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials 2022, 280, 121320. [Google Scholar] [CrossRef]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.T.; Skupien-Rabian, B.; Chen, X.; et al. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef]
- Jha, B.S.; Farnoodian, M.; Bharti, K. Regulatory considerations for developing a phase I investigational new drug application for autologous induced pluripotent stem cells-based therapy product. Stem Cells Transl. Med. 2021, 10, 198–208. [Google Scholar] [CrossRef]
- Nakagawa, M.; Taniguchi, Y.; Senda, S.; Takizawa, N.; Ichisaka, T.; Asano, K.; Morizane, A.; Doi, D.; Takahashi, J.; Nishizawa, M.; et al. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci. Rep. 2014, 4, 3594. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.E.; Diol, N.R.; Propson, N.E.; et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Kaini, R.R.; Shen-Gunther, J.; Cleland, J.M.; Greene, W.A.; Wang, H.C. Recombinant Xeno-Free Vitronectin Supports Self-Renewal and Pluripotency in Protein-Induced Pluripotent Stem Cells. Tissue Eng. Part C Methods 2016, 22, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Isobe, T.; Nakatsuji, N.; Suemori, H. Efficient Adhesion Culture of Human Pluripotent Stem Cells Using Laminin Fragments in an Uncoated Manner. Sci. Rep. 2017, 7, 41165. [Google Scholar] [CrossRef] [PubMed]
- Rodin, S.; Antonsson, L.; Niaudet, C.; Simonson, O.E.; Salmela, E.; Hansson, E.M.; Domogatskaya, A.; Xiao, Z.; Damdimopoulou, P.; Sheikhi, M.; et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat. Commun. 2014, 5, 3195. [Google Scholar] [CrossRef]
- Dayem, A.A.; Won, J.; Goo, H.G.; Yang, G.M.; Seo, D.S.; Jeon, B.M.; Choi, H.Y.; Park, S.E.; Lim, K.M.; Jang, S.H.; et al. The immobilization of fibronectin- and fibroblast growth factor 2-derived peptides on a culture plate supports the attachment and proliferation of human pluripotent stem cells. Stem Cell Res. 2020, 43, 101700. [Google Scholar] [CrossRef]
- Schvartz, I.; Seger, D.; Shaltiel, S. Vitronectin. Int. J. Biochem. Cell Biol. 1999, 31, 539–544. [Google Scholar] [CrossRef]
- Preissner, K.T. Structure and biological role of vitronectin. Annu. Rev. Cell Biol. 1991, 7, 275–310. [Google Scholar] [CrossRef]
- Nagaoka, M.; Kobayashi, M.; Kawai, C.; Mallanna, S.K.; Duncan, S.A. Design of a Vitronectin-Based Recombinant Protein as a Defined Substrate for Differentiation of Human Pluripotent Stem Cells into Hepatocyte-Like Cells. PLoS ONE 2015, 10, e0136350. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Chang, C.H.; Hocking, D.C. Expression, production, and characterization of full-length vitronectin in Escherichia coli. Protein Expr. Purif. 2004, 36, 131–138. [Google Scholar] [CrossRef]
- Kueng, W.; Silber, E.; Eppenberger, U. Quantification of cells cultured on 96-well plates. Anal. Biochem. 1989, 182, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Taooka, Y.; Chen, J.; Yednock, T.; Sheppard, D. The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J. Cell Biol. 1999, 145, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Leavesley, D.I.; Kashyap, A.S.; Croll, T.; Sivaramakrishnan, M.; Shokoohmand, A.; Hollier, B.G.; Upton, Z. Vitronectin-Master controller or micromanager? IUBMB Life 2013, 65, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Kolhar, P.; Kotamraju, V.R.; Hikita, S.T.; Clegg, D.O.; Ruoslahti, E. Synthetic surfaces for human embryonic stem cell culture. J. Biotechnol. 2010, 146, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, Z.C.; Liu, Y.; Chen, Y.R.; Deng, R.H.; Zhang, Z.N.; Yu, J.K.; Yuan, F.Z. Function and Mechanism of RGD in Bone and Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 773636. [Google Scholar] [CrossRef]
- Ye, H.; Wang, Q. Efficient Generation of Non-Integration and Feeder-Free Induced Pluripotent Stem Cells from Human Peripheral Blood Cells by Sendai Virus. Cell Physiol. Biochem. 2018, 50, 1318–1331. [Google Scholar] [CrossRef]
- Braam, S.R.; Zeinstra, L.; Litjens, S.; Ward-van Oostwaard, D.; van den Brink, S.; van Laake, L.; Lebrin, F.; Kats, P.; Hochstenbach, R.; Passier, R.; et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells 2008, 26, 2257–2265. [Google Scholar] [CrossRef]
- de Marco, A. Strategies for successful recombinant expression of disulfide bond-dependent proteins in Escherichia coli. Microb. Cell Fact. 2009, 8, 26. [Google Scholar] [CrossRef]
- Berkmen, M. Production of disulfide-bonded proteins in Escherichia coli. Protein Expr. Purif. 2012, 82, 240–251. [Google Scholar] [CrossRef]
- Kamikubo, Y.; Okumura, Y.; Loskutoff, D.J. Identification of the disulfide bonds in the recombinant somatomedin B domain of human vitronectin. J. Biol. Chem. 2002, 277, 27109–27119. [Google Scholar] [CrossRef] [PubMed]
- Deinsberger, J.; Reisinger, D.; Weber, B. Global trends in clinical trials involving pluripotent stem cells: A systematic multi-database analysis. NPJ Regen. Med. 2020, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Aly, R.M. Current state of stem cell-based therapies: An overview. Stem Cell Investig. 2020, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Rivera, T.; Zhao, Y.; Ni, Y.; Wang, J. Human-Induced Pluripotent Stem Cell Culture Methods Under cGMP Conditions. Curr. Protoc. Stem Cell Biol. 2020, 54, e117. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Trujillo, S.; Gonzalez-Garcia, C.; Rico, P.; Reid, A.; Windmill, J.; Dalby, M.J.; Salmeron-Sanchez, M. Engineered 3D hydrogels with full-length fibronectin that sequester and present growth factors. Biomaterials 2020, 252, 120104. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Perr, E.; Naqvi, T.; Galitz, D.; Andersen, M.; Grabowski, D.; Person, A.; Kalyuzhny, A.; Flynn, K.C. A Novel Recombinant Vitronectin Variant Supports the Expansion and Differentiation of Pluripotent Stem Cells in Defined Animal-Free Workflows. Cells 2024, 13, 1566. https://doi.org/10.3390/cells13181566
Lu X, Perr E, Naqvi T, Galitz D, Andersen M, Grabowski D, Person A, Kalyuzhny A, Flynn KC. A Novel Recombinant Vitronectin Variant Supports the Expansion and Differentiation of Pluripotent Stem Cells in Defined Animal-Free Workflows. Cells. 2024; 13(18):1566. https://doi.org/10.3390/cells13181566
Chicago/Turabian StyleLu, Xi, Eli Perr, Tahmina Naqvi, David Galitz, Marnelle Andersen, David Grabowski, Anthony Person, Alex Kalyuzhny, and Kevin C. Flynn. 2024. "A Novel Recombinant Vitronectin Variant Supports the Expansion and Differentiation of Pluripotent Stem Cells in Defined Animal-Free Workflows" Cells 13, no. 18: 1566. https://doi.org/10.3390/cells13181566
APA StyleLu, X., Perr, E., Naqvi, T., Galitz, D., Andersen, M., Grabowski, D., Person, A., Kalyuzhny, A., & Flynn, K. C. (2024). A Novel Recombinant Vitronectin Variant Supports the Expansion and Differentiation of Pluripotent Stem Cells in Defined Animal-Free Workflows. Cells, 13(18), 1566. https://doi.org/10.3390/cells13181566






