Modulation of Microglial Function by ATP-Gated P2X7 Receptors: Studies in Rat, Mice and Human
Abstract
1. Introduction
P2X Receptors
2. Microglia
2.1. Microglia Express Multiple Subtypes of Purinergic Receptors
2.2. Healthy Prenatal CNS
2.3. Healthy Postnatal CNS
2.4. Infection and Disease
3. P2X7R and Microglia
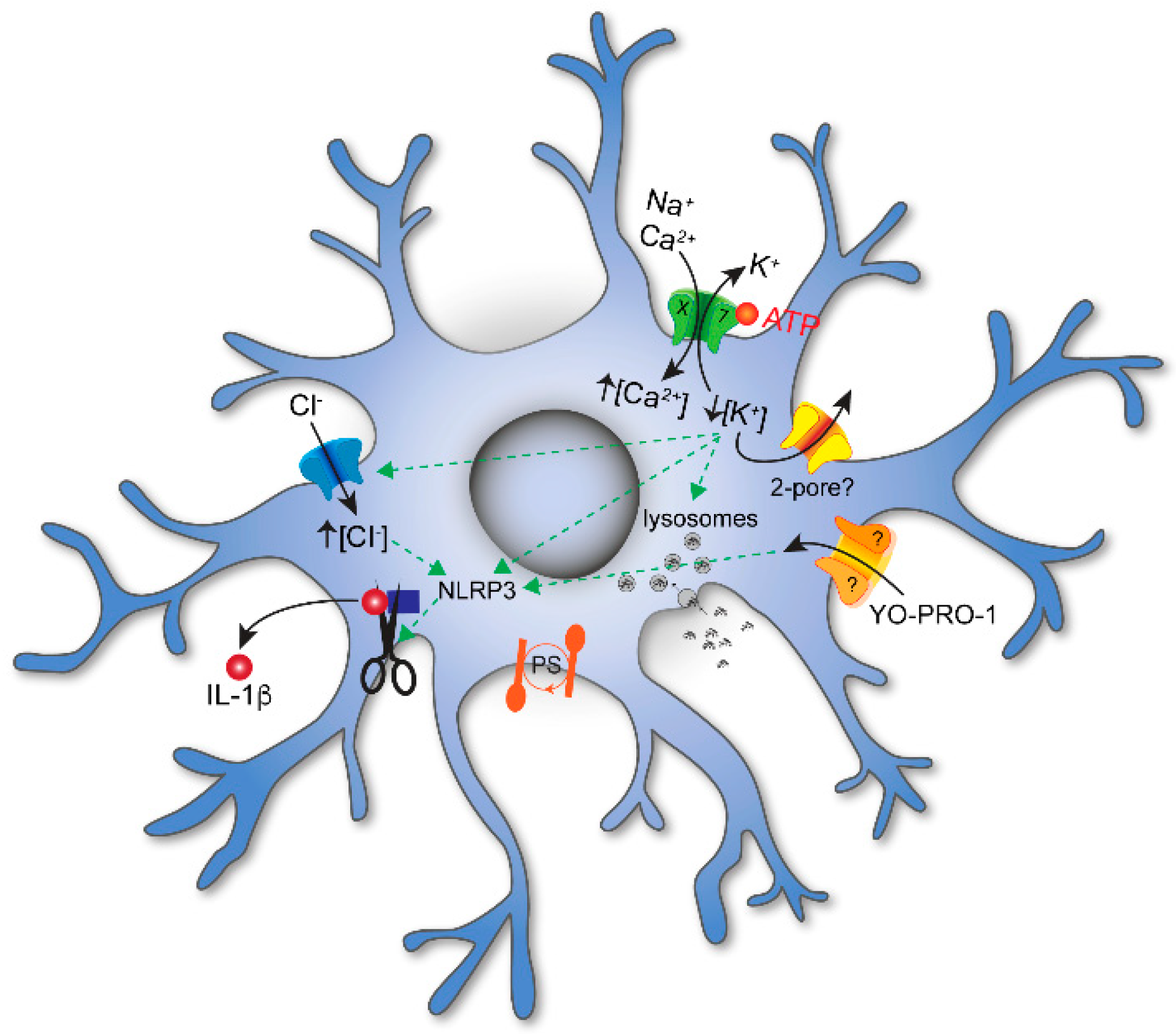
3.1. Membrane Permeabilization and Cell Lysis
3.2. Membrane Blebbing and Microvesicular Shedding
3.3. Cytokines and Reactive Oxygen Species (ROS)
3.4. Tumor Microenvironment
3.5. Cell Death and Disease
3.6. Oxygen Glucose Deprivation
4. Disease States
4.1. Alzheimer’s Disease
4.2. Parkinson’s Disease
4.3. Epilepsy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rajendran, M.; Dane, E.; Conley, J.; Tantama, M. Imaging Adenosine Triphosphate (ATP). Biol. Bull. 2016, 231, 73–84. [Google Scholar] [CrossRef]
- Khakh, B.S.; Burnstock, G. The Double Life of ATP. Sci. Am. 2009, 301, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.L.; Sarti, A.C.; Di Virgilio, F. Extracellular Nucleotides and Nucleosides as Signalling Molecules. Immunol. Lett. 2019, 205, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. A Basis for Distinguishing Two Types of Purinergic Receptor. In Cell Membrane Receptors for Drugs and Hormones A Multidisciplinary Approach; Raven Press: New York, NY, USA, 1978. [Google Scholar]
- Burnstock, G.; Kennedy, C. Is There a Basis for Distinguishing Two Types of P2-Purinoceptor? Gen. Pharmacol. 1985, 16, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Hammarberg, C.; Schulte, G.; Fredholm, B.B. Evidence for Functional Adenosine A3 Receptors in Microglia Cells. J. Neurochem. 2003, 86, 1051–1054. [Google Scholar] [CrossRef]
- Synowitz, M.; Glass, R.; Färber, K.; Markovic, D.; Kronenberg, G.; Herrmann, K.; Schnermann, J.; Nolte, C.; van Rooijen, N.; Kiwit, J.; et al. A1 Adenosine Receptors in Microglia Control Glioblastoma-Host Interaction. Cancer Res. 2006, 66, 8550–8557. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.G.; Orr, A.L.; Li, X.-J.; Gross, R.E.; Traynelis, S.F.; Neurosci, N. Adenosine A 2A Receptor Mediates Microglial Process Retraction HHS Public Access Author Manuscript. Nat. Neurosci. 2009, 12, 872–878. [Google Scholar] [CrossRef]
- Haselkorn, M.L.; Shellington, D.K.; Jackson, E.K.; Vagni, V.A.; Janesko-Feldman, K.; Dubey, R.K.; Gillespie, D.G.; Cheng, D.; Bell, M.J.; Jenkins, L.W.; et al. Adenosine A1 Receptor Activation as a Brake on the Microglial Response after Experimental Traumatic Brain Injury in Mice. J. Neurotrauma 2010, 27, 901–910. [Google Scholar] [CrossRef]
- Koscsó, B.; Csóka, B.; Selmeczy, Z.; Himer, L.; Pacher, P.; Virág, L.; Haskó, G. Adenosine Augments IL-10 Production by Microglial Cells through an A2B Adenosine Receptor-Mediated Process. J. Immunol. 2012, 188, 445–453. [Google Scholar] [CrossRef]
- Ohsawa, K.; Sanagi, T.; Nakamura, Y.; Suzuki, E.; Inoue, K.; Kohsaka, S. Adenosine A3 Receptor is Involved in ADP-Induced Microglial Process Extension and Migration. J. Neurochem. 2012, 121, 217–227. [Google Scholar] [CrossRef]
- Merighi, S.; Bencivenni, S.; Vincenzi, F.; Varani, K.; Borea, P.A.; Gessi, S. A 2B Adenosine Receptors Stimulate IL-6 Production in Primary Murine Microglia through p38 MAPK Kinase Pathway. Pharmacol. Res. 2017, 117, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-Derived ATP Induces Vesicle Shedding and IL-1β Release from Microglia. J. Immunol. 2005, 174, 7268–7277. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Burnstock, G. Expression of P2X Receptors on Rat Microglial Cells during Early Development. Glia 2005, 52, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Calovi, S.; Mut-Arbona, P.; Sperlágh, B. Microglia and the Purinergic Signaling System. Neuroscience 2019, 405, 137–147. [Google Scholar] [CrossRef] [PubMed]
- von Kügelgen, I. Pharmacology of P2Y Receptors. Brain Res. Bull. 2019, 151, 12–24. [Google Scholar] [CrossRef]
- Hidetoshi, T.-S.; Makoto, T.; Inoue, K. P2Y Receptors in Microglia and Neuroinflammation. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 493–501. [Google Scholar] [CrossRef]
- Swiatkowski, P.; Murugan, M.; Eyo, U.; Wang, Y.; Rangaraju, S.; Oh, S.; Wu, L.-J. Activation of Microglial P2Y12 Receptor Is Required for Outward Potassium Currents in Response to Neuronal Injury. Neuroscience 2016, 318, 22–33. [Google Scholar] [CrossRef]
- Madry, C.; Kyrargyri, V.; Arancibia-Cárcamo, I.L.; Jolivet, R.; Kohsaka, S.; Bryan, R.M.; Attwell, D. Microglial Ramification, Surveillance, and Interleukin-1β Release Are Regulated by the Two-Pore Domain K+ Channel THIK-1. Neuron 2018, 97, 299–312.e6. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef]
- Egan, T.M.; Samways, D.S.K.; Li, Z. Biophysics of P2X Receptors. Pflüg. Arch. Eur. J. Physiol. 2006, 452, 501–512. [Google Scholar] [CrossRef]
- Lê, K.-T.; Paquet, M.; Nouel, D.; Babinski, K.; Séguéla, P. Primary Structure and Expression of a Naturally Truncated Human P2X ATP Receptor Subunit from Brain and Immune System. FEBS Lett. 1997, 418, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Bo, X.; Jiang, L.-H.; Wilson, H.L.; Kim, M.; Burnstock, G.; Surprenant, A.; North, R.A. Pharmacological and Biophysical Properties of the Human P2X5 Receptor. Mol. Pharmacol. 2003, 63, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Ormond, S.J.; Barrera, N.P.; Qureshi, O.S.; Henderson, R.M.; Edwardson, J.M.; Murrell-Lagnado, R.D. An Uncharged Region within the N Terminus of the P2X6 Receptor Inhibits Its Assembly and Exit from the Endoplasmic Reticulum. Mol. Pharmacol. 2006, 69, 1692–1700. [Google Scholar] [CrossRef]
- Murrell-Lagnado, R.D.; Qureshi, O.S. Assembly and Trafficking of P2X Purinergic Receptors (Review). Mol. Membr. Biol. 2008, 25, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Torres, G.E.; Egan, T.M.; Voigt, M.M. Hetero-Oligomeric Assembly of P2X Receptor Subunits. Specificities Exist with Regard to Possible Partners. J. Biol. Chem. 1999, 274, 6653–6659. [Google Scholar] [CrossRef] [PubMed]
- Saul, A.; Hausmann, R.; Kless, A.; Nicke, A. Heteromeric Assembly of P2X Subunits. Front. Cell. Neurosci. 2013, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Nicke, A. Homotrimeric Complexes Are the Dominant Assembly State of Native P2X7 Subunits. Biochem. Biophys. Res. Commun. 2008, 377, 803–808. [Google Scholar] [CrossRef]
- Cheewatrakoolpong, B.; Gilchrest, H.; Anthes, J.C.; Greenfeder, S. Identification and Characterization of Splice Variants of the Human P2X7 ATP Channel. Biochem. Biophys. Res. Commun. 2005, 332, 17–27. [Google Scholar] [CrossRef]
- Sluyter, R. The P2X7 Receptor. Adv. Exp. Med. Biol. 2017, 19, 17–53. [Google Scholar]
- Liang, X.; Samways, D.S.K.; Wolf, K.; Bowles, E.A.; Richards, J.P.; Bruno, J.; Dutertre, S.; DiPaolo, R.J.; Egan, T.M. Quantifying Ca2+ Current and Permeability in ATP-Gated P2X7 Receptors. J. Biol. Chem. 2015, 290, 7930–7942. [Google Scholar] [CrossRef]
- Surprenant, A.; Rassendren, F.; Kawashima, E.; North, R.A.; Buell, G. The Cytolytic P2Z Receptor for Extracellular ATP Identified as a P2X Receptor (P2X7). Science 1996, 272, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Habermacher, C.; Dunning, K.; Chataigneau, T.; Grutter, T. Molecular Structure and Function of P2X Receptors. Neuropharmacology 2016, 104, 18–30. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.E.; Yoshioka, C.; Mansoor, S.E. Full-Length P2X7 Structures Reveal How Palmitoylation Prevents Channel Desensitization. Cell 2019, 179, 659–670.e13. [Google Scholar] [CrossRef] [PubMed]
- Costa-Junior, H.M.; Vieira, F.S.; Coutinho-Silva, R. C Terminus of the P2X7 Receptor: Treasure Hunting. Purinergic Signal. 2011, 7, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulos, J.M.; Delarasse, C. Pleiotropic Roles of P2X7 in the Central Nervous System. Front. Cell. Neurosci. 2019, 13, 401. [Google Scholar] [CrossRef] [PubMed]
- Kopp, R.; Krautloher, A.; Ramírez-Fernández, A.; Nicke, A. P2X7 Interactions and Signaling—Making Head or Tail of It. Front. Mol. Neurosci. 2019, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-H. Inhibition of P2X7 Receptors by Divalent Cations: Old Action and New Insight. Eur. Biophys. J. 2009, 38, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Nicke, A.; Kuan, Y.-H.; Masin, M.; Rettinger, J.; Marquez-Klaka, B.; Bender, O.; Górecki, D.C.; Murrell-Lagnado, R.D.; Soto, F. A Functional P2X7 Splice Variant with an Alternative Transmembrane Domain 1 Escapes Gene Inactivation in P2X7 Knock-out Mice. J. Biol. Chem. 2009, 284, 25813–25822. [Google Scholar] [CrossRef]
- Zimmermann, H. Extracellular Metabolism of ATP and Other Nucleotides. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 362, 299–309. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Boeynaems, J.-M.; Robson, S.C. Extracellular Nucleotides as Negative Modulators of Immunity. Curr. Opin. Pharmacol. 2009, 9, 507–513. [Google Scholar] [CrossRef]
- Trautmann, A. Extracellular ATP in the Immune System: More than Just a “Danger Signal”. Sci. Signal. 2009, 2, pe6. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F. ATP as a Death Factor. BioFactors 1998, 8, 301–303. [Google Scholar] [CrossRef]
- Linden, J.; Koch-Nolte, F.; Dahl, G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu. Rev. Immunol. 2019, 37, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.L. Extracellular ATP: Effects, Sources and Fate. Biochem. J. 1986, 233, 309–319. [Google Scholar] [CrossRef]
- Guerra, A.N.; Gavala, M.L.; Chung, H.S.; Bertics, P.J. Nucleotide Receptor Signalling and the Generation of Reactive Oxygen Species. Purinergic Signal. 2007, 3, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Pellegatti, P.; Raffaghello, L.; Bianchi, G.; Piccardi, F.; Pistoia, V.; Di Virgilio, F. Increased Level of Extracellular ATP at Tumor Sites: In Vivo Imaging with Plasma Membrane Luciferase. PLoS ONE 2008, 3, e2599. [Google Scholar] [CrossRef]
- McLarnon, J.G. Roles of Purinergic P2X7 Receptor in Glioma and Microglia in Brain Tumors. Cancer Lett. 2017, 402, 93–99. [Google Scholar] [CrossRef]
- Morciano, G.; Sarti, A.C.; Marchi, S.; Missiroli, S.; Falzoni, S.; Raffaghello, L.; Pistoia, V.; Giorgi, C.; Di Virgilio, F.; Pinton, P. Use of Luciferase Probes to Measure ATP in Living Cells and Animals. Nat. Protoc. 2017, 12, 1542–1562. [Google Scholar] [CrossRef]
- Rodrigues, R.J.; Tomé, A.R.; Cunha, R.A. ATP as a Multi-Target Danger Signal in the Brain. Front. Neurosci. 2015, 9, 148. [Google Scholar] [CrossRef]
- Honda, S.; Sasaki, Y.; Ohsawa, K.; Imai, Y.; Nakamura, Y.; Inoue, K.; Kohsaka, S. Extracellular ATP or ADP Induce Chemotaxis of Cultured Microglia through Gi/o-Coupled P2Y Receptors. J. Neurosci. 2001, 21, 1975–1982. [Google Scholar] [CrossRef]
- Haynes, S.E.; Hollopeter, G.; Yang, G.; Kurpius, D.; Dailey, M.E.; Gan, W.-B.; Julius, D. The P2Y12 Receptor Regulates Microglial Activation by Extracellular Nucleotides. Nat. Neurosci. 2006, 9, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides Released by Apoptotic Cells Act as a Find-Me Signal to Promote Phagocytic Clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef]
- Ravichandran, K.S. Beginnings of a Good Apoptotic Meal: The Find-Me and Eat-Me Signaling Pathways. Immunity 2011, 35, 445–455. [Google Scholar] [CrossRef] [PubMed]
- von Kügelgen, I. Structure, Pharmacology and Roles in Physiology of the P2Y12 Receptor. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1051. [Google Scholar] [CrossRef]
- Courageot, M.-P.; Lépine, S.; Hours, M.; Giraud, F.; Sulpice, J.-C. Involvement of Sodium in Early Phosphatidylserine Exposure and Phospholipid Scrambling Induced by P2X7 Purinoceptor Activation in Thymocytes. J. Biol. Chem. 2004, 279, 21815–21823. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, R.; Shemon, A.N.; Wiley, J.S. P2X7 Receptor Activation Causes Phosphatidylserine Exposure in Human Erythrocytes. Biochem. Biophys. Res. Commun. 2007, 355, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Meng, L.; He, B.; Chen, J.; Liu, P.; Zhao, J.; Zhang, Y.; Li, M.; An, D. The Role of P2X7 Receptor in ATP-Mediated Human Leukemia Cell Death: Calcium Influx-Independent. Acta Biochim. Biophys. Sin. 2009, 41, 362–369. [Google Scholar] [CrossRef]
- Janks, L.; Sharma, C.V.R.; Egan, T.M. A Central Role for P2X7 Receptors in Human Microglia. J. Neuroinflamm. 2018, 15, 325. [Google Scholar] [CrossRef]
- Gu, B.J.; Wiley, J.S. P2X7 as a Scavenger Receptor for Innate Phagocytosis in the Brain. Br. J. Pharmacol. 2018, 175, 4195–4208. [Google Scholar] [CrossRef]
- Gu, B.J.; Zhang, W.Y.; Bendall, L.J.; Chessell, I.P.; Buell, G.N.; Wiley, J.S. Expression of P2X7 Purinoceptors on Human Lymphocytes and Monocytes: Evidence for Nonfunctional P2X7 receptors. Am. J. Physiol. Cell Physiol. 2000, 279, C1189–C1197. [Google Scholar] [CrossRef]
- Barden, J.A. Non-Functional P2X7: A Novel and Ubiquitous Target in Human Cancer. J. Clin. Cell. Immunol. 2014, 5, 4. [Google Scholar] [CrossRef]
- Gilbert, S.; Oliphant, C.; Hassan, S.; Peille, A.; Bronsert, P.; Falzoni, S.; Di Virgilio, F.; McNulty, S.; Lara, R. ATP in the Tumour Microenvironment Drives Expression of nfP2X7, a Key Mediator of Cancer Cell Survival. Oncogene 2019, 38, 194–208. [Google Scholar] [CrossRef]
- Azevedo, F.A.C.; Carvalho, L.R.B.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.L.; Leite, R.E.P.; Filho, W.J.; Lent, R.; Herculano-Houzel, S. Equal Numbers of Neuronal and Nonneuronal Cells Make the Human Brain an Isometrically Scaled-Up Primate Brain. J. Comp. Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef]
- Fan, X.; Agid, Y. At the Origin of the History of Glia. Neuroscience 2018, 385, 255–271. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as Architects of Central Nervous System Formation and Function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and Macrophages in Brain Homeostasis and Disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Bahney, J.; Herculano-Houzel, S. The Search for True Numbers of Neurons and Glial Cells in the Human Brain: A Review of 150 Years of Cell Counting. J. Comp. Neurol. 2016, 524, 3865–3895. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Brioschi, S.; Zhou, Y.; Colonna, M. Brain Parenchymal and Extraparenchymal Macrophages in Development, Homeostasis, and Disease. J. Immunol. 2020, 204, 294–305. [Google Scholar] [CrossRef]
- Verney, C.; Monier, A.; Fallet-Bianco, C.; Gressens, P. Early Microglial Colonization of the Human Forebrain and Possible Involvement in Periventricular White-Matter Injury of Preterm Infants. J. Anat. 2010, 217, 436–448. [Google Scholar] [CrossRef]
- Menassa, D.A.; Gomez-Nicola, D. Microglial Dynamics during Human Brain Development. Front. Immunol. 2018, 9, 1014. [Google Scholar] [CrossRef]
- Smolders, S.M.-T.; Kessels, S.; Vangansewinkel, T.; Rigo, J.-M.; Legendre, P.; Brône, B. Microglia: Brain Cells on the Move. Prog. Neurobiol. 2019, 178, 101612. [Google Scholar] [CrossRef] [PubMed]
- Bennett, F.C.; Bennett, M.L.; Yaqoob, F.; Mulinyawe, S.B.; Grant, G.A.; Gephart, M.H.; Plowey, E.D.; Barres, B.A. A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron 2018, 98, 1170–1183.e8. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Z.; Zhou, L.; Darmanis, S.; Neff, N.F.; Okamoto, J.; Gulati, G.; Bennett, M.L.; Sun, L.O.; Clarke, L.E.; et al. Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 2019, 101, 207–223.e10. [Google Scholar] [CrossRef]
- Hickman, S.E.; Kingery, N.D.; Ohsumi, T.K.; Borowsky, M.L.; Wang, L.-C.; Means, T.K.; El Khoury, J. The Microglial Sensome Revealed by Direct RNA Sequencing. Nat. Neurosci. 2013, 16, 1896–1905. [Google Scholar] [CrossRef]
- Lai, A.Y.; Dhami, K.S.; Dibal, C.D.; Todd, K.G. Neonatal Rat Microglia Derived from different Brain Regions Have Distinct Activation Responses. Neuron Glia Biol. 2012, 7, 5–16. [Google Scholar] [CrossRef]
- Grabert, K.; Michoel, T.; Karavolos, M.H.; Clohisey, S.; Baillie, J.K.; Stevens, M.P.; Freeman, T.C.; Summers, K.M.; McColl, B.W. Microglial Brain Region–Dependent Diversity and Selective Regional Sensitivities to Aging. Nat. Neurosci. 2016, 19, 504–516. [Google Scholar] [CrossRef]
- De Biase, L.M.; Schuebel, K.E.; Fusfeld, Z.H.; Jair, K.; Hawes, I.A.; Cimbro, R.; Zhang, H.-Y.; Liu, Q.-R.; Shen, H.; Xi, Z.-X.; et al. Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia. Neuron 2017, 95, 341–356.e6. [Google Scholar] [CrossRef]
- Ayata, P.; Badimon, A.; Strasburger, H.J.; Duff, M.K.; Montgomery, S.E.; Loh, Y.-H.E.; Ebert, A.; Pimenova, A.A.; Ramirez, B.R.; Chan, A.T.; et al. Epigenetic Regulation of Brain Region-Specific Microglia Clearance Activity. Nat. Neurosci. 2018, 21, 1049–1060. [Google Scholar] [CrossRef]
- Tan, Y.-L.; Yuan, Y.; Tian, L. Microglial Regional Heterogeneity and Its Role in the Brain. Mol. Psychiatry 2020, 25, 351–367. [Google Scholar] [CrossRef]
- Böttcher, C.; Psy, N.; Schlickeiser, S.; Sneeboer, M.A.M.; Kunkel, D.; Knop, A.; Paza, E.; Fidzinski, P.; Kraus, L.; Snijders, G.J.L.; et al. Human Microglia Regional Heterogeneity and Phenotypes Determined by Multiplexed Single-Cell Mass Cytometry. Nat. Neurosci. 2019, 22, 78–90. [Google Scholar] [CrossRef]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Böttcher, C.; Amann, L.; Sagar, S.; Scheiwe, C.; Nessler, S.; Kunz, P.; Van Loo, G.; et al. Spatial and Temporal Heterogeneity of Mouse and Human Microglia at Single-Cell Resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef] [PubMed]
- van der Poel, M.; Ulas, T.; Mizee, M.R.; Hsiao, C.-C.; Miedema, S.S.M.; Adelia, N.; Schuurman, K.G.; Helder, B.; Tas, S.W.; Schultze, J.L.; et al. Transcriptional Profiling of Human Microglia Reveals Grey–White Matter Heterogeneity and Multiple Sclerosis-Associated Changes. Nat. Commun. 2019, 10, 1139. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Sholar, P.W.; Bilbo, S.D. Sex Differences in Microglial Colonization of the Developing Rat Brain. J. Neurochem. 2012, 120, 948–963. [Google Scholar] [CrossRef]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e6. [Google Scholar] [CrossRef] [PubMed]
- Kodama, L.; Gan, L. Do Microglial Sex Differences Contribute to Sex Differences in Neurodegenerative Diseases? Trends Mol. Med. 2019, 25, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Della Torre, S.; Maggi, A. Sexual Differentiation of Microglia. Front. Neuroendocr. 2019, 52, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Hanamsagar, R.; Alter, M.D.; Block, C.S.; Sullivan, H.; Bolton, J.L.; Bilbo, S.D. Generation of a Microglial Developmental Index in Mice and in Humans Reveals a Sex Difference in Maturation and Immune Reactivity. Glia 2017, 65, 1504–1520. [Google Scholar] [CrossRef]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and Differentiation of Microglia. Front. Cell Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef]
- Askew, K.; Li, K.; Olmos-Alonso, A.; Garcia-Moreno, F.; Liang, Y.; Richardson, P.; Tipton, T.; Chapman, M.A.; Riecken, K.; Beccari, S.; et al. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep. 2017, 18, 391–405. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Z.; Xiong, S.; Sun, F.; Qin, G.; Hu, G.; Wang, J.; Zhao, L.; Liang, Y.-X.; Wu, T.; et al. Repopulated Microglia Are Solely Derived from the Proliferation of Residual Microglia after Acute Depletion. Nat. Neurosci. 2018, 21, 530–540. [Google Scholar] [CrossRef]
- Dubbelaar, M.L.; Kracht, L.; Eggen, B.J.L.; Boddeke, E.W.G.M. The Kaleidoscope of Microglial Phenotypes. Front. Immunol. 2018, 9, 1753. [Google Scholar] [CrossRef]
- Tay, T.L.; Mai, D.; Dautzenberg, J.; Fernandez-Klett, F.; Lin, G.; Sagar, S.; Datta, M.; Drougard, A.; Stempfl, T.; Ardura-Fabregat, A.; et al. A New Fate Mapping System Reveals Context-Dependent Random or Clonal Expansion of Microglia. Nat. Neurosci. 2017, 20, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Réu, P.; Khosravi, A.; Bernard, S.; Mold, J.E.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017, 20, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J.; Sisodia, S.S.; Ransohoff, R.M. Heterogeneity of CNS Myeloid Cells and Their Roles in Neurodegeneration. Nat. Neurosci. 2011, 14, 1227–1235. [Google Scholar] [CrossRef]
- Bennett, M.L.; Bennett, F.C.; Liddelow, S.A.; Ajami, B.; Zamanian, J.L.; Fernhoff, N.B.; Mulinyawe, S.B.; Bohlen, C.J.; Adil, A.; Tucker, A.; et al. New Tools for Studying Microglia in the Mouse and Human CNS. Proc. Natl. Acad. Sci. USA 2016, 113, E1738–E1746. [Google Scholar] [CrossRef] [PubMed]
- Antel, J.P.; Becher, B.; Ludwin, S.K.; Prat, A.; Quintana, F.J. Glial Cells as Regulators of Neuroimmune Interactions in the Central Nervous System. J. Immunol. 2020, 204, 251–255. [Google Scholar] [CrossRef]
- Senatorov, V.V.; Friedman, A.R.; Milikovsky, D.Z.; Ofer, J.; Saar-Ashkenazy, R.; Charbash, A.; Jahan, N.; Chin, G.; Mihaly, E.; Lin, J.M.; et al. Blood-Brain Barrier Dysfunction in Aging Induces Hyperactivation of TGFβ Signaling and Chronic Yet Reversible Neural Dysfunction. Sci. Transl. Med. 2019, 11, eaaw8283. [Google Scholar] [CrossRef]
- Bohlen, C.J.; Bennett, F.C.; Tucker, A.F.; Collins, H.Y.; Mulinyawe, S.B.; Barres, B.A. Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron 2017, 94, 759–773.e8. [Google Scholar] [CrossRef]
- Geirsdottir, L.; David, E.; Keren-Shaul, H.; Weiner, A.; Bohlen, S.C.; Neuber, J.; Balic, A.; Giladi, A.; Sheban, F.; Dutertre, C.-A.; et al. Cross-Species Single-Cell Analysis Reveals Divergence of the Primate Microglia Program. Cell 2019, 179, 1609–1622.e16. [Google Scholar] [CrossRef]
- Smith, A.M.; Dragunow, M. The Human Side of Microglia. Trends Neurosci. 2014, 37, 125–135. [Google Scholar] [CrossRef]
- Volonté, C.; D’ambrosi, N. Membrane compartments and purinergic signalling: The Purinome, a Complex Interplay among Ligands, Degrading Enzymes, Receptors and Transporters. FEBS J. 2009, 276, 318–329. [Google Scholar] [CrossRef]
- Burnstock, G. Introduction to Purinergic Signalling in the Brain. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1202. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular Function and Molecular Structure of Ecto-Nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G. Enzymes Involved in Metabolism of Extracellular Nucleotides and Nucleosides: Functional Implications and Measurement of Activities. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Taylor, N.; Fourgeaud, L.; Bhattacharya, A. The Role of Microglial P2X7: Modulation of Cell Death and Cytokine Release. J. Neuroinflamm. 2017, 14, 135. [Google Scholar] [CrossRef]
- Sperlágh, B.; Illes, P. Purinergic Modulation of Microglial Cell Activation. Purinergic Signal. 2007, 3, 117–127. [Google Scholar] [CrossRef]
- Domercq, M.; Vázquez-Villoldo, N.; Matute, C. Neurotransmitter Signaling in the Pathophysiology of Microglia. Front. Cell. Neurosci. 2013, 7, 49. [Google Scholar] [CrossRef]
- Luongo, L.; Guida, F.; Imperatore, R.; Napolitano, F.; Gatta, L.; Cristino, L.; Giordano, C.; Siniscalco, D.; Di Marzo, V.; Bellini, G.; et al. The A1 Adenosine Receptor as a New Player in Microglia Physiology. Glia 2014, 62, 122–132. [Google Scholar] [CrossRef]
- Milior, G.; Morin-Brureau, M.; Chali, F.; Le Duigou, C.; Savary, E.; Huberfeld, G.; Rouach, N.; Pallud, J.; Capelle, L.; Navarro, V.; et al. Distinct P2Y Receptors Mediate Extension and Retraction of Microglial Processes in Epileptic and Peritumoral Human Tissue. J. Neurosci. 2020, 40, 1373–1388. [Google Scholar] [CrossRef]
- Avignone, E.; Ulmann, L.; Levavasseur, F.; Rassendren, F.; Audinat, E. Status Epilepticus Induces a Particular Microglial Activation State Characterized by Enhanced Purinergic Signaling. J. Neurosci. 2008, 28, 9133–9144. [Google Scholar] [CrossRef]
- Matyash, M.; Zabiegalov, O.; Wendt, S.; Matyash, V.; Kettenmann, H. The Adenosine Generating Enzymes CD39/CD73 Control Microglial Processes Ramification in the Mouse Brain. PLoS ONE 2017, 12, e0175012. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S.; Shigemoto-Mogami, Y.; Nasu-Tada, K.; Shinozaki, Y.; Ohsawa, K.; Tsuda, M.; Joshi, B.V.; Jacobson, K.A.; Kohsaka, S.; Inoue, K. UDP Acting at P2Y6 Receptors is a Mediator of Microglial Phagocytosis. Nature 2007, 446, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Tsuda, M. P2X4 Receptors of Microglia in Neuropathic Pain. CNS Neurol. Disord.—Drug Targets 2012, 11, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Tsuda, M. Microglia in Neuropathic Pain: Cellular and Molecular Mechanisms and Therapeutic Potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Chessell, I.P.; Hatcher, J.P.; Bountra, C.; Michel, A.D.; Hughes, J.P.; Green, P.; Egerton, J.; Murfin, M.; Richardson, J.; Peck, W.L.; et al. Disruption of the P2X7 Purinoceptor Gene Abolishes Chronic Inflammatory and Neuropathic Pain. Pain 2005, 114, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Luchting, B.; Heyn, J.; Woehrle, T.; Rachinger-Adam, B.; Kreth, S.; Hinske, L.C.; Azad, S.C. Differential Expression of P2X7 Receptor and IL-1β in Nociceptive and Neuropathic Pain. J. Neuroinflamm. 2016, 13, 100. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Zhu, Z.-M.; Liu, Z.-X. The Role and Pharmacological Properties of the P2X7 Receptor in Neuropathic Pain. Brain Res. Bull. 2020, 155, 19–28. [Google Scholar] [CrossRef]
- Di Virgilio, F. Liaisons Dangereuses: P2X7 and the Inflammasome. Trends Pharmacol. Sci. 2007, 28, 465–472. [Google Scholar] [CrossRef]
- Tewari, M.; Khan, M.; Verma, M.; Coppens, J.; Kemp, J.M.; Bucholz, R.; Mercier, P.; Egan, T.M. Physiology of Cultured Human Microglia Maintained in a Defined Culture Medium. ImmunoHorizons 2021, 5, 257–272. [Google Scholar] [CrossRef]
- Hoshiko, M.; Arnoux, I.; Avignone, E.; Yamamoto, N.; Audinat, E. Deficiency of the Microglial Receptor CX3CR1 Impairs Postnatal Functional Development of Thalamocortical Synapses in the Barrel Cortex. J. Neurosci. 2012, 32, 15106–15111. [Google Scholar] [CrossRef]
- Wolf, Y.; Yona, S.; Kim, K.-W.; Jung, S. Microglia, Seen from the CX3CR1 Angle. Front. Cell. Neurosci. 2013, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, N.; Smolders, S.; Avila, A.; Notelaers, K.; Paesen, R.; Ameloot, M.; Brône, B.; Legendre, P.; Rigo, J. Complex Invasion Pattern of the Cerebral Cortex Bymicroglial Cells during Development of the Mouse Embryo. Glia 2013, 61, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.L.; Schafer, D.P. Microglia: Architects of the Developing Nervous System. Trends Cell Biol. 2016, 26, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.A.; Boddeke, H.W.G.M.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.; Petri, W.A. Microglia: Immune Regulators of Neurodevelopment. Front. Immunol. 2018, 9, 2576. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.L.; Martínez-Cerdeño, V.; Noctor, S.C. Microglia Regulate the Number of Neural Precursor Cells in the Developing Cerebral Cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef]
- Antony, J.M.; Paquin, A.; Nutt, S.L.; Kaplan, D.R.; Miller, F.D. Endogenous Microglia Regulate Development of Embryonic Cortical Precursor Cells. J. Neurosci. Res. 2011, 89, 286–298. [Google Scholar] [CrossRef]
- Rymo, S.F.; Gerhardt, H.; Sand, F.W.; Lang, R.; Uv, A.; Betsholtz, C. A Two-Way Communication between Microglial Cells and Angiogenic Sprouts Regulates Angiogenesis in Aortic Ring Cultures. PLoS ONE 2011, 6, e15846. [Google Scholar] [CrossRef]
- Reemst, K.; Noctor, S.C.; Lucassen, P.J.; Hol, E.M. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front. Hum. Neurosci. 2016, 10, 566. [Google Scholar] [CrossRef]
- Thion, M.S.; Ginhoux, F.; Garel, S. Microglia and Early Brain Development: An Intimate Journey. Science 2018, 362, 185–189. [Google Scholar] [CrossRef]
- Nelson, L.H.; Saulsbery, A.I.; Lenz, K.M. Small Cells with Big Implications: Microglia and Sex Differences in Brain Development, Plasticity and Behavioral Health. Prog. Neurobiol. 2019, 176, 103–119. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Casano, A.M.; Albert, M.; Peri, F. Developmental Apoptosis Mediates Entry and Positioning of Microglia in the Zebrafish Brain. Cell Rep. 2016, 16, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.-B. ATP Mediates Rapid Microglial Response to Local Brain Injury In Vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Rathnasamy, G.; Ling, E.-A. Biology of Microglia in the Developing Brain. J. Neuropathol. Exp. Neurol. 2017, 76, 736–753. [Google Scholar] [CrossRef]
- Sominsky, L.; De Luca, S.; Spencer, S.J. Microglia: Key Players in Neurodevelopment and Neuronal Plasticity. Int. J. Biochem. Cell Biol. 2018, 94, 56–60. [Google Scholar] [CrossRef]
- Cengiz, P.; Zafer, D.; Chandrashekhar, J.H.; Chanana, V.; Bogost, J.; Waldman, A.; Novak, B.; Kintner, D.B.; Ferrazzano, P.A. Developmental Differences in Microglia Morphology and Gene Expression during Normal Brain Development and in Response to Hypoxia-Ischemia. Neurochem. Int. 2019, 127, 137–147. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Kierdorf, K.; Prinz, M. Microglia in Steady State. J. Clin. Investig. 2017, 127, 3201–3209. [Google Scholar] [CrossRef]
- Madry, C.; Attwell, D. Receptors, Ion Channels, and Signaling Mechanisms Underlying Microglial Dynamics. J. Biol. Chem. 2015, 290, 12443–12450. [Google Scholar] [CrossRef]
- Bernier, L.-P.; Bohlen, C.J.; York, E.M.; Choi, H.B.; Kamyabi, A.; Dissing-Olesen, L.; Hefendehl, J.K.; Collins, H.Y.; Stevens, B.; Barres, B.A.; et al. Nanoscale Surveillance of the Brain by Microglia via cAMP-Regulated Filopodia. Cell Rep. 2019, 27, 2895–2908.e4. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.Ě.; Lowery, R.L.; Majewska, A.K. Microglial Interactions with Synapses Are Modulated by Visual Experience. PLoS Biol. 2010, 8, e1000527. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Schafer, D.P.; Stevens, B. Microglia Function in Central Nervous System Development and Plasticity. Cold Spring Harb. Perspect. Biol. 2015, 7, a020545. [Google Scholar] [CrossRef] [PubMed]
- Cserép, C.; Pósfai, B.; Orsolits, B.; Molnár, G.; Heindl, S.; Lénárt, N.; Fekete, R.; László, Z.I.; Lele, Z.; Schwarcz, A.D.; et al. Microglia Monitor and Protect Neuronal Function Via Specialized Somatic Purinergic Junctions in an Activity-Dependent Manner. SSRN Electron. J. 2019, 367, 528–537. [Google Scholar] [CrossRef]
- Hammond, T.R.; Robinton, D.; Stevens, B. Microglia and the Brain: Complementary Partners in Development and Disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 523–544. [Google Scholar] [CrossRef] [PubMed]
- Mody, M.; Cao, Y.; Cui, Z.; Tay, K.-Y.; Shyong, A.; Shimizu, E.; Pham, K.; Schultz, P.; Welsh, D.; Tsien, J.Z. Genome-Wide Gene Expression Profiles of the Developing Mouse Hippocampus. Proc. Natl. Acad. Sci. USA 2001, 98, 8862–8867. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef]
- Györffy, B.A.; Kun, J.; Török, G.; Bulyáki, É.; Borhegyi, Z.; Gulyássy, P.; Kis, V.; Szocsics, P.; Micsonai, A.; Matkó, J.; et al. Local Apoptotic-like Mechanisms Underlie Complement-Mediated Synaptic Pruning. Proc. Natl. Acad. Sci. USA 2018, 115, 6303–6308. [Google Scholar] [CrossRef]
- Stephan, A.H.; Barres, B.A.; Stevens, B. The Complement System: An Unexpected Role in Synaptic Pruning during Development and Disease. Annu. Rev. Neurosci. 2012, 35, 369–389. [Google Scholar] [CrossRef]
- Thion, M.S.; Garel, S. Microglia Under the Spotlight: Activity and Complement-Dependent Engulfment of Synapses. Trends Neurosci. 2018, 41, 332–334. [Google Scholar] [CrossRef]
- Li, W.; Ma, L.; Yang, G.; Gan, W.-B. REM Sleep Selectively Prunes and Maintains New Synapses in Development and Learning. Nat. Neurosci. 2017, 20, 427–437. [Google Scholar] [CrossRef]
- Seibt, J.; Frank, M.G. Primed to Sleep: The Dynamics of Synaptic Plasticity Across Brain States. Front. Syst. Neurosci. 2019, 13, 2. [Google Scholar] [CrossRef]
- Choudhury, M.E.; Miyanishi, K.; Takeda, H.; Islam, A.; Matsuoka, N.; Kubo, M.; Matsumoto, S.; Kunieda, T.; Nomoto, M.; Yano, H.; et al. Phagocytic Elimination of Synapses by Microglia during Sleep. Glia 2020, 68, 44–59. [Google Scholar] [CrossRef]
- Stowell, R.D.; Sipe, G.O.; Dawes, R.P.; Batchelor, H.N.; Lordy, K.A.; Whitelaw, B.S.; Stoessel, M.B.; Bidlack, J.M.; Brown, E.; Sur, M.; et al. Noradrenergic Signaling in the Wakeful State Inhibits Microglial Surveillance and Synaptic Plasticity in the Mouse Visual Cortex. Nat. Neurosci. 2019, 22, 1782–1792. [Google Scholar] [CrossRef]
- Wang, C.; Yue, H.; Hu, Z.; Shen, Y.; Ma, J.; Li, J.; Wang, X.-D.; Wang, L.; Sun, B.; Shi, P.; et al. Microglia Mediate Forgetting via Complement-Dependent Synaptic Elimination. Science 2020, 367, 688–694. [Google Scholar] [CrossRef]
- Ferrini, F.; De Koninck, Y. Microglia Control Neuronal Network Excitability via BDNF Signalling. Neural Plast. 2013, 2013, 429815. [Google Scholar] [CrossRef]
- Pöyhönen, S.; Er, S.; Domanskyi, A.; Airavaara, M. Effects of Neurotrophic Factors in Glial Cells in the Central Nervous System: Expression and Properties in Neurodegeneration and Injury. Front. Physiol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Akiyoshi, R.; Wake, H.; Kato, D.; Horiuchi, H.; Ono, R.; Ikegami, A.; Haruwaka, K.; Omori, T.; Tachibana, Y.; Moorhouse, A.J.; et al. Microglia Enhance Synapse Activity to Promote Local Network Synchronization. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Branchi, I.; Alboni, S.; Maggi, L. The Role of Microglia in Mediating the Effect of the Environment in Brain Plasticity and Behavior. Front. Cell. Neurosci. 2014, 8, 390. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.; Lopes-Araújo, A.; Santos-Sacramento, L.; Takeda, P.Y.; Anthony, D.C.; Malva, J.O.; Crespo-Lopez, M.E. What Do Microglia Really Do in Healthy Adult Brain? Cells 2019, 8, 1293. [Google Scholar] [CrossRef]
- Mariani, M.M.; Kielian, T. Microglia in Infectious Diseases of the Central Nervous System. J. Neuroimmune Pharmacol. 2009, 4, 448–461. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef]
- Bsibsi, M.; Ravid, R.; Gveric, D.; van Noort, J.M. Broad Expression of Toll-Like Receptors in the Human Central Nervous System. J. Neuropathol. Exp. Neurol. 2002, 61, 1013–1021. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Batista, C.R.A.; Saliba, S.W.; Yousif, N.M.; de Oliveira, A.C.P. Role of Microglia TLRs in Neurodegeneration. Front. Cell. Neurosci. 2018, 12, 329. [Google Scholar] [CrossRef]
- Kielian, T. Toll-like Receptors in Central Nervous System Glial Inflammation and Homeostasis. J. Neurosci. Res. 2006, 83, 711–730. [Google Scholar] [CrossRef]
- Nie, L.; Cai, S.-Y.; Shao, J.-Z.; Chen, J. Toll-Like Receptors, Associated Biological Roles, and Signaling Networks in Non-Mammals. Front. Immunol. 2018, 9, 1523. [Google Scholar] [CrossRef]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release Mechanisms of Major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef]
- Wright-Jin, E.C.; Gutmann, D.H. Microglia as Dynamic Cellular Mediators of Brain Function. Trends Mol. Med. 2019, 25, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Wake, H.; Moorhouse, A.J.; Miyamoto, A.; Nabekura, J. Microglia: Actively Surveying and Shaping Neuronal Circuit Structure and Function. Trends Neurosci. 2013, 36, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Song, W.M.; Colonna, M. The Identity and Function of Microglia in Neurodegeneration. Nat. Immunol. 2018, 19, 1048–1058. [Google Scholar] [CrossRef]
- Chen, X.; Firulyova, M.; Manis, M.; Herz, J.; Smirnov, I.; Aladyeva, E.; Wang, C.; Bao, X.; Finn, M.B.; Hu, H.; et al. Microglia-Mediated T Cell Infiltration Drives Neurodegeneration in Tauopathy. Nature 2023, 615, 668–677. [Google Scholar] [CrossRef]
- Wang, S.; Colonna, M. Microglia in Alzheimer’s Disease: A Target for Immunotherapy. J. Leukoc. Biol. 2019, 106, 219–227. [Google Scholar] [CrossRef]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef]
- Friedman, B.A.; Srinivasan, K.; Ayalon, G.; Meilandt, W.J.; Lin, H.; Huntley, M.A.; Cao, Y.; Lee, S.-H.; Haddick, P.C.; Ngu, H.; et al. Diverse Brain Myeloid Expression Profiles Reveal Distinct Microglial Activation States and Aspects of Alzheimer’s Disease Not Evident in Mouse Models. Cell Rep. 2018, 22, 832–847. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sudan, R.; Peng, V.; Zhou, Y.; Du, S.; Yuede, C.M.; Lei, T.; Hou, J.; Cai, Z.; Cella, M.; et al. TREM2 Drives Microglia Response to Amyloid-β via SYK-Dependent and -Independent Pathways. Cell 2022, 185, 4153–4169.e19. [Google Scholar] [CrossRef]
- Poliani, P.L.; Wang, Y.; Fontana, E.; Robinette, M.L.; Yamanishi, Y.; Gilfillan, S.; Colonna, M. TREM2 Sustains Microglial Expansion during Aging and Response to Demyelination. J. Clin. Investig. 2015, 125, 2161–2170. [Google Scholar] [CrossRef]
- Ulland, T.K.; Colonna, M. TREM2—A Key Player in Microglial Biology and Alzheimer Disease. Nat. Rev. Neurol. 2018, 14, 667–675. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.; Li, X.; Jiang, L.-L.; Gui, X.; Liu, Y.; Sun, Y.; Zhu, B.; Piña-Crespo, J.C.; Zhang, M.; et al. TREM2 Is a Receptor for β-Amyloid that Mediates Microglial Function. Neuron 2018, 97, 1023–1031.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Wang, Z.; Wang, D.; Wang, Z.; Martens, Y.A.; Wu, L.; Xu, Y.; Wang, K.; Li, J.; Huang, R.; et al. Amyloid-Beta Modulates Microglial Responses by Binding to the Triggering Receptor Expressed on Myeloid Cells 2 (TREM2). Mol. Neurodegener. 2018, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Lewis, C.A.; Ulrich, J.D.; Holtzman, D.M. Chronic TREM2 Activation Exacerbates Aβ-Associated Tau Seeding and Spreading. J. Exp. Med. 2022, 220, e20220654. [Google Scholar] [CrossRef]
- Nacmias, B.; Tedde, A.; Latorraca, S.; Piacentini, S.; Bracco, L.; Amaducci, L.; Guarnieri, B.M.; Petruzzi, C.; Ortenzi, L.; Sorbi, S. Apolipoprotein E and α1-Antichymotrypsin Polymorphism in Alzheimer’s Disease. Ann. Neurol. 1996, 40, 678–680. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.-C.; Bu, G. Apolipoprotein E and Alzheimer Disease: Pathobiology and Targeting Strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.E9. [Google Scholar] [CrossRef]
- Jurga, A.M.; Piotrowska, A.; Makuch, W.; Przewlocka, B.; Mika, J. Blockade of P2X4 Receptors Inhibits Neuropathic Pain-Related Behavior by Preventing MMP-9 Activation and, Consequently, Pronociceptive Interleukin Release in a Rat Model. Front. Pharmacol. 2017, 8, 48. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Sarti, A.C. Microglia P2X4 Receptors as Pharmacological Targets for Demyelinating Diseases. EMBO Mol. Med. 2018, 10, e9369. [Google Scholar] [CrossRef]
- Zabala, A.; Vazquez-Villoldo, N.; Rissiek, B.; Gejo, J.; Martin, A.; Palomino, A.; Perez-Samartín, A.; Pulagam, K.R.; Lukowiak, M.; Capetillo-Zarate, E.; et al. P2X4 Receptor Controls Microglia Activation and Favors Remyelination in Autoimmune Encephalitis. EMBO Mol. Med. 2018, 10, e8743. [Google Scholar] [CrossRef]
- Long, T.; He, W.; Pan, Q.; Zhang, S.; Zhang, Y.; Liu, C.; Liu, Q.; Qin, G.; Chen, L.; Zhou, J. Microglia P2X4 Receptor Contributes to Central Sensitization Following Recurrent Nitroglycerin Stimulation. J. Neuroinflamm. 2018, 15, 245. [Google Scholar] [CrossRef]
- Nörenberg, W.; Langosch, J.; Gebicke-Haerter, P.; Illes, P. Characterization and Possible Function of Adenosine 5′-Triphosphate Receptors in Activated Rat Microglia. Br. J. Pharmacol. 1994, 111, 942–950. [Google Scholar] [CrossRef]
- Walz, W.; Ilschner, S.; Ohlemeyer, C.; Banati, R.; Kettenmann, H. Extracellular ATP Activates a Cation Conductance and a K+ Conductance in Cultured Microglial Cells from Mouse Brain. J. Neurosci. 1993, 13, 4403–4411. [Google Scholar] [CrossRef] [PubMed]
- Illes, P.; Nörenberg, W.; Gebicke-Haerter, P.J. Molecular mechanisms of microglial activation. B. Voltage- and purinoceptor-operated channels in microglia. Neurochem. Int. 1996, 29, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Toulme, E.; Garcia, A.; Samways, D.; Egan, T.M.; Carson, M.J.; Khakh, B.S. P2X4 Receptors in Activated C8-B4 Cells of Cerebellar Microglial Origin. J. Gen. Physiol. 2010, 135, 333–353. [Google Scholar] [CrossRef]
- McLarnon, J.G. Purinergic Mediated Changes in Ca2+ Mobilization and Functional Responses in Microglia: Effects of Low Levels of ATP. J. Neurosci. Res. 2005, 81, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Brockhaus, J.; Verkhratsky, A.; Kettenmann, H. ATP-Induced Membrane Currents in Ameboid Microglia Acutely Isolated from Mouse Brain Slices. Neuroscience 1996, 75, 257–261. [Google Scholar] [CrossRef]
- Schilling, T.; Eder, C. Microglial K+ Channel Expression in Young Adult and Aged Mice. Glia 2015, 63, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Nörenberg, W.; Gebicke-Haerter, P.J.; Illes, P. Voltage-Dependent Potassium Channels in Activated Rat Microglia. J. Physiol. 1994, 475, 15–32. [Google Scholar] [CrossRef]
- Bianco, F.; Ceruti, S.; Colombo, A.; Fumagalli, M.; Ferrari, D.; Pizzirani, C.; Matteoli, M.; Di Virgilio, F.; Abbracchio, M.P.; Verderio, C. A Role for P2X7 in Microglial Proliferation. J. Neurochem. 2006, 99, 745–758. [Google Scholar] [CrossRef]
- Hide, I.; Tanaka, M.; Inoue, A.; Nakajima, K.; Kohsaka, S.; Inoue, K.; Nakata, Y. Extracellular ATP Triggers Tumor Necrosis Factor-α Release from Rat Microglia. J. Neurochem. 2000, 75, 965–972. [Google Scholar] [CrossRef]
- Inoue, K.; Nakajima, K.; Morimoto, T.; Kikuchi, Y.; Koizumi, S.; Illes, P.; Kohsaka, S. ATP stimulation of Ca2+-dependent plasminogen release from cultured microglia. Br. J. Pharmacol. 1998, 123, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Stroh, C.; Schulze-Osthoff, K. P2X7/P2Z Purinoreceptor-mediated Activation of Transcription Factor NFAT in Microglial Cells. J. Biol. Chem. 1999, 274, 13205–13210. [Google Scholar] [CrossRef]
- Mackenzie, A.B.; Young, M.T.; Adinolfi, E.; Surprenant, A. Pseudoapoptosis Induced by Brief Activation of ATP-gated P2X7 Receptors. J. Biol. Chem. 2005, 280, 33968–33976. [Google Scholar] [CrossRef] [PubMed]
- Parvathenani, L.K.; Tertyshnikova, S.; Greco, C.R.; Roberts, S.B.; Robertson, B.; Posmantur, R. P2X7 Mediates Superoxide Production in Primary Microglia and Is Up-Regulated in a Transgenic Mouse Model of Alzheimer’s Disease. J. Biol. Chem. 2003, 278, 13309–13317. [Google Scholar] [CrossRef]
- Pétrilli, V.; Papin, S.; Dostert, C.; Mayor, A.; Martinon, F.; Tschopp, J. Activation of the NALP3 Inflammasome is Triggered by Low Intracellular Potassium Concentration. Cell Death Differ. 2007, 14, 1583–1589. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Drinkall, S.; Lawrence, C.B.; Ossola, B.; Russell, S.; Bender, C.; Brice, N.B.; Dawson, L.A.; Harte, M.; Brough, D. The Two Pore potassium Channel THIK-1 Regulates NLRP3 Inflammasome Activation. Glia 2022, 70, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Ossola, B.; Rifat, A.; Rowland, A.; Hunter, H.; Drinkall, S.; Bender, C.; Hamlischer, M.; Teall, M.; Burley, R.; Barker, D.F.; et al. Characterisation of C101248: A Novel Selective THIK-1 Channel Inhibitor for the Modulation of Microglial NLRP3-Inflammasome. Neuropharmacology 2023, 224, 109330. [Google Scholar] [CrossRef]
- Di, A.; Xiong, S.; Ye, Z.; Malireddi, R.S.; Kometani, S.; Zhong, M.; Mittal, M.; Hong, Z.; Kanneganti, T.-D.; Rehman, J.; et al. The TWIK2 Potassium Efflux Channel in Macrophages Mediates NLRP3 Inflammasome-Induced Inflammation. Immunity 2018, 49, 56–65.e4. [Google Scholar] [CrossRef]
- Chessell, I.P.; Michel, A.D.; Humphrey, P.P.A. Properties of the Pore-Forming P2x7 Purinoceptor in Mouse NTW8 Microglial Cells. Br. J. Pharmacol. 1997, 121, 1429–1437. [Google Scholar] [CrossRef]
- Raouf, R.; Chabot-Doré, A.-J.; Ase, A.R.; Blais, D.; Séguéla, P. Differential Regulation of Microglial P2X4 and P2X7 ATP Receptors following LPS-Induced Activation. Neuropharmacology 2007, 53, 496–504. [Google Scholar] [CrossRef]
- Steinberg, T.; Silverstein, S. Extracellular ATP4-Promotes Cation Fluxes in the J774 Mouse Macrophage Cell Line. J. Biol. Chem. 1987, 262, 3118–3122. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, A.; Capece, M.; Chiozzi, P.; Falzoni, S.; Sanz, J.M.; Sarti, A.C.; Bonora, M.; Pinton, P.; Di Virgilio, F. The P2X7 Receptor Directly Interacts with the NLRP3 Inflammasome Scaffold Protein. FASEB J. 2015, 29, 2450–2461. [Google Scholar] [CrossRef]
- Virginio, C.; MacKenzie, A.; North, R.A.; Surprenant, A. Kinetics of Cell Lysis, Dye Uptake and Permeability Changes in Cells Expressing the Rat P2X7 Receptor. J. Physiol. 1999, 519, 335–346. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Schmalzing, G.; Markwardt, F. The Elusive P2X7 Macropore. Trends Cell Biol. 2018, 28, 392–404. [Google Scholar] [CrossRef]
- Peverini, L.; Beudez, J.; Dunning, K.; Chataigneau, T.; Grutter, T. New Insights Into Permeation of Large Cations Through ATP-Gated P2X Receptors. Front. Mol. Neurosci. 2018, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Rozengurt, E.; Heppel, L.A. A Specific Effect of External ATP on the Permeability of Transformed 3T3 Cells. Biochem. Biophys. Res. Commun. 1975, 67, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, S.; Gomperts, B.D. ATP Induces Nucleotide Permeability in Rat Mast Cells. Nature 1979, 279, 541–542. [Google Scholar] [CrossRef]
- Steinberg, T.H.; Newman, A.S.; Swanson, J.A.; Silverstein, S.C. ATP4-Permeabilizes the Plasma Membrane of Mouse Macrophages to Fluorescent Dyes. J. Biol. Chem. 1987, 262, 8884–8888. [Google Scholar] [CrossRef]
- Buisman, H.P.; Steinberg, T.H.; Fischbarg, J.; Silverstein, S.C.; Vogelzang, S.A.; Ince, C.; Ypey, D.L.; Leijh, P.C. Extracellular ATP Induces a Large Nonselective Conductance in Macrophage Plasma Membranes. Proc. Natl. Acad. Sci. USA 1988, 85, 7988–7992. [Google Scholar] [CrossRef]
- Ferrari, D.; Villalba, M.; Chiozzi, P.; Falzoni, S.; Ricciardi-Castagnoli, P.; Di Virgilio, F. Mouse Microglial Cells Express a Plasma Membrane Pore Gated by Extracellular ATP. J. Immunol. 1996, 156, 1531–1539. [Google Scholar] [CrossRef]
- Khakh, B.S.; Bao, X.R.; Labarca, C.; Lester, H.A. Neuronal P2X Transmitter-Gated Cation Channels Change Their Ion Selectivity in Seconds. Nat. Neurosci. 1999, 2, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Virginio, C.; MacKenzie, A.; Rassendren, F.A.; North, R.A.; Surprenant, A. Pore Dilation of Neuronal P2X Receptor Channels. Nat. Neurosci. 1999, 2, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Harkat, M.; Peverini, L.; Cerdan, A.H.; Dunning, K.; Beudez, J.; Martz, A.; Calimet, N.; Specht, A.; Cecchini, M.; Chataigneau, T.; et al. On the Permeation of Large Organic Cations through the Pore of ATP-Gated P2X Receptors. Proc. Natl. Acad. Sci. USA 2017, 114, E3786–E3795. [Google Scholar] [CrossRef]
- Chung, M.-K.; Güler, A.D.; Caterina, M.J. TRPV1 Shows Dynamic Ionic Selectivity during Agonist Stimulation. Nat. Neurosci. 2008, 11, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, A.; Michalski, K.; Mikhelzon, P.; Kawate, T. The P2X7 Receptor Forms a Dye-Permeable Pore Independent of Its Intracellular Domain but Dependent on Membrane Lipid Composition. eLife 2017, 6, e31186. [Google Scholar] [CrossRef]
- Pelegrin, P.; Surprenant, A. Pannexin-1 Mediates Large Pore Formation and Interleukin-1β Release by the ATP-Gated P2X7 Receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, P.; Surprenant, A. The P2X7 Receptor–Pannexin Connection to Dye Uptake and IL-1β Release. Purinergic Signal. 2009, 5, 129–137. [Google Scholar] [CrossRef]
- Janks, L.; Sprague, R.S.; Egan, T.M. ATP-Gated P2X7 Receptors Require Chloride Channels to Promote Inflammation in Human Macrophages. J. Immunol. 2019, 202, 883–898. [Google Scholar] [CrossRef]
- Duan, S.; Anderson, C.M.; Keung, E.C.; Chen, Y.; Chen, Y.; Swanson, R.A. P2X7 Receptor-Mediated Release of Excitatory Amino Acids from Astrocytes. J. Neurosci. 2003, 23, 1320–1328. [Google Scholar] [CrossRef]
- Marques-Da-Silva, C.; Chaves, M.M.; Rodrigues, J.C.; Corte-Real, S.; Coutinho-Silva, R.; Persechini, P.M. Differential Modulation of ATP-Induced P2X7-Associated Permeabilities to Cations and Anions of Macrophages by Infection with Leishmania amazonensis. PLoS ONE 2011, 6, e25356. [Google Scholar] [CrossRef][Green Version]
- Ugur, M.; Ugur, Ö. A Mechanism-Based Approach to P2X7 Receptor Action. Mol. Pharmacol. 2019, 95, 442–450. [Google Scholar] [CrossRef]
- Schachter, J.; Motta, A.P.; Zamorano, A.d.S.; da Silva-Souza, H.A.; Guimarães, M.Z.P.; Persechini, P.M. ATP-Induced P2X7-Associated Uptake of Large Molecules Involves Distinct Mechanisms for Cations and Anions in Macrophages. J. Cell Sci. 2008, 121, 3261–3270. [Google Scholar] [CrossRef]
- Ferrari, D.; Chiozzi, P.; Falzoni, S.; Susino, M.D.; Collo, G.; Buell, G.; Di Virgilio, F. ATP-Mediated Cytotoxicity in Microglial Cells. Neuropharmacology 1997, 36, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Guerra, G.P.; Rubin, M.A.; Mello, C.F. Modulation of Learning and Memory by Natural Polyamines. Pharmacol. Res. 2016, 112, 99–118. [Google Scholar] [CrossRef]
- Charras, G.T.; Coughlin, M.; Mitchison, T.J.; Mahadevan, L. Life and Times of a Cellular Bleb. Biophys. J. 2008, 94, 1836–1853. [Google Scholar] [CrossRef] [PubMed]
- Weng, N.J.-H.; Talbot, P. The P2X7 Receptor is an Upstream Regulator of Dynamic Blebbing and a Pluripotency Marker in Human Embryonic Stem Cells. Stem Cell Res. 2017, 23, 39–49. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, A.; Wilson, H.L.; Kiss-Toth, E.; Dower, S.K.; North, R.A.; Surprenant, A. Rapid Secretion of Interleukin-1β by Microvesicle Shedding. Immunity 2001, 15, 825–835. [Google Scholar] [CrossRef]
- Bianco, F.; Perrotta, C.; Novellino, L.; Francolini, M.; Riganti, L.; Menna, E.; Saglietti, L.; Schuchman, E.H.; Furlan, R.; Clementi, E.; et al. Acid Sphingomyelinase Activity Triggers Microparticle Release from Glial Cells. EMBO J. 2009, 28, 1043–1054. [Google Scholar] [CrossRef]
- Charras, G.; Paluch, E. Blebs Lead the Way: How to Migrate without Lamellipodia. Nat. Rev. Mol. Cell Biol. 2008, 9, 730–736. [Google Scholar] [CrossRef]
- Babiychuk, E.B.; Monastyrskaya, K.; Potez, S.; Draeger, A. Blebbing Confers Resistance against Cell Lysis. Cell Death Differ. 2011, 18, 80–89. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and INFLAMMATION. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef]
- Inoue, K. Microglial Activation by Purines and Pyrimidines. Glia 2002, 40, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Shieh, C.; Heinrich, A.; Serchov, T.; van Calker, D.; Biber, K. P2X7-Dependent, but Differentially Regulated Release of IL-6, CCL2, and TNF-α in Cultured Mouse Microglia. Glia 2014, 62, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Savio, L.E.B.; de Andrade Mello, P.; Da Silva, C.G.; Coutinho-Silva, R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.M.; Di Virgilio, F. Kinetics and Mechanism of ATP-Dependent IL-1β Release from Microglial Cells. J. Immunol. 2000, 164, 4893–4898. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hide, I.; Ido, K.; Kohsaka, S.; Inoue, K.; Nakata, Y. Production and Release of Neuroprotective Tumor Necrosis Factor by P2X7 Receptor-Activated Microglia. J. Neurosci. 2004, 24, 1–7. [Google Scholar] [CrossRef]
- Facci, L.; Barbierato, M.; Zusso, M.; Skaper, S.D.; Giusti, P. Serum Amyloid A Primes Microglia for ATP-Dependent Interleukin-1β Release. J. Neuroinflamm. 2018, 15, 164. [Google Scholar] [CrossRef]
- Ferrari, D.; Pizzirani, C.; Adinolfi, E.; Lemoli, R.M.; Curti, A.; Idzko, M.; Panther, E.; Di Virgilio, F. The P2X7 Receptor: A Key Player in IL-1 Processing and Release. J. Immunol. 2006, 176, 3877–3883. [Google Scholar] [CrossRef]
- Munoz, F.M.; Patel, P.A.; Gao, X.; Mei, Y.; Xia, J.; Gilels, S.; Hu, H. Reactive Oxygen Species Play a Role in P2X7 Receptor-Mediated IL-6 Production in Spinal Astrocytes. Purinergic Signal. 2020, 16, 97–107. [Google Scholar] [CrossRef]
- Kim, S.Y.; Moon, J.H.; Lee, H.G.; Kim, S.U.; Lee, Y.B. ATP Released from β-Amyloid-Stimulated Microglia Induces Reactive Oxygen Species Production in an Autocrine Fashion. Exp. Mol. Med. 2007, 39, 820–827. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; De Marchi, E.; Orioli, E.; Pegoraro, A.; Di Virgilio, F. Role of the P2X7 Receptor in Tumor-Associated Inflammation. Curr. Opin. Pharmacol. 2019, 47, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Amoroso, F.; Capece, M.; Rotondo, A.; Cangelosi, D.; Ferracin, M.; Franceschini, A.; Raffaghello, L.; Pistoia, V.; Varesio, L.; Adinolfi, E. The P2X7 Receptor is a Key Modulator of the PI3K/GSK3β/VEGF Signaling Network: Evidence in Experimental Neuroblastoma. Oncogene 2015, 34, 5240–5251. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Adinolfi, E. Extracellular Purines, Purinergic Receptors and Tumor Growth. Oncogene 2017, 36, 293–303. [Google Scholar] [CrossRef]
- Scarpellino, G.; Genova, T.; Munaron, L. Purinergic P2X7 Receptor: A Cation Channel Sensitive to Tumor Microenvironment. Recent Patents Anti-Cancer Drug Discov. 2019, 14, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Arnaud-Sampaio, V.F.; Rabelo, I.L.A.; Ulrich, H.; Lameu, C. The P2X7 Receptor in the Maintenance of Cancer Stem Cells, Chemoresistance and Metastasis. Stem Cell Rev. Rep. 2019, 16, 288–300. [Google Scholar] [CrossRef]
- Hui, L.; Chen, Y. Tumor Microenvironment: Sanctuary of the Devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar] [CrossRef]
- Bianchi, G.; Vuerich, M.; Pellegatti, P.; Marimpietri, D.; Emionite, L.; Marigo, I.; Bronte, V.; Di Virgilio, F.; Pistoia, V.; Raffaghello, L. ATP/P2X7 Axis Modulates Myeloid-Derived Suppressor Cell Functions in Neuroblastoma Microenvironment. Cell Death Dis. 2014, 5, e1135. [Google Scholar] [CrossRef]
- Adinolfi, E.; Raffaghello, L.; Giuliani, A.L.; Cavazzini, L.; Capece, M.; Chiozzi, P.; Bianchi, G.; Kroemer, G.; Pistoia, V.; Di Virgilio, F. Expression of P2X7 Receptor Increases In Vivo Tumor Growth. Cancer Res 2012, 72, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, L.S.; Capece, M.; Salaro, E.; Sarti, A.C.; Falzoni, S.; Pereira, M.S.L.; De Bastiani, M.A.; Scholl, J.N.; Battastini, A.M.O.; Di Virgilio, F. Role of the P2X7 Receptor in In Vitro and In Vivo Glioma Tumor Growth. Oncotarget 2019, 10, 4840–4856. [Google Scholar] [CrossRef]
- Virgilio, F.D. Purines, Purinergic Receptors, and Cancer. Cancer Res. 2012, 72, 5441–5447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, F.; Wang, L.; Lou, Y. A438079 Affects Colorectal Cancer Cell Proliferation, migration, apoptosis, and pyroptosis by inhibiting the P2X7 receptor. Biochem. Biophys. Res. Commun. 2021, 558, 147–153. [Google Scholar] [CrossRef]
- Kan, L.K.; Williams, D.; Drummond, K.; O’Brien, T.; Monif, M. The Role of Microglia and P2X7 Receptors in Gliomas. J. Neuroimmunol. 2019, 332, 138–146. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Apetoh, L.; Tesniere, A.; Aymeric, L.; Ma, Y.; Ortiz, C.; Vermaelen, K.; Panaretakis, T.; Mignot, G.; Ullrich, E.; et al. Activation of the NLRP3 inflammasome in Dendritic Cells Induces IL-1β–Dependent Adaptive Immunity against Tumors. Nat. Med. 2009, 15, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Capece, M.; Franceschini, A.; Falzoni, S.; Giuliani, A.L.; Rotondo, A.; Sarti, A.C.; Bonora, M.; Syberg, S.; Corigliano, D.; et al. Accelerated Tumor Progression in Mice Lacking the ATP Receptor P2X7. Cancer Res 2015, 75, 635–644. [Google Scholar] [CrossRef]
- Hoelzinger, D.B.; Demuth, T.; Berens, M.E. Autocrine Factors That Sustain Glioma Invasion and Paracrine Biology in the Brain Microenvironment. JNCI J. Natl. Cancer Inst. 2007, 99, 1583–1593. [Google Scholar] [CrossRef]
- Cekic, C.; Day, Y.-J.; Sag, D.; Linden, J. Myeloid Expression of Adenosine A2A Receptor Suppresses T and NK Cell Responses in the Solid Tumor Microenvironment. Cancer Res 2014, 74, 7250–7259. [Google Scholar] [CrossRef]
- Tarassishin, L.; Lim, J.; Weatherly, D.B.; Angeletti, R.H.; Lee, S.C. Interleukin-1-Induced Changes in the Glioblastoma Secretome Suggest Its Role in Tumor Progression. J. Proteom. 2014, 99, 152–168. [Google Scholar] [CrossRef]
- Illes, P. P2X7 Receptors Amplify CNS Damage in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 5996. [Google Scholar] [CrossRef]
- Tewari, M.; Seth, P. Emerging Role of P2X7 Receptors in CNS Health and Disease. Ageing Res. Rev. 2015, 24, 328–342. [Google Scholar] [CrossRef]
- Hogquist, K.A.; Nett, M.A.; Unanue, E.R.; Chaplin, D.D. Interleukin 1 is Processed and Released during Apoptosis. Proc. Natl. Acad. Sci. USA 1991, 88, 8485–8489. [Google Scholar] [CrossRef]
- Brough, D.; Le Feuvre, R.A.; Iwakura, Y.; Rothwell, N.J. Purinergic (P2X7) Receptor Activation of Microglia Induces Cell Death via an Interleukin-1-Independent Mechanism. Mol. Cell. Neurosci. 2002, 19, 272–280. [Google Scholar] [CrossRef]
- Mehta, V.B.; Hart, J.; Wewers, M.D. ATP-stimulated Release of Interleukin (IL)-1β and IL-18 Requires Priming by Lipopolysaccharide and Is Independent of Caspase-1 Cleavage. J. Biol. Chem. 2001, 276, 3820–3826. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, L.C.; Fisette, P.L.; Sommer, J.A.; Watters, J.J.; Prabhu, U.; Dubyak, G.R.; Proctor, R.A.; Bertics, P.J. Cutting Edge: The Nucleotide Receptor P2X7 Contains Multiple Protein- and Lipid-Interaction Motifs Including a Potential Binding Site for Bacterial Lipopolysaccharide. J. Immunol. 2001, 167, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.; Yerbury, J.J.; Sluyter, R. P2X7 Receptor Activation Induces Reactive Oxygen Species Formation and Cell Death in Murine EOC13 Microglia. Mediat. Inflamm. 2013, 2013, 271813. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.V.; Moura-Neto, L.I.; Martins, C.S.; Silva, P.I.M.; Lopes, M.B.; Leitão, R.F.C.; Coelho-Aguiar, J.M.; Moura-Neto, V.; Warren, C.A.; Costa, D.V.; et al. Role of Pannexin-1-P2X7R Signaling on Cell Death and Pro-Inflammatory Mediator Expression Induced by Clostridioides Difficile Toxins in Enteric Glia. Front. Immunol. 2022, 13, 956340. [Google Scholar] [CrossRef]
- Cavaliere, F.; Dinkel, K.; Reymann, K. Microglia Response and P2 Receptor Participation in Oxygen/Glucose Deprivation-Induced Cortical Damage. Neuroscience 2005, 136, 615–623. [Google Scholar] [CrossRef]
- Cavaliere, F.; D’Ambrosi, N.; Sancesario, G.; Bernardi, G.; Volonté, C. Hypoglycaemia-Induced Cell Death: Features of Neuroprotection by the P2 Receptor Antagonist Basilen Blue. Neurochem. Int. 2000, 38, 199–207. [Google Scholar] [CrossRef]
- Eyo, U.B.; Miner, S.A.; Ahlers, K.E.; Wu, L.-J.; Dailey, M.E. P2X7 Receptor Activation Regulates Microglial Cell Death during Oxygen-Glucose Deprivation. Neuropharmacology 2013, 73, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Xie, L.; Liu, Y.; Liu, T.; Yang, P.; Hu, J.; Peng, Z.; Luo, K.; Du, M.; Chen, C. Astragalus polysaccharide (APS) Exerts Protective Effect against Acute Ischemic Stroke (AIS) through Enhancing M2 Micoglia Polarization by Regulating Adenosine Triphosphate (ATP)/Purinergic Receptor (P2X7R) Axis. Bioengineered 2022, 13, 4468–4480. [Google Scholar] [CrossRef]
- Lopez, J.A.S.; González, H.M.; Léger, G.C. Alzheimer’s Disease. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 167, pp. 231–255. [Google Scholar]
- De-Paula, V.J.; Radanovic, M.; Diniz, B.S.; Forlenza, O.V. Alzheimer’s Disease. Subcell. Biochem. 2012, 65, 329–352. [Google Scholar]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of Pro-Inflammatory Cytokines Released from Microglia in Alzheimer’s Disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar]
- Nizami, S.; Hall-Roberts, H.; Warrier, S.; Cowley, S.A.; Di Daniel, E. Microglial Inflammation and Phagocytosis in Alzheimer’s Disease: Potential Therapeutic Targets. Br. J. Pharmacol. 2019, 176, 3515–3532. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.; Phasuk, S.; Liu, I. Modulation of Microglia Activation and Alzheimer’s Disease: CX3 Chemokine Ligand 1/CX3CR and P2X7R Signaling. Tzu Chi Med. J. 2021, 33, 1–6. [Google Scholar] [CrossRef] [PubMed]
- McLarnon, J.G.; Ryu, J.K.; Walker, D.G.; Choi, H.B. Upregulated Expression of Purinergic P2X(7) Receptor in Alzheimer Disease and Amyloid-Beta Peptide-Treated Microglia and in Peptide-Injected Rat Hippocampus. J. Neuropathol. Exp. Neurol. 2006, 65, 1090–1097. [Google Scholar] [CrossRef]
- Lee, H.G.; Won, S.M.; Gwag, B.J.; Lee, Y.B. Microglial P2X Receptor Expression Is Accompanied by Neuronal Damage in the Cerebral Cortex of the APPswe/PS1dE9 Mouse Model of Alzheimer’s Disease. Exp. Mol. Med. 2011, 43, 7–14. [Google Scholar] [CrossRef]
- Sanz, J.M.; Chiozzi, P.; Ferrari, D.; Colaianna, M.; Idzko, M.; Falzoni, S.; Fellin, R.; Trabace, L.; Di Virgilio, F. Activation of Microglia by Amyloid β Requires P2X7 Receptor Expression. J. Immunol. 2009, 182, 4378–4385. [Google Scholar] [CrossRef]
- Chiozzi, P.; Sarti, A.C.; Sanz, J.M.; Giuliani, A.L.; Adinolfi, E.; Vultaggio-Poma, V.; Falzoni, S.; Di Virgilio, F. Amyloid β-Dependent Mitochondrial Toxicity in Mouse Microglia Requires P2X7 Receptor Expression and Is Prevented by Nimodipine. Sci. Rep. 2019, 9, 6475. [Google Scholar] [CrossRef]
- Thawkar, B.S.; Kaur, G. Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 Pathway in Microglia: Novel Therapeutic Opportunities in Neuroinflammation Induced Early-Stage Alzheimer’s Disease. J. Neuroimmunol. 2019, 326, 62–74. [Google Scholar] [CrossRef]
- Francistiová, L.; Bianchi, C.; Di Lauro, C.; Sebastián-Serrano, Á.; De Diego-García, L.; Kobolák, J.; Dinnyés, A.; Díaz-Hernández, M. The Role of P2X7 Receptor in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 94. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lin, R.-R.; Tao, Q.-Q. The role of P2X7R in Neuroinflammation and Implications in Alzheimer’s Disease. Life Sci. 2021, 271, 119187. [Google Scholar] [CrossRef]
- Carvalho, K.; Martin, E.; Ces, A.; Sarrazin, N.; Lagouge-Roussey, P.; Nous, C.; Boucherit, L.; Youssef, I.; Prigent, A.; Faivre, E.; et al. P2X7-Deficiency Improves Plasticity and Cognitive Abilities in a Mouse Model of Tauopathy. Prog. Neurobiol. 2021, 206, 102139. [Google Scholar] [CrossRef]
- Jeon, S.G.; Kang, M.; Kim, Y.-S.; Kim, D.-H.; Nam, D.W.; Song, E.J.; Mook-Jung, I.; Moon, M. Intrahippocampal Injection of a Lentiviral Vector Expressing Neurogranin Enhances Cognitive Function in 5XFAD Mice. Exp. Mol. Med. 2018, 50, e461. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Amar, M.; Dalle, C.; Youssef, I.; Boucher, C.; Le Duigou, C.; Brückner, M.; Prigent, A.; Sazdovitch, V.; Halle, A.; et al. New Role of P2X7 Receptor in an Alzheimer’s disease Mouse Model. Mol. Psychiatry 2019, 24, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Frailes, C.; Di Lauro, C.; Bianchi, C.; de Diego-García, L.; Sebastián-Serrano, Á.; Boscá, L.; Díaz-Hernández, M. Amyloid Peptide Induced Neuroinflammation Increases the P2X7 Receptor Expression in Microglial Cells, Impacting on Its Functionality. Front. Cell. Neurosci. 2019, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.M.; Santana, I.; Swerdlow, R.H.; Oliveira, C.R. Mitochondria Dysfunction of Alzheimer’s Disease Cybrids Enhances Aβ Toxicity. J. Neurochem. 2004, 89, 1417–1426. [Google Scholar] [CrossRef]
- Chen, J.X.; Yan, S.D. Amyloid-β-Induced Mitochondrial Dysfunction. J. Alzheimer’s Dis. 2007, 12, 177–184. [Google Scholar] [CrossRef]
- Tobore, T.O. On the Central Role of Mitochondria Dysfunction and Oxidative Stress in Alzheimer’s Disease. Neurol. Sci. 2019, 40, 1527–1540. [Google Scholar] [CrossRef]
- Sanz, J.M.; Falzoni, S.; Rizzo, R.; Cipollone, F.; Zuliani, G.; Di Virgilio, F. Possible Protective Role of the 489C>T P2X7R Polymorphism in Alzheimer’s Disease. Exp. Gerontol. 2014, 60, 117–119. [Google Scholar] [CrossRef]
- Islam, J.; Cho, J.-A.; Kim, J.-Y.; Park, K.-S.; Koh, Y.-J.; Chung, C.Y.; Lee, E.-J.; Nam, S.J.; Lee, K.; Kim, S.-H.; et al. GPCR19 Regulates P2X7R-Mediated NLRP3 Inflammasomal Activation of Microglia by Amyloid β in a Mouse Model of Alzheimer’s Disease. Front. Immunol. 2022, 13, 766919. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jiang, X.; Fang, X.; Wang, Y.; Liu, P.; Ling, J.; Yu, L.; Jiang, M.; Tang, C. Transauricular Vagal Nerve Stimulation at 40 Hz Inhibits Hippocampal P2X7R/NLRP3/Caspase-1 Signaling and Improves Spatial Learning and Memory in 6-Month-Old APP/PS1 Mice. Neuromodul. Technol. Neural Interface 2023, 26, 589–600. [Google Scholar] [CrossRef]
- Doorn, K.J.; Moors, T.; Drukarch, B.; van de Berg, W.D.; Lucassen, P.J.; van Dam, A.-M. Microglial Phenotypes and Toll-like Receptor 2 in the Substantia Nigra and Hippocampus of Incidental Lewy Body Disease Cases and Parkinson’s Disease Patients. Acta Neuropathol. Commun. 2014, 2, 90. [Google Scholar] [CrossRef] [PubMed]
- Joers, V.; Tansey, M.G.; Mulas, G.; Carta, A.R. Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog. Neurobiol. 2017, 155, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Carmo, M.R.; Menezes, A.P.F.; Nunes, A.C.L.; Pliássova, A.; Rolo, A.P.; Palmeira, C.M.; Cunha, R.A.; Canas, P.M.; Andrade, G.M. The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology 2014, 81, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Van Weehaeghe, D.; Koole, M.; Schmidt, M.E.; Deman, S.; Jacobs, A.H.; Souche, E.; Serdons, K.; Sunaert, S.; Bormans, G.; Vandenberghe, W.; et al. [11C]JNJ54173717, a Novel P2X7 Receptor Radioligand as Marker for Neuroinflammation: Human Biodistribution, Dosimetry, Brain Kinetic Modelling and Quantification of Brain P2X7 Receptors in Patients with Parkinson’s Disease and Healthy Volunteers. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2051–2064. [Google Scholar] [CrossRef]
- Ren, C.; Li, L.-X.; Dong, A.-Q.; Zhang, Y.-T.; Hu, H.; Mao, C.-J.; Wang, F.; Liu, C.-F. Depression Induced by Chronic Unpredictable Mild Stress Increases Susceptibility to Parkinson’s Disease in Mice via Neuroinflammation Mediated by P2X7 Receptor. ACS Chem. Neurosci. 2021, 12, 1262–1272. [Google Scholar] [CrossRef]
- Jiang, T.; Hoekstra, J.; Heng, X.; Kang, W.; Ding, J.; Liu, J.; Chen, S.; Zhang, J. P2X7 Receptor is Critical in α-Synuclein–Mediated Microglial NADPH Oxidase Activation. Neurobiol. Aging 2015, 36, 2304–2318. [Google Scholar] [CrossRef]
- Crabbé, M.; Van der Perren, A.; Bollaerts, I.; Kounelis, S.; Baekelandt, V.; Bormans, G.; Casteels, C.; Moons, L.; Van Laere, K. Increased P2X7 Receptor Binding Is Associated with Neuroinflammation in Acute but Not Chronic Rodent Models for Parkinson’s Disease. Front. Neurosci. 2019, 13, 799. [Google Scholar] [CrossRef]
- Kumar, S.; Mishra, A.; Krishnamurthy, S. Purinergic Antagonism Prevents Mitochondrial Dysfunction and Behavioral Deficits Associated with Dopaminergic Toxicity Induced by 6-OHDA in Rats. Neurochem. Res. 2017, 42, 3414–3430. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Xie, X.; Luo, X.-G.; Shang, H.; He, Z.-Y. Inhibiting Purinergic P2X7 Receptors with the Antagonist Brilliant Blue G is Neuroprotective in an Intranigral Lipopolysaccharide Animal Model of Parkinson’s Disease. Mol. Med. Rep. 2017, 15, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Xu, C.; Gao, S.; Zhang, J.; Zheng, J.; Wu, X.; Lu, Q.; Cao, L.; Yang, D.; Xu, J.; et al. Cathepsin L-Containing Exosomes from α-Synuclein-Activated Microglia Induce Neurotoxicity through the P2X7 Receptor. npj Park. Dis. 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lou, Y.; Liu, G.; Wang, X.; Qian, Y.; Ding, J.; Chen, S.; Xiao, Q. Microglia P2Y6 Receptor Is Related to Parkinson’s Disease through Neuroinflammatory Process. J. Neuroinflamm. 2017, 14, 38. [Google Scholar] [CrossRef]
- Oliveira-Giacomelli, Á.; Albino, C.M.; de Souza, H.D.N.; Corrêa-Velloso, J.; Santos, A.P.d.J.; Baranova, J.; Ulrich, H. P2Y6 and P2X7 Receptor Antagonism Exerts Neuroprotective/Neuroregenerative Effects in an Animal Model of Parkinson’s Disease. Front. Cell. Neurosci. 2019, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in Adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A Practical Clinical Definition of Epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef]
- Boison, D. Adenosine Dysfunction and Adenosine Kinase in Epileptogenesis. Open Neurosci. J. 2010, 4, 93–101. [Google Scholar] [CrossRef]
- Eyo, U.B.; Murugan, M.; Wu, L. Microglia–Neuron Communication in Epilepsy. Glia 2017, 65, 5–18. [Google Scholar] [CrossRef]
- Chen, F.; He, X.; Luan, G.; Li, T. Role of DNA Methylation and Adenosine in Ketogenic Diet for Pharmacoresistant Epilepsy: Focus on Epileptogenesis and Associated Comorbidities. Front. Neurol. 2019, 10, 119. [Google Scholar] [CrossRef]
- Alyu, F.; Dikmen, M. Inflammatory Aspects of epileptogenesis: Contribution of Molecular Inflammatory Mechanisms. Acta Neuropsychiatr. 2017, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Abiega, O.; Beccari, S.; Diaz-Aparicio, I.; Nadjar, A.; Layé, S.; Leyrolle, Q.; Gómez-Nicola, D.; Domercq, M.; Pérez-Samartín, A.; Sánchez-Zafra, V.; et al. Neuronal Hyperactivity Disturbs ATP Microgradients, Impairs Microglial Motility, and Reduces Phagocytic Receptor Expression Triggering Apoptosis/Microglial Phagocytosis Uncoupling. PLOS Biol. 2016, 14, e1002466. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Alves, M.; Sheedy, C.; Henshall, D.C. ATPergic Signalling during Seizures and Epilepsy. Neuropharmacology 2016, 104, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Deng, X.; Xie, Y.; Chen, Y. Astaxanthin Attenuates Neuroinflammation in Status Epilepticus Rats by Regulating the ATP-P2X7R Signal. Drug Des. Dev. Ther. 2020, 14, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Dale, N.; Frenguelli, B.G. Release of Adenosine and ATP during Ischemia and Epilepsy. Curr. Neuropharmacol. 2009, 7, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Weissberg, I.; Reichert, A.; Heinemann, U.; Friedman, A. Blood-Brain Barrier Dysfunction in Epileptogenesis of the Temporal Lobe. Epilepsy Res. Treat. 2011, 2011, 143908. [Google Scholar] [CrossRef]
- Kim, S.Y.; Buckwalter, M.; Soreq, H.; Vezzani, A.; Kaufer, D. Blood-Brain Barrier Dysfunction-Induced Inflammatory Signaling in Brain Pathology and Epileptogenesis. Epilepsia 2012, 53 (Suppl. S6), 37–44. [Google Scholar] [CrossRef]
- Hong, S.; Xin, Y.; JiaWen, W.; ShuQin, Z.; GuiLian, Z.; HaiQin, W.; Zhen, G.; HongWei, R.; YongNan, L. The P2X7 Receptor in Activated Microglia Promotes Depression- and Anxiety-like Behaviors in Lithium -Pilocarpine Induced Epileptic Rats. Neurochem. Int. 2020, 138, 104773. [Google Scholar] [CrossRef]
- Lee, D.-S.; Kim, J.-E. Protein Disulfide Isomerase-Mediated S-Nitrosylation Facilitates Surface Expression of P2X7 Receptor Following Status Epilepticus. J. Neuroinflamm. 2021, 18, 14. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Feng, B.; Zhang, Q.; Tong, J.; Wang, M.; Lu, C.; Peng, S. Seizures in PPT1 Knock-In Mice Are Associated with Inflammatory Activation of Microglia. Int. J. Mol. Sci. 2022, 23, 5586. [Google Scholar] [CrossRef]
- Smith, J.; Méndez, A.M.; Alves, M.; Parras, A.; Conte, G.; Bhattacharya, A.; Ceusters, M.; Nicke, A.; Henshall, D.C.; Jimenez-Mateos, E.M.; et al. The P2X7 Receptor Contributes to Seizures and Inflammation-Driven Long-Lasting Brain Hyperexcitability Following Hypoxia in Neonatal Mice. Br. J. Pharmacol. 2023, 180, 1710–1729. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tewari, M.; Michalski, S.; Egan, T.M. Modulation of Microglial Function by ATP-Gated P2X7 Receptors: Studies in Rat, Mice and Human. Cells 2024, 13, 161. https://doi.org/10.3390/cells13020161
Tewari M, Michalski S, Egan TM. Modulation of Microglial Function by ATP-Gated P2X7 Receptors: Studies in Rat, Mice and Human. Cells. 2024; 13(2):161. https://doi.org/10.3390/cells13020161
Chicago/Turabian StyleTewari, Manju, Stephanie Michalski, and Terrance M. Egan. 2024. "Modulation of Microglial Function by ATP-Gated P2X7 Receptors: Studies in Rat, Mice and Human" Cells 13, no. 2: 161. https://doi.org/10.3390/cells13020161
APA StyleTewari, M., Michalski, S., & Egan, T. M. (2024). Modulation of Microglial Function by ATP-Gated P2X7 Receptors: Studies in Rat, Mice and Human. Cells, 13(2), 161. https://doi.org/10.3390/cells13020161






