Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles as Natural Nanocarriers in the Treatment of Nephrotoxic Injury In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vivo: Obtaining the Umbilical Cord-Derived Mesenchymal Stem Cell (UC-MSCs)
2.2. In Vitro Analyses: Umbilical Cord-Derived Mesenchymal Stem Cell (UC-MSCs)
2.3. In Vitro Analyses: Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles (UC-EVs)
2.4. In Vitro Analyses: Tubular Cells Exposed to Nephrotoxic Injury and Simultaneously Treated with UC-EVs Carrying miRNA-126 or Anti-miRNA-126 Sequences
2.5. Statistical Analyses
3. Results
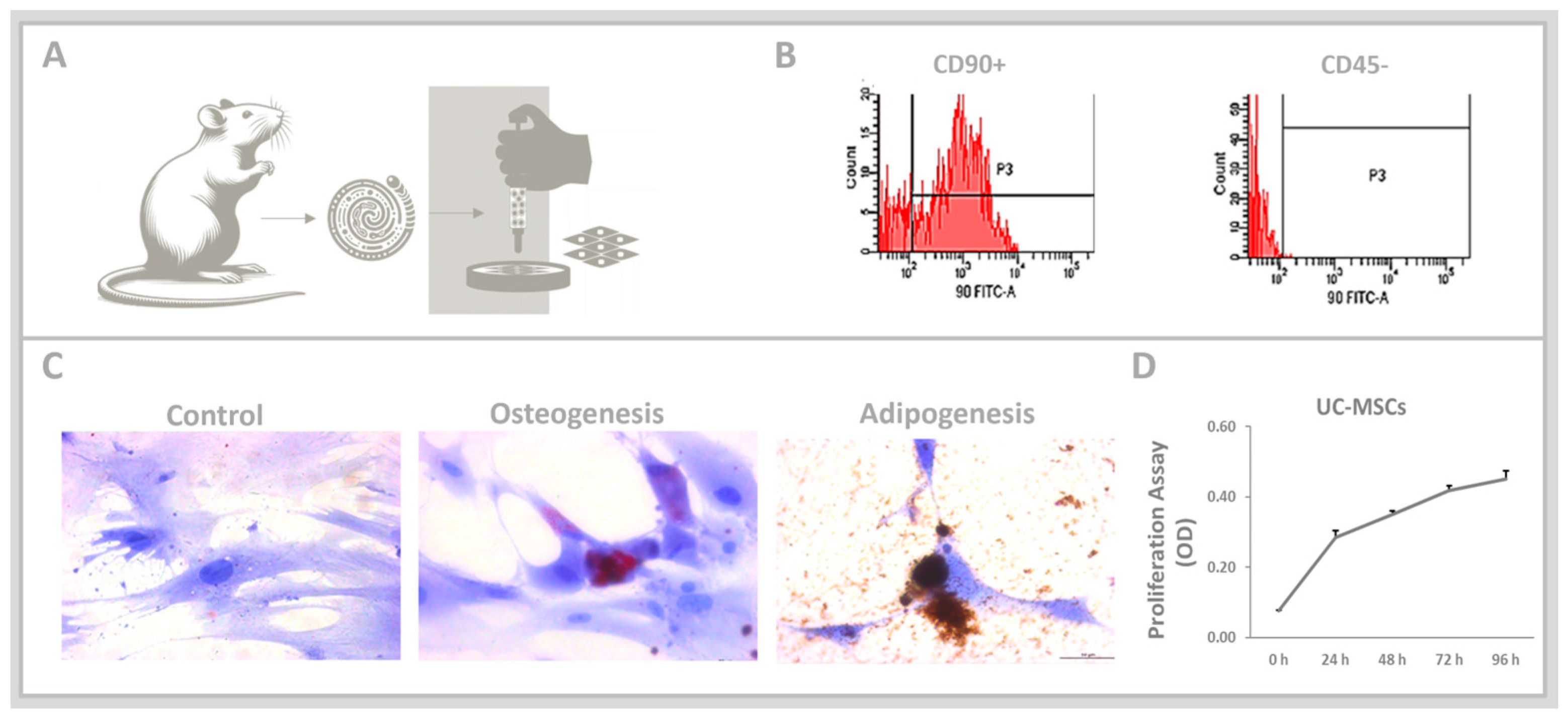
3.1. Umbilical Cord-Derived Mesenchymal Stem Cells (UC-MSCs)
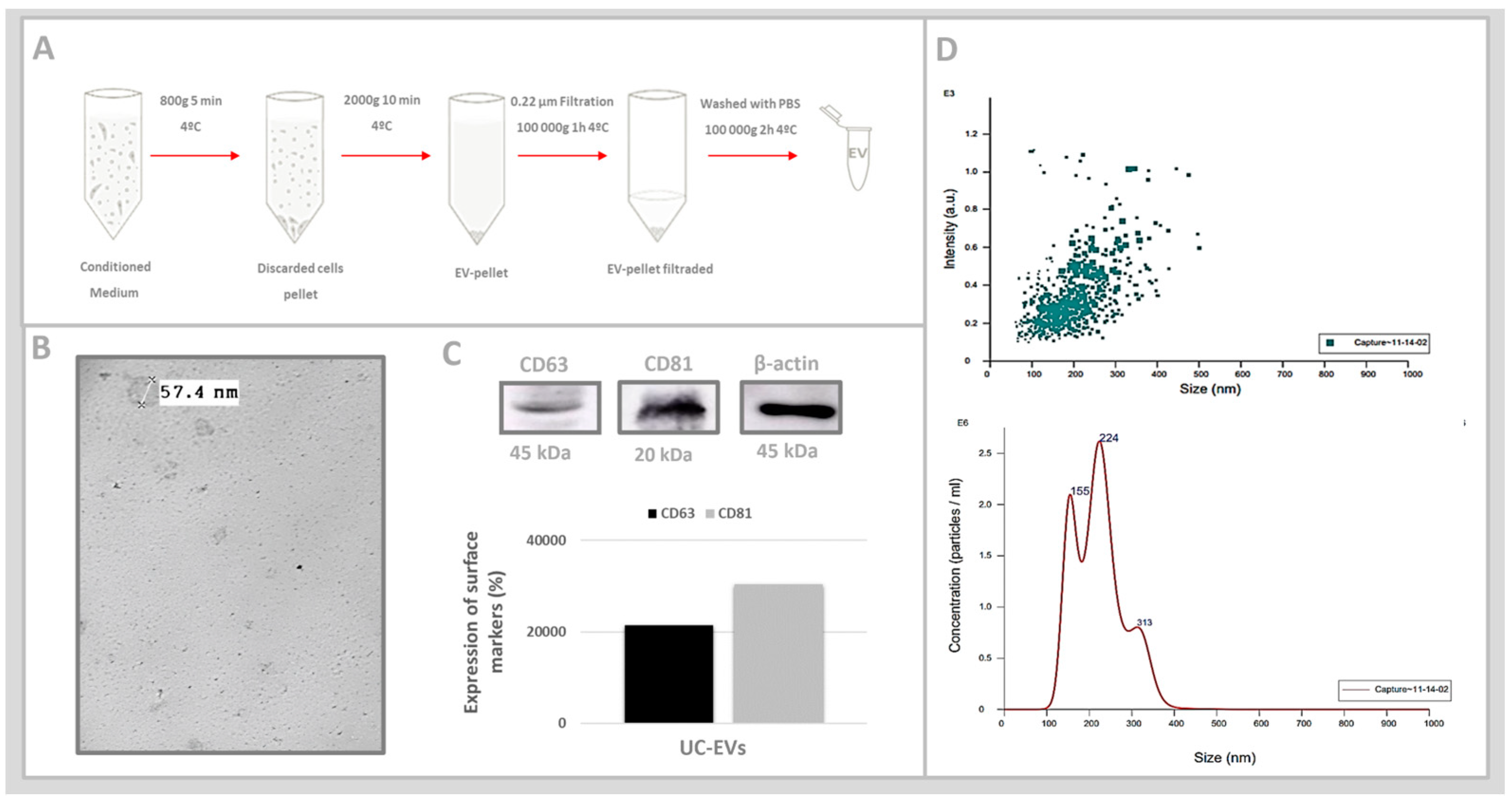
3.2. Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles (UC-EVs)
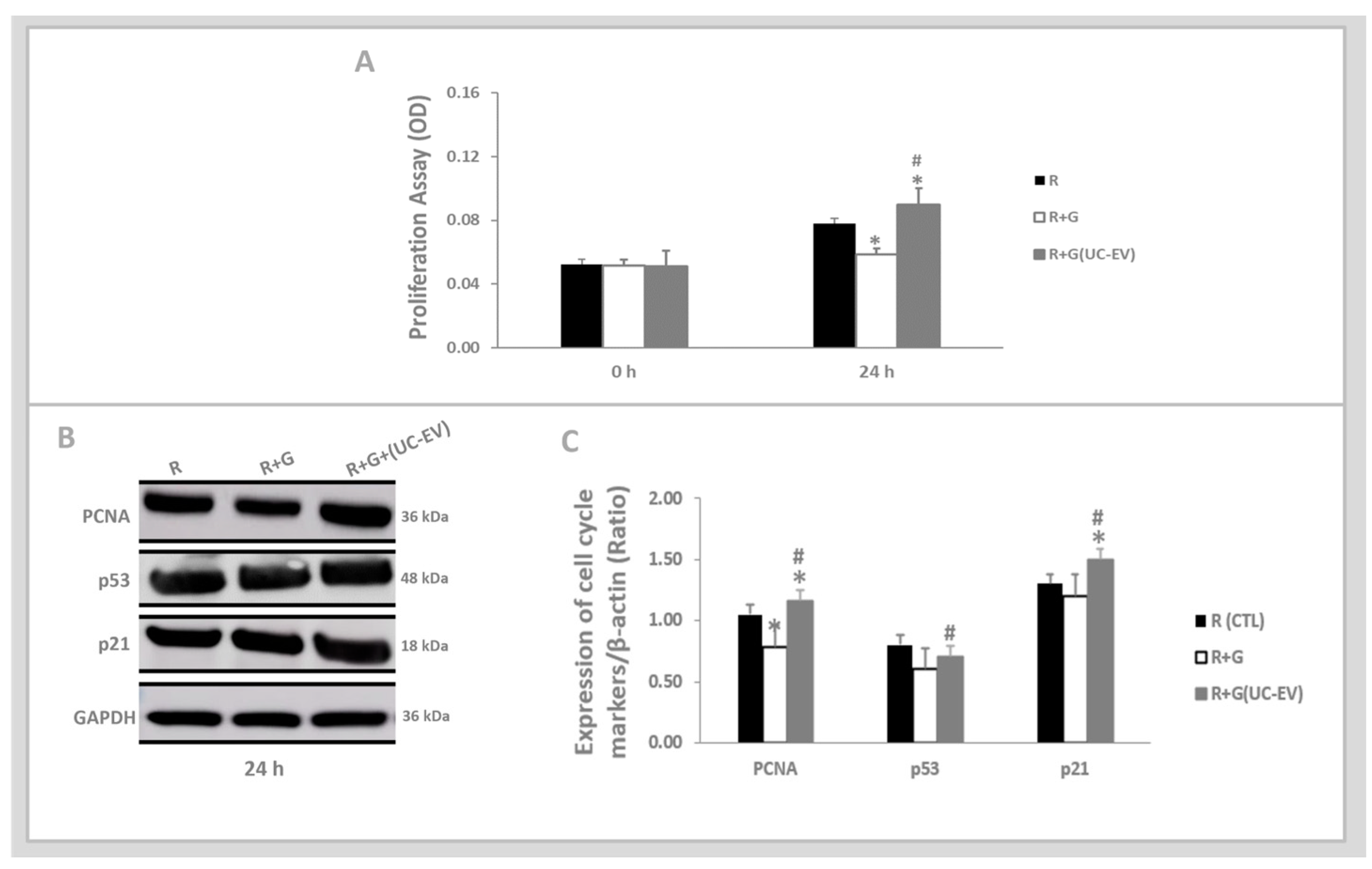
3.3. Tubular Cells Exposed to Nephrotoxic Injury and Simultaneously Treated with UC-EVs Carrying miRNA-126 Sequences at 24 h
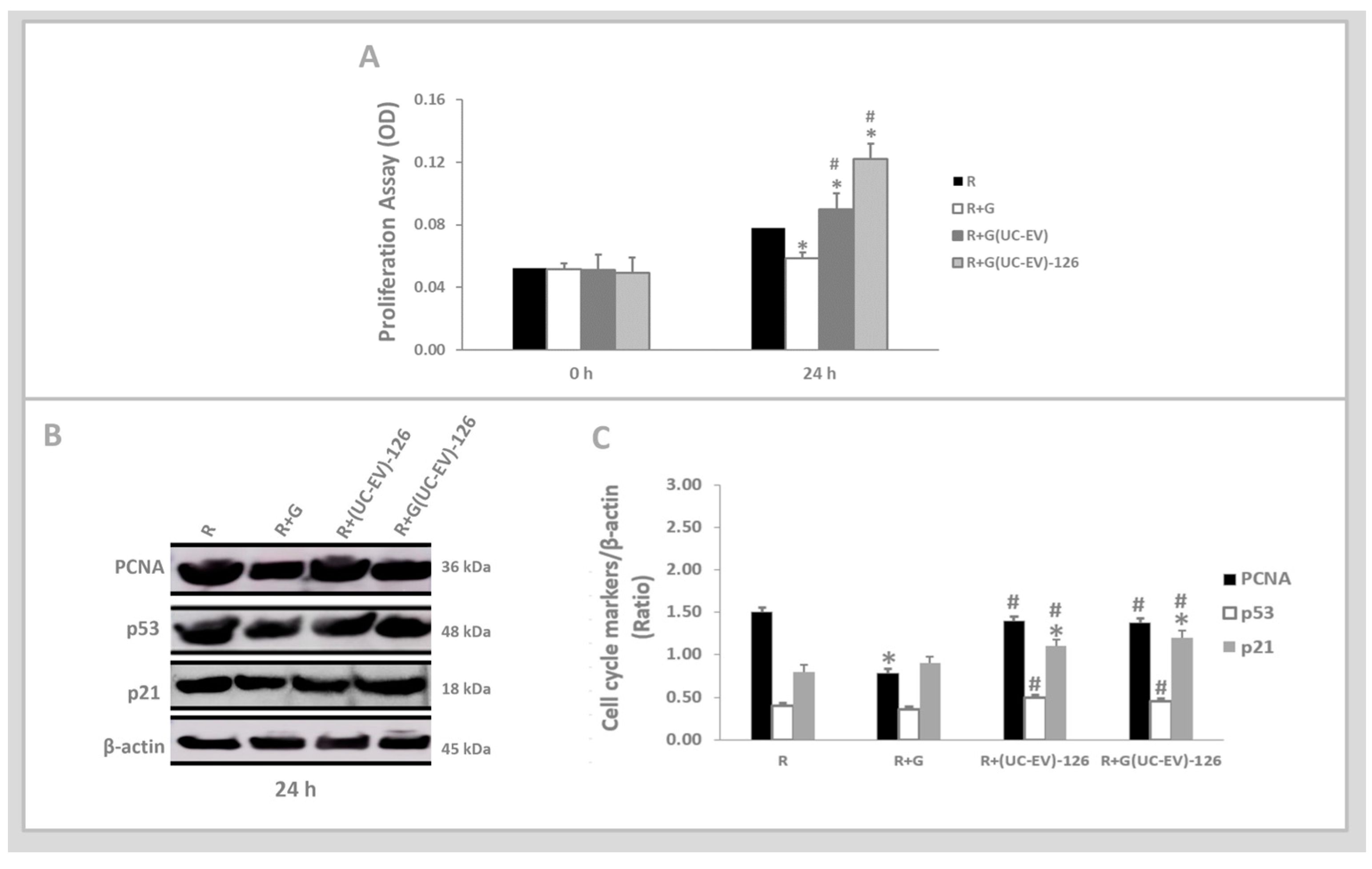
3.4. Tubular Cells Exposed to Nephrotoxic Injury and Simultaneously Treated with UC-EVs Carrying Anti-miRNA-126 Sequences at 24 h
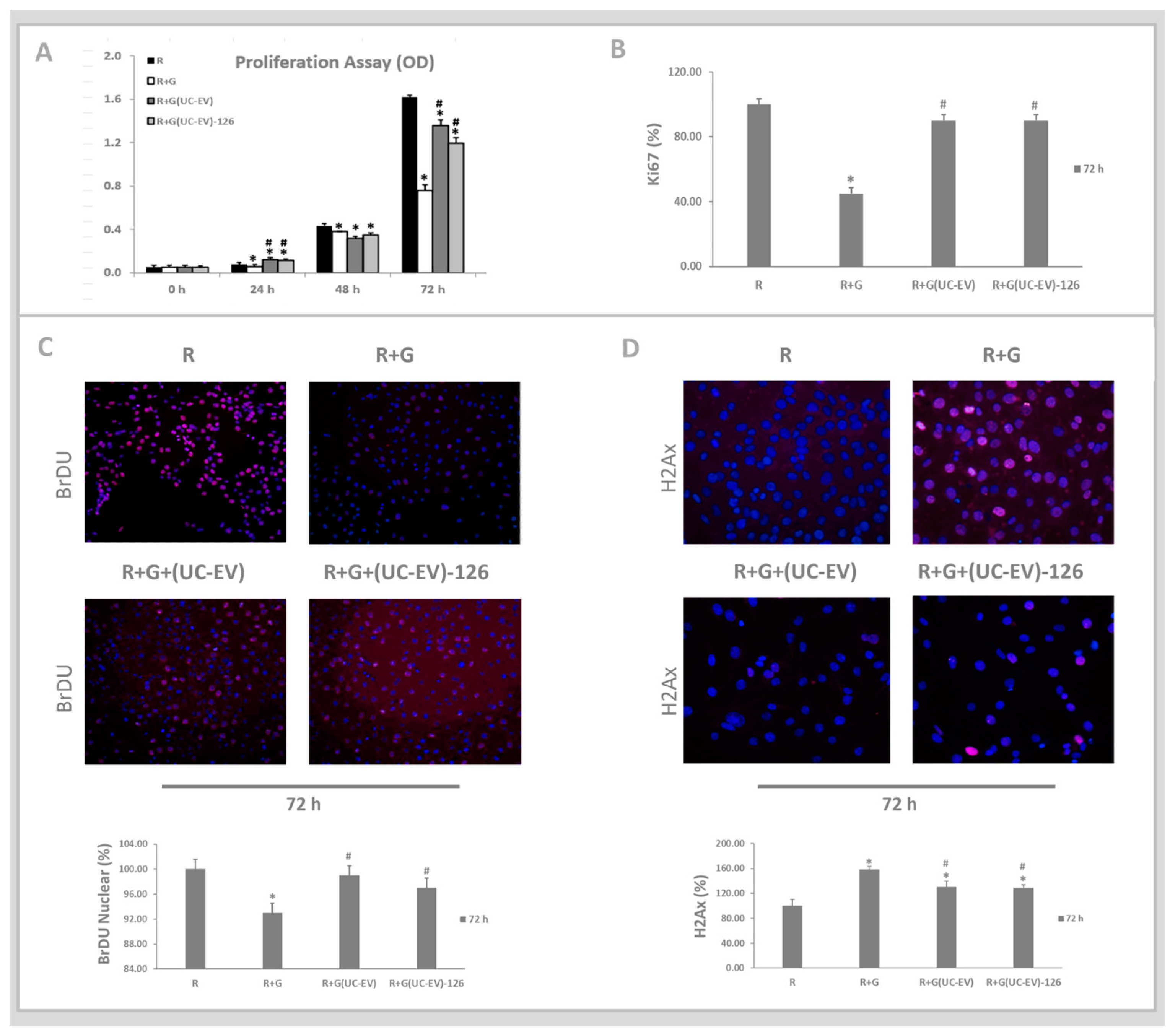
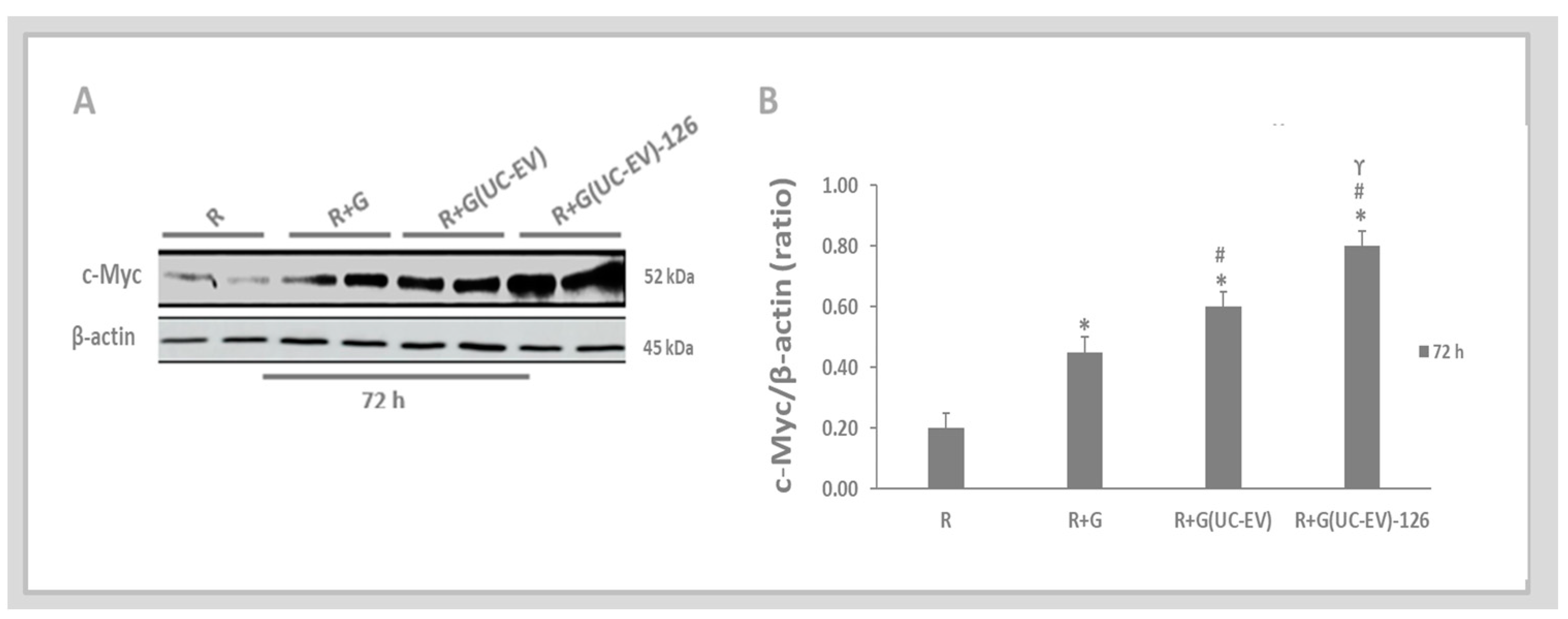
3.5. Tubular Cells Exposed to Nephrotoxic Injury and Simultaneously Treated with UC-EVs Carrying miRNA 126 or Anti-miRNA-126 Sequences at 72 h
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mennan, C.; Wright, K.; Bhattacharjee, A.; Balain, B.; Richardson, J.; Roberts, S. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. BioMed Res. Int. 2013, 2013, 916136. [Google Scholar] [CrossRef]
- Maguire, G. Stem cell therapy without the cells. Commun. Integr. Biol. 2013, 6, e26631. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef]
- González-González, A.; García-Sánchez, D.; Dotta, M.; Rodríguez-Rey, J.C.; Pérez-Campo, F.M. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J. Stem Cells 2020, 12, 1529–1552. [Google Scholar] [CrossRef]
- Amiri, A.; Bagherifar, R.; Ansari Dezfouli, E.; Kiaie, S.H.; Jafari, R.; Ramezani, R. Exosomes as bio-inspired nanocarriers for RNA delivery: Preparation and applications. J. Transl. Med. 2022, 20, 125. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.; Zhao, M.; Wang, C.; Li, L.; Yuan, Y.; Li, L.; Liao, G.; Bresette, W.; Zhang, J.; et al. Injectable extracellular vesicle-released self-assembling peptide nanofiber hydrogel as an enhanced cell-free therapy for tissue regeneration. J. Control. Release 2019, 316, 93–104. [Google Scholar] [CrossRef]
- Schindler, C.; Collinson, A.; Matthews, C.; Pointon, A.; Jenkinson, L.; Minter, R.R.; Vaughan, T.J.; Tigue, N.J. Exosomal delivery of doxorubicin enables rapid cell entry and enhanced in vitro potency. PLoS ONE 2019, 14, e0214545. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, Y.; Wang, X.; Wang, S. An integrated hypothesis for miR-126 in vascular disease. Med Res. Arch. 2020, 8, 2133. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776. [Google Scholar] [CrossRef]
- Quiros, Y.; Vicente-Vicente, L.; Morales, A.I.; López-Novoa, J.M.; López-Hernández, F.J. An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicol. Sci. 2011, 119, 245–256. [Google Scholar] [CrossRef]
- Motshwari, D.D.; Matshazi, D.M.; Erasmus, R.T.; Kengne, A.P.; Matsha, T.E.; George, C. MicroRNAs Associated with Chronic Kidney Disease in the General Population and High-Risk Subgroups—A Systematic Review. Int. J. Mol. Sci. 2023, 24, 1792. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, F.; Zarch, S.M.A.; Jahan-Mihan, A.; Kalantar, S.M.; Mehrjardi, M.Y.V.; Fallahzadeh, H.; Hosseinzadeh, M.; Rahmanian, M.; Mozaffari-Khosravi, H. Circulating microRNA-122, microRNA-126-3p and microRNA-146a are associated with inflammation in patients with pre-diabetes and type 2 diabetes mellitus: A case control study. PLoS ONE 2021, 16, e0251697. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.F.; Bekele, S.; O’Dwyer, M.J.; Prowle, J.R. MicroRNAs in Acute Kidney Injury. Nephron 2018, 140, 124–128. [Google Scholar] [CrossRef]
- Aguado-Fraile, E.; Ramos, E.; Conde, E.; Rodríguez, M.; Martín-Gómez, L.; Lietor, A.; Candela, Á.; Ponte, B.; Liaño, F.; García-Bermejo, M.L. A Pilot Study Identifying a Set of microRNAs As Precise Diagnostic Biomarkers of Acute Kidney Injury. PLoS ONE 2015, 10, e0127175. [Google Scholar] [CrossRef]
- Florijn, B.W.; Duijs, J.M.; Levels, J.H.; Dallinga-Thie, G.M.; Wang, Y.; Boing, A.N.; Yuana, Y.; Stam, W.; Limpens, R.W.; Au, Y.W.; et al. Diabetic Nephropathy Alters the Distribution of Circulating Angiogenic MicroRNAs Among Extracellular Vesicles, HDL, and Ago-2. Diabetes 2019, 68, 2287–2300, published correction appears in Diabetes 2020, 69, 1855. [Google Scholar] [CrossRef]
- Reis, M.; Mavin, E.; Nicholson, L.; Green, K.; Dickinson, A.M.; Wang, X.N. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function. Front. Immunol. 2018, 9, 2538. [Google Scholar] [CrossRef]
- Wen, Y.-H.; Lin, H.-Y.; Lin, J.-N.; Tseng, G.-F.; Hwang, C.-F.; Lin, C.-C.; Hsu, C.-J.; Wu, H.-P. 2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-glucoside ameliorates gentamicin-induced ototoxicity by modulating autophagy via Sesn2/AMPK/mTOR signaling. Int. J. Mol. Med. 2022, 49, 71. [Google Scholar] [CrossRef]
- Bijkerk, R.; van Solingen, C.; de Boer, H.C.; van der Pol, P.; Khairoun, M.; de Bruin, R.G.; van Oeveren-Rietdijk, A.M.; Lievers, E.; Schlagwein, N.; van Gijlswijk, D.J.; et al. Hematopoietic microRNA-126 protects against renal ischemia/reperfusion injury by promoting vascular integrity. J. Am. Soc. Nephrol. 2014, 25, 1710–1722. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Wu, Y.; Zuo, L.; Zhang, S.; Zhou, Q.; Wei, W.; Wang, Y.; Zhu, H. MicroRNA-126 attenuates palmitate-induced apoptosis by targeting TRAF7 in HUVECs. Mol. Cell. Biochem. 2015, 399, 123–130. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Gatti, S.; Medica, D.; Figliolini, F.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012, 82, 412–427. [Google Scholar] [CrossRef]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.-F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.R.; Srivastava, D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, Y.; Li, Y.; Zhao, Y.; Jiang, H. Sirt5 Attenuates Cisplatin-Induced Acute Kidney Injury through Regulation of Nrf2/HO-1 and Bcl-2. Biomed Res. Int. 2019, 2019, 4745132. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Peasley, K.D.; Cargill, K.R.; Maringer, K.V.; Bharathi, S.S.; Mukherjee, E.; Zhang, Y.; Holtz, A.; Basisty, N.; Yagobian, S.D.; et al. Sirtuin 5 Regulates Proximal Tubule Fatty Acid Oxidation to Protect against AKI. J. Am. Soc. Nephrol. 2019, 30, 2384–2398. [Google Scholar] [CrossRef]
- Satoh, K.; Wakui, H.; Komatsuda, A.; Nakamoto, Y.; Miura, A.B.; Itoh, H.; Tashima, Y. Induction and altered localization of 90-kDa heat-shock protein in rat kidneys with cisplatin-induced acute renal failure. Ren. Fail. 1994, 16, 313–323. [Google Scholar] [CrossRef]
- Marques, R.G.; Morales, M.M.; Petroianu, A. Brazilian law for scientific use of animals. Acta Cir. Bras. 2009, 24, 69–74. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Convento, M.B.; de Oliveira, A.S.; Boim, M.A.; Borges, F.T. Mesenchymal Stromal Cells Nanovesicles Carry microRNA with Nephroprotective Proprieties Regardless of Aging. Curr. Aging Sci. 2024, 17, 118–126. [Google Scholar] [CrossRef]
- de Oliveira, A.S.; Convento, M.B.; Razvickas, C.V.; Castino, B.; Leme, A.M.; Luiz, R.d.S.; da Silva, W.H.; da Glória, M.A.; Guirão, T.P.; Bondan, E.; et al. The Nephroprotective Effects of the Allogeneic Transplantation with Mesenchymal Stromal Cells Were Potentiated by ω3 Stimulating Up-Regulation of the PPAR-γ. Pharmaceuticals 2023, 16, 1484. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Deng, J.; Luo, K.; Xu, P.; Jiang, Q.; Wang, Y.; Yao, Y.; Chen, X.; Cheng, F.; Xie, D.; Deng, H. High-efficiency c-Myc-mediated induction of functional hepatoblasts from the human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2021, 12, 375. [Google Scholar] [CrossRef] [PubMed]
- Min, D.-J.; Ezponda, T.; Kim, M.K.; Will, C.M.; Martinez-Garcia, E.; Popovic, R.; Basrur, V.; Elenitoba-Johnson, K.S.; Licht, J.D. MMSET stimulates myeloma cell growth through microRNA-mediated modulation of c-MYC. Leukemia 2013, 27, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Goldie, B.J.; Dun, M.D.; Lin, M.; Smith, N.D.; Verrills, N.M.; Dayas, C.V.; Cairns, M.J. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014, 42, 9195–9208. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.A.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef]
- Pessoa, E.; Convento, M.; Silva, R.; Oliveira, A.; Borges, F.; Schor, N. Gentamicin-induced preconditioning of proximal tubular LLC-PK1 cells stimulates nitric oxide production but not the synthesis of heat shock protein. Braz. J. Med. Biol. Res. 2009, 42, 614–620. [Google Scholar] [CrossRef]
- Revitt-Mills, S.A.; Wright, E.K.; Vereker, M.; O’Flaherty, C.; McPherson, F.; Dawson, C.; van Oijen, A.M.; Robinson, A. Defects in DNA double-strand break repair resensitize antibiotic-resistant Escherichia coli to multiple bactericidal antibiotics. Microbiologyopen 2022, 11, e1316. [Google Scholar] [CrossRef]
- Paunesku, T.; Mittal, S.; Protić, M.; Oryhon, J.; Korolev, S.V.; Joachimiak, A.; Woloschak, G.E. Proliferating cell nuclear antigen (PCNA): Ringmaster of the genome. Int. J. Radiat. Biol. 2001, 77, 1007–1021. [Google Scholar] [CrossRef]
- Canaud, G.; Bonventre, J.V. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol. Dial. Transplant. 2015, 30, 575–583. [Google Scholar] [CrossRef]
- De Chiara, L.; Conte, C.; Antonelli, G.; Lazzeri, E. Tubular Cell Cycle Response upon AKI: Revising Old and New Paradigms to Identify Novel Targets for CKD Prevention. Int. J. Mol. Sci. 2021, 22, 11093. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Liu, J.; Pang, P.; Krautzberger, A.M.; Reginensi, A.; Akiyama, H.; Schedl, A.; Humphreys, B.D.; McMahon, A.P. Sox9 Activation Highlights a Cellular Pathway of Renal Repair in the Acutely Injured Mammalian Kidney. Cell Rep. 2015, 12, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, T.; Lalli, M.; Kramann, R.; Kobayashi, A.; Humphreys, B.D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. USA 2014, 111, 1527–1532. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Valerius, M.T.; Kobayashi, A.; Mugford, J.W.; Soeung, S.; Duffield, J.S.; McMahon, A.P.; Bonventre, J.V. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2008, 2, 284–291. [Google Scholar] [CrossRef]
- Chang, S.C.; Gopal, P.; Lim, S.; Wei, X.; Chandramohan, A.; Mangadu, R.; Smith, J.; Ng, S.; Gindy, M.; Phan, U.; et al. Targeted degradation of PCNA outperforms stoichiometric inhibition to result in programed cell death. Cell Chem. Biol. 2022, 29, 1601–1615. [Google Scholar] [CrossRef]
- Williams, A.B.; Schumacher, B. p53 in the DNA-Damage-Repair Process. Cold Spring Harb. Perspect. Med. 2016, 6, a026070. [Google Scholar] [CrossRef]
- Park, J.H.; Zhuang, J.; Li, J.; Hwang, P.M. p53 as guardian of the mitochondrial genome. FEBS Lett. 2016, 590, 924–934. [Google Scholar] [CrossRef]
- Amarh, V.; Arthur, P.K. DNA double-strand break formation and repair as targets for novel antibiotic combination chemotherapy. Future Sci. OA 2019, 5, FSO411. [Google Scholar] [CrossRef]
- Thomasova, D.; Anders, H.J. Cell cycle control in the kidney. Nephrol. Dial. Transplant. 2015, 30, 1622–1630. [Google Scholar] [CrossRef]
- Megyesi, J.; Andrade, L.; Vieira, J.M., Jr.; Safirstein, R.L.; Price, P.M. Positive effect of the induction of p21WAF1/CIP1 on the course of ischemic acute renal failure. Kidney Int. 2001, 60, 2164–2172. [Google Scholar] [CrossRef]
- Nishioka, S.; Nakano, D.; Kitada, K.; Sofue, T.; Ohsaki, H.; Moriwaki, K.; Hara, T.; Ohmori, K.; Kohno, M.; Nishiyama, A. The cyclin-dependent kinase inhibitor p21 is essential for the beneficial effects of renal ischemic preconditioning on renal ischemia/reperfusion injury in mice. Kidney Int. 2014, 85, 871–879. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, R.E.; Arsenault, M.G.; Esparza Gonzalez, B.P.; Patriquen, A.; Hartwig, S. Three Optimized Methods for In Situ Quantification of Progenitor Cell Proliferation in Embryonic Kidneys Using BrdU, EdU, and PCNA. Can. J. Kidney Health Dis. 2019, 6, 2054358119871936. [Google Scholar] [CrossRef]
- Kuo, L.J.; Yang, L.X. Gamma-H2AX—A novel biomarker for DNA double-strand breaks. In Vivo 2008, 22, 305–309. [Google Scholar]
- Sander, V.; Naylor, R.W.; Davidson, A.J. Mind the gap: Renal tubule responses to injury and the role of Cxcl12 and Myc. Ann. Transl. Med. 2019, 7, S30. [Google Scholar] [CrossRef]
- Gandarillas, A.; Watt, F.M. c-Myc promotes differentiation of human epidermal stem cells. Genes Dev. 1997, 11, 2869–2882. [Google Scholar] [CrossRef]
- Sears, R.C. The life cycle of C-myc: From synthesis to degradation. Cell Cycle 2004, 3, 1133–1137. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, C.; Chen, X.; Tao, C.; Cheng, H.; Lu, X. Suppression of tumor cell proliferation and migration by human umbilical cord mesenchymal stem cells: A possible role for apoptosis and Wnt signaling. Oncol. Lett. 2018, 15, 8536–8544. [Google Scholar] [CrossRef]
- Knoepfler, P.S. Why Myc? An unexpected ingredient in the stem cell cocktail. Cell Stem Cell 2008, 2, 18–21. [Google Scholar] [CrossRef]
- Lee, S.A.; Choi, C.; Yoo, T.H. Extracellular vesicles in kidneys and their clinical potential in renal diseases. Kidney Res. Clin. Pract. 2021, 40, 194–207. [Google Scholar] [CrossRef]
- Miyasaki, D.M.; Senegaglia, A.C.; de Moura, S.A.B.; Leitolis, A.; Capriglione, L.G.A.; Fracaro, L.; Boldrini Leite, L.M.; Utumi, P.H.; Fragoso, F.Y.I.; Meyer, F.; et al. Treatment of Chronic Kidney Disease with Extracellular Vesicles from Mesenchymal Stem Cells and CD133+ Expanded Cells: A Comparative Preclinical Analysis. Int. J. Mol. Sci. 2022, 23, 2521. [Google Scholar] [CrossRef]
- Quaglia, M.; Merlotti, G.; Colombatto, A.; Bruno, S.; Stasi, A.; Franzin, R.; Castellano, G.; Grossini, E.; Fanelli, V.; Cantaluppi, V. Stem Cell-Derived Extracellular Vesicles as Potential Therapeutic Approach for Acute Kidney Injury. Front. Immunol. 2022, 13, 849891. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Bussolati, B. Extracellular vesicles in kidney disease. Nat. Rev. Nephrol. 2022, 18, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Yudintceva, N.; Bobkov, D.; Sulatsky, M.; Mikhailova, N.; Oganesyan, E.; Vinogradova, T.; Muraviov, A.; Remezova, A.; Bogdanova, E.; Garapach, I.; et al. Mesenchymal stem cells-derived extracellular vesicles for therapeutics of renal tuberculosis. Sci. Rep. 2024, 14, 4495. [Google Scholar] [CrossRef]
- Shimbo, K.; Miyaki, S.; Ishitobi, H.; Kato, Y.; Kubo, T.; Shimose, S.; Ochi, M. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem. Biophys. Res. Commun. 2014, 445, 381–387. [Google Scholar] [CrossRef]
- Munoz, J.L.; Bliss, S.A.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol. Ther.-Nucleic Acids. 2013, 2, e126. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Biagiotti, S.; Abbas, F.; Montanari, M.; Barattini, C.; Rossi, L.; Magnani, M.; Papa, S.; Canonico, B. Extracellular Vesicles as New Players in Drug Delivery: A Focus on Red Blood Cells-Derived EVs. Pharmaceutics 2023, 15, 365. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, H.; Liu, J.; Chen, J.; Cui, Y.; Wang, S.; Zhang, X.; Yang, Z. Extracellular Vesicles: A New Star for Gene Drug Delivery. Int. J. Nanomed. 2024, 19, 2241–2264. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef]
- Poinsot, V.; Pizzinat, N.; Ong-Meang, V. Engineered and Mimicked Extracellular Nanovesicles for Therapeutic Delivery. Nanomaterials 2024, 14, 639. [Google Scholar] [CrossRef]
- Bi, Y.; Chen, J.; Li, Q.; Li, Y.; Zhang, L.; Zhida, L.; Yuan, F.; Zhang, R. Tumor-derived extracellular vesicle drug delivery system for chemo-photothermal-immune combination cancer treatment. iScience 2024, 27, 108833. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Xavier, J.; Kumar, N.; Ahmad, M.Z.; Ranjan, O.P. Exosomes as natural nanocarrier-based drug delivery system: Recent insights and future perspectives. 3 Biotech 2023, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Draguet, F.; Dubois, N.; Bouland, C.; Pieters, K.; Bron, D.; Meuleman, N.; Stamatopoulos, B.; Lagneaux, L. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stromal Cells as an Efficient Nanocarrier to Deliver siRNA or Drug to Pancreatic Cancer Cells. Cancers 2023, 15, 2901. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Convento, M.B.; de Oliveira, A.S.; Boim, M.A.; Borges, F.T. Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles as Natural Nanocarriers in the Treatment of Nephrotoxic Injury In Vitro. Cells 2024, 13, 1658. https://doi.org/10.3390/cells13191658
Convento MB, de Oliveira AS, Boim MA, Borges FT. Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles as Natural Nanocarriers in the Treatment of Nephrotoxic Injury In Vitro. Cells. 2024; 13(19):1658. https://doi.org/10.3390/cells13191658
Chicago/Turabian StyleConvento, Márcia Bastos, Andreia Silva de Oliveira, Mirian Aparecida Boim, and Fernanda Teixeira Borges. 2024. "Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles as Natural Nanocarriers in the Treatment of Nephrotoxic Injury In Vitro" Cells 13, no. 19: 1658. https://doi.org/10.3390/cells13191658






