GDF15 Modulates the Zoledronic-Acid-Induced Hyperinflammatory Mechanoresponse of Periodontal Ligament Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Stimulation with Zoledronic Acid (ZOL)
2.3. MTT Assay
2.4. Trypan Blue Staining
2.5. Immunofluorescent Staining
2.6. RNA Extraction and cDNA Synthesis
2.7. Quantitative Polymerase Chain Reaction (PCR)
2.8. Alkaline Phospatase Activity Analysis
2.9. TUNEL Assay
2.10. β-Galactosidase Staining
2.11. Mechanical Compression
2.12. siRNA-Mediated Knockdown of GDF15
2.13. THP1 Activation Assay
2.14. Osteoclast Activation Assay and Tartrate-Resistant Acid Phosphatase Staining
2.15. Enzyme-Linked Immunosorbent Assay (ELISA)
2.16. Microscopy and Image Analysis
2.17. Statistics
3. Results
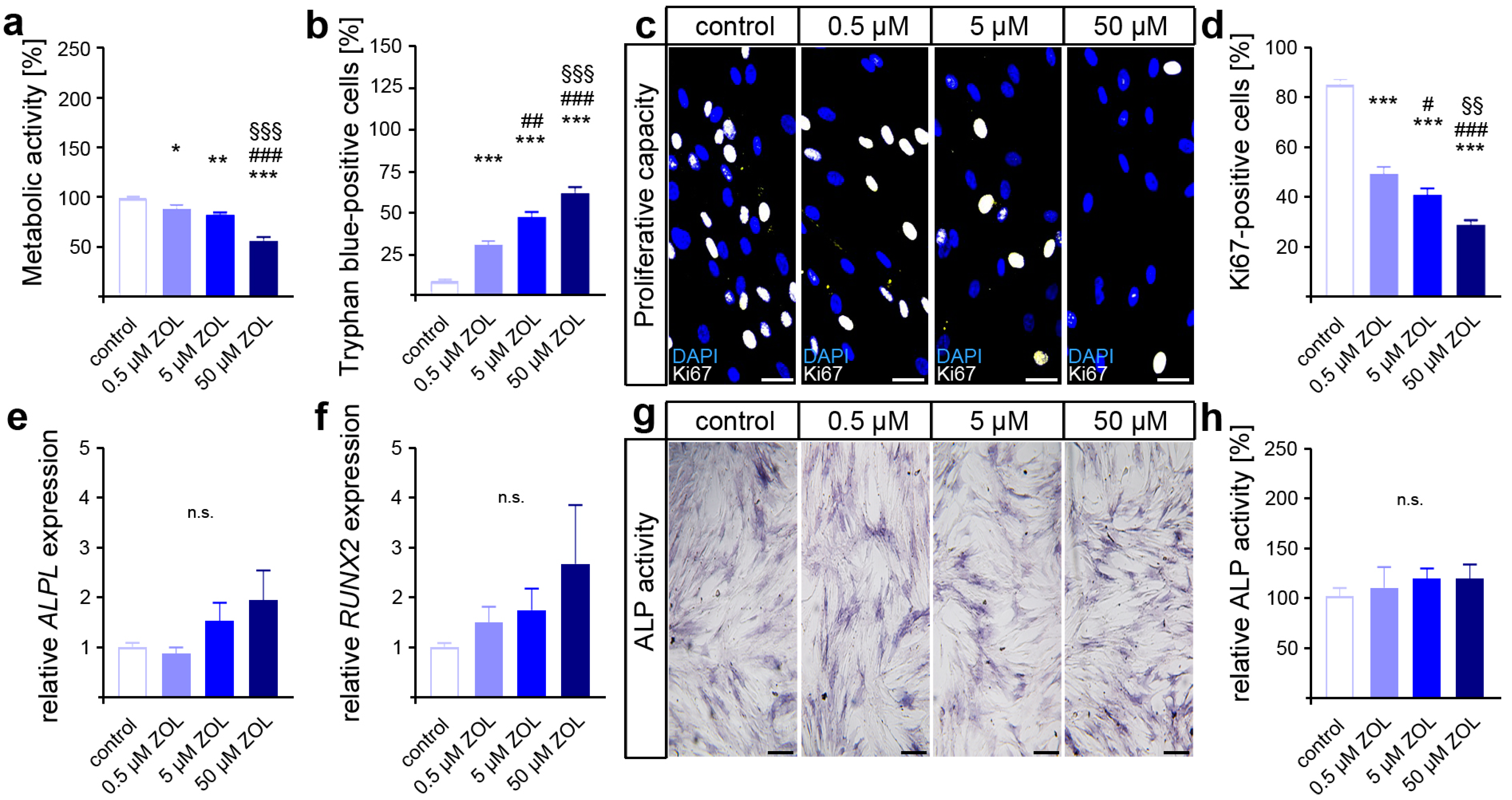
3.1. Zoledronic Acid Affects the Viability and Proliferation of hPdLFs but Not Their Differentiation
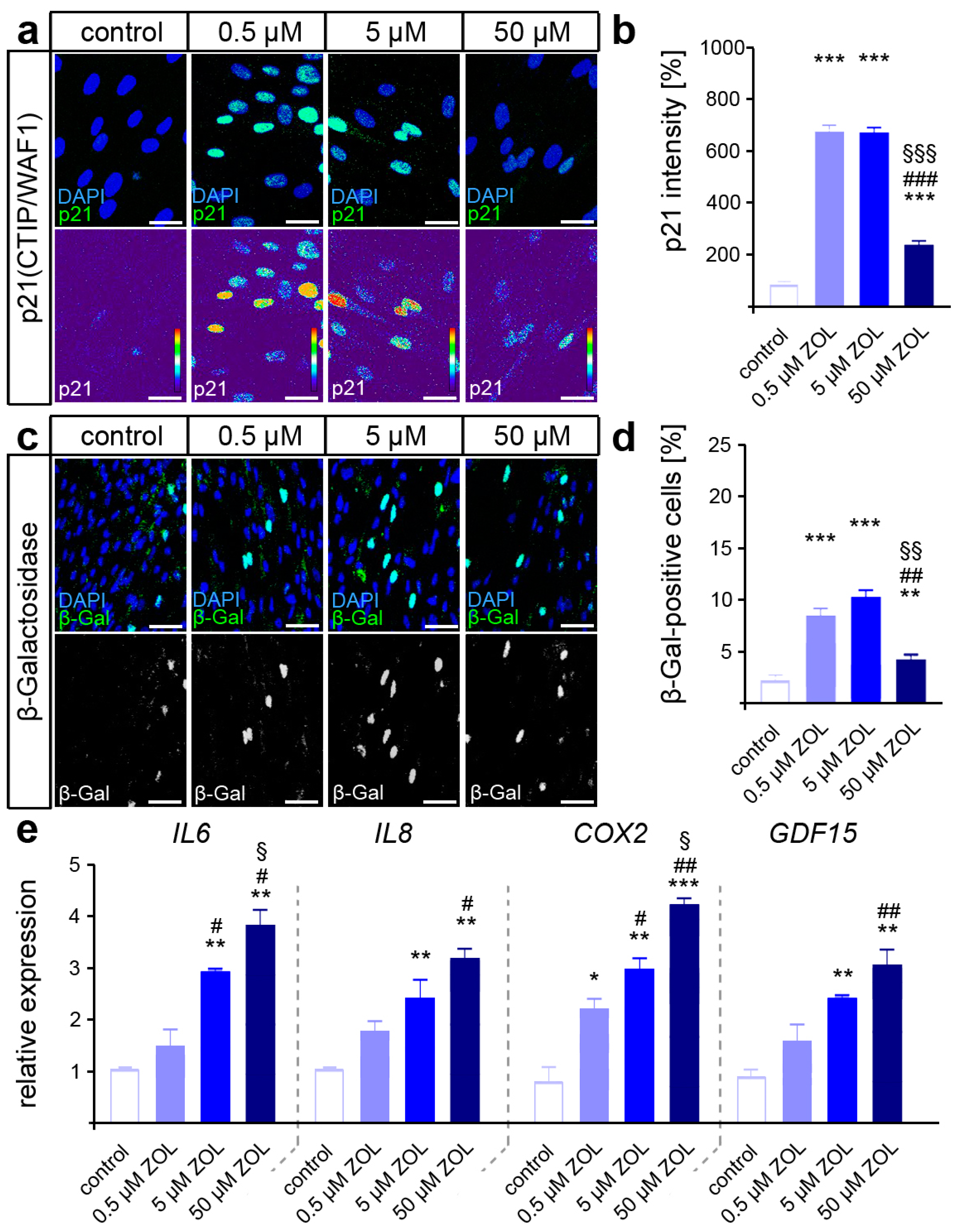
3.2. Zoledronate Activate DNA Damage Response and Promote Cellular Senescence in hPdLFs
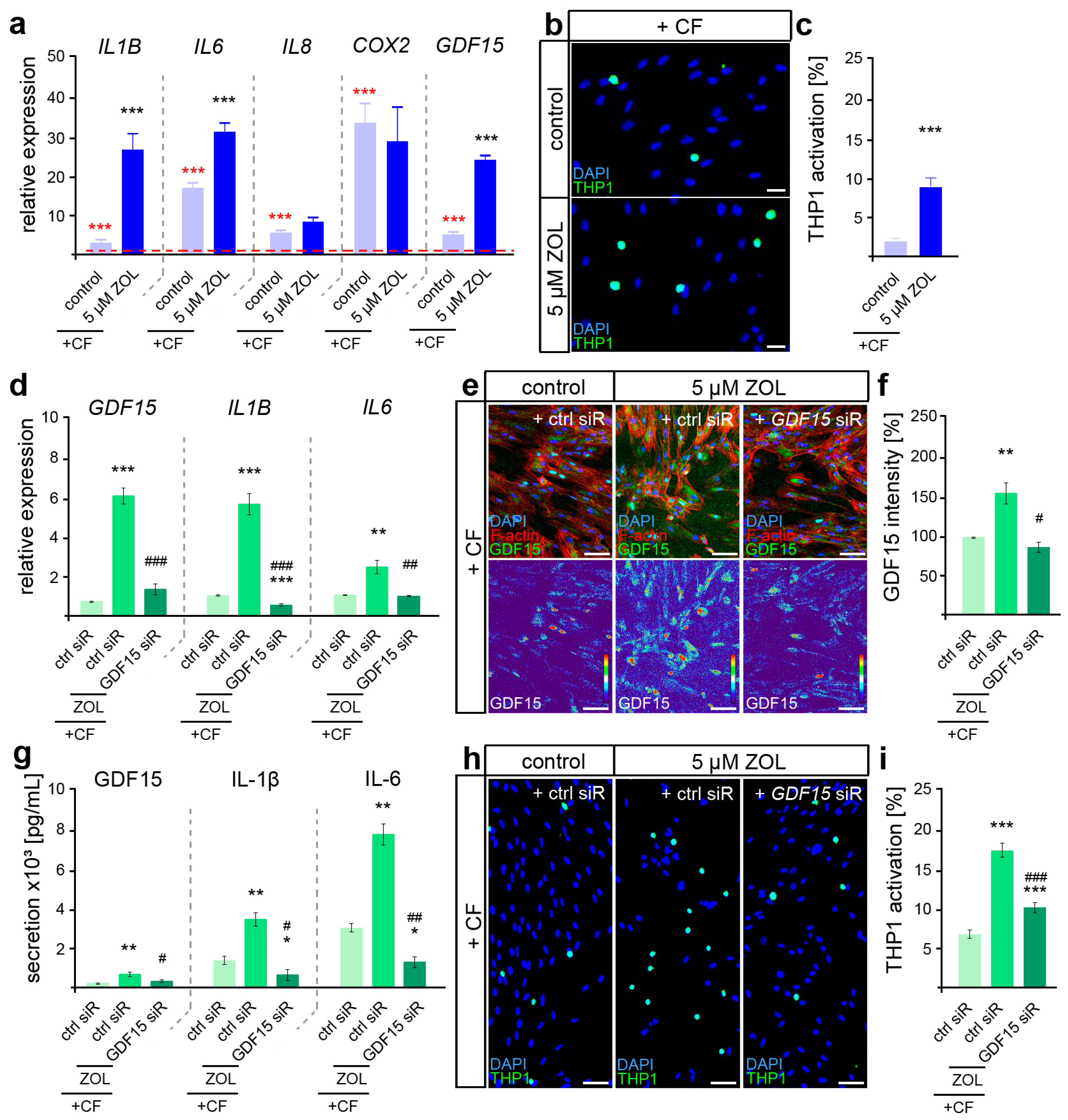
3.3. Zoledronate Induces a Hyperinflammatory Mechanoresponse but Restrains Osteoclast Activation in Part Modulated by GDF15
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Jacox, L.A.; Little, S.H.; Ko, C.C. Orthodontic tooth movement: The biology and clinical implications. Kaohsiung J. Med. Sci. 2018, 34, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Symmank, J.; Zimmermann, S.; Goldschmitt, J.; Schiegnitz, E.; Wolf, M.; Wehrbein, H.; Jacobs, C. Mechanically-induced gdf15 secretion by periodontal ligament fibroblasts regulates osteogenic transcription. Sci. Rep. 2019, 9, 11516. [Google Scholar] [CrossRef]
- Nanci, A.; Bosshardt, D.D. Structure of periodontal tissues in health and disease. Periodontol. 2000 2006, 40, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Basdra, E.K.; Komposch, G. Osteoblast-like properties of human periodontal ligament cells: An in vitro analysis. Eur. J. Orthod. 1997, 19, 615–621. [Google Scholar] [CrossRef]
- Somerman, M.J.; Young, M.F.; Foster, R.A.; Moehring, J.M.; Imm, G.; Sauk, J.J. Characteristics of human periodontal ligament cells in vitro. Arch. Oral Biol. 1990, 35, 241–247. [Google Scholar] [CrossRef]
- Arceo, N.; Sauk, J.J.; Moehring, J.; Foster, R.A.; Somerman, M.J. Human periodontal cells initiate mineral-like nodules in vitro. J. Periodontol. 1991, 62, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Wise, G.E.; King, G.J. Mechanisms of tooth eruption and orthodontic tooth movement. J. Dent. Res. 2008, 87, 414–434. [Google Scholar] [CrossRef] [PubMed]
- Andrade, I., Jr; Taddei, S.R.A.; Souza, P.E.A. Inflammation and tooth movement: The role of cytokines, chemokines, and growth factors. Semin. Orthod. 2012, 18, 257–269. [Google Scholar] [CrossRef]
- Ullrich, N.; Schroder, A.; Jantsch, J.; Spanier, G.; Proff, P.; Kirschneck, C. The role of mechanotransduction versus hypoxia during simulated orthodontic compressive strain-an in vitro study of human periodontal ligament fibroblasts. Int. J. Oral Sci. 2019, 11, 33. [Google Scholar] [CrossRef]
- Vansant, L.; Cadenas De Llano-Perula, M.; Verdonck, A.; Willems, G. Expression of biological mediators during orthodontic tooth movement: A systematic review. Arch. Oral Biol. 2018, 95, 170–186. [Google Scholar] [CrossRef]
- Brooks, P.J.; Nilforoushan, D.; Manolson, M.F.; Simmons, C.A.; Gong, S.G. Molecular markers of early orthodontic tooth movement. Angle Orthod. 2009, 79, 1108–1113. [Google Scholar] [CrossRef]
- Rody, W.J., Jr.; King, G.J.; Gu, G. Osteoclast recruitment to sites of compression in orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2001, 120, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Sokos, D.; Everts, V.; de Vries, T.J. Role of periodontal ligament fibroblasts in osteoclastogenesis: A review. J. Periodontal Res. 2015, 50, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Stemmler, A.; Symmank, J.; Steinmetz, J.; von Brandenstein, K.; Hennig, C.L.; Jacobs, C. Gdf15 supports the inflammatory response of pdl fibroblasts stimulated by P. gingivalis lps and concurrent compression. Int. J. Mol. Sci. 2021, 22, 13608. [Google Scholar] [CrossRef]
- Losch, L.; Stemmler, A.; Fischer, A.; Steinmetz, J.; Schuldt, L.; Hennig, C.L.; Symmank, J.; Jacobs, C. Gdf15 promotes the osteogenic cell fate of periodontal ligament fibroblasts, thus affecting their mechanobiological response. Int. J. Mol. Sci. 2023, 24, 10011. [Google Scholar] [CrossRef]
- Li, S.; Li, Q.; Zhu, Y.; Hu, W. Gdf15 induced by compressive force contributes to osteoclast differentiation in human periodontal ligament cells. Exp. Cell Res. 2020, 387, 111745. [Google Scholar] [CrossRef] [PubMed]
- Bauskin, A.R.; Brown, D.A.; Kuffner, T.; Johnen, H.; Luo, X.W.; Hunter, M.; Breit, S.N. Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Res. 2006, 66, 4983–4986. [Google Scholar] [CrossRef]
- Breit, S.N.; Johnen, H.; Cook, A.D.; Tsai, V.W.; Mohammad, M.G.; Kuffner, T.; Zhang, H.P.; Marquis, C.P.; Jiang, L.; Lockwood, G.; et al. The tgf-beta superfamily cytokine, mic-1/gdf15: A pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factors 2011, 29, 187–195. [Google Scholar] [CrossRef]
- Hinoi, E.; Ochi, H.; Takarada, T.; Nakatani, E.; Iezaki, T.; Nakajima, H.; Fujita, H.; Takahata, Y.; Hidano, S.; Kobayashi, T.; et al. Positive regulation of osteoclastic differentiation by growth differentiation factor 15 upregulated in osteocytic cells under hypoxia. J. Bone Miner. Res. 2012, 27, 938–949. [Google Scholar] [CrossRef]
- Vanhara, P.; Lincova, E.; Kozubik, A.; Jurdic, P.; Soucek, K.; Smarda, J. Growth/differentiation factor-15 inhibits differentiation into osteoclasts—A novel factor involved in control of osteoclast differentiation. Differ. Res. Biol. Divers. 2009, 78, 213–222. [Google Scholar]
- Westhrin, M.; Moen, S.H.; Holien, T.; Mylin, A.K.; Heickendorff, L.; Olsen, O.E.; Sundan, A.; Turesson, I.; Gimsing, P.; Waage, A.; et al. Growth differentiation factor 15 (gdf15) promotes osteoclast differentiation and inhibits osteoblast differentiation and high serum gdf15 levels are associated with multiple myeloma bone disease. Haematologica 2015, 100, e511–e514. [Google Scholar] [CrossRef]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. Mic-1, a novel macrophage inhibitory cytokine, is a divergent member of the tgf-beta superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef]
- Wedel, S.; Martic, I.; Guerrero Navarro, L.; Ploner, C.; Pierer, G.; Jansen-Durr, P.; Cavinato, M. Depletion of growth differentiation factor 15 (gdf15) leads to mitochondrial dysfunction and premature senescence in human dermal fibroblasts. Aging Cell 2023, 22, e13752. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, C.H.; Jeong, J.H.; Park, M.; Kim, K.S. Gdf15 contributes to radiation-induced senescence through the ros-mediated p16 pathway in human endothelial cells. Oncotarget 2016, 7, 9634–9644. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Martinez, R.; Khosla, S.; Farr, J.N.; Monroe, D.G. Periodontal disease and senescent cells: New players for an old oral health problem? Int. J. Mol. Sci. 2020, 21, 7441. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Martinez, R.; Rowsey, J.L.; Fraser, D.G.; Eckhardt, B.A.; Khosla, S.; Farr, J.N.; Monroe, D.G. Lps-induced premature osteocyte senescence: Implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone 2020, 132, 115220. [Google Scholar] [CrossRef]
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Carducci, M.; Smith, M.; Damiao, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Goldvaser, H.; Amir, E. Role of bisphosphonates in breast cancer therapy. Curr. Treat. Options Oncol. 2019, 20, 26. [Google Scholar] [CrossRef]
- Flanagan, A.M.; Chambers, T.J. Inhibition of bone resorption by bisphosphonates: Interactions between bisphosphonates, osteoclasts, and bone. Calcif. Tissue Int. 1991, 49, 407–415. [Google Scholar] [CrossRef]
- Wang, Z.P.; Eisenberger, M.A.; Carducci, M.A.; Partin, A.W.; Scher, H.I.; Ts’o, P.O. Identification and characterization of circulating prostate carcinoma cells. Cancer 2000, 88, 2787–2795. [Google Scholar] [CrossRef]
- Yamashita, J.; Sawa, N.; Sawa, Y.; Miyazono, S. Effect of bisphosphonates on healing of tooth extraction wounds in infectious osteomyelitis of the jaw. Bone 2021, 143, 115611. [Google Scholar] [CrossRef] [PubMed]
- Mucke, T.; Krestan, C.R.; Mitchell, D.A.; Kirschke, J.S.; Wutzl, A. Bisphosphonate and medication-related osteonecrosis of the jaw: A review. Semin. Musculoskelet. Radiol. 2016, 20, 305–314. [Google Scholar] [PubMed]
- Wong, L.; Ryan, F.S.; Christensen, L.R.; Cunningham, S.J. Factors influencing satisfaction with the process of orthodontic treatment in adult patients. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 362–370. [Google Scholar] [CrossRef]
- Jacobs, C.; Walter, C.; Ziebart, T.; Dirks, I.; Schramm, S.; Grimm, S.; Krieger, E.; Wehrbein, H. Mechanical loading influences the effects of bisphosphonates on human periodontal ligament fibroblasts. Clin. Oral Investig. 2015, 19, 699–708. [Google Scholar] [CrossRef]
- Di, W.; Shuai, Y.; Bo, W.; Wei, T.; Jinpeng, H.; Qian, G.; Deng, Y. A bifunctional zoledronate sustained-release system in scaffold: Tumor therapy and bone repair. Colloids Surf. B Biointerfaces 2023, 222, 113064. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Park, J.S.; Righesso, L.; Pabst, A.M.; Al-Nawas, B.; Kwon, Y.D.; Walter, C. Effects of an oral bisphosphonate and three intravenous bisphosphonates on several cell types in vitro. Clin. Oral Investig. 2018, 22, 2527–2534. [Google Scholar] [CrossRef]
- Samakkarnthai, P.; Saul, D.; Zhang, L.; Aversa, Z.; Doolittle, M.L.; Sfeir, J.G.; Kaur, J.; Atkinson, E.J.; Edwards, J.R.; Russell, G.G.; et al. In Vitro and in vivo effects of zoledronic acid on senescence and senescence-associated secretory phenotype markers. Aging 2023, 15, 3331–3355. [Google Scholar] [CrossRef]
- Bayram, M.; Soyer, C.; Kadioglu, E.; Sardas, S. Assessment of DNA damage in postmenopausal women under osteoporosis therapy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 127, 227–230. [Google Scholar] [CrossRef]
- Sirisoontorn, I.; Hotokezaka, H.; Hashimoto, M.; Gonzales, C.; Luppanapornlarp, S.; Darendeliler, M.A.; Yoshida, N. Orthodontic tooth movement and root resorption in ovariectomized rats treated by systemic administration of zoledronic acid. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 563–573. [Google Scholar] [CrossRef]
- Kaipatur, N.R.; Wu, Y.; Adeeb, S.; Stevenson, T.R.; Major, P.W.; Doschak, M.R. Impact of bisphosphonate drug burden in alveolar bone during orthodontic tooth movement in a rat model: A pilot study. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Adachi, H.; Mitani, H.; Shinoda, H. Inhibitory effect of the topical administration of a bisphosphonate (risedronate) on root resorption incident to orthodontic tooth movement in rats. J. Dent. Res. 1996, 75, 1644–1649. [Google Scholar] [PubMed]
- Karras, J.C.; Miller, J.R.; Hodges, J.S.; Beyer, J.P.; Larson, B.E. Effect of alendronate on orthodontic tooth movement in rats. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 843–847. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, F.R.N.; de Sousa Ferreira, V.C.; da Silva Martins, C.; Dantas, H.V.; de Sousa, F.B.; Girao-Carmona, V.C.C.; Goes, P.; de Castro Brito, G.A.; de Carvalho Leitao, R.F. The effect of high concentration of zoledronic acid on tooth induced movement and its repercussion on root, periodontal ligament and alveolar bone tissues in rats. Sci. Rep. 2021, 11, 7672. [Google Scholar] [CrossRef]
- Liu, L.; Igarashi, K.; Kanzaki, H.; Chiba, M.; Shinoda, H.; Mitani, H. Clodronate inhibits pge(2) production in compressed periodontal ligament cells. J. Dent. Res. 2006, 85, 757–760. [Google Scholar] [CrossRef]
- Kim, T.W.; Yoshida, Y.; Yokoya, K.; Sasaki, T. An ultrastructural study of the effects of bisphosphonate administration on osteoclastic bone resorption during relapse of experimentally moved rat molars. Am. J. Orthod. Dentofac. Orthop. 1999, 115, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Ajwa, N. The role of bisphosphonates in orthodontic tooth movement-a review. J. Fam. Med. Prim. Care 2019, 8, 3783–3788. [Google Scholar] [CrossRef]
- Schuldt, L.; Reimann, M.; von Brandenstein, K.; Steinmetz, J.; Doding, A.; Schulze-Spate, U.; Jacobs, C.; Symmank, J. Palmitate-triggered cox2/pge2-related hyperinflammation in dual-stressed pdl fibroblasts is mediated by repressive h3k27 trimethylation. Cells 2022, 11, 955. [Google Scholar] [CrossRef]
- Schuldt, L.; von Brandenstein, K.; Jacobs, C.; Symmank, J. Oleic acid-related anti-inflammatory effects in force-stressed pdl fibroblasts are mediated by h3 lysine acetylation associated with altered il10 expression. Epigenetics 2022, 17, 1892–1904. [Google Scholar] [CrossRef]
- Symmank, J.; Chorus, M.; Appel, S.; Marciniak, J.; Knaup, I.; Bastian, A.; Hennig, C.L.; Doding, A.; Schulze-Spate, U.; Jacobs, C.; et al. Distinguish fatty acids impact survival, differentiation and cellular function of periodontal ligament fibroblasts. Sci. Rep. 2020, 10, 15706. [Google Scholar] [CrossRef]
- Symmank, J.; Appel, S.; Bastian, J.A.; Knaup, I.; Marciniak, J.; Hennig, C.L.; Doding, A.; Schulze-Spate, U.; Jacobs, C.; Wolf, M. Hyperlipidemic conditions impact force-induced inflammatory response of human periodontal ligament fibroblasts concomitantly challenged with P. gingivalis-lps. Int. J. Mol. Sci. 2021, 22, 6069. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nagai, Y.; Dohdoh, M.; Oizumi, T.; Ohki, A.; Kuroishi, T.; Sugawara, S.; Endo, Y. In Vitro cytotoxicity of zoledronate (nitrogen-containing bisphosphonate: Nbp) and/or etidronate (non-nbp) in tumour cells and periodontal cells. Arch. Oral Biol. 2013, 58, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Agis, H.; Blei, J.; Watzek, G.; Gruber, R. Is zoledronate toxic to human periodontal fibroblasts? J. Dent. Res. 2010, 89, 40–45. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, M.S.; Lee, K.H.; Koh, J.S.; Jung, W.G.; Kong, C.B. Zoledronic acid is an effective radiosensitizer in the treatment of osteosarcoma. Oncotarget 2016, 7, 70869–70880. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.J.; Choi, Y.; Bae, M.K.; Hwang, D.S.; Shin, S.H.; Lee, J.Y. Zoledronate enhances osteocyte-mediated osteoclast differentiation by il-6/rankl axis. Int. J. Mol. Sci. 2019, 20, 1467. [Google Scholar] [CrossRef]
- Huang, K.C.; Huang, T.W.; Chuang, P.Y.; Yang, T.Y.; Chang, S.F. Zoledronate induces cell cycle arrest and differentiation by upregulating p21 in mouse mc3t3-e1 preosteoblasts. Int. J. Med. Sci. 2019, 16, 751–756. [Google Scholar] [CrossRef] [PubMed]
- de Barros Silva, P.G.; Ferreira Junior, A.E.C.; de Oliveira, C.C.; Brizeno, L.A.C.; Wong, D.V.T.; Lima Junior, R.C.P.; Sousa, F.B.; Mota, M.R.L.; Alves, A. Chronic treatment with zoledronic acid increases inflammatory markers in periodontium of rats. J. Oral Pathol. Med. 2017, 46, 1046–1053. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The mtt assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blazquez-Castro, A. Tetrazolium salts and formazan products in cell biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef]
- Ohnuki, H.; Izumi, K.; Terada, M.; Saito, T.; Kato, H.; Suzuki, A.; Kawano, Y.; Nozawa-Inoue, K.; Takagi, R.; Maeda, T. Zoledronic acid induces s-phase arrest via a DNA damage response in normal human oral keratinocytes. Arch. Oral Biol. 2012, 57, 906–917. [Google Scholar] [CrossRef]
- Iguchi, T.; Miyakawa, Y.; Saito, K.; Nakabayashi, C.; Nakanishi, M.; Saya, H.; Ikeda, Y.; Kizaki, M. Zoledronate-induced s phase arrest and apoptosis accompanied by DNA damage and activation of the atm/chk1/cdc25 pathway in human osteosarcoma cells. Int. J. Oncol. 2007, 31, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Misra, J.; Mohanty, S.T.; Madan, S.; Fernandes, J.A.; Hal Ebetino, F.; Russell, R.G.; Bellantuono, I. Zoledronate attenuates accumulation of DNA damage in mesenchymal stem cells and protects their function. Stem Cells 2016, 34, 756–767. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, K.; Almasan, A. Histone h2ax phosphorylation: A marker for DNA damage. Methods Mol. Biol. 2012, 920, 613–626. [Google Scholar] [PubMed]
- Siddiqui, M.S.; Francois, M.; Fenech, M.F.; Leifert, W.R. Persistent gammah2ax: A promising molecular marker of DNA damage and aging. Mutat. Res. Rev. Mutat. Res. 2015, 766, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Valieva, Y.; Ivanova, E.; Fayzullin, A.; Kurkov, A.; Igrunkova, A. Senescence-associated beta-galactosidase detection in pathology. Diagnostics 2022, 12, 2309. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.; Mundethu, A.; Symmank, J.; Hennig, C.; Walter, C.; Reichardt, E.; Wehrbein, H.; Jacobs, C. Compressive force strengthened the pro-inflammatory effect of zoledronic acid on il-1ss stimulated human periodontal fibroblasts. Clin. Oral Investig. 2020, 25, 3453–3461. [Google Scholar] [CrossRef]
- Mohd Yasin, Z.N.; Mohd Idrus, F.N.; Hoe, C.H.; Yvonne-Tee, G.B. Macrophage polarization in thp-1 cell line and primary monocytes: A systematic review. Differ. Res. Biol. Divers. 2022, 128, 67–82. [Google Scholar] [CrossRef]
- Seifi, M.; Asefi, S.; Hatamifard, G.; Lotfi, A. Effect of local injection of zolena, zoledronic acid made in iran, on orthodontic tooth movement and root and bone resorption in rats. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 257–264. [Google Scholar]
- Li, Z.H.; Si, Y.; Xu, G.; Chen, X.M.; Xiong, H.; Lai, L.; Zheng, Y.Q.; Zhang, Z.G. High-dose pma with rankl and mcsf induces thp1 cell differentiation into human functional osteoclasts in vitro. Mol. Med. Rep. 2017, 16, 8380–8384. [Google Scholar] [CrossRef] [PubMed]
- Hattinger, C.M.; Patrizio, M.P.; Luppi, S.; Magagnoli, F.; Picci, P.; Serra, M. Current understanding of pharmacogenetic implications of DNA damaging drugs used in osteosarcoma treatment. Expert Opin. Drug Metab. Toxicol. 2019, 15, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Osaki, M.; Onuma, K.; Ishikawa, M.; Ryoke, K.; Kodani, I.; Okada, F. Bisphosphonate-induced reactive oxygen species inhibit proliferation and migration of oral fibroblasts: A pathogenesis of bisphosphonate-related osteonecrosis of the jaw. J. Periodontol. 2020, 91, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, A.; Chiarella, E.; Baudi, F.; Scardamaglia, P.; Antonelli, A.; Giudice, D.; Barni, T.; Fortunato, L.; Giudice, A. Dose-dependent effects of zoledronic acid on human periodontal ligament stem cells: An in vitro pilot study. Cell Transplant. 2020, 29, 963689720948497. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Boran, T.; Oztas, E.; Jannuzzi, A.T.; Ozden, S.; Ozhan, G. Zoledronic acid-induced oxidative damage and endoplasmic reticulum stress-mediated apoptosis in human embryonic kidney (hek-293) cells. J. Biochem. Mol. Toxicol. 2022, 36, e23083. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Sakai, K.; Urata, Y.; Toyama, N.; Nakamichi, E.; Hibi, H. Extracellular vesicles of stem cells to prevent bronj. J. Dent. Res. 2020, 99, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.H.; Huang, P.H.; Chang, W.C.; Tsai, H.Y.; Lin, C.P.; Leu, H.B.; Wu, T.C.; Chen, J.W.; Lin, S.J. Zoledronate inhibits ischemia-induced neovascularization by impairing the mobilization and function of endothelial progenitor cells. PLoS ONE 2012, 7, e41065. [Google Scholar] [CrossRef]
- Konstantonis, D.; Papadopoulou, A.; Makou, M.; Eliades, T.; Basdra, E.K.; Kletsas, D. Senescent human periodontal ligament fibroblasts after replicative exhaustion or ionizing radiation have a decreased capacity towards osteoblastic differentiation. Biogerontology 2013, 14, 741–751. [Google Scholar] [CrossRef]
- Huang, K.C.; Cheng, C.C.; Chuang, P.Y.; Yang, T.Y. The effects of zoledronate on the survival and function of human osteoblast-like cells. BMC Musculoskelet. Disord. 2015, 16, 355. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Patel, C.G.; Yee, A.J.; Scullen, T.A.; Nemani, N.; Santo, L.; Richardson, P.G.; Laubach, J.P.; Ghobrial, I.M.; Schlossman, R.L.; Munshi, N.C.; et al. Biomarkers of bone remodeling in multiple myeloma patients to tailor bisphosphonate therapy. Clin. Cancer Res. 2014, 20, 3955–3961. [Google Scholar] [CrossRef]
- Mattia, L.; Gossiel, F.; Walsh, J.S.; Eastell, R. Effect of age and gender on serum growth differentiation factor 15 and its relationship to bone density and bone turnover. Bone Rep. 2023, 18, 101676. [Google Scholar] [CrossRef]
- Schiegnitz, E.; Kammerer, P.W.; Rode, K.; Schorn, T.; Brieger, J.; Al-Nawas, B. Growth differentiation factor 15 as a radiation-induced marker in oral carcinoma increasing radiation resistance. J. Oral Pathol. Med. 2016, 45, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Brockhaus, J.; Craveiro, R.B.; Azraq, I.; Niederau, C.; Schroder, S.K.; Weiskirchen, R.; Jankowski, J.; Wolf, M. In vitro compression model for orthodontic tooth movement modulates human periodontal ligament fibroblast proliferation, apoptosis and cell cycle. Biomolecules 2021, 11, 932. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, X.; Chen, J.; Wang, Q.; Wang, G.; Ai, X.; Wang, X.; Pan, J. Zoledronic acid regulates the synthesis and secretion of il-1beta through histone methylation in macrophages. Cell Death Discov. 2020, 6, 47. [Google Scholar] [CrossRef]
- Kaneko, J.; Okinaga, T.; Hikiji, H.; Ariyoshi, W.; Yoshiga, D.; Habu, M.; Tominaga, K.; Nishihara, T. Zoledronic acid exacerbates inflammation through m1 macrophage polarization. Inflamm. Regen. 2018, 38, 16. [Google Scholar] [CrossRef] [PubMed]
- Wrana, J.L.; Attisano, L. The smad pathway. Cytokine Growth Factor Rev. 2000, 11, 5–13. [Google Scholar] [CrossRef]
- Komatsu, Y.; Ibi, M.; Chosa, N.; Kyakumoto, S.; Kamo, M.; Shibata, T.; Sugiyama, Y.; Ishisaki, A. Zoledronic acid suppresses transforming growth factor-beta-induced fibrogenesis by human gingival fibroblasts. Int. J. Mol. Med. 2016, 38, 139–147. [Google Scholar] [CrossRef]
- Min, K.W.; Liggett, J.L.; Silva, G.; Wu, W.W.; Wang, R.; Shen, R.F.; Eling, T.E.; Baek, S.J. Nag-1/gdf15 accumulates in the nucleus and modulates transcriptional regulation of the smad pathway. Oncogene 2016, 35, 377–388. [Google Scholar] [CrossRef]


| Gene | Gene Symbol | NCBI Gene ID | Primer Sequence |
|---|---|---|---|
| Alkaline phosphatase | ALPL | 249 | ACTGCAGACATTCTCAAA GAGTGAGTGAGTGAGCA |
| C-X-C motif chemokine ligand 8 | IL8 | 3576 | TTGGCAGCCTTCCTGATTTCT GGTCCACTCTCAATCATCTCA |
| Growth differentiation factor 15 | GDF15 | 3576 | CCGAAGACTCCAGATTCCGA CCCGAGAGATACGCAGGTG |
| Interleukin 1 beta | IL1B | 3553 | CGAATCTCCGACCACCACTA AGCCTCGTTATCCCATGTGT |
| Interleukin 6 | IL6 | 3569 | CATCCTCGACGGCATCTCAG TCACCAGGCAAGTCTCCTCA |
| Prostaglandinendoperoxide synthase 2 | PTGS2 (alias COX2) | 4743 | GATGATTGCCCGACTCCCTT GGCCCTCGCTTATGATCTGT |
| RUNX family transcription factor 2 | RUNX2 | 6146 | CCCACGAATGCACTATCC GGACATACCGAGGGACA |
| TNF receptor superfamily member 11b | TNFRSF11B (alias OPG) | 4982 | GAAGGGCGCTACCTTGA GCAAACTGTATTTCGCTC |
| TNF superfamily member 11 | TNFSF11 (alias RANKL) | 8600 | ATCACAGCACATCAGACAGA TCATTTATGGAACAGATGGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitzsche, A.; Hennig, C.-L.; von Brandenstein, K.; Döding, A.; Schulze-Späte, U.; Symmank, J.; Jacobs, C. GDF15 Modulates the Zoledronic-Acid-Induced Hyperinflammatory Mechanoresponse of Periodontal Ligament Fibroblasts. Cells 2024, 13, 147. https://doi.org/10.3390/cells13020147
Nitzsche A, Hennig C-L, von Brandenstein K, Döding A, Schulze-Späte U, Symmank J, Jacobs C. GDF15 Modulates the Zoledronic-Acid-Induced Hyperinflammatory Mechanoresponse of Periodontal Ligament Fibroblasts. Cells. 2024; 13(2):147. https://doi.org/10.3390/cells13020147
Chicago/Turabian StyleNitzsche, Ann, Christoph-Ludwig Hennig, Katrin von Brandenstein, Annika Döding, Ulrike Schulze-Späte, Judit Symmank, and Collin Jacobs. 2024. "GDF15 Modulates the Zoledronic-Acid-Induced Hyperinflammatory Mechanoresponse of Periodontal Ligament Fibroblasts" Cells 13, no. 2: 147. https://doi.org/10.3390/cells13020147
APA StyleNitzsche, A., Hennig, C.-L., von Brandenstein, K., Döding, A., Schulze-Späte, U., Symmank, J., & Jacobs, C. (2024). GDF15 Modulates the Zoledronic-Acid-Induced Hyperinflammatory Mechanoresponse of Periodontal Ligament Fibroblasts. Cells, 13(2), 147. https://doi.org/10.3390/cells13020147







