Abstract
Liver cancer represents a substantial global health challenge, contributing significantly to worldwide morbidity and mortality. It has long been understood that tumors are not composed solely of cancerous cells, but also include a variety of normal cells within their structure. These tumor-associated normal cells encompass vascular endothelial cells, fibroblasts, and various inflammatory cells, including neutrophils, monocytes, macrophages, mast cells, eosinophils, and lymphocytes. Additionally, tumor cells engage in complex interactions with stromal cells and elements of the extracellular matrix (ECM). Initially, the components of what is now known as the tumor microenvironment (TME) were thought to be passive bystanders in the processes of tumor proliferation and local invasion. However, recent research has significantly advanced our understanding of the TME’s active role in tumor growth and metastasis. Tumor progression is now known to be driven by an intricate imbalance of positive and negative regulatory signals, primarily influenced by specific growth factors produced by both inflammatory and neoplastic cells. This review article explores the latest developments and future directions in understanding how the TME modulates liver cancer, with the aim of informing the design of novel therapies that target critical components of the TME.
1. Introduction
Cancer development is a multistep process regulated by various intrinsic and extrinsic factors, such as mutations in tumor suppressor genes, protooncogenes, infections, environmental factors, diet, and lifestyle. Tumor growth is supported by various types of non-cancerous cells in the tumor microenvironment (TME). The TME is critical for tumor growth and progression. The TME is a dynamic system composed of immunological, neoplastic, stromal, and extracellular matrix cells [1]. Figure 1 depicts the main components of the TME. The TME is highly immunosuppressive and supports cancer growth and metastasis [2,3].
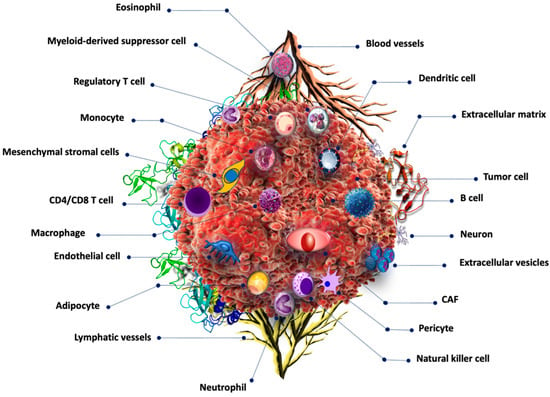
Figure 1.
Tumor microenvironment components. Cancer cells are surrounded by numerous non-cancerous cells including those related to the immune system such as B cells, T-cells, dendritic cells, monocytes, eosinophils, and basophils, among others. Cancer-associated fibroblasts are also common in the TME.
Hypoxia is common in solid tumors, including liver cancer, and it induces hypoxia-inducible factors (HIFs). HIF signaling in innate immune cells and cancer cells activates pro-tumorigenic immune cells and inhibits anti-tumor immune cells, enabling immune evasion [4]. Thus, HIFs are potential therapeutic targets for decreasing immunosuppression and cancer progression. Cancer-associated fibroblasts (CAF) and the extracellular matrix (ECM) protect cancer cells from immune surveillance, as well as from therapeutic antibodies and drugs. CAFs also promote tumor growth by secreting tumor-promoting and immune-suppressing cytokines, chemokines, and growth factors and contribute to resistance to chemotherapy and immunotherapy [5].
Herein, we describe the components of TME and review their unique roles in cancer. Furthermore, the roles of hypoxia, immune cells, and ECM in immune invasion and future directions are also discussed.
2. Tumor Microenvironment
The tumor microenvironment is particularly challenging for immune cell infiltration, activation, and effector function due to its acidic pH, hypoxic conditions, and limited nutrient availability. Tumor cells predominantly rely on aerobic glycolysis for their proliferation; however, there is significant heterogeneity in the glycolytic and oxidative capacities among different tumor cells [6]. Initially, it was thought that glycolysis served as the primary mechanism for ATP production in tumors due to the belief that cancer cells possessed impaired mitochondria. However, recent findings have demonstrated that mitochondria in cancer cells are not merely passive entities but are actively involved in processes beyond conventional mitochondrial respiration [7]. Given the high energy demands of rapidly proliferating tumor cells, they utilize both aerobic glycolysis and oxidative phosphorylation, depleting essential nutrients from the surrounding environment. This results in a tumor microenvironment characterized by low glucose levels, hypoxia, and acidic pH [8].
2.1. Extracellular pH
Cancer’s extensive metabolic reprogramming is complex and may involve metabolic cooperation between cancer cells and the surrounding stroma [9]. One key aspect of this reprogramming is the acidity of the tumor microenvironment (TME), which has been a focal point in research on cancer-related metabolic changes. Acidosis plays a critical role in the development of malignancy and somatic evolution [10]. It influences malignant behavior, metastasis, and invasion rates, and impacts the immunosurveillance mechanism by inducing the polarization of tumor-associated macrophages (TAMs) to the M2 phenotype under hypoxic conditions [11]. In poorly oxygenated regions, such as the tumor core, cells shift their energy production from the efficient, oxygen-dependent oxidative phosphorylation to the less efficient, oxygen-independent anaerobic glycolysis. This shift leads to a buildup of lactic acid, creating a more acidic environment within the tumor [12]. Acidosis is no longer considered a mere byproduct of tumor growth; it is now recognized as a critical regulator of tumor progression, closely linked to extracellular lactic acid accumulation and hypoxia [13]. The hyperglycolytic phenotype of cancer can lead to an accumulation of lactate and protons, which must be expelled into the extracellular environment to maintain intracellular pH and essential cellular functions. As a result, cancer cells exhibit a higher intracellular pH compared to healthy cells, while their extracellular pH is more acidic than that of normal tissues [14]. Extracellular tumor acidosis is associated with aggressive tumor growth and invasion, neoangiogenesis, and metastasis [15]. Elevated levels of carbonic anhydrases, monocarboxylate transporters 1 and 4, and the Na+-H+ exchanger 1 in tumors facilitate the release of protons and lactate into the extracellular environment [16]. The acidic extracellular pH in the TME can serve as a biomarker for oncologic imaging to detect the effects of increased glycolysis and as a therapeutic target to overcome resistance mechanisms to chemotherapy or radiation. Numerous studies have linked tumor acidity to many aspects of cancer growth and progression, including distant metastatic spread and local tumor invasion. Researchers have recently discovered that lowering the pH in the TME might promote cancer cell motility and create changes in cytoskeletal dynamics that affect macrophage and fibroblast polarization and function [13] (Figure 2).
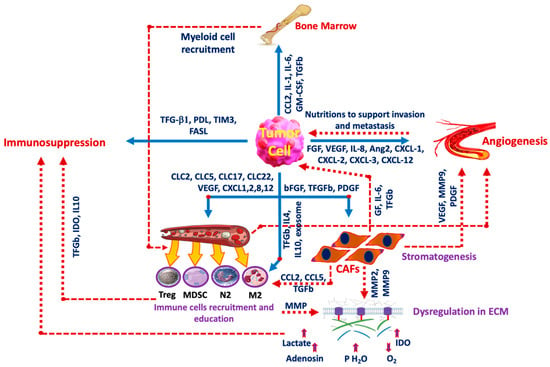
Figure 2.
Tumor cell interaction with the microenvironment. The interplay between cancer cells and The TME is the main factor in cancer progression. Some inducers are released from the tumor cells to affect other components such as the FGF family, VEGF, and IL-8 among others which induce angiogenesis, and other factors including TGF-b1 and FASL induce immunosuppression. Tumor cells also release IL-4 and IL-19 to induce immune cell recruitment and education and release CCL2, IL-1, and IL-6 to activate bone marrow to take part in the production of myeloid cell recruitment. GM-CSF: Granulocyte-macrophage colony-stimulating factor, FGF: Fibroblast growth factor, Ang2: Angiopoietin-2, PDGF: Platelet-derived growth factor, PD-L1: Programmed Cell Death Ligand-1, TIM3: T-cell immunoglobulin domain and mucin domain 3, FASL: Fas ligand, CXCL: chemokines, CAF: cancer-associated fibroblasts, IL: Interleukin, M2: M2 macrophage, N2: Neutrophil, Treg: T-regulatory lymphocyte, MDSC: myeloid-derived suppressor cell, MMP: Matrix metalloproteinase, GF: Growth factor, IDO: Indolamine 2,3 dioxygenase.
One of the key metabolites produced during glycolysis is lactate, which is transported out of the cell by monocarboxylate transporters (MCTs) [17]. Elevated levels of lactate and kynurenine in the TME are known to enhance the immunosuppressive functions of regulatory T-cells, enabling them to dominate over CD8 effector cells [18]. Additionally, studies have shown that the acidification caused by the efflux of lactic acid and protons suppresses the production of inflammatory cytokines in CD8 T-cells and natural killer (NK) cells, thereby inhibiting NFAT activation and reducing cytotoxicity [19]. Inhibitors of MCTs have been employed to block lactate export from tumor cells, thereby reducing acidosis within the TME. AZD3965, a potent MCT1 inhibitor currently in clinical trials (NCT01791595), has been shown to inhibit the growth of various cell lines in culture and to limit glycolysis by causing an accumulation of intracellular lactate [20]. Recent studies have further demonstrated that combining metformin with the inhibition of lactate transporters MCT1 and MCT4 is synthetically lethal for cancer cells in culture [21]. Figure 2 illustrates the interaction between the TME and cancer cells.
LAMP2 (lysosome-associated membrane protein 2) plays a crucial role in helping cancer cells survive in acidic environments [15]. It functions by protecting lysosomal membranes from acidic degradation during cancer progression. The increased acidity in the TME can trigger the induction of the autophagy regulator autophagy-related gene 5 (ATG5) in pre-invasive cancer cells [22]. Additionally, cells that are exposed to low pH conditions for prolonged periods exhibit elevated levels of autophagy markers such as ATG5 and BNIP3, a member of the BCL-2 family [23]. Autophagy plays a dual role in cancer, promoting tumor survival by recycling damaged components under stress, while in other contexts, it can suppress tumor growth by removing harmful mutations and leading to autophagic cell death [24,25]. Despite these observations, the precise mechanisms underlying these changes remain largely unknown. Tumor acidosis is increasingly recognized as a promising therapeutic target for developing new cancer treatments [26]. When designing strategies to target tumor acidosis, it is crucial to consider the metabolic vulnerabilities associated with acidosis, the potential for neutralizing acidity using buffers, and approaches to inhibit hydrogen ion production.
2.2. Hypoxia
Hypoxia is a critical element of the tumor microenvironment (TME), arising from uncontrolled proliferation and poor vascularization, which leaves tumors utilizing partial metabolic respiration in a highly oxygen-deprived environment. Oxygen levels within tumors are often heterogeneous, leading to varied responses to immunotherapy across different tumor regions [27]. Hypoxia can suppress T-cell receptor (TCR) signaling and increase the prevalence of regulatory T-cells [28]. Interestingly, metastases due to a lack of a robust immune response have been found to exhibit increased oxidative phosphorylation pathways. Inhibiting mitochondrial respiration in such cases has been shown to improve survival in both murine tumor implantation models and spontaneous brain metastasis models [29]. While the impact of tumor glycolysis on limiting anti-tumor responses is well-documented [30], the roles of hypoxia and mitochondrial respiration are emerging as promising therapeutic targets.
Hypoxia contributes to tumor progression by promoting endothelial-to-mesenchymal transition (EMT) and facilitating metastasis through the upregulation of growth factors, such as hypoxia-inducible factor (HIF)-1 [31,32]. Moreover, hypoxia fosters the expression of immune checkpoints, activates regulatory T-cells, and polarizes macrophages toward an anti-inflammatory, pro-tumorigenic M2 phenotype. Hypoxia is a pervasive, continuous, and complex condition affecting both malignant and stromal cells. It is frequently associated with poor prognosis and contributes to cancer progression by influencing several aspects of cancer biology, including tumor growth, stemness, dormancy, redox adaptation, intercellular communication, and resistance to therapy [33]. Cancer cells depend heavily on the upregulation of hypoxia-inducible factors (HIFs) and HIF signaling to adapt to low-oxygen environments. Intra-tumoral hypoxia results from the imbalance between cancer cell expansion and oxygen supply, which is further compounded by metabolic shifts within the cancer cells. Additionally, hypoxia drives angiogenesis by increasing VEGF production and activating vascular endothelial cells, all of which significantly influence the TME and the effectiveness of therapies [34,35].
The genes SLC2A1, VEGFA, ENO1, LDHA, TUBB6, ALDOA, TP11, ADM, NDRG1, MIF, P4HA1, MRPS1, CDKN3, PGAM1, and ACOT7 are among the top-ranked hypoxia-associated genes [36,37,38]. In their exploration of hypoxia in cancer, Bhandari et al. [39] analyzed 1188 tumors from 27 categories, encompassing both solid and hematologic malignancies. Their findings reveal that hypoxia displays both intra- and inter-tumor heterogeneity among different cancer types and even among patients with the same type of cancer. For instance, lung and cervical squamous cell carcinomas are among the most hypoxic cancer types, while thyroid adenocarcinoma and chronic lymphocytic leukemia exhibit the lowest hypoxia scores [40]. Moreover, the hypoxic niche is associated with a higher mutational burden of somatic variants and alterations in key oncogenes and tumor suppressors, including TP53, PTEN, and MYC [32,41].
The acidic niche is closely connected with the hypoxic niche and the broader metabolic microenvironment, particularly in relation to lactate metabolism, which plays a key role in its formation through processes such as lactate production and CO2 hydration [42,43,44]. Hypoxia can drive increased lactate production and proton accumulation in hypoxic zones, leading to the acidification of the TME and enhancing the adaptability of tumor cells [42]. This acidic environment not only facilitates tumor invasion and metastasis but also interacts with lactate metabolism to establish a favorable milieu for cancer progression. This interplay, termed lactate-based metabolic symbiosis, was first recognized in this context. Notably, the acidic microenvironment has been shown to enhance oxidative phosphorylation, EMT, and the invasiveness of melanoma cells [45]. Imaging hypoxia within the TME prior to therapy could potentially help in identifying and assessing the tumor’s hypoxic state and monitoring changes induced by treatment. However, hypoxia imaging techniques have yet to be integrated into clinical practice.
2.3. ROS in TME
Reactive oxygen species (ROS) are tightly regulated in healthy cells, acting as secondary messengers in response to various environmental stimuli. However, in tumor cells, aberrant ROS accumulation and signaling cascades contribute to the oncogenic phenotype. ROS not only impacts tumor epithelial cells but also influences the surrounding cells in the tumor microenvironment (TME). Elevated ROS levels trigger inflammation, promote the differentiation of fibroblasts into myofibroblasts, and enhance tumor angiogenesis. Chronic oxidative stress significantly alters the function of these fibroblast subtypes, driving tumor growth and metastatic spread [46]. In the TME, ROS plays a pivotal role in maintaining redox homeostasis and regulating oxidative stress. Due to the TME’s capacity for rapid proliferation and its unique metabolic patterns, ROS are involved in nearly all complex physiological processes, influencing protein alteration, signal transduction, metabolism, and energy production across various tumors [47].
Understanding the dynamic and multicomponent changes in ROS within the TME is crucial for elucidating the specific mechanisms underlying tumor proliferation and metastasis [48]. Additionally, ROS are linked to tumor-induced immunosuppression and serve as critical signaling mediators within the immune system. They are regulated by cytokines, amino acid metabolism, and enzymatic activity [49]. By releasing ROS, immunosuppressive cells accumulate in the TME, leading to T-cell apoptosis and functional suppression. Consequently, controlling ROS levels may be key to prolonging T-cell survival and enhancing antitumor activity [49].
Notably, elevated ROS levels have been associated with carcinogenesis, tumor immunity, and the reprogramming of the TME [50]. Under hypoxic conditions, mitochondrial ROS stabilize hypoxia-inducible factors (HIF), which may promote autophagy and enhance tumorigenicity [51,52]. Both tumor and stromal cells within the TME generate ROS, which can influence cancer cell development. As cancer cells evolve, they become tolerant to ROS accumulation, a condition termed ROS addiction [53].
Furthermore, ROS play a role in the renewal of cancer stem cells (CSCs) and the epithelial–mesenchymal transition (EMT), contributing to drug resistance in various cancers, including liver cancer [54]. The immunosuppression linked to increased ROS production can undermine the ability of immune cells to control tumor growth [55]. Therefore, targeting ROS represents a potential strategy for cancer prevention and treatment. However, the challenge lies in the dual role of ROS; while elevated levels can promote antioxidant capacity and redox balance, they can also drive tumor growth and metastasis. The sources and functions of ROS may vary at different stages of tumor development, making the heterogeneous TME a complex target for therapeutic intervention [56].
2.4. TME Reprogramming
The TME encompasses immune cells that engage in both anti-tumor and tumor-promoting interactions with stromal and non-tumor cells. Enhancing treatment efficacy can be achieved by modifying the tumor’s immune microenvironment, making any tumor a viable target through TME reprogramming [57,58]. Various strategies have been suggested, including photodynamic therapy (PDT) and nitric oxide (NO) therapy, which, when combined, amplify the antitumor immune response [59].
The hypoxic niche pervades nearly the entire tumor and its surrounding environment, triggering a hypoxia-induced cascade that affects not only cancer cells but also other specialized microenvironments within the TME. Notably, the immune system, lactate and ROS metabolism, and the acidic niche are significantly impacted. According to the theories of metabolic symbiosis and the glycolytic switch, oxidative cancer cells favor lactate over glucose, with MCT1 facilitating lactate exchange in tumors [60,61,62,63]. In this process, hypoxic cells utilize glucose to produce lactate, which diffuses according to the concentration gradient, while oxidative cancer cells absorb lactate via MCT1.
Cancer-associated fibroblasts (CAFs) mediate bidirectional communication between mechanical and hypoxic environments [64,65,66]. They play a key role in extracellular matrix (ECM) remodeling and the creation of a hypoxic microenvironment. For instance, in breast cancer, CAFs become elongated and spindle-shaped, secrete more type I collagen, enhance matrix adherence and mesenchymal morphology, and produce ECM with increased stiffness and aligned collagen fibers, thereby facilitating cancer cell invasion and migration [66,67]. CAFs are also crucial in the TME of cancers such as the liver and pancreas, where they support tumor growth and are associated with poorer outcomes [68]. Despite their potential as therapeutic targets, the diversity of CAFs and the lack of specific markers present challenges in developing effective cancer treatments [69].
Understanding and recapturing the hypoxic niche while exploring these mechanisms could enhance fundamental research and aid in translating strategies into clinical settings, ultimately leading to better outcomes with novel therapeutic approaches (reviewed in [70]).
2.5. Extracellular Matrix
The extracellular matrix (ECM) is a non-cellular tissue component that provides crucial structural and biochemical support to cells. It possesses physiological properties similar to those of living cells [71], influencing cell communication, adhesion, and proliferation rather than merely serving as an intercellular filler. The ECM is composed of water, fibrous proteins, proteoglycans, and minerals [72,73,74]. The unique composition of the ECM is attributed to the biochemical and biophysical interactions between cellular components and the microenvironment where tissues develop. ECM components vary depending on the resident cells and the specific needs of the tissue. Recent studies have shown that the ECM plays a significant role in the formation of various fibrous tissues [75].
Cancer cells secrete several growth factors, including platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), VEGF, basic fibroblast growth factor (bFGF), and interleukins, which regulate the TME [76]. The increased expression of these mediators often leads to the production of proteolytic enzymes by tumor cells, exerting autocrine effects and triggering the release of these substances from stromal cells, such as fibroblasts.
Glucose, in addition to being an energy source, contributes to ECM formation, providing structural and biochemical support to tumor cells and other cells within the TME [77,78]. Along with collagen and elastin, proteoglycans, a subclass of glycoproteins, constitute a significant portion of the ECM. These are connected to a core protein by at least one glycosaminoglycan chain and are found in the cytoplasm, on the cell surface, and within the ECM [79]. The connection between tumor growth and ECM is described in detail in the work of Arneth [80].
Several tumors, particularly liver cancer, express high levels of Glypican-3 (GPC-3), a marker associated with poor prognosis. GPC-3, an oncofetal protein overexpressed in lung, breast, colon, liver, and head and neck cancers, is a promising drug target [81]. Its roles in cancer progression include recruiting and polarizing macrophages toward an M2 phenotype, promoting EMT, and stimulating Wnt signaling to advance tumor growth [82,83,84]. Anti-GPC-3 chimeric antigen receptor (CAR) T-cells, combined with soluble IL-15, have shown significant efficacy in preclinical mouse models of hepatocellular carcinoma (HCC), highlighting GPC-3’s therapeutic potential. Due to its high tumor-to-liver ratio, GPC-3 is also ideal for targeted imaging in liver cancer.
The breakdown of ECM and cell surface proteins is critically dependent on matrix metalloproteinases (MMPs), bone morphogenic protein 1 (BMP1), tissue serine proteinases, and adamalysin-related membrane proteinases [85]. A recent study demonstrated that both stromal and tumor cells can modify the ECM to create an environment conducive to tumor cell microinvasion [86]. Modifications to the ECM can release new molecular fragments and expose cryptic protein sites, which significantly impact migratory and angiogenic properties. For example, fibronectin, a component of the ECM, contains cryptic protein domains that are often folded and hidden. Proteolytic enzymes can expose these domains, revealing new integrin-binding sites and anti-angiogenic sequences [87].
Hyaluronan, a major glycosaminoglycan in the ECM, promotes cell proliferation and migration [88,89]. It also increases ECM tension in the TME [90], leading to growth-induced solid stress and significant mechanical load on blood vessels. Lymphatic endothelial cells uniquely express the hyaluronan receptor LYVE-1 [91], which induces lymphangiogenesis and angiogenesis in tumors. The role of LYVE-1 receptors in tumor-associated lymphangiogenesis remains unclear. However, recent studies have shown that low molecular weight hyaluronan can bind to LYVE-1, promoting lymphatic endothelial cell migration, proliferation, and tube formation [92,93]. Dynamic ECM remodeling and the interaction between cells and their substrates are essential components of tumor-mediated lymphangiogenesis.
Research has also shown that the ECM can function as a reservoir for TGF-β [94]. Typically, TGF-β regulates epithelial cell growth, and mutations in TGF-β signaling molecules, such as TGF-βRII and SMAD4, are associated with tumor formation in the gastrointestinal tract [95]. However, in hepatocellular carcinoma, SMAD4, a common SMAD for TGF-β and BMP signaling, has been shown to promote tumor growth via a non-canonical signaling mechanism [96]. TGF-β is crucial for maintaining tissue homeostasis and preventing tumor formation in nearly all human cells. However, genetically unstable cancer cells can evade the tumor-suppressive effects of the TGF-β pathway in the TME. They achieve this by deactivating key components of the TGF-β pathway, such as TGF-β receptors, or by inhibiting other components that suppress tumor growth [97]. Tumors that produce TGF-β induce immune suppression by converting T-cells to regulatory T-cells (Tregs), promoting the expansion of myeloid-derived suppressor cells (MDSCs), and polarizing macrophages to an M2 phenotype. Tumor-secreted TGF-β1 also recruits stromal cells, such as myofibroblasts and osteoclasts, which contribute to tumorigenesis.
The composition and biomechanical properties of the ECM influence integrin signaling, which affects key cancer formation processes, including the Hippo pathway and EMT [97]. Integrins, which cells use to bind to the ECM, are crucial for promoting epithelial differentiation and cell development [98]. Additionally, the loss of integrin subunits, such as β2 and β6, may accelerate tumor growth. Syndecans, which bind ECM proteins such as collagen and laminin, are necessary for integrin function and activity.
3. Inflammation and Immune Evasion
The tumor microenvironment (TME) in liver cancer is composed of hepatic stellate cells, endothelial cells, and neighboring immune cells. The TME creates a hostile setting for immune cell infiltration due to the presence of dense stroma and competition for nutrients, leading to immune suppression and tumor advancement. Cancer cells heavily rely on aerobic glycolysis for energy. Similarly, proinflammatory M1 macrophages and activated T-cells shift their metabolism to an active state, enhancing nutrient absorption, glutaminolysis, and aerobic glycolysis (oxidative phosphorylation) to fuel their effector functions [99]. However, in the TME, cancer cells often outcompete local immune cells for nutrients, thereby gaining a survival advantage [100] (Figure 3).
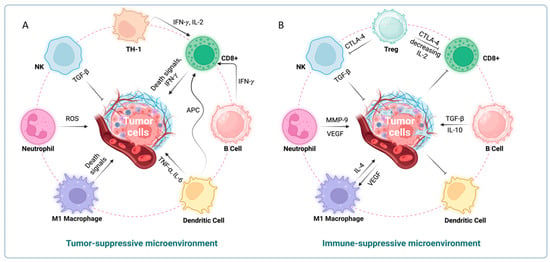
Figure 3.
Immunological mechanisms regulating tumor growth. (A) Tumor suppressive microenvironment. Tumor cell proliferation is inhibited by activated CD4+, CD8+, NK, M1 macrophages, and neutrophils. (B) Immunosuppressive microenvironment. Tumor cells that secrete factors such as TGFβ1, G-CSF, etc. promote MDSC, Treg cells, and M2 macrophages, which inhibit anti-tumor T-cells and NK cells.
Inflammatory stimuli polarize macrophages toward an M1-like phenotype, resulting in the production of pro-inflammatory cytokines [101]. Conversely, anti-inflammatory stimuli induce polarization toward an M2-like phenotype, which has immunosuppressive properties. Prolonged inflammation, such as that seen in chronic viral hepatitis, increases the prevalence of immunosuppressive M2-like macrophages [102,103]. Tumor-derived lactate plays a crucial role in this process by enhancing VEGF expression and promoting M2 polarization of TAMs, which in turn foster angiogenesis and immunosuppression [104]. Moreover, lactate accumulation and the acidic TME reduce interferon-gamma expression in CD8 T-cells and NK cells, further weakening the immune response [105,106]. M2-like TAMs are also associated with poor overall survival in hepatocellular carcinoma (HCC) and can drive EMT and chemoresistance [107]. These findings underscore the importance of targeting the immune microenvironment with novel therapeutic strategies to improve patient outcomes.
4. Cancer-Associated Fibroblasts (CAFs)
There is increasing evidence that fibroblasts play a crucial role in cancer progression [108,109]. Cancer-associated fibroblasts (CAFs) are spindle-shaped mesenchymal cells, sharing characteristics with both fibroblasts and smooth muscle cells [110], though they originate from different sources. Immunohistochemically, CAFs can be distinguished from typical stromal fibroblasts by their higher levels of smooth muscle actin, vimentin, desmin, and fibroblast-activating protein (FAP), using a combination of these markers [111]. Within the tumor microenvironment (TME), CAFs are heterogeneous, exhibiting diverse origins, functions (either pro-tumor or anti-tumor), and surface markers such as alpha-smooth muscle actin (α-SMA), myosin light chain 9 (MYL9), myosin light chain kinase (MYLK), matrix metalloproteinase 2 (MMP2), decorin (DCN), and collagen type I alpha 2 (COL1A2) [112,113].
Cancer-associated fibroblasts (CAFs) play a pivotal role in the tumor microenvironment (TME), contributing significantly to tumor formation and progression through the synthesis of growth factors, cytokines, extracellular matrix (ECM) proteins (including collagen and fibronectin), and matrix metalloproteinases (MMPs) [114]. Notably, CAFs exhibit elevated levels of transforming growth factor-beta 1 (TGF-β1), insulin-like growth factor-binding protein 2, tumor necrosis factor superfamily member 4, and heparin-binding EGF-like growth factor compared to normal fibroblasts [115,116]. CAFs also secrete growth factors such as hepatocyte growth factor (HGF) and ECM glycoproteins such as tenascin-C (TNC). Conversely, tumor cells produce TGF-β1 and platelet-derived growth factor (PDGF), which are critical in mediating interactions between tumors and fibroblasts. TGF-β1 induces the differentiation of fibroblasts and myofibroblasts into CAFs [117,118], while CAFs indirectly promote tumor growth and metastasis by recruiting immune cells, such as myeloid-derived suppressor cells (MDSCs) and regulatory T-cells (Tregs), through cytokines and chemokines, including IL-6, IL-8, and TGF-β1 [119]. Other TME components, including TAMs, MDSCs, and Tregs, further support tumor growth by suppressing anti-tumor CD8+ T-cells and NK cells [120].
CAFs also release stromal-derived factor 1 (SDF1), also known as C-X-C motif chemokine ligand 12 (CXCL12), which aids in recruiting endothelial progenitor cells (EPCs) to tumors. C-X-C chemokine receptor type 4 (CXCR4), expressed on cancer cells, binds to CXCL12 and promotes tumor growth [121]. Additionally, in several cancer types, CAFs express podoplanin, which correlates with the number of CD31+ blood vessels within tumors and VEGF-C expression in tumor cells. Interestingly, increased podoplanin expression in CAFs is associated with peri-tumoral microvessels and LYVE-1 positive lymphatic vessels, though it does not correlate with VEGF-A or VEGF-D expression in tumor cells [122].
CAFs significantly contribute to lymphangiogenesis and metastasis in the TME by activating Th2 T-cells, recruiting immunosuppressive cells, and releasing growth factors. However, the precise mechanisms remain incompletely understood. CAFs are instrumental in cancer development by reducing apoptosis and enhancing the proliferation, motility, and viability of cancer cells in close proximity to healthy cells [123]. They regulate cancer cell metabolism and growth by activating the autophagic pathway in response to oxidative stress. Moreover, CAFs can nourish cancer cells by producing cytokines and nutrients, such as ketones, which promote mitochondrial biogenesis and autophagy in nearby cancer cells [124]. CAF-derived cytokines and chemokines (e.g., CCL5, IL-6, and CXCL10) drive cancer cell metabolism by increasing the phosphorylation of phosphoglucomutase 1 and stimulating glycogen mobilization, NADPH synthesis, and the tricarboxylic acid (TCA) cycle, thereby supporting the growth and metastasis of ovarian cancer cells in vivo [125].
Recent advancements in cancer models, particularly 3D models, have provided insights into how tumor cells selectively control CAF functions. A study using organoid and mouse models of pancreatic ductal adenocarcinoma revealed that tumor-secreted ligands such as TGF-β and interleukin-1a (IL-1a) have opposing roles in producing two distinct CAF subtypes—myofibroblastic and inflammatory [126]. Inflammatory CAFs arise through IL-1a, leukemia inhibitory factor (LIF), Janus kinase (JAK), and signal transducers and activators of transcription (STAT) signaling, while TGF-β signaling blocks this process by enhancing myofibroblast differentiation and reducing IL-1 receptor type I (IL-1R1) expression [126].
The CAF classification system that emerged includes vascular CAFs (vCAFs), matrix CAFs (mCAFs), interferon-response CAFs (ifnCAFs), tumor-like CAFs (tCAFs), inflammatory CAFs (iCAFs), dividing CAFs (dCAFs), reticular-like CAFs (rCAFs), and antigen-presenting CAFs (apCAFs). This system was developed based on marker genes, biological functions, spatial distribution within the TME, and cellular interactions [127].
Innovative platforms, such as the tissue roll for the analysis of the cellular environment and response (TRACER), have enabled the study of tumor-stroma interactions in 3D systems. For example, co-culturing CAFs with FaDu cells (derived from hypopharyngeal squamous carcinoma) on the TRACER platform increased proliferation and invasive migration after 24 and 48 h of culture, although it did not affect radiation resistance [128]. Additionally, an in vitro organotypic microfluidic chip was used to investigate the interactions between tumor cells and CAFs by co-culturing breast cancer cells with patient-derived fibroblasts in 3D tumor and stroma zones. This 3D model revealed that CAFs accelerate breast cancer cell invasion and migration by inducing the expression of the glycoprotein nonmetastatic B (GPNMB) gene [129,130].
Pirfenidone (PFD) has shown anti-fibrotic and anti-inflammatory effects by downregulating TGF-β1, collagenase 1, IL-18, SDF1a, and bFGF. PFD can inhibit tumor cell growth mediated by CAFs, resulting in cell death in a 3D culture model of 4T1 tumor cells and CAFs. In vivo, PFD combined with doxorubicin can also inhibit lung metastasis and tumor growth [131]. Another approach to inhibiting CAFs involves using polyclonal rabbit anti-CAF antibodies obtained by immunizing rabbits with bFGF-activated fibroblasts. These polyclonal antibodies have been shown to slow tumor growth in murine models of CT26 colon cancer [132].
Inhibiting autophagy in CAFs presents another strategy for curbing cancer cell proliferation. Drugs such as metformin and gemcitabine have been reported to induce autophagy in CAFs. Chemotherapeutic agents, such as cyano-4-hydroxycinnamate (CHC) alone or in combination with metformin, have been shown to inhibit autophagic flux in CAFs and reduce tumor cell proliferation in both in vitro and syngeneic pancreatic cancer models, regardless of the chemotherapeutic agents used [133].
5. Interactions between Immune System, TME and Tumor Cells
Clinical data from prognostic studies [134] demonstrate that lymphocytes can be highly effective in combating malignant cells. However, this effectiveness is not universal across all tumors.
TAMs are known to produce proinflammatory molecules, including EGF, MMP9, MT1-MMP, MMP2, IL-1β, and TNFα [135]. Notably, in cancer patients, regulatory T-cells (Tregs) can suppress anti-tumor immune responses. Reducing Treg cells through antibodies or chemotherapy has been shown to enhance T-cell responses in patients undergoing immunotherapy [136]. Additionally, it has been observed that lactic acid in glycolytic tumors upregulates PD-1 on Treg cells, contributing to resistance to anti-PD-1 therapy in patients.
5.1. Dendritic Cells
Dendritic cells (DCs) are the most potent antigen-presenting cells and are classified into several subsets [137]. They play a crucial role in tumor immunity by uptaking tumor antigens and presenting them to T-cells in draining lymph nodes, a critical step in initiating the anti-tumor immune response [137,138]. However, the significance of DCs in the tumor microenvironment (TME), their activation, and their role in tolerance induction is complex and sometimes contradictory. DCs are essential for T-cell-mediated cancer immunity as they regulate adaptive immune responses. Specifically, antitumor responses rely on a subset of conventional DCs that carry tumor antigens to the lymph nodes, activating cytotoxic T-cells. Although mature DCs provide costimulatory signals to T-cells, their ability to produce robust immunity is often compromised by inhibitory processes that affect DC maturation within tumors [139,140,141]. Overcoming these inhibitory pathways or directly activating DCs can enhance T-cell responses [142]. Despite limited clinical success, such as the FDA-approved DC vaccine for prostate cancer, therapeutic targeting of DCs holds promise in combination therapies [143].
DCs are naturally anti-tumorigenic, as they internalize and present antigens to T-cells in secondary lymphoid organs, bridging adaptive and innate immunity and initiating antigen-specific T-cell responses. However, the TME can alter DCs to support tumor progression. Tumor-associated DCs (TADCs) can release several growth factors and other molecules, including TGF-β, granulocyte-macrophage colony-stimulating factor (GM-CSF), CXCL12, and TNFα, which are all proangiogenic factors. Interestingly, VEGFs can also influence DCs by restricting their maturation through the suppression of NF-kB transcription. For example, an anti-VEGF-R3 antibody has been shown to prevent DC migration to draining lymph nodes in the eye [144]. Additionally, DCs can pick up antigens and migrate to draining lymph nodes via a CCL21 gradient, but certain tumor cells can exploit this mechanism by expressing the CCL21 receptor CCR7, enabling them to enter lymphatic capillaries [145].
Tumor cells also secrete TGF-β1, which induces the conversion of naïve T-cells into regulatory T-cells (Tregs). Tregs express CTLA-4, which interacts with CD80 and CD86 on antigen-presenting cells such as DCs, downregulating these co-stimulatory molecules and converting DCs into tolerogenic DCs [146]. These tolerogenic DCs within the TME then inhibit anti-tumor immunity by promoting the differentiation of Tregs, further suppressing the immune response against the tumor.
5.2. Macrophages
Macrophages play a critical role in tumor hemangiogenesis and lymphangiogenesis [147]. TAMs respond to hypoxic conditions within tumor tissue by secreting various factors, including VEGFs, basic fibroblast growth factor (bFGF), thymidine phosphorylase [83], and multiple matrix metalloproteinases (MMPs), such as MMP-2, MMP-7, MMP-9, and MMP-12 [148]. TAMs can also transdifferentiate into lymphatic endothelial cells, expressing pro-lymphangiogenic factors such as VEGF-C, VEGF-D, and VEGF-R3 [149]. Additionally, TAMs have been reported to express the lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) [150]. TAMs frequently exhibit an M2 phenotype, which is associated with immunosuppressive functions. For instance, in uveal melanoma, M2 macrophages, predominantly characterized by CD68+ CD163+ markers, are linked to poor prognosis [149]. Elevated levels of VEGF-C produced by TAMs are associated with increased peri-tumoral lymphatic vessel density in various cancers, including liver cancer and cutaneous squamous cell carcinoma [151]. Moreover, both TAMs and tumor cells secrete VEGF-A, VEGF-C, and MMP-9, which contribute to perineural lymphangiogenesis, further promoting tumor progression [152]. A study by Liu et al. [153] highlighted the potential for functional reprogramming of Kupffer cells (KCs), the largest population of hepatic macrophages, to treat liver cancer. The study utilized the CRISPR-Cas9 gene editing system to target specific genes in KCs, confirming their critical role in controlling tumor progression. The loss of KCs in metastatic tumors was found to correlate with a failure to control tumor growth, underscoring their importance in tumor immunity. TAMs also exert indirect proangiogenic effects by inhibiting the maturation of dendritic cells (DCs), which contributes to immunological tolerance and tumor progression [154]. An increased presence of immature DCs within tumor tissues is associated with enhanced tumor vascularization [155]. This inhibitory and immunosuppressive effect is primarily driven by interleukin 10 (IL-10), prostaglandin E2 (PGE2), and transforming growth factor-beta (TGF-β) secreted by TAMs [156].
5.3. T-Cells
T lymphocytes are central to cellular immunity, acting as primary effector cells that release cytokines during immune responses to mediate inflammation and regulate other immune cells. Understanding cytokine regulation and T-cell function has paved the way for novel treatments across various human disorders, including cancer [157]. Recent advancements in research have enhanced our comprehension of adaptive immune cells’ roles within the tumor microenvironment (TME). Notably, the first therapeutic option aimed at stimulating T-cell functions, ipilimumab, was approved by the US FDA on 28 March 2011. However, tumor-specific CD8+ T-cells, when exposed to persistent antigenic stimulation, often enter a dysfunctional state known as “exhaustion”, leading to diminished effector function. Unfortunately, most patients exhibit limited efficacy to immune checkpoint inhibitors (ICIs) due to this exhaustion [158].
The TME imposes significant barriers on the metabolism and activity of tumor-infiltrating lymphocytes, with T-cells requiring substantial nutrient uptake to mount an effective immune response. Inadequate nutrient acquisition can severely impair effector T-cell differentiation and function [159]. Gamma delta (γδ) T-cells, known for their strong cytotoxic and pro-inflammatory activities, can kill a wide range of tumor cells, and the presence of tumor-infiltrating γδ T-cells is considered a positive prognostic marker in many studies [160]. Thus, they represent a promising target for cancer therapy. Conversely, regulatory T-cells (Tregs) are key immunosuppressive cells that promote tumor growth by inhibiting the effector immune response. Targeting Tregs, either alone or in combination with other immunotherapies, is being explored in clinical settings to enhance anti-tumor effects [161].
Tregs share signaling pathways with other T-cells and play a crucial role in maintaining immunological tolerance within the body. A significant challenge in cancer therapy is the difficulty in selectively blocking Tregs within the TME without disrupting self-tolerance [162]. Despite this, recent studies have focused on manipulating Treg cells as a therapeutic approach for liver cancer [163]. While immunotherapy has the potential to enhance anti-tumor immunity, its efficacy is often limited by the stromal cell barrier that restricts T-cell entry into the tumor [164,165]. Recent research by Mariathasan et al. [166] and Tauriello et al. [167] suggests that pre-existing immunity and tumor mutation burden are correlated with responses to anti-PD-L1 therapy. However, the presence of transforming growth factor-beta (TGF-β), particularly TGF-β1 secreted by tumor and stromal cells within the TME, is responsible for immune exclusion and resistance to anti-PD-L1 antibodies in metastatic cancers.
5.4. B-Cells
Invading immune cells can significantly influence carcinogenesis by modulating cancer formation and anticancer responses. While many aspects of hepatocellular carcinoma (HCC)-related T lymphocytes have been extensively studied, the role of B lymphocytes remains less explored [168]. B cells are traditionally known for generating antibodies against target antigens. However, recent advances in B cell biology have revealed that B cells also secrete a wide range of cytokines and, like T helper cells, can be divided into subsets based on their cytokine profiles. One such subset, regulatory B cells (Bregs), has been discovered to play a critical role in maintaining the balance necessary for immune tolerance [169].
B lymphocytes are commonly found in draining lymph nodes, lymphoid structures surrounding the tumor microenvironment (TME), and at the invasive tumor border [170,171]. These cells play key roles in regulating tumor cell survival and proliferation and in the development of treatment resistance. Additionally, other studies have associated B cells with promoting immune escape mechanisms [172,173]. Due to the various underlying mechanisms, the precise role and impact of B cells in cancer development and tumor suppression remain critical areas of ongoing research [174,175,176].
Controlling B cells within the TME is essential for preventing cancer-induced immunosuppressive processes, such as the TGF-β-dependent conversion of FoxP3+ Treg cells, which support and promote metastasis [168,177,178]. In liver cancer patients, there is notably higher infiltration of TIM-1+ Breg cells in tumor tissue compared to peritumoral tissue. Tumor-derived exosomes have been shown to stimulate B cells, which then exert suppressive effects on CD8+ T-cells [158]. Moreover, B-cell infiltration in HCCs has been associated with longer patient survival and a unique immunoglobulin profile, which correlates with improved patient outcomes. B lymphocytes contribute to local tumor control by secreting higher levels of anticancer immunoglobulins [179].
5.5. Neutrophils
Neutrophils play a crucial role in the early immune response to infections through mechanisms such as phagocytosis and the formation of extracellular traps (NETs). These cells enhance the inflammatory response by producing cytokines and help resolve inflammation by phagocytizing dead cells, presenting antigens, and thus contributing to the termination of the inflammatory process [180]. However, in the context of cancer, neutrophils can adopt pro-tumoral roles by secreting factors such as MMP9, HGF, and VEGF, which promote angiogenesis. In fact, reducing neutrophil numbers has been shown to halt the angiogenic switch, a critical step in tumor progression, which is why inhibiting IL-8 can slow tumor growth [181].
In solid tumors such as liver cancer, neutrophil infiltration is typically linked to poor prognosis. A higher neutrophil-to-lymphocyte ratio in the bloodstream is often indicative of worse outcomes in various cancers [182,183]. The dominance of neutrophils over macrophages in tumors is largely driven by chemokines such as IL-8, produced by tumor cells, inflammation, and necrosis. Despite their transient nature, neutrophils can impact tumor progression in two distinct ways. They can function as anti-tumor neutrophils (N1 neutrophils), particularly in the presence of IL-12 and TNF-α, with CD8+ T-cells playing a crucial role in this activity. The dynamic behavior of neutrophils within the tumor microenvironment highlights their complex and multifaceted roles in cancer progression and immune responses [181,184].
5.6. Eosinophils
Eosinophils, a subset of white blood cells, play a crucial role in the immune system by defending vertebrates against multicellular parasites and certain infections [185]. Within the tumor microenvironment (TME), eosinophils secrete a range of soluble mediators and effector molecules that can have significant immunoregulatory effects. As such, eosinophils are considered potent immune effectors and modulators within the TME [186]. Although eosinophil infiltration in tumors has been recognized for some time, their role in the TME has only recently gained significant attention [187]. Upon infiltrating tumors, eosinophils exert pleiotropic effects through at least two non-exclusive mechanisms: complex crosstalk with lymphocytes and direct interactions with tumor cells [188]. Eosinophils are highly responsive to various stimuli and can rapidly release soluble mediators that not only accelerate tumor growth but also promote angiogenesis and matrix remodeling, further influencing tumor progression [189].
5.7. Mast Cells
Mast cells (MCs) play a pivotal role in the interaction between inflammatory and tumor cells by producing both conventional and unconventional proangiogenic mediators, which are crucial in regulating tumor angiogenesis. The density of MCs in human malignancies is closely correlated with tumor angiogenesis [190]. One of the most potent angiogenic mediators released by MCs is tryptase, a protease that stimulates human vascular endothelium and promotes tumor cell proliferation, invasion, and metastasis in a paracrine manner. Given its significant proangiogenic activity, tryptase has been proposed as a promising therapeutic target in cancer treatment [191]. Beyond their role in angiogenesis, MCs significantly influence both innate and adaptive immune responses. They express Toll-like receptors (TLRs) 1 through 7, 9, and Fc receptors, which are involved in mucosal barrier immune defense. Additionally, MCs attract various immune cells, including neutrophils, eosinophils, CD8+ T-cells, and natural killer lymphocytes (NK LTs), by releasing inflammatory mediators or cytokines from their granules. MCs also play a role in stimulating dendritic cells (DCs), presenting antigens via MHC class I or II molecules, and supporting angiogenesis [192].
Typically, MCs are tissue-resident innate immune cells that regulate inflammation and homeostasis. However, they proliferate within the tumor stroma of various human cancers. The impact of MC density on prognosis varies depending on the tumor type and stage; higher MC density can be associated with either favorable or poor outcomes, as MCs modulate cell proliferation, survival, angiogenesis, invasiveness, and metastasis within the tumor microenvironment (TME) [193,194]. Tumor-associated mast cells influence the TME through interactions with invading cells [195]. Although the significance of MCs in the TME is increasingly recognized, their exact role as modulators within the TME remains unclear and is a subject of ongoing research [196].
5.8. Natural Killer Cells
Natural killer (NK) cells are cytotoxic lymphocytes with the innate ability to recognize and eliminate tumor cells, making them a promising candidate for cancer therapy. The adoptive transfer of autologous or allogeneic NK cells has emerged as a potential therapeutic approach. However, the tumor microenvironment (TME) can impair NK cell function, phenotype, activation, and persistence, leading to their exhaustion [197,198]. To counteract these challenges, activation strategies using cytokines or their analogs have been tested to enhance NK cell persistence, activation, and cytolytic activity [199]. Additionally, the incorporation of chimeric antigen receptors (CARs) has improved the targeting selectivity of NK cells, while checkpoint blockade has shown promise in rejuvenating NK cell function [199].
Significant efforts are being made to fully exploit the anti-tumor potential of NK cells in clinical settings, given their role as potential effector cells in cell-based cancer immunotherapy [200]. Various approaches include large-scale NK cell expansion for adoptive transfer, creating a supportive environment for NK cell activity, redirecting NK cells against tumor cells, and overcoming inhibitory signals that limit NK cell effectiveness [201].
Immune escape is a hallmark of cancer, contributing to tumor progression and metastasis [202]. NK cells, as key effector cells of innate immunity, are highly heterogeneous within their microenvironment, making them crucial targets for therapeutic intervention [203]. Most current immunotherapies focus on enhancing T-cell immunity by modulating inhibitory signals; however, the limited success of T-cell-based therapies underscores the need for alternative approaches, such as utilizing NK cells. Tumors can adapt to resist NK cell-induced cytotoxicity, but cytokine supplementation, blockade of suppressive molecules, and genetic engineering of NK cells are showing promise in both solid and hematological malignancies [204].
Furthermore, the development of CAR NK cells, which redirect NK cells to target tumor cells expressing specific antigens, has significantly advanced cancer treatment options [205]. CAR NK cells hold the potential to be used as universal CAR cells without the need for matching or prior exposure to tumor-associated antigens. Recent clinical trials have demonstrated the feasibility of CAR NK cells as “off-the-shelf” anti-cancer immunotherapies, offering new avenues for treatment [205].
6. Conclusions
Future perspectives on understanding and targeting the TME in liver cancer could revolutionize cancer therapy. As our knowledge deepens, it becomes clear that the TME is not just a passive bystander but an active participant in cancer progression, influencing tumor growth, immune evasion, and metastasis. Future research should focus on unraveling the intricate interactions between tumor cells and their microenvironment, particularly in the context of hypoxia, immune suppression, and metabolic reprogramming. Innovative therapeutic strategies that target both tumor cells and the TME, such as modulating immune responses or disrupting metabolic symbiosis, hold great promise. Additionally, developing precise imaging techniques to monitor TME dynamics and integrating these with personalized treatment approaches could lead to more effective, tailored therapies. By targeting the TME alongside tumor cells, we may significantly improve treatment outcomes and offer new hope for patients with liver cancer.
Author Contributions
H.S. and B.A. together conceptualized the idea and wrote the first draft; S.A.-G., E.F.M. and A.H.G. created the ideas of the figures and drew them; P.S., D.M.S., R.B. and A.E. revised the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
An ethics statement is not applicable because this study is based exclusively on published literature.
Data Availability Statement
All data generated or analyzed during this study are included in this article and will be available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
α-SMA: Alpha-smooth muscle actin; Ang2: Angiopoietin-2; ATG5: Autophagy-related gene 5; bFGF: Basic fibroblast growth factor; BMP1: Bone morphogenic protein 1; CAF: Cancer-associated fibroblasts; CARs: Chimeric antigen receptors; CCL5: Chemokine ligand 5; CHC: Cyano-4-hydroxycinnamate; CSC: Cancer stem cell; CXCL: Chemokines; CXCL10: C-X-C motif chemokine 10; CXCL12: C-X-C motif chemokine ligand 12; CXCR4: CXC-chemokine receptor 4; DCs: Dendritic cells; ECM: Extracellular matrix; EMT: Epithelial-to-mesenchymal transition; FAP: Fibroblast-activating protein; FASL: Fas ligand; FGF: Fibroblast growth factor; GPNMB: Glycoprotein nonmetastatic B; GF: Growth factor; GM-CSF: Granulocyte-macrophage colony-stimulating factor; GPC-3: Glypican-3; HCC: Hepatocellular carcinoma; HGF: Hepatocyte growth factor; HIFs: Hypoxia-inducible factor; HNSCC: Head and neck squamous cell carcinoma; ICC: Intrahepatic cholangiocarcinoma; ICIs: Immune checkpoint inhibitors; IDO: Indolamine 2,3-dioxygenase; KCs: Kupffer cells; LAMP2: Lysosome-associated membrane protein 2; MCs: Mast cells; M2: M2 macrophage; MDSC: Myeloid-derived suppressor cell; MMP: Matrix metalloproteinase; NADPH: Nicotinamide adenine dinucleotide phosphate; N2: Neutrophil; NETs: Extracellular trapping; NK: Natural killer; NKT: Natural killer T-cells; PD-1: Programmed cell death protein 1; PDGF: Platelet-derived growth factor; PD-L1: Programmed cell death ligand-1; PFD: Pirfenidone; PGE2: Prostaglandin E2; PLC: Primary liver cancer; ROS: Reactive oxygen species; TAM: Tumor-associated macrophages; TGF-β: Transforming growth factor-beta; TIM3: T-cell immunoglobulin domain and mucin domain 3; TME: Tumor microenvironment; TNC: Tenascin-C; TNBC: Triple negative breast cancer; Treg: T-regulatory lymphocyte; VEGF: Vascular endothelial growth factor.
References
- Zeng, D.; Wu, J.; Luo, H.; Li, Y.; Xiao, J.; Peng, J.; Ye, Z.; Zhou, R.; Yu, Y.; Wang, G.; et al. Tumor microenvironment evaluation promotes precise checkpoint immunotherapy of advanced gastric cancer. J. Immunother. Cancer 2021, 9, e002467. [Google Scholar] [CrossRef] [PubMed]
- Massalha, H.; Bahar Halpern, K.; Abu-Gazala, S.; Jana, T.; Massasa, E.E.; Moor, A.E.; Buchauer, L.; Rozenberg, M.; Pikarsky, E.; Amit, I.; et al. A single cell atlas of the human liver tumor microenvironment. Mol. Syst. Biol. 2020, 16, e9682. [Google Scholar] [CrossRef] [PubMed]
- Muppala, S. Significance of the Tumor Microenvironment in Liver Cancer Progression. Crit. Rev. Oncog. 2020, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yuen, V.W.; Wong, C.C. Hypoxia-inducible factors and innate immunity in liver cancer. J. Clin. Investig. 2020, 130, 5052–5062. [Google Scholar] [CrossRef] [PubMed]
- Kaps, L.; Schuppan, D. Targeting Cancer Associated Fibroblasts in Liver Fibrosis and Liver Cancer Using Nanocarriers. Cells 2020, 9, 2027. [Google Scholar] [CrossRef]
- Warmoes, M.O.; Locasale, J.W. Heterogeneity of glycolysis in cancers and therapeutic opportunities. Biochem. Pharmacol. 2014, 92, 12–21. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Peiris-Pages, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 113. [Google Scholar] [CrossRef]
- Li, M.; Hao, B.; Zhang, M.; Reiter, R.J.; Lin, S.; Zheng, T.; Chen, X.; Ren, Y.; Yue, L.; Abay, B.; et al. Melatonin enhances radiofrequency-induced NK antitumor immunity, causing cancer metabolism reprogramming and inhibition of multiple pulmonary tumor development. Signal Transduct. Target. Ther. 2021, 6, 330. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- He, Z.; Zhang, S. Tumor-Associated Macrophages and Their Functional Transformation in the Hypoxic Tumor Microenvironment. Front. Immunol. 2021, 12, 741305. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Zhang, C.; Le, A. Glucose Metabolism in Cancer: The Warburg Effect and Beyond. Adv. Exp. Med. Biol. 2021, 1311, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Mayer, A. Hypoxia in tumors: Pathogenesis-related classification, characterization of hypoxia subtypes, and associated biological and clinical implications. Adv. Exp. Med. Biol. 2014, 812, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Commun. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Damaghi, M.; Tafreshi, N.K.; Lloyd, M.C.; Sprung, R.; Estrella, V.; Wojtkowiak, J.W.; Morse, D.L.; Koomen, J.M.; Bui, M.M.; Gatenby, R.A.; et al. Chronic acidosis in the Tumor microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat. Commun. 2015, 6, 8752. [Google Scholar] [CrossRef]
- Zheng, G.; Peng, C.; Jia, X.; Gu, Y.; Zhang, Z.; Deng, Y.; Wang, C.; Li, N.; Yin, J.; Liu, X.; et al. ZEB1 transcriptionally regulated carbonic anhydrase 9 mediates the chemoresistance of tongue cancer via maintaining intracellular pH. Mol. Cancer 2015, 14, 84. [Google Scholar] [CrossRef]
- Rogatzki, M.J.; Ferguson, B.S.; Goodwin, M.L.; Gladden, L.B. Lactate is always the end product of glycolysis. Front. Neurosci. 2015, 9, 22. [Google Scholar] [CrossRef]
- Michalek, R.D.; Gerriets, V.A.; Jacobs, S.R.; Macintyre, A.N.; MacIver, N.J.; Mason, E.F.; Sullivan, S.A.; Nichols, A.G.; Rathmell, J.C. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011, 186, 3299–3303. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Curtis, N.J.; Mooney, L.; Hopcroft, L.; Michopoulos, F.; Whalley, N.; Zhong, H.; Murray, C.; Logie, A.; Revill, M.; Byth, K.F.; et al. Pre-clinical pharmacology of AZD3965, a selective inhibitor of MCT1: DLBCL, NHL and Burkitt’s lymphoma anti-tumor activity. Oncotarget 2017, 8, 69219–69236. [Google Scholar] [CrossRef]
- Benjamin, D.; Robay, D.; Hindupur, S.K.; Pohlmann, J.; Colombi, M.; El-Shemerly, M.Y.; Maira, S.M.; Moroni, C.; Lane, H.A.; Hall, M.N. Dual Inhibition of the Lactate Transporters MCT1 and MCT4 Is Synthetic Lethal with Metformin due to NAD+ Depletion in Cancer Cells. Cell Rep. 2018, 25, 3047–3058 e3044. [Google Scholar] [CrossRef] [PubMed]
- Morell, C.; Bort, A.; Vara-Ciruelos, D.; Ramos-Torres, Á.; Altamirano-Dimas, M.; Díaz-Laviada, I.; Rodríguez-Henche, N. Up-Regulated Expression of LAMP2 and Autophagy Activity during Neuroendocrine Differentiation of Prostate Cancer LNCaP Cells. PLoS ONE 2016, 11, e0162977. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowiak, J.W.; Rothberg, J.M.; Kumar, V.; Schramm, K.J.; Haller, E.; Proemsey, J.B.; Lloyd, M.C.; Sloane, B.F.; Gillies, R.J. Chronic autophagy is a cellular adaptation to tumor acidic pH microenvironments. Cancer Res. 2012, 72, 3938–3947. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Bogdanov, A.; Bogdanov, A.; Chubenko, V.; Volkov, N.; Moiseenko, F.; Moiseyenko, V. Tumor acidity: From hallmark of cancer to target of treatment. Front. Oncol. 2022, 12, 979154. [Google Scholar] [CrossRef]
- Scharping, N.E.; Menk, A.V.; Whetstone, R.D.; Zeng, X.; Delgoffe, G.M. Efficacy of PD-1 Blockade Is Potentiated by Metformin-Induced Reduction of Tumor Hypoxia. Cancer Immunol. Res. 2017, 5, 9–16. [Google Scholar] [CrossRef]
- McNamee, E.N.; Korns Johnson, D.; Homann, D.; Clambey, E.T. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol. Res. 2013, 55, 58–70. [Google Scholar] [CrossRef]
- Fischer, G.M.; Jalali, A.; Kircher, D.A.; Lee, W.C.; McQuade, J.L.; Haydu, L.E.; Joon, A.Y.; Reuben, A.; de Macedo, M.P.; Carapeto, F.C.L.; et al. Molecular Profiling Reveals Unique Immune and Metabolic Features of Melanoma Brain Metastases. Cancer Discov. 2019, 9, 628–645. [Google Scholar] [CrossRef]
- Gupta, S.; Roy, A.; Dwarakanath, B.S. Metabolic Cooperation and Competition in the Tumor Microenvironment: Implications for Therapy. Front. Oncol. 2017, 7, 68. [Google Scholar] [CrossRef]
- Bao, M.H.; Wong, C.C. Hypoxia, Metabolic Reprogramming, and Drug Resistance in Liver Cancer. Cells 2021, 10, 1715. [Google Scholar] [CrossRef] [PubMed]
- Romero, Y.; Aquino-Gálvez, A. Hypoxia in Cancer and Fibrosis: Part of the Problem and Part of the Solution. Int. J. Mol. Sci. 2021, 22, 8335. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.M.; Gupta, K.; Palma, A.M.; Yekelchyk, M.; Fisher, P.B.; Grossman, S.R.; Won, K.J.; Madan, E.; Moreno, E.; Gogna, R. Cell competition in intratumoral and tumor microenvironment interactions. EMBO J. 2021, 40, e107271. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. Tumor refractoriness to anti-VEGF therapy. Oncotarget 2016, 7, 46668–46677. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Li, S.; Meng, Y.; Huang, G.; Liang, S.; Du, J.; Liu, Q.; Cheng, B. UDCA Inhibits Hypoxic Hepatocellular Carcinoma Cell-Induced Angiogenesis Through Suppressing HIF-1α/VEGF/IL-8 Intercellular Signaling. Front. Pharmacol. 2021, 12, 755394. [Google Scholar] [CrossRef]
- Liu, K.; Chen, S.; Lu, R. Identification of important genes related to ferroptosis and hypoxia in acute myocardial infarction based on WGCNA. Bioengineered 2021, 12, 7950–7963. [Google Scholar] [CrossRef]
- Xu, S.; Tang, L.; Liu, Z.; Luo, C.; Cheng, Q. Hypoxia-Related lncRNA Correlates With Prognosis and Immune Microenvironment in Lower-Grade Glioma. Front. Immunol. 2021, 12, 731048. [Google Scholar] [CrossRef]
- Tan, L.; Cheng, D.; Wen, J.; Huang, K.; Zhang, Q. Identification of prognostic hypoxia-related genes signature on the tumor microenvironment in esophageal cancer. Math. Biosci. Eng. 2021, 18, 7743–7758. [Google Scholar] [CrossRef]
- Bhandari, V.; Li, C.H.; Bristow, R.G.; Boutros, P.C. Divergent mutational processes distinguish hypoxic and normoxic Tumors. Nat. Commun. 2020, 11, 737. [Google Scholar] [CrossRef]
- Ma, L.; Hernandez, M.O.; Zhao, Y.; Mehta, M.; Tran, B.; Kelly, M.; Rae, Z.; Hernandez, J.M.; Davis, J.L.; Martin, S.P.; et al. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell 2019, 36, 418–430.e416. [Google Scholar] [CrossRef]
- Bouleftour, W.; Rowinski, E.; Louati, S.; Sotton, S.; Wozny, A.S.; Moreno-Acosta, P.; Mery, B.; Rodriguez-Lafrasse, C.; Magne, N. A Review of the Role of Hypoxia in Radioresistance in Cancer Therapy. Med. Sci. Monit. 2021, 27, e934116. [Google Scholar] [CrossRef] [PubMed]
- Corbet, C.; Feron, O. Tumor acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Padda, J.; Khalid, K.; Kakani, V.; Cooper, A.C.; Jean-Charles, G. Metabolic Acidosis in Leukemia. Cureus 2021, 13, e17732. [Google Scholar] [CrossRef] [PubMed]
- Adoor, D.; Tariq, H.; Rashidi, A. Metabolic Acidosis and Hyponatremia in a Patient With Metastatic Melanoma. Am. J. Kidney Dis. 2021, 78, A16–A18. [Google Scholar] [CrossRef] [PubMed]
- Peppicelli, S.; Toti, A.; Giannoni, E.; Bianchini, F.; Margheri, F.; Del Rosso, M.; Calorini, L. Metformin is also effective on lactic acidosis-exposed melanoma cells switched to oxidative phosphorylation. Cell Cycle 2016, 15, 1908–1918. [Google Scholar] [CrossRef]
- Costa, A.; Scholer-Dahirel, A.; Mechta-Grigoriou, F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin. Cancer Biol. 2014, 25, 23–32. [Google Scholar] [CrossRef]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef]
- Wu, C.; Mao, Y.; Wang, X.; Li, P.; Tang, B. Deep-Tissue Fluorescence Imaging Study of Reactive Oxygen Species in a Tumor Microenvironment. Anal. Chem. 2022, 94, 165–176. [Google Scholar] [CrossRef]
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive Oxygen Species Regulate T Cell Immune Response in the Tumor Microenvironment. Oxid. Med. Cell Longev. 2016, 2016, 1580967. [Google Scholar] [CrossRef]
- Elia, I.; Rossi, M.; Stegen, S.; Broekaert, D.; Doglioni, G.; van Gorsel, M.; Boon, R.; Escalona-Noguero, C.; Torrekens, S.; Verfaillie, C.; et al. Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature 2019, 568, 117–121. [Google Scholar] [CrossRef]
- Fuhrmann, D.C.; Brüne, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Steffen, J.B.M.; Haider, F.; Sokolov, E.P.; Bock, C.; Sokolova, I.M. Mitochondrial capacity and reactive oxygen species production during hypoxia and reoxygenation in the ocean quahog, Arctica islandica. J. Exp. Biol. 2021, 224. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Y.; Jin, M.Z.; Chen, J.F.; Zhou, H.H.; Jin, W.L. Live or let die: Neuroprotective and anti-cancer effects of nutraceutical antioxidants. Pharmacol. Ther. 2018, 183, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Huang, T.; Shen, Y.; Liu, Y.; Zhou, F.; Jin, Y.; Sattar, H.; Wei, Y. Reactive Oxygen Species-Mediated Tumor Microenvironment Transformation: The Mechanism of Radioresistant Gastric Cancer. Oxid. Med. Cell Longev. 2018, 2018, 5801209. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kuang, Z.; Zhang, D.; Gao, Y.; Ying, M.; Wang, T. Reactive oxygen species in immune cells: A new antitumor target. Biomed. Pharmacother. 2021, 133, 110978. [Google Scholar] [CrossRef]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. Reactive oxygen species and cancer: A complex interaction. Cancer Lett. 2019, 452, 132–143. [Google Scholar] [CrossRef]
- Liu, K.; Cui, J.-J.; Zhan, Y.; Ouyang, Q.-Y.; Lu, Q.-S.; Yang, D.-H.; Li, X.-P.; Yin, J.-Y. Reprogramming the tumor microenvironment by genome editing for precision cancer therapy. Mol. Cancer 2022, 21, 98. [Google Scholar] [CrossRef]
- Kim, M.; Lee, N.K.; Wang, C.-P.J.; Lim, J.; Byun, M.J.; Kim, T.-H.; Park, W.; Park, D.-H.; Kim, S.-N.; Park, C.G. Reprogramming the tumor microenvironment with biotechnology. Biomater. Res. 2023, 27, 5. [Google Scholar] [CrossRef]
- Zou, Y.-M.; Li, R.-T.; Yu, L.; Huang, T.; Peng, J.; Meng, W.; Sun, B.; Zhang, W.-H.; Jiang, Z.-H.; Chen, J.; et al. Reprogramming of the tumor microenvironment using a PCN-224@IrNCs/d-Arg nanoplatform for the synergistic PDT, NO, and radiosensitization therapy of breast cancer and improving anti-tumor immunity. Nanoscale 2023, 15, 10715–10729. [Google Scholar] [CrossRef]
- Horikawa, N.; Abiko, K.; Matsumura, N.; Baba, T.; Hamanishi, J.; Yamaguchi, K.; Murakami, R.; Taki, M.; Ukita, M.; Hosoe, Y.; et al. Anti-VEGF therapy resistance in ovarian cancer is caused by GM-CSF-induced myeloid-derived suppressor cell recruitment. Br. J. Cancer 2020, 122, 778–788. [Google Scholar] [CrossRef]
- Tamura, R.; Tanaka, T.; Akasaki, Y.; Murayama, Y.; Yoshida, K.; Sasaki, H. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: Perspectives for therapeutic implications. Med. Oncol. 2019, 37, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Brekken, R.A. Direct and indirect regulation of the tumor immune microenvironment by VEGF. J. Leukoc. Biol. 2022, 111, 1269–1286. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Mukherjee, A.; Saha, D.; Dash, J.; Chatterjee, T.K. Poly-l-Lysine inhibits VEGF and c-Myc mediated tumor-angiogenesis and induces apoptosis in 2D and 3D tumor microenvironment of both MDA-MB-231 and B16F10 induced mice model. Int. J. Biol. Macromol. 2021, 183, 528–548. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G. CAFs Interacting With TAMs in Tumor Microenvironment to Enhance Tumorigenesis and Immune Evasion. Front. Oncol. 2021, 11, 668349. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact. Mater. 2021, 6, 1973–1987. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Li, C.; Teixeira, A.F.; Zhu, H.J.; Ten Dijke, P. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol. Cancer 2021, 20, 154. [Google Scholar] [CrossRef]
- Peng, H.; Zhu, E.; Zhang, Y. Advances of cancer-associated fibroblasts in liver cancer. Biomark. Res. 2022, 10, 59. [Google Scholar] [CrossRef]
- Ying, F.; Chan, M.S.M.; Lee, T.K.W. Cancer-Associated Fibroblasts in Hepatocellular Carcinoma and Cholangiocarcinoma. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 985–999. [Google Scholar] [CrossRef]
- Walsh, J.C.; Lebedev, A.; Aten, E.; Madsen, K.; Marciano, L.; Kolb, H.C. The clinical importance of assessing tumor hypoxia: Relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid. Redox Signal 2014, 21, 1516–1554. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Piperigkou, Z.; Passi, A.; Götte, M.; Rousselle, P.; Vlodavsky, I. Extracellular matrix-based cancer targeting. Trends Mol. Med. 2021, 27, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ha, S.E.; Wu, M.; Zogg, H.; Ronkon, C.F.; Lee, M.Y.; Ro, S. Extracellular Matrix Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 9185. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, J.; Li, H.; Yu, Y.; Wang, X.; Lu, L.; Lv, C.; Chang, B.; Jin, W.; Guo, W.; et al. Extracellular matrix protein-1 secretory isoform promotes ovarian cancer through increasing alternative mRNA splicing and stemness. Nat. Commun. 2021, 12, 4230. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, S.; Li, C.; Li, T.; Huang, Y. Remodeling tumor microenvironment with natural products to overcome drug resistance. Front. Immunol. 2022, 13, 1051998. [Google Scholar] [CrossRef]
- Jang, M.; Oh, S.W.; Lee, Y.; Kim, J.Y.; Ji, E.S.; Kim, P. Targeting extracellular matrix glycation to attenuate fibroblast activation. Acta Biomater. 2022, 141, 255–263. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Li, Y.; Ren, W.; Wang, L. Etomidate ameliorated advanced glycation end-products (AGEs)-induced reduction of extracellular matrix genes expression in chondrocytes. Bioengineered 2021, 12, 4191–4200. [Google Scholar] [CrossRef]
- Kerever, A.; Arikawa-Hirasawa, E. Optimal Extracellular Matrix Niches for Neurogenesis: Identifying Glycosaminoglycan Chain Composition in the Subventricular Neurogenic Zone. Front. Neuroanat. 2021, 15, 764458. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Moek, K.L.; Fehrmann, R.S.N.; van der Vegt, B.; de Vries, E.G.E.; de Groot, D.J.A. Glypican 3 Overexpression across a Broad Spectrum of Tumor Types Discovered with Functional Genomic mRNA Profiling of a Large Cancer Database. Am. J. Pathol. 2018, 188, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Bai, Y.; Liu, T.; Zhang, G.; Han, Y.; Chen, L.; Gao, H.; Wei, W.; Wang, M. Evaluation of Glypican-3 Expression in Hepatocellular Carcinoma by Using IDEAL IQ Magnetic Resonance Imaging. Acad. Radiol. 2021, 28, e227–e234. [Google Scholar] [CrossRef] [PubMed]
- Makkouk, A.; Yang, X.C.; Barca, T.; Lucas, A.; Turkoz, M.; Wong, J.T.S.; Nishimoto, K.P.; Brodey, M.M.; Tabrizizad, M.; Gundurao, S.R.Y.; et al. Off-the-shelf Vδ1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Zhang, H.; Jin, S.; Yan, C.; Li, Z.; Tao, L.; Yu, H. The prognostic value of arginase-1 and glypican-3 expression levels in patients after surgical intrahepatic cholangiocarcinoma resection. World J. Surg. Oncol. 2021, 19, 316. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Lodillinsky, C.; Fuhrmann, L.; Irondelle, M.; Pylypenko, O.; Li, X.Y.; Bonsang-Kitzis, H.; Reyal, F.; Vacher, S.; Calmel, C.; De Wever, O.; et al. Metastasis-suppressor NME1 controls the invasive switch of breast cancer by regulating MT1-MMP surface clearance. Oncogene 2021, 40, 4019–4032. [Google Scholar] [CrossRef]
- Arai, Y.; Choi, B.; Kim, B.J.; Park, S.; Park, H.; Moon, J.J.; Lee, S.H. Cryptic ligand on collagen matrix unveiled by MMP13 accelerates bone tissue regeneration via MMP13/Integrin α3/RUNX2 feedback loop. Acta Biomater. 2021, 125, 219–230. [Google Scholar] [CrossRef]
- Caon, I.; Bartolini, B.; Parnigoni, A.; Caravà, E.; Moretto, P.; Viola, M.; Karousou, E.; Vigetti, D.; Passi, A. Revisiting the hallmarks of cancer: The role of hyaluronan. Semin. Cancer Biol. 2020, 62, 9–19. [Google Scholar] [CrossRef]
- Garantziotis, S.; Savani, R.C. Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biol. 2019, 78–79, 1–10. [Google Scholar] [CrossRef]
- Mills, C.D.; Lenz, L.L.; Harris, R.A. A Breakthrough: Macrophage-Directed Cancer Immunotherapy. Cancer Res. 2016, 76, 513–516. [Google Scholar] [CrossRef]
- Courtwright, A.M.; Lamattina, A.M.; Louis, P.H.; Trindade, A.J.; Burkett, P.; Imani, J.; Shrestha, S.; Divo, M.; Keller, S.; Rosas, I.O.; et al. Hyaluronan and LYVE-1 and allograft function in lung transplantation recipients. Sci. Rep. 2019, 9, 9003. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Cho, W.; Stump, B.; Imani, J.; Lamattina, A.M.; Louis, P.H.; Pazzanese, J.; Rosas, I.O.; Visner, G.; Perrella, M.A.; et al. FK506 induces lung lymphatic endothelial cell senescence and downregulates LYVE-1 expression, with associated decreased hyaluronan uptake. Mol. Med. 2020, 26, 75. [Google Scholar] [CrossRef] [PubMed]
- Stanly, T.A.; Fritzsche, M.; Banerji, S.; Shrestha, D.; Schneider, F.; Eggeling, C.; Jackson, D.G. The cortical actin network regulates avidity-dependent binding of hyaluronan by the lymphatic vessel endothelial receptor LYVE-1. J. Biol. Chem. 2020, 295, 5036–5050. [Google Scholar] [CrossRef] [PubMed]
- Taipale, J.; Saharinen, J.; Keski-Oja, J. Extracellular matrix-associated transforming growth factor-beta: Role in cancer cell growth and invasion. Adv. Cancer Res. 1998, 75, 87–134. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, L.M.; Roberts, A.B. TGF-beta signaling: Positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 2002, 12, 22–29. [Google Scholar] [CrossRef]
- Hernanda, P.Y.; Chen, K.; Das, A.M.; Sideras, K.; Wang, W.; Li, J.; Cao, W.; Bots, S.J.; Kodach, L.L.; de Man, R.A.; et al. SMAD4 exerts a tumor-promoting role in hepatocellular carcinoma. Oncogene 2015, 34, 5055–5068. [Google Scholar] [CrossRef]
- Korneev, K.V.; Atretkhany, K.N.; Drutskaya, M.S.; Grivennikov, S.I.; Kuprash, D.V.; Nedospasov, S.A. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 2017, 89, 127–135. [Google Scholar] [CrossRef]
- Cassim, S.; Pouyssegur, J. Tumor Microenvironment: A Metabolic Player that Shapes the Immune Response. Int. J. Mol. Sci. 2019, 21, 157. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Pearce, E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016, 213, 15–23. [Google Scholar] [CrossRef]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- Colegio, O.R.; Chu, N.Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of Tumor-associated macrophages by Tumor-derived lactic acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, M.; Kfuri-Rubens, R.; Pruessmann, J.N.; Ozga, A.J.; Messemaker, M.; Cadilha, B.L.; Sivakumar, R.; Cianciaruso, C.; Warner, R.D.; Marangoni, F.; et al. CXCR6 positions cytotoxic T cells to receive critical survival signals in the tumor microenvironment. Cell 2021, 184, 4512–4530.e4522. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.; Bröckelmann, P.J.; Iaccarino, I.; Garcia-Marquez, M.; Borchmann, S.; Jochims, F.; Kotrova, M.; Pal, K.; Brüggemann, M.; Hartmann, E.; et al. Tumor and microenvironment response but no cytotoxic T-cell activation in classic Hodgkin lymphoma treated with anti-PD1. Blood 2020, 136, 2851–2863. [Google Scholar] [CrossRef]
- Shirabe, K.; Mano, Y.; Muto, J.; Matono, R.; Motomura, T.; Toshima, T.; Takeishi, K.; Uchiyama, H.; Yoshizumi, T.; Taketomi, A.; et al. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg. Today 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Denton, A.E.; Roberts, E.W.; Fearon, D.T. Stromal Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2018, 1060, 99–114. [Google Scholar] [CrossRef]
- Steenbrugge, J.; De Jaeghere, E.A.; Meyer, E.; Denys, H.; De Wever, O. Splenic Hematopoietic and Stromal Cells in Cancer Progression. Cancer Res. 2021, 81, 27–34. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in Tumor development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc. Natl. Acad. Sci. USA 1990, 87, 7235–7239. [Google Scholar] [CrossRef] [PubMed]
- Nurmik, M.; Ullmann, P.; Rodriguez, F.; Haan, S.; Letellier, E. In search of definitions: Cancer-associated fibroblasts and their markers. Int. J. Cancer 2020, 146, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Bagordakis, E.; Sawazaki-Calone, I.; Macedo, C.C.; Carnielli, C.M.; de Oliveira, C.E.; Rodrigues, P.C.; Rangel, A.L.; Dos Santos, J.N.; Risteli, J.; Graner, E.; et al. Secretome profiling of oral squamous cell carcinoma-associated fibroblasts reveals organization and disassembly of extracellular matrix and collagen metabolic process signatures. Tumor Biol. 2016, 37, 9045–9057. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Li, W.; Chen, R.; Wang, J.; Lu, X.; Li, J. Stromal POSTN induced by TGF-β1 facilitates the migration and invasion of ovarian cancer. Gynecol. Oncol. 2021, 160, 530–538. [Google Scholar] [CrossRef]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.; Iglesias, M.; Céspedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.; et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 2012, 22, 571–584. [Google Scholar] [CrossRef]
- Cho, H.; Seo, Y.; Loke, K.M.; Kim, S.W.; Oh, S.M.; Kim, J.H.; Soh, J.; Kim, H.S.; Lee, H.; Kim, J.; et al. Cancer-Stimulated CAFs Enhance Monocyte Differentiation and Protumoral TAM Activation via IL6 and GM-CSF Secretion. Clin. Cancer Res. 2018, 24, 5407–5421. [Google Scholar] [CrossRef]
- Ringuette Goulet, C.; Bernard, G.; Tremblay, S.; Chabaud, S.; Bolduc, S.; Pouliot, F. Exosomes Induce Fibroblast Differentiation into Cancer-Associated Fibroblasts through TGFβ Signaling. Mol. Cancer Res. 2018, 16, 1196–1204. [Google Scholar] [CrossRef]
- Morgan, A.; Griffin, M.; Kameni, L.; Wan, D.C.; Longaker, M.T.; Norton, J.A. Medical Biology of Cancer-Associated Fibroblasts in Pancreatic Cancer. Biology 2023, 12, 1044. [Google Scholar] [CrossRef]
- Lindau, D.; Gielen, P.; Kroesen, M.; Wesseling, P.; Adema, G.J. The immunosuppressive Tumor network: Myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 2013, 138, 105–115. [Google Scholar] [CrossRef]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Venning, F.A.; Zornhagen, K.W.; Wullkopf, L.; Sjölund, J.; Rodriguez-Cupello, C.; Kjellman, P.; Morsing, M.; Hajkarim, M.C.; Won, K.J.; Erler, J.T.; et al. Deciphering the temporal heterogeneity of cancer-associated fibroblast subpopulations in breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 175. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, X.; Wang, X.; Zhao, Z.; Hu, W.; Zeng, S.; Wei, J.; Yang, X.; Qian, L.; Zhou, S.; et al. The effects and the mechanisms of autophagy on the cancer-associated fibroblasts in cancer. J. Exp. Clin. Cancer Res. 2019, 38, 171. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.; Kenny, H.A.; Ashcroft, B.; Mukherjee, A.; Johnson, A.; Zhang, Y.; Helou, Y.; Batlle, R.; Liu, X.; Gutierrez, N.; et al. Fibroblasts Mobilize Tumor Cell Glycogen to Promote Proliferation and Metastasis. Cell Metab. 2019, 29, 141–155.e149. [Google Scholar] [CrossRef]
- Biffi, G.; Oni, T.E.; Spielman, B.; Hao, Y.; Elyada, E.; Park, Y.; Preall, J.; Tuveson, D.A. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019, 9, 282–301. [Google Scholar] [CrossRef]
- Cords, L.; Tietscher, S.; Anzeneder, T.; Langwieder, C.; Rees, M.; de Souza, N.; Bodenmiller, B. Cancer-associated fibroblast classification in single-cell and spatial proteomics data. Nat. Commun. 2023, 14, 4294. [Google Scholar] [CrossRef]
- Young, M.; Rodenhizer, D.; Dean, T.; D’Arcangelo, E.; Xu, B.; Ailles, L.; McGuigan, A.P. A TRACER 3D Co-Culture Tumor model for head and neck cancer. Biomaterials 2018, 164, 54–69. [Google Scholar] [CrossRef]
- Ferrari, E.; Ugolini, G.S.; Piutti, C.; Marzorati, S.; Rasponi, M. Plasma-enhanced protein patterning in a microfluidic compartmentalized platform for multi-organs-on-chip: A liver-tumor model. Biomed. Mater. 2021, 16, 045032. [Google Scholar] [CrossRef]
- Harrison, S.P.; Baumgarten, S.F.; Verma, R.; Lunov, O.; Dejneka, A.; Sullivan, G.J. Liver Organoids: Recent Developments, Limitations and Potential. Front. Med. 2021, 8, 574047. [Google Scholar] [CrossRef]
- Takai, K.; Le, A.; Weaver, V.M.; Werb, Z. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget 2016, 7, 82889–82901. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, F.; Xu, X.; Hu, S. Polyclonal Rabbit Anti-Cancer-Associated Fibroblasts Globulins Induce Cancer Cells Apoptosis and Inhibit Tumor Growth. Int. J. Biol. Sci. 2018, 14, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Schönrogge, M.; Eichberg, J.; Wendt, E.H.U.; Kumstel, S.; Stenzel, J.; Lindner, T.; Jaster, R.; Krause, B.J.; Vollmar, B.; et al. Blocking Autophagy in Cancer-Associated Fibroblasts Supports Chemotherapy of Pancreatic Cancer Cells. Front. Oncol. 2018, 8, 590. [Google Scholar] [CrossRef] [PubMed]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Brassart-Pasco, S.; Brézillon, S.; Brassart, B.; Ramont, L.; Oudart, J.-B.; Monboisse, J.C. Tumor Microenvironment: Extracellular Matrix Alterations Influence Tumor Progression. Front. Oncol. 2020, 10, 397. [Google Scholar] [CrossRef]
- Kumagai, S.; Koyama, S.; Itahashi, K.; Tanegashima, T.; Lin, Y.T.; Togashi, Y.; Kamada, T.; Irie, T.; Okumura, G.; Kono, H.; et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell 2022, 40, 201–218.e209. [Google Scholar] [CrossRef]
- Gardner, A.; de Mingo Pulido, Á.; Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Cabeza-Cabrerizo, M.; Cardoso, A.; Minutti, C.M.; Pereira da Costa, M.; Reis e Sousa, C. Dendritic Cells Revisited. Annu. Rev. Immunol. 2021, 39, 131–166. [Google Scholar] [CrossRef]
- Del Prete, A.; Salvi, V.; Soriani, A.; Laffranchi, M.; Sozio, F.; Bosisio, D.; Sozzani, S. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell. Mol. Immunol. 2023, 20, 432–447. [Google Scholar] [CrossRef]
- Garris, C.S.; Luke, J.J. Dendritic Cells, the T-cell-inflamed Tumor Microenvironment, and Immunotherapy Treatment Response. Clin. Cancer Res. 2020, 26, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Alfei, F.; Ho, P.-C.; Lo, W.-L. DCision-making in tumors governs T cell anti-tumor immunity. Oncogene 2021, 40, 5253–5261. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Ruffell, B. Dendritic Cells and Cancer Immunity. Trends Immunol. 2016, 37, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, J.; Liu, B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Tiberio, L.; Del Prete, A.; Schioppa, T.; Sozio, F.; Bosisio, D.; Sozzani, S. Chemokine and chemotactic signals in dendritic cell migration. Cell. Mol. Immunol. 2018, 15, 346–352. [Google Scholar] [CrossRef]
- Oderup, C.; Cederbom, L.; Makowska, A.; Cilio, C.M.; Ivars, F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology 2006, 118, 240–249. [Google Scholar] [CrossRef]
- Bieniasz-Krzywiec, P.; Martín-Pérez, R.; Ehling, M.; García-Caballero, M.; Pinioti, S.; Pretto, S.; Kroes, R.; Aldeni, C.; Di Matteo, M.; Prenen, H.; et al. Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell Metab. 2019, 30, 917–936.e910. [Google Scholar] [CrossRef]
- Riabov, V.; Gudima, A.; Wang, N.; Mickley, A.; Orekhov, A.; Kzhyshkowska, J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front. Physiol. 2014, 5, 75. [Google Scholar] [CrossRef]
- Schlereth, S.L.; Refaian, N.; Iden, S.; Cursiefen, C.; Heindl, L.M. Impact of the prolymphangiogenic crosstalk in the tumor microenvironment on lymphatic cancer metastasis. BioMed Res. Int. 2014, 2014, 639058. [Google Scholar] [CrossRef]
- Brezovakova, V.; Jadhav, S. Identification of Lyve-1 positive macrophages as resident cells in meninges of rats. J. Comp. Neurol. 2020, 528, 2021–2032. [Google Scholar] [CrossRef]
- Deng, L.; He, K.; Pan, Y.; Wang, H.; Luo, Y.; Xia, Q. The role of tumor-associated macrophages in primary hepatocellular carcinoma and its related targeting therapy. Int. J. Med. Sci. 2021, 18, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Kim, J.W.; Ylaya, K.; Chung, E.J.; Kitano, H.; Perry, C.; Hanaoka, J.; Fukuoka, J.; Chung, J.Y.; Hewitt, S.M. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J. Transl. Med. 2020, 18, 443. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, X.; Yao, Q.; Chen, C.; Zhang, Q.; Ding, K.; Li, L.; Zeng, Z. In situ expansion and reprogramming of Kupffer cells elicit potent tumoricidal immunity against liver metastasis. J. Clin. Investig. 2023, 133, e157937. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, M.; Zhang, Y.; Ge, S.; Zhong, F.; Xia, G.; Sun, C. Tumor-Associated Macrophages: A Potential Target for Cancer Therapy. Front. Oncol. 2021, 11, 693517. [Google Scholar] [CrossRef] [PubMed]
- Brencicova, E.; Jagger, A.L.; Evans, H.G.; Georgouli, M.; Laios, A.; Attard Montalto, S.; Mehra, G.; Spencer, J.; Ahmed, A.A.; Raju-Kankipati, S.; et al. Interleukin-10 and prostaglandin E2 have complementary but distinct suppressive effects on Toll-like receptor-mediated dendritic cell activation in ovarian carcinoma. PLoS ONE 2017, 12, e0175712. [Google Scholar] [CrossRef] [PubMed]
- Dong, C. Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef]
- Jiang, W.; He, Y.; He, W.; Wu, G.; Zhou, X.; Sheng, Q.; Zhong, W.; Lu, Y.; Ding, Y.; Lu, Q.; et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front. Immunol. 2020, 11, 622509. [Google Scholar] [CrossRef]
- Lim, A.R.; Rathmell, W.K.; Rathmell, J.C. The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy. Elife 2020, 9. [Google Scholar] [CrossRef]
- Imbert, C.; Olive, D. γδ T Cells in Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 91–104. [Google Scholar] [CrossRef]
- Scott, E.N.; Gocher, A.M.; Workman, C.J.; Vignali, D.A.A. Regulatory T Cells: Barriers of Immune Infiltration Into the Tumor Microenvironment. Front. Immunol. 2021, 12, 702726. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tan, M.; Chen, X.; Liu, Y.; Huang, J.; Ou, J.; Deng, X. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. medRxiv 2020. [Google Scholar] [CrossRef]
- Yu, J.; Green, M.D.; Li, S.; Sun, Y.; Journey, S.N.; Choi, J.E.; Rizvi, S.M.; Qin, A.; Waninger, J.J.; Lang, X.; et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 2021, 27, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.K.; Yuen, V.W.; Cheu, J.W.; Wei, L.L.; Ting, V.; Fehlings, M.; Sumatoh, H.; Nardin, A.; Newell, E.W.; Ng, I.O.; et al. Hepatocellular Carcinoma Cells Up-regulate PVRL1, Stabilizing PVR and Inhibiting the Cytotoxic T-Cell Response via TIGIT to Mediate Tumor Resistance to PD1 Inhibitors in Mice. Gastroenterology 2020, 159, 609–623. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Xiang, J.; Long, L.; Green, S.; Yang, Z.; Zimdahl, B.; Lu, J.; Cheng, N.; Horan, L.H.; et al. Targeting Alpha-Fetoprotein (AFP)-MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clin. Cancer Res. 2017, 23, 478–488. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel Iii, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFbeta attenuates Tumor response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Canellas, A.; Hernando-Momblona, X.; et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef]
- Qin, M.; Wang, D.; Fang, Y.; Zheng, Z.; Liu, X.; Wu, F.; Wang, L.; Li, X.; Hui, B.; Ma, S.; et al. Current Perspectives on B Lymphocytes in the Immunobiology of Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 647854. [Google Scholar] [CrossRef]
- Filippone, E.J.; Farber, J.L. The Implications of B-lineage Cells in Kidney Allografts. Transplantation 2020, 104, 2011–2023. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Bader, J.E.; Voss, K.; Rathmell, J.C. Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol. Cell 2020, 78, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Simiczyjew, A.; Dratkiewicz, E.; Mazurkiewicz, J.; Ziętek, M.; Matkowski, R.; Nowak, D. The Influence of Tumor Microenvironment on Immune Escape of Melanoma. Int. J. Mol. Sci. 2020, 21, 8359. [Google Scholar] [CrossRef]
- Sharonov, G.V.; Serebrovskaya, E.O.; Yuzhakova, D.V.; Britanova, O.V.; Chudakov, D.M. B cells, plasma cells and antibody repertoires in the Tumor microenvironment. Nat. Rev. Immunol. 2020, 20, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T. Regulatory and effector B cells: Friends or foes? J. Dermatol. Sci. 2019, 93, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.; Steward, C.R.; Mirlekar, B.; Pylayeva-Gupta, Y. Regulatory B cells in cancer. Immunol. Rev. 2021, 299, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Alhabbab, R.Y.; Nova-Lamperti, E.; Aravena, O.; Burton, H.M.; Lechler, R.I.; Dorling, A.; Lombardi, G. Regulatory B cells: Development, phenotypes, functions, and role in transplantation. Immunol. Rev. 2019, 292, 164–179. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, L.; Goswami, S.; Ma, J.; Zheng, B.; Duan, M.; Liu, L.; Zhang, L.; Shi, J.; Dong, L.; et al. Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma. Oncoimmunology 2019, 8, e1571388. [Google Scholar] [CrossRef]
- Ju, M.; Jiang, L.; Wei, Q.; Yu, L.; Chen, L.; Wang, Y.; Hu, B.; Qian, P.; Zhang, M.; Zhou, C.; et al. A Immune-Related Signature Associated with TME Can Serve as a Potential Biomarker for Survival and Sorafenib Resistance in Liver Cancer. Onco Targets Ther. 2021, 14, 5065–5083. [Google Scholar] [CrossRef]
- Brunner, S.M.; Itzel, T.; Rubner, C.; Kesselring, R.; Griesshammer, E.; Evert, M.; Teufel, A.; Schlitt, H.J.; Fichtner-Feigl, S. Tumor-infiltrating B cells producing antitumor active immunoglobulins in resected HCC prolong patient survival. Oncotarget 2017, 8, 71002–71011. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Qin, F.; Liu, X.; Chen, J.; Huang, S.; Wei, W.; Zou, Y.; Liu, X.; Deng, K.; Mo, S.; Chen, J.; et al. Anti-TGF-β attenuates tumor growth via polarization of tumor associated neutrophils towards an anti-tumor phenotype in colorectal cancer. J. Cancer 2020, 11, 2580–2592. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Shan, J.; Zhou, X.; Liu, H.; Sun, X. Neutrophil-lymphocyte ratio as a prognostic factor for stereotactic body radiotherapy treatment of metastatic liver tumors. Transl. Cancer Res. 2020, 9, 5566–5573. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jung, H.I.; Kwon, S.H.; Bae, S.H.; Kim, H.C.; Baek, M.J.; Lee, M.S. Preoperative neutrophil-lymphocyte ratio and CEA is associated with poor prognosis in patients with synchronous colorectal cancer liver metastasis. Ann. Surg. Treat. Res. 2019, 96, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Chu, Y.; Hu, J.; Ding, X.; Liu, Z.; Fu, D.; Yuan, Y.; Deng, Y.; Wang, G.; Wang, L.; et al. Tumor-associated neutrophils secrete AGR2 to promote colorectal cancer metastasis via its receptor CD98hc-xCT. Gut 2022. [Google Scholar] [CrossRef]
- Uhm, T.G.; Kim, B.S.; Chung, I.Y. Eosinophil Development, Regulation of Eosinophil-Specific Genes, and Role of Eosinophils in the Pathogenesis of Asthma. Allergy Asthma Immunol. Res. 2012, 4, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Reichman, H.; Karo-Atar, D.; Munitz, A. Emerging Roles for Eosinophils in the Tumor Microenvironment. Trends Cancer 2016, 2, 664–675. [Google Scholar] [CrossRef]
- Mattei, F.; Andreone, S.; Marone, G.; Gambardella, A.R.; Loffredo, S.; Varricchi, G.; Schiavoni, G. Eosinophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 1–28. [Google Scholar] [CrossRef]
- Grisaru-Tal, S.; Rothenberg, M.E.; Munitz, A. Eosinophil-lymphocyte interactions in the tumor microenvironment and cancer immunotherapy. Nat. Immunol. 2022, 23, 1309–1316. [Google Scholar] [CrossRef]
- Grisaru-Tal, S.; Itan, M.; Klion, A.D.; Munitz, A. A new dawn for eosinophils in the Tumor microenvironment. Nat. Rev. Cancer 2020, 20, 594–607. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Redegeld, F.A. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin. Rev. Allergy Immunol. 2020, 58, 313–325. [Google Scholar] [CrossRef]
- Ammendola, M.; Leporini, C.; Marech, I.; Gadaleta, C.D.; Scognamillo, G.; Sacco, R.; Sammarco, G.; De Sarro, G.; Russo, E.; Ranieri, G. Targeting mast cells tryptase in tumor microenvironment: A potential antiangiogenetic strategy. Biomed. Res. Int. 2014, 2014, 154702. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. Mast cells, angiogenesis, and Tumor growth. Biochim. Biophys. Acta 2012, 1822, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhao, Y.; Wang, X.; Chen, N.; Mao, F.; Teng, Y.; Wang, T.; Peng, L.; Zhang, J.; Cheng, P.; et al. Increased intratumoral mast cells foster immune suppression and gastric cancer progression through TNF-α-PD-L1 pathway. J. ImmunoTherapy Cancer 2019, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Lichterman, J.N.; Reddy, S.M. Mast Cells: A New Frontier for Cancer Immunotherapy. Cells 2021, 10, 1270. [Google Scholar] [CrossRef] [PubMed]
- Aponte-López, A.; Muñoz-Cruz, S. Mast Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Zhao, J.; Yang, Z.; Li, D.; Katirai, F.; Huang, B. Mast cell: Insight into remodeling a tumor microenvironment. Cancer Metastasis Rev. 2011, 30, 177–184. [Google Scholar] [CrossRef]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef]
- Du, N.; Guo, F.; Wang, Y.; Cui, J. NK Cell Therapy: A Rising Star in Cancer Treatment. Cancers 2021, 13, 4129. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Y.; Shi, C. Targeting Natural Killer Cells for Tumor Immunotherapy. Front. Immunol. 2020, 11, 60. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, Z.G.; Zhang, C. Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacol. Sin. 2018, 39, 167–176. [Google Scholar] [CrossRef]
- Karagiannis, P.; Kim, S.I. iPSC-Derived Natural Killer Cells for Cancer Immunotherapy. Mol. Cells 2021, 44, 541–548. [Google Scholar] [CrossRef]
- Seliger, B.; Koehl, U. Underlying mechanisms of evasion from NK cells as rationale for improvement of NK cell-based immunotherapies. Front. Immunol. 2022, 13, 910595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheng, L.; Liu, L.; Li, X. NK cells are never alone: Crosstalk and communication in Tumor microenvironments. Mol. Cancer 2023, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Fu, T.; Jiang, Y.Z.; Shao, Z.M. Natural killer cells in cancer biology and therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Cui, H.; Caligiuri, M.A.; Yu, J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).