Intestinal Motility Dysfunction in Goto-Kakizaki Rats: Role of the Myenteric Plexus
Abstract
:1. Introduction
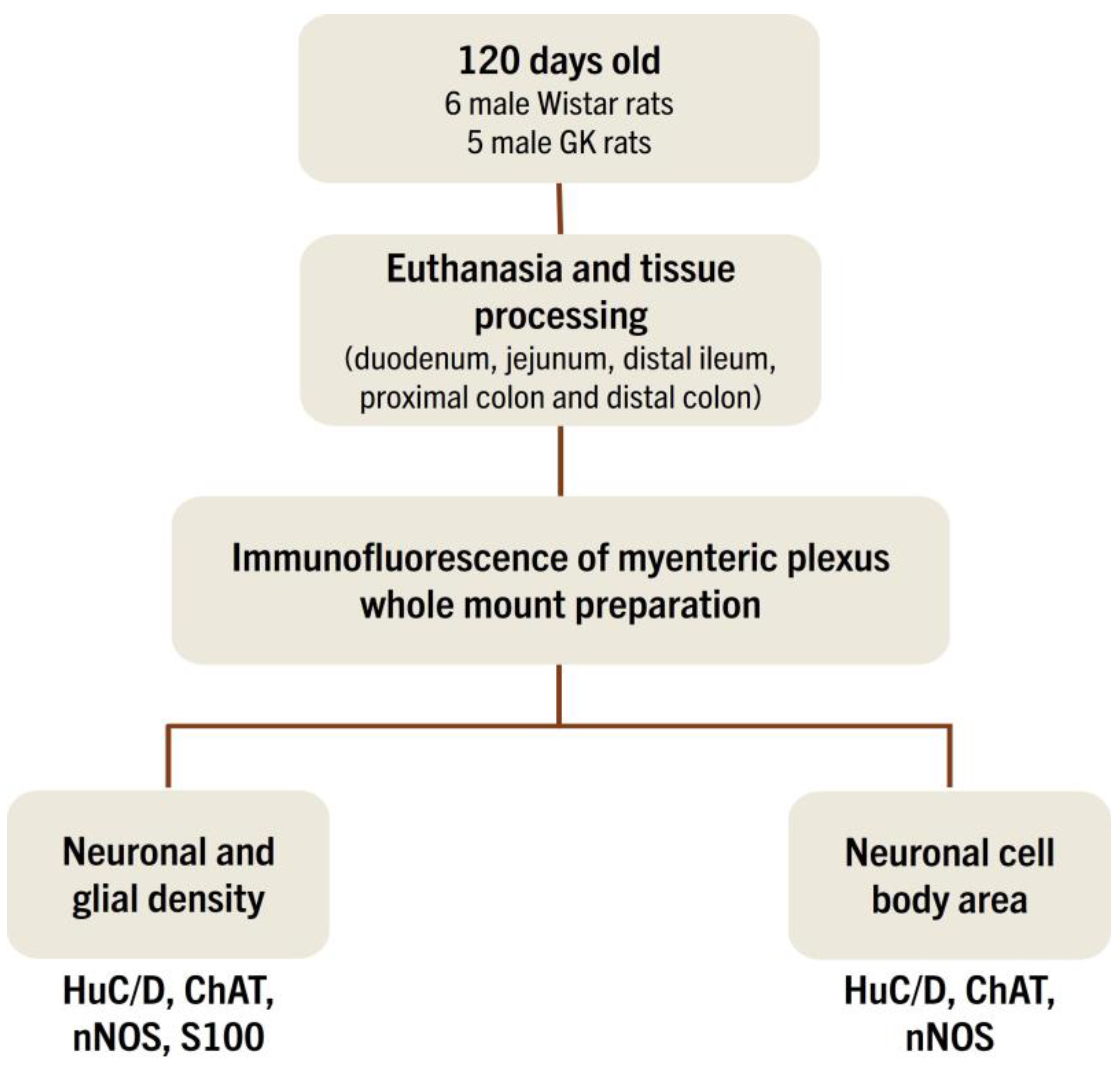
2. Materials and Methods
2.1. Animals
2.2. Characterization of Non-Obese T2DM Model
2.3. Collection of Intestinal Segments
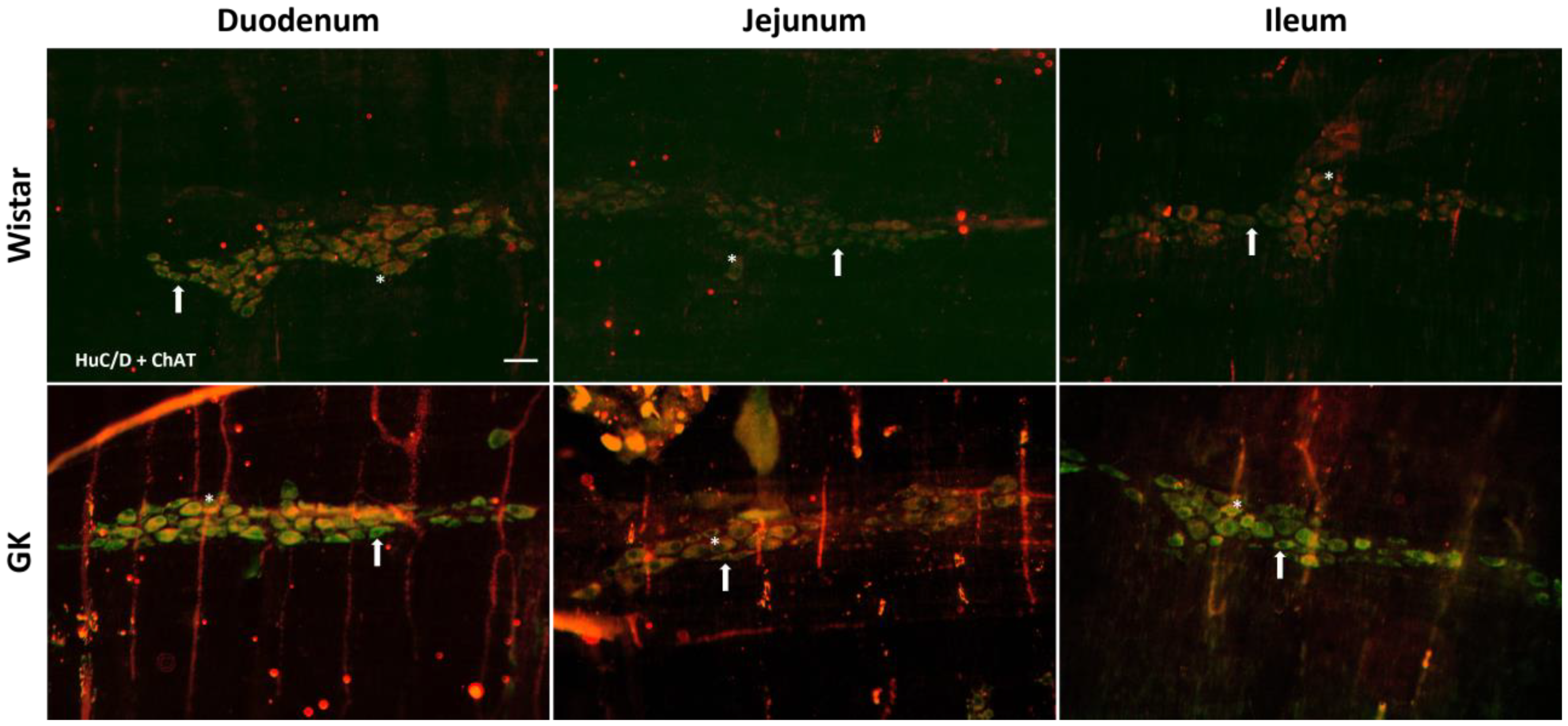
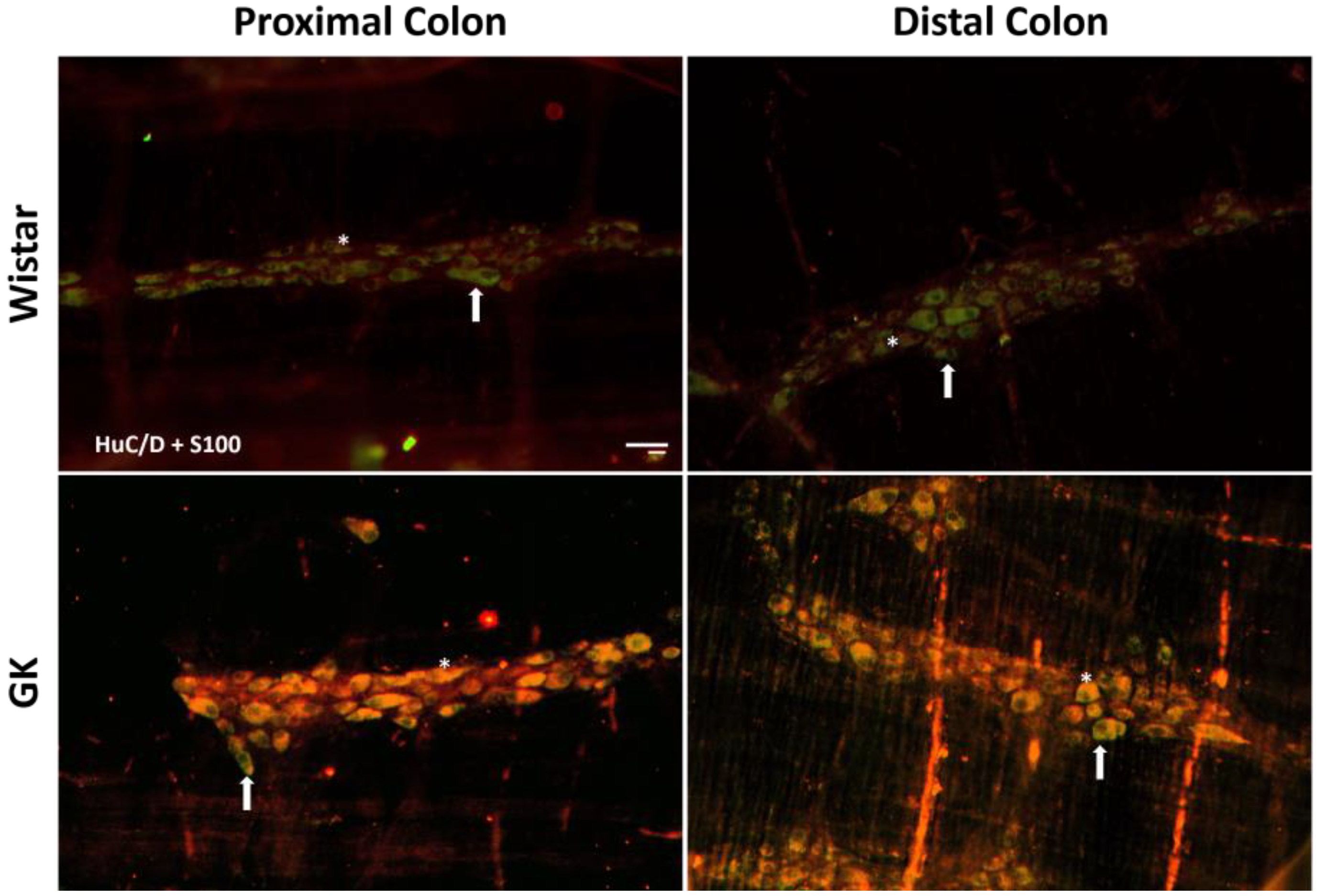
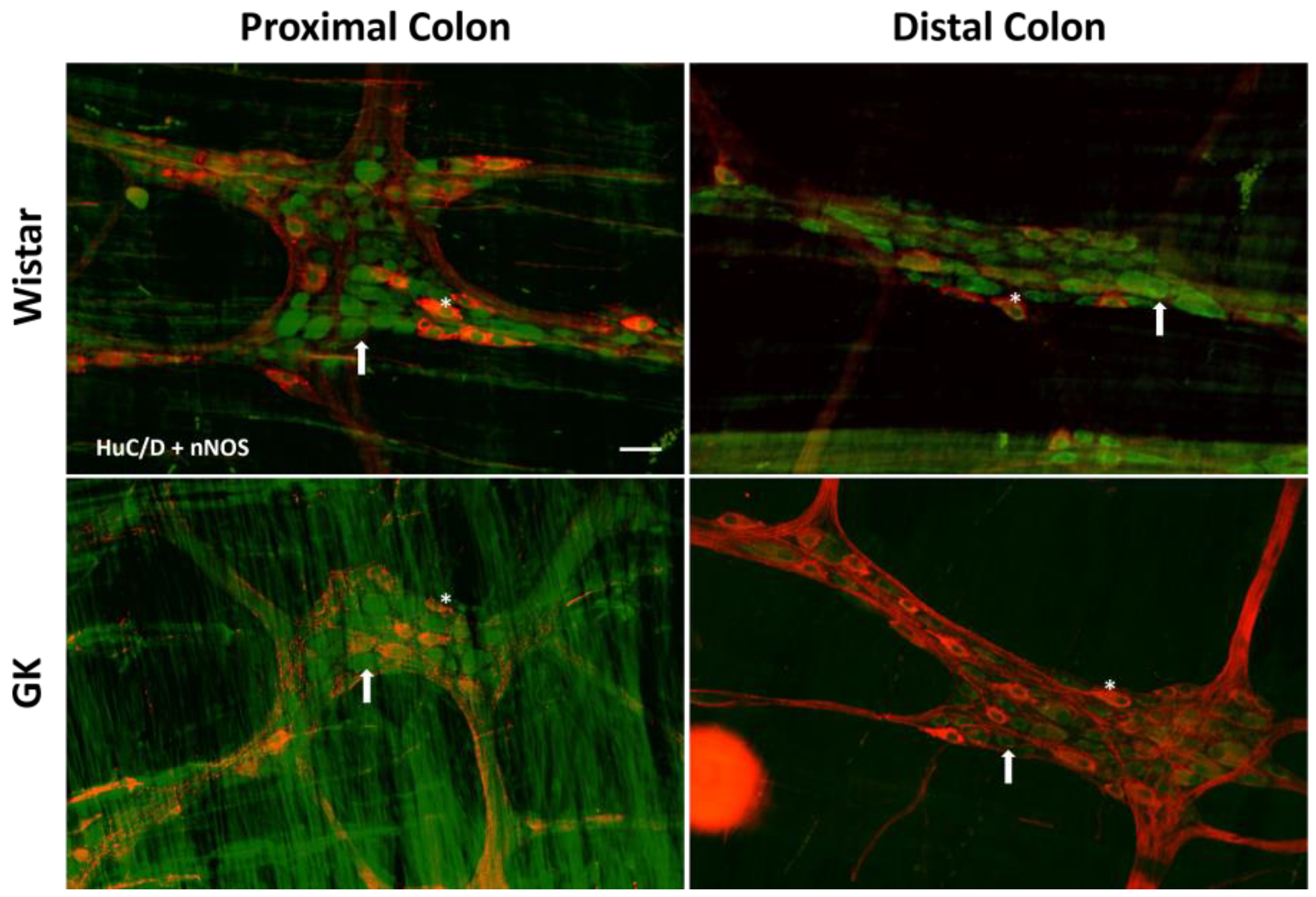
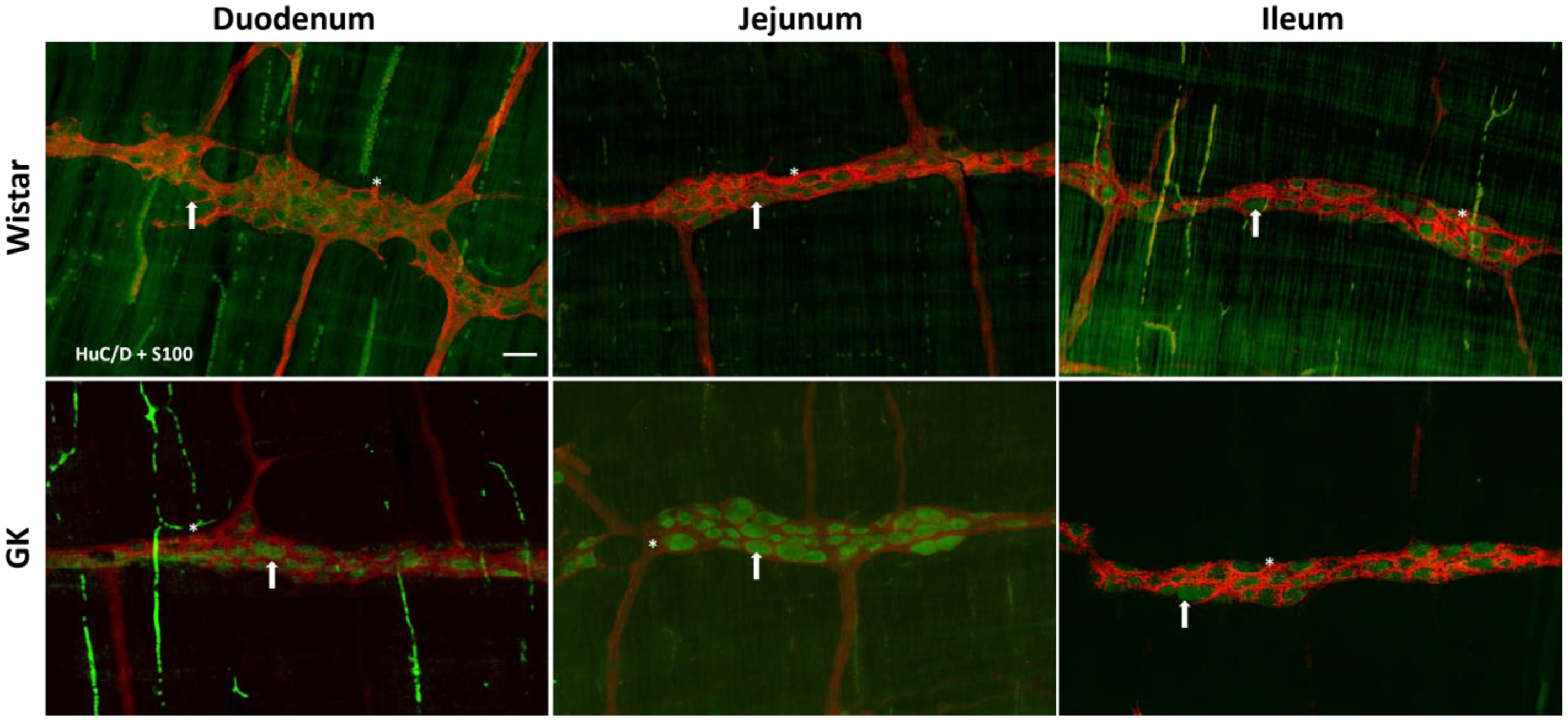
2.4. Immunofluorescence
2.5. Quantitative Analysis of Myenteric Plexus
2.6. Morphometric Analysis of the Myenteric Plexus
2.7. Statistical Analysis
3. Results
3.1. Neuronal and Glial Ganglia Density
3.2. Morphometric Analysis of Neuronal Cell Body Area
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S17–S38. [Google Scholar] [CrossRef]
- Aroda, V.R.; Krause-Steinrauf, H.; Kazemi, E.J.; Buse, J.B.; Gulanski, B.I.; Florez, H.J.; Ahmann, A.J.; Loveland, A.; Kuhn, A.; Lonier, J.Y.; et al. Clinical and Metabolic Characterization of Adults With Type 2 Diabetes by Age in the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE) Cohort. Diabetes Care 2022, 45, 1512–1521. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; IDF Diabetes Atlas: Brussels, Belgium, 2021; Volume 10th edn. [Google Scholar]
- Acosta-Martinez, M.; Cabail, M.Z. The PI3K/Akt Pathway in Meta-Inflammation. Int. J. Mol. Sci. 2022, 23, 15330. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Justin Milner, J.; Makowski, L. The Inflammation Highway: Metabolism Accelerates Inflammatory Traffic in Obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef]
- Russo, S.; Kwiatkowski, M.; Govorukhina, N.; Bischoff, R.; Melgert, B.N. Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites. Front. Immunol. 2021, 12, 746151. [Google Scholar] [CrossRef]
- Goto, Y.; Kakizaki, M.; Masaki, N. Spontaneous Diabetes Produced by Selective Breeding of Normal Wistar Rats. Proc. Jpn. Acad. 1975, 51, 80–85. [Google Scholar] [CrossRef]
- Goto, Y.; Kakizaki, M.; Masaki, N. Production of Spontaneous Diabetic Rats by Repetition of Selective Breeding. Tohoku J. Exp. Med. 1976, 119, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, W.M.T.; Panveloski-Costa, A.C.; Yokota, C.N.F.; Pereira, J.N.B.; Filho, J.M.; Torres, R.P.; Hirabara, S.M.; Curi, R.; Alba-Loureiro, T.C. Comparison of Goto-Kakizaki Rats and High Fat Diet-Induced Obese Rats: Are They Reliable Models to Study Type 2 Diabetes Mellitus? PLoS ONE 2017, 12, e0189622. [Google Scholar] [CrossRef]
- Serdan, T.D.A.; Masi, L.N.; Pereira, J.N.B.; Rodrigues, L.E.; Alecrim, A.L.; Scervino, M.V.M.; Diniz, V.L.S.; dos Santos, A.A.C.; Filho, C.P.B.S.; Alba-Loureiro, T.C.; et al. Impaired Brown Adipose Tissue Is Differentially Modulated in Insulin-Resistant Obese Wistar and Type 2 Diabetic Goto-Kakizaki Rats. Biomed. Pharmacother. 2021, 142, 112019. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.C.O.; Oliveira, V.A.B.; Serdan, T.D.A.; Silva, F.L.R.; Santos, C.S.; Pauferro, J.R.B.; Ribas, A.S.F.; Manoel, R.; Pereira, A.C.G.; Correa, I.S.; et al. Brain Glucose Hypometabolism and Hippocampal Inflammation in Goto-Kakizaki Rats. Braz. J. Med. Biol. Res. 2023, 56, e12742. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, G.M.; Santana, G.O.; Scervino, M.V.M.; Curi, R.; Pereira, J.N.B. A Short Review on the Features of the Non-Obese Diabetic Goto-Kakizaki Rat Intestine. Braz. J. Med. Biol. Res. 2022, 55, e11910. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.N.B.; Murata, G.M.; Sato, F.T.; Marosti, A.R.; Carvalho, C.R.d.O.; Curi, R. Small Intestine Remodeling in Male Goto–Kakizaki Rats. Physiol. Rep. 2021, 9, e14755. [Google Scholar] [CrossRef]
- Ouyang, X.; Li, S.; Tan, Y.; Lin, L.; Yin, J.; Chen, J.D.Z. Intestinal Electrical Stimulation Attenuates Hyperglycemia and Prevents Loss of Pancreatic β Cells in Type 2 Diabetic Goto–Kakizaki Rats. Nutr. Diabetes 2019, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, B.; Anitha, M.; Blatt, R.; Shahnavaz, N.; Staley, C.; Mwangi, S.; Jones, D.P.; Sitaraman, S.V. Colonic Motor Dysfunction in Human Diabetes Is Associated with Enteric Neuronal Loss and Increased Oxidative Stress. Neurogastroenterol. Motil. 2012, 23, 131–138.e26. [Google Scholar] [CrossRef] [PubMed]
- Maisey, A. A Practical Approach to Gastrointestinal Complications of Diabetes. Diabetes Ther. 2016, 7, 379–386. [Google Scholar] [CrossRef]
- Krishnan, B.; Babu, S.; Walker, J.; Walker, A.B.; Pappachan, J.M. Gastrointestinal Complications of Diabetes Mellitus. World J. Diabetes 2013, 4, 51. [Google Scholar] [CrossRef]
- Meldgaard, T.; Brock, C. Diabetes and the Gastrointestinal Tract. Medicine 2019, 47, 454–459. [Google Scholar] [CrossRef]
- Furness, J.B.; Stebbing, M.J. The First Brain: Species Comparisons and Evolutionary Implications for the Enteric and Central Nervous Systems. Neurogastroenterol. Motil. 2018, 30, e13234. [Google Scholar] [CrossRef]
- Furness, J.B. Types of Neurons in the Enteric Nervous System. J. Auton. Nerv. Syst. 2000, 81, 87–96. [Google Scholar] [CrossRef]
- Furness, J.B. The Organisation of the Autonomic Nervous System: Peripheral Connections. Auton. Neurosci. 2006, 130, 1–5. [Google Scholar] [CrossRef]
- Furness, J.B. The Enteric Nervous System and Neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.; Holmgren, S. The Control of Gut Motility. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 479–501. [Google Scholar] [CrossRef] [PubMed]
- Abot, A.; Lucas, A.; Bautzova, T.; Bessac, A.; Fournel, A.; Le-Gonidec, S.; Valet, P.; Moro, C.; Cani, P.D.; Knauf, C. Galanin Enhances Systemic Glucose Metabolism through Enteric Nitric Oxide Synthase-Expressed Neurons. Mol. Metab. 2018, 10, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, K.A.; Mawe, G.M. The Enteric Nervous System. Physiol. Rev. 2023, 103, 1487–1564. [Google Scholar] [CrossRef]
- Coelho-Aguiar, J.d.M.; Bon-Frauches, A.C.; Gomes, A.L.T.; Veríssimo, C.P.; Aguiar, D.P.; Matias, D.; Thomasi, B.B.d.M.; Gomes, A.S.; Brito, G.A.d.C.; Moura-Neto, V. The Enteric Glia: Identity and Functions. Glia 2015, 63, 921–935. [Google Scholar] [CrossRef]
- Bessac, A.; Cani, P.D.; Meunier, E.; Dietrich, G.; Knauf, C. Inflammation and Gut-Brain Axis during Type 2 Diabetes: Focus on the Crosstalk between Intestinal Immune Cells and Enteric Nervous System. Front. Neurosci. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- McMenamin, C.A.; Clyburn, C.; Browning, K.N. High-Fat Diet During the Perinatal Period Induces Loss of Myenteric Nitrergic Neurons and Increases Enteric Glial Density, Prior to the Development of Obesity. Neuroscience 2018, 393, 369–380. [Google Scholar] [CrossRef]
- Brasileiro, A.D.; Garcia, L.P.; de Carvalho da Silva, S.; Rocha, L.B.; Pedrosa, A.L.; Vieira, A.S.; da Silva, V.J.D.; Rodrigues, A.R.A. Effects of Diabetes Mellitus on Myenteric Neuronal Density and Sodium Channel Expression in the Rat Ileum. Brain Res. 2019, 1708, 1–9. [Google Scholar] [CrossRef]
- Fournel, A.; Drougard, A.; Duparc, T.; Marlin, A.; Brierley, S.M.; Castro, J.; Le-Gonidec, S.; Masri, B.; Colom, A.; Lucas, A.; et al. Apelin Targets Gut Contraction to Control Glucose Metabolism via the Brain. Gut 2017, 66, 258–269. [Google Scholar] [CrossRef]
- McGarrity, T.J.; Peiffer, L.P.; Colony, P.C. Cellular Proliferation in Proximal and Distal Rat Colon during 1,2-Dimethylhydrazine-Induced Carcinogenesis. Gastroenterology 1988, 95, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Trevizan, A.R.; Schneider, L.C.L.; Araújo, E.J.d.A.; Garcia, J.L.; Buttow, N.C.; Nogueira-Melo, G.d.A.; Sant’Ana, D.d.M.G. Acute Toxoplasma Gondii Infection Alters the Number of Neurons and the Proportion of Enteric Glial Cells in the Duodenum in Wistar Rats. Neurogastroenterol. Motil. 2019, 31, e13523. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, C.; Bessard, A.; Aubert, P.; Joussain, C.; Giuliano, F.; Behr-Roussel, D.; Perrouin-Verbe, M.-A.; Perrouin-Verbe, B.; Brochard, C.; Neunlist, M. Enteric Nervous System Remodeling in a Rat Model of Spinal Cord Injury: A Pilot Study. Neurotrauma Rep. 2020, 1, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.G.; Bendtsen, T.F.; Korbo, L.; Marcussen, N.; Møller, A.; Nielsen, K.; Nyengaard, J.R.; Pakkenberg, B.; Sørensen, F.B.; Vesterby, A.; et al. Some New, Simple and Efficient Stereological Methods and Their Use in Pathological Research and Diagnosis. APMIS 1988, 96, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.N.B.; Mari, R.B.; Stabille, S.R.; De Faria, H.G.; Mota, T.F.M.; Ferreira, W.M. Benefits of Caloric Restriction in the Myenteric Neuronal Plasticity in Aging Rats. An. Acad. Bras. Cienc. 2014, 86, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Stenkamp-Strahm, C.M.; Kappmeyer, A.J.; Schmalz, J.T.; Gericke, M.; Balemba, O. High-Fat Diet Ingestion Correlates with Neuropathy in the Duodenum Myenteric Plexus of Obese Mice with Symptoms of Type 2 Diabetes. Cell Tissue Res. 2013, 354, 381–394. [Google Scholar] [CrossRef]
- Beraldi, E.J.; Soares, A.; Borges, S.C.; de Souza, A.C.d.S.; Natali, M.R.M.; Bazotte, R.B.; Buttow, N.C. High-Fat Diet Promotes Neuronal Loss in the Myenteric Plexus of the Large Intestine in Mice. Dig. Dis. Sci. 2015, 60, 841–849. [Google Scholar] [CrossRef]
- Izbéki, F.; Wittman, T.; Rosztóczy, A.; Linke, N.; Bódi, N.; Fekete, É.; Bagyánszki, M. Immediate Insulin Treatment Prevents Gut Motility Alterations and Loss of Nitrergic Neurons in the Ileum and Colon of Rats with Streptozotocin-Induced Diabetes. Diabetes Res. Clin. Pract. 2008, 80, 192–198. [Google Scholar] [CrossRef]
- Bagyánszki, M. Diabetes-Related Alterations in the Enteric Nervous System and Its Microenvironment. World J. Diabetes 2012, 3, 80. [Google Scholar] [CrossRef] [PubMed]
- Nyavor, Y.; Brands, C.R.; May, G.; Kuther, S.; Nicholson, J.; Tiger, K.; Tesnohlidek, A.; Yasuda, A.; Starks, K.; Litvinenko, D.; et al. High-Fat Diet–Induced Alterations to Gut Microbiota and Gut-Derived Lipoteichoic Acid Contributes to the Development of Enteric Neuropathy. Neurogastroenterol. Motil. 2020, 32, e13838. [Google Scholar] [CrossRef]
- Demedts, I.; Masaoka, T.; Kindt, S.; De Hertogh, G.; Geboes, K.; Farré, R.; Berghe, P.V.; Tack, J. Gastrointestinal Motility Changes and Myenteric Plexus Alterations in Spontaneously Diabetic Biobreeding Rats. J. Neurogastroenterol. Motil. 2013, 19, 161–170. [Google Scholar] [CrossRef]
- Gros, M.; Gros, B.; Mesonero, J.E.; Latorre, E. Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management. J. Clin. Med. 2021, 10, 3429. [Google Scholar] [CrossRef] [PubMed]
- Deb, B.; Prichard, D.O.; Bharucha, A.E. Constipation and Fecal Incontinence in the Elderly. Curr. Gastroenterol. Rep. 2020, 22, 54. [Google Scholar] [CrossRef] [PubMed]
- Fornai, M.; Pellegrini, C.; Antonioli, L.; Segnani, C.; Ippolito, C.; Barocelli, E.; Ballabeni, V.; Vegezzi, G.; Al Harraq, Z.; Blandini, F.; et al. Enteric Dysfunctions in Experimental Parkinsons Disease: Alterations of Excitatory Cholinergic Neurotransmission Regulating Colonic Motility in Rats. J. Pharmacol. Exp. Ther. 2016, 356, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, L.; Chen, X.; Le, W. New Understanding on the Pathophysiology and Treatment of Constipation in Parkinson’s Disease. Front. Aging Neurosci. 2022, 14, 917499. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.A.; Stavely, R.; Pan, W.; Ott, L.; Ohishi, K.; Ohkura, T.; Han, C.; Hotta, R.; Goldstein, A.M. Optogenetic Activation of Cholinergic Enteric Neurons Reduces Inflammation in Experimental Colitis. CMGH 2024, 17, 907–921. [Google Scholar] [CrossRef]
- Spear, E.T.; Mawe, G.M. MINI-REVIEW Neurogastroenterology and Motility Enteric Neuroplasticity and Dysmotility in Inflammatory Disease: Key Players and Possible Therapeutic Targets. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, 853–861. [Google Scholar] [CrossRef]
- Lomax, A.E.; Fernández, E.; Sharkey, K.A. Plasticity of the Enteric Nervous System during Intestinal Inflammation. Neurogastroenterol. Motil. 2005, 17, 4–15. [Google Scholar] [CrossRef]
- Vasina, V.; Barbara, G.; Talamonti, L.; Stanghellini, V.; Corinaldesi, R.; Tonini, M.; De Ponti, F.; De Giorgio, R. Enteric Neuroplasticity Evoked by Inflammation. Auton. Neurosci. 2006, 126–127, 264–272. [Google Scholar] [CrossRef]
- Ferreira, P.E.B.; Beraldi, E.J.; Borges, S.C.; Natali, M.R.M.; Buttow, N.C. Resveratrol Promotes Neuroprotection and Attenuates Oxidative and Nitrosative Stress in the Small Intestine in Diabetic Rats. Biomed. Pharmacother. 2018, 105, 724–733. [Google Scholar] [CrossRef] [PubMed]
- de Souza, S.R.G.; Neto, M.H.d.M.; Perles, J.V.C.M.; Frez, F.C.V.; Zignani, I.; Ramalho, F.V.; Hermes-Uliana, C.; Bossolani, G.D.P.; Zanoni, J.N. Antioxidant Effects of the Quercetin in the Jejunal Myenteric Innervation of Diabetic Rats. Front. Med. 2017, 4, 4–11. [Google Scholar] [CrossRef]
- Sehaber-Sierakowski, C.C.; Vieira-Frez, F.C.; Hermes-Uliana, C.; Martins, H.A.; Bossolani, G.D.P.; Lima, M.M.; Blegniski, F.P.; Guarnier, F.A.; Baracat, M.M.; Perles, J.V.C.M.; et al. Protective Effects of Quercetin-Loaded Microcapsules on the Enteric Nervous System of Diabetic Rats. Auton. Neurosci. 2021, 230, 102759. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; D’Antongiovanni, V.; Pellegrini, C.; Fornai, M.; Benvenuti, L.; di Carlo, A.; van den Wijngaard, R.; Caputi, V.; Cerantola, S.; Giron, M.C.; et al. Colonic Dysmotility Associated with High-fat Diet-induced Obesity: Role of Enteric Glia. FASEB J. 2020, 34, 5512–5524. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Cortes, F.; Turco, F.; Linan-Rico, A.; Soghomonyan, S.; Whitaker, E.; Wehner, S.; Cuomo, R.; Christofi, F.L. Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Turco, F.; Steardo, L.; Cuomo, R. S100B Protein in the Gut: The Evidence for Enteroglialsustained Intestinal Inflammation. World J. Gastroenterol. 2011, 17, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Linan-Rico, A.; Ochoa-Cortes, F.; Schneider, R.; Christofi, F.L. Mini-Review: Enteric Glial Cell Reactions to Inflammation and Potential Therapeutic Implications for GI Diseases, Motility Disorders, and Abdominal Pain. Neurosci. Lett. 2023, 812, 137395. [Google Scholar] [CrossRef]
- Pochard, C.; Coquenlorge, S.; Freyssinet, M.; Naveilhan, P.; Bourreille, A.; Neunlist, M.; Rolli-Derkinderen, M. The Multiple Faces of Inflammatory Enteric Glial Cells: Is Crohn’s Disease a Gliopathy? Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G1–G11. [Google Scholar] [CrossRef]
- Murakami, M.; Ohta, T.; Ito, S. Lipopolysaccharides Enhance the Action of Bradykinin in Enteric Neurons via Secretion of Interleukin-1β from Enteric Glial Cells. J. Neurosci. Res. 2009, 87, 2095–2104. [Google Scholar] [CrossRef]
- Costa, D.V.S.; Moura-Neto, V.; Bolick, D.T.; Guerrant, R.L.; Fawad, J.A.; Shin, J.H.; Medeiros, P.H.Q.S.; Ledwaba, S.E.; Kolling, G.L.; Martins, C.S.; et al. S100B Inhibition Attenuates Intestinal Damage and Diarrhea Severity During Clostridioides Difficile Infection by Modulating Inflammatory Response. Front. Cell Infect. Microbiol. 2021, 11, 739874. [Google Scholar] [CrossRef]
- Chen, P.-M.; Gregersen, H.; Zhao, J.-B. Advanced Glycation End-Product Expression Is Upregulated in the Gastrointestinal Tract of Type 2 Diabetic Rats. World J. Diabetes 2015, 6, 662. [Google Scholar] [CrossRef]
- López-Gómez, L.; Szymaszkiewicz, A.; Zielińska, M.; Abalo, R. Nutraceuticals and Enteric Glial Cells. Molecules 2021, 26, 3762. [Google Scholar] [CrossRef]
- Meldgaard, T.; Olesen, S.S.; Farmer, A.D.; Krogh, K.; Wendel, A.A.; Brock, B.; Drewes, A.M.; Brock, C. Diabetic Enteropathy: From Molecule to Mechanism-Based Treatment. J. Diabetes Res. 2018, 2018, 3827301. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-T.; Seino, S.; Kirchgessner, A.L. Identification and Characterization of Glucoresponsive Neurons in the Enteric Nervous System. J. Neurosci. 1999, 19, 10305–10317. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.W.; Al-Rammahi, M.A.; Batchelor, D.J.; Bravo, D.M.; Shirazi-Beechey, S.P. Glucagon-Like Peptide-2 and the Enteric Nervous System Are Components of Cell-Cell Communication Pathway Regulating Intestinal Na+/Glucose Co-Transport. Front. Nutr. 2018, 5, 101. [Google Scholar] [CrossRef]
- Meldgaard, T.; Keller, J.; Olesen, A.E.; Olesen, S.S.; Krogh, K.; Borre, M.; Farmer, A.; Brock, B.; Brock, C.; Drewes, A.M. Pathophysiology and Management of Diabetic Gastroenteropathy. Therap. Adv. Gastroenterol. 2019, 12, 1756284819852047. [Google Scholar] [CrossRef]
- Abdu Seid, M.; Diress, M.; Mohammed, A.; Sinamaw, D. Chronic Constipation and Its Associated Factors in Patients with Type-2 Diabetes: A Multicenter Cross-Sectional Study. Diabetes Res. Clin. Pract. 2023, 204, 110905. [Google Scholar] [CrossRef]




| Antibodies | Dilution | Source | Identifier |
|---|---|---|---|
| Mouse anti-HuC/D | 1:500 | Invitrogen, Carlsbad, CA, USA | CAT#: A21271 |
| Rabbit anti-nNOS | 1:500 | Abcam, Cambridge, UK | CAT#: ab76067 |
| Goat anti-ChAT | 1:40 | Millipore, MA, USA | CAT#: AB144P |
| Rabbit anti-S100 beta | 1:1000 | Abcam, Cambridge, UK | CAT#: ab52642 |
| Alexa Fluor 488 donkey anti-mouse IgG | 1:500 | Invitrogen, Carlsbad, CA, USA | CAT#: A21202 |
| Alexa Fluor 596 goat anti-rabbit IgG | 1:500 | Abcam, Cambridge, UK | CAT#: ab150080 |
| Alexa Fluor 546 donkey anti-goat IgG | 1:500 | Invitrogen, Carlsbad, CA, USA | CAT#: A11056 |
| Duodenum | Jejunum | Ileum | Proximal Colon | Distal Colon | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wistar | GK | Wistar | GK | Wistar | GK | Wistar | GK | Wistar | GK | |
| HuC/D | 36 ± 2 | 32 ± 1.4 | 32 (31; 36.5) | 33 (30.5; 40) | 37 ± 1.1 | 36 ± 1 | 41.8 ± 1.2 | 43.8 ± 2.3 | 42.6 ± 3.4 | 46.4 ± 2.7 |
| S100 | 16.3 ± 2.5 | 14.7 ± 1.4 | 13.4 ± 1.1 | 13.5 ± 0.7 | 12.9 ± 0.5 | 17.9 ± 1 ** | 25.2 ± 1.3 | 22.4 ± 1.2 | 20.5 ± 1.4 | 22.3 ± 2 |
| ChAT | 31.7 ± 2 | 19.4 ± 0.8 *** | 24.4 ± 1.2 | 19.4 ± 0.75 ** | 26.9 ± 1.1 | 20.9 ± 0.7 ** | 28.6 ± 1.3 | 34.4 ± 1.3 * | 31 ± 2.8 | 30.9 ± 2.1 |
| nNOS | 6.1 ± 0.45 | 6.3 ± 0.26 | 6.8 ± 0.44 | 8.2 ± 0.59 | 8.1 ± 0.7 | 7.7 ± 0.8 | 8.5 ± 0.6 | 8.2 ± 0.9 | 9.6 ± 1.3 | 10.3 ± 0.9 |
| Duodenum | Jejunum | Ileum | Proximal Colon | Distal Colon | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wistar | GK | Wistar | GK | Wistar | GK | Wistar | GK | Wistar | GK | |
| HuC/D | 268 ± 35.6 | 315.6 ± 15.5 | 282 ± 28.3 | 366 ± 10.5 * | 255.7 ± 22.8 | 323.4 ± 14.8 ** | 339.5 ± 12.8 | 318 ± 10.3 | 336 ± 19.9 | 284 ± 11.6 |
| ChAT | 322 ± 21 | 300 ± 12.8 | 277 ± 21.5 | 318 ± 12.2 | 280 ± 15.9 | 296 ± 12.5 | 329 ± 25.3 | 325 ± 13.9 | 327 ± 20.8 | 306 ± 13.9 |
| nNOS | 285 ± 9.3 | 262 ± 14 | 312 ± 20.2 | 302 ± 21.6 | 295 ± 8.3 | 338 ± 27.4 | 420 ± 24.5 | 361.5± 29.3 | 437 (403.5; 468.5) | 290 (272; 460.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimenes, G.M.; Pereira, J.N.B.; Borges da Silva, E.; Santos, A.A.C.d.; Rodrigues, T.M.; Santana, G.d.O.; Scervino, M.V.M.; Pithon-Curi, T.C.; Hirabara, S.M.; Gorjão, R.; et al. Intestinal Motility Dysfunction in Goto-Kakizaki Rats: Role of the Myenteric Plexus. Cells 2024, 13, 1626. https://doi.org/10.3390/cells13191626
Gimenes GM, Pereira JNB, Borges da Silva E, Santos AACd, Rodrigues TM, Santana GdO, Scervino MVM, Pithon-Curi TC, Hirabara SM, Gorjão R, et al. Intestinal Motility Dysfunction in Goto-Kakizaki Rats: Role of the Myenteric Plexus. Cells. 2024; 13(19):1626. https://doi.org/10.3390/cells13191626
Chicago/Turabian StyleGimenes, Gabriela Mandú, Joice Naiara Bertaglia Pereira, Eliane Borges da Silva, Alef Aragão Carneiro dos Santos, Thais Martins Rodrigues, Giovanna de Oliveira Santana, Maria Vitoria Martins Scervino, Tania Cristina Pithon-Curi, Sandro Massao Hirabara, Renata Gorjão, and et al. 2024. "Intestinal Motility Dysfunction in Goto-Kakizaki Rats: Role of the Myenteric Plexus" Cells 13, no. 19: 1626. https://doi.org/10.3390/cells13191626






