Administration of Essential Phospholipids Prevents Drosophila Melanogaster Oocytes from Responding to Change in Gravity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Stiffness Measurements Using Atomic Force Microscopy
2.3. Determination of the Relative Contents of Cholesterol and Neutral Lipids
2.4. Statistical Analysis
3. Results
3.1. Oocytes’ Stiffness
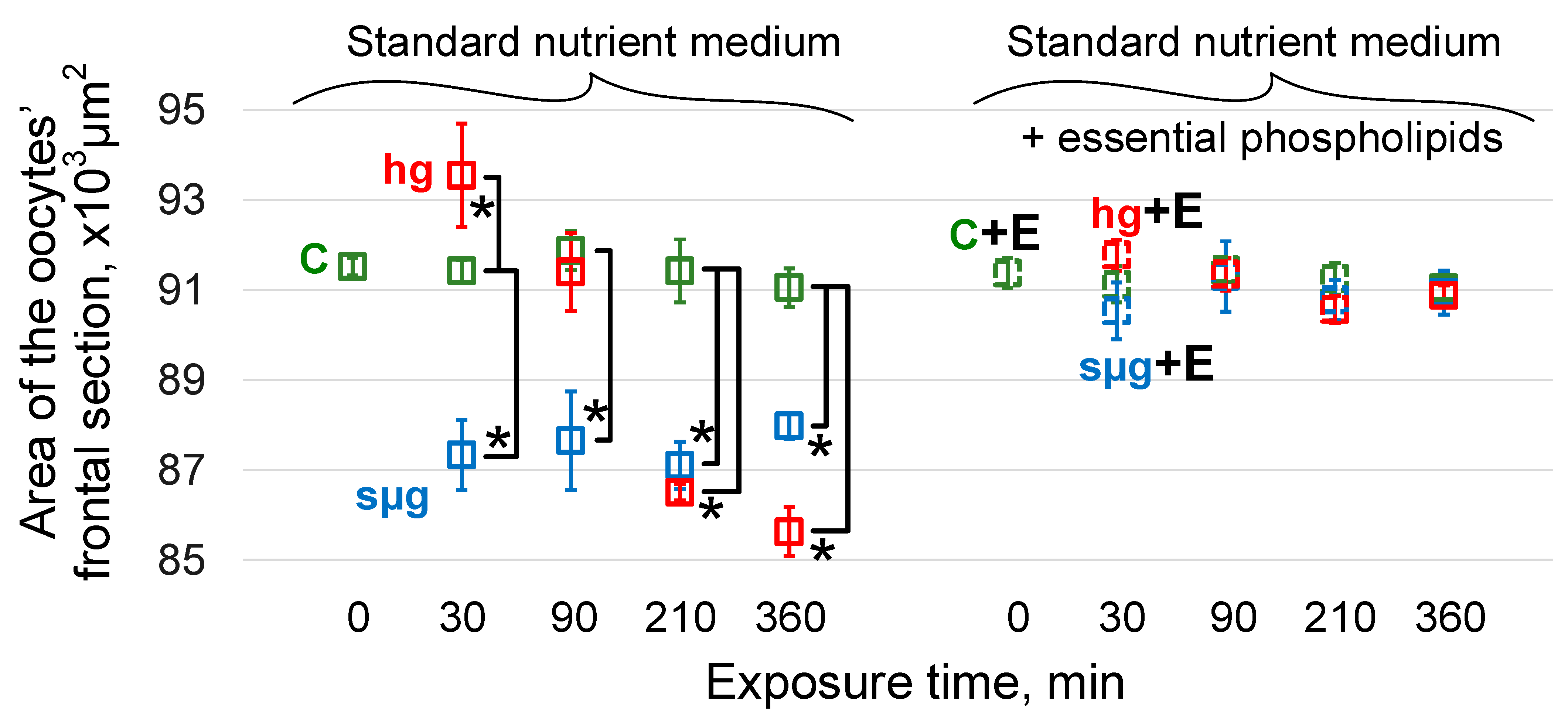
3.2. Maximum Cross-Sectional Area of Oocytes
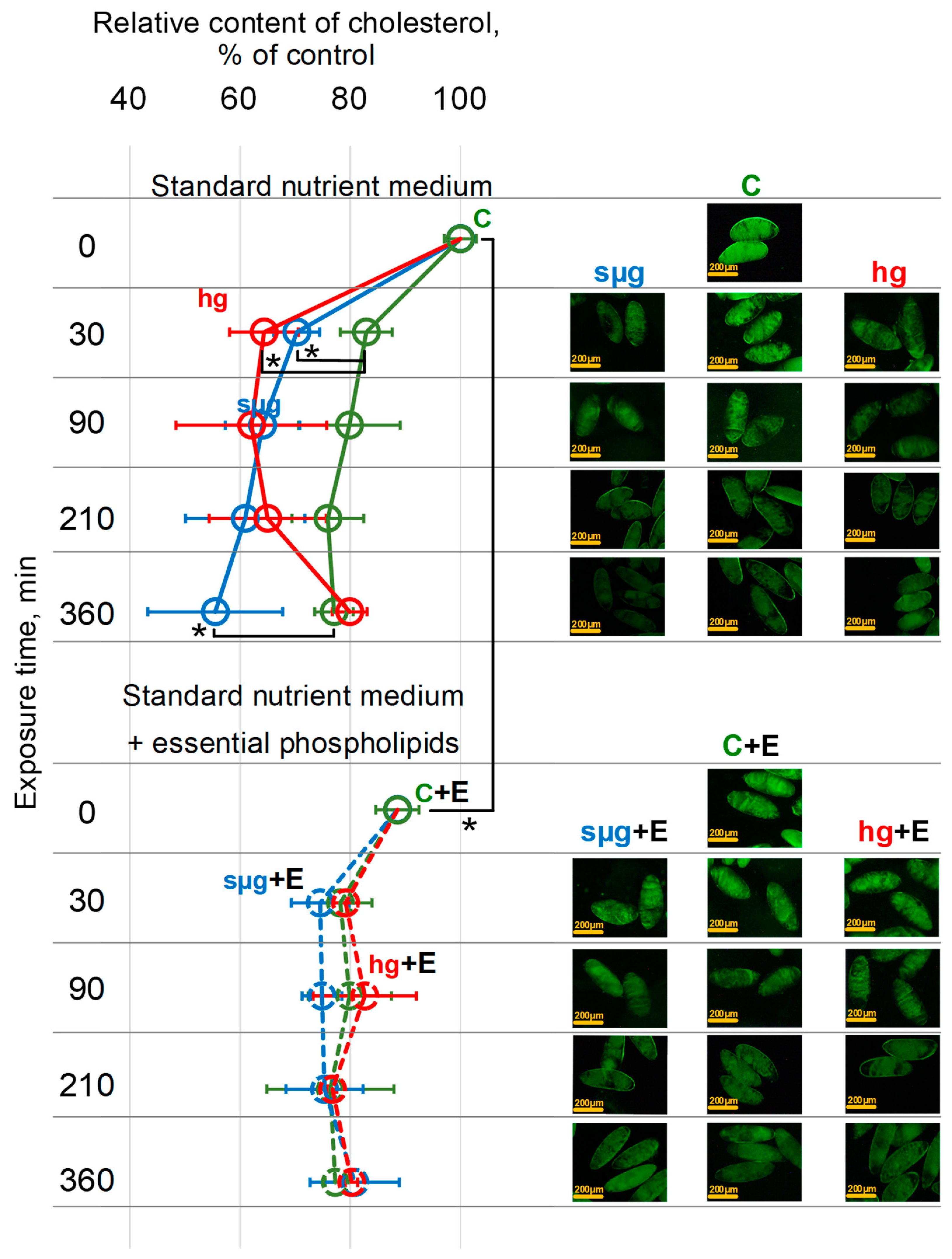
3.3. Relative Cholesterol Content
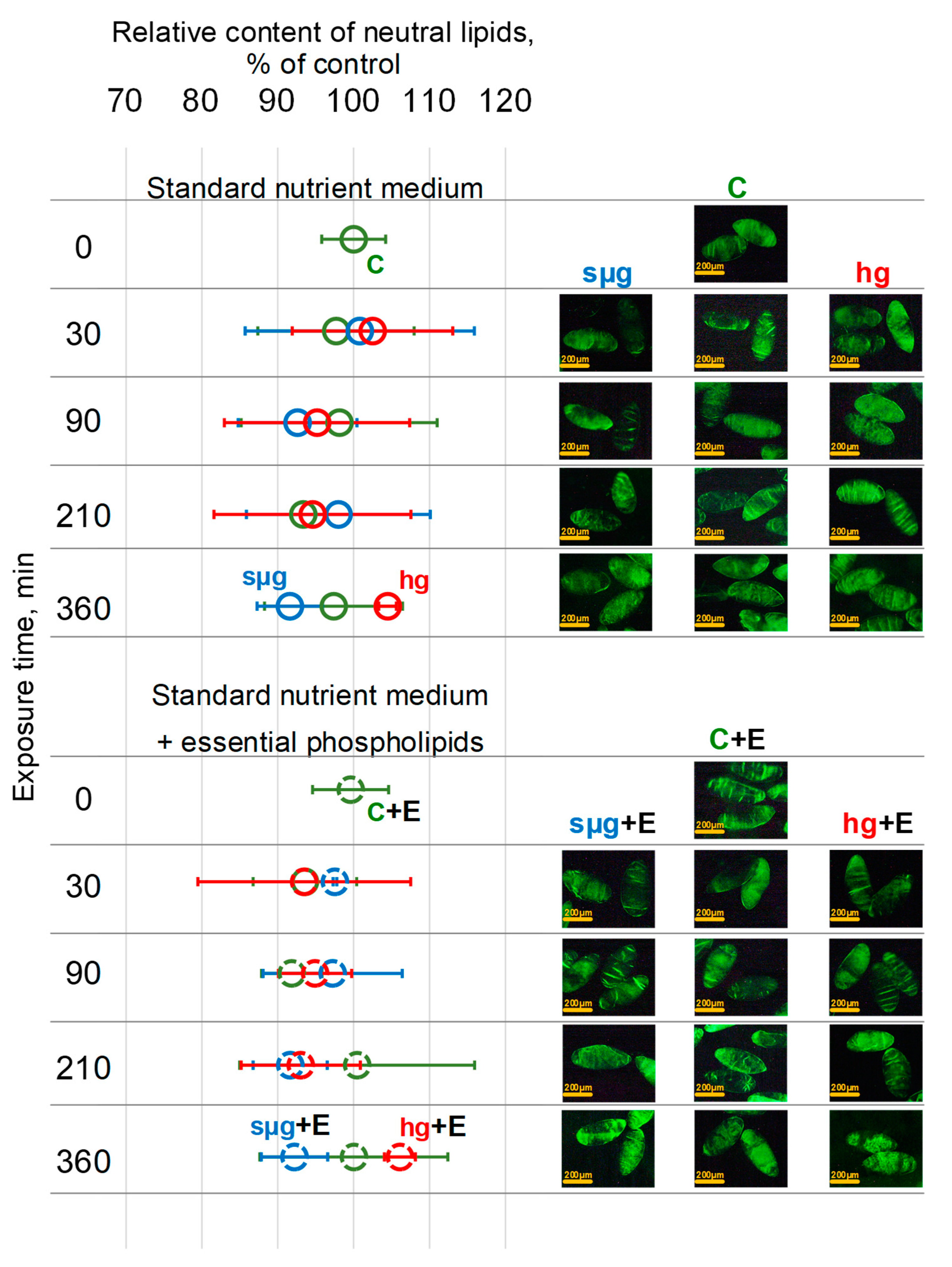
3.4. Relative Content of Neutral Lipids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hargens, A.R.; Watenpaugh, D.E. Cardiovascular adaptation to spaceflight. Med. Sci. Sports Exerc. 1996, 28, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Baevsky, R.; Moser, M.; Nikulina, G.; Polyakov, V.; Funtova, I.; Chernikova, A. Autonomic regulation of circulation and cardiac contractility during a 14-month space flight. Acta Astronaut. 1998, 42, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Delp, M.D.; Charvat, J.M.; Limoli, C.L.; Globus, R.K.; Ghosh, P. Apollo Lunar Astronauts Show Higher Cardiovascular Disease Mortality: Possible Deep Space Radiation Effects on the Vascular Endothelium. Sci. Rep. 2016, 6, 29901. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; Marchal, S.; Campos, S.G.; Rehnberg, E.; Tabury, K.; Baselet, B.; Wehland, M.; Grimm, D.; Baatout, S. The Cardiovascular System in Space: Focus on In Vivo and In Vitro Studies. Biomedicines 2021, 10, 59. [Google Scholar] [CrossRef]
- Sofronova, S.I.; Tarasova, O.S.; Gaynullina, D.; Borzykh, A.A.; Behnke, B.J.; Stabley, J.N.; McCullough, D.J.; Maraj, J.J.; Hanna, M.; Muller-Delp, J.M.; et al. Spaceflight on the Bion-M1 biosatellite alters cerebral artery vasomotor and mechanical properties in mice. J. Appl. Physiol. 2015, 118, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.W.; Nishiyama, N.C.; Byrum, S.D.; Stanbouly, S.; Jones, T.; Holley, J.; Sridharan, V.; Boerma, M.; Tackett, A.J.; Willey, J.S.; et al. Spaceflight induces oxidative damage to blood-brain barrier integrity in a mouse model. FASEB J. 2020, 34, 15516–15530. [Google Scholar] [CrossRef]
- Oganov, V.S. Modern analysis of bone loss mechanisms in microgravity. J. Gravit. Physiol. 2004, 11, P143–P146. [Google Scholar]
- Ohira, T.; Kawano, F.; Ohira, T.; Goto, K.; Ohira, Y. Responses of skeletal muscles to gravitational unloading and/or reloading. J. Physiol. Sci. 2015, 65, 293–310. [Google Scholar] [CrossRef]
- Vico, L.; Hargens, A. Skeletal changes during and after spaceflight. Nat. Rev. Rheumatol. 2018, 14, 229–245. [Google Scholar] [CrossRef]
- Ohira, T.; Kawano, F.; Goto, K.; Kaji, H.; Ohira, Y. Responses of neuromuscular properties to unloading and potential countermeasures during space exploration missions. Neurosci. Biobehav. Rev. 2022, 136, 104617. [Google Scholar] [CrossRef]
- Lee, P.H.U.; Chung, M.; Ren, Z.; Mair, D.B.; Kim, D.-H. Factors mediating spaceflight-induced skeletal muscle atrophy. Am. J. Physiol. Physiol. 2022, 322, C567–C580. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, I.B. Countermeasures for long-term space flights, lessons learned from the Russian space program. J. Gravit. Physiol. 2002, 9, P313–P317. [Google Scholar] [PubMed]
- Smith, S.M.; A Heer, M.; Shackelford, L.C.; Sibonga, J.D.; Ploutz-Snyder, L.; Zwart, S.R. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J. Bone Miner. Res. 2012, 27, 1896–1906. [Google Scholar] [CrossRef]
- Schlüter, K.; Piper, H.M. Regulation of growth in the adult cardiomyocytes. FASEB J. 1999, 13, S17–S22. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; Trappe, S.W.; Costill, D.L.; Gallagher, P.M.; Creer, A.C.; Colloton, P.A.; Peters, J.R.; Romatowski, J.G.; Bain, J.L.; Riley, D.A. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J. Physiol. 2010, 588, 3567–3592. [Google Scholar] [CrossRef] [PubMed]
- Ogneva, I.V.; Maximova, M.V.; Larina, I.M. Structure of cortical cytoskeleton in fibers of mouse muscle cells after being exposed to a 30-day space flight on board the BION-M1 biosatellite. J. Appl. Physiol. 2014, 116, 1315–1323. [Google Scholar] [CrossRef]
- Ogneva, I.V.; Gnyubkin, V.; Laroche, N.; Maximova, M.V.; Larina, I.M.; Vico, L. Structure of the cortical cytoskeleton in fibers of postural muscles and cardiomyocytes of mice after 30-day 2-g centrifugation. J. Appl. Physiol. 2015, 118, 613–623. [Google Scholar] [CrossRef]
- Walls, S.; Diop, S.; Birse, R.; Elmen, L.; Gan, Z.; Kalvakuri, S.; Pineda, S.; Reddy, C.; Taylor, E.; Trinh, B.; et al. Prolonged Exposure to Microgravity Reduces Cardiac Contractility and Initiates Remodeling in Drosophila. Cell Rep. 2020, 33, 108445. [Google Scholar] [CrossRef]
- Iandolo, D.; Strigini, M.; Guignandon, A.; Vico, L. Osteocytes and Weightlessness. Curr. Osteoporos. Rep. 2021, 19, 626–636. [Google Scholar] [CrossRef]
- Kohn, F.P.M.; Ritzmann, R. Gravity and neuronal adaptation, in vitro and in vivo—from neuronal cells up to neuromuscular responses: A first model. Eur. Biophys. J. 2017, 47, 97–107. [Google Scholar] [CrossRef]
- Sundaresan, A.; Mann, V.; Chaganti, M. Cellular changes in the nervous system when exposed to gravitational variation. Neurol. India 2019, 67, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Thiel, C.S.; de Zélicourt, D.; Tauber, S.; Adrian, A.; Franz, M.; Simmet, D.M.; Schoppmann, K.; Hauschild, S.; Krammer, S.; Christen, M.; et al. Rapid adaptation to microgravity in mammalian macrophage cells. Sci. Rep. 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Thiel, C.S.; Tauber, S.; Lauber, B.; Polzer, J.; Seebacher, C.; Uhl, R.; Neelam, S.; Zhang, Y.; Levine, H.; Ullrich, O. Rapid Morphological and Cytoskeletal Response to Microgravity in Human Primary Macrophages. Int. J. Mol. Sci. 2019, 20, 2402. [Google Scholar] [CrossRef] [PubMed]
- Schatten, H.; Lewis, M.L.; Chakrabarti, A. Spaceflight and clinorotation cause cytoskeleton and mitochondria changes and increases in apoptosis in cultured cells. Acta Astronaut 2001, 49, 399–418. [Google Scholar] [CrossRef]
- Uva, B.M.; Masini, M.A.; Sturla, M.; Prato, P.; Passalacqua, M.; Giuliani, M.; Tagliafierro, G.; Strollo, F. Clinorotation-induced weightlessness influences the cytoskeleton of glial cells in culture. Brain Res. 2002, 934, 132–139. [Google Scholar] [CrossRef]
- Gaboyard, S.; Blanchard, M.P.; Travo, C.; Viso, M.; Sans, A.; Lehouelleur, J. Weightlessness affects cytoskeleton of rat utricular hair cells during maturation in vitro. NeuroReport 2002, 13, 2139–2142. [Google Scholar] [CrossRef]
- Kacena, M.A.; Todd, P.; Landis, W.J. Osteoblasts subjected to spaceflight and simulated space shuttle launch conditions. In Vitro Cell Dev. Biol. Anim. 2003, 39, 454–459. [Google Scholar] [CrossRef]
- Crawford-Young, S.J. Effects of microgravity on cell cytoskeleton and embryogenesis. Int. J. Dev. Biol. 2006, 50, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Corydon, T.J.; Kopp, S.; Wehland, M.; Braun, M.; Schütte, A.; Mayer, T.; Hülsing, T.; Oltmann, H.; Schmitz, B.; Hemmersbach, R.; et al. Alterations of the cytoskeleton in human cells in space proved by life-cell imaging. Sci. Rep. 2016, 6, 20043. [Google Scholar] [CrossRef]
- Ogneva, I.V.; Golubkova, M.A.; Biryukov, N.S.; Kotov, O.V. Drosophila melanogaster Oocytes after Space Flight: The Early Period of Adaptation to the Force of Gravity. Cells 2022, 11, 3871. [Google Scholar] [CrossRef]
- Ogneva, I.V. Cell Mechanosensitivity: Mechanical Properties and Interaction with Gravitational Field. BioMed Res. Int. 2013, 2013, 598461. [Google Scholar] [CrossRef] [PubMed]
- Ogneva, I.V. Single Cell in a Gravity Field. Life 2022, 12, 1601. [Google Scholar] [CrossRef] [PubMed]
- Ogneva, I.V. The Mechanoreception in Drosophila melanogaster Oocyte under Modeling Micro- and Hypergravity. Cells 2023, 12, 1819. [Google Scholar] [CrossRef] [PubMed]
- Morachevskaya, E.; Sudarikova, A.; Negulyaev, Y. Mechanosensitive channel activity and F-actin organization in cholesterol-depleted human leukaemia cells. Cell Biol. Int. 2007, 31, 374–381. [Google Scholar] [CrossRef]
- Chubinskiy-Nadezhdin, V.I.; Negulyaev, Y.A.; Morachevskaya, E.A. Cholesterol depletion-induced inhibition of stretch-activated channels is mediated via actin rearrangement. Biochem. Biophys. Res. Commun. 2011, 412, 80–85. [Google Scholar] [CrossRef]
- Jayaraman, T.; Kannappan, S.; Ravichandran, M.K.; Anuradha, C.V. Impact of Essentiale L on ethanol-induced changes in rat brain and erythrocytes. Singapore Med. J. 2008, 49, 320–327. [Google Scholar]
- Sventitskaya, M.A.; Ogneva, I.V. Reorganization of the mouse oocyte’ cytoskeleton after cultivation under simulated weightlessness. Life Sci. Space Res. 2024, 40, 8–18. [Google Scholar] [CrossRef]
- Tran, S.L.; Welte, M.A. In-vivo centrifugation of Drosophila embryos. J. Vis. Exp. 2010, 23, 2005. [Google Scholar] [CrossRef]
- Harder, T.; Simons, K. Clusters of glycolipid and glycosylphosphatidylinositol-anchored proteins in lymphoid cells: Accumulation of actin regulated by local tyrosine phosphorylation. Eur. J. Immunol. 1999, 29, 556–562. [Google Scholar] [CrossRef]
- Brown, D.A.; London, E. Structure and Function of Sphingolipid- and Cholesterol-rich Membrane Rafts. J. Biol. Chem. 2000, 275, 17221–17224. [Google Scholar] [CrossRef]
- Brown, D.A. Lipid Rafts, Detergent-Resistant Membranes, and Raft Targeting Signals. Physiology 2006, 21, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.; Quiroz, A.; Santander, N.; Morselli, E.; Busso, D. Implications of High-Density Cholesterol Metabolism for Oocyte Biology and Female Fertility. Front. Cell Dev. Biol. 2022, 10, 941539. [Google Scholar] [CrossRef] [PubMed]
- Juhl, A.D.; Wüstner, D. Pathways and Mechanisms of Cellular Cholesterol Efflux—Insight From Imaging. Front. Cell Dev. Biol. 2022, 10, 834408. [Google Scholar] [CrossRef]
- Ayling, L.J.; Briddon, S.J.; Halls, M.L.; Hammond, G.R.V.; Vaca, L.; Pacheco, J.; Hill, S.J.; Cooper, D.M.F. Adenylyl cyclase AC8 directly controls its micro-environment by recruiting the actin cytoskeleton in a cholesterol-rich milieu. J. Cell Sci. 2012, 125, 869–886. [Google Scholar] [CrossRef] [PubMed]
- Bukowiecka-Matusiak, M.; Burzynska-Pedziwiatr, I.; Sansone, A.; Malachowska, B.; Zurawska-Klis, M.; Ferreri, C.; Chatgilialoglu, C.; Ochedalski, T.; Cypryk, K.; Wozniak, L.A. Lipid profile changes in erythrocyte membranes of women with diagnosed GDM. PLoS ONE 2018, 13, e0203799. [Google Scholar] [CrossRef]
- Nishino, M.; Matsuzaki, I.; Musangile, F.Y.; Takahashi, Y.; Iwahashi, Y.; Warigaya, K.; Kinoshita, Y.; Kojima, F.; Murata, S.-I. Measurement and visualization of cell membrane surface charge in fixed cultured cells related with cell morphology. PLoS ONE 2020, 15, e0236373. [Google Scholar] [CrossRef]
- Abdolahad, M.; Shashaani, H.; Janmaleki, M.; Mohajerzadeh, S. Silicon nanograss based impedance biosensor for label free detection of rare metastatic cells among primary cancerous colon cells, suitable for more accurate cancer staging. Biosens. Bioelectron. 2014, 59, 151–159. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Solomatin, D.V.; Kosenok, V.K. Analysis of the lipid profile of saliva in ovarian and endometrial cancer by IR fourier spectroscopy. Vib. Spectrosc. 2019, 104, 102944. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gogichaeva, K.K.; Ogneva, I.V. Administration of Essential Phospholipids Prevents Drosophila Melanogaster Oocytes from Responding to Change in Gravity. Cells 2024, 13, 1593. https://doi.org/10.3390/cells13181593
Gogichaeva KK, Ogneva IV. Administration of Essential Phospholipids Prevents Drosophila Melanogaster Oocytes from Responding to Change in Gravity. Cells. 2024; 13(18):1593. https://doi.org/10.3390/cells13181593
Chicago/Turabian StyleGogichaeva, Ksenia K., and Irina V. Ogneva. 2024. "Administration of Essential Phospholipids Prevents Drosophila Melanogaster Oocytes from Responding to Change in Gravity" Cells 13, no. 18: 1593. https://doi.org/10.3390/cells13181593




