RhoBTB3 Functions as a Novel Regulator of Autophagy by Suppressing AMBRA1 Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids and siRNA
2.2. Antibodies and Reagents
2.3. Cell Culture and Transfection
2.4. Immunoprecipitation (IP) and Immunoblotting
2.5. Mass Spectrometric (MS) Analysis
2.6. In Vivo Ubiquitination Assay
2.7. Immunofluorescence Microscopy
2.8. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
2.9. Statistical Analyses
3. Results
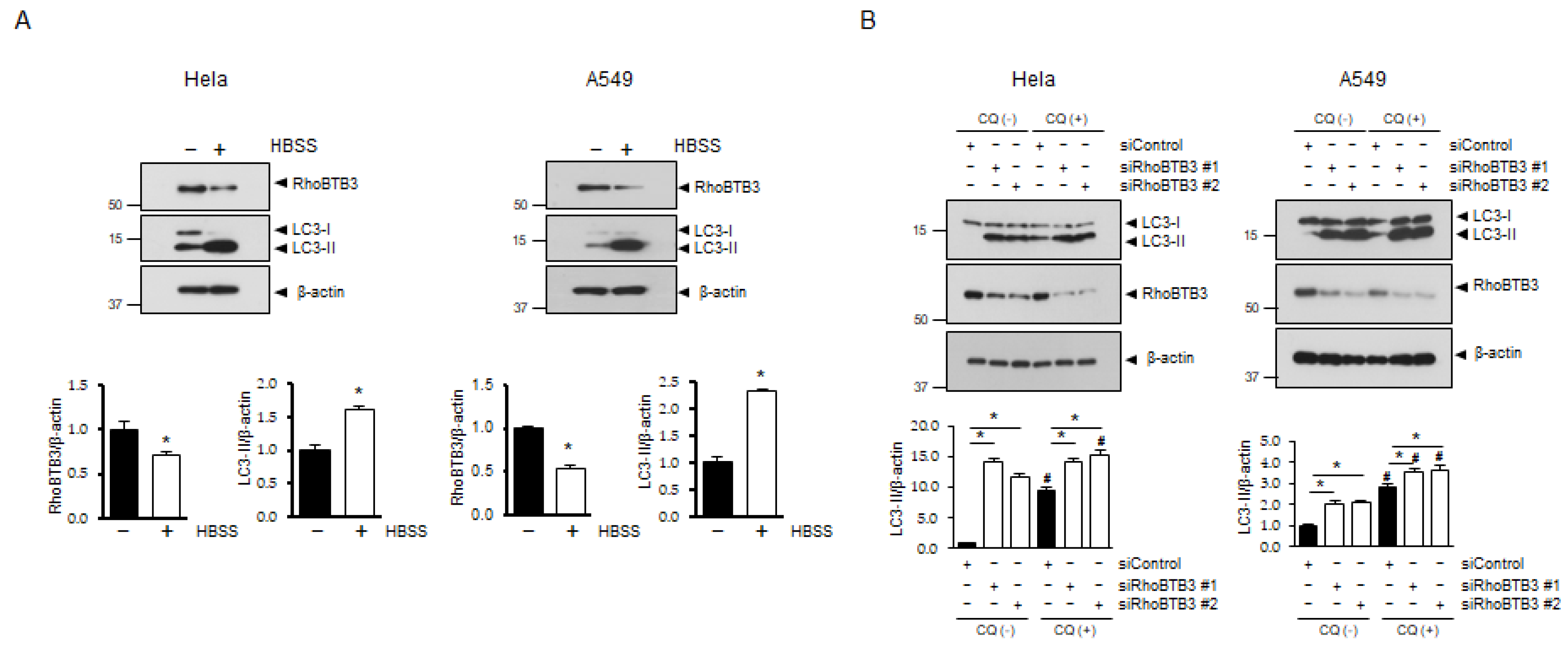
3.1. RhoBTB3 Regulates Autophagy
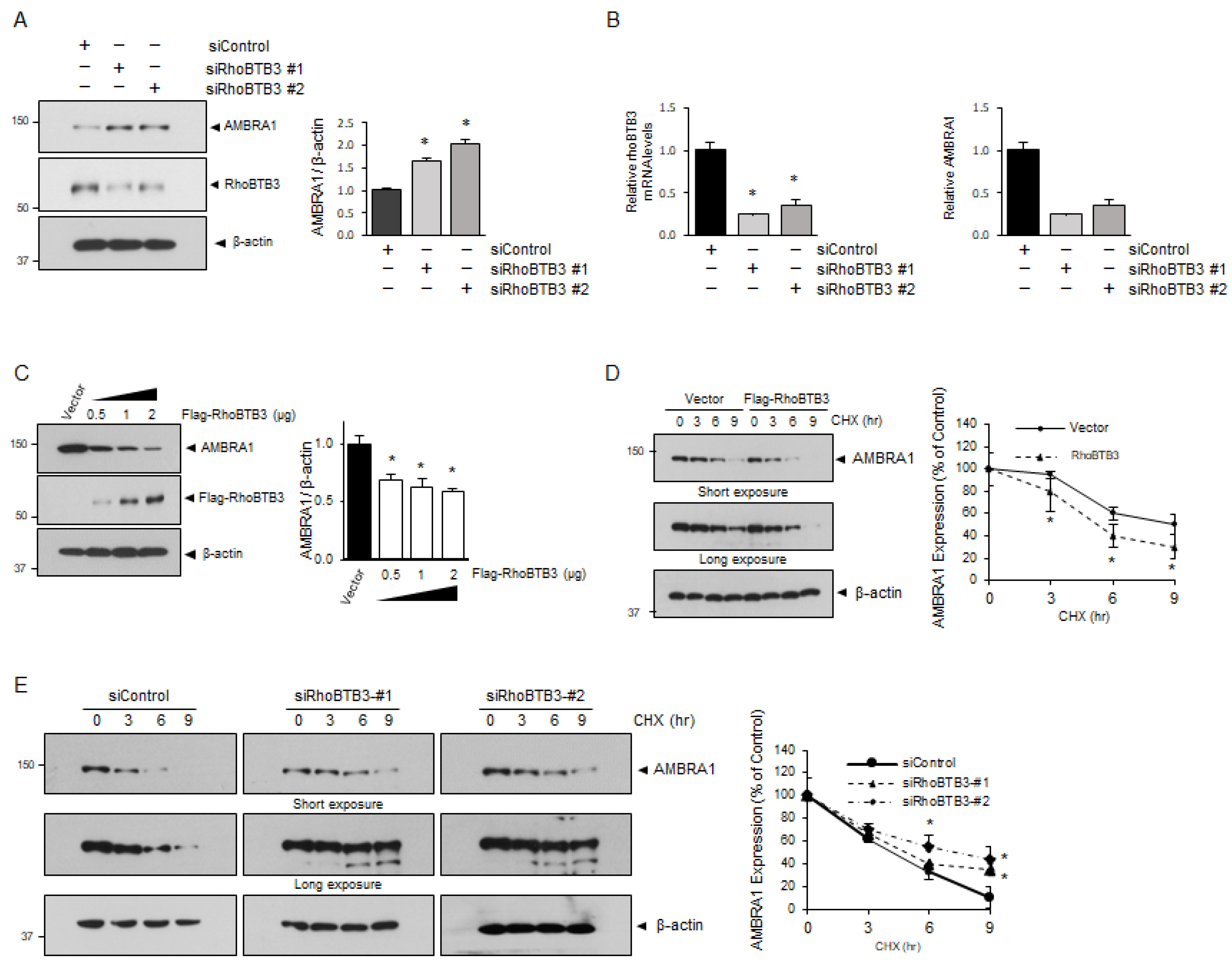
3.2. AMBRA1 Is a Substrate of RhoBTB3
3.3. RhoBTB3 Regulates AMBRA1 Stability
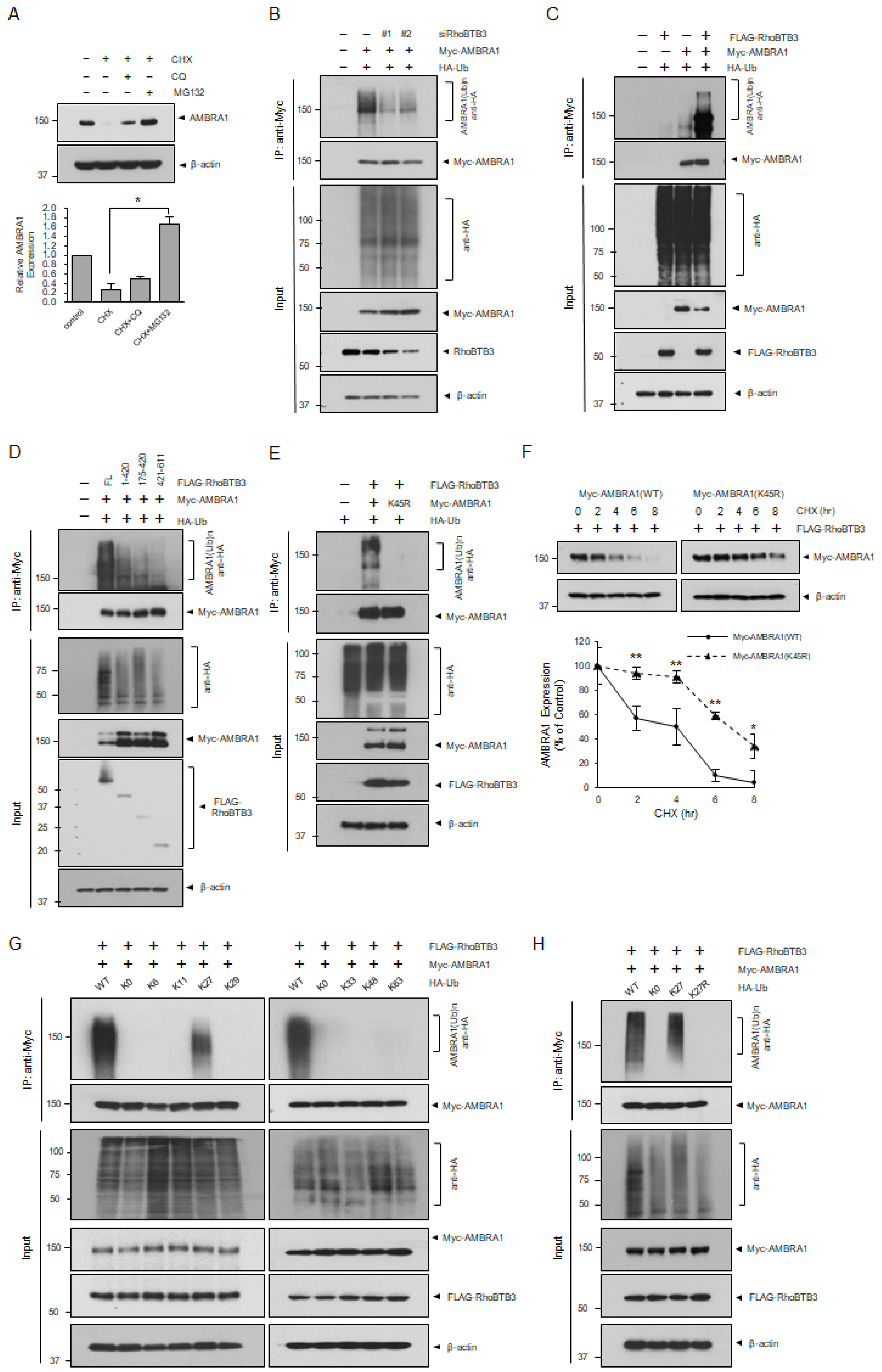
3.4. RhoBTB3 Induces the Ubiquitination and Proteasome-Mediated Degradation of AMBRA1
3.5. RhoBTB3-Induced Autophagy Inhibition Is Dependent on AMBRA1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, Y.; Chen, Y.; Wu, X.; Chen, J.; Zhou, Q.; Liu, B.; Zhang, L.; Yi, C. Interplay of energy metabolism and autophagy. Autophagy 2024, 20, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Kim, J. Autophagy: An essential degradation program for cellular homeostasis and life. Cells 2018, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Ichimiya, T.; Yamakawa, T.; Hirano, T.; Yokoyama, Y.; Hayashi, Y.; Hirayama, D.; Wagatsuma, K.; Itoi, T.; Nakase, H. Autophagy and autophagy-related diseases: A review. Int. J. Mol. Sci. 2020, 21, 8974. [Google Scholar] [CrossRef]
- Grasso, D.; Renna, F.J.; Vaccaro, M.I. Initial Steps in Mammalian Autophagosome Biogenesis. Front. Cell Dev. Biol. 2018, 6, 146. [Google Scholar] [CrossRef]
- Hurley, J.H.; Young, L.N. Mechanisms of Autophagy Initiation. Annu. Rev. Biochem. 2017, 86, 225–244. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Yang, K.; Cao, D. An overview of autophagy: Mechanism, regulation and research progress. Bull. Cancer 2021, 108, 304–322. [Google Scholar] [CrossRef]
- Fujioka, Y.; Suzuki, S.W.; Yamamoto, H.; Kondo-Kakuta, C.; Kimura, Y.; Hirano, H.; Akada, R.; Inagaki, F.; Ohsumi, Y.; Noda, N.N. Structural basis of starvation-induced assembly of the autophagy initiation complex. Nat. Struct. Mol. Biol. 2014, 21, 513–521. [Google Scholar] [CrossRef]
- Banerjee, C.; Puchner, E.M.; Kim, D.-H. ULK1 seen at the single-molecule level during autophagy initiation. Autophagy 2024, 20, 707–708. [Google Scholar] [CrossRef]
- Abrahamsen, H.; Stenmark, H.; Platta, H.W. Ubiquitination and phosphorylation of Beclin 1 and its binding partners: Tuning class III phosphatidylinositol 3-kinase activity and tumor suppression. FEBS Lett. 2012, 586, 1584–1591. [Google Scholar] [CrossRef]
- Hill, S.M.; Wrobel, L.; Ashkenazi, A.; Fernandez-Estevez, M.; Tan, K.; Burli, R.W.; Rubinsztein, D.C. VCP/p97 regulates Beclin-1-dependent autophagy initiation. Nat. Chem. Biol. 2021, 17, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-M.; Seo, M.; Jung, C.H.; Grunwald, D.; Stone, M.; Otto, N.M.; Toso, E.; Ahn, Y.; Kyba, M.; Griffin, T.J. ULK1 phosphorylates Ser30 of BECN1 in association with ATG14 to stimulate autophagy induction. Autophagy 2018, 14, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-M.; Jung, C.H.; Seo, M.; Otto, N.M.; Grunwald, D.; Kim, K.H.; Moriarity, B.; Kim, Y.-M.; Starker, C.; Nho, R.S. The ULK1 complex mediates MTORC1 signaling to the autophagy initiation machinery via binding and phosphorylating ATG14. Autophagy 2016, 12, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Osawa, T.; Fujioka, Y.; Noda, N.N. Structural biology of the core autophagy machinery. Curr. Opin. Struct. Biol. 2017, 43, 10–17. [Google Scholar] [CrossRef]
- Kawamata, T.; Kamada, Y.; Kabeya, Y.; Sekito, T.; Ohsumi, Y. Organization of the pre-autophagosomal structure responsible for autophagosome formation. Mol. Biol. Cell 2008, 19, 2039–2050. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Piacentini, M.; Fimia, G.M. A TRIM32-AMBRA1-ULK1 complex initiates the autophagy response in atrophic muscle cells. Autophagy 2019, 15, 1674–1676. [Google Scholar] [CrossRef]
- Hawkins, K.; Watt, M.; Gillotin, S.; Hanspal, M.; Helley, M.; Richardson, J.; Corbett, N.; Brownlees, J. Disrupting the interaction between AMBRA1 and DLC1 is a promising therapeutic strategy for neurodegeneration that prevents apoptosis while enhancing autophagy and mitophagy. bioRxiv 2023. preprint. [Google Scholar]
- Behura, A.; Dhiman, R. Beclin1-associated complex in autophagy and regulation. In Autophagy Processes and Mechanisms; Academic Pree: Cambridge, MA, USA, 2023; pp. 53–79. [Google Scholar]
- Chaikovsky, A.C.; Li, C.; Jeng, E.E.; Loebell, S.; Lee, M.C.; Murray, C.W.; Cheng, R.; Demeter, J.; Swaney, D.L.; Chen, S.H.; et al. The AMBRA1 E3 ligase adaptor regulates the stability of cyclin D. Nature 2021, 592, 794–798. [Google Scholar] [CrossRef]
- Nazio, F.; Strappazzon, F.; Antonioli, M.; Bielli, P.; Cianfanelli, V.; Bordi, M.; Gretzmeier, C.; Dengjel, J.; Piacentini, M.; Fimia, G.M.; et al. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat. Cell Biol. 2013, 15, 406–416. [Google Scholar] [CrossRef]
- Antonioli, M.; Albiero, F.; Nazio, F.; Vescovo, T.; Perdomo, A.B.; Corazzari, M.; Marsella, C.; Piselli, P.; Gretzmeier, C.; Dengjel, J. AMBRA1 interplay with cullin E3 ubiquitin ligases regulates autophagy dynamics. Dev. Cell 2014, 31, 734–746. [Google Scholar] [CrossRef]
- Aspenstrom, P.; Ruusala, A.; Pacholsky, D. Taking Rho GTPases to the next level: The cellular functions of atypical Rho GTPases. Exp. Cell Res. 2007, 313, 3673–3679. [Google Scholar] [CrossRef] [PubMed]
- Berthold, J.; Schenkova, K.; Rivero, F. Rho GTPases of the RhoBTB subfamily and tumorigenesis. Acta Pharmacol. Sin. 2008, 29, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Rivero, F. Atypical Rho GTPases of the RhoBTB Subfamily: Roles in Vesicle Trafficking and Tumorigenesis. Cells 2016, 5, 28. [Google Scholar] [CrossRef]
- Blondelle, J.; Biju, A.; Lange, S. The Role of Cullin-RING Ligases in Striated Muscle Development, Function, and Disease. Int. J. Mol. Sci. 2020, 21, 7936. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.J.; Calero, M.; Sridevi, K.; Pfeffer, S.R. RhoBTB3: A Rho GTPase-family ATPase required for endosome to Golgi transport. Cell 2009, 137, 938–948. [Google Scholar] [CrossRef]
- Kim, K.; Kim, Y.J. RhoBTB3 Regulates Proliferation and Invasion of Breast Cancer Cells via Col1a1. Mol. Cells 2022, 45, 631–639. [Google Scholar] [CrossRef]
- Zhang, C.S.; Liu, Q.; Li, M.; Lin, S.Y.; Peng, Y.; Wu, D.; Li, T.Y.; Fu, Q.; Jia, W.; Wang, X.; et al. RHOBTB3 promotes proteasomal degradation of HIFalpha through facilitating hydroxylation and suppresses the Warburg effect. Cell Res. 2015, 25, 1025–1042. [Google Scholar] [CrossRef]
- Lu, A.; Pfeffer, S.R. Golgi-associated RhoBTB3 targets cyclin E for ubiquitylation and promotes cell cycle progression. J. Cell Biol. 2013, 203, 233–250. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, B.C.; Yeo, H.Y.; Kim, K.-H.; Yoo, B.C.; Park, J.W.; Chang, H.J. Deleted in malignant brain tumor 1 is a novel prognostic marker in colorectal cancer. Oncol. Rep. 2018, 39, 2279–2287. [Google Scholar] [CrossRef]
- Pintard, L.; Willems, A.; Peter, M. Cullin-based ubiquitin ligases: Cul3-BTB complexes join the family. EMBO J. 2004, 23, 1681–1687. [Google Scholar] [CrossRef]
- Pei, J.; Wang, G.; Feng, L.; Zhang, J.; Jiang, T.; Sun, Q.; Ouyang, L. Targeting lysosomal degradation pathways: New strategies and techniques for drug discovery. J. Med. Chem. 2021, 64, 3493–3507. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Padhy, A.A.; Kumari, V.; Mishra, P. Role of ubiquitin–proteasome and autophagy-lysosome pathways in α-synuclein aggregate clearance. Mol. Neurobiol. 2022, 59, 5379–5407. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Wang, S.; Huang, G.; Du, Y.; Zhu, P.; Li, M.; Fan, Z. RNF2 is recruited by WASH to ubiquitinate AMBRA1 leading to downregulation of autophagy. Cell Res. 2014, 24, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Popelka, H.; Lei, Y.; Yang, Y.; Klionsky, D.J. The Roles of Ubiquitin in Mediating Autophagy. Cells 2020, 9, 2025. [Google Scholar] [CrossRef]
- Kaur, S.; Changotra, H. The beclin 1 interactome: Modification and roles in the pathology of autophagy-related disorders. Biochimie 2020, 175, 34–49. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Teng, F.; Mai, X.; Wang, X.; Su, M.-Y.; Stjepanovic, G. Structure of the DDB1-AMBRA1 E3 ligase receptor complex linked to cell cycle regulation. Nat. Commun. 2023, 14, 7631. [Google Scholar] [CrossRef]
- Wang, Y.; Stjepanovic, G. AMBRA1: Orchestrating Cell Cycle Control and Autophagy for Cellular Homeostasis. J. Cancer Immunol. 2024, 6, 44–50. [Google Scholar] [CrossRef]
- Dunn, W.A., Jr. Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends in cell biology 1994, 4, 139–143. [Google Scholar] [CrossRef]
- Eskelinen, E.-L.; Saftig, P. Autophagy: A lysosomal degradation pathway with a central role in health and disease. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2009, 1793, 664–673. [Google Scholar] [CrossRef]
- Cianfanelli, V.; Nazio, F.; Cecconi, F. Connecting autophagy: AMBRA1 and its network of regulation. Mol. Cell Oncol. 2015, 2, e970059. [Google Scholar] [CrossRef]
- Di Bartolomeo, S.; Corazzari, M.; Nazio, F.; Oliverio, S.; Lisi, G.; Antonioli, M.; Pagliarini, V.; Matteoni, S.; Fuoco, C.; Giunta, L.; et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J. Cell Biol. 2010, 191, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Danglot, L.; Galli, T. Bric-a-brac at the Golgi. Dev. Cell 2009, 16, 775–776. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, V.; Matarrese, P.; Antonioli, M.; Gambardella, L.; Vescovo, T.; Gretzmeier, C.; Longo, A.; Capozzi, A.; Recalchi, S.; Riitano, G. Raft-like lipid microdomains drive autophagy initiation via AMBRA1-ERLIN1 molecular association within MAMs. Autophagy 2021, 17, 2528–2548. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, E.; Tirpák, F.; Hamilton, L.E.; Zigo, M.; Kerns, K.; Sutovsky, M.; Kim, J.; Volkmann, D.; Jovine, L.; Taylor, J.F. A non-synonymous point mutation in a WD-40 domain repeat of EML5 leads to decreased bovine sperm quality and fertility. Front. Cell Dev. Biol. 2022, 10, 872740. [Google Scholar] [CrossRef]
- Cianfanelli, V.; De Zio, D.; Di Bartolomeo, S.; Nazio, F.; Strappazzon, F.; Cecconi, F. Ambra1 at a glance. J. Cell Sci. 2015, 128, 2003–2008. [Google Scholar] [CrossRef]
- Ikeda, F.; Dikic, I. Atypical ubiquitin chains: New molecular signals. EMBO Rep. 2008, 9, 536–542. [Google Scholar] [CrossRef]
- Akutsu, M.; Dikic, I.; Bremm, A. Ubiquitin chain diversity at a glance. J. Cell Sci. 2016, 129, 875–880. [Google Scholar] [CrossRef]
- Tracz, M.; Bialek, W. Beyond K48 and K63: Non-canonical protein ubiquitination. Cell. Mol. Biol. Lett. 2021, 26, 1. [Google Scholar] [CrossRef]
- Pan, M.; Zheng, Q.; Ding, S.; Zhang, L.; Qu, Q.; Wang, T.; Hong, D.; Ren, Y.; Liang, L.; Chen, C.; et al. Chemical Protein Synthesis Enabled Mechanistic Studies on the Molecular Recognition of K27-linked Ubiquitin Chains. Angew. Chem. Int. Ed. Engl. 2019, 58, 2627–2631. [Google Scholar] [CrossRef]
- Castaneda, C.A.; Dixon, E.K.; Walker, O.; Chaturvedi, A.; Nakasone, M.A.; Curtis, J.E.; Reed, M.R.; Krueger, S.; Cropp, T.A.; Fushman, D. Linkage via K27 Bestows Ubiquitin Chains with Unique Properties among Polyubiquitins. Structure 2016, 24, 423–436. [Google Scholar] [CrossRef]
- Van Huizen, M.; Kikkert, M. The Role of Atypical Ubiquitin Chains in the Regulation of the Antiviral Innate Immune Response. Front. Cell Dev. Biol. 2019, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Gao, R.; Jeevan-Raj, B.; Wyss, C.B.; Kalathur, R.K.R.; Piscuoglio, S.; Ng, C.K.Y.; Hindupur, S.K.; Nuciforo, S.; Dazert, E.; et al. LATS1 but not LATS2 represses autophagy by a kinase-independent scaffold function. Nat. Commun. 2019, 10, 5755. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.-W.; Feng, L.; Liu, R.; Lin, H.; Shu, H.-B.; Li, S. The membrane-associated ubiquitin ligases MARCH2 and MARCH3 target IL-5 receptor alpha to negatively regulate eosinophilic airway inflammation. Cell. Mol. Immunol. 2022, 19, 1117–1129. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Kim, D.-G.; Kim, Y.-J. RhoBTB3 Functions as a Novel Regulator of Autophagy by Suppressing AMBRA1 Stability. Cells 2024, 13, 1659. https://doi.org/10.3390/cells13191659
Kim K, Kim D-G, Kim Y-J. RhoBTB3 Functions as a Novel Regulator of Autophagy by Suppressing AMBRA1 Stability. Cells. 2024; 13(19):1659. https://doi.org/10.3390/cells13191659
Chicago/Turabian StyleKim, Kyungho, Dong-Gun Kim, and Youn-Jae Kim. 2024. "RhoBTB3 Functions as a Novel Regulator of Autophagy by Suppressing AMBRA1 Stability" Cells 13, no. 19: 1659. https://doi.org/10.3390/cells13191659
APA StyleKim, K., Kim, D.-G., & Kim, Y.-J. (2024). RhoBTB3 Functions as a Novel Regulator of Autophagy by Suppressing AMBRA1 Stability. Cells, 13(19), 1659. https://doi.org/10.3390/cells13191659





