Innovative Strategies for Liver Transplantation: The Role of Mesenchymal Stem Cells and Their Cell-Free Derivatives
Abstract
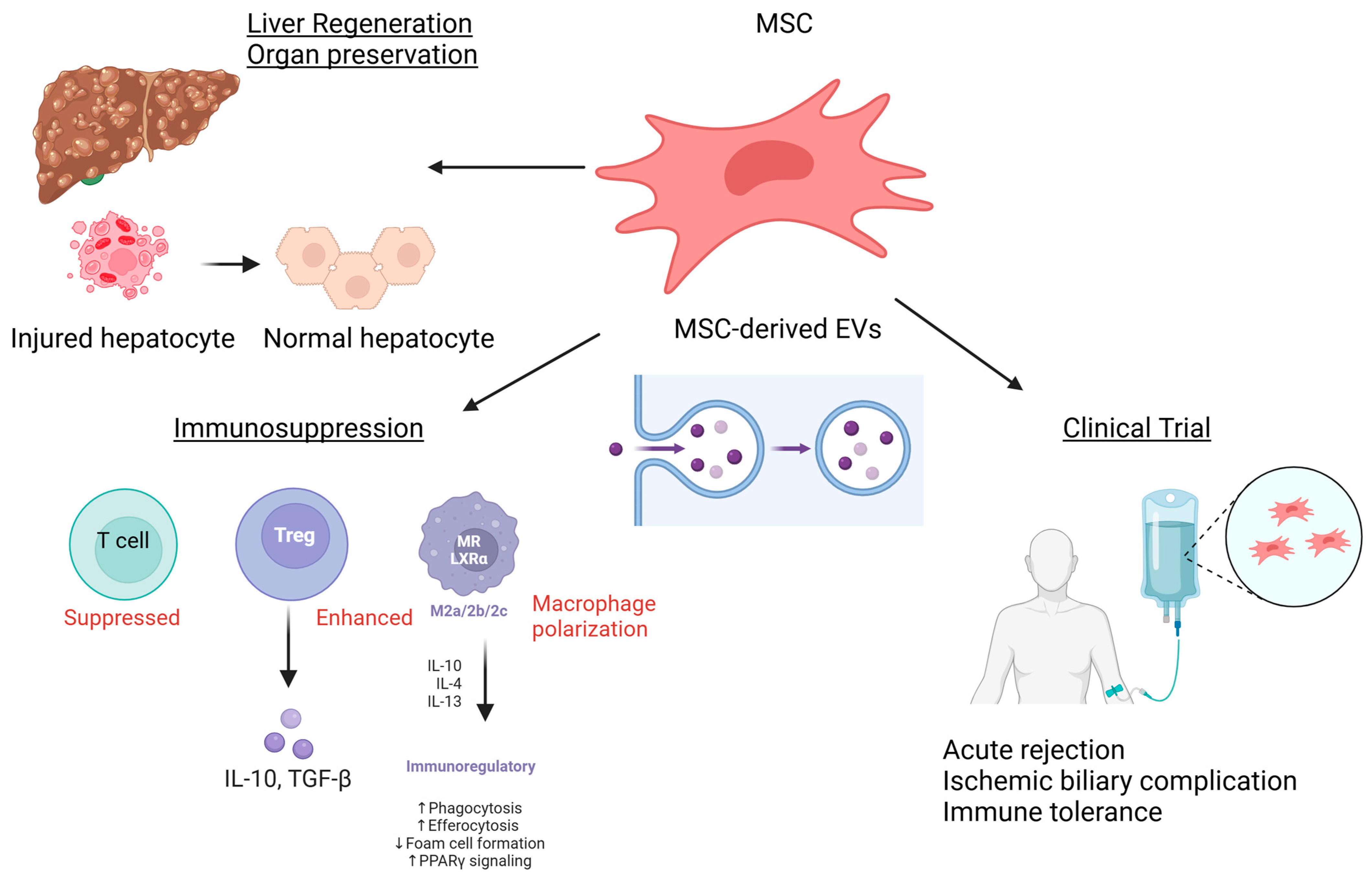
:1. Introduction
2. MSCs in Liver Regeneration
3. Ischemia–Reperfusion Injury and Immunosuppression Therapy
4. Organ Preservation and Restoration of Organ Function
5. Current Research and Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, C.A.; Sinha, S.; Fitzpatrick, E.; Dhawan, A. Hepatocyte transplantation and advancements in alternative cell sources for liver-based regenerative medicine. J. Mol. Med. 2018, 96, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Fan, L.; Zhang, F.; Li, L. The clinical application of mesenchymal stem cells in liver disease: The current situation and potential future. Ann. Transl. Med. 2020, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Deo, D.; Marchioni, M.; Rao, P. Mesenchymal Stem/Stromal Cells in Organ Transplantation. Pharmaceutics 2022, 14, 791. [Google Scholar] [CrossRef]
- Hora, S.; Wuestefeld, T. Liver Injury and Regeneration: Current Understanding, New Approaches, and Future Perspectives. Cells 2023, 12, 2129. [Google Scholar] [CrossRef]
- Miceli, M.; Baldi, D.; Cavaliere, C.; Soricelli, A.; Salvatore, M.; Napoli, C. Peripheral artery disease: The new frontiers of imaging techniques to evaluate the evolution of regenerative medicine. Expert Rev. Cardiovasc. Ther. 2019, 17, 511–532. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Okita, K.; Mochiduki, Y.; Takizawa, N.; Yamanaka, S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008, 26, 101–106. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, L.; Li, L. Current understanding of adipose-derived mesenchymal stem cell-based therapies in liver diseases. Stem Cell Res. Ther. 2019, 10, 199. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, J.; Zhou, Q.; Xin, J.; Jiang, J.; Jiang, L.; Wu, T.; Li, J.; Ding, W.; Li, J.; et al. Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut 2017, 66, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Cao, H.; Pan, X.; Li, J.; He, J.; Pan, Q.; Xin, J.; Yu, X.; Li, J.; Wang, Y.; et al. Adipogenic placenta-derived mesenchymal stem cells are not lineage restricted by withdrawing extrinsic factors: Developing a novel visual angle in stem cell biology. Cell Death Dis. 2016, 7, e2141. [Google Scholar] [CrossRef] [PubMed]
- Lindenmair, A.; Hatlapatka, T.; Kollwig, G.; Hennerbichler, S.; Gabriel, C.; Wolbank, S.; Redl, H.; Kasper, C. Mesenchymal stem or stromal cells from amnion and umbilical cord tissue and their potential for clinical applications. Cells 2012, 1, 1061–1088. [Google Scholar] [CrossRef]
- Chen, L.; Qu, J.; Cheng, T.; Chen, X.; Xiang, C. Menstrual blood-derived stem cells: Toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem Cell Res. Ther. 2019, 10, 406. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Kurtz, A. Mesenchymal stem cell delivery routes and fate. Int. J. Stem Cells 2008, 1, 1–7. [Google Scholar] [CrossRef]
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies—Bridging scientific observations and regulatory viewpoints. Cytotherapy 2013, 15, 753–759. [Google Scholar] [CrossRef]
- Prockop, D.J.; Brenner, M.; Fibbe, W.E.; Horwitz, E.; Le Blanc, K.; Phinney, D.G.; Simmons, P.J.; Sensebe, L.; Keating, A. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy 2010, 12, 576–578. [Google Scholar] [CrossRef]
- Miura, M.; Miura, Y.; Padilla-Nash, H.M.; Molinolo, A.A.; Fu, B.; Patel, V.; Seo, B.M.; Sonoyama, W.; Zheng, J.J.; Baker, C.C.; et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells 2006, 24, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Perez, S.A.; Salagianni, M.; Baxevanis, C.N.; Papamichail, M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 2006, 24, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Furlani, D.; Ugurlucan, M.; Ong, L.; Bieback, K.; Pittermann, E.; Westien, I.; Wang, W.; Yerebakan, C.; Li, W.; Gaebel, R.; et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc. Res. 2009, 77, 370–376. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Luk, F.; Dahlke, M.H.; Hoogduijn, M.J. The life and fate of mesenchymal stem cells. Front. Immunol. 2014, 5, 148. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef]
- Beer, L.; Mildner, M.; Ankersmit, H.J. Cell secretome based drug substances in regenerative medicine: When regulatory affairs meet basic science. Ann. Transl. Med. 2017, 5, 170. [Google Scholar] [CrossRef]
- Bermudez, M.A.; Sendon-Lago, J.; Seoane, S.; Eiro, N.; Gonzalez, F.; Saa, J.; Vizoso, F.; Perez-Fernandez, R. Anti-inflammatory effect of conditioned medium from human uterine cervical stem cells in uveitis. Exp. Eye Res. 2016, 149, 84–92. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, A.J.; Woffindale, C.A.; Wood, M.J. Exosomes and the emerging field of exosome-based gene therapy. Curr. Gene Ther. 2012, 12, 262–274. [Google Scholar] [CrossRef]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, M.Y.; Eom, Y.W.; Baik, S.K. Mesenchymal Stem Cells for the Treatment of Liver Disease: Present and Perspectives. Gut Liver 2020, 14, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Lightner, A.L.; Fujiki, M.; Elshawy, M.; Dadgar, N.; Barnoski, A.; Osman, M.; Fulmer, C.G.; Vaidya, A. Mesenchymal Stem Cell Extracellular Vesicles as a New Treatment Paradigm in Solid Abdominal Organ Transplantation: A Case Series. Stem Cells Dev. 2024, 33, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Hua, T.; Ouyang, T.; Qian, H.; Yu, B. Applications of Mesenchymal Stem Cells in Liver Fibrosis: Novel Strategies, Mechanisms, and Clinical Practice. Stem Cells Int. 2021, 2021, 6546780. [Google Scholar] [CrossRef] [PubMed]
- Serras, A.S.M.; Cipriano, M.Z.d.R.F.; da Graça Silva, P.M.; Miranda, J.P.G. Challenges for deriving hepatocyte-like cells from umbilical cord mesenchymal stem cells for in vitro toxicology applications. In Novel Perspectives of Stem Cell Manufacturing and Therapies; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Klyushnenkova, E.; Mosca, J.D.; Zernetkina, V.; Majumdar, M.K.; Beggs, K.J.; Simonetti, D.W.; Deans, R.J.; McIntosh, K.R. T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. J. Biomed. Sci. 2005, 12, 47–57. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. Improvement of mesenchymal stromal cells and their derivatives for treating acute liver failure. J. Mol. Med. 2019, 97, 1065–1084. [Google Scholar] [CrossRef]
- Tietze, L.; Christ, M.; Yu, J.; Stock, P.; Nickel, S.; Schulze, A.; Bartels, M.; Tautenhahn, H.M.; Christ, B. Approaching Thrombospondin-1 as a Potential Target for Mesenchymal Stromal Cells to Support Liver Regeneration after Partial Hepatectomy in Mouse and Humans. Cells 2024, 13, 529. [Google Scholar] [CrossRef]
- Dai, L.J.; Li, H.Y.; Guan, L.X.; Ritchie, G.; Zhou, J.X. The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res. 2009, 2, 16–25. [Google Scholar] [CrossRef]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef]
- Hyenne, V.; Apaydin, A.; Rodriguez, D.; Spiegelhalter, C.; Hoff-Yoessle, S.; Diem, M.; Tak, S.; Lefebvre, O.; Schwab, Y.; Goetz, J.G.; et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J. Cell Biol. 2015, 211, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.F.; Otoc, N.; Sethi, J.K.; Gupta, A.; Antes, T.J. Integrated systems for exosome investigation. Methods 2015, 87, 31–45. [Google Scholar] [CrossRef]
- Lai, R.C.; Yeo, R.W.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015, 40, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhu, Y.L.; Zhou, Y.Y.; Liang, G.F.; Wang, Y.Y.; Hu, F.H.; Xiao, Z.D. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 2014, 289, 22258–22267. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Chen, T.S.; Lim, S.K. Mesenchymal stem cell exosome: A novel stem cell-based therapy for cardiovascular disease. Regen. Med. 2011, 6, 481–492. [Google Scholar] [CrossRef]
- Kim, H.S.; Choi, D.Y.; Yun, S.J.; Choi, S.M.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D.W. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J. Proteome Res. 2012, 11, 839–849. [Google Scholar] [CrossRef]
- Angulski, A.B.; Capriglione, L.G.; Batista, M.; Marcon, B.H.; Senegaglia, A.C.; Stimamiglio, M.A.; Correa, A. The Protein Content of Extracellular Vesicles Derived from Expanded Human Umbilical Cord Blood-Derived CD133(+) and Human Bone Marrow-Derived Mesenchymal Stem Cells Partially Explains Why both Sources are Advantageous for Regenerative Medicine. Stem Cell Rev. Rep. 2017, 13, 244–257. [Google Scholar] [CrossRef]
- Anderson, J.D.; Johansson, H.J.; Graham, C.S.; Vesterlund, M.; Pham, M.T.; Bramlett, C.S.; Montgomery, E.N.; Mellema, M.S.; Bardini, R.L.; Contreras, Z.; et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells 2016, 34, 601–613. [Google Scholar] [CrossRef]
- La Greca, A.; Solari, C.; Furmento, V.; Lombardi, A.; Biani, M.C.; Aban, C.; Moro, L.; García, M.; Guberman, A.S.; Sevlever, G.E.; et al. Extracellular vesicles from pluripotent stem cell-derived mesenchymal stem cells acquire a stromal modulatory proteomic pattern during differentiation. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; de Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int. J. Proteom. 2012, 2012, 971907. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Woollard, J.R.; Tang, H.; Dasari, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci. Rep. 2016, 6, 36120. [Google Scholar] [CrossRef] [PubMed]
- Raghav, A.; Khan, Z.A.; Upadhayay, V.K.; Tripathi, P.; Gautam, K.A.; Mishra, B.K.; Ahmad, J.; Jeong, G.B. Mesenchymal Stem Cell-Derived Exosomes Exhibit Promising Potential for Treating SARS-CoV-2-Infected Patients. Cells 2021, 10, 587. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, L.; Shi, H.; Pan, Z.; Wu, L.; Yan, Y.; Zhang, X.; Mao, F.; Qian, H.; Xu, W. Exosomes from Human Umbilical Cord Mesenchymal Stem Cells: Identification, Purification, and Biological Characteristics. Stem Cells Int. 2016, 2016, 1929536. [Google Scholar] [CrossRef]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl. Med. 2013, 2, 455–463. [Google Scholar] [CrossRef]
- Bortolotti, F.; Ukovich, L.; Razban, V.; Martinelli, V.; Ruozi, G.; Pelos, B.; Dore, F.; Giacca, M.; Zacchigna, S. In vivo therapeutic potential of mesenchymal stromal cells depends on the source and the isolation procedure. Stem Cell Rep. 2015, 4, 332–339. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, Q.; Que, H.; Wang, N.; Gong, P.; Gu, J. Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Agent for the Treatment of Liver Diseases. Int. J. Mol. Sci. 2022, 23, 10972. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wei, C.; Cheng, K.; Han, B.; Yan, J.; Zhang, M.; Peng, C.; Liu, Y. Mesenchymal stem cell-conditioned medium reduces liver injury and enhances regeneration in reduced-size rat liver transplantation. J. Surg. Res. 2013, 183, 907–915. [Google Scholar] [CrossRef]
- Huang, B.; Cheng, X.; Wang, H.; Huang, W.; la Ga Hu, Z.; Wang, D.; Zhang, K.; Zhang, H.; Xue, Z.; Da, Y.; et al. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J. Transl. Med. 2016, 14, 45. [Google Scholar] [CrossRef]
- Korkida, F.; Stamatopoulou, A.; Roubelakis, M.G. Recent Advances in Mesenchymal Stem/Stromal Cell-Based Therapy for Alcohol-Associated Liver Disease and Non-alcoholic Fatty Liver Disease. Stem Cells Transl. Med. 2024, 13, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ezquer, F.; Bruna, F.; Calligaris, S.; Conget, P.; Ezquer, M. Multipotent mesenchymal stromal cells: A promising strategy to manage alcoholic liver disease. World J. Gastroenterol. 2016, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Nikokiraki, C.; Psaraki, A.; Roubelakis, M.G. The potential clinical use of stem/progenitor cells and organoids in liver diseases. Cells 2022, 11, 1410. [Google Scholar] [CrossRef]
- Nickel, S.; Christ, M.; Schmidt, S.; Kosacka, J.; Kühne, H.; Roderfeld, M.; Longerich, T.; Tietze, L.; Bosse, I.; Hsu, M.-J. Human mesenchymal stromal cells resolve lipid load in high fat diet-induced non-alcoholic steatohepatitis in mice by mitochondria donation. Cells 2022, 11, 1829. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-J.; Karkossa, I.; Schäfer, I.; Christ, M.; Kühne, H.; Schubert, K.; Rolle-Kampczyk, U.E.; Kalkhof, S.; Nickel, S.; Seibel, P. Mitochondrial transfer by human mesenchymal stromal cells ameliorates hepatocyte lipid load in a mouse model of NASH. Biomedicines 2020, 8, 350. [Google Scholar] [CrossRef]
- Tamura, R.; Uemoto, S.; Tabata, Y. Immunosuppressive effect of mesenchymal stem cell-derived exosomes on a concanavalin A-induced liver injury model. Inflamm. Regen. 2016, 36, 26. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, S.; Hu, H.; Yang, J.; Wang, X.; Ma, Y.; Jiang, J.; Wang, J.; Zhong, L.; Chen, M.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages. Biochem. Biophys. Res. Commun. 2019, 508, 735–741. [Google Scholar] [CrossRef]
- Mebarki, M.; Abadie, C.; Larghero, J.; Cras, A. Human umbilical cord-derived mesenchymal stem/stromal cells: A promising candidate for the development of advanced therapy medicinal products. Stem Cell Res. Ther. 2021, 12, 152. [Google Scholar] [CrossRef]
- Du, Y.; Li, D.; Han, C.; Wu, H.; Xu, L.; Zhang, M.; Zhang, J.; Chen, X. Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (hiPSC-MSCs) Protect Liver against Hepatic Ischemia/Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway. Cell Physiol. Biochem. 2017, 43, 611–625. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, B.; Wang, X.; Xiang, C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res. Ther. 2017, 8, 9. [Google Scholar] [CrossRef]
- Salomon, C.; Ryan, J.; Sobrevia, L.; Kobayashi, M.; Ashman, K.; Mitchell, M.; Rice, G.E. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS ONE 2013, 8, e68451. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, L.; Impola, U.; Sankkila, L.; Ritamo, I.; Aatonen, M.; Kilpinen, S.; Tuimala, J.; Valmu, L.; Levijoki, J.; Finckenberg, P.; et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J. Extracell. Vesicles 2013, 2, 21927. [Google Scholar] [CrossRef]
- Zhu, L.P.; Tian, T.; Wang, J.Y.; He, J.N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.X.; Qiu, X.T.; Li, C.C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.P.; Wei, Z.; Wei, L. Preconditioning strategy in stem cell transplantation therapy. Transl. Stroke Res. 2013, 4, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, W.; Meng, W.; Jegga, A.G.; Wang, Y.; Cai, W.; Kim, H.W.; Pasha, Z.; Wen, Z.; Rao, F.; et al. Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: A critical role for HSF1/miR-34a/HSP70 pathway. Stem Cells 2014, 32, 462–472. [Google Scholar] [CrossRef]
- Hawkins, K.E.; Sharp, T.V.; McKay, T.R. The role of hypoxia in stem cell potency and differentiation. Regen. Med. 2013, 8, 771–782. [Google Scholar] [CrossRef]
- Das, R.; Jahr, H.; van Osch, G.J.; Farrell, E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: Considerations for regenerative medicine approaches. Tissue Eng. Part B Rev. 2010, 16, 159–168. [Google Scholar] [CrossRef]
- Ong, H.-T.; Federspiel, M.J.; Guo, C.M.; Ooi, L.L.; Russell, S.J.; Peng, K.-W.; Hui, K.M. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J. Hepatol. 2013, 59, 999–1006. [Google Scholar] [CrossRef]
- Zhao, W.; Ren, G.; Zhang, L.; Zhang, Z.; Liu, J.; Kuang, P.; Yin, Z.; Wang, X. Efficacy of mesenchymal stem cells derived from human adipose tissue in inhibition of hepatocellular carcinoma cells in vitro. Cancer Biother. Radiopharm. 2012, 27, 606–613. [Google Scholar] [CrossRef]
- Pelagalli, A.; Nardelli, A.; Fontanella, R.; Zannetti, A. Inhibition of AQP1 hampers osteosarcoma and hepatocellular carcinoma progression mediated by bone marrow-derived mesenchymal stem cells. Int. J. Mol. Sci. 2016, 17, 1102. [Google Scholar] [CrossRef]
- Fontanella, R.; Pelagalli, A.; Nardelli, A.; D’Alterio, C.; Ieranò, C.; Cerchia, L.; Lucarelli, E.; Scala, S.; Zannetti, A. A novel antagonist of CXCR4 prevents bone marrow-derived mesenchymal stem cell-mediated osteosarcoma and hepatocellular carcinoma cell migration and invasion. Cancer Lett. 2016, 370, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.-A.; Duggal, S.; Coulston, N.; Millar, D.; Melki, J.; Shahdadfar, A.; Brinchmann, J.E.; Collas, P. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int. J. Dev. Biol. 2008, 52, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Røsland, G.V.; Svendsen, A.; Torsvik, A.; Sobala, E.; McCormack, E.; Immervoll, H.; Mysliwietz, J.; Tonn, J.-C.; Goldbrunner, R.; Lønning, P.E. Long-term cultures of bone marrow–derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009, 69, 5331–5339. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, J.; Zhu, W.; Guan, Y.; Zou, C.; Chen, Z.; Zhang, Y.A. Spontaneous transformation of adult mesenchymal stem cells from cynomolgus macaques in vitro. Exp. Cell Res. 2011, 317, 2950–2957. [Google Scholar] [CrossRef]
- Wakitani, S.; Okabe, T.; Horibe, S.; Mitsuoka, T.; Saito, M.; Koyama, T.; Nawata, M.; Tensho, K.; Kato, H.; Uematsu, K. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J. Tissue Eng. Regen. Med. 2011, 5, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.; Yin, J.; Xu, J.; Wei, J.; Zhang, Y. Efficacy of Mesenchymal Stem Cell Therapy for Steroid-Refractory Acute Graft-Versus-Host Disease following Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0136991. [Google Scholar] [CrossRef]
- Dotoli, G.M.; De Santis, G.C.; Orellana, M.D.; de Lima Prata, K.; Caruso, S.R.; Fernandes, T.R.; Rensi Colturato, V.A.; Kondo, A.T.; Hamerschlak, N.; Simões, B.P.; et al. Mesenchymal stromal cell infusion to treat steroid-refractory acute GvHD III/IV after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017, 52, 859–862. [Google Scholar] [CrossRef]
- Fan, X.; Guo, D.; Cheung, A.M.S.; Poon, Z.Y.; Yap, C.S.; Goh, S.E.; Guo, D.; Li, H.; Bari, S.; Li, S.; et al. Mesenchymal Stromal Cell (MSC)-Derived Combination of CXCL5 and Anti-CCL24 Is Synergistic and Superior to MSC and Cyclosporine for the Treatment of Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2018, 24, 1971–1980. [Google Scholar] [CrossRef]
- Muroi, K.; Miyamura, K.; Okada, M.; Yamashita, T.; Murata, M.; Ishikawa, T.; Uike, N.; Hidaka, M.; Kobayashi, R.; Imamura, M.; et al. Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: A phase II/III study. Int. J. Hematol. 2016, 103, 243–250. [Google Scholar] [CrossRef]
- Wolff, D.; Schleuning, M.; von Harsdorf, S.; Bacher, U.; Gerbitz, A.; Stadler, M.; Ayuk, F.; Kiani, A.; Schwerdtfeger, R.; Vogelsang, G.B.; et al. Consensus Conference on Clinical Practice in Chronic GVHD: Second-Line Treatment of Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2011, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.; Jiménez-Castro, M.B.; Gracia-Sancho, J. Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J. Hepatol. 2013, 59, 1094–1106. [Google Scholar] [CrossRef]
- Resch, T.; Cardini, B.; Oberhuber, R.; Weissenbacher, A.; Dumfarth, J.; Krapf, C.; Boesmueller, C.; Oefner, D.; Grimm, M.; Schneeberger, S. Transplanting Marginal Organs in the Era of Modern Machine Perfusion and Advanced Organ Monitoring. Front. Immunol. 2020, 11, 631. [Google Scholar] [CrossRef]
- Rowart, P.; Erpicum, P.; Detry, O.; Weekers, L.; Grégoire, C.; Lechanteur, C.; Briquet, A.; Beguin, Y.; Krzesinski, J.M.; Jouret, F. Mesenchymal Stromal Cell Therapy in Ischemia/Reperfusion Injury. J. Immunol. Res. 2015, 2015, 602597. [Google Scholar] [CrossRef] [PubMed]
- Boteon, Y.L.; Afford, S.C.; Mergental, H. Pushing the Limits: Machine Preservation of the Liver as a Tool to Recondition High-Risk Grafts. Curr. Transplant. Rep. 2018, 5, 113–120. [Google Scholar] [CrossRef]
- Cao, H.; Yang, L.; Hou, B.; Sun, D.; Lin, L.; Song, H.L.; Shen, Z.Y. Retraction Note: Heme oxygenase-1-modified bone marrow mesenchymal stem cells combined with normothermic machine perfusion to protect donation after circulatory death liver grafts. Stem Cell Res. Ther. 2022, 13, 510. [Google Scholar] [CrossRef] [PubMed]
- Lonati, C.; Bassani, G.A.; Brambilla, D.; Leonardi, P.; Carlin, A.; Maggioni, M.; Zanella, A.; Dondossola, D.; Fonsato, V.; Grange, C.; et al. Mesenchymal stem cell-derived extracellular vesicles improve the molecular phenotype of isolated rat lungs during ischemia/reperfusion injury. J. Heart Lung Transplant. 2019, 38, 1306–1316. [Google Scholar] [CrossRef]
- Vandermeulen, M.; Grégoire, C.; Briquet, A.; Lechanteur, C.; Beguin, Y.; Detry, O. Rationale for the potential use of mesenchymal stromal cells in liver transplantation. World J. Gastroenterol. 2014, 20, 16418–16432. [Google Scholar] [CrossRef] [PubMed]
- Kojima, L.; Akabane, M.; Murray, M.; Fruscione, M.; Soma, D.; Snyder, A.; McVey, J.; Firl, D.J.; Hernandez-Alejandro, R.; Kubal, C.A.; et al. Reappraisal of tacrolimus levels post liver transplant for hepatocellular carcinoma: A multicenter study toward personalized immunosuppression regimen. Liver Transpl. 2024. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cantero, M.J.; Bueloni, B.; Gonzalez Llamazares, L.; Fiore, E.; Lameroli, L.; Atorrasagasti, C.; Mazzolini, G.; Malvicini, M.; Bayo, J.; García, M.G. Modified mesenchymal stromal cells by in vitro transcribed mRNA: A therapeutic strategy for hepatocellular carcinoma. Stem Cell Res. Ther. 2024, 15, 208. [Google Scholar] [CrossRef]
- Shi, M.; Liu, Z.; Wang, Y.; Xu, R.; Sun, Y.; Zhang, M.; Yu, X.; Wang, H.; Meng, L.; Su, H.; et al. A Pilot Study of Mesenchymal Stem Cell Therapy for Acute Liver Allograft Rejection. Stem Cells Transl. Med. 2017, 6, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Yi, H.; Zheng, J.; Cai, J.; Chen, W.; Lu, T.; Chen, L.; Du, C.; Liu, J.; et al. A novel MSC-based immune induction strategy for ABO-incompatible liver transplantation: A phase I/II randomized, open-label, controlled trial. Stem Cell Res. Ther. 2021, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Maugeri, M.; Garre, E.; Nawaz, M.; Wahlgren, J.; Papadimitriou, A.; Lundqvist, C.; Lindfors, L.; Collén, A.; Sunnerhagen, P.; et al. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS ONE 2018, 13, e0195969. [Google Scholar] [CrossRef] [PubMed]
- Lopez, K.; Lai, S.W.T.; Lopez Gonzalez, E.J.; Dávila, R.G.; Shuck, S.C. Extracellular vesicles: A dive into their role in the tumor microenvironment and cancer progression. Front. Cell Dev. Biol. 2023, 11, 1154576. [Google Scholar] [CrossRef]
- Lee, H.; Li, C.; Zhang, Y.; Zhang, D.; Otterbein, L.E.; Jin, Y. Caveolin-1 selectively regulates microRNA sorting into microvesicles after noxious stimuli. J. Exp. Med. 2019, 216, 2202–2220. [Google Scholar] [CrossRef]
- Gregson, A.L.; Hoji, A.; Injean, P.; Poynter, S.T.; Briones, C.; Palchevskiy, V.; Weigt, S.S.; Shino, M.Y.; Derhovanessian, A.; Sayah, D.; et al. Altered Exosomal RNA Profiles in Bronchoalveolar Lavage from Lung Transplants with Acute Rejection. Am. J. Respir. Crit. Care Med. 2015, 192, 1490–1503. [Google Scholar] [CrossRef]
- Delaura, I.F.; Gao, Q.; Anwar, I.J.; Abraham, N.; Kahan, R.; Hartwig, M.G.; Barbas, A.S. Complement-targeting therapeutics for ischemia-reperfusion injury in transplantation and the potential for ex vivo delivery. Front. Immunol. 2022, 13, 1000172. [Google Scholar] [CrossRef]
- Matsui, F.; Babitz, S.K.; Rhee, A.; Hile, K.L.; Zhang, H.; Meldrum, K.K. Mesenchymal stem cells protect against obstruction-induced renal fibrosis by decreasing STAT3 activation and STAT3-dependent MMP-9 production. Am. J. Physiol. Ren. Physiol. 2017, 312, F25–F32. [Google Scholar] [CrossRef]
- Hu, C.; Wu, Z.; Li, L. Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. Int. J. Biol. Sci. 2020, 16, 893–903. [Google Scholar] [CrossRef]
- Wu, R.; Fan, X.; Wang, Y.; Shen, M.; Zheng, Y.; Zhao, S.; Yang, L. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Liver Immunity and Therapy. Front. Immunol. 2022, 13, 833878. [Google Scholar] [CrossRef]
- Hamaoui, K.; Gowers, S.; Boutelle, M.; Cook, T.H.; Hanna, G.; Darzi, A.; Smith, R.; Dorling, A.; Papalois, V. Organ Pretreatment With Cytotopic Endothelial Localizing Peptides to Ameliorate Microvascular Thrombosis and Perfusion Deficits in Ex Vivo Renal Hemoreperfusion Models. Transplantation 2016, 100, e128–e139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Liu, W.; Fu, B.S.; Wang, G.Y.; Li, H.B.; Yi, H.M.; Jiang, N.; Wang, G.; Zhang, J.; Yi, S.H.; et al. Therapeutic potentials of umbilical cord-derived mesenchymal stromal cells for ischemic-type biliary lesions following liver transplantation. Cytotherapy 2017, 19, 194–199. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akabane, M.; Imaoka, Y.; Kawashima, J.; Endo, Y.; Schenk, A.; Sasaki, K.; Pawlik, T.M. Innovative Strategies for Liver Transplantation: The Role of Mesenchymal Stem Cells and Their Cell-Free Derivatives. Cells 2024, 13, 1604. https://doi.org/10.3390/cells13191604
Akabane M, Imaoka Y, Kawashima J, Endo Y, Schenk A, Sasaki K, Pawlik TM. Innovative Strategies for Liver Transplantation: The Role of Mesenchymal Stem Cells and Their Cell-Free Derivatives. Cells. 2024; 13(19):1604. https://doi.org/10.3390/cells13191604
Chicago/Turabian StyleAkabane, Miho, Yuki Imaoka, Jun Kawashima, Yutaka Endo, Austin Schenk, Kazunari Sasaki, and Timothy M. Pawlik. 2024. "Innovative Strategies for Liver Transplantation: The Role of Mesenchymal Stem Cells and Their Cell-Free Derivatives" Cells 13, no. 19: 1604. https://doi.org/10.3390/cells13191604
APA StyleAkabane, M., Imaoka, Y., Kawashima, J., Endo, Y., Schenk, A., Sasaki, K., & Pawlik, T. M. (2024). Innovative Strategies for Liver Transplantation: The Role of Mesenchymal Stem Cells and Their Cell-Free Derivatives. Cells, 13(19), 1604. https://doi.org/10.3390/cells13191604







