The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection
Abstract
:1. Introduction
2. Subnuclear Bodies and Their Importance during Viral Infections
2.1. Phosphatidylinositol Phosphates in the Formation of Nuclear Condensates
2.2. Cajal Bodies
2.3. Promyelocytic Leukemia Bodies
2.4. Paraspeckles
2.5. Nuclear Speckles
2.6. Super-Enhancers
2.7. The Nucleolus: Basic Composition and Principal Function
2.7.1. Transcription of rDNA
2.7.2. Ribosomal RNA Processing
2.7.3. Subunit Assembly
2.7.4. The Nucleolus as a Multifaceted Nuclear Compartment
3. Involvement of Nucleolus Components during Viral Infections
3.1. Viral Proteins and Their Interaction with Nucleolus Components
3.2. Modulation of Host Genes and rDNA Transcription during Viral Infections
4. The Role of Non-Coding RNAs in Viral Infections
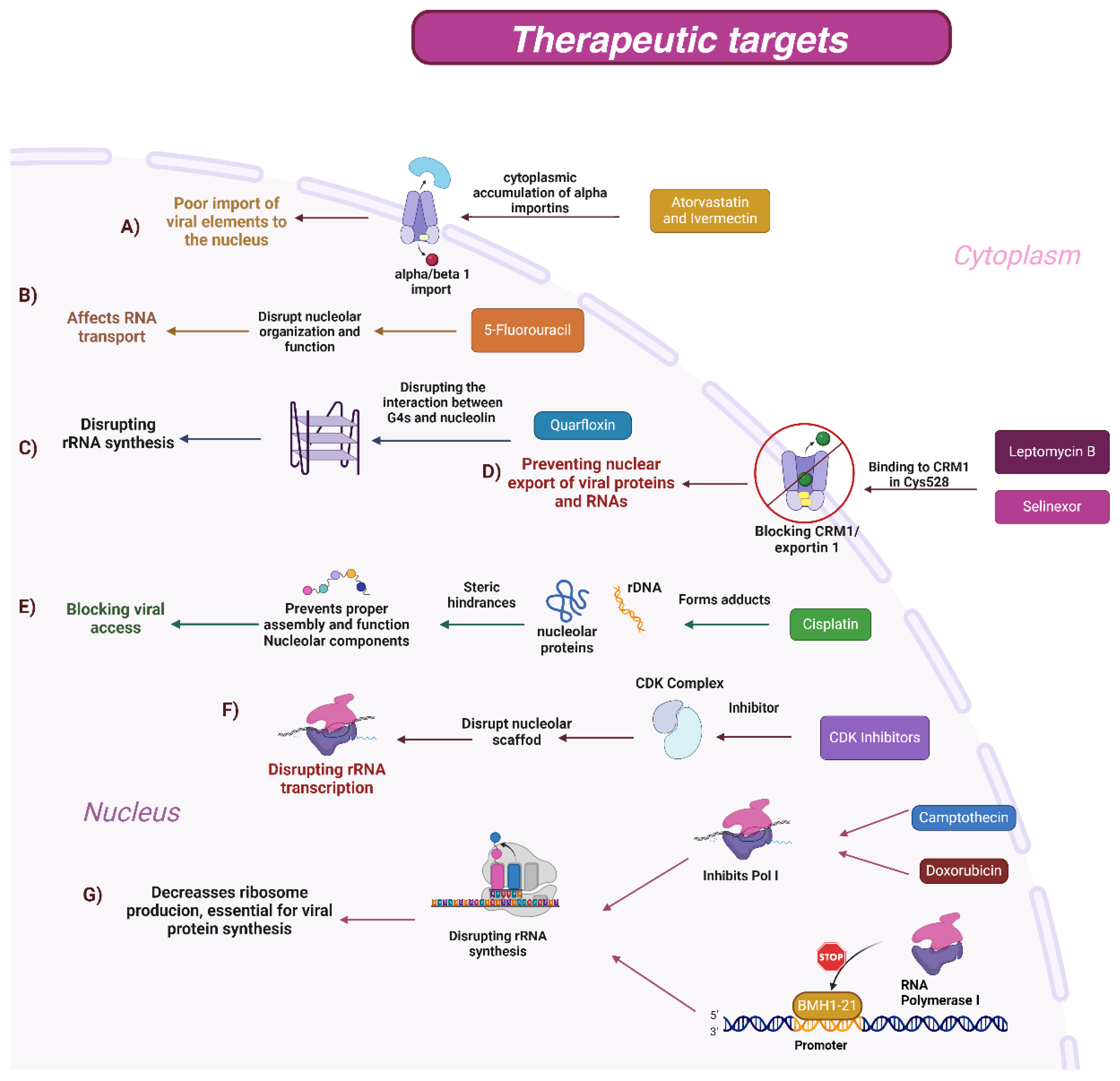
5. Therapeutic Targets for Viral Replication: Nucleolus and Cytoplasm–Nucleus Traffic
5.1. Mechanism of Action of Drugs
5.1.1. BMH-21
5.1.2. CDK Inhibitors
5.1.3. Cisplatin
5.1.4. 5-Fluorouracil (5-FU)
5.1.5. Camptothecin
5.1.6. Doxorubicin
5.1.7. Atorvastatin and Ivermectin
5.1.8. Leptomycin B
5.1.9. Selinexor
5.1.10. Quarfloxin (CX-3543)
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kvansakul, M. Viral Infection and Apoptosis. Viruses 2017, 9, 356. [Google Scholar] [CrossRef]
- Stern-Ginossar, N.; Thompson, S.R.; Mathews, M.B.; Mohr, I. Translational Control in Virus-Infected Cells. Cold Spring Harb. Perspect. Biol. 2019, 11, a033001. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, S.M.; Moseley, G.W. The Nucleolar Interface of RNA Viruses. Cell. Microbiol. 2015, 17, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, S.M.; Zhao, T.; Rozario, A.M.; Rootes, C.L.; McMillan, P.J.; Purcell, A.W.; Woon, A.; Marsh, G.A.; Lieu, K.G.; Wang, L.-F.; et al. Viral Regulation of Host Cell Biology by Hijacking of the Nucleolar DNA-Damage Response. Nat. Commun. 2018, 9, 3057. [Google Scholar] [CrossRef] [PubMed]
- Rattay, S.; Hufbauer, M.; Hoboth, P.; Sztacho, M.; Akgül, B. Viruses and Phospholipids: Friends and Foes during Infection. J Med. Virol. 2023, 95, e28658. [Google Scholar] [CrossRef] [PubMed]
- Desfarges, S.; Ciuffi, A. Viral Integration and Consequences on Host Gene Expression. Viruses Essent. Agents Life 2012, XVI, 147–175. [Google Scholar] [CrossRef]
- Michieletto, D.; Lusic, M.; Marenduzzo, D.; Orlandini, E. Physical Principles of Retroviral Integration in the Human Genome. Nat. Commun. 2019, 10, 575. [Google Scholar] [CrossRef]
- Le Sage, V.; Mouland, A.J. Viral Subversion of the Nuclear Pore Complex. Viruses 2013, 5, 2019–2042. [Google Scholar] [CrossRef]
- Li, Y.-J.; Macnaughton, T.; Gao, L.; Lai, M.M.C. RNA-Templated Replication of Hepatitis Delta Virus: Genomic and Antigenomic RNAs Associate with Different Nuclear Bodies. J. Virol. 2006, 80, 6478–6486. [Google Scholar] [CrossRef]
- Amorim, M.J.; Digard, P. Influenza A Virus and the Cell Nucleus. Vaccine 2006, 24, 6651–6655. [Google Scholar] [CrossRef]
- Dubois, M.L.; Boisvert, F.M. The Nucleolus: Structure and Function. In The Functional Nucleus; Bazett-Jones, D., Dellaire, G., Eds.; Springer: Cham, Switzerland, 2016; pp. 29–49, VI. [Google Scholar] [CrossRef]
- Olson, M.O.; Dundr, M. Nucleolus: Structure and Function. In eLS; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–9. ISBN 978-0-470-01590-2. [Google Scholar]
- Lafontaine, D.L.J.; Riback, J.A.; Bascetin, R.; Brangwynne, C.P. The Nucleolus as a Multiphase Liquid Condensate. Nat. Rev. Mol. Cell Biol. 2021, 22, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Hoboth, P.; Sztacho, M.; Hozák, P. Nuclear Patterns of Phosphatidylinositol 4,5- and 3,4-Bisphosphate Revealed by Super-Resolution Microscopy Differ between the Consecutive Stages of RNA Polymerase II Transcription. FEBS J. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sztacho, M.; Sobol, M.; Balaban, C.; Lopes, S.E.E.; Hozák, P. Nuclear Phosphoinositides and Phase Separation: Important Players in Nuclear Compartmentalization. Adv. Biol. Regul. 2019, 71, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Dumelie, J.G.; Chen, Q.; Miller, D.; Attarwala, N.; Gross, S.S.; Jaffrey, S.R. Biomolecular Condensates Create Phospholipid-Enriched Microenvironments. Nat. Chem. Biol. 2024, 20, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.; Sztacho, M.; Antiga, L.; Miladinović, A.; Harata, M.; Hozák, P. PIP2-Effector Protein MPRIP Regulates RNA Polymerase II Condensation and Transcription. Biomolecules 2023, 13, 426. [Google Scholar] [CrossRef]
- Lafarga, M.; Hervás, J.P.; Santa-Cruz, M.C.; Villegas, J.; Crespo, D. The “Accessory Body” of Cajal in the Neuronal Nucleus. A Light and Electron Microscopic Approach. Anat. Embryol. 1983, 166, 19–30. [Google Scholar] [CrossRef]
- Logan, M.K.; Lett, K.E.; Hebert, M.D. The Cajal Body Protein Coilin Is a Regulator of the miR-210 hypoxamiR and Influences MIR210HG Alternative Splicing. J. Cell Sci. 2021, 134, jcs258575. [Google Scholar] [CrossRef]
- Bergstrand, S.; O’Brien, E.M.; Farnebo, M. The Cajal Body Protein WRAP53β Prepares the Scene for Repair of DNA Double-Strand Breaks by Regulating Local Ubiquitination. Front. Mol. Biosci. 2019, 6, 51. [Google Scholar] [CrossRef]
- Logan, M.K.; McLaurin, D.M.; Hebert, M.D. Synergistic Interactions between Cajal Bodies and the miRNA Processing Machinery. Mol. Biol. Cell 2020, 31, 1561–1569. [Google Scholar] [CrossRef]
- Sawyer, I.A.; Sturgill, D.; Sung, M.-H.; Hager, G.L.; Dundr, M. Cajal Body Function in Genome Organization and Transcriptome Diversity. Bioessays 2016, 38, 1197–1208. [Google Scholar] [CrossRef]
- Lettin, L.; Erbay, B.; Blair, G.E. Viruses and Cajal Bodies: A Critical Cellular Target in Virus Infection? Viruses 2023, 15, 2311. [Google Scholar] [CrossRef] [PubMed]
- Young, P.J.; Jensen, K.T.; Burger, L.R.; Pintel, D.J.; Lorson, C.L. Minute Virus of Mice NS1 Interacts with the SMN Protein, and They Colocalize in Novel Nuclear Bodies Induced by Parvovirus Infection. J. Virol. 2002, 76, 3892–3904. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.; Martins, C.; Ferreira, F. Early Intranuclear Replication of African Swine Fever Virus Genome Modifies the Landscape of the Host Cell Nucleus. Virus Res. 2015, 210, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fortes, P.; Lamond, A.I.; Ortín, J. Influenza Virus NS1 Protein Alters the Subnuclear Localization of Cellular Splicing Components. J. Gen. Virol. 1995, 76 Pt 4, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Coyaud, E.; Ranadheera, C.; Cheng, D.; Gonçalves, J.; Dyakov, B.J.A.; Laurent, E.M.N.; St-Germain, J.; Pelletier, L.; Gingras, A.-C.; Brumell, J.H.; et al. Global Interactomics Uncovers Extensive Organellar Targeting by Zika Virus. Mol. Cell Proteom. 2018, 17, 2242–2255. [Google Scholar] [CrossRef]
- White, L.; Erbay, B.; Blair, G.E. The Cajal Body Protein P80-Coilin Forms a Complex with the Adenovirus L4-22K Protein and Facilitates the Nuclear Export of Adenovirus mRNA. mBio 2023, 14, e0145923. [Google Scholar] [CrossRef]
- Hoppe, A.; Beech, S.J.; Dimmock, J.; Leppard, K.N. Interaction of the Adenovirus Type 5 E4 Orf3 Protein with Promyelocytic Leukemia Protein Isoform II Is Required for ND10 Disruption. J. Virol. 2006, 80, 3042–3049. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Dong, H.; Yang, Z.; Zhou, L.; Xu, P. PML Body Component Sp100A Restricts Wild-Type Herpes Simplex Virus 1 Infection. J. Virol. 2022, 96, e0027922. [Google Scholar] [CrossRef]
- Scherer, M.; Schilling, E.-M.; Stamminger, T. The Human CMV IE1 Protein: An Offender of PML Nuclear Bodies. Adv. Anat. Embryol. Cell Biol. 2017, 223, 77–94. [Google Scholar] [CrossRef]
- Staněk, D.; Fox, A.H. Nuclear Bodies: News Insights into Structure and Function. Curr. Opin. Cell Biol. 2017, 46, 94–101. [Google Scholar] [CrossRef]
- Ilık, İ.A.; Aktaş, T. Nuclear Speckles: Dynamic Hubs of Gene Expression Regulation. FEBS J. 2022, 289, 7234–7245. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Vijayakumar, U.; Zhang, Y.; Fullwood, M.J. Super-Enhancers, Phase-Separated Condensates, and 3D Genome Organization in Cancer. Cancers 2022, 14, 2866. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Huang, S. Nucleolar Components Involved in Ribosome Biogenesis Cycle between the Nucleolus and Nucleoplasm in Interphase Cells. J. Cell Biol. 2001, 153, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Liu, Y.; Yu, X.-Y.; Pan, X.; Zhang, Y.; Tu, J.; Song, Y.-H.; Li, Y. Ribosome Biogenesis in Disease: New Players and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Aubert, M.; O’Donohue, M.-F.; Lebaron, S.; Gleizes, P.-E. Pre-Ribosomal RNA Processing in Human Cells: From Mechanisms to Congenital Diseases. Biomolecules 2018, 8, 123. [Google Scholar] [CrossRef]
- Yao, R.-W.; Xu, G.; Wang, Y.; Shan, L.; Luan, P.-F.; Wang, Y.; Wu, M.; Yang, L.-Z.; Xing, Y.-H.; Yang, L.; et al. Nascent Pre-rRNA Sorting via Phase Separation Drives the Assembly of Dense Fibrillar Components in the Human Nucleolus. Mol. Cell 2019, 76, 767–783.e11. [Google Scholar] [CrossRef]
- Sloan, K.E.; Bohnsack, M.T.; Watkins, N.J. The 5S RNP Couples P53 Homeostasis to Ribosome Biogenesis and Nucleolar Stress. Cell Rep. 2013, 5, 237–247. [Google Scholar] [CrossRef]
- Woolford, J.L., Jr.; Baserga, S.J. Ribosome Biogenesis in the Yeast Saccharomyces Cerevisiae. Genetics 2013, 195, 643–681. [Google Scholar] [CrossRef]
- Correll, C.C.; Rudloff, U.; Schmit, J.D.; Ball, D.A.; Karpova, T.S.; Balzer, E.; Dundr, M. Crossing Boundaries of Light Microscopy Resolution Discerns Novel Assemblies in the Nucleolus. Histochem. Cell Biol. 2024, 162, 161–183. [Google Scholar] [CrossRef]
- Pfister, A.S. Emerging Role of the Nucleolar Stress Response in Autophagy. Front. Cell. Neurosci. 2019, 13, 156. [Google Scholar] [CrossRef]
- Lindström, M.S.; Jurada, D.; Bursac, S.; Orsolic, I.; Bartek, J.; Volarevic, S. Nucleolus as an Emerging Hub in Maintenance of Genome Stability and Cancer Pathogenesis. Oncogene 2018, 37, 2351–2366. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, D.; Brown-Suedel, A.; Bouchier-Hayes, L. Chapter Seven—The Role of the Nucleolus in Regulating the Cell Cycle and the DNA Damage Response. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Control of Cell Cycle & Cell Proliferation; Academic Press: Cambridge, MA, USA, 2023; Volume 135, pp. 203–241. [Google Scholar]
- Desterro, J.M.P.; Keegan, L.P.; Lafarga, M.; Berciano, M.T.; O’Connell, M.; Carmo-Fonseca, M. Dynamic Association of RNA-Editing Enzymes with the Nucleolus. J. Cell Sci. 2003, 116, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Klump, B.M.; Perez, G.I.; Patrick, E.M.; Adams-Boone, K.; Cohen, S.B.; Han, L.; Yu, K.; Schmidt, J.C. TCAB1 Prevents Nucleolar Accumulation of the Telomerase RNA to Facilitate Telomerase Assembly. Cell Rep. 2023, 42, 112577. [Google Scholar] [CrossRef] [PubMed]
- Boulon, S.; Westman, B.J.; Hutten, S.; Boisvert, F.-M.; Lamond, A.I. The Nucleolus under Stress. Mol. Cell 2010, 40, 216–227. [Google Scholar] [CrossRef]
- Okuwaki, M. The Structure and Functions of NPM1/Nucleophsmin/B23, a Multifunctional Nucleolar Acidic Protein. J. Biochem. 2008, 143, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Höfler, S.; Lukat, P.; Blankenfeldt, W.; Carlomagno, T. High-Resolution Structure of Eukaryotic Fibrillarin Interacting with Nop56 Amino-Terminal Domain. RNA 2021, 27, 496–512. [Google Scholar] [CrossRef]
- Salvetti, A.; Couté, Y.; Epstein, A.; Arata, L.; Kraut, A.; Navratil, V.; Bouvet, P.; Greco, A. Nuclear Functions of Nucleolin through Global Proteomics and Interactomic Approaches. J. Proteome Res. 2016, 15, 1659–1669. [Google Scholar] [CrossRef]
- Gautier, T.; Bergès, T.; Tollervey, D.; Hurt, E. Nucleolar KKE/D Repeat Proteins Nop56p and Nop58p Interact with Nop1p and Are Required for Ribosome Biogenesis. Mol. Cell Biol. 1997, 17, 7088–7098. [Google Scholar] [CrossRef]
- Dobbyn, H.C.; O’keefe, R.T. Analysis of Snu13p Mutations Reveals Differential Interactions with the U4 snRNA and U3 snoRNA. RNA 2004, 10, 308–320. [Google Scholar] [CrossRef]
- Mitrea, D.M.; Cika, J.A.; Guy, C.S.; Ban, D.; Banerjee, P.R.; Stanley, C.B.; Nourse, A.; Deniz, A.A.; Kriwacki, R.W. Nucleophosmin Integrates within the Nucleolus via Multi-Modal Interactions with Proteins Displaying R-Rich Linear Motifs and rRNA. eLife 2016, 5, e13571. [Google Scholar] [CrossRef]
- Zatsepina, O.V.; Rousselet, A.; Chan, P.K.; Olson, M.O.; Jordan, E.G.; Bornens, M. The Nucleolar Phosphoprotein B23 Redistributes in Part to the Spindle Poles during Mitosis. J. Cell Sci. 1999, 112 Pt 4, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Okuwaki, M.; Tsujimoto, M.; Nagata, K. The RNA Binding Activity of a Ribosome Biogenesis Factor, Nucleophosmin/B23, Is Modulated by Phosphorylation with a Cell Cycle-Dependent Kinase and by Association with Its Subtype. MBoC 2002, 13, 2016–2030. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, A.; Olson, M.O. Nucleolar Protein B23 Has Molecular Chaperone Activities. Protein Sci. 1999, 8, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Shubina, M.Y.; Musinova, Y.R.; Sheval, E.V. Proliferation, Cancer, and Aging—Novel Functions of the Nucleolar Methyltransferase Fibrillarin? Cell Biol. Int. 2018, 42, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Shubina, M.Y.; Musinova, Y.R.; Sheval, E.V. Nucleolar Methyltransferase Fibrillarin: Evolution of Structure and Functions. Biochemistry 2016, 81, 941–950. [Google Scholar] [CrossRef]
- Lechertier, T.; Grob, A.; Hernandez-Verdun, D.; Roussel, P. Fibrillarin and Nop56 Interact before Being Co-Assembled in Box C/D snoRNPs. Exp. Cell Res. 2009, 315, 928–942. [Google Scholar] [CrossRef]
- Pereira-Santana, A.; Gamboa-Tuz, S.D.; Zhao, T.; Schranz, M.E.; Vinuesa, P.; Bayona, A.; Rodríguez-Zapata, L.C.; Castano, E. Fibrillarin Evolution through the Tree of Life: Comparative Genomics and Microsynteny Network Analyses Provide New Insights into the Evolutionary History of Fibrillarin. PLoS Comput. Biol. 2020, 16, e1008318. [Google Scholar] [CrossRef]
- Angelov, D.; Bondarenko, V.A.; Almagro, S.; Menoni, H.; Mongélard, F.; Hans, F.; Mietton, F.; Studitsky, V.M.; Hamiche, A.; Dimitrov, S.; et al. Nucleolin Is a Histone Chaperone with FACT-like Activity and Assists Remodeling of Nucleosomes. EMBO J. 2006, 25, 1669–1679. [Google Scholar] [CrossRef]
- Kobayashi, J.; Fujimoto, H.; Sato, J.; Hayashi, I.; Burma, S.; Matsuura, S.; Chen, D.J.; Komatsu, K. Nucleolin Participates in DNA Double-Strand Break-Induced Damage Response through MDC1-Dependent Pathway. PLoS ONE 2012, 7, e49245. [Google Scholar] [CrossRef]
- Shefer, K.; Boulos, A.; Gotea, V.; Arafat, M.; Ben Chaim, Y.; Muharram, A.; Isaac, S.; Eden, A.; Sperling, J.; Elnitski, L.; et al. A Novel Role for Nucleolin in Splice Site Selection. RNA Biol. 2022, 19, 333–352. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D.; Liu, S.; Huang, J. The Roles of NOP56 in Cancer and SCA36. Pathol. Oncol. Res. 2023, 29, 1610884. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Malla, S.; Lyons, S.M. snoRNPs: Functions in Ribosome Biogenesis. Biomolecules 2020, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, D.; Fafard-Couture, É.; Scott, M.S. Small Nucleolar RNAs: Continuing Identification of Novel Members and Increasing Diversity of Their Molecular Mechanisms of Action. Biochem. Soc. Trans. 2020, 48, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Du, Y.; Wen, J.; Lu, B.; Zhao, Y. snoRNAs: Functions and Mechanisms in Biological Processes, and Roles in Tumor Pathophysiology. Cell Death Discov. 2022, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Falaleeva, M.; Welden, J.R.; Duncan, M.J.; Stamm, S. C/D-Box snoRNAs Form Methylating and Non-Methylating Ribonucleoprotein Complexes: Old Dogs Show New Tricks. Bioessays 2017, 39, 1600264. [Google Scholar] [CrossRef]
- Lykke-Andersen, S.; Ardal, B.K.; Hollensen, A.K.; Damgaard, C.K.; Jensen, T.H. Box C/D snoRNP Autoregulation by a Cis-Acting snoRNA in the NOP56 Pre-mRNA. Mol. Cell 2018, 72, 99–111.e5. [Google Scholar] [CrossRef]
- Lafontaine, D.L.; Tollervey, D. Nop58p Is a Common Component of the Box C+D snoRNPs That Is Required for snoRNA Stability. RNA 1999, 5, 455–467. [Google Scholar] [CrossRef]
- Marmier-Gourrier, N.; Cléry, A.; Senty-Ségault, V.; Charpentier, B.; Schlotter, F.; Leclerc, F.; Fournier, R.; Branlant, C. A Structural, Phylogenetic, and Functional Study of 15.5-kD/Snu13 Protein Binding on U3 Small Nucleolar RNA. RNA 2003, 9, 821–838. [Google Scholar] [CrossRef]
- Höfler, S.; Lukat, P.; Blankenfeldt, W.; Carlomagno, T. Eukaryotic Box C/D Methylation Machinery Has Two Non-Symmetric Protein Assembly Sites. Sci. Rep. 2021, 11, 17561. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Zhang, D.; Liu, G. Ribosomal Control in RNA Virus-Infected Cells. Front. Microbiol. 2022, 13, 1026887. [Google Scholar] [CrossRef]
- Pyper, J.M.; Clements, J.E.; Zink, M.C. The Nucleolus Is the Site of Borna Disease Virus RNA Transcription and Replication. J. Virol. 1998, 72, 7697–7702. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Speiseder, T.; Dobner, T.; Gonzalez, R.A. DNA Virus Replication Compartments. J. Virol. 2014, 88, 1404–1420. [Google Scholar] [CrossRef]
- Hiscox, J.A. The Nucleolus—A Gateway to Viral Infection? Arch. Virol. 2002, 147, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Iarovaia, O.V.; Ioudinkova, E.S.; Velichko, A.K.; Razin, S.V. Manipulation of Cellular Processes via Nucleolus Hijaking in the Course of Viral Infection in Mammals. Cells 2021, 10, 1597. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; MacFarlane, S.; Kalinina, N.O.; Rakitina, D.V.; Ryabov, E.V.; Gillespie, T.; Haupt, S.; Brown, J.W.S.; Taliansky, M. Interaction of a Plant Virus-Encoded Protein with the Major Nucleolar Protein Fibrillarin Is Required for Systemic Virus Infection. Proc. Natl. Acad. Sci. USA 2007, 104, 11115–11120. [Google Scholar] [CrossRef] [PubMed]
- Decle-Carrasco, S.; Rodríguez-Piña, A.L.; Rodríguez-Zapata, L.C.; Castano, E. Current Research on Viral Proteins That Interact with Fibrillarin. Mol. Biol. Rep. 2023, 50, 4631–4643. [Google Scholar] [CrossRef]
- Dove, B.K.; You, J.-H.; Reed, M.L.; Emmett, S.R.; Brooks, G.; Hiscox, J.A. Changes in Nucleolar Morphology and Proteins during Infection with the Coronavirus Infectious Bronchitis Virus. Cell. Microbiol. 2006, 8, 1147–1157. [Google Scholar] [CrossRef]
- Mattola, S.; Leclerc, S.; Hakanen, S.; Aho, V.; Parrish, C.R.; Vihinen-Ranta, M. Parvovirus Infection Alters the Nucleolar Structure. bioRxiv 2022. [Google Scholar] [CrossRef]
- Rausch, J.W.; Grice, S.F.J.L. HIV Rev Assembly on the Rev Response Element (RRE): A Structural Perspective. Viruses 2015, 7, 3053–3075. [Google Scholar] [CrossRef]
- Jarboui, M.A.; Bidoia, C.; Woods, E.; Roe, B.; Wynne, K.; Elia, G.; Hall, W.W.; Gautier, V.W. Nucleolar Protein Trafficking in Response to HIV-1 Tat: Rewiring the Nucleolus. PLoS ONE 2012, 7, e48702. [Google Scholar] [CrossRef]
- Love, D.C.; Sweitzer, T.D.; Hanover, J.A. Reconstitution of HIV-1 Rev Nuclear Export: Independent Requirements for Nuclear Import and Export. Proc. Natl. Acad. Sci. USA 1998, 95, 10608–10613. [Google Scholar] [CrossRef] [PubMed]
- Deffrasnes, C.; Marsh, G.A.; Foo, C.H.; Rootes, C.L.; Gould, C.M.; Grusovin, J.; Monaghan, P.; Lo, M.K.; Tompkins, S.M.; Adams, T.E.; et al. Genome-Wide siRNA Screening at Biosafety Level 4 Reveals a Crucial Role for Fibrillarin in Henipavirus Infection. PLoS Pathog. 2016, 12, e1005478. [Google Scholar] [CrossRef] [PubMed]
- Melén, K.; Tynell, J.; Fagerlund, R.; Roussel, P.; Hernandez-Verdun, D.; Julkunen, I. Influenza A H3N2 Subtype Virus NS1 Protein Targets into the Nucleus and Binds Primarily via Its C-Terminal NLS2/NoLS to Nucleolin and Fibrillarin. Virol. J. 2012, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Murayama, R.; Harada, Y.; Shibata, T.; Kuroda, K.; Hayakawa, S.; Shimizu, K.; Tanaka, T. Influenza A Virus Non-Structural Protein 1 (NS1) Interacts with Cellular Multifunctional Protein Nucleolin during Infection. Biochem. Biophys. Res. Commun. 2007, 362, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Du, Y.; Wang, G.; Li, K. Non-Structural Protein 1 of H3N2 Influenza A Virus Induces Nucleolar Stress via Interaction with Nucleolin. Sci. Rep. 2017, 7, 17761. [Google Scholar] [CrossRef]
- Hutzinger, R.; Feederle, R.; Mrazek, J.; Schiefermeier, N.; Balwierz, P.J.; Zavolan, M.; Polacek, N.; Delecluse, H.-J.; Hüttenhofer, A. Expression and Processing of a Small Nucleolar RNA from the Epstein-Barr Virus Genome. PLoS Pathog. 2009, 5, e1000547. [Google Scholar] [CrossRef]
- Lung, R.W.-M.; Tong, J.H.-M.; To, K.-F. Emerging Roles of Small Epstein-Barr Virus Derived Non-Coding RNAs in Epithelial Malignancy. Int. J. Mol. Sci. 2013, 14, 17378–17409. [Google Scholar] [CrossRef]
- Lee, N. The Many Ways Epstein-Barr Virus Takes Advantage of the RNA Tool Kit. RNA Biol. 2021, 18, 759–766. [Google Scholar] [CrossRef]
- Yang, K.; Wang, M.; Zhao, Y.; Sun, X.; Yang, Y.; Li, X.; Zhou, A.; Chu, H.; Zhou, H.; Xu, J.; et al. A Redox Mechanism Underlying Nucleolar Stress Sensing by Nucleophosmin. Nat. Commun. 2016, 7, 13599. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Fontanes, V.; Barat, B.; Dasgupta, A. Activation of Ribosomal RNA Transcription by Hepatitis C Virus Involves Upstream Binding Factor Phosphorylation Via Induction of Cyclin D1. Cancer Res. 2009, 69, 2057–2064. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-Coding RNAs in Disease: From Mechanisms to Therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and Their Integrated Networks. J. Integr. Bioinform. 2019, 16, 20190027. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. The Opening of Pandora’s Box: An Emerging Role of Long Noncoding RNA in Viral Infections. Front. Immunol. 2019, 9, 3138. [Google Scholar] [CrossRef] [PubMed]
- Ginn, L.; La Montagna, M.; Wu, Q.; Shi, L. Diverse Roles of Long Non-coding RNAs in Viral Diseases. Rev. Med. Virol. 2021, 31, e2198. [Google Scholar] [CrossRef]
- Rossetto, C.C.; Pari, G.S. PAN’s Labyrinth: Molecular Biology of Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) PAN RNA, a Multifunctional Long Noncoding RNA. Viruses 2014, 6, 4212–4226. [Google Scholar] [CrossRef]
- Naipauer, J.; García Solá, M.E.; Salyakina, D.; Rosario, S.; Williams, S.; Coso, O.; Abba, M.C.; Mesri, E.A.; Lacunza, E. A Non-Coding RNA Network Involved in KSHV Tumorigenesis. Front. Oncol. 2021, 11, 687629. [Google Scholar] [CrossRef]
- Rossetto, C.C.; Pari, G. KSHV PAN RNA Associates with Demethylases UTX and JMJD3 to Activate Lytic Replication through a Physical Interaction with the Virus Genome. PLoS Pathog. 2012, 8, e1002680. [Google Scholar] [CrossRef]
- Zhou, X.; Yuan, Q.; Zhang, C.; Dai, Z.; Du, C.; Wang, H.; Li, X.; Yang, S.; Zhao, A. Inhibition of Japanese Encephalitis Virus Proliferation by Long Non-Coding RNA SUSAJ1 in PK-15 Cells. Virol. J. 2021, 18, 29. [Google Scholar] [CrossRef]
- Schuessler, A.; Funk, A.; Lazear, H.M.; Cooper, D.A.; Torres, S.; Daffis, S.; Jha, B.K.; Kumagai, Y.; Takeuchi, O.; Hertzog, P.; et al. West Nile Virus Noncoding Subgenomic RNA Contributes to Viral Evasion of the Type I Interferon-Mediated Antiviral Response. J. Virol. 2012, 86, 5708–5718. [Google Scholar] [CrossRef]
- Fan, Y.-H.; Nadar, M.; Chen, C.-C.; Weng, C.-C.; Lin, Y.-T.; Chang, R.-Y. Small Noncoding RNA Modulates Japanese Encephalitis Virus Replication and Translation in Trans. Virol. J. 2011, 8, 492. [Google Scholar] [CrossRef] [PubMed]
- Yetming, K.D.; Lupey-Green, L.N.; Biryukov, S.; Hughes, D.J.; Marendy, E.M.; Miranda, J.L.; Sample, J.T. The BHLF1 Locus of Epstein-Barr Virus Contributes to Viral Latency and B-Cell Immortalization. J. Virol. 2020, 94, e01215-20. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.; Kerr, K.; Camiolo, S.; Nightingale, K.; Gu, Q.; Antrobus, R.; Suárez, N.M.; Loney, C.; Stanton, R.J.; Weekes, M.P.; et al. Human Cytomegalovirus RNA2.7 Is Required for Upregulating Multiple Cellular Genes to Promote Cell Motility and Viral Spread Late in Lytic Infection. J. Virol. 2021, 95, e00698-21. [Google Scholar] [CrossRef]
- Ding, M.; Wu, J.; Sun, R.; Yan, L.; Bai, L.; Shi, J.; Feng, H.; Zhang, Y.; Lan, K.; Wang, X. Androgen Receptor Transactivates KSHV Noncoding RNA PAN to Promote Lytic Replication–Mediated Oncogenesis: A Mechanism of Sex Disparity in KS. PLOS Pathog. 2021, 17, e1009947. [Google Scholar] [CrossRef]
- Zou, W.; Xiong, M.; Deng, X.; Engelhardt, J.F.; Yan, Z.; Qiu, J. A Comprehensive RNA-Seq Analysis of Human Bocavirus 1 Transcripts in Infected Human Airway Epithelium. Viruses 2019, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, C.C.; Pari, G.S. Kaposi’s Sarcoma-Associated Herpesvirus Noncoding Polyadenylated Nuclear RNA Interacts with Virus- and Host Cell-Encoded Proteins and Suppresses Expression of Genes Involved in Immune Modulation. J. Virol. 2011, 85, 13290–13297. [Google Scholar] [CrossRef] [PubMed]
- De Jesús-González, L.A.; Palacios-Rápalo, S.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Gutiérrez-Escolano, A.L.; del Ángel, R.M. The Nuclear Pore Complex Is a Key Target of Viral Proteases to Promote Viral Replication. Viruses 2021, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, M.; Miyamoto, Y.; Suzuki, T.; Otani, M.; Inuki, S.; Esaki, T.; Nagao, C.; Mizuguchi, K.; Ohno, H.; Yoneda, Y.; et al. Novel Anti-Flavivirus Drugs Targeting the Nucleolar Distribution of Core Protein. Virology 2020, 541, 41–51. [Google Scholar] [CrossRef]
- De Jesús-González, L.A.; Palacios-Rápalo, S.N.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Farfán-Morales, C.N.; Cordero-Rivera, C.D.; Cisneros, B.; Gutiérrez-Escolano, A.L.; del Ángel, R.M. Nucleo-Cytoplasmic Transport of ZIKV Non-Structural 3 Protein Is Mediated by Importin-α/β and Exportin CRM-1. J. Virol. 2022, 97, e01773-22. [Google Scholar] [CrossRef]
- Peltonen, K.; Colis, L.; Liu, H.; Jäämaa, S.; Zhang, Z.; Af Hällström, T.; Moore, H.M.; Sirajuddin, P.; Laiho, M. Small Molecule BMH-Compounds That Inhibit RNA Polymerase I and Cause Nucleolar Stress. Mol. Cancer Ther. 2014, 13, 2537–2546. [Google Scholar] [CrossRef]
- Potapova, T.A.; Unruh, J.R.; Conkright-Fincham, J.; Banks, C.A.; Florens, L.; Schneider, D.A.; Gerton, J.L. Distinct States of Nucleolar Stress Induced by Anticancer Drugs. eLife 2023, 12, RP88799. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, P.; Pecoraro, A.; Palma, G.; Russo, G.; Russo, A. Therapeutic Approaches Targeting Nucleolus in Cancer. Cells 2019, 8, 1090. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Rápalo, S.N.; Farfan-Morales, C.N.; Cordero-Rivera, C.D.; Jesús-González, L.A.D.; Reyes-Ruiz, J.M.; Meraz-Ríos, M.A.; Ángel, R.M.D. An Ivermectin—Atorvastatin Combination Impairs Nuclear Transport Inhibiting Dengue Infection in Vitro and in Vivo. iScience 2023, 26, 108294. [Google Scholar] [CrossRef] [PubMed]
- Deutzmann, A.; Sullivan, D.K.; Dhanasekaran, R.; Li, W.; Chen, X.; Tong, L.; Mahauad-Fernandez, W.D.; Bell, J.; Mosley, A.; Koehler, A.N.; et al. Nuclear to Cytoplasmic Transport Is a Druggable Dependency in MYC-Driven Hepatocellular Carcinoma. Nat. Commun. 2024, 15, 963. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hurley, L.H. A First-in-Class Clinical G-Quadruplex-Targeting Drug. The Bench-to-Bedside Translation of the Fluoroquinolone QQ58 to CX-5461 (Pidnarulex). Bioorganic Med. Chem. Lett. 2022, 77, 129016. [Google Scholar] [CrossRef] [PubMed]
- Sirri, V.; Hernandez-Verdun, D.; Roussel, P. Cyclin-Dependent Kinases Govern Formation and Maintenance of the Nucleolus. J. Cell Biol. 2002, 156, 969–981. [Google Scholar] [CrossRef]
- Zhai, W.; Tuan, J.A.; Comai, L. SV40 Large T Antigen Binds to the TBP-TAF(I) Complex SL1 and Coactivates Ribosomal RNA Transcription. Genes Dev. 1997, 11, 1605–1617. [Google Scholar] [CrossRef]
- Zhai, W.; Comai, L. A Kinase Activity Associated with Simian Virus 40 Large T Antigen Phosphorylates Upstream Binding Factor (UBF) and Promotes Formation of a Stable Initiation Complex between UBF and SL1. Mol. Cell Biol. 1999, 19, 2791–2802. [Google Scholar] [CrossRef]
- Forte, I.M.; Indovina, P.; Montagnaro, S.; Costa, A.; Iannuzzi, C.A.; Capone, F.; Camerlingo, R.; Malfitano, A.M.; Pentimalli, F.; Ferrara, G.; et al. The Oncolytic Caprine Herpesvirus 1 (CpHV-1) Induces Apoptosis and Synergizes with Cisplatin in Mesothelioma Cell Lines: A New Potential Virotherapy Approach. Viruses 2021, 13, 2458. [Google Scholar] [CrossRef]
- Ahmad, S.I. 5-Fluorouracil in Combination with Deoxyribonucleosides and Deoxyribose as Possible Therapeutic Options for the Coronavirus, COVID-19 Infection. Med. Hypotheses 2020, 142, 109754. [Google Scholar] [CrossRef]
- Wu, K.X.; Chu, J.J.-H. Antiviral Screen Identifies EV71 Inhibitors and Reveals Camptothecin-Target, DNA Topoisomerase 1 as a Novel EV71 Host Factor. Antivir. Res. 2017, 143, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Yin, L.; Wang, J.; Shen, X.; Dai, Y.; Zhao, R.; Hu, X.; Hou, H.; Zhang, D.; Wang, G.; et al. Antiviral and Virucidal Activities of Camptothecin on Fowl Adenovirus Serotype 4 by Blocking Virus Replication. Front. Cell. Infect. Microbiol. 2022, 12, 823820. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-J.; Liu, C.-H.; Pan, Y.-C.; Wong, S.H.; Tai, C.-J.; Richardson, C.D.; Lin, L.-T. Chemovirotherapeutic Treatment Using Camptothecin Enhances Oncolytic Measles Virus-Mediated Killing of Breast Cancer Cells. Sci. Rep. 2019, 9, 6767. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, S.J.F.; De Burghgraeve, T.; Froeyen, M.; Pastorino, B.; Alen, M.M.F.; Mondotte, J.A.; Herdewijn, P.; Jacobs, M.; de Lamballerie, X.; Schols, D.; et al. A Derivate of the Antibiotic Doxorubicin Is a Selective Inhibitor of Dengue and Yellow Fever Virus Replication In Vitro. Antimicrob. Agents Chemother. 2010, 54, 5269–5280. [Google Scholar] [CrossRef] [PubMed]
- Sajid Jamal, Q.M.; Alharbi, A.H.; Ahmad, V. Identification of Doxorubicin as a Potential Therapeutic against SARS-CoV-2 (COVID-19) Protease: A Molecular Docking and Dynamics Simulation Studies. J. Biomol. Struct. Dyn. 2022, 40, 7960–7974. [Google Scholar] [CrossRef]
- Nakano, K.; Watanabe, T. HTLV-1 Rex Tunes the Cellular Environment Favorable for Viral Replication. Viruses 2016, 8, 58. [Google Scholar] [CrossRef]
- Fleta-Soriano, E.; Martinez, J.P.; Hinkelmann, B.; Gerth, K.; Washausen, P.; Diez, J.; Frank, R.; Sasse, F.; Meyerhans, A. The Myxobacterial Metabolite Ratjadone a Inhibits HIV Infection by Blocking the Rev/CRM1-Mediated Nuclear Export Pathway. Microb. Cell Factories 2014, 13, 17. [Google Scholar] [CrossRef]
- Perwitasari, O.; Johnson, S.; Yan, X.; Howerth, E.; Shacham, S.; Landesman, Y.; Baloglu, E.; McCauley, D.; Tamir, S.; Tompkins, S.M.; et al. Verdinexor, a Novel Selective Inhibitor of Nuclear Export, Reduces Influenza a Virus Replication In Vitro and In Vivo. J. Virol. 2014, 88, 10228–10243. [Google Scholar] [CrossRef]
- Jorquera, P.A.; Mathew, C.; Pickens, J.; Williams, C.; Luczo, J.M.; Tamir, S.; Ghildyal, R.; Tripp, R.A. Verdinexor (KPT-335), a Selective Inhibitor of Nuclear Export, Reduces Respiratory Syncytial Virus Replication In Vitro. J. Virol. 2019, 93, e01684-18. [Google Scholar] [CrossRef]
- Kashyap, T.; Murray, J.; Walker, C.J.; Chang, H.; Tamir, S.; Hou, B.; Shacham, S.; Kauffman, M.G.; Tripp, R.A.; Landesman, Y. Selinexor, a Novel Selective Inhibitor of Nuclear Export, Reduces SARS-CoV-2 Infection and Protects the Respiratory System in Vivo. Antiviral Res. 2021, 192, 105115. [Google Scholar] [CrossRef]
- Norseen, J.; Johnson, F.B.; Lieberman, P.M. Role for G-Quadruplex RNA Binding by Epstein-Barr Virus Nuclear Antigen 1 in DNA Replication and Metaphase Chromosome Attachment. J. Virol. 2009, 83, 10336–10346. [Google Scholar] [CrossRef] [PubMed]
- Artusi, S.; Perrone, R.; Lago, S.; Raffa, P.; Di Iorio, E.; Palù, G.; Richter, S.N. Visualization of DNA G-Quadruplexes in Herpes Simplex Virus 1-Infected Cells. Nucleic Acids Res. 2016, 44, 10343–10353. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-C.; Ravichandran, S.; Park, D.; Lee, G.M.; Kim, Y.-E.; Choi, Y.; Song, M.J.; Kim, K.K.; Ahn, J.-H. G-Quadruplexes Formed by Varicella-Zoster Virus Reiteration Sequences Suppress Expression of Glycoprotein C and Regulate Viral Cell-to-Cell Spread. PLoS Pathog. 2023, 19, e1011095. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Zhao, C.; Liu, Y.; Zhang, C.; Yang, G.; Yang, J.; Wang, Z.; Wang, C.; Tu, C.; Guo, Z.; et al. RNA G-Quadruplex Formed in SARS-CoV-2 Used for COVID-19 Treatment in Animal Models. Cell Discov. 2022, 8, 86. [Google Scholar] [CrossRef]
- Belachew, B.; Gao, J.; Byrd, A.K.; Raney, K.D. Hepatitis C Virus Nonstructural Protein NS3 Unfolds Viral G-Quadruplex RNA Structures. J. Biol. Chem. 2022, 298, 102486. [Google Scholar] [CrossRef]
- Ravichandran, S.; Kim, Y.-E.; Bansal, V.; Ghosh, A.; Hur, J.; Subramani, V.K.; Pradhan, S.; Lee, M.K.; Kim, K.K.; Ahn, J.-H. Genome-Wide Analysis of Regulatory G-Quadruplexes Affecting Gene Expression in Human Cytomegalovirus. PLoS Pathog. 2018, 14, e1007334. [Google Scholar] [CrossRef]
- Lv, L.; Cui, H.; Chen, Z.; Zhou, Y.; Zhang, L. G-Quadruplex Ligands Inhibit Chikungunya Virus Replication. J. Med. Virol. 2022, 94, 2519–2527. [Google Scholar] [CrossRef]
- Terrell, J.R.; Le, T.T.; Paul, A.; Brinton, M.A.; Wilson, W.D.; Poon, G.M.K.; Germann, M.W.; Siemer, J.L. Structure of an RNA G-Quadruplex from the West Nile Virus Genome. Nat. Commun. 2024, 15, 5428. [Google Scholar] [CrossRef]
- Amrane, S.; Jaubert, C.; Bedrat, A.; Rundstadler, T.; Recordon-Pinson, P.; Aknin, C.; Guédin, A.; De Rache, A.; Bartolucci, L.; Diene, I.; et al. Deciphering RNA G-Quadruplex Function during the Early Steps of HIV-1 Infection. Nucleic Acids Res. 2022, 50, 12328–12343. [Google Scholar] [CrossRef]
- Gemmill, D.L.; Nelson, C.R.; Badmalia, M.D.; Pereira, H.S.; Kerr, L.; Wolfinger, M.T.; Patel, T.R. The 3’ Terminal Region of Zika Virus RNA Contains a Conserved G-Quadruplex and Is Unfolded by Human DDX17. Biochem. Cell Biol. 2024, 102, 96–105. [Google Scholar] [CrossRef]
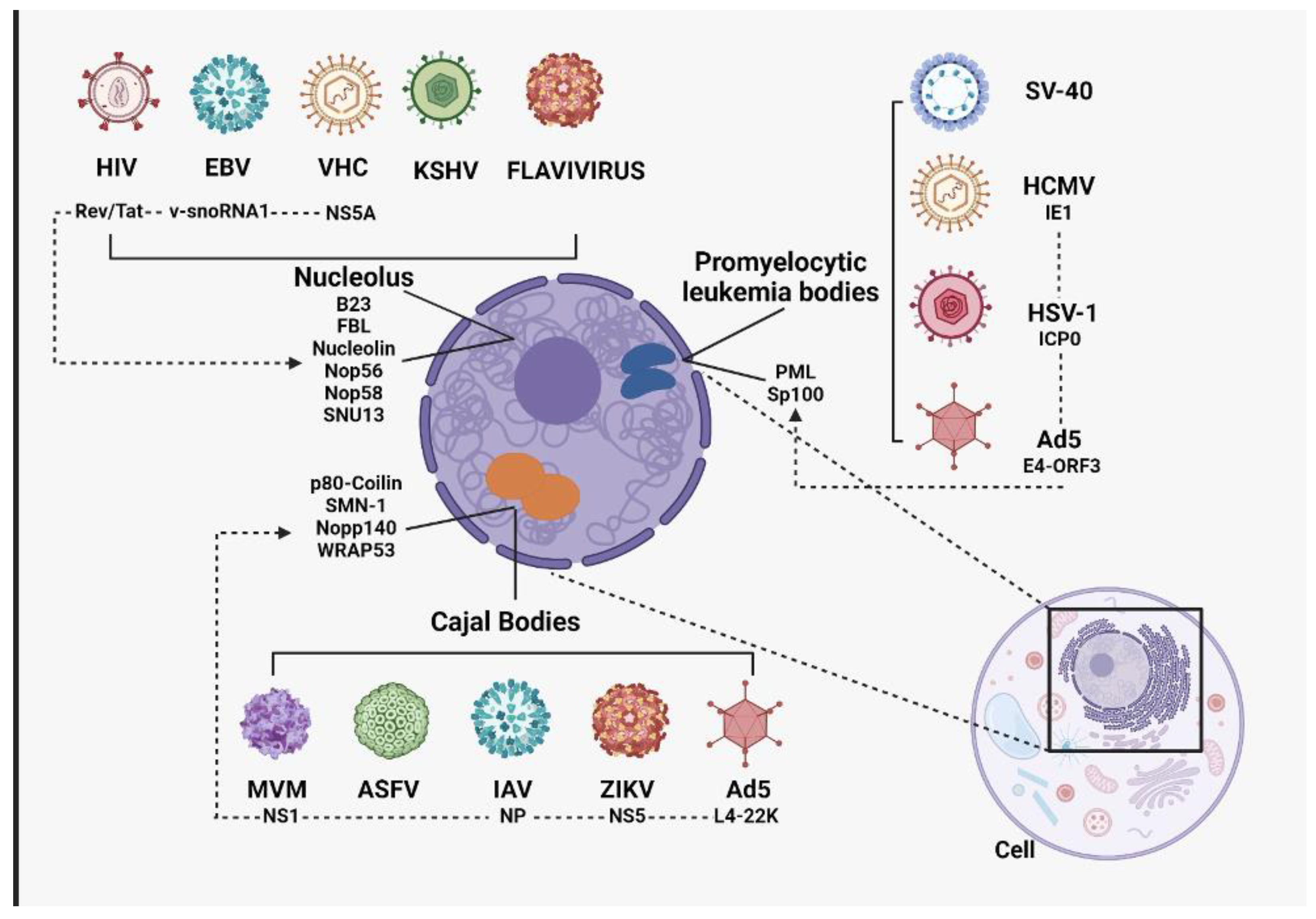
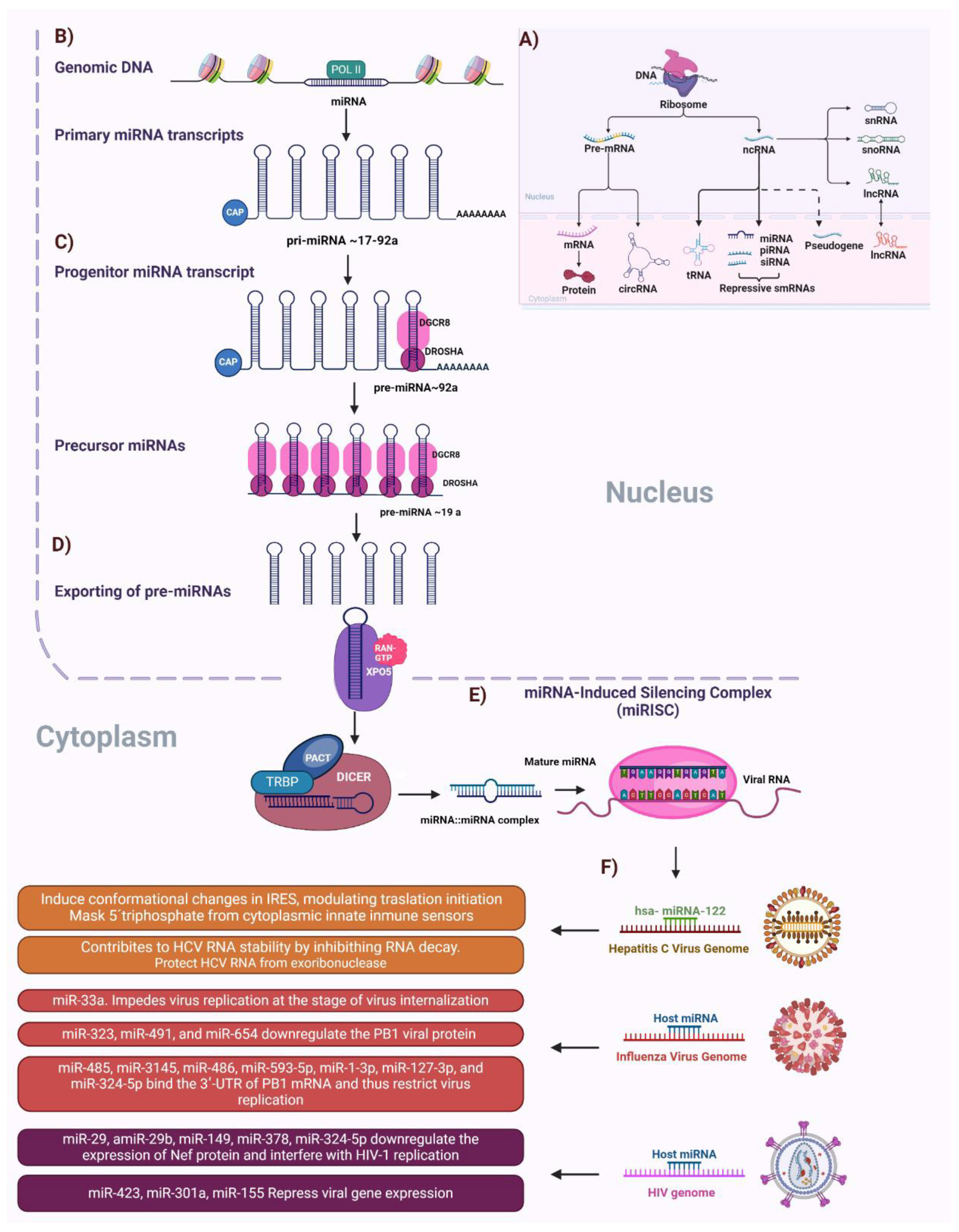
| Nucleolar Protein | Function | References |
|---|---|---|
| B23 (Nucleophosmin) | Participates in ribosome assembly, nucleocytoplasmic transport, and regulation of ribosomal DNA transcription. Has ribonuclease activity and prevents the formation of protein aggregates. Considered a core protein of the C/D box small nucleolar ribonucleoprotein (snoRNP) particles. | [48] |
| Fibrillarin (FBL) | S-adenosylmethionine-dependent methyltransferase that catalyzes ribosomal RNA methylation. Acts as a transcriptional regulator, cellular stress sensor, and ribonuclease. Considered a core protein of the C/D box small nucleolar ribonucleoprotein (snoRNP) particles. | [49] |
| Nucleolin | Involved in DNA and RNA metabolism, chromatin remodeling, DNA repair and replication, transcription, splicing, and transport of messenger RNAs. | [50] |
| Nop56 | Participates in ribosomal RNA processing and the formation of small nucleolar ribonucleoprotein complexes (snoRNPs). Necessary for SUMOylation and nucleolar localization. Considered a core protein of the C/D box small nucleolar ribonucleoprotein (snoRNP) particles. | [51] |
| Nop58 | Forms heteromers with Nop56 to stabilize ribosomal RNA in the C/D Box snoRNP complex, allowing ribosomal RNA methylation by fibrillarin. Considered a core protein of the C/D box small nucleolar ribonucleoprotein (snoRNP) particles | [51] |
| SNU13 | Binds to RNA to form rotational structures, promoting the formation of C/D box motifs and facilitating ribosomal RNA methylation. Considered a core protein of the C/D box small nucleolar ribonucleoprotein (snoRNP) particles. | [52] |
| Drug | Mechanism of Action | Target and Effects | References |
|---|---|---|---|
| BMH-21 | Inhibition of rRNA transcription. | Decreases RNAm production, essential for viral protein synthesis. | [113] |
| CDK Inhibitors | Disrupt nucleolar scaffold, causing nucleolar dissolution and affecting rRNA transcription. | Impairs nucleolar function and nucleus–cytoplasm trafficking. | [114] |
| Cisplatin | Forms adducts with rDNA and nucleolar proteins, creating steric hindrances. | Prevents proper assembly and function of nucleolar components, blocking viral access. | [115] |
| 5-Fluorouracil (5-FU) | Targets nucleolar structures, disrupting nucleolar organization and function. | Affects the transport of rRNA and other molecules. | [115] |
| Camptothecin | Inhibits Pol I, reducing rRNA synthesis. | Decreases ribosome production, essential for viral protein synthesis. | [114] |
| Doxorubicin | Inhibits Pol I, reducing rRNA synthesis. | Decreases ribosome production, essential for viral protein synthesis. | [114] |
| Atorvastatin and Ivermectin | Disrupts nuclear–cytoplasmic transport of viral proteins. | Impairs trafficking in Dengue virus and ZIKV. | [116] |
| Leptomycin B | Inhibits CRM1/exportin 1, preventing nuclear export of viral proteins and RNAs. | Blocks replication of HIV, Influenza, and other viruses. | [116] |
| Selinexor | Inhibits CRM1/exportin 1, blocking nuclear export of viral components. | Used in cancers and viral infections. | [117] |
| Quarfloxin (CX-3543) | Inhibits RNA polymerase I (Pol I), reduces ribosomal RNA (rRNA) synthesis. | Disrupts the nucleolin–rDNA complex | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulloa-Aguilar, J.M.; Herrera Moro Huitron, L.; Benítez-Zeferino, R.Y.; Cerna-Cortes, J.F.; García-Cordero, J.; León-Reyes, G.; Guzman-Bautista, E.R.; Farfan-Morales, C.N.; Reyes-Ruiz, J.M.; Miranda-Labra, R.U.; et al. The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection. Cells 2024, 13, 1591. https://doi.org/10.3390/cells13181591
Ulloa-Aguilar JM, Herrera Moro Huitron L, Benítez-Zeferino RY, Cerna-Cortes JF, García-Cordero J, León-Reyes G, Guzman-Bautista ER, Farfan-Morales CN, Reyes-Ruiz JM, Miranda-Labra RU, et al. The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection. Cells. 2024; 13(18):1591. https://doi.org/10.3390/cells13181591
Chicago/Turabian StyleUlloa-Aguilar, José Manuel, Luis Herrera Moro Huitron, Rocío Yazmin Benítez-Zeferino, Jorge Francisco Cerna-Cortes, Julio García-Cordero, Guadalupe León-Reyes, Edgar Rodrigo Guzman-Bautista, Carlos Noe Farfan-Morales, José Manuel Reyes-Ruiz, Roxana U. Miranda-Labra, and et al. 2024. "The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection" Cells 13, no. 18: 1591. https://doi.org/10.3390/cells13181591
APA StyleUlloa-Aguilar, J. M., Herrera Moro Huitron, L., Benítez-Zeferino, R. Y., Cerna-Cortes, J. F., García-Cordero, J., León-Reyes, G., Guzman-Bautista, E. R., Farfan-Morales, C. N., Reyes-Ruiz, J. M., Miranda-Labra, R. U., De Jesús-González, L. A., & León-Juárez, M. (2024). The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection. Cells, 13(18), 1591. https://doi.org/10.3390/cells13181591








