Neddylation and Its Target Cullin 3 Are Essential for Adipocyte Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, CRISPR/Cas9 Gene Targeting, Lentivirus Production and Infection, and 3T3-L1 Adipocyte Differentiation
2.2. Isolation of Mouse Embryonic Fibroblasts (MEF) for Adipocyte Differentiation
2.3. Isolation of Human Stromal Vascular Cells from Adipose Tissue and Adipocyte Differentiation
2.4. FACS Analysis
2.5. Triglyceride (TG) Contents and Oil Red O (ORO) Staining
2.6. Real-Time Quantitative PCR
2.7. Immunoblot Analysis
2.8. Statistical Analysis
3. Results
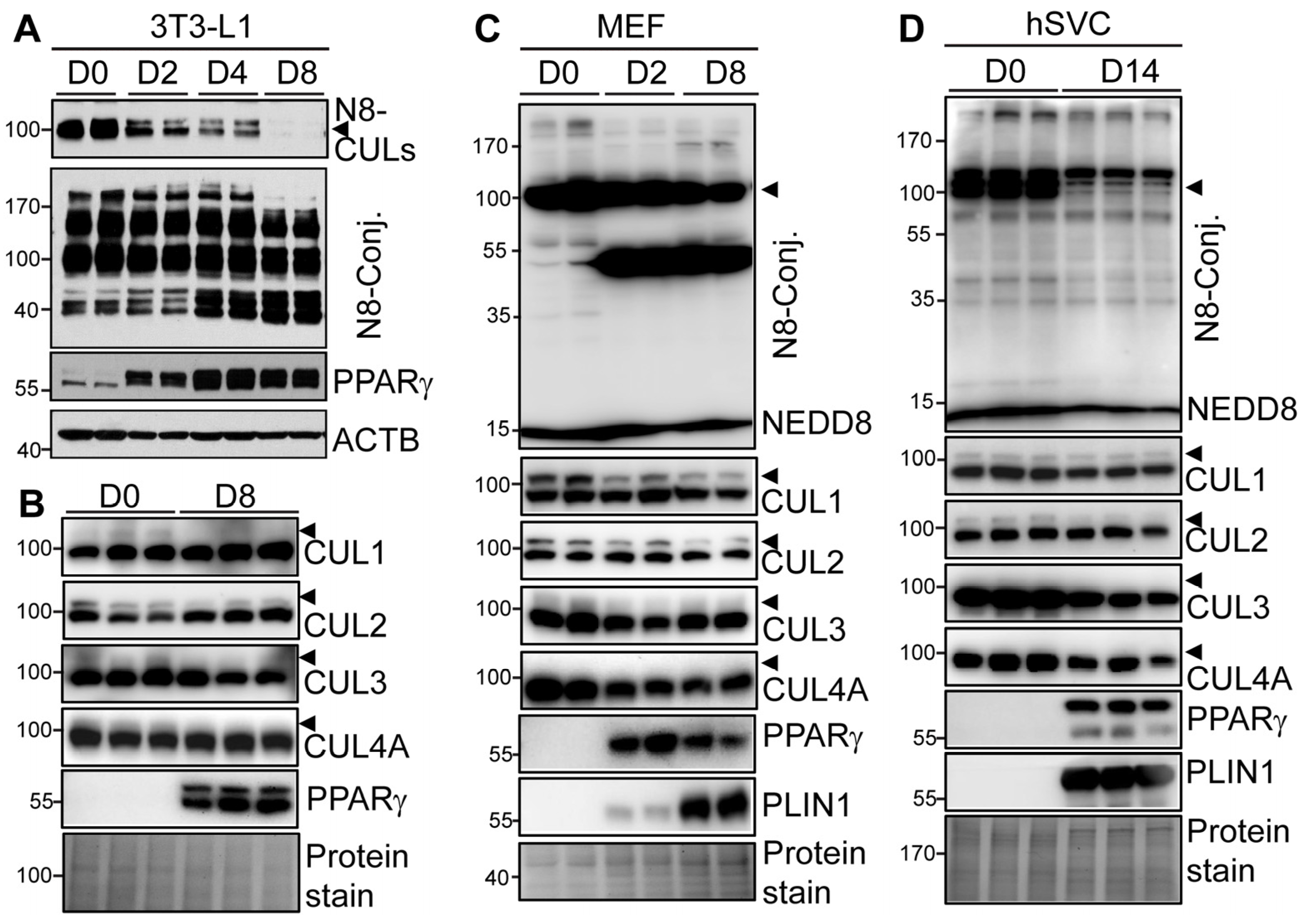
3.1. Dysregulated Neddylation and Its Targets CULs during Mouse and Human Adipogenesis
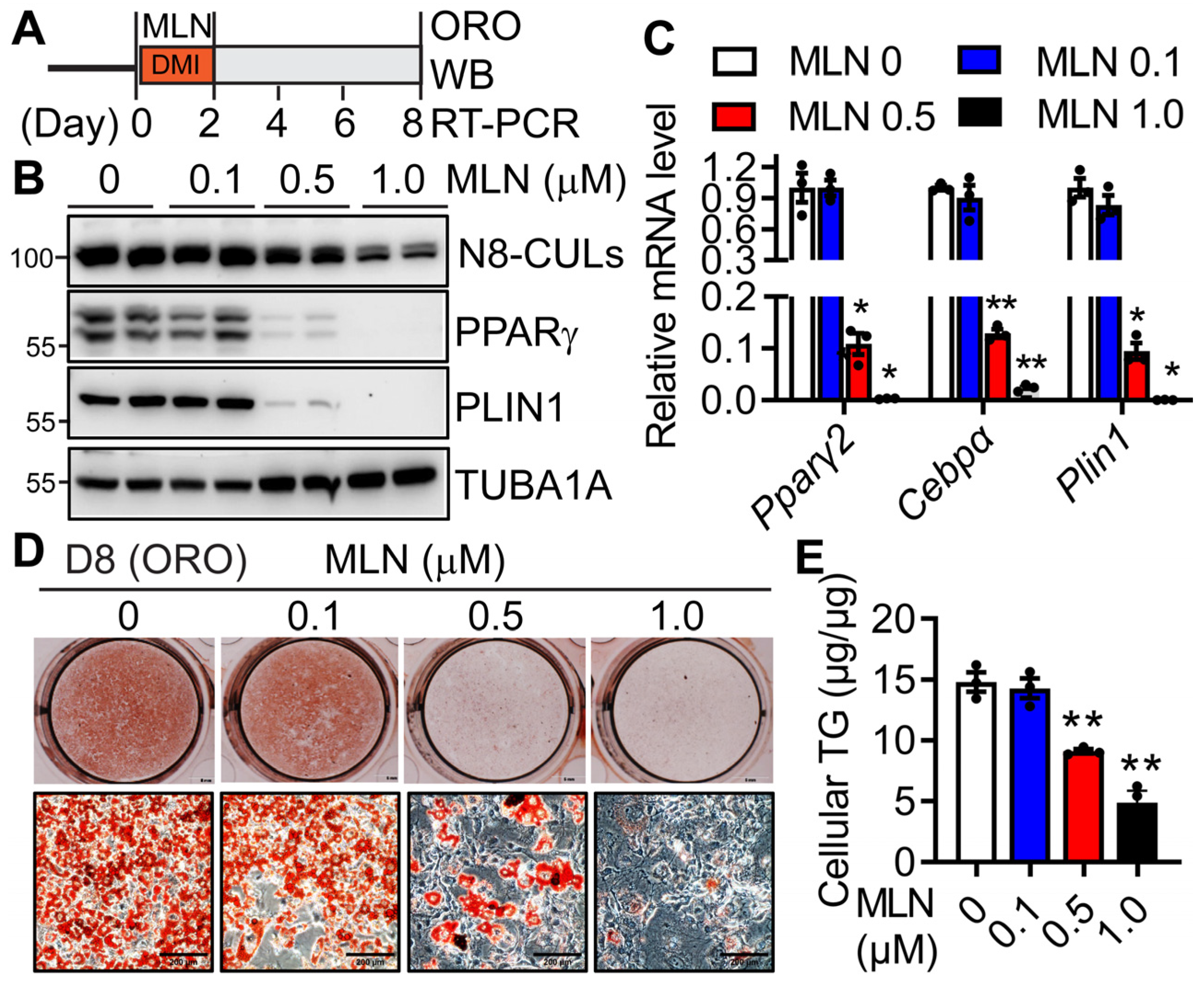
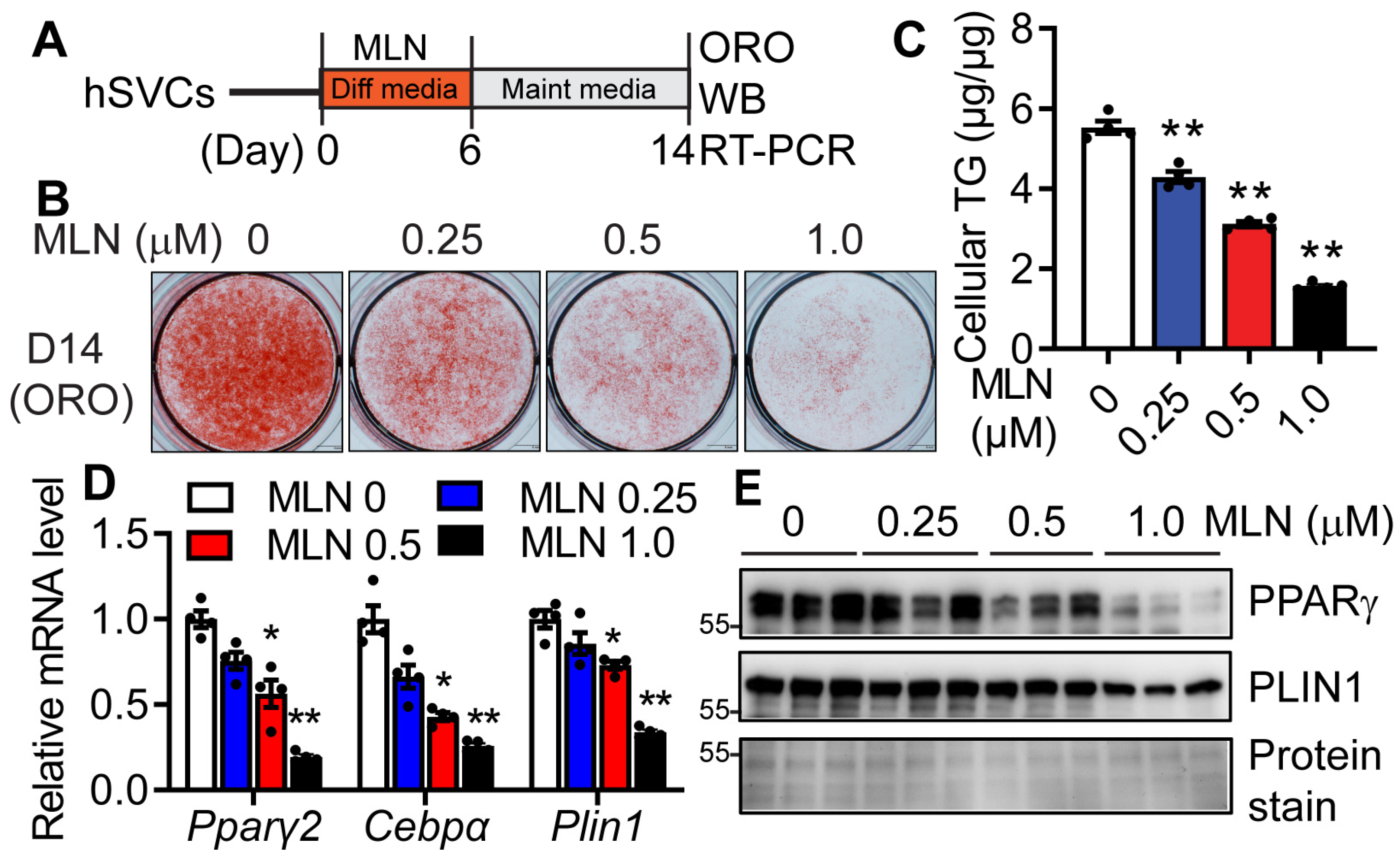
3.2. Pharmacologic Inhibition of Neddylation Blocks Mouse and Human Adipogenesis
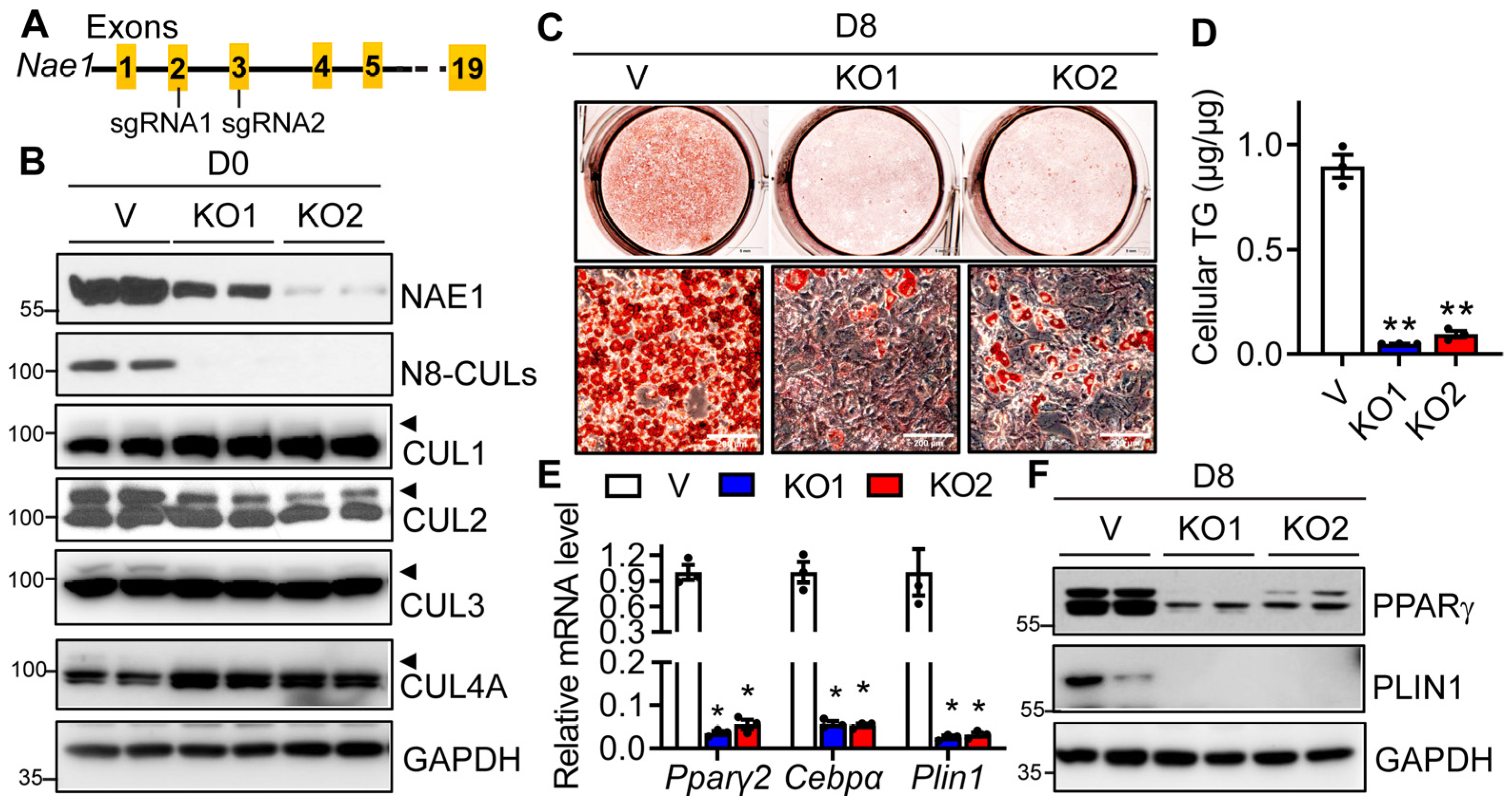
3.3. Genetic Deletion of NAE1 Blocks Adipogenesis
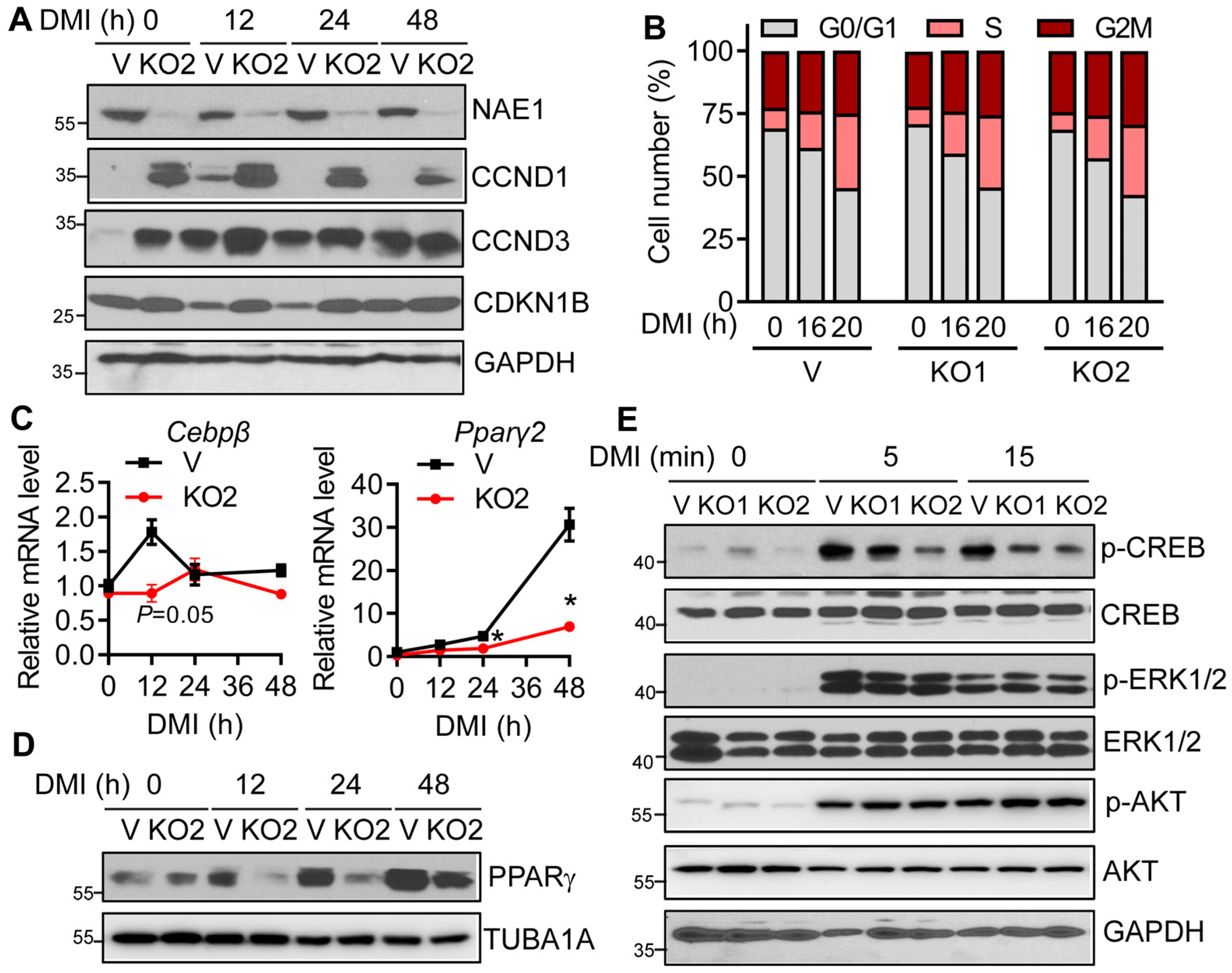
3.4. Neddylation Deficiency Does Not Affect Mitotic Clone Expansion but Impairs CREB/CEBPβ/PPARγ Signaling
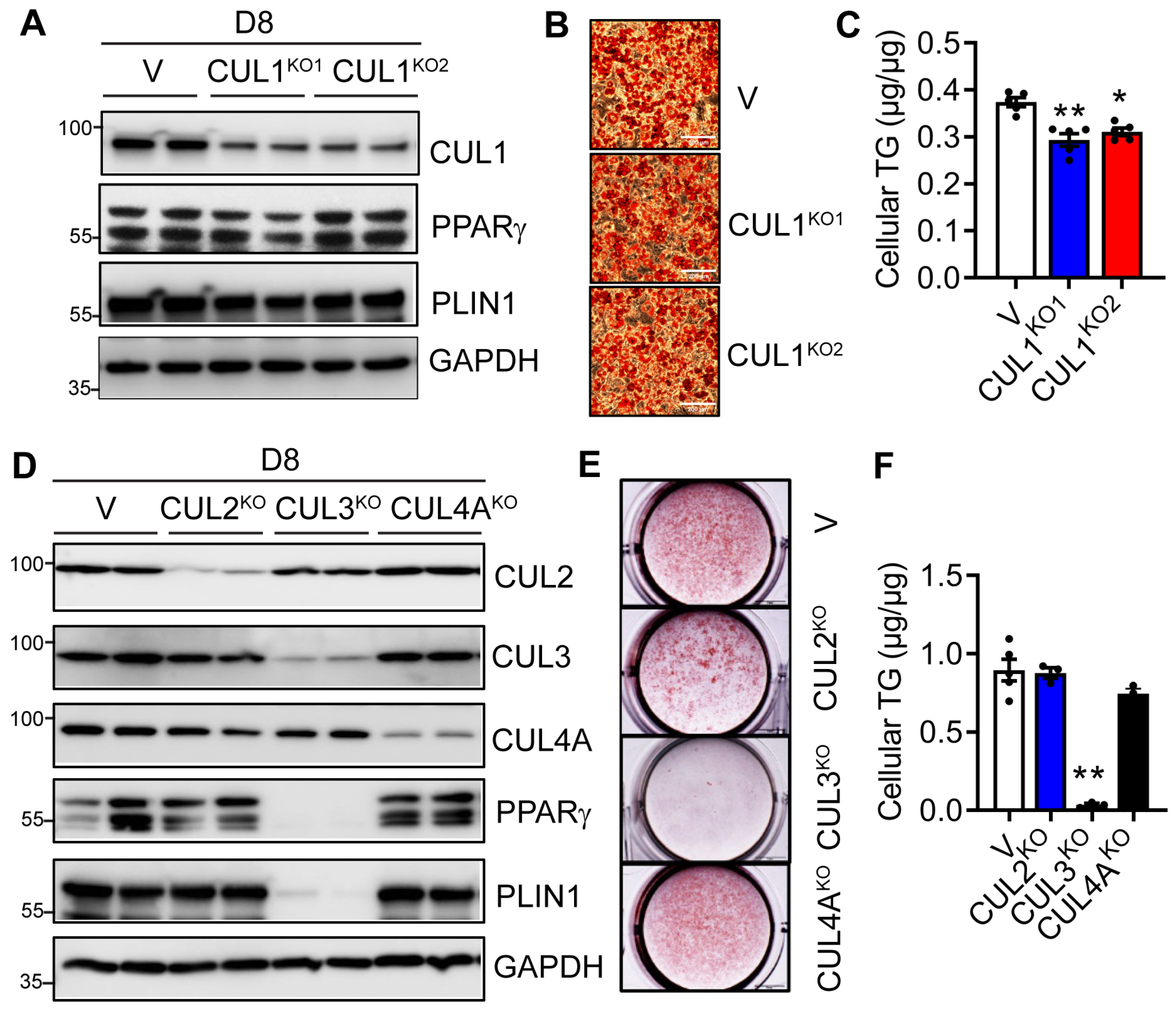
3.5. Deletion of CUL3, but Not CUL1, 2 and 4A Blocks Adipogenesis
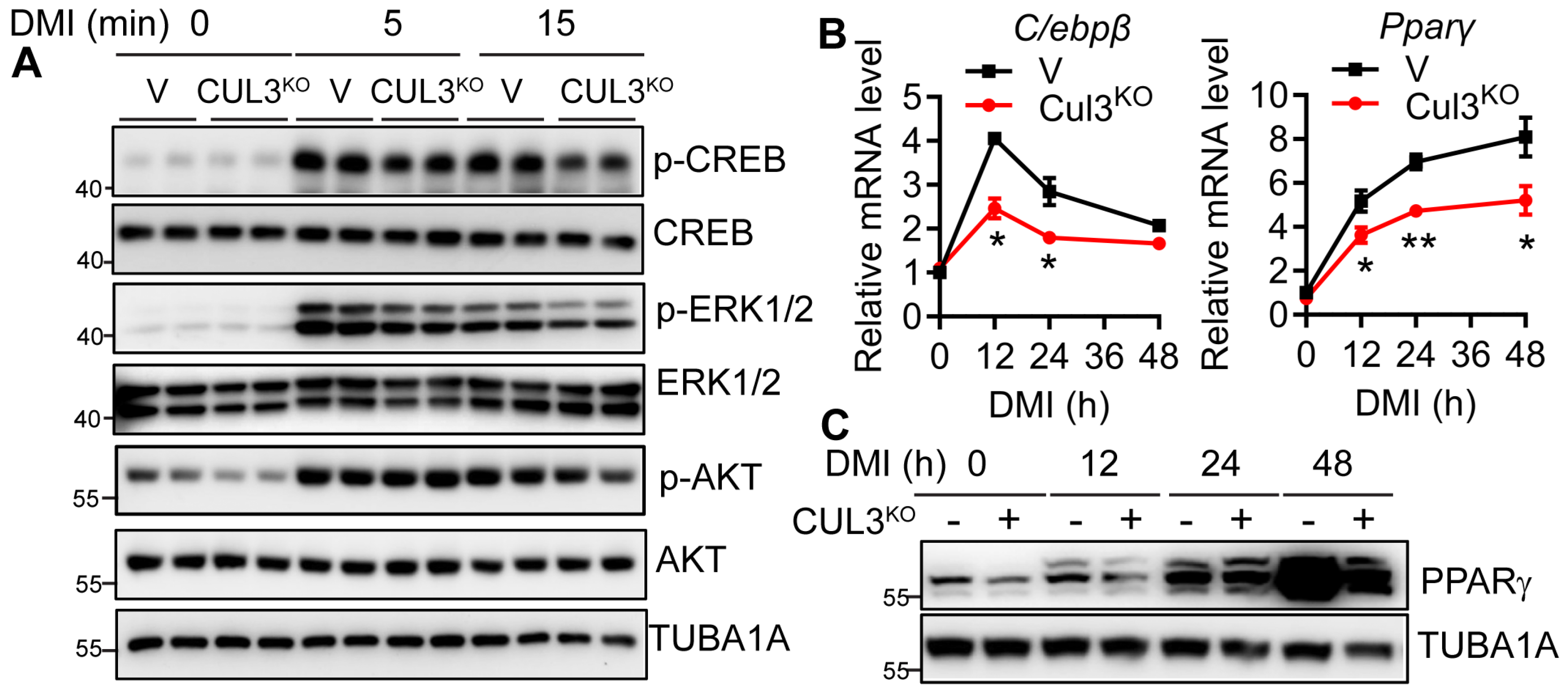
3.6. CUL3 Deletion Led to Impaired CREB/CEBPβ/PPARγ Signaling and Adipogenic Deficiency
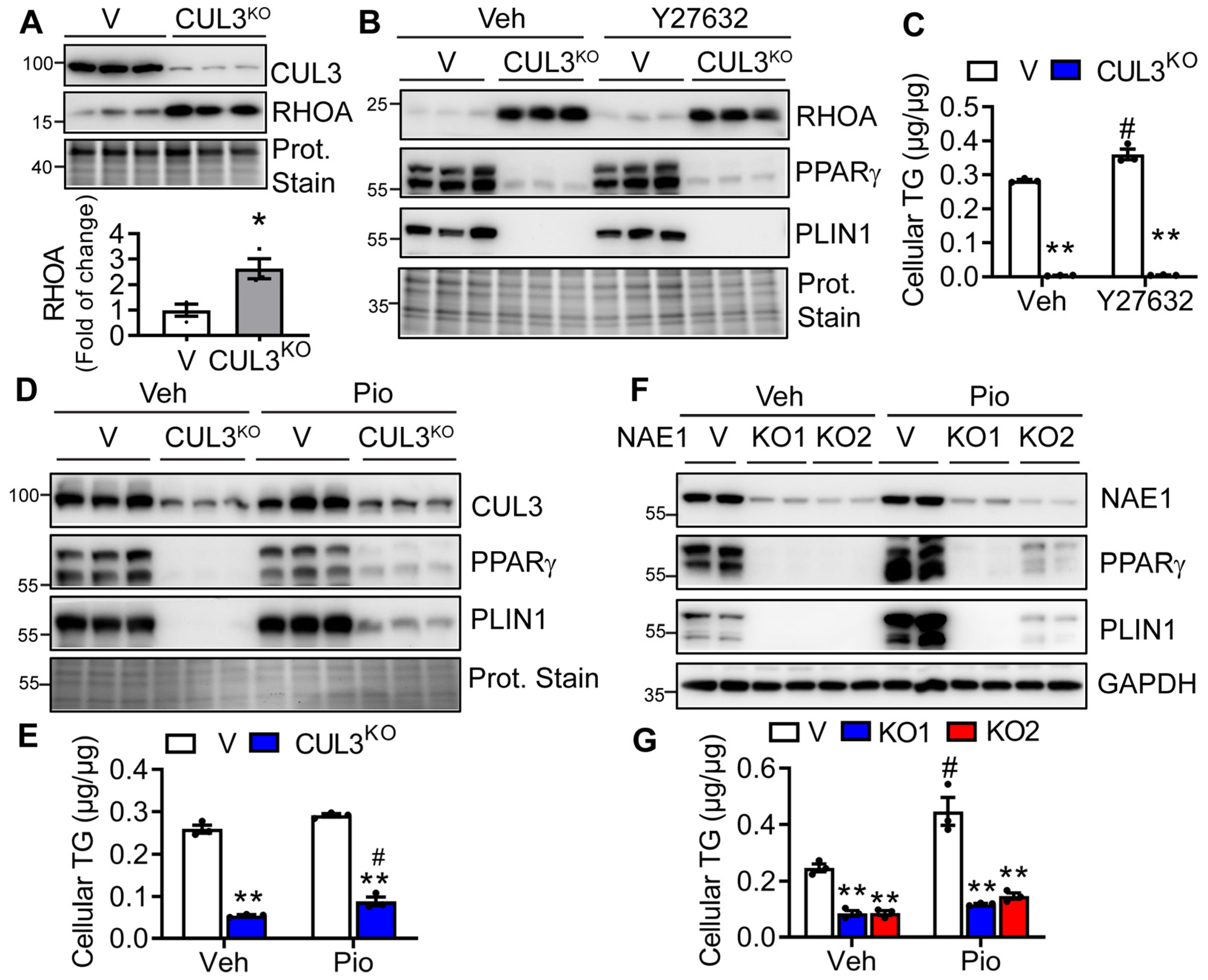
3.7. RHOA/ROCK Inhibitor Fails to Restore, but Pioglitazone Minimally Rescues Adipogenesis in CUL3KO and NAE1KO Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Tanaka, T.; Yoshida, N.; Kishimoto, T.; Akira, S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997, 16, 7432–7443. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Zhang, Y.; Steger, D.J.; Schupp, M.; Schug, J.; Cristancho, A.; Feng, D.; Zhuo, D.; Stoeckert, C.J., Jr.; Liu, X.S.; et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008, 22, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Terauchi, Y.; Miki, H.; Tamemoto, H.; Yamauchi, T.; Komeda, K.; Satoh, S.; Nakano, R.; Ishii, C.; Sugiyama, T.; et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 1999, 4, 597–609. [Google Scholar] [CrossRef]
- Fox, K.E.; Fankell, D.M.; Erickson, P.F.; Majka, S.M.; Crossno, J.T., Jr.; Klemm, D.J. Depletion of cAMP-response element-binding protein/ATF1 inhibits adipogenic conversion of 3T3-L1 cells ectopically expressing CCAAT/enhancer-binding protein (C/EBP) alpha, C/EBP beta, or PPAR gamma 2. J. Biol. Chem. 2006, 281, 40341–40353. [Google Scholar] [CrossRef]
- Reusch, J.E.; Colton, L.A.; Klemm, D.J. CREB activation induces adipogenesis in 3T3-L1 cells. Mol. Cell. Biol. 2000, 20, 1008–1020. [Google Scholar] [CrossRef]
- Zhang, J.W.; Klemm, D.J.; Vinson, C.; Lane, M.D. Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein beta gene during adipogenesis. J. Biol. Chem. 2004, 279, 4471–4478. [Google Scholar] [CrossRef]
- Kumar, S.; Yoshida, Y.; Noda, M. Cloning of a cDNA which encodes a novel ubiquitin-like protein. Biochem. Biophys. Res. Commun. 1993, 195, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Yeh, E.T. Identification of the activating and conjugating enzymes of the NEDD8 conjugation pathway. J. Biol. Chem. 1999, 274, 12036–12042. [Google Scholar] [CrossRef] [PubMed]
- Lipkowitz, S.; Weissman, A.M. RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat. Rev. Cancer 2011, 11, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Oved, S.; Mosesson, Y.; Zwang, Y.; Santonico, E.; Shtiegman, K.; Marmor, M.D.; Kochupurakkal, B.S.; Katz, M.; Lavi, S.; Cesareni, G.; et al. Conjugation to Nedd8 instigates ubiquitylation and down-regulation of activated receptor tyrosine kinases. J. Biol. Chem. 2006, 281, 21640–21651. [Google Scholar] [CrossRef]
- Petroski, M.D.; Deshaies, R.J. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2005, 6, 9–20. [Google Scholar] [CrossRef]
- Broderick, S.R.; Golas, B.J.; Pham, D.; Towe, C.W.; Talbot, S.G.; Kaufman, A.; Bains, S.; Huryn, L.A.; Yonekawa, Y.; Carlson, D.; et al. SCCRO promotes glioma formation and malignant progression in mice. Neoplasia 2010, 12, 476–484. [Google Scholar] [CrossRef]
- Lee, M.H.; Zhao, R.; Phan, L.; Yeung, S.C. Roles of COP9 signalosome in cancer. Cell Cycle 2011, 10, 3057–3066. [Google Scholar] [CrossRef]
- Su, H.; Li, J.; Menon, S.; Liu, J.; Kumarapeli, A.R.; Wei, N.; Wang, X. Perturbation of cullin deneddylation via conditional Csn8 ablation impairs the ubiquitin-proteasome system and causes cardiomyocyte necrosis and dilated cardiomyopathy in mice. Circ. Res. 2011, 108, 40–50. [Google Scholar] [CrossRef]
- Park, H.S.; Ju, U.I.; Park, J.W.; Song, J.Y.; Shin, D.H.; Lee, K.H.; Jeong, L.S.; Yu, J.; Lee, H.W.; Cho, J.Y.; et al. PPARgamma neddylation essential for adipogenesis is a potential target for treating obesity. Cell Death Differ. 2016, 23, 1296–1311. [Google Scholar] [CrossRef]
- Soucy, T.A.; Smith, P.G.; Milhollen, M.A.; Berger, A.J.; Gavin, J.M.; Adhikari, S.; Brownell, J.E.; Burke, K.E.; Cardin, D.P.; Critchley, S.; et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 2009, 458, 732–736. [Google Scholar] [CrossRef]
- Li, P.; Song, Y.; Zan, W.; Qin, L.; Han, S.; Jiang, B.; Dou, H.; Shao, C.; Gong, Y. Lack of CUL4B in Adipocytes Promotes PPARgamma-Mediated Adipose Tissue Expansion and Insulin Sensitivity. Diabetes 2017, 66, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.Q.; Kentsis, A.; Dias, D.C.; Yamoah, K.; Wu, K. Nedd8 on cullin: Building an expressway to protein destruction. Oncogene 2004, 23, 1985–1997. [Google Scholar] [CrossRef]
- Furukawa, M.; He, Y.J.; Borchers, C.; Xiong, Y. Targeting of protein ubiquitination by BTB-Cullin 3-Roc1 ubiquitin ligases. Nat. Cell Biol. 2003, 5, 1001–1007. [Google Scholar] [CrossRef]
- Pintard, L.; Willis, J.H.; Willems, A.; Johnson, J.L.; Srayko, M.; Kurz, T.; Glaser, S.; Mains, P.E.; Tyers, M.; Bowerman, B.; et al. The BTB protein MEL-26 is a substrate-specific adaptor of the CUL-3 ubiquitin-ligase. Nature 2003, 425, 311–316. [Google Scholar] [CrossRef]
- Genschik, P.; Sumara, I.; Lechner, E. The emerging family of CULLIN3-RING ubiquitin ligases (CRL3s): Cellular functions and disease implications. EMBO J. 2013, 32, 2307–2320. [Google Scholar] [CrossRef]
- Boyden, L.M.; Choi, M.; Choate, K.A.; Nelson-Williams, C.J.; Farhi, A.; Toka, H.R.; Tikhonova, I.R.; Bjornson, R.; Mane, S.M.; Colussi, G.; et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 2012, 482, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, F.R.; Siew, K.; Zhang, J.; Johnson, C.; Wood, N.; Cleary, S.E.; Al Maskari, R.S.; Ferryman, J.T.; Hardege, I.; Yasmin; et al. Characterisation of the Cullin-3 mutation that causes a severe form of familial hypertension and hyperkalaemia. EMBO Mol. Med. 2015, 7, 1285–1306. [Google Scholar] [CrossRef] [PubMed]
- Dubiel, D.; Bintig, W.; Kahne, T.; Dubiel, W.; Naumann, M. Cul3 neddylation is crucial for gradual lipid droplet formation during adipogenesis. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1405–1412. [Google Scholar] [CrossRef]
- Zhou, H.; Su, H.; Chen, W. Neddylation Regulates Class IIa and III Histone Deacetylases to Mediate Myoblast Differentiation. Int. J. Mol. Sci. 2021, 22, 9509. [Google Scholar] [CrossRef]
- Chen, W.; Yechoor, V.K.; Chang, B.H.; Li, M.V.; March, K.L.; Chan, L. The human lipodystrophy gene product Berardinelli-Seip congenital lipodystrophy 2/seipin plays a key role in adipocyte differentiation. Endocrinology 2009, 150, 4552–4561. [Google Scholar] [CrossRef]
- Xu, J. Preparation, culture, and immortalization of mouse embryonic fibroblasts. Curr. Protoc. Mol. Biol. 2005, 70, 28.1.1–28.1.8. [Google Scholar] [CrossRef]
- Zhou, H.; Lei, X.; Yan, Y.; Lydic, T.; Li, J.; Weintraub, N.L.; Su, H.; Chen, W. Targeting ATGL to rescue BSCL2 lipodystrophy and its associated cardiomyopathy. JCI Insight 2019, 5, e129781. [Google Scholar] [CrossRef]
- Lee, M.J.; Fried, S.K. Optimal protocol for the differentiation and metabolic analysis of human adipose stromal cells. Methods Enzymol. 2014, 538, 49–65. [Google Scholar]
- Wu, M.H.; Lee, C.Y.; Huang, T.J.; Huang, K.Y.; Tang, C.H.; Liu, S.H.; Kuo, K.L.; Kuan, F.C.; Lin, W.C.; Shi, C.S. MLN4924, a Protein Neddylation Inhibitor, Suppresses the Growth of Human Chondrosarcoma through Inhibiting Cell Proliferation and Inducing Endoplasmic Reticulum Stress-Related Apoptosis. Int. J. Mol. Sci. 2018, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Frescas, D.; Pagano, M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: Tipping the scales of cancer. Nat. Rev. Cancer 2008, 8, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, K.; Omata, M.; Tanaka, K.; Chiba, T. The NEDD8 system is essential for cell cycle progression and morphogenetic pathway in mice. J. Cell Biol. 2001, 155, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef]
- Petersen, R.K.; Madsen, L.; Pedersen, L.M.; Hallenborg, P.; Hagland, H.; Viste, K.; Doskeland, S.O.; Kristiansen, K. Cyclic AMP (cAMP)-mediated stimulation of adipocyte differentiation requires the synergistic action of Epac- and cAMP-dependent protein kinase-dependent processes. Mol. Cell. Biol. 2008, 28, 3804–3816. [Google Scholar] [CrossRef]
- Ji, Y.; Cao, M.; Liu, J.; Chen, Y.; Li, X.; Zhao, J.; Qu, C. Rock signaling control PPARgamma expression and actin polymerization during adipogenesis. Saudi J. Biol. Sci. 2017, 24, 1866–1870. [Google Scholar] [CrossRef]
- Emont, M.P.; Jacobs, C.; Essene, A.L.; Pant, D.; Tenen, D.; Colleluori, G.; Di Vincenzo, A.; Jorgensen, A.M.; Dashti, H.; Stefek, A.; et al. A single-cell atlas of human and mouse white adipose tissue. Nature 2022, 603, 926–933. [Google Scholar] [CrossRef]
- Lignitto, L.; Carlucci, A.; Sepe, M.; Stefan, E.; Cuomo, O.; Nistico, R.; Scorziello, A.; Savoia, C.; Garbi, C.; Annunziato, L.; et al. Control of PKA stability and signalling by the RING ligase praja2. Nat. Cell Biol. 2011, 13, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Delle Donne, R.; Catalanotti, B.; Torres-Quesada, O.; Enzler, F.; Moraca, F.; Nistico, R.; Chiuso, F.; Piccinin, S.; Bachmann, V.; et al. Feedback inhibition of cAMP effector signaling by a chaperone-assisted ubiquitin system. Nat. Commun. 2019, 10, 2572. [Google Scholar] [CrossRef]
- Rinaldi, L.; Sepe, M.; Donne, R.D.; Feliciello, A. A dynamic interface between ubiquitylation and cAMP signaling. Front. Pharmacol. 2015, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, J.Y.; Xu, Z.; Kho, D.H.; Zhuang, Z.; Raz, A.; Wu, G.S. The role of Cullin3-mediated ubiquitination of the catalytic subunit of PP2A in TRAIL signaling. Cell Cycle 2014, 13, 3750–3758. [Google Scholar] [CrossRef]
- Sundqvist, A.; Bengoechea-Alonso, M.T.; Ye, X.; Lukiyanchuk, V.; Jin, J.; Harper, J.W.; Ericsson, J. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab. 2005, 1, 379–391. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Tajima, K.; Verkerke, A.R.P.; Taxin, Z.H.; Hou, Z.; Cole, J.B.; Li, F.; Wong, J.; Abe, I.; et al. Post-translational control of beige fat biogenesis by PRDM16 stabilization. Nature 2022, 609, 151–158. [Google Scholar] [CrossRef]
- Groh, B.S.; Yan, F.; Smith, M.D.; Yu, Y.; Chen, X.; Xiong, Y. The antiobesity factor WDTC1 suppresses adipogenesis via the CRL4WDTC1 E3 ligase. EMBO Rep. 2016, 17, 638–647. [Google Scholar] [CrossRef]
- Huang, X.; Dubiel, W. The COP9 Signalosome Controls Adipocyte Differentiation by Regulating CHOP Protein Stability. Engineering 2013, 4, 192. [Google Scholar] [CrossRef]
| Gene Name | gRNA Sequence |
|---|---|
| mNae1 gRNA1 | TATAGGCTGTGGGGTGATCA |
| mNae1 gRNA2 | GAACCGAGCTCAAGCTGCCA |
| mCul1 gRNA1 | CCAACAAACTGAGCTCCCCC |
| mCul1 gRNA2 | TGCATCAGTCCAACCAAGCC |
| mCul2 gRNA1 | ACTATATGGACTGCTTATAT |
| mCul3 gRNA1 | GACCACTGTTATTCTTACGC |
| mCul3 gRNA2 | AACTTCTCCTTTCCGCTCTC |
| mCul4 gRNA1 | GCTCTACAAGCAGCTGCGCC |
| Gene Name | 5′ Forward Primer Sequence | 3′ Reverse Primer Sequence |
|---|---|---|
| mCebpa | GAACAGCAACGAGTACCGGGTA | GCCATGGCCTTGACCAAGGAG |
| mCebpb | CAAGCTGAGCGACGAGTACA | CAGCTGCTCCACCTTCTTCT |
| mPlin1 | CACCTGCGGCTGTGCTGG | CGATGTCTCGGAATTCGCT |
| mPparγ2 | TCTCCTGTTGACCCAGAGCA | GTGGAGCAGAAATGCTGGAG |
| mPpia | CTGTTTGCAGACAAAGTTCCA | AGGATGAAGTTCTCATCCTCA |
| mRplp0 | CGCTTTCTGGAGGGTGTCCGC | TGCCAGGACGCGCTTGTACC |
| hRPLP0 | GCAATGTTGCCAGTGTCTGT | AGATGGATCAGCCAAGAAGG |
| hPPARγ | TCCATGCTGTTATGGGTGAA | ACGGAGCTGATCCCAAAGT |
| hPlin1 | TCCCTCCAGACAAGGAAGAG | CATGGTCTGCACGGTGTATC |
| Antibody | Supplier (Catalog No.) | RRID |
|---|---|---|
| ACTB | Sigma-Millipore (MAB1501) | RRID:AB_2223041 |
| AKT | Cell signaling technology (9272) | RRID:AB_329827 |
| Phospho-AKT (ser473) | Cell signaling technology (4060) | RRID:AB_2315049 |
| CCND1 (Cyclin D1) | Cell signaling technology (2978) | RRID:AB_2259616 |
| CCND3 (Cyclin D3) | Cell signaling technology (2936) | RRID:AB_2070801 |
| CDKN1B (p27) | Cell signaling technology (3698) | RRID:AB_2077832 |
| CREB (48H2) | Cell signaling technology (9197) | RRID:AB_331277 |
| Phospho-CREB (Ser133) | Cell signaling technology (9198) | RRID:AB_2561044 |
| CUL1 | Proteintech (12895-1-AP) | RRID:AB_2086291 |
| CUL2 | Abcam (ab166917) | |
| CUL3 | Cell signaling technology (2759) | RRID:AB_2086432 |
| CUL4A | Proteintech (14851-1-AP) | RRID:AB_2261175 |
| ERK1/2 | Cell signaling technology (9102) | RRID:AB_330744 |
| Phospho-ERK1/2 | Cell signaling technology (4370) | RRID:AB_2315112 |
| GAPDH | Proteintech (60004-1-IG) | RRID:AB_2107436 |
| NAE1 | Cell signaling technology (14321) | RRID:AB_2798448 |
| NEDD8 | Cell signaling technology (2754) | RRID:AB_659972 |
| PLIN1 | Cell signaling technology (9349) | RRID:AB_10829911 |
| PPARγ | Cell signaling technology (2435) | RRID:AB_2166051 |
| RHOA | Proteintech (10749-1-AP) | RRID:AB_2285104 |
| TUBA1A | Proteintech (66031-1-Ig) | RRID:AB_11042766 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Patel, V.; Rice, R.; Lee, R.; Kim, H.W.; Weintraub, N.L.; Su, H.; Chen, W. Neddylation and Its Target Cullin 3 Are Essential for Adipocyte Differentiation. Cells 2024, 13, 1654. https://doi.org/10.3390/cells13191654
Zhou H, Patel V, Rice R, Lee R, Kim HW, Weintraub NL, Su H, Chen W. Neddylation and Its Target Cullin 3 Are Essential for Adipocyte Differentiation. Cells. 2024; 13(19):1654. https://doi.org/10.3390/cells13191654
Chicago/Turabian StyleZhou, Hongyi, Vijay Patel, Robert Rice, Richard Lee, Ha Won Kim, Neal L. Weintraub, Huabo Su, and Weiqin Chen. 2024. "Neddylation and Its Target Cullin 3 Are Essential for Adipocyte Differentiation" Cells 13, no. 19: 1654. https://doi.org/10.3390/cells13191654
APA StyleZhou, H., Patel, V., Rice, R., Lee, R., Kim, H. W., Weintraub, N. L., Su, H., & Chen, W. (2024). Neddylation and Its Target Cullin 3 Are Essential for Adipocyte Differentiation. Cells, 13(19), 1654. https://doi.org/10.3390/cells13191654









