Allogeneic CAR-T Therapy Technologies: Has the Promise Been Met?
Abstract
:1. Introduction
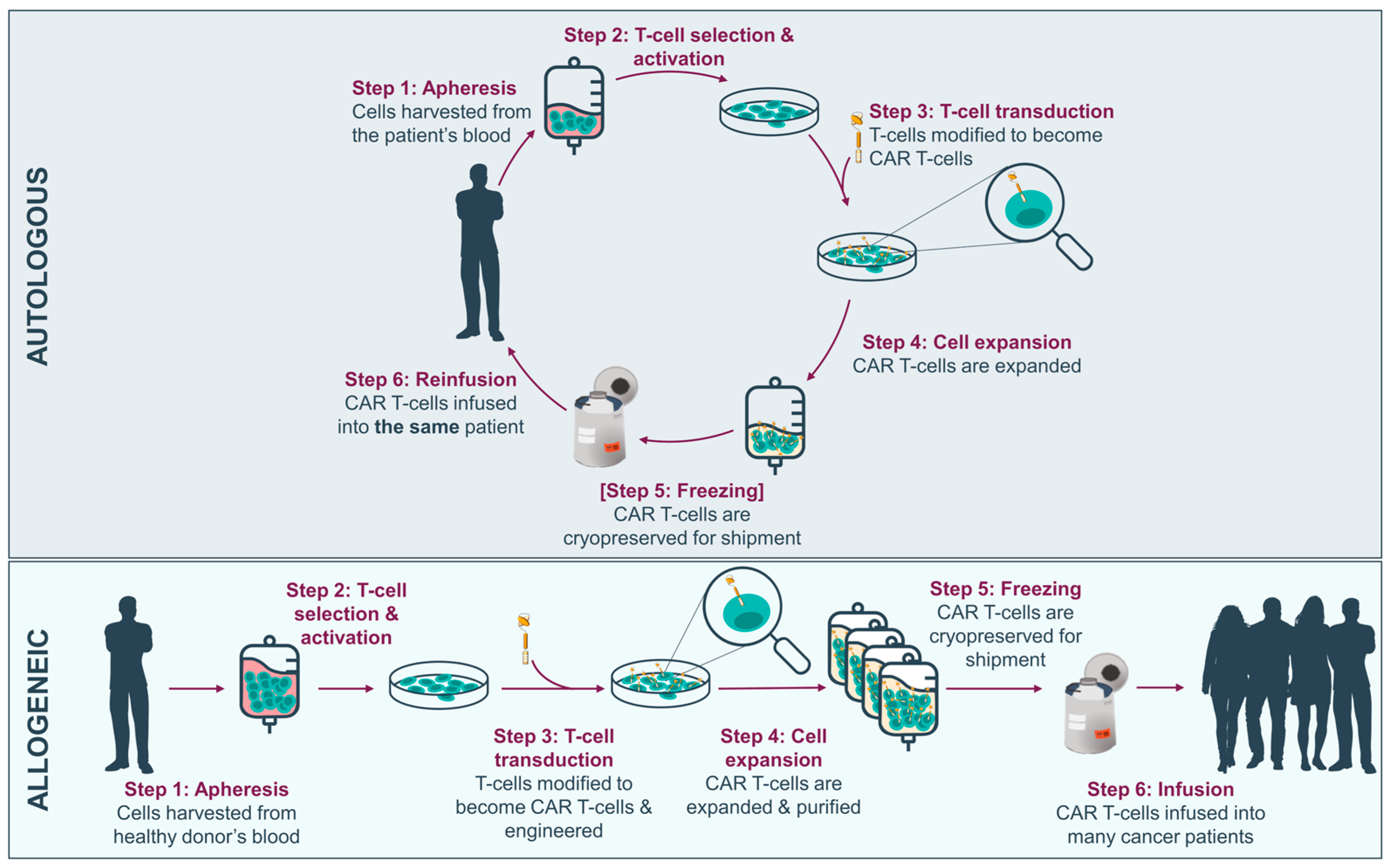
2. Source of Allogeneic Cells
2.1. PBMCs
2.2. UCB
2.3. Induced Pluripotent Stem Cells (iPSCs)
3. How to Prevent Alloreactivity in CAR-Ts by Selecting the Right Cell Population?
3.1. Infusion of Allogeneic CAR-Ts Post or Prior to an Allogeneic Transplantation
3.2. Memory T-Cells
3.3. T-Cell Sub-Populations
3.3.1. Double-Negative T-Cells (DNTs)
3.3.2. Invariant Natural Killer T-Cells (iNKTs)
3.3.3. Cytokine-Induced Killer (CIK)-Cells
3.3.4. Mucosal-Associated Invariant T (MAIT)-Cells
3.3.5. γδT-Cells
4. ‘Off-the-Shelf’ Allogeneic CAR-Ts
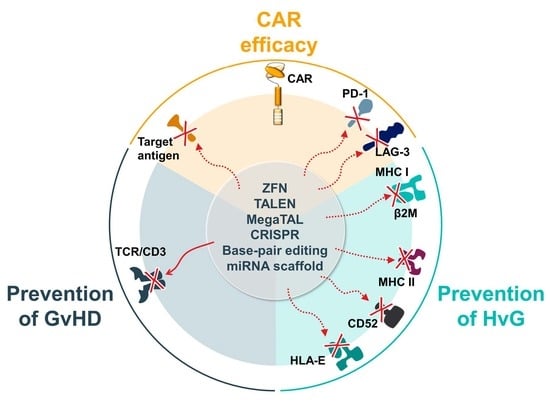
4.1. Methods to Engineer ‘Off-the-Shelf’ Allogeneic CAR-Ts
4.1.1. Gene-Editing Technology
4.1.2. Non-Gene Editing
4.2. Clinical Experience with ‘Off-the-Shelf’ Allogeneic CAR-Ts
4.2.1. Successes to Date
4.2.2. Challenges to Overcome
5. Conclusions
Funding
Conflicts of Interest
References
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef]
- Zimmer, J.; Jurišić, V. Special Issue “New Developments in Natural Killer Cells for Immunotherapy”. Cells 2023, 12, 1496. [Google Scholar] [CrossRef]
- Mitra, A.; Barua, A.; Huang, L.; Ganguly, S.; Feng, Q.; He, B. From Bench to Bedside: The History and Progress of CAR T Cell Therapy. Front. Immunol. 2023, 14, 1188049. [Google Scholar] [CrossRef]
- Cappell, K.M.; Kochenderfer, J.N. Long-Term Outcomes Following CAR T Cell Therapy: What We Know so Far. Nat. Rev. Clin. Oncol. 2023, 20, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A.; Frigault, M.J.; Ansari, A.; Gliner, B.; Heery, C.; Shah, B. Dose–Response Correlation for CAR-T Cells: A Systematic Review of Clinical Studies. J. Immunother. Cancer 2022, 10, e005678. [Google Scholar] [CrossRef] [PubMed]
- Ruella, M.; Korell, F.; Porazzi, P.; Maus, M.V. Mechanisms of Resistance to Chimeric Antigen Receptor-T Cells in Haematological Malignancies. Nat. Rev. Drug Discov. 2023, 1–20. [Google Scholar] [CrossRef]
- Daei Sorkhabi, A.; Mohamed Khosroshahi, L.; Sarkesh, A.; Mardi, A.; Aghebati-Maleki, A.; Aghebati-Maleki, L.; Baradaran, B. The Current Landscape of CAR T-Cell Therapy for Solid Tumors: Mechanisms, Research Progress, Challenges, and Counterstrategies. Front. Immunol. 2023, 14, 1113882. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cibrian, N.; Español-Rego, M.; Pascal, M.; Delgado, J.; Ortiz-Maldonado, V. Practical Aspects of Chimeric Antigen Receptor T-Cell Administration: From Commercial to Point-of-Care Manufacturing. Front. Immunol. 2022, 13, 1005457. [Google Scholar] [CrossRef]
- Abraham-Miranda, J.; Menges, M.; Atkins, R.; Mattie, M.; Kanska, J.; Turner, J.; Hidalgo-Vargas, M.J.; Locke, F.L. CAR-T Manufactured from Frozen PBMC Yield Efficient Function with Prolonged in Vitro Production. Front. Immunol. 2022, 13, 1007042. [Google Scholar] [CrossRef]
- Michaux, A.; Mauën, S.; Breman, E.; Dheur, M.-S.; Twyffels, L.; Saerens, L.; Jacques-Hespel, C.; Gauthy, E.; Agaugué, S.; Gilham, D.E.; et al. Clinical Grade Manufacture of CYAD-101, a NKG2D-Based, First in Class, Non-Gene-Edited Allogeneic CAR T-Cell Therapy. J. Immunother. 2022, 45, 150–161. [Google Scholar] [CrossRef]
- Künkele, A.; Brown, C.; Beebe, A.; Mgebroff, S.; Johnson, A.J.; Taraseviciute, A.; Rolczynski, L.S.; Chang, C.A.; Finney, O.C.; Park, J.R.; et al. Manufacture of Chimeric Antigen Receptor T Cells from Mobilized Cyropreserved Peripheral Blood Stem Cell Units Depends on Monocyte Depletion. Biol. Blood Marrow Transplant. 2019, 25, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Balint, M.T.; Lemajić, N.; Jurišić, V.; Pantelić, S.; Stanisavljević, D.; Kurtović, N.K.; Balint, B. An Evidence-Based and Risk-Adapted GSF versus GSF plus Plerixafor Mobilization Strategy to Obtain a Sufficient CD34+ Cell Yield in the Harvest for Autologous Stem Cell Transplants. Transl. Oncol. 2024, 39, 101811. [Google Scholar] [CrossRef]
- Tang, T.C.Y.; Xu, N.; Nordon, R.; Haber, M.; Micklethwaite, K.; Dolnikov, A. Donor T Cells for CAR T Cell Therapy. Biomark. Res. 2022, 10, 14. [Google Scholar] [CrossRef]
- Eapen, M.; Rocha, V.; Sanz, G.; Scaradavou, A.; Zhang, M.-J.; Arcese, W.; Sirvent, A.; Champlin, R.E.; Chao, N.; Gee, A.P.; et al. Effect of Graft Source on Unrelated Donor Haemopoietic Stem-Cell Transplantation in Adults with Acute Leukaemia: A Retrospective Analysis. Lancet Oncol. 2010, 11, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.; Cartledge, K.; Cao, H.; Evtimov, V.; Pupovac, A.; Trounson, A.; Boyd, R. “Off-the-Shelf” Immunotherapy: Manufacture of CD8+ T Cells Derived from Hematopoietic Stem Cells. Cells 2021, 10, 2631. [Google Scholar] [CrossRef]
- Van Caeneghem, Y.; De Munter, S.; Tieppo, P.; Goetgeluk, G.; Weening, K.; Verstichel, G.; Bonte, S.; Taghon, T.; Leclercq, G.; Kerre, T.; et al. Antigen Receptor-Redirected T Cells Derived from Hematopoietic Precursor Cells Lack Expression of the Endogenous TCR/CD3 Receptor and Exhibit Specific Antitumor Capacities. OncoImmunology 2017, 6, e1283460. [Google Scholar] [CrossRef]
- Kwoczek, J.; Riese, S.B.; Tischer, S.; Bak, S.; Lahrberg, J.; Oelke, M.; Maul, H.; Blasczyk, R.; Sauer, M.; Eiz-Vesper, B. Cord Blood-Derived T Cells Allow the Generation of a More Naïve Tumor-Reactive Cytotoxic T-Cell Phenotype. Transfusion 2018, 58, 88–99. [Google Scholar] [CrossRef]
- Liu, D.-D.; Hong, W.-C.; Qiu, K.-Y.; Li, X.-Y.; Liu, Y.; Zhu, L.-W.; Lai, W.-X.; Chen, H.; Yang, H.-Q.; Xu, L.-H.; et al. Umbilical Cord Blood: A Promising Source for Allogeneic CAR-T Cells. Front. Oncol. 2022, 12, 944248. [Google Scholar] [CrossRef]
- Kadereit, S.; Mohammad, S.F.; Miller, R.E.; Woods, K.D.; Listrom, C.D.; McKinnon, K.; Alali, A.; Bos, L.S.; Iacobucci, M.L.; Sramkoski, M.R.; et al. Reduced NFAT1 Protein Expression in Human Umbilical Cord Blood T Lymphocytes. Blood 1999, 94, 3101–3107. [Google Scholar] [CrossRef]
- Themeli, M.; Kloss, C.C.; Ciriello, G.; Fedorov, V.D.; Perna, F.; Gonen, M.; Sadelain, M. Generation of Tumor-Targeted Human T Lymphocytes from Induced Pluripotent Stem Cells for Cancer Therapy. Nat. Biotechnol. 2013, 31, 928–933. [Google Scholar] [CrossRef]
- Themeli, M.; Rivière, I.; Sadelain, M. New Cell Sources for T Cell Engineering and Adoptive Immunotherapy. Cell Stem Cell 2015, 16, 357–366. [Google Scholar] [CrossRef]
- Ueda, T.; Kaneko, S. In Vitro Differentiation of T Cell: From CAR-Modified T-IPSC. Methods Mol. Biol. 2019, 2048, 85–91. [Google Scholar] [CrossRef]
- Wang, B.; Iriguchi, S.; Waseda, M.; Ueda, N.; Ueda, T.; Xu, H.; Minagawa, A.; Ishikawa, A.; Yano, H.; Ishi, T.; et al. Generation of Hypoimmunogenic T Cells from Genetically Engineered Allogeneic Human Induced Pluripotent Stem Cells. Nat. Biomed. Eng. 2021, 5, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Mahdizadeh, H.; Šarić, T.; Kim, J.; Harati, J.; Shahsavarani, H.; Greber, B.; Moore, J.B. Research and Therapy with Induced Pluripotent Stem Cells (IPSCs): Social, Legal, and Ethical Considerations. Stem Cell Res. Ther. 2019, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Iriguchi, S.; Yasui, Y.; Kawai, Y.; Arima, S.; Kunitomo, M.; Sato, T.; Ueda, T.; Minagawa, A.; Mishima, Y.; Yanagawa, N.; et al. A Clinically Applicable and Scalable Method to Regenerate T-Cells from IPSCs for off-the-Shelf T-Cell Immunotherapy. Nat. Commun. 2021, 12, 430. [Google Scholar] [CrossRef]
- Wood, K.J.; Goto, R. Mechanisms of Rejection: Current Perspectives. Transplantation 2012, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alhaj Hussen, K.; Michonneau, D.; Biajoux, V.; Keita, S.; Dubouchet, L.; Nelson, E.; Setterblad, N.; Le Buanec, H.; Bouaziz, J.-D.; Guimiot, F.; et al. CD4+CD8+ T-Lymphocytes in Xenogeneic and Human Graft-versus-Host Disease. Front. Immunol. 2020, 11, 579776. [Google Scholar] [CrossRef] [PubMed]
- Ringdén, O.; Karlsson, H.; Olsson, R.; Omazic, B.; Uhlin, M. The Allogeneic Graft-versus-Cancer Effect. Br. J. Haematol. 2009, 147, 614–633. [Google Scholar] [CrossRef]
- Champlin, R.E.; Passweg, J.R.; Zhang, M.J.; Rowlings, P.A.; Pelz, C.J.; Atkinson, K.A.; Barrett, A.J.; Cahn, J.Y.; Drobyski, W.R.; Gale, R.P.; et al. T-Cell Depletion of Bone Marrow Transplants for Leukemia from Donors Other than HLA-Identical Siblings: Advantage of T-Cell Antibodies with Narrow Specificities. Blood 2000, 95, 3996–4003. [Google Scholar]
- Abdelhakim, H.; Abdel-Azim, H.; Saad, A. Role of Aβ T Cell Depletion in Prevention of Graft versus Host Disease. Biomedicines 2017, 5, 35. [Google Scholar] [CrossRef]
- Biernacki, M.A.; Sheth, V.S.; Bleakley, M. T Cell Optimization for Graft-versus-Leukemia Responses. JCI Insight 2020, 5, e134939. [Google Scholar] [CrossRef] [PubMed]
- Rådestad, E.; Wikell, H.; Engström, M.; Watz, E.; Sundberg, B.; Thunberg, S.; Uzunel, M.; Mattsson, J.; Uhlin, M. Alpha/Beta T-Cell Depleted Grafts as an Immunological Booster to Treat Graft Failure after Hematopoietic Stem Cell Transplantation with HLA-Matched Related and Unrelated Donors. J. Immunol. Res. 2014, 2014, 578741. [Google Scholar] [CrossRef] [PubMed]
- Korngold, R.; Sprent, J. Lethal Graft-versus-Host Disease after Bone Marrow Transplantation across Minor Histocompatibility Barriers in Mice. Prevention by Removing Mature T Cells from Marrow. J. Exp. Med. 1978, 148, 1687–1698. [Google Scholar] [CrossRef] [PubMed]
- Maraninchi, D.; Gluckman, E.; Blaise, D.; Guyotat, D.; Rio, B.; Pico, J.L.; Leblond, V.; Michallet, M.; Dreyfus, F.; Ifrah, N. Impact of T-Cell Depletion on Outcome of Allogeneic Bone-Marrow Transplantation for Standard-Risk Leukaemias. Lancet Lond. Engl. 1987, 2, 175–178. [Google Scholar] [CrossRef]
- Ho, V.T.; Soiffer, R.J. The History and Future of T-Cell Depletion as Graft-versus-Host Disease Prophylaxis for Allogeneic Hematopoietic Stem Cell Transplantation. Blood 2001, 98, 3192–3204. [Google Scholar] [CrossRef] [PubMed]
- Anwer, F.; Shaukat, A.-A.; Zahid, U.; Husnain, M.; McBride, A.; Persky, D.; Lim, M.; Hasan, N.; Riaz, I.B. Donor Origin CAR T Cells: Graft versus Malignancy Effect without GVHD, a Systematic Review. Immunotherapy 2017, 9, 123–130. [Google Scholar] [CrossRef]
- Felix, N.J.; Allen, P.M. Specificity of T-Cell Alloreactivity. Nat. Rev. Immunol. 2007, 7, 942–953. [Google Scholar] [CrossRef]
- Mangum, D.S.; Caywood, E. A Clinician’s Guide to HLA Matching in Allogeneic Hematopoietic Stem Cell Transplant. Hum. Immunol. 2022, 83, 687–694. [Google Scholar] [CrossRef]
- Yeung, M.Y.; Coates, P.T.; Li, P.K.-T. Kidney Organ Allocation System: How to Be Fair. Semin. Nephrol. 2022, 42, 151274. [Google Scholar] [CrossRef]
- Brudno, J.N.; Somerville, R.P.T.; Shi, V.; Rose, J.J.; Halverson, D.C.; Fowler, D.H.; Gea-Banacloche, J.C.; Pavletic, S.Z.; Hickstein, D.D.; Lu, T.L.; et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J. Clin. Oncol. 2016, 34, 1112–1121. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Dudley, M.E.; Carpenter, R.O.; Kassim, S.H.; Rose, J.J.; Telford, W.G.; Hakim, F.T.; Halverson, D.C.; Fowler, D.H.; Hardy, N.M.; et al. Donor-Derived CD19-Targeted T Cells Cause Regression of Malignancy Persisting after Allogeneic Hematopoietic Stem Cell Transplantation. Blood 2013, 122, 4129–4139. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.R.Y.; Micklethwaite, K.P.; Savoldo, B.; Ramos, C.A.; Lam, S.; Ku, S.; Diouf, O.; Liu, E.; Barrett, A.J.; Ito, S.; et al. Infusion of Donor-Derived CD19-Redirected Virus-Specific T Cells for B-Cell Malignancies Relapsed after Allogeneic Stem Cell Transplant: A Phase 1 Study. Blood 2013, 122, 2965–2973. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Cao, Y.; Wang, L.; Sun, R.; Cheng, L.; He, X.; Xiao, X.; Jiang, Y.; Li, Q.; Zhang, H.; et al. HLA-Matched and HLA-Haploidentical Allogeneic CD19-Directed Chimeric Antigen Receptor T-Cell Infusions Are Feasible in Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia before Hematopoietic Stem Cell Transplantation. Leukemia 2020, 34, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Melenhorst, J.J.; Leen, A.M.; Bollard, C.M.; Quigley, M.F.; Price, D.A.; Rooney, C.M.; Brenner, M.K.; Barrett, A.J.; Heslop, H.E. Allogeneic Virus-Specific T Cells with HLA Alloreactivity Do Not Produce GVHD in Human Subjects. Blood 2010, 116, 4700–4702. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, R.J.; Prockop, S.; Hasan, A.N.; Koehne, G.; Doubrovina, E. Virus-Specific T-Cell Banks for “off the Shelf” Adoptive Therapy of Refractory Infections. Bone Marrow Transplant. 2016, 51, 1163–1172. [Google Scholar] [CrossRef]
- Savoldo, B.; Rooney, C.M.; Di Stasi, A.; Abken, H.; Hombach, A.; Foster, A.E.; Zhang, L.; Heslop, H.E.; Brenner, M.K.; Dotti, G. Epstein Barr Virus Specific Cytotoxic T Lymphocytes Expressing the Anti-CD30 Artificial Chimeric T-Cell Receptor for Immunotherapy of Hodgkin Disease. Blood 2007, 110, 2620–2630. [Google Scholar] [CrossRef] [PubMed]
- Curran, K.J.; Sauter, C.S.; Kernan, N.A.; Prockop, S.E.; Boulad, F.; Perales, M.; Giralt, S.A.; Riviere, I.; Wang, X.; Boelens, J.-J.; et al. Durable Remission Following “Off-the-Shelf” Chimeric Antigen Receptor (CAR) T-Cells in Patients with Relapse/Refractory (R/R) B-Cell Malignancies. Biol. Blood Marrow Transplant. 2020, 26, S89. [Google Scholar] [CrossRef]
- Wang, X.; Diamond, D.J.; Forman, S.J.; Nakamura, R. Development of CMV-CD19 Bi-Specific CAR T Cells with Post-Infusion in Vivo Boost Using an Anti-CMV Vaccine. Int. J. Hematol. 2021, 114, 544–553. [Google Scholar] [CrossRef]
- Young, K.J.; Kay, L.S.; Phillips, M.J.; Zhang, L. Antitumor Activity Mediated by Double-Negative T Cells. Cancer Res. 2003, 63, 8014–8021. [Google Scholar]
- Lee, J.-B.; Vasic, D.; Kang, H.; Fang, K.K.-L.; Zhang, L. State-of-Art of Cellular Therapy for Acute Leukemia. Int. J. Mol. Sci. 2021, 22, 4590. [Google Scholar] [CrossRef]
- Chen, B.; Lee, J.B.; Kang, H.; Minden, M.D.; Zhang, L. Targeting Chemotherapy-Resistant Leukemia by Combining DNT Cellular Therapy with Conventional Chemotherapy. J. Exp. Clin. Cancer Res. CR 2018, 37, 88. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Kang, H.; Fang, L.; D’Souza, C.; Adeyi, O.; Zhang, L. Developing Allogeneic Double-Negative T Cells as a Novel Off-the-Shelf Adoptive Cellular Therapy for Cancer. Clin. Cancer Res. 2019, 25, 2241–2253. [Google Scholar] [CrossRef]
- Lee, J.; Minden, M.D.; Chen, W.C.; Streck, E.; Chen, B.; Kang, H.; Arruda, A.; Ly, D.; Der, S.D.; Kang, S.; et al. Allogeneic Human Double Negative T Cells as a Novel Immunotherapy for Acute Myeloid Leukemia and Its Underlying Mechanisms. Clin. Cancer Res. 2018, 24, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Vasic, D.; Lee, J.B.; Leung, Y.; Khatri, I.; Na, Y.; Abate-Daga, D.; Zhang, L. Allogeneic Double-Negative CAR-T Cells Inhibit Tumor Growth without off-Tumor Toxicities. Sci. Immunol. 2022, 7, eabl3642. [Google Scholar] [CrossRef]
- Baolin, T.; Lee, J.; Cheng, S.; Yao, W.; Wang, D.; Tu, M.; Xiang, Z.; Geng, L.; Wang, M.; Qiang, P.; et al. Safety and Efficacy of Ex Vivo Expanded Healthy Donor-Derived Double Negative T Cells for the Treatment of AML Relapsed after Allogeneic Stem Cell Transplantation: A First in-Human Phase I/IIa Clinical Trial. Blood 2020, 136, 1–2. [Google Scholar] [CrossRef]
- Kang, H.; Lee, J.B.; Khatri, I.; Na, Y.; D’Souza, C.; Arruda, A.; Minden, M.D.; Zhang, L. Enhancing Therapeutic Efficacy of Double Negative T Cells against Acute Myeloid Leukemia Using Idelalisib. Cancers 2021, 13, 5039. [Google Scholar] [CrossRef]
- Breman, E.; Demoulin, B.; Agaugué, S.; Mauën, S.; Michaux, A.; Springuel, L.; Houssa, J.; Huberty, F.; Jacques-Hespel, C.; Marchand, C.; et al. Overcoming Target Driven Fratricide for T Cell Therapy. Front. Immunol. 2018, 9, 2940. [Google Scholar] [CrossRef]
- Leveson-Gower, D.B.; Olson, J.A.; Sega, E.I.; Luong, R.H.; Baker, J.; Zeiser, R.; Negrin, R.S. Low Doses of Natural Killer T Cells Provide Protection from Acute Graft-versus-Host Disease via an IL-4-Dependent Mechanism. Blood 2011, 117, 3220–3229. [Google Scholar] [CrossRef]
- Schneidawind, D.; Baker, J.; Pierini, A.; Buechele, C.; Luong, R.H.; Meyer, E.H.; Negrin, R.S. Third-Party CD4+ Invariant Natural Killer T Cells Protect from Murine GVHD Lethality. Blood 2015, 125, 3491–3500. [Google Scholar] [CrossRef]
- Yang, J.; Gao, L.; Liu, Y.; Ren, Y.; Xie, R.; Fan, H.; Qian, K. Adoptive Therapy by Transfusing Expanded Donor Murine Natural Killer T Cells Can Suppress Acute Graft-versus-Host Disease in Allogeneic Bone Marrow Transplantation. Transfusion 2010, 50, 407–417. [Google Scholar] [CrossRef]
- Bendelac, A.; Savage, P.B.; Teyton, L. The Biology of NKT Cells. Annu. Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef]
- Salio, M.; Silk, J.D.; Jones, E.Y.; Cerundolo, V. Biology of CD1- and MR1-Restricted T Cells. Annu. Rev. Immunol. 2014, 32, 323–366. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, A.; Caputo, V.S.; Holubova, M.; Baxan, N.; Dubois, O.; Chaudhry, M.S.; Xiao, X.; Goudevenou, K.; Pitcher, D.S.; Petevi, K.; et al. Enhanced Anti-Lymphoma Activity of CAR19-INKT Cells Underpinned by Dual CD19 and CD1d Targeting. Cancer Cell 2018, 34, 596–610.e11. [Google Scholar] [CrossRef] [PubMed]
- Cappuzzello, E.; Vigolo, E.; D’Accardio, G.; Astori, G.; Rosato, A.; Sommaggio, R. How Can Cytokine-Induced Killer Cells Overcome CAR-T Cell Limits. Front. Immunol. 2023, 14, 1229540. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Luo, F.; Chu, Y. Strategies for Overcoming Bottlenecks in Allogeneic CAR-T Cell Therapy. Front. Immunol. 2023, 14, 1199145. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Baker, J.; Beilhack, A.; Zeiser, R.; Olson, J.A.; Sega, E.I.; Karimi, M.; Negrin, R.S. In Vivo Trafficking and Survival of Cytokine-Induced Killer Cells Resulting in Minimal GVHD with Retention of Antitumor Activity. Blood 2008, 112, 2563–2574. [Google Scholar] [CrossRef]
- Sangiolo, D.; Martinuzzi, E.; Todorovic, M.; Vitaggio, K.; Vallario, A.; Jordaney, N.; Carnevale-Schianca, F.; Capaldi, A.; Geuna, M.; Casorzo, L.; et al. Alloreactivity and Anti-Tumor Activity Segregate within Two Distinct Subsets of Cytokine-Induced Killer (CIK) Cells: Implications for Their Infusion across Major HLA Barriers. Int. Immunol. 2008, 20, 841–848. [Google Scholar] [CrossRef]
- Wu, X.; Schmidt-Wolf, I.G.H. An Alternative Source for Allogeneic CAR T Cells With a High Safety Profile. Front. Immunol. 2022, 13, 913123. [Google Scholar] [CrossRef]
- Rettinger, E.; Huenecke, S.; Bonig, H.; Merker, M.; Jarisch, A.; Soerensen, J.; Willasch, A.; Bug, G.; Schulz, A.; Klingebiel, T.; et al. Interleukin-15-Activated Cytokine-Induced Killer Cells May Sustain Remission in Leukemia Patients after Allogeneic Stem Cell Transplantation: Feasibility, Safety and First Insights on Efficacy. Haematologica 2016, 101, e153–e156. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Liu, C.; Jiao, X.; Liu, D.; Du, W.; He, Y.; Zhang, Z.; Wu, X.; Wang, J.; et al. Human Leukocyte Antigen-Haploidentical Donor-Derived Cytokine-Induced Killer Cells Are Safe and Prolong the Survival of Patients with Advanced Non-Small Cell Lung Cancer. Oncol. Lett. 2014, 8, 2727–2733. [Google Scholar] [CrossRef]
- Introna, M.; Lussana, F.; Algarotti, A.; Gotti, E.; Valgarsdottir, R.; Micò, C.; Grassi, A.; Pavoni, C.; Ferrari, M.L.; Delaini, F.; et al. Phase II Study of Sequential Infusion of DLI and Cytokine Induced Killer Cells for Patients Relapsed after AlloHSCT. Biol. Blood Marrow Transplant. 2017, 23, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Magnani, C.F.; Gaipa, G.; Lussana, F.; Belotti, D.; Gritti, G.; Napolitano, S.; Matera, G.; Cabiati, B.; Buracchi, C.; Borleri, G.; et al. Sleeping Beauty-Engineered CAR T Cells Achieve Antileukemic Activity without Severe Toxicities. J. Clin. Investig. 2020, 130, 6021–6033. [Google Scholar] [CrossRef] [PubMed]
- Legoux, F.; Salou, M.; Lantz, O. MAIT Cell Development and Functions: The Microbial Connection. Immunity 2020, 53, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Treiner, E.; Duban, L.; Bahram, S.; Radosavljevic, M.; Wanner, V.; Tilloy, F.; Affaticati, P.; Gilfillan, S.; Lantz, O. Selection of Evolutionarily Conserved Mucosal-Associated Invariant T Cells by MR1. Nature 2003, 422, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Treiner, E.; Duban, L.; Guerri, L.; Laude, H.; Toly, C.; Premel, V.; Devys, A.; Moura, I.C.; Tilloy, F.; et al. Stepwise Development of MAIT Cells in Mouse and Human. PLoS Biol. 2009, 7, e54. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, H.; D’Souza, C.; Sun, S.; Kostenko, L.; Eckle, S.B.G.; Meehan, B.S.; Jackson, D.C.; Strugnell, R.A.; Cao, H.; et al. Mucosal-Associated Invariant T-Cell Activation and Accumulation after in Vivo Infection Depends on Microbial Riboflavin Synthesis and Co-Stimulatory Signals. Mucosal Immunol. 2017, 10, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Tourret, M.; Talvard-Balland, N.; Lambert, M.; Ben Youssef, G.; Chevalier, M.F.; Bohineust, A.; Yvorra, T.; Morin, F.; Azarnoush, S.; Lantz, O.; et al. Human MAIT Cells Are Devoid of Alloreactive Potential: Prompting Their Use as Universal Cells for Adoptive Immune Therapy. J. Immunother. Cancer 2021, 9, e003123. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-R.; Zhou, K.; Wilson, M.; Kramer, A.; Zhu, Y.; Dawson, N.; Yang, L. Mucosal-Associated Invariant T Cells for Cancer Immunotherapy. Mol. Ther. 2023, 31, 631–646. [Google Scholar] [CrossRef]
- Bohineust, A.; Tourret, M.; Derivry, L.; Caillat-Zucman, S. Mucosal-Associated Invariant T (MAIT) Cells, a New Source of Universal Immune Cells for Chimeric Antigen Receptor (CAR)-Cell Therapy. Bull. Cancer 2021, 108, S92–S95. [Google Scholar] [CrossRef]
- Li, Y.-R.; Brown, J.; Yu, Y.; Lee, D.; Zhou, K.; Dunn, Z.S.; Hon, R.; Wilson, M.; Kramer, A.; Zhu, Y.; et al. Targeting Immunosuppressive Tumor-Associated Macrophages Using Innate T Cells for Enhanced Antitumor Reactivity. Cancers 2022, 14, 2749. [Google Scholar] [CrossRef]
- Kabelitz, D.; Serrano, R.; Kouakanou, L.; Peters, C.; Kalyan, S. Cancer Immunotherapy with Γδ T Cells: Many Paths Ahead of Us. Cell. Mol. Immunol. 2020, 17, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Mensurado, S.; Blanco-Domínguez, R.; Silva-Santos, B. The Emerging Roles of Γδ T Cells in Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2023, 20, 178–191. [Google Scholar] [CrossRef]
- Jhita, N.; Raikar, S.S. Allogeneic Gamma Delta T Cells as Adoptive Cellular Therapy for Hematologic Malignancies. Explor. Immunol. 2022, 2, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Saura-Esteller, J.; de Jong, M.; King, L.A.; Ensing, E.; Winograd, B.; de Gruijl, T.D.; Parren, P.W.H.I.; van der Vliet, H.J. Gamma Delta T-Cell Based Cancer Immunotherapy: Past-Present-Future. Front. Immunol. 2022, 13, 915837. [Google Scholar] [CrossRef]
- Rozenbaum, M.; Meir, A.; Aharony, Y.; Itzhaki, O.; Schachter, J.; Bank, I.; Jacoby, E.; Besser, M.J. Gamma-Delta CAR-T Cells Show CAR-Directed and Independent Activity Against Leukemia. Front. Immunol. 2020, 11, 1347. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, K.P.; Barca, T.; Azameera, A.; Makkouk, A.; Romero, J.M.; Bai, L.; Brodey, M.M.; Kennedy-Wilde, J.; Shao, H.; Papaioannou, S.; et al. Allogeneic CD20-Targeted Γδ T Cells Exhibit Innate and Adaptive Antitumor Activities in Preclinical B-Cell Lymphoma Models. Clin. Transl. Immunol. 2022, 11, e1373. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Feng, Y.; Zhou, Z. A Close Look at Current Γδ T-Cell Immunotherapy. Front. Immunol. 2023, 14, 1140623. [Google Scholar] [CrossRef]
- Xu, Y.; Xiang, Z.; Alnaggar, M.; Kouakanou, L.; Li, J.; He, J.; Yang, J.; Hu, Y.; Chen, Y.; Lin, L.; et al. Allogeneic Vγ9Vδ2 T-Cell Immunotherapy Exhibits Promising Clinical Safety and Prolongs the Survival of Patients with Late-Stage Lung or Liver Cancer. Cell. Mol. Immunol. 2021, 18, 427–439. [Google Scholar] [CrossRef]
- Kakimi, K.; Matsushita, H.; Murakawa, T.; Nakajima, J. Γδ T Cell Therapy for the Treatment of Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2014, 3, 23. [Google Scholar]
- López-Cantillo, G.; Urueña, C.; Camacho, B.A.; Ramírez-Segura, C. CAR-T Cell Performance: How to Improve Their Persistence? Front. Immunol. 2022, 13, 878209. [Google Scholar] [CrossRef]
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. “Off-the-Shelf” Allogeneic CAR T Cells: Development and Challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Duygu, B.; Olieslagers, T.I.; Groeneweg, M.; Voorter, C.E.M.; Wieten, L. HLA Class I Molecules as Immune Checkpoints for NK Cell Alloreactivity and Anti-Viral Immunity in Kidney Transplantation. Front. Immunol. 2021, 12, 680480. [Google Scholar] [CrossRef] [PubMed]
- Jasinski-Bergner, S.; Eckstein, M.; Taubert, H.; Wach, S.; Fiebig, C.; Strick, R.; Hartmann, A.; Seliger, B. The Human Leukocyte Antigen G as an Immune Escape Mechanism and Novel Therapeutic Target in Urological Tumors. Front. Immunol. 2022, 13, 811200. [Google Scholar] [CrossRef] [PubMed]
- Gornalusse, G.G.; Hirata, R.K.; Funk, S.E.; Riolobos, L.; Lopes, V.S.; Manske, G.; Prunkard, D.; Colunga, A.G.; Hanafi, L.-A.; Clegg, D.O.; et al. HLA-E-Expressing Pluripotent Stem Cells Escape Allogeneic Responses and Lysis by NK Cells. Nat. Biotechnol. 2017, 35, 765–772. [Google Scholar] [CrossRef] [PubMed]
- van Duijn, A.; Van der Burg, S.H.; Scheeren, F.A. CD47/SIRPα Axis: Bridging Innate and Adaptive Immunity. J. Immunother. Cancer 2022, 10, e004589. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Su, H.; Shen, X.; Du, J.; Zhang, X.; Zhao, Y. The Immunological Function of CD52 and Its Targeting in Organ Transplantation. Inflamm. Res. 2017, 66, 571–578. [Google Scholar] [CrossRef]
- Hu, X.; Manner, K.; DeJesus, R.; White, K.; Gattis, C.; Ngo, P.; Bandoro, C.; Tham, E.; Chu, E.Y.; Young, C.; et al. Hypoimmune Anti-CD19 Chimeric Antigen Receptor T Cells Provide Lasting Tumor Control in Fully Immunocompetent Allogeneic Humanized Mice. Nat. Commun. 2023, 14, 2020. [Google Scholar] [CrossRef]
- Domagała, A.; Kurpisz, M. CD52 Antigen—A Review. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2001, 7, 325–331. [Google Scholar]
- Weaver, T.A.; Kirk, A.D. Alemtuzumab. Transplantation 2007, 84, 1545. [Google Scholar] [CrossRef]
- Zent, C.S.; Secreto, C.R.; LaPlant, B.R.; Bone, N.D.; Call, T.G.; Shanafelt, T.D.; Jelinek, D.F.; Tschumper, R.C.; Kay, N.E. Direct and Complement Dependent Cytotoxicity in CLL Cells from Patients with High-Risk Early-Intermediate Stage Chronic Lymphocytic Leukemia (CLL) Treated with Alemtuzumab and Rituximab. Leuk. Res. 2008, 32, 1849–1856. [Google Scholar] [CrossRef]
- Golay, J.; Manganini, M.; Rambaldi, A.; Introna, M. Effect of Alemtuzumab on Neoplastic B Cells. Haematologica 2004, 89, 1476–1483. [Google Scholar] [PubMed]
- Panowski, S.H.; Srinivasan, S.; Tan, N.; Tacheva-Grigorova, S.K.; Smith, B.; Mak, Y.S.L.; Ning, H.; Villanueva, J.; Wijewarnasuriya, D.; Lang, S.; et al. Preclinical Development and Evaluation of Allogeneic CAR T Cells Targeting CD70 for the Treatment of Renal Cell Carcinoma. Cancer Res. 2022, 82, 2610–2624. [Google Scholar] [CrossRef]
- Lauron, E.J.; Zhang, K.; Sanchez, J.; Ramanathan, V.; Nguyen, D.; Nguyen, G.; Sasu, B.; Sommer, C. 279 Preclinical Evaluation of Allogeneic CD19 CAR T Cells Expressing an Anti-Rejection CD70 CAR. J. Immunother. Cancer 2023, 11. [Google Scholar] [CrossRef]
- Holling, T.M.; van der Stoep, N.; Quinten, E.; van den Elsen, P.J. Activated Human T Cells Accomplish MHC Class II Expression through T Cell-Specific Occupation of Class II Transactivator Promoter III. J. Immunol. 2002, 168, 763–770. [Google Scholar] [CrossRef]
- Ali, J.M.; Bolton, E.M.; Bradley, J.A.; Pettigrew, G.J. Allorecognition Pathways in Transplant Rejection and Tolerance. Transplantation 2013, 96, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Ramia, E.; Chiaravalli, A.M.; Bou Nasser Eddine, F.; Tedeschi, A.; Sessa, F.; Accolla, R.S.; Forlani, G. CIITA-Related Block of HLA Class II Expression, Upregulation of HLA Class I, and Heterogeneous Expression of Immune Checkpoints in Hepatocarcinomas: Implications for New Therapeutic Approaches. Oncoimmunology 2018, 8, 1548243. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, L.P.; Chandrasegaran, S. Functional Domains in Fok I Restriction Endonuclease. Proc. Natl. Acad. Sci. USA 1992, 89, 4275–4279. [Google Scholar] [CrossRef]
- Perez, E.E.; Wang, J.; Miller, J.C.; Jouvenot, Y.; Kim, K.A.; Liu, O.; Wang, N.; Lee, G.; Bartsevich, V.V.; Lee, Y.-L.; et al. Establishment of HIV-1 Resistance in CD4+ T Cells by Genome Editing Using Zinc-Finger Nucleases. Nat. Biotechnol. 2008, 26, 808–816. [Google Scholar] [CrossRef]
- Urnov, F.D.; Rebar, E.J.; Holmes, M.C.; Zhang, H.S.; Gregory, P.D. Genome Editing with Engineered Zinc Finger Nucleases. Nat. Rev. Genet. 2010, 11, 636–646. [Google Scholar] [CrossRef]
- Chattong, S.; Chaikomon, K.; Chaiya, T.; Tangkosakul, T.; Palavutitotai, N.; Anusornvongchai, T.; Manotham, K. Efficient ZFN-Mediated Stop Codon Integration into the CCR5 Locus in Hematopoietic Stem Cells: A Possible Source for Intrabone Marrow Cell Transplantation. AIDS Res. Hum. Retroviruses 2018, 34, 575–579. [Google Scholar] [CrossRef]
- Brown, C.E.; Rodriguez, A.; Palmer, J.; Ostberg, J.R.; Naranjo, A.; Wagner, J.R.; Aguilar, B.; Starr, R.; Weng, L.; Synold, T.W.; et al. Off-the-Shelf, Steroid-Resistant, IL13Rα2-Specific CAR T Cells for Treatment of Glioblastoma. Neuro-Oncol. 2022, 24, 1318–1330. [Google Scholar] [CrossRef]
- Bogdanove, A.J.; Voytas, D.F. TAL Effectors: Customizable Proteins for DNA Targeting. Science 2011, 333, 1843–1846. [Google Scholar] [CrossRef] [PubMed]
- Beurdeley, M.; Bietz, F.; Li, J.; Thomas, S.; Stoddard, T.; Juillerat, A.; Zhang, F.; Voytas, D.F.; Duchateau, P.; Silva, G.H. Compact Designer TALENs for Efficient Genome Engineering. Nat. Commun. 2013, 4, 1762. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; DeAngelo, D.J.; Pemmaraju, N.; Dinner, S.; Gill, S.; Olin, R.L.; Wang, E.S.; Stark, E.; Korngold, A.; Figliola, C.; et al. AMELI-01: A Phase I Trial of UCART123v1.2, an Anti-CD123 Allogeneic CAR-T Cell Product, in Adult Patients with Relapsed or Refractory (R/R) CD123+ Acute Myeloid Leukemia (AML). Mol. Ther. 2023, 31, 51. [Google Scholar] [CrossRef]
- Aranda-Orgilles, B.; Chion-Sotinel, I.; Skinner, J.; Grudman, S.; Mumford, B.; Dixon, C.; Postigo Fernandez, J.; Erler, P.; Duchateau, P.; Gouble, A.; et al. Preclinical Evidence of an Allogeneic Dual CD20xCD22 CAR to Target a Broad Spectrum of Patients with B-Cell Malignancies. Cancer Immunol. Res. 2023, 11, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Korst, C.L.B.M.; Bruins, W.S.C.; Cosovic, M.; Verkleij, C.P.M.; Twickler, I.; Le Clerre, D.; Chion-Sotinel, I.; Zweegman, S.; Galetto, R.; Mutis, T.; et al. Preclinical Activity of Allogeneic CS1-Specific CAR T-Cells (UCARTCS1) in Multiple Myeloma. Blood 2022, 140, 4215–4216. [Google Scholar] [CrossRef]
- Zaslavskiy, M.; Bertonati, C.; Duchateau, P.; Duclert, A.; Silva, G.H. Efficient Design of Meganucleases Using a Machine Learning Approach. BMC Bioinform. 2014, 15, 191. [Google Scholar] [CrossRef]
- Werther, R.; Hallinan, J.P.; Lambert, A.R.; Havens, K.; Pogson, M.; Jarjour, J.; Galizi, R.; Windbichler, N.; Crisanti, A.; Nolan, T.; et al. Crystallographic Analyses Illustrate Significant Plasticity and Efficient Recoding of Meganuclease Target Specificity. Nucleic Acids Res. 2017, 45, 8621–8634. [Google Scholar] [CrossRef]
- MacLeod, D.T.; Antony, J.; Martin, A.J.; Moser, R.J.; Hekele, A.; Wetzel, K.J.; Brown, A.E.; Triggiano, M.A.; Hux, J.A.; Pham, C.D.; et al. Integration of a CD19 CAR into the TCR Alpha Chain Locus Streamlines Production of Allogeneic Gene-Edited CAR T Cells. Mol. Ther. 2017, 25, 949–961. [Google Scholar] [CrossRef]
- Boissel, S.; Jarjour, J.; Astrakhan, A.; Adey, A.; Gouble, A.; Duchateau, P.; Shendure, J.; Stoddard, B.L.; Certo, M.T.; Baker, D.; et al. MegaTALs: A Rare-Cleaving Nuclease Architecture for Therapeutic Genome Engineering. Nucleic Acids Res. 2014, 42, 2591–2601. [Google Scholar] [CrossRef]
- Poirot, L.; Philip, B.; Schiffer-Mannioui, C.; Le Clerre, D.; Chion-Sotinel, I.; Derniame, S.; Potrel, P.; Bas, C.; Lemaire, L.; Galetto, R.; et al. Multiplex Genome-Edited T-Cell Manufacturing Platform for “Off-the-Shelf” Adoptive T-Cell Immunotherapies. Cancer Res. 2015, 75, 3853–3864. [Google Scholar] [CrossRef] [PubMed]
- Hussain, W.; Mahmood, T.; Hussain, J.; Ali, N.; Shah, T.; Qayyum, S.; Khan, I. CRISPR/Cas System: A Game Changing Genome Editing Technology, to Treat Human Genetic Diseases. Gene 2019, 685, 70–75. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-Guided Human Genome Engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Georgiadis, C.; Preece, R.; Nickolay, L.; Etuk, A.; Petrova, A.; Ladon, D.; Danyi, A.; Humphryes-Kirilov, N.; Ajetunmobi, A.; Kim, D.; et al. Long Terminal Repeat CRISPR-CAR-Coupled “Universal” T Cells Mediate Potent Anti-Leukemic Effects. Mol. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, X.; Fang, C.; Jiang, S.; June, C.H.; Zhao, Y. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clin. Cancer Res. 2017, 23, 2255–2266. [Google Scholar] [CrossRef] [PubMed]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-Fidelity CRISPR–Cas9 Nucleases with No Detectable Genome-Wide off-Target Effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Slaymaker, I.M.; Gao, L.; Zetsche, B.; Scott, D.A.; Yan, W.X.; Zhang, F. Rationally Engineered Cas9 Nucleases with Improved Specificity. Science 2016, 351, 84–88. [Google Scholar] [CrossRef]
- Dimitri, A.; Herbst, F.; Fraietta, J.A. Engineering the Next-Generation of CAR T-Cells with CRISPR-Cas9 Gene Editing. Mol. Cancer 2022, 21, 78. [Google Scholar] [CrossRef]
- Li, D.; Zhou, H.; Zeng, X. Battling CRISPR-Cas9 off-Target Genome Editing. Cell Biol. Toxicol. 2019, 35, 403–406. [Google Scholar] [CrossRef]
- Wen, W.; Zhang, X.-B. CRISPR–Cas9 Gene Editing Induced Complex on-Target Outcomes in Human Cells. Exp. Hematol. 2022, 110, 13–19. [Google Scholar] [CrossRef]
- Tsuchida, C.A.; Brandes, N.; Bueno, R.; Trinidad, M.; Mazumder, T.; Yu, B.; Hwang, B.; Chang, C.; Liu, J.; Sun, Y.; et al. Mitigation of Chromosome Loss in Clinical CRISPR-Cas9-Engineered T Cells. Cell 2023, 186, 4567–4582.e20. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Li, J.; Liu, Y.; Wu, Q. Precise and Predictable CRISPR Chromosomal Rearrangements Reveal Principles of Cas9-Mediated Nucleotide Insertion. Mol. Cell 2018, 71, 498–509.e4. [Google Scholar] [CrossRef] [PubMed]
- Webber, B.R.; Lonetree, C.; Kluesner, M.G.; Johnson, M.J.; Pomeroy, E.J.; Diers, M.D.; Lahr, W.S.; Draper, G.M.; Slipek, N.J.; Smeester, B.A.; et al. Highly Efficient Multiplex Human T Cell Engineering without Double-Strand Breaks Using Cas9 Base Editors. Nat. Commun. 2019, 10, 5222. [Google Scholar] [CrossRef] [PubMed]
- Nishimasu, H.; Nureki, O. Structures and Mechanisms of CRISPR RNA-Guided Effector Nucleases. Curr. Opin. Struct. Biol. 2017, 43, 68–78. [Google Scholar] [CrossRef]
- Shmakov, S.; Smargon, A.; Scott, D.; Cox, D.; Pyzocha, N.; Yan, W.; Abudayyeh, O.O.; Gootenberg, J.S.; Makarova, K.S.; Wolf, Y.I.; et al. Diversity and Evolution of Class 2 CRISPR–Cas Systems. Nat. Rev. Microbiol. 2017, 15, 169–182. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, H.; Ahn, W.-C.; Park, K.-H.; Woo, E.-J.; Lee, D.-H.; Lee, S.-G. Efficient Transcriptional Gene Repression by Type V-A CRISPR-Cpf1 from Eubacterium Eligens. ACS Synth. Biol. 2017, 6, 1273–1282. [Google Scholar] [CrossRef]
- Campa, C.C.; Weisbach, N.R.; Santinha, A.J.; Incarnato, D.; Platt, R.J. Multiplexed Genome Engineering by Cas12a and CRISPR Arrays Encoded on Single Transcripts. Nat. Methods 2019, 16, 887–893. [Google Scholar] [CrossRef]
- Jasin, M.; Rothstein, R. Repair of Strand Breaks by Homologous Recombination. Cold Spring Harb. Perspect. Biol. 2013, 5, a012740. [Google Scholar] [CrossRef]
- Schmid-Burgk, J.L.; Höning, K.; Ebert, T.S.; Hornung, V. CRISPaint Allows Modular Base-Specific Gene Tagging Using a Ligase-4-Dependent Mechanism. Nat. Commun. 2016, 7, 12338. [Google Scholar] [CrossRef]
- Suzuki, K.; Tsunekawa, Y.; Hernandez-Benitez, R.; Wu, J.; Zhu, J.; Kim, E.J.; Hatanaka, F.; Yamamoto, M.; Araoka, T.; Li, Z.; et al. In Vivo Genome Editing via CRISPR/Cas9 Mediated Homology-Independent Targeted Integration. Nature 2016, 540, 144–149. [Google Scholar] [CrossRef]
- Madison, B.B.; Patil, D.; Richter, M.; Li, X.; Tong, M.; Cranert, S.; Wang, X.; Martin, R.; Xi, H.; Tan, Y.; et al. Cas-CLOVER Is a Novel High-Fidelity Nuclease for Safe and Robust Generation of TSCM-Enriched Allogeneic CAR-T Cells. Mol. Ther. Nucleic Acids 2022, 29, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Arnould, S.; Chames, P.; Perez, C.; Lacroix, E.; Duclert, A.; Epinat, J.-C.; Stricher, F.; Petit, A.-S.; Patin, A.; Guillier, S.; et al. Engineering of Large Numbers of Highly Specific Homing Endonucleases That Induce Recombination on Novel DNA Targets. J. Mol. Biol. 2006, 355, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Glaser, V.; Flugel, C.; Kath, J.; Du, W.; Drosdek, V.; Franke, C.; Stein, M.; Pruß, A.; Schmueck-Henneresse, M.; Volk, H.-D.; et al. Combining Different CRISPR Nucleases for Simultaneous Knock-in and Base Editing Prevents Translocations in Multiplex-Edited CAR T Cells. Genome Biol. 2023, 24, 89. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable Editing of a Target Base in Genomic DNA without Double-Stranded DNA Cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome Editing with CRISPR-Cas Nucleases, Base Editors, Transposases and Prime Editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef]
- Diorio, C.; Murray, R.; Naniong, M.; Barrera, L.; Camblin, A.; Chukinas, J.; Coholan, L.; Edwards, A.; Fuller, T.; Gonzales, C.; et al. Cytosine Base Editing Enables Quadruple-Edited Allogeneic CART Cells for T-ALL. Blood 2022, 140, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, R.; Georgiadis, C.; Syed, F.; Zhan, H.; Etuk, A.; Gkazi, S.A.; Preece, R.; Ottaviano, G.; Braybrook, T.; Chu, J.; et al. Base-Edited CAR7 T Cells for Relapsed T-Cell Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2023, 389, 899–910. [Google Scholar] [CrossRef]
- Grünewald, J.; Zhou, R.; Garcia, S.P.; Iyer, S.; Lareau, C.A.; Aryee, M.J.; Joung, J.K. Transcriptome-Wide off-Target RNA Editing Induced by CRISPR-Guided DNA Base Editors. Nature 2019, 569, 433–437. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, Y.; Yan, R.; Liu, Y.; Zuo, E.; Gu, C.; Han, L.; Wei, Y.; Hu, X.; Zeng, R.; et al. Off-Target RNA Mutation Induced by DNA Base Editing and Its Elimination by Mutagenesis. Nature 2019, 571, 275–278. [Google Scholar] [CrossRef]
- Zuo, E.; Sun, Y.; Wei, W.; Yuan, T.; Ying, W.; Sun, H.; Yuan, L.; Steinmetz, L.M.; Li, Y.; Yang, H. Cytosine Base Editor Generates Substantial Off-Target Single-Nucleotide Variants in Mouse Embryos. Science 2019, 364, 289–292. [Google Scholar] [CrossRef]
- Jin, S.; Zong, Y.; Gao, Q.; Zhu, Z.; Wang, Y.; Qin, P.; Liang, C.; Wang, D.; Qiu, J.-L.; Zhang, F.; et al. Cytosine, but Not Adenine, Base Editors Induce Genome-Wide off-Target Mutations in Rice. Science 2019, 364, 292–295. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeong, Y.K.; Hur, J.K.; Kim, J.-S.; Bae, S. Adenine Base Editors Catalyze Cytosine Conversions in Human Cells. Nat. Biotechnol. 2019, 37, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.K.; Song, B.; Bae, S. Current Status and Challenges of DNA Base Editing Tools. Mol. Ther. 2020, 28, 1938–1952. [Google Scholar] [CrossRef]
- Prenen, H.; Dekervel, J.; Hendlisz, A.; Anguille, S.; Awada, A.; Cerf, E.; Lonez, C.; Breman, E.; Dheur, M.-S.; Alcantar-Orozco, E.; et al. Updated Data from AlloSHRINK Phase I First-in-Human Study Evaluating CYAD-101, an Innovative Non-Gene Edited Allogeneic CAR-T in MCRC. J. Clin. Oncol. 2021, 39, 74. [Google Scholar] [CrossRef]
- Lonez, C.; Bolsee, J.; Huberty, F.; Nguyen, T.; Jacques-Hespel, C.; Demoulin, B.; Flament, A.; Breman, E. 285 Proof-of-Concept of a Non-Gene Editing Technology Using ShRNA down-Regulation to Engineer and Optimize CAR T-Cell Functionality. J. Immunother. Cancer 2023, 11. [Google Scholar] [CrossRef]
- Wang, X.; Xue, L.; Li, S.; Fan, Q.; Liu, K.; Jin, R.; Yang, X.; Wang, T.; He, L.; Li, J. Preliminary Analyses of a Non-Gene-Editing Allogentic CAR-T in CD19+ Relapsed or Refractory Non-Hodgin’s Lymphoma. EHA Libr. 2022, 357128, S264. [Google Scholar] [CrossRef]
- Liu, Y.P.; Haasnoot, J.; ter Brake, O.; Berkhout, B.; Konstantinova, P. Inhibition of HIV-1 by Multiple SiRNAs Expressed from a Single MicroRNA Polycistron. Nucleic Acids Res. 2008, 36, 2811–2824. [Google Scholar] [CrossRef]
- Choi, J.-G.; Bharaj, P.; Abraham, S.; Ma, H.; Yi, G.; Ye, C.; Dang, Y.; Manjunath, N.; Wu, H.; Shankar, P. Multiplexing Seven MiRNA-Based ShRNAs to Suppress HIV Replication. Mol. Ther. 2015, 23, 310–320. [Google Scholar] [CrossRef]
- Wang, T.; Xie, Y.; Tan, A.; Li, S.; Xie, Z. Construction and Characterization of a Synthetic MicroRNA Cluster for Multiplex RNA Interference in Mammalian Cells. ACS Synth. Biol. 2016, 5, 1193–1200. [Google Scholar] [CrossRef]
- Du, X.; Cai, Y.; Xi, W.; Zhang, R.; Jia, L.; Yang, A.; Zhao, J.; Yan, B. Multi-target Inhibition by Four Tandem ShRNAs Embedded in Homo- or Hetero-miRNA Backbones. Mol. Med. Rep. 2018, 17, 307–314. [Google Scholar] [CrossRef]
- Rossi, M.; Steklov, M.; Huberty, F.; Nguyen, T.; Marijsse, J.; Jacques-Hespel, C.; Najm, P.; Lonez, C.; Breman, E. Efficient ShRNA-Based Knockdown of Multiple Target Genes for Cell Therapy Using a Chimeric MiRNA Cluster Platform. Mol. Ther. Nucleic Acids 2023, 34, 102038. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.; Graham, C.; Yallop, D.; Jozwik, A.; Mirci-Danicar, O.C.; Lucchini, G.; Pinner, D.; Jain, N.; Kantarjian, H.; Boissel, N.; et al. Genome-Edited, Donor-Derived Allogeneic Anti-CD19 Chimeric Antigen Receptor T Cells in Paediatric and Adult B-Cell Acute Lymphoblastic Leukaemia: Results of Two Phase 1 Studies. Lancet 2020, 396, 1885–1894. [Google Scholar] [CrossRef]
- Benjamin, R.; Jain, N.; Maus, M.V.; Boissel, N.; Graham, C.; Jozwik, A.; Yallop, D.; Konopleva, M.; Frigault, M.J.; Teshima, T.; et al. UCART19, a First-in-Class Allogeneic Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy for Adults with Relapsed or Refractory B-Cell Acute Lymphoblastic Leukaemia (CALM): A Phase 1, Dose-Escalation Trial. Lancet Haematol. 2022, 9, e833–e843. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Nath, R.; Munoz, J.; Tees, M.; Miklos, D.B.; Frank, M.J.; Malik, S.A.; Stevens, D.; Shin, C.R.; Balakumaran, A.; et al. ALPHA Study: ALLO-501 Produced Deep and Durable Responses in Patients with Relapsed/Refractory Non-Hodgkin’s Lymphoma Comparable to Autologous CAR T. Blood 2021, 138, 3878. [Google Scholar] [CrossRef]
- Lekakis, L.J.; Locke, F.L.; Tees, M.; Neelapu, S.S.; Malik, S.A.; Hamadani, M.; Frank, M.J.; Popplewell, L.L.; Abramson, J.S.; de Vos, S.; et al. ALPHA2 Study: ALLO-501A Allogeneic CAR T in LBCL, Updated Results Continue to Show Encouraging Safety and Efficacy with Consolidation Dosing. Blood 2021, 138, 649. [Google Scholar] [CrossRef]
- Mailankody, S.; Matous, J.V.; Chhabra, S.; Liedtke, M.; Sidana, S.; Oluwole, O.O.; Malik, S.; Nath, R.; Anwer, F.; Cruz, J.C.; et al. Allogeneic BCMA-Targeting CAR T Cells in Relapsed/Refractory Multiple Myeloma: Phase 1 UNIVERSAL Trial Interim Results. Nat. Med. 2023, 29, 422–429. [Google Scholar] [CrossRef]
- Srour, S.; Kotecha, R.; Curti, B.; Chahoud, J.; Drakaki, A.; Tang, L.; Goyal, L.; Prashad, S.; Szenes, V.; Norwood, K.; et al. Abstract CT011: A Phase 1 Multicenter Study (TRAVERSE) Evaluating the Safety and Efficacy of ALLO-316 Following Conditioning Regimen in Pts with Advanced or Metastatic Clear Cell Renal Cell Carcinoma (CcRCC). Cancer Res. 2023, 83, CT011. [Google Scholar] [CrossRef]
- Boissel, N.; Chevallier, P.; Curran, K.; Schiller, G.; Liu, H.; Larson, R.; Deangelo, D.J.; Mear, J.-B.; Grupp, S.; Baruchel, A.; et al. P1408: Updated Results of the Phase I BALLI-01 Trial of UCART22, an Anti-CD22 Allogeneic CAR-t Cell Product, in Patients with Relapsed or Refractory (r/r) CD22+ B-Cell Acute Lymphoblastic Leukemia (B-ALL). HemaSphere 2023, 7, e323373f. [Google Scholar] [CrossRef]
- Cellectis Cellectis to Showcase Clinical Data from AMELI-01 and Preclinical Data from UCARTCS1 at ASH 2022. Available online: https://www.cellectis.com/en/press/cellectis-to-showcase-clinical-data-from-ameli-01-and-preclinical-data-from-ucartcs1-at-ash-2022/ (accessed on 22 November 2023).
- Patel, K.K.; Bharathan, M.; Esteva, F.J.; Siegel, D.; Rossi, A.; Frattini, M.G.; Smith, J.; Brownstein, C. UCARTCS1A, an Allogeneic CAR T-Cell Therapy Targeting CS1 in Patients with Relapsed/Refractory Multiple Myeloma (RRMM): Preliminary Translational Results from a First-in-Human Phase I Trial (MELANI-01). Mol. Ther. 2021, 29, 59. [Google Scholar]
- Precision BioSciences Provides Update on Allogeneic CAR T Programs and Regulatory Path Forward. Available online: https://investor.precisionbiosciences.com/node/9926/pdf (accessed on 22 November 2023).
- Precision Biosciences Corporate Presentation 2022. Available online: https://investor.precisionbiosciences.com/static-files/717d82d7-d304-4428-ba22-1a19c965d8d5 (accessed on 22 November 2023).
- Precision BioSciences Reports Third Quarter 2021 Financial Results and Provides Business Update. Available online: https://investor.precisionbiosciences.com/node/8986/pdf (accessed on 22 November 2023).
- Caribou Biosciences Corporate Presentation. November 2023. Available online: https://investor.cariboubio.com/static-files/3168b9f6-48dd-4e44-8103-df5ff9013b6b (accessed on 22 November 2023).
- McGuirk, J.P.; Tam, C.S.; Kröger, N.; Riedell, P.A.; Murthy, H.S.; Ho, P.J.; Maakaron, J.E.; Waller, E.K.; Awan, F.T.; Shaughnessy, P.J.; et al. CTX110 Allogeneic CRISPR-Cas9-Engineered CAR T Cells in Patients (Pts) with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL): Results from the Phase 1 Dose Escalation Carbon Study. Blood 2022, 140, 10303–10306. [Google Scholar] [CrossRef]
- CRISPR Therapeutics Corporate Presentation. June 2022. Available online: https://crisprtx.gcs-web.com/static-files/5c256eb5-4982-4e47-b463-79eff954e622 (accessed on 23 November 2023).
- Pal, S.; Tran, B.; Haanen, J.; Hurwitz, M.; Sacher, A.; Agarwal, N.; Tannir, N.; Budde, E.; Harrison, S.; Klobuch, S.; et al. 558 CTX130 Allogeneic CRISPR-Cas9–Engineered Chimeric Antigen Receptor (CAR) T Cells in Patients with Advanced Clear Cell Renal Cell Carcinoma: Results from the Phase 1 COBALT-RCC Study. J. Immunother. Cancer 2022, 10, A584. [Google Scholar] [CrossRef]
- Iyer, S.P.; Sica, R.A.; Ho, P.J.; Hu, B.; Zain, J.; Prica, A.; Weng, W.-K.; Kim, Y.H.; Khodadoust, M.S.; Palomba, M.L.; et al. Horwitz The COBALT-LYM Study of Ctx130: A Phase 1 Dose Escalation Study of CD70-Targeted Allogeneic CRISPR-Cas9–Engineered CAR T Cells in Patients with Relapsed/Refractory (r/r) T-Cell Malignancies. EHA Libr. 2022, 357126, S262. [Google Scholar]
- Hu, Y.; Zhou, Y.; Zhang, M.; Ge, W.; Li, Y.; Yang, L.; Wei, G.; Han, L.; Wang, H.; Yu, S.; et al. CRISPR/Cas9-Engineered Universal CD19/CD22 Dual-Targeted CAR-T Cell Therapy for Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2021, 27, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yuan, Z.; Liu, L.; Li, Y.; Martina, S.; Liu, J.; Li, Z.; Wang, X.; He, J.; Zhao, W.; et al. Early Results of a Safety and Efficacy Study of Allogeneic TRuUCARTM GC502 in Patients with Relapsed/Refractory b-Cell Acute Lymphoblastic Leukemia (R/R B-ALL). EHA Libr. 2022, 357233, P370. [Google Scholar]
- Ghobadi, A.; Aldoss, I.; Maude, S.; S Wayne, A.; Bhojwani, D.; Bajel, A.; Dholaria, B.; Faramand, R.; Mattison, R.; Rettig, M.; et al. P356: Phase 1/2 Dose-Escalation Study of Anti-CD7 Allogenic CAR-T Cell in Relapsed or Refractory (r/r) T-Cell Acute Lymphoblastic Leukemia/Lymphoblastic Lymphoma (T-ALL/LBL). HemaSphere 2023, 7, e1789302. [Google Scholar] [CrossRef]
- Dholaria, B.; Kocoglu, M.H.; Kin, A.; Asch, A.S.; Ramakrishnan, A.; Bachier, C.; Rodriguez, T.E.; Shune, L.; McArthur, K.; McCaigue, J.; et al. Early Safety Results of P-BCMA-ALLO1, a Fully Allogeneic Chimeric Antigen Receptor T-Cell (CAR-T), in Patients with Relapsed / Refractory Multiple Myeloma (RRMM). Blood 2023, 142, 3479. [Google Scholar] [CrossRef]
- Oh, D.; Henry, J.; Baranda, J.C.; Dumbrava, E.E.; Cohen, E.; Eskew, J.D.; Belani, R.; McCaigue, J.; Namini, H.; Martin, C.; et al. Development of an Allogeneic CAR-T Targeting MUC1-C (MUC1, Cell Surface Associated, C-Terminal) for Epithelial Derived Tumors. Immuno-Oncol. Technol. 2022, 16, 46P. [Google Scholar] [CrossRef]
- Prenen, H.; Dekervel, J.; Anguille, S.; Hendlisz, A.; Michaux, A.; Sotiropoulou, P.A.; Gilham, D.E.; Mauen, S.; Snykers, S.; Cerf, E.; et al. CYAD-101: An Innovative Non-Gene Edited Allogeneic CAR-T for Solid Tumor Cancer Therapy. J. Clin. Oncol. 2020, 38, 3032. [Google Scholar] [CrossRef]
- Al-Homsi, A.-S.; Anguille, S.; Deeren, D.; Nishihori, T.; Meuleman, N.; Abdul-Hay, M.; Morgan, G.J.; Brayer, J.; Braun, N.; Lonez, C.; et al. Immunicy-1: Targeting BCMA with Cyad-211 to Establish Proof of Concept of an ShRNA-Based Allogeneic CAR T Cell Therapy Platform. Blood 2021, 138, 2817. [Google Scholar] [CrossRef]
- Mehta, A.; Farooq, U.; Chen, A.; McGuirk, J.P.; Ly, T.; Wong, L.; Cooley, S.; Valamehr, B.; Elstrom, R.; Chu, Y.-W.; et al. Interim Phase I Clinical Data of FT819-101, a Study of the First-Ever, Off-the-Shelf, IPSC-Derived TCR-Less CD19 CAR T-Cell Therapy for Patients with Relapsed/Refractory B-Cell Malignancies. Blood 2022, 140, 4577–4578. [Google Scholar] [CrossRef]
- Qasim, W.; Zhan, H.; Samarasinghe, S.; Adams, S.; Amrolia, P.; Stafford, S.; Butler, K.; Rivat, C.; Wright, G.; Somana, K.; et al. Molecular Remission of Infant B-ALL after Infusion of Universal TALEN Gene-Edited CAR T Cells. Sci. Transl. Med. 2017, 9, eaaj2013. [Google Scholar] [CrossRef] [PubMed]
- Allogene Therapeutics Presents Updated ALLO-501/501A Phase 1 Data in Large B Cell Lymphoma at the American Society of Clinical Oncology (ASCO) Annual Meeting|Allogene Therapeutics. Available online: https://ir.allogene.com/news-releases/news-release-details/allogene-therapeutics-presents-updated-allo-501501a-phase-1-data/ (accessed on 24 November 2023).
- Dupouy, S.; Marchiq, I.; Derippe, T.; Almena-Carrasco, M.; Jozwik, A.; Fouliard, S.; Adimy, Y.; Geronimi, J.; Graham, C.; Jain, N.; et al. Clinical Pharmacology and Determinants of Response to UCART19, an Allogeneic Anti-CD19 CAR-T Cell Product, in Adult B-Cell Acute Lymphoblastic Leukemia. Cancer Res. Commun. 2022, 2, 1520–1531. [Google Scholar] [CrossRef]
- FDA. FDA Investigating Serious Risk of T-Cell Malignancy Following BCMA-Directed or CD19-Directed Autologous Chimeric Antigen Receptor (CAR) T Cell Immunotherapies. Available online: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-investigating-serious-risk-t-cell-malignancy-following-bcma-directed-or-cd19-directed-autologous (accessed on 8 January 2024).
| ZFN | TALEN | CRISPR/Cas9 | CRISPR/Cas12a | Base-Editing | |
|---|---|---|---|---|---|
| Recognition site | Zinc finger protein | RVD tandem repeat region TALE protein | Two RNA molecules (guideRNA and tracrRNA) | Single-stranded guide RNA | CRISPR/Cas dependent (Cas sequence + base-editor mRNA) |
| Modification pattern | Fok1 nuclease | Fok1 nuclease | Cas9 nuclease | Cas12a nuclease | Four possible transition mutations: C→T A→G T→C G→A |
| Target sequence size | 9–18 bp | 14–20 bp | 20 bp- guide + PAM sequence | 20 bp- guide + PAM sequence | CRISPR/Cas dependent |
| Specificity | Small number of positional mismatches | Small number of positional mismatches | Positional/multiple consecutive mismatches | Positional/multiple consecutive mismatches | CRISPR/Cas dependent |
| Targeting limitations | Difficult to target non-G-rich sites | 5′ targeted base must be a T for each TALEN monomer | Recognizes 3′ G-rich Must precede a PAM sequence of 3–5 nt | Recognizes 5′ T-rich Must precede a PAM sequence of 3–4 nt | CRISPR/Cas dependent |
| Engineering | Requires substantial protein engineering | Requires complex molecular cloning methods | Uses standard cloning procedures | Uses standard cloning procedures | Uses standard cloning procedures |
| Delivery | Easy due to small size | Difficult due to large size | Moderate to difficult due to large size of SpCas9 | Moderate to difficult due to large size of FnCas12a | Difficult due to large site and added complexity |
| Allogeneic Engineering Technology | Target Antigen | Strategy for GvHD | Strategy for HvG | Product Name | Developers | Trial Names, Phase and Number |
|---|---|---|---|---|---|---|
| αβ T-cells (from PBMCs) | ||||||
| TALEN | CD19 | Disruption of TRAC | Disruption of CD52 and use of anti-CD52 | ALLO-501/UCART19 | Cellectis (Paris, France); Allogene Therapeutics (San Francisco, CA, USA) | CALM Phase 1 [162,163] NCT02746952 PALL Phase 1 [162] NCT02808442 ALPHA Phase 1 [164] NCT03939026 |
| CD19 | Disruption of TRAC | Disruption of CD52 and use of anti-CD52 | ALLO-501A | Cellectis (Paris, France); Allogene Therapeutics (San Francisco, CA, USA) | ALPHA 2 Phase 1/2 [165] NCT04416984 | |
| BCMA | Disruption of TRAC | Disruption of CD52 and use of anti-CD52 | ALLO-715 | Allogene Therapeutics (San Francisco, CA, USA); Cellectis (Paris, France) | UNIVERSAL Phase 1 [166] NCT04093596 | |
| CD70 | Disruption of TRAC | Disruption of CD52 and use of anti-CD52 CD70 CAR designed to avoid fratricide | ALLO-316 | Allogene Therapeutics (San Francisco, CA, USA); Cellectis (Paris, France) | TRAVERSE Phase 1 [167] NCT04696731 | |
| CD123 | Disruption of TRAC | Disruption of CD52 and use of anti-CD52 | UCART123 | Cellectis (Paris, France) | AMELI-01 Phase 1 [114] NCT03190278 Phase 1 NCT04106076 ABC123 Phase 1 NCT03203369 | |
| CD22 | Disruption of TRAC | Disruption of CD52 and use of anti-CD52 | UCART22 | Cellectis (Paris, France) | BALLI-01 Phase 1 [168] NCT04150497 | |
| SLAMF7 | Disruption of TRAC | Disruption of CS1 gene to avoid fratricide | UCARTCS1 | Cellectis (Paris, France) | MELANI-01 Phase 1 [169,170] NCT04142619 | |
| ARCUS | CD19 | Disruption of TCR | - | PBCAR0191/Azercabtagene zapreleucel | Precision BioSciences (Durham, NC, USA) | Phase 1/2 [171] NCT03666000 |
| CD19 | Disruption of TCR | shRNA against β2M and HLA-E transgene | PBCAR19B | Precision BioSciences (Durham, NC, USA) | Phase 1 [171] NCT04649112 | |
| BCMA | Disruption of TCR | - | PBCAR269A | Precision BioSciences (Durham, NC, USA) | Phase 1 [172] NCT04171843 | |
| CD20 | Disruption of TCR | - | PBCAR20A | Precision BioSciences (Durham, NC, USA) | Phase 1/2 [173] NCT04030195 | |
| CRISPR/Cas9 | CD19 | Disruption of TRAC | - | CB-010 | Caribou Biosciences (Berkeley, CA, USA) | ANTLER Phase 1 [174] NCT04637763 |
| CD19 | Disruption of TRAC | Disruption of β2M | CTX110 | CRISPR Therapeutics (Zug, Switzerland) | CARBON Phase 1/2 [175] NCT04035434 | |
| BCMA | Disruption of TRAC | Disruption of β2M | CTX120 | CRISPR Therapeutics (Zug, Switzerland) | Phase 1 [176] NCT04244656 | |
| CD70 | Disruption of TRAC | Disruption of β2M + CD70 disruption to avoid fratricide | CTX130 | CRISPR Therapeutics (Zug, Switzerland) | COBALT-RCC Phase 1 [177] NCT04438083 COBALT-LYM Phase 1 [178] NCT04502446 | |
| CD19 | Disruption of TRAC | Disruption of CD52 and use of anti-CD52 | CTA101 | Nanjing Bioheng Biotech (Nanjing, China) | Phase 1 [179] NCT04154709 NCT04227015 | |
| CD19/CD7 | Disruption of TRAC | CD7 disruption to avoid fratricide | GC502 | Gracell Biotechnologies (Suzhou, China) | Early Phase 1 [180] NCT05105867 | |
| CD7 | Disruption of TRAC | CD7 disruption to avoid fratricide | WU CART 007 | Wugen (St Louis, MO, USA) | Phase 1/2 [181] NCT04984356 | |
| Cas-CLOVER™ | BCMA | Disruption of TCR beta chain 1 | Disruption of β2M | P-BCMA-ALLO1 | Poseida Therapeutics (San Diego, CA, USA) | Phase 1 [182] NCT04960579 |
| FKBP12; MUC1-C | Disruption of TCR | Disruption of β2M | P-MUC1C-ALLO1 | Poseida Therapeutics (San Diego, CA, USA) | Phase 1 [183] NCT05239143 | |
| Base-pair editing | CD7 | Disruption of TRAC | Disruption of CD52 and CD7 to avoid fratricide | BE-CAR7 | Great Ormond Street Hospital (London, UK) | Phase 1 [147] ISRCTN15323014 |
| Peptide-based (TIM8) | NKG2DL | Negative competition with CD3ζ | - | CYAD-101 | Celyad Oncology (Mont-Saint-Guibert, Belgium) | alloSHRINK Phase 1 [154,184] NCT03692429 CYAD-101-002 Phase 1 NCT04991948 |
| miRNA-based shRNA | BCMA | Knock-down of CD3ζ | - | CYAD-211 | Celyad Oncology (Mont-Saint-Guibert, Belgium) | IMMUNICY-1 Phase 1 [185] NCT04613557 |
| Non-gene editing | CD19 | Intracellular retention of TCR/CD3 complex via KDEL-tagged anti-CD3 scFv | Decreasing surface HLA-A and HLA-B by HCMV US11 protein | ThisCART19 cells | Fundamenta Therapeutics (Suzhou, China) | Phase 1 [156] NCT04384393 |
| cytolytic T-lymphocytes (from PBMCs) | ||||||
| Zinc Finger Nuclease | IL13-zetakine | Disruption of the glucocorticoid receptor | Use of dexamethasone | GRm13Z40-2 | City of Hope (Duarte, CA, USA) | Phase 1 [111] NCT01082926 |
| αβ T-cells (from iPSCs) | ||||||
| CRISPR/Cas | CD19 | Disruption of TRAC | - | FT819 | Fate Therapeutics (San Diego, CA, USA) | Phase 1 [186] NCT04629729 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lonez, C.; Breman, E. Allogeneic CAR-T Therapy Technologies: Has the Promise Been Met? Cells 2024, 13, 146. https://doi.org/10.3390/cells13020146
Lonez C, Breman E. Allogeneic CAR-T Therapy Technologies: Has the Promise Been Met? Cells. 2024; 13(2):146. https://doi.org/10.3390/cells13020146
Chicago/Turabian StyleLonez, Caroline, and Eytan Breman. 2024. "Allogeneic CAR-T Therapy Technologies: Has the Promise Been Met?" Cells 13, no. 2: 146. https://doi.org/10.3390/cells13020146
APA StyleLonez, C., & Breman, E. (2024). Allogeneic CAR-T Therapy Technologies: Has the Promise Been Met? Cells, 13(2), 146. https://doi.org/10.3390/cells13020146