Development of Innate-Immune-Cell-Based Immunotherapy for Adult T-Cell Leukemia–Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Derivation of γδ T Cells and NK Cells
2.2. Flow Cytometric Analysis
2.3. Patient Characterization and Outcome
2.4. Cytotoxicity Assay Using Time-Resolved Fluorescence Spectroscopy
2.5. Statistical Analysis
3. Results
3.1. Expansion of γδ T Cells and NK Cells Derived from HDs
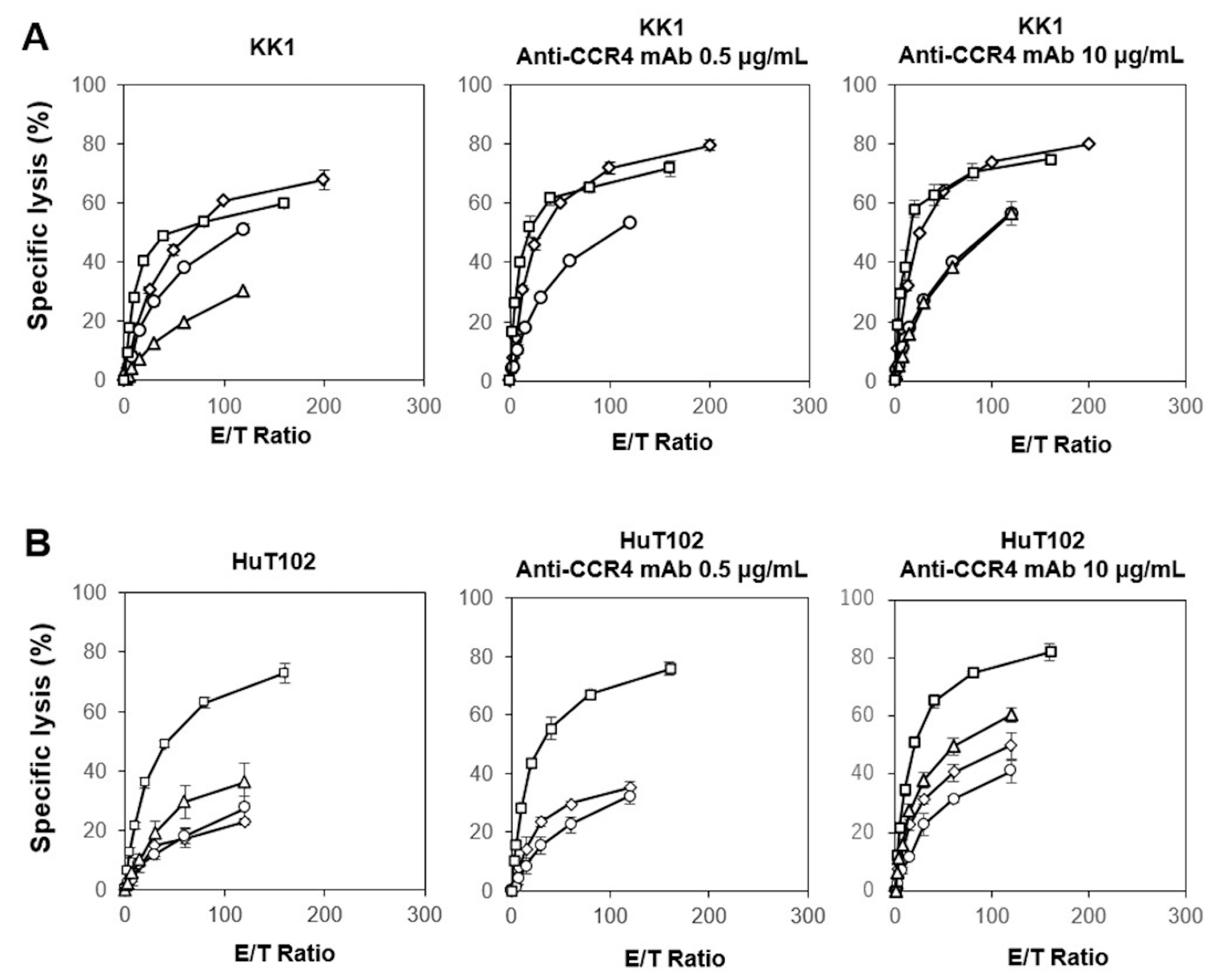
3.2. Cytotoxicity Exhibited by γδ T Cells and NK Cells Derived from HDs against ATL Cell Lines In Vitro
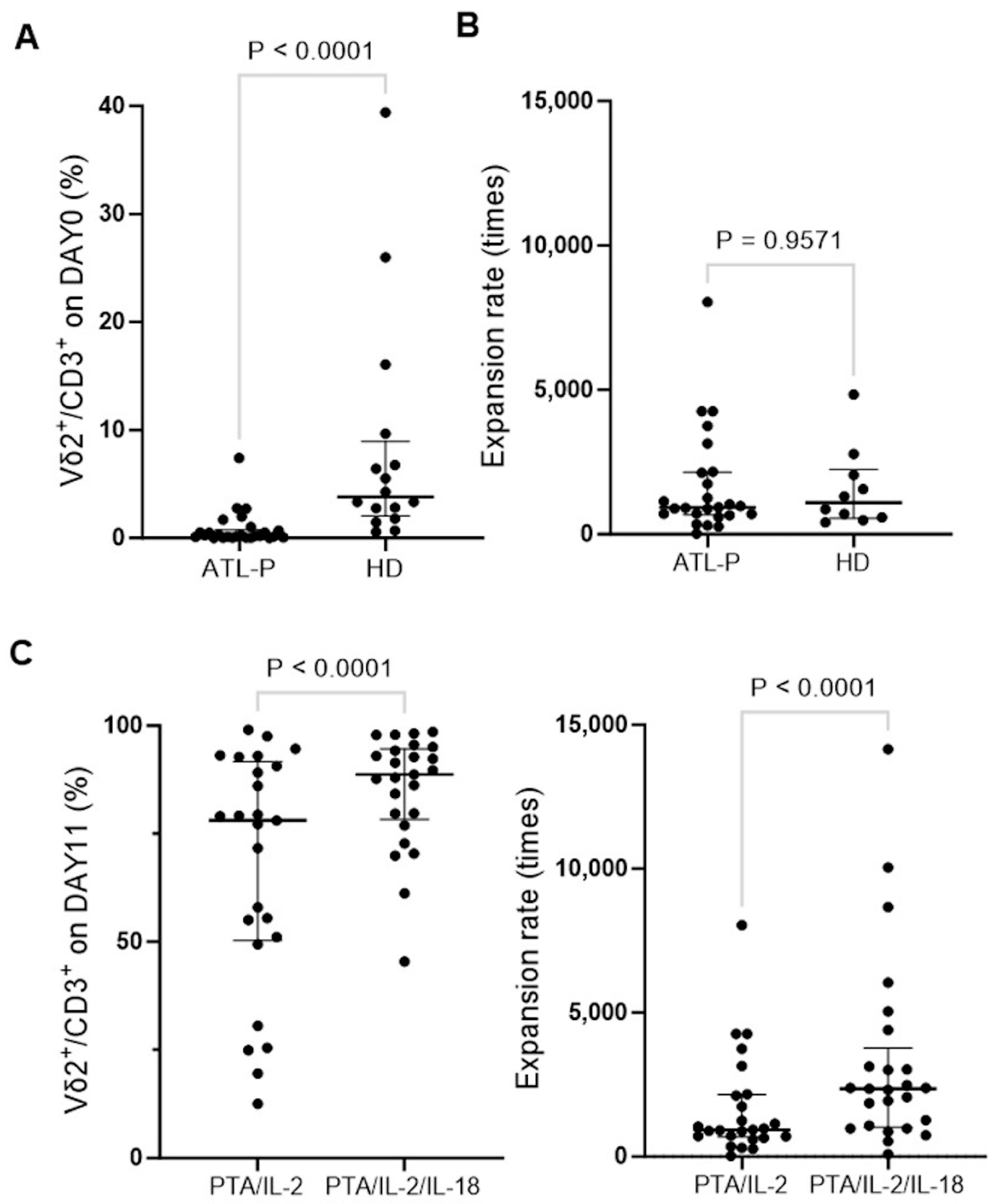
3.3. Effect of IL-18 on the Expansion of γδ T Cells Derived from ATL Patients
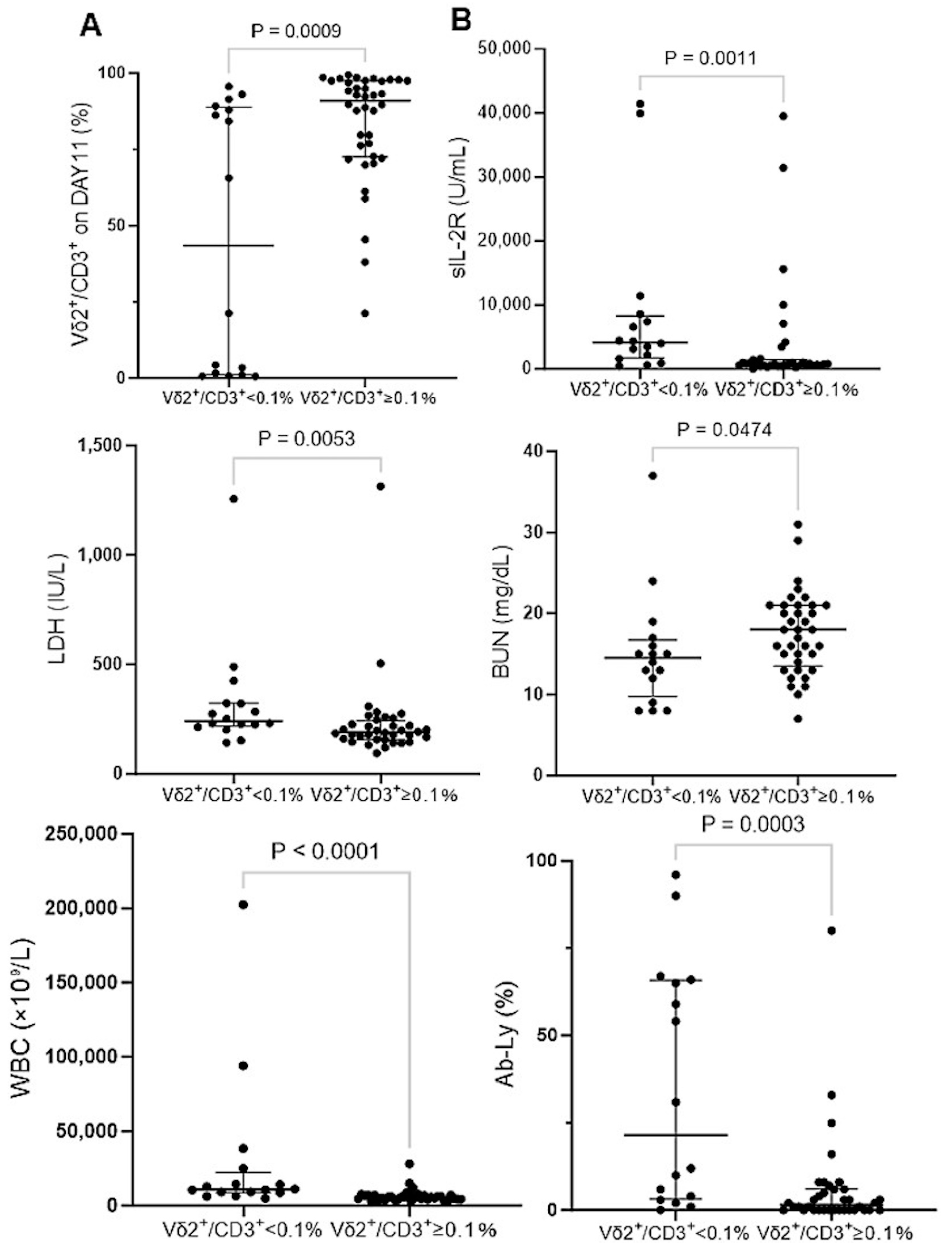
3.4. IL-2/IL-18-Mediated Expansion of NK Cells Derived from ATL Patients
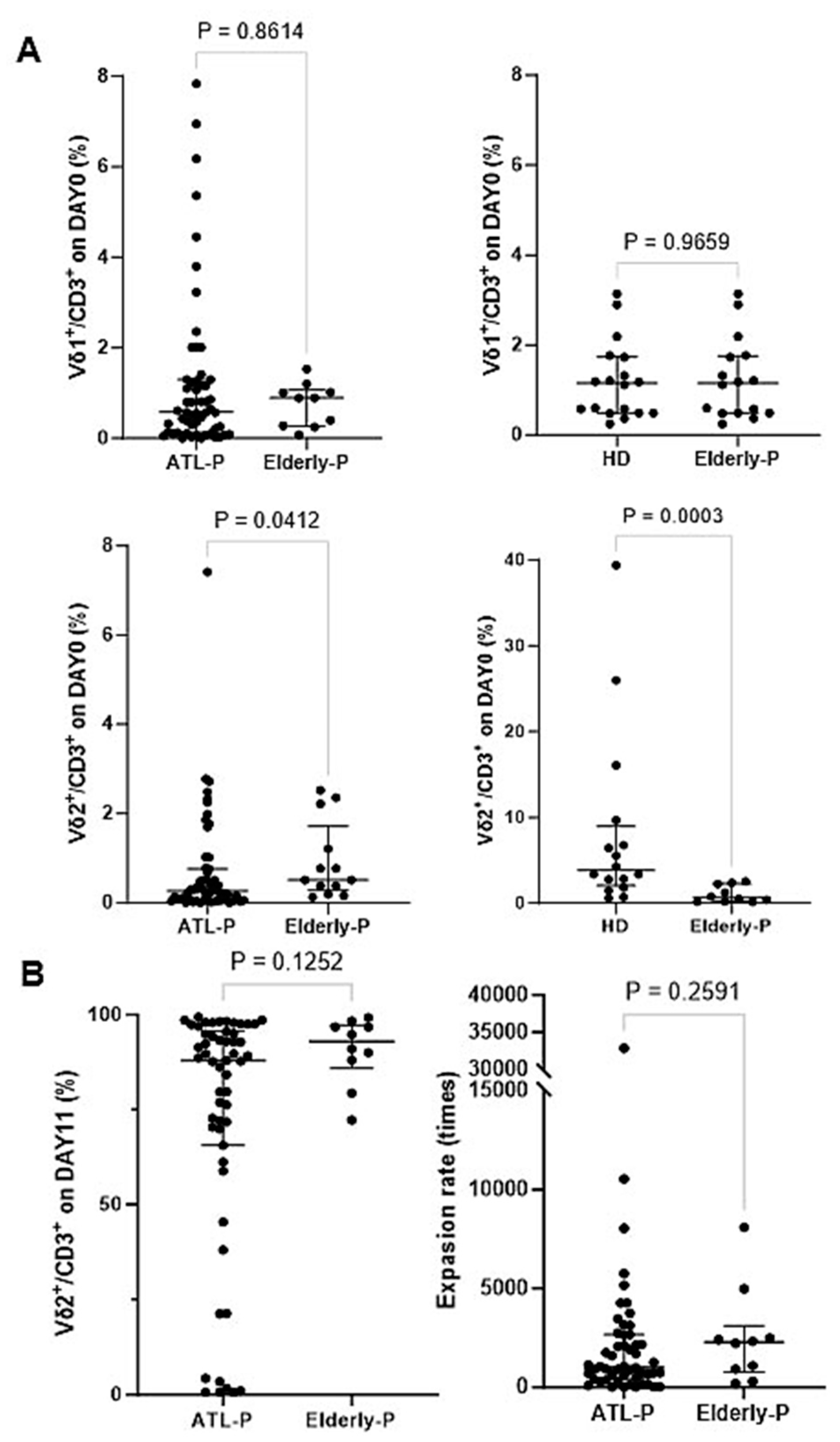
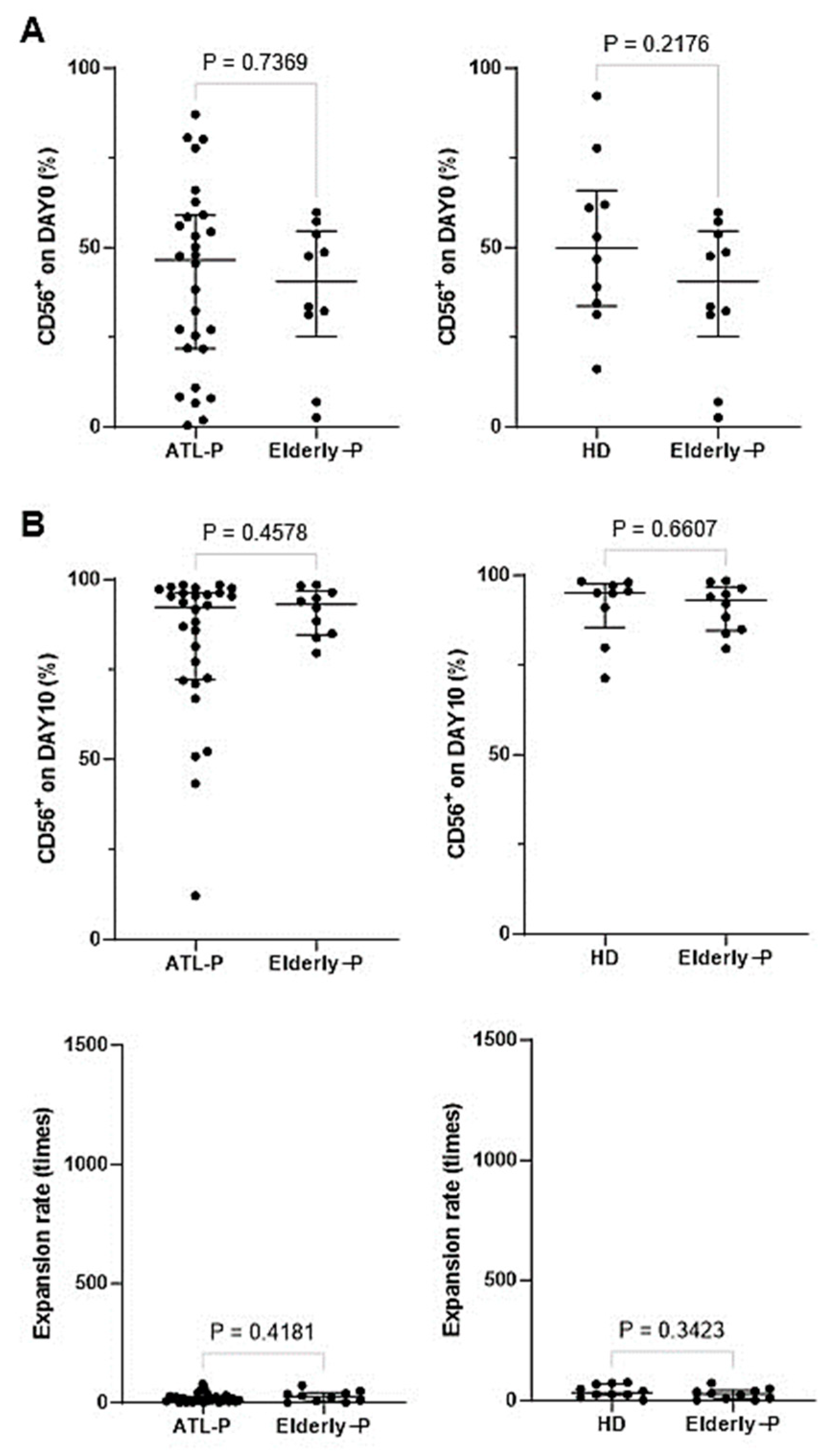
3.5. Effect of Aging on the Phenotype and Immunological Properties of γδ T Cells and NK Cells
3.6. Cytotoxic Activity Exhibited by γδ T Cells and NK Cells Derived from ATL Patients against ATL Cell Lines In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uchiyama, T.; Yodoi, J.; Sagawa, K.; Takatsuki, K.; Uchino, H. Adult T-cell leukemia: Clinical and hematologic features of 16 cases. Blood 1977, 50, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, K. Discovery of adult T-cell leukemia. Retrovirology 2005, 2, 16. [Google Scholar] [CrossRef]
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Yamaguchi, K.; Tadokoro, K. Current prevalence of HTLV-1 in Japan as determined by screening of blood donors. J Med Virol. 2012, 84, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Iwanaga, M.; Sagara, Y.; Watanabe, T.; Okuma, K.; Hamaguchi, I. Incidence of human T-lymphotropic virus 1 infection in adolescent and adult blood donors in Japan: A nationwide retrospective cohort analysis. Lancet Infect. Dis. 2016, 16, 1246–1254. [Google Scholar] [CrossRef]
- Ito, S.; Iwanaga, M.; Nosaka, K.; Imaizumi, Y.; Ishitsuka, K.; Amano, M.; Utsunomiya, A.; Tokura, Y.; Watanabe, T.; Uchimaru, K.; et al. Epidemiology of adult T-cell leukemia-lymphoma in Japan: An updated analysis, 2012–2013. Cancer Sci. 2021, 112, 4346–4354. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M. Epidemiology of HTLV-1 infection and ATL in Japan: An update. Front. Microbiol. 2020, 29, 1124. [Google Scholar] [CrossRef]
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef]
- Tsukasaki, K.; Hermine, O.; Bazarbachi, A.; Ratner, L.; Ramos, J.C.; Harrington, W., Jr.; O’Mahony, D.; Janik, J.E.; Bittencourt, A.L.; Taylor, G.P.; et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: A proposal from an international consensus meeting. J. Clin. Oncol. 2009, 27, 453–459. [Google Scholar] [CrossRef]
- Ishida, T.; Itoh, A.; Tokura, Y.; Tanaka, J.; Uike, N.; Tobinai, K.; Tsukasaki, K. An integrated manual for hematologists and dermatologists to access the guidelines for the management of adult T-cell leukemia-lymphoma (2014). Rinsho Ketsueki 2014, 55, 2257–2261. [Google Scholar]
- Utsunomiya, A.; Miyazaki, Y.; Takatsuka, Y.; Hanada, S.; Uozumi, K.; Yashiki, S.; Tara, M.; Kawano, F.; Saburi, Y.; Kikuchi, H.; et al. Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 2001, 27, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, A.; Choi, I.; Chihara, D.; Seto, M. Recent advances in the treatment of adult T-cell leukemia-lymphomas. Cancer Sci. 2015, 106, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, T.; Nosaka, K.; Tatetsu, H.; Higuchi, Y.; Endo, S.; Inoue, Y.; Toyoda, K.; Kikukawa, Y.; Kawakita, T.; Yasunaga, J.; et al. Beneficial impact of first-line mogamulizumab-containing chemotherapy in adult T-cell leukaemia-lymphoma. Br. J. Hematol. 2022, 198, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, J.; Imaizumi, Y.; Taniguchi, H.; Ando, K.; Iwanaga, M.; Itonaga, H.; Sato, S.; Sawayama, Y.; Hata, T.; Yoshida, S.; et al. Clinical factors to predict outcome following mogamulizumab in adult T-cell leukemia-lymphoma. Int. J. Hematol. 2018, 108, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Imaizumi, Y.; Uike, N.; Asou, N.; Utsunomiya, A.; Uchida, T.; Aoki, T.; Tsukasaki, K.; Taguchi, J.; Choi, I.; et al. Lenalidomide in relapsed adult T-cell leukemia-lymphoma or peripheral T-cell lymphoma (ATLL-001): A phase 1, multicentre, dose-escalation study. Lancet Hematol. 2016, 3, 107–118. [Google Scholar] [CrossRef]
- Imaizumi, Y.; Iwanaga, M.; Nosaka, K.; Ishitsuka, K.; Ishizawa, K.; Ito, S.; Amano, M.; Ishida, T.; Uike, N.; Utsunomiya, A.; et al. Prognosis of patients with adult T-cell leukemia/lymphoma in Japan: A nationwide hospital-based study. Cancer Sci. 2020, 111, 4567–4580. [Google Scholar] [CrossRef]
- Hishizawa, M.; Kanda, J.; Utsunomiya, A.; Taniguchi, S.; Eto, T.; Moriuchi, Y.; Tanosaki, R.; Kawano, F.; Miyazaki, Y.; Masuda, M.; et al. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: A nationwide retrospective study. Blood 2010, 116, 1369–1376. [Google Scholar] [CrossRef]
- Nosaka, K.; Iwanaga, M.; Imaizumi, Y.; Ishitsuka, K.; Ishizawa, K.; Ishida, Y.; Amano, M.; Ishida, T.; Uike, N.; Utsunomiya, A.; et al. Epidemiological and clinical features of adult T-cell leukemia-lymphoma in Japan, 2010–2011, a nationwide survey. Cancer Sci. 2017, 108, 2478–2486. [Google Scholar] [CrossRef]
- Kinosada, H.; Yasunaga, J.I.; Shimura, K.; Miyazato, P.; Onishi, C.; Iyoda, T.; Inaba, K.; Matsuoka, M. HTLV-1 bZIP Factor Enhances T-Cell Proliferation by Impeding the Suppressive Signaling of Co-inhibitory Receptors. PLoS Pathog. 2017, 13, 1006120. [Google Scholar]
- Watanabe, T. Adult T-cell leukemia: Molecular basis for clonal expansion and transformation of HTLV-1-infected T cells. Blood 2017, 129, 1071–1081. [Google Scholar] [CrossRef]
- Yamagishi, M.; Fujikawa, D.; Watanabe, T.; Uchimaru, K. HTLV-1-Mediated Epigenetic Pathway to Adult T-Cell Leukemia-Lymphoma. Front. Microbiol. 2018, 9, 1686. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, M.; Yasunaga, J.; Iwami, S.; Nakaoka, S.; Koizumi, Y.; Shimura, K.; Matsuoka, M. Sporadic on/off switching of HTLV-1 Tax expression is crucial to maintain the whole population of virus-induced leukemic cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1269–E1278. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Koya, J. Clinical application of genomic aberrations in adult T-cell leukemia/lymphoma. J. Clin. Exp. Hematol. 2020, 60, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Azakami, K.; Sato, T.; Araya, N.; Utsunomiya, A.; Kubota, R.; Suzuki, K.; Hasegawa, D.; Izumi, T.; Fujita, H.; Aratani, S.; et al. Severe loss of invariant NKT cells exhibiting anti–HTLV-1 activity in patients with HTLV-1-associated disorders. Blood 2009, 114, 3208–3215. [Google Scholar] [CrossRef]
- Ogura, M.; Ishida, T.; Tsukasaki, K.; Takahashi, T.; Utsunomiya, A. Effects of first-line chemotherapy on natural killer cells in adult T-cell leukemia–lymphoma and peripheral T-cell lymphoma. Cancer Chemother. Pharmacol. 2016, 78, 199–207. [Google Scholar] [CrossRef]
- Nagai, K.; Nagai, S. Effectiveness of Amplified Natural Killer (ANK) Therapy for Adult T-cell Leukemia/Lymphoma (ATL) and Future Prospects of ANK Therapy. Cancer Med. J. 2022, 5, 27–33. [Google Scholar] [PubMed]
- Wu, Y.; Tian, Z.; Wei, H. Developmental and Functional Control of Natural Killer Cells by Cytokines. Front. Immunol. 2017, 8, 930. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lim, E.J.; Kim, S.W.; Moon, Y.W.; Parket, K.S.; An, H.J. IL-27 enhances IL-15/IL-18-mediated activation of human natural killer cells. J. Immunother. Cancer 2019, 7, 168. [Google Scholar] [CrossRef]
- Wrona, E.; Borowiec, M.; Potemski, P. CAR-NK Cells in the Treatment of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 5899. [Google Scholar] [CrossRef]
- Conlon, K.C.; Potter, E.L.; Pittaluga, S.; Lee, C.R.; Miljkovic, M.D.; Fleisher, T.A.; Dubois, S.; Bryant, B.R.; Petrus, M.; Perera, L.P.; et al. IL15 by Continuous Intravenous Infusion to Adult Patients with Solid Tumors in a Phase I Trial Induced Dramatic NK-Cell Subset Expansion. Clin. Cancer Res. 2019, 25, 4945–4954. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitaions and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, M.R.; Poggi, A. Role of gammadelta T lymphocytes in tumor defense. Front. Biosci. 2004, 9, 2588–2604. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, D.; Wesch, D.; Pitters, E.; Zöller, M. Characterization of Tumor Reactivity of Human Vγ9Vδ2 γδ T Cells In Vitro and in SCID Mice In Vivo. J. Immunol. 2004, 173, 6767–6776. [Google Scholar] [CrossRef]
- Kabelitz, D.; Wesch, D.; He, W. Perspectives of γδ T Cells in Tumor Immunology. Cancer Res. 2007, 67, 5–8. [Google Scholar] [CrossRef]
- Hayday, A.C. γδ CELLS: A Right Time and a Right Place for a Conserved Third Way of Protection. Annu. Rev. Immunol. 2000, 18, 975–1026. [Google Scholar] [CrossRef]
- Fournie, J.J.; Sicard, H.; Poupot, M.; Bezombes, C.; Blanc, A.; Romagné, F.; Ysebaert, L.; Laurent, G. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell. Mol. Immunol. 2013, 10, 35–41. [Google Scholar] [CrossRef]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. γδ T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef]
- Bonneville, M.; Scotet, E. Human Vgamma9Vdelta2 T cells: Promising new leads for immunotherapy of infections and tumors. Curr. Opin. Immunol. 2006, 18, 539–546. [Google Scholar] [CrossRef]
- Scotet, E.; Martinez, L.O.; Grant, E.; Barbaras, R.; Jenö, P. Tumor Recognition following Vγ9Vδ2 T Cell Receptor Interactions with a Surface F1-ATPase-Related Structure and Apolipoprotein A-I. Immunity 2005, 22, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santos, B.; Serre, K.; Norell, H. γδ T cells in cancer. Nat. Rev. Immunol. 2015, 15, 683–691. [Google Scholar] [CrossRef]
- Mensurado, S.; Blanco-Domínguez, R.; Silva-Santos, B. The emerging roles of γδ T cells in cancer immunotherapy. Nat. Rev. Clin. Oncol. 2023, 20, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Cerapio, J.P.; Perrier, M.; Balança, C.C.; Gravellea, P.; Pont, F. Phased differentiation of γδ T and T CD8 tumor-infiltrating lymphocytes revealed by single-cell transcriptomics of human cancers. Oncoimmunology 2021, 10, e1939518. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.; Lamb, J.R. γδ T cells as immune effectors against high-grade gliomas. Immunol. Res. 2009, 45, 85–95. [Google Scholar]
- Porcelli, S.; Brenner, M.B.; Band, H. Biology of human γδ T cell receptor. Immunol. Rev. 1991, 120, 137–183. [Google Scholar] [CrossRef]
- Constant, P.; Davodeau, F.; Peyrat, M.A.; Poquet, Y.; Puzo, G.; Bonneville, M.; Fournié, J.J. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science 1994, 264, 267–270. [Google Scholar] [CrossRef]
- Tanaka, Y.; Morita, C.T.; Tanaka, Y.; Nieves, E.; Brenner, M.B.; Bloom, B.R. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 1995, 375, 155–158. [Google Scholar] [CrossRef]
- Puan, K.J.; Jin, C.; Wang, H.; Sarikonda, G.; Raker, A.M.; Lee, H.K.; Samuelson, M.I.; Märker-Hermann, E.; Pasa-Tolic, L.; Nieves, E.; et al. Preferential recognition of a microbial metabolite by human Vg2Vd2 T cells. Int. Immunol. 2007, 19, 657–673. [Google Scholar] [CrossRef]
- Tanaka, Y.; Murata-Hirai, K.; Iwasaki, M.; Matsumoto, K.; Hayashi, K.; Kumagai, A.; Nada, M.H.; Wang, H.; Kobayashi, H.; Kamitakahara, H.; et al. Expansion of human γδ T cells for adoptive immunotherapy using a bisphosphonate prodrug. Cancer Sci. 2018, 109, 587–599. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.-W.; Sheard, M.A.; Sposto, R.; Somanchi, S.S.; Cooper, L.J.N.; Lee, D.A.; Seeger, R.C. Growth and activation of natural killer cells ex vivo from children with neuroblastoma for adoptive therapy. Clin. Cancer Res. 2013, 19, 2132–2143. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yamamoto, H.; Kubo, S.; Okamura, H. Modulation of innate immunity by IL-18. J. Rep. Immunol. 2009, 83, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Utsunomiya, A.; Iida, S.; Inagaki, H.; Takatsuka, Y.; Kusumoto, S.; Takeuchi, G.; Shimizu, S.; Ito, M.; Komatsu, H.; et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: Its close association with skin involvement and unfavorable outcome. Clin. Cancer Res. 2003, 9, 3625–3634. [Google Scholar]
- Ishii, T.; Ishida, T.; Utsunomiya, A.; Inagaki, A.; Yano, H.; Komatsu, H.; Iida, S.; Imada, K.; Uchiyama, T.; Akinaga, S.; et al. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immuno-therapeutic agent for adult T-cell leukemia/lymphoma. Clin. Cancer Res. 2010, 16, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Fujiwara, H.; Ochi, F.; Tanimoto, K.; Casey, N.; Okamoto, S.; Mineno, J.; Kuzushima, K.; Shiku, H.; Sugiyama, T.; et al. Development of Engineered T Cells Expressing a Chimeric CD16-CD3ζ Receptor to Improve the Clinical Efficacy of Mogamulizumab Therapy Against Adult T-Cell Leukemia. Clin. Cancer Res. 2016, 22, 4405–4416. [Google Scholar] [CrossRef]
- Couzi, L.; Pitard, V.; Sicard, X.; Garrigue, I.; Hawchar, O.; Merville, P.; Moreau, J.F.; Déchanet-Merville, J. Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (Fcγ RIIIa). Blood 2012, 119, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Capietto, A.H.; Martinet, L.; Fournie, J.J. Stimulated γδ T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. J. Immunol. 2011, 187, 1031–1038. [Google Scholar] [CrossRef]
- Besser, M.J.; Shoham, T.; Fournie, J.J.; Harari-Steinberg, O.; Zabari, N.; Ortenberg, R.; Yakirevitch, A.; Nagler, A.; Loewenthal, R.; Schachter, J.; et al. Development of allogeneic NK cell adoptive transfer therapy in metastatic melanoma patients: In vitro preclinical optimization studies. PLoS ONE 2013, 8, e57922. [Google Scholar]
- Yssel, H.; De Vries, J.E.; Koken, M.; Blitterswijk, W.V.; Spits, H. Serum-free medium for generation and propagation of functional human cytotoxic and helper T cell clones. J. Immunol. Methods 1984, 72, 219–227. [Google Scholar] [CrossRef]
- Han, S.; Georgiev, P.; Ringel, A.E.; Sharpe, A.H.; Haigis, M.C. Age-associated remodeling of T cell immunity and metabolism. Cell Metab. 2023, 35, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, K.; Beetz, S.; Welte, S.; Gruen, J.; Oberg, H.H.; Wesch, D.; Kabelitz, D. Toll-like receptor expression and function in subsets of human gammadelta T lymphocytes. Scand J. Immunol. 2009, 70, 245–255. [Google Scholar] [CrossRef]
- Rincon-Orozco, B.; Kunzmann, V.; Wrobel, P.; Kabelitz, D.; Steinle, A.; Herrmann, T. Activation of V gamma 9 V delta 2 T cells by NKG2D. J. Immunol. 2005, 175, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Gertner-Dardenne, J.; Bonnafous, C.; Bezombes, C.; Capietto, A.H.; Scaglione, V.; Ingoure, S.; Cendron, D.; Gross, E.; Lepage, J.F.; Quillet-Mary, A.; et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood 2009, 113, 4875–4884. [Google Scholar] [CrossRef] [PubMed]
- Toutirais, O.; Cabillic, F.; Le Friec, G.; Salot, S.; Loyer, P.; Gallo, M.L.; Desille, M.; LaPintie`re, C.T.; Daniel, P.; Bouet, F.; et al. DNAX accessory molecule-1 (CD226) promotes human hepatocellular carcinoma cell lysis by Vgamma9Vdelta2 T cells. Eur. J. Immunol. 2009, 39, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, A.; Correia, M.P.; Cerwenka, A. The NKG2D/NKG2DL Axis in the Crosstalk Between Lymphoid and Myeloid Cells in Health and Disease. Front. Immunol. 2018, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, P.; Shojaei, H.; Schittek, B.; Gieseler, F.; Wollenberg, B.; Kalthoff, H.; Kabelitz, D.; Wesch, D. Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: Involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand. J. Immunol. 2007, 66, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Tanaka, Y.; Kobayashi, H.; Murata-Hirai, K.; Miyabe, H.; Sugie, T.; Toi, M.; Minato, N. Expression and function of PD-1 in human γδ T cells that recognize phosphoantigens. Eur. J. Immunol. 2011, 41, 345–355. [Google Scholar] [CrossRef]
- Maeda, M.; Tanabe-Shibuya, J.; Miyazato, P.; Masutani, H.; Yasunaga, J.; Usami, K.; Shimizu, A.; Matsuoka, M. IL-2/IL-2 Receptor Pathway Plays a Crucial Role in the Growth and Malignant Transformation of HTLV-1-Infected T Cells to Develop Adult T-Cell Leukemia. Front. Microbiol. 2020, 11, 356. [Google Scholar] [CrossRef]
- Niehrs, A.; Altfeld, M. Regulation of NK-Cell Function by HLA Class II. Front. Cell. Infect. Microbiol. 2020, 10, 55. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, R.; Chen, H.; Guo, W.; Li, Z.; Xu, K.; Chen, W. CD86 Is Associated with Immune Infiltration and Immunotherapy Signatures in AML and Promotes Its Progression. J. Oncol. 2023, 2023, 9988405. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Mizuta, S.; Kumagai, A.; Tagod, M.S.O.; Senju, H.; Nakamura, T.; Morita, C.T.; Tanaka, Y. Live cell labeling with terpyridine derivative proligands to measure cytotoxicity mediated by immune cells. ChemMedChem 2017, 12, 2006–2013. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.G.; Ponnusamy, K.; Ren, H.; Taylor, G.P.; Cook, L.B.M.; Karadimitris, A. CAR-iNKT cells targeting clonal TCRVβ chains as a precise strategy to treat T cell lymphoma. Front. Immunol. 2023, 14, 118681. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, V.; Wilhelm, M. Anti-lymphoma effect of gammadelta T cells. Leuk Lymphoma 2005, 46, 671–680. [Google Scholar] [CrossRef]
- Robertson, M.J.; Kline, J.; Struemper, H.; Koch, K.M.; Bauman, J.W.; Gardner, O.S.; Murray, S.C.; Germaschewski, F.; Weisenbach, J.; Jonak, Z.; et al. A dose-escalation study of recombinant human interleukin-18 in combination with rituximab in patients with non-Hodgkin lymphoma. J. Immunother. 2013, 36, 331–341. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakashima, M.; Tanaka, Y.; Okamura, H.; Kato, T.; Imaizumi, Y.; Nagai, K.; Miyazaki, Y.; Murota, H. Development of Innate-Immune-Cell-Based Immunotherapy for Adult T-Cell Leukemia–Lymphoma. Cells 2024, 13, 128. https://doi.org/10.3390/cells13020128
Nakashima M, Tanaka Y, Okamura H, Kato T, Imaizumi Y, Nagai K, Miyazaki Y, Murota H. Development of Innate-Immune-Cell-Based Immunotherapy for Adult T-Cell Leukemia–Lymphoma. Cells. 2024; 13(2):128. https://doi.org/10.3390/cells13020128
Chicago/Turabian StyleNakashima, Maho, Yoshimasa Tanaka, Haruki Okamura, Takeharu Kato, Yoshitaka Imaizumi, Kazuhiro Nagai, Yasushi Miyazaki, and Hiroyuki Murota. 2024. "Development of Innate-Immune-Cell-Based Immunotherapy for Adult T-Cell Leukemia–Lymphoma" Cells 13, no. 2: 128. https://doi.org/10.3390/cells13020128




