The Endogenous Expression of BMI1 in Adult Human Eyes
Abstract
:1. Introduction
Objectives
2. Materials and Methods
2.1. Study Subjects
Selection Process
2.2. Eye Dissection and Tissue Lysis
2.3. Meso Scale Discovery (MSD) Assay
2.4. Immunohistochemistry
2.5. Immunofluorescence Assay
2.6. Data Analysis
3. Results
3.1. Distribution of BMI1 Expression Based on Sex
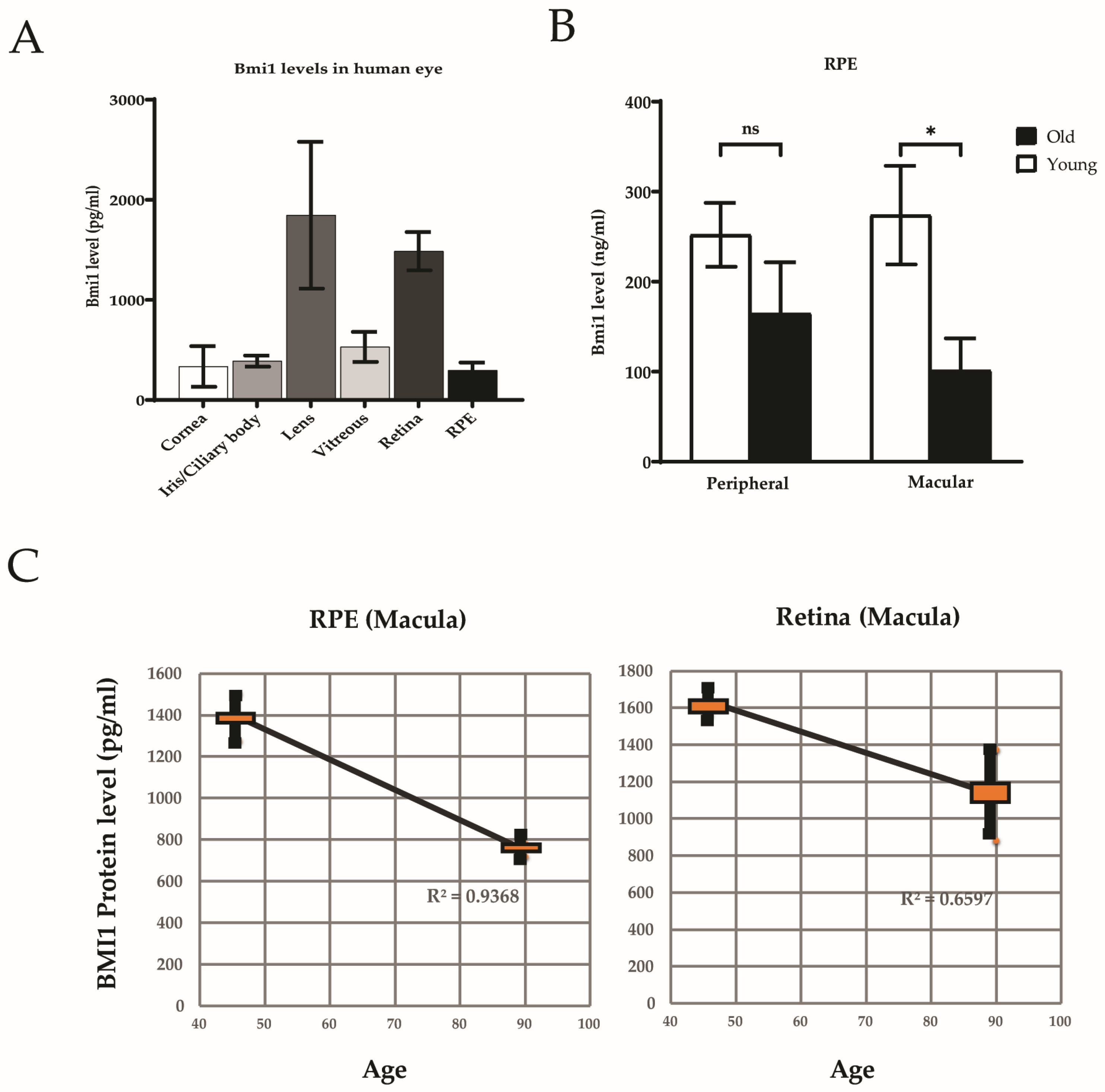
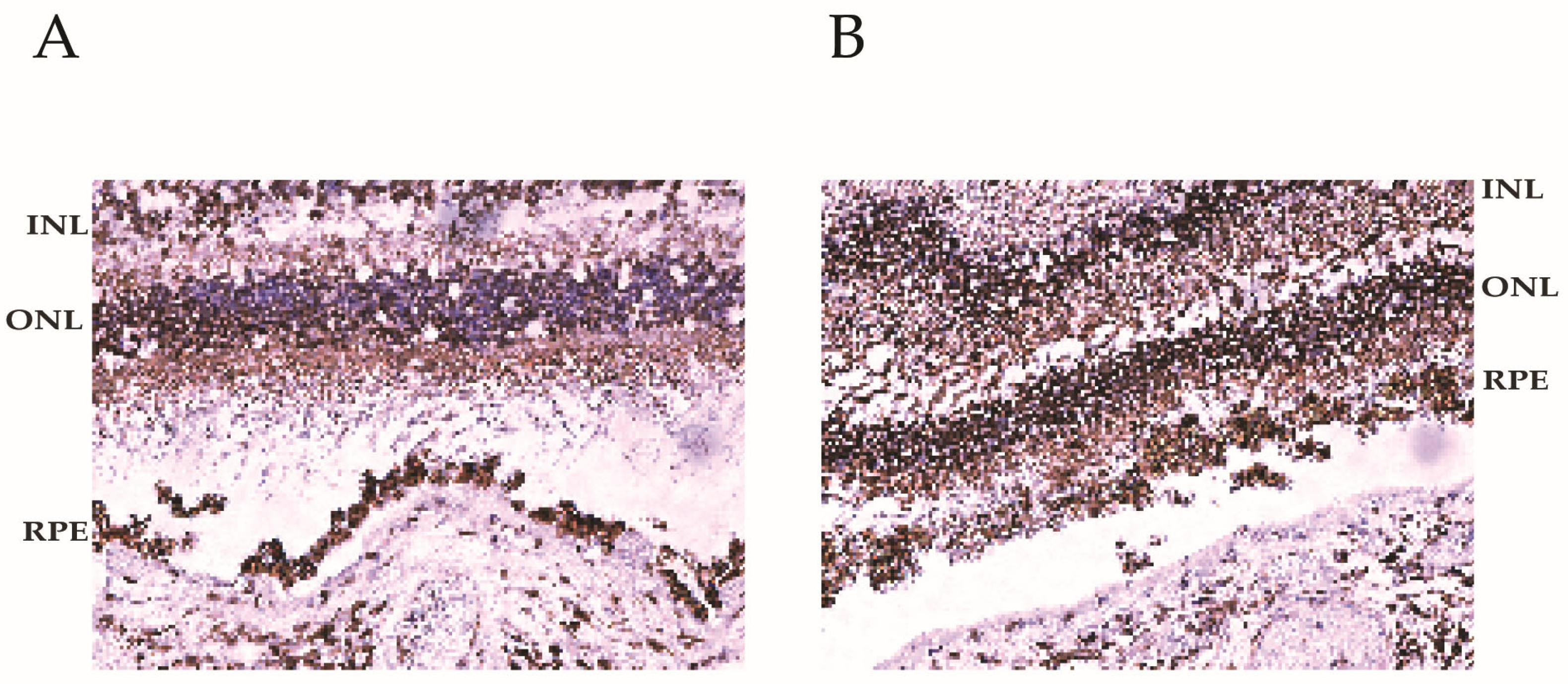
3.2. BMI1 Expression in the Human Eye
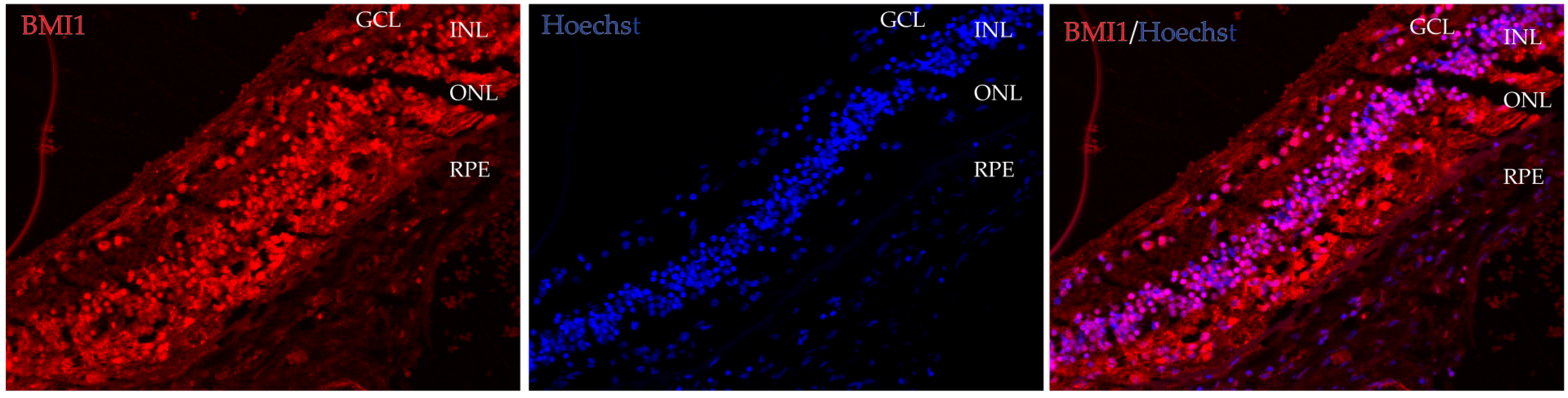
3.3. BMI1 Co-Localization in the Retina
3.4. BMI1 Levels in Young and Aged Retinas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bringmann, A.; Iandiev, I.; Pannicke, T.; Wurm, A.; Hollborn, M.; Wiedemann, P.; Osborne, N.N.; Reichenbach, A. Cellular signaling and factors involved in Muller cell gliosis: Neuroprotective and detrimental effects. Prog. Retin. Eye Res. 2009, 28, 423–451. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J.; Nowicki, M.O.; Bronisz, A.; Williams, S.; Otsuki, A.; Nuovo, G.; Raychaudhury, A.; Newton, H.B.; Chiocca, E.A.; Lawler, S. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008, 68, 9125–9130. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.V.; Pardal, R.; Iwashita, T.; Park, I.K.; Clarke, M.F.; Morrison, S.J. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 2003, 425, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Y.; Nepal, N.; Li, G.; Yang, N.; Chen, H.; Lin, Q.; Ji, X.; Zhang, S.; Jin, S. Dental pulp stem cells overexpressing hepatocyte growth factor facilitate the repair of DSS-induced ulcerative colitis. Stem Cell Res. Ther. 2021, 12, 30. [Google Scholar] [CrossRef]
- Chatoo, W.; Abdouh, M.; David, J.; Champagne, M.P.; Ferreira, J.; Rodier, F.; Bernier, G. The polycomb group gene Bmi1 regulates antioxidant defenses in neurons by repressing p53 pro-oxidant activity. J. Neurosci. 2009, 29, 529–542. [Google Scholar] [CrossRef]
- Chatoo, W.; Abdouh, M.; Duparc, R.H.; Bernier, G. Bmi1 distinguishes immature retinal progenitor/stem cells from the main progenitor cell population and is required for normal retinal development. Stem Cells 2010, 28, 1412–1423. [Google Scholar] [CrossRef]
- Ramkumar, H.; Lu, Z.; Morales, M.G.; Ramkumar, R. The Endogenous Expression of BMI1 in Adult Human Eyes. Investig. Ophthalmol. Vis. Sci. 2024, 65, 207. [Google Scholar]
- Abdouh, M.; Chatoo, W.; El Hajjar, J.; David, J.; Ferreira, J.; Bernier, G. Bmi1 Is Down-Regulated in the Aging Brain and Displays Antioxidant and Protective Activities in Neurons. PLoS ONE 2012, 7, e31870. [Google Scholar] [CrossRef]
- Barabino, A.; Plamondon, V.; Abdouh, M.; Chatoo, W.; Flamier, A.; Hanna, R.; Zhou, S.; Motoyama, N.; Hebert, M.; Lavoie, J.; et al. Loss of Bmi1 causes anomalies in retinal development and degeneration of cone photoreceptors. Development 2016, 143, 1571–1584. [Google Scholar] [CrossRef]
- Zencak, D.; Schouwey, K.; Chen, D.; Ekstrom, P.; Tanger, E.; Bremner, R.; van Lohuizen, M.; Arsenijevic, Y. Retinal degeneration depends on Bmi1 function and reactivation of cell cycle proteins. Proc. Natl. Acad. Sci. USA 2013, 110, E593–E601. [Google Scholar] [CrossRef]
- Flamier, A.; El Hajjar, J.; Adjaye, J.; Fernandes, K.J.; Abdouh, M.; Bernier, G. Modeling Late-Onset Sporadic Alzheimer’s Disease through BMI1 Deficiency. Cell Rep. 2018, 23, 2653–2666. [Google Scholar] [CrossRef]
- Abd El Hafez, A.; El-Hadaad, H.A. Immunohistochemical expression and prognostic relevance of Bmi-1, a stem cell factor, in epithelial ovarian cancer. Ann. Diagn. Pathol. 2014, 18, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.F.; He, W.P.; Cai, M.Y.; He, L.R.; Luo, J.H.; Deng, H.X.; Guan, X.Y.; Zeng, M.S.; Zeng, Y.X.; Xie, D. Intensive expression of Bmi-1 is a new independent predictor of poor outcome in patients with ovarian carcinoma. BMC Cancer 2010, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xue, X.; Wang, Y.; Zhao, H.; Zhang, Y.; Wang, H.; Miao, D. BMI1 Deficiency Results in Female Infertility by Activating p16/p19 Signaling and Increasing Oxidative Stress. Int. J. Biol. Sci. 2019, 15, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Shen, L.; Bai, L.; Gao, J.; Marshall, C.; Wu, T.; Ding, J.; Miao, D.; Xiao, M. Heterozygous knockout of the Bmi-1 gene causes an early onset of phenotypes associated with brain aging. Age 2014, 36, 129–139. [Google Scholar] [CrossRef]
- Masuzzo, A.; Dinet, V.; Cavanagh, C.; Mascarelli, F.; Krantic, S. Amyloidosis in Retinal Neurodegenerative Diseases. Front. Neurol. 2016, 7, 127. [Google Scholar] [CrossRef]
- Banerjee Mustafi, S.; Aznar, N.; Dwivedi, S.K.; Chakraborty, P.K.; Basak, R.; Mukherjee, P.; Ghosh, P.; Bhattacharya, R. Mitochondrial BMI1 maintains bioenergetic homeostasis in cells. FASEB J. 2016, 30, 4042–4055. [Google Scholar] [CrossRef]
- Ganapathi, M.; Boles, N.C.; Charniga, C.; Lotz, S.; Campbell, M.; Temple, S.; Morse, R.H. Effect of Bmi1 over-expression on gene expression in adult and embryonic murine neural stem cells. Sci. Rep. 2018, 8, 7464. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Chaum, E.; Johnson, D.A.; Johnson, L.R. Age-related susceptibility to apoptosis in human retinal pigment epithelial cells is triggered by disruption of p53-Mdm2 association. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8350–8366. [Google Scholar] [CrossRef]
- Ren, R.; Liu, W.; Huang, L.; Liu, D.T.; Choy, K.W.; Shi, J.; Zhao, J.; Zhao, B.; Guan, M.; Shields, C.L.; et al. Role of B lymphoma Mo-MLV insertion region 1 in the oncogenic behavior of retinoblastomas. Mol. Vis. 2013, 19, 561–574. [Google Scholar]
- Zhang, Q.; Li, J.; Li, Y.; Che, H.; Chen, Y.; Dong, J.; Xian, C.J.; Miao, D.; Wang, L.; Ren, Y. Bmi1 deficiency causes oxidative stress and intervertebral disc degeneration which can be alleviated by antioxidant treatment. J. Cell. Mol. Med. 2020, 24, 8950–8961. [Google Scholar] [CrossRef]
- Justilien, V.; Pang, J.J.; Renganathan, K.; Zhan, X.; Crabb, J.W.; Kim, S.R.; Sparrow, J.R.; Hauswirth, W.W.; Lewin, A.S. SOD2 knockdown mouse model of early AMD. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4407–4420. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-Related Macular Degeneration: Role of Oxidative Stress and Blood Vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattarai, S.; Ara, H.; Sun, G.; St Clair, D.K.; Bhuiyan, M.S.; Kevil, C.; Watts, M.N.; Dominic, P.; Shimizu, T.; et al. SOD2 deficiency in cardiomyocytes defines defective mitochondrial bioenergetics as a cause of lethal dilated cardiomyopathy. Redox Biol. 2020, 37, 101740. [Google Scholar] [CrossRef]
- Pujol-Lereis, L.M.; Schafer, N.; Kuhn, L.B.; Rohrer, B.; Pauly, D. Interrelation Between Oxidative Stress and Complement Activation in Models of Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2016, 854, 87–93. [Google Scholar] [CrossRef]
- Vuong, L.; Conley, S.M.; Al-Ubaidi, M.R. Expression and role of p53 in the retina. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1362–1371. [Google Scholar] [CrossRef]
- Chatoo, W.; Abdouh, M.; Bernier, G. p53 Pro-Oxidant Activity in the Central Nervous System: Implication in Aging and Neurodegenerative Diseases. Antioxid. Redox Signal. 2011, 15, 1729–1737. [Google Scholar] [CrossRef]
- Liu, J.; Cao, L.; Chen, J.; Song, S.; Lee, I.H.; Quijano, C.; Liu, H.; Keyvanfar, K.; Chen, H.; Cao, L.Y.; et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 2009, 459, 387–392. [Google Scholar] [CrossRef]
- Ressler, S.; Bartkova, J.; Niederegger, H.; Bartek, J.; Scharffetter-Kochanek, K.; Jansen-Durr, P.; Wlaschek, M. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 2006, 5, 379–389. [Google Scholar] [CrossRef]
- Maeda, T.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Trends of Stem Cell Therapies in Age-Related Macular Degeneration. J. Clin. Med. 2021, 10, 1785. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mustafi, S.B.; Street, M.; Dey, A.; Dwivedi, S.K. Bmi-1: At the crossroads of physiological and pathological biology. Genes Dis. 2015, 2, 225–239. [Google Scholar] [CrossRef]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal Diseases Associated with Oxidative Stress and the Effects of a Free Radical Scavenger (Edaravone). Oxid. Med. Cell Longev. 2017, 2017, 9208489. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Morales, M.G.; Liu, S.; Ramkumar, H.L. The Endogenous Expression of BMI1 in Adult Human Eyes. Cells 2024, 13, 1672. https://doi.org/10.3390/cells13191672
Lu Z, Morales MG, Liu S, Ramkumar HL. The Endogenous Expression of BMI1 in Adult Human Eyes. Cells. 2024; 13(19):1672. https://doi.org/10.3390/cells13191672
Chicago/Turabian StyleLu, Zhongyang, Maria G. Morales, Shufeng Liu, and Hema L. Ramkumar. 2024. "The Endogenous Expression of BMI1 in Adult Human Eyes" Cells 13, no. 19: 1672. https://doi.org/10.3390/cells13191672





