Current Advances in Corneal Stromal Stem Cell Biology and Therapeutic Applications
Abstract
:1. Introduction
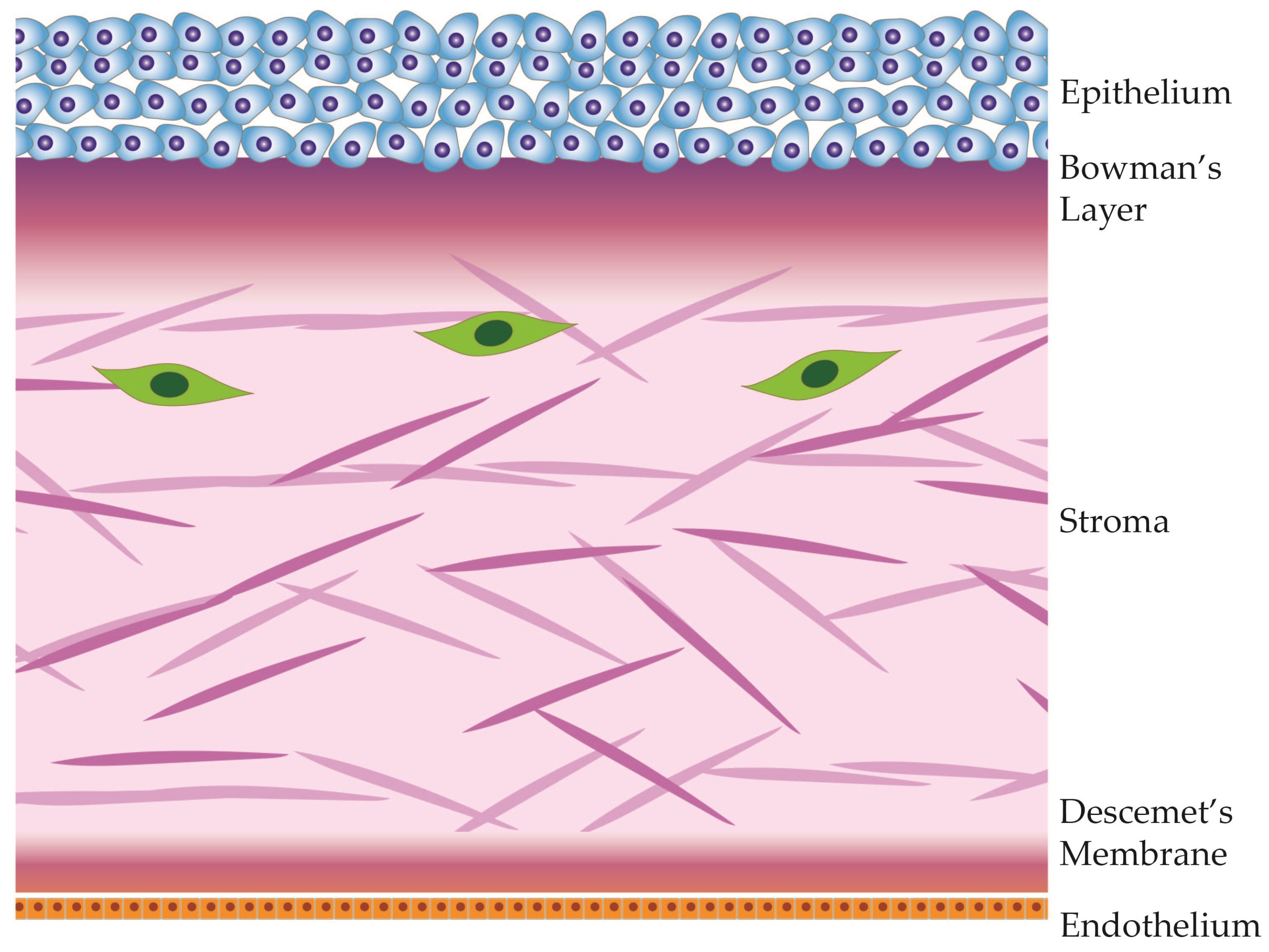
2. Corneal Anatomy
3. Identity of Corneal Stromal Stem Cells
3.1. Origins and Potency
3.2. Molecular Markers
3.3. Location
3.4. Homeostasis and Tissue Integrity
3.5. Regeneration of Keratocytes
3.6. Maintenance of Corneal Clarity
4. Interactions within the Stromal Stem Cell Niche
5. Stromal Stem Cell Defects
6. Stromal Stem Cell Therapy—Potential for Exosome Therapy
7. Stromal Stem Cell Rejuvenation
8. Future Research and Therapy Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lucas, R.M.; Norval, M.; Wright, C.Y. Solar ultraviolet radiation in Africa: A systematic review and critical evaluation of the health risks and use of photoprotection. Photochem. Photobiol. Sci. 2016, 15, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Kong, X.; Wolle, M.; Gasquet, N.; Ssekasanvu, J.; Mariotti, S.P.; Bourne, R.; Taylor, H.; Resnikoff, S.; West, S. Global Trends in Blindness and Vision Impairment Resulting from Corneal Opacity 1984–2020: A Meta-analysis. Ophthalmology 2023, 130, 863–871. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e130–e143. [Google Scholar] [CrossRef]
- Bilchut, A.H.; Burroughs, H.R.; Oldenburg, C.E.; Lietman, T.M. Trachoma Control: A Glass Half Full? Am. J. Trop. Med. Hyg. 2023, 108, 237–238. [Google Scholar] [CrossRef]
- He, J.; Chen, A.; Zou, M.; Young, C.A.; Jin, L.; Zheng, D.; Jin, G.; Congdon, N. Time trends and heterogeneity in the disease burden of trachoma, 1990–2019: A global analysis. Br. J. Ophthalmol. 2023, 107, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.S.; Schottman, T.; Gulati, M. Turning the tide of corneal blindness. Indian J. Ophthalmol. 2012, 60, 423–427. [Google Scholar] [CrossRef]
- Tidke, S.C.; Tidake, P. A Review of Corneal Blindness: Causes and Management. Cureus 2022, 14, e30097. [Google Scholar] [CrossRef]
- Liu, S.; Wong, Y.L.; Walkden, A. Current Perspectives on Corneal Transplantation. Clin. Ophthalmol. 2022, 16, 631–646. [Google Scholar] [CrossRef]
- Wong, Y.L.; Liu, S.; Walkden, A. Current Perspectives on Corneal Transplantation (Part 2). Clin. Ophthalmol. 2022, 16, 647–659. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Cursiefen, C.; Hos, D. Cutting Edge: Novel Treatment Options Targeting Corneal Neovascularization to Improve High-Risk Corneal Graft Survival. Cornea 2021, 40, 1512–1518. [Google Scholar] [CrossRef]
- Tran, T.M.; Duong, H.; Bonnet, C.; Kashanchi, A.; Buckshey, A.; Aldave, A.J. Corneal Blindness in Asia: A Systematic Review and Meta-Analysis to Identify Challenges and Opportunities. Cornea 2020, 39, 1196–1205. [Google Scholar] [CrossRef]
- Mathews, P.M.; Lindsley, K.; Aldave, A.J.; Akpek, E.K. Etiology of Global Corneal Blindness and Current Practices of Corneal Transplantation: A Focused Review. Cornea 2018, 37, 1198–1203. [Google Scholar] [CrossRef]
- Lucas, R.M.; Yazar, S.; Young, A.R.; Norval, M.; de Gruijl, F.R.; Takizawa, Y.; Rhodes, L.E.; Sinclair, C.A.; Neale, R.E. Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate. Photochem. Photobiol. Sci. 2019, 18, 641–680. [Google Scholar] [CrossRef]
- Matthaei, M.; Sandhaeger, H.; Hermel, M.; Adler, W.; Jun, A.S.; Cursiefen, C.; Heindl, L.M. Changing Indications in Penetrating Keratoplasty: A Systematic Review of 34 Years of Global Reporting. Transplantation 2017, 101, 1387–1399. [Google Scholar] [CrossRef]
- Volatier, T.; Schumacher, B.; Cursiefen, C.; Notara, M. UV Protection in the Cornea: Failure and Rescue. Biology 2022, 11, 278. [Google Scholar] [CrossRef]
- Wright, C.Y.; Norval, M. Health Risks Associated with Excessive Exposure to Solar Ultraviolet Radiation among Outdoor Workers in South Africa: An Overview. Front. Public Health 2021, 9, 678680. [Google Scholar] [CrossRef] [PubMed]
- Shortt, A.J.; Secker, G.A.; Notara, M.D.; Limb, G.A.; Khaw, P.T.; Tuft, S.J.; Daniels, J.T. Transplantation of ex vivo cultured limbal epithelial stem cells: A review of techniques and clinical results. Surv. Ophthalmol. 2007, 52, 483–502. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.R.; Daniels, J.T. Concise review: Limbal epithelial stem cell therapy: Controversies and challenges. Stem Cells 2011, 29, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Sheha, H.; Li, J.; Tseng, S.C. Limbal stem cell transplantation: New progresses and challenges. Eye 2009, 23, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Shortt, A.J.; Secker, G.A.; Rajan, M.S.; Meligonis, G.; Dart, J.K.; Tuft, S.J.; Daniels, J.T. Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmology 2008, 115, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.D.; Kaplan, D.R. Mobilizing endogenous stem cells for repair and regeneration: Are we there yet? Cell Stem Cell 2012, 10, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Brown, J.; Kanarek, A.; Rajagopal, J.; Melton, D.A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008, 455, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, K.; Sotozono, C.; Kinoshita, S. Current Advancements in Corneal Cell-Based Therapy. Asia-Pac. J. Ophthalmol. 2022, 11, 335–345. [Google Scholar] [CrossRef]
- Kitazawa, K.; Hikichi, T.; Nakamura, T.; Mitsunaga, K.; Tanaka, A.; Nakamura, M.; Yamakawa, T.; Furukawa, S.; Takasaka, M.; Goshima, N.; et al. OVOL2 Maintains the Transcriptional Program of Human Corneal Epithelium by Suppressing Epithelial-to-Mesenchymal Transition. Cell Rep. 2016, 15, 1359–1368. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, K. Stem cell-derived exosome versus stem cell therapy. Nat. Rev. Bioeng. 2023, 1, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Yan, J.; Jiang, Y.; Wu, L.; Li, M.; Fan, X. Exploring Cutting-Edge Approaches to Potentiate Mesenchymal Stem Cell and Exosome Therapy for Myocardial Infarction. J. Cardiovasc. Transl. Res. 2023, 1–20. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, T.; Yao, C.; Zhang, J.; Sun, C.; Chen, S.; Chen, M. Effects of stem cell-derived exosome therapy on erectile dysfunction: A systematic review and meta-analysis of preclinical studies. Sex. Med. 2023, 11, qfac019. [Google Scholar] [CrossRef]
- Oh, S.; Jung, J.H.; Ahn, K.J.; Jang, A.Y.; Byun, K.; Yang, P.C.; Chung, W.J. Stem Cell and Exosome Therapy in Pulmonary Hypertension. Korean Circ. J. 2022, 52, 110–122. [Google Scholar] [CrossRef]
- Izadi, M.; Dehghan Marvast, L.; Rezvani, M.E.; Zohrabi, M.; Aliabadi, A.; Mousavi, S.A.; Aflatoonian, B. Mesenchymal Stem-Cell Derived Exosome Therapy as a Potential Future Approach for Treatment of Male Infertility Caused by Chlamydia Infection. Front. Microbiol. 2021, 12, 785622. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liu, W.; Lian, L.; Xu, Y.; Bai, X.; Xu, S.; Zhang, J. Stroke treatment: Is exosome therapy superior to stem cell therapy? Biochimie 2020, 179, 190–204. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Maurice, D.M. The structure and transparency of the cornea. J. Physiol. 1957, 136, 263–286. [Google Scholar] [CrossRef]
- Tong, L.; Corrales, R.M.; Chen, Z.; Villarreal, A.L.; De Paiva, C.S.; Beuerman, R.; Li, D.Q.; Pflugfelder, S.C. Expression and regulation of cornified envelope proteins in human corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, B.; Wan, P.; Liang, X.; Wang, X.; Liu, Y.; Zhou, Q.; Wang, Z. Roles of limbal microvascular net and limbal stroma in regulating maintenance of limbal epithelial stem cells. Cell Tissue Res. 2015, 359, 547–563. [Google Scholar] [CrossRef]
- Selvarajah, K.; Tan, J.J.; Shaharuddin, B. Corneal Epithelial Development and the Role of Induced Pluripotent Stem Cells in Regeneration. Curr. Stem Cell Res. Ther. 2024, 19, 292–306. [Google Scholar] [CrossRef]
- Pellegrini, G.; Rama, P.; Mavilio, F.; De Luca, M. Epithelial stem cells in corneal regeneration and epidermal gene therapy. J. Pathol. 2009, 217, 217–228. [Google Scholar] [CrossRef]
- Kruse, F.E. Stem cells and corneal epithelial regeneration. Eye 1994, 8, 170–183. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Saint-Geniez, M.; Hamrah, P.; Jin, Y.; Rashid, S.; Pytowski, B.; Persaud, K.; Wu, Y.; Streilein, J.W.; et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc. Natl. Acad. Sci. USA 2006, 103, 11405–11410. [Google Scholar] [CrossRef] [PubMed]
- Clahsen, T.; Hadrian, K.; Notara, M.; Schlereth, S.L.; Howaldt, A.; Prokosch, V.; Volatier, T.; Hos, D.; Schroedl, F.; Kaser-Eichberger, A.; et al. The novel role of lymphatic vessels in the pathogenesis of ocular diseases. Prog. Retin. Eye Res. 2023, 96, 101157. [Google Scholar] [CrossRef]
- Hayashi, S.; Osawa, T.; Tohyama, K. Comparative observations on corneas, with special reference to Bowman’s layer and Descemet’s membrane in mammals and amphibians. J. Morphol. 2002, 254, 247–258. [Google Scholar] [CrossRef]
- Kokot, J.; Wylegala, A.; Wowra, B.; Wojcik, L.; Dobrowolski, D.; Wylegala, E. Corneal confocal sub-basal nerve plexus evaluation: A review. Acta Ophthalmol. 2018, 96, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Kowtharapu, B.S.; Winter, K.; Marfurt, C.; Allgeier, S.; Kohler, B.; Hovakimyan, M.; Stahnke, T.; Wree, A.; Stachs, O.; Guthoff, R.F. Comparative quantitative assessment of the human corneal sub-basal nerve plexus by in vivo confocal microscopy and histological staining. Eye 2017, 31, 481–490. [Google Scholar] [CrossRef] [PubMed]
- White, T.L.; Lewis, P.N.; Young, R.D.; Kitazawa, K.; Inatomi, T.; Kinoshita, S.; Meek, K.M. Elastic microfibril distribution in the cornea: Differences between normal and keratoconic stroma. Exp. Eye Res. 2017, 159, 40–48. [Google Scholar] [CrossRef]
- Morishige, N.; Takagi, Y.; Chikama, T.; Takahara, A.; Nishida, T. Three-dimensional analysis of collagen lamellae in the anterior stroma of the human cornea visualized by second harmonic generation imaging microscopy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 911–915. [Google Scholar] [CrossRef]
- Teng, C.C. Fine structure of the human cornea: Epithelium and stroma. Am. J. Ophthalmol. 1962, 54, 969–1002. [Google Scholar] [CrossRef]
- Daxer, A.; Misof, K.; Grabner, B.; Ettl, A.; Fratzl, P. Collagen fibrils in the human corneal stroma: Structure and aging. Investig. Ophthalmol. Vis. Sci. 1998, 39, 644–648. [Google Scholar]
- Akhtar, S.; Petrovski, G.; Albert, R.; Alkanaan, A.; Kirat, O.; Khan, A.D.; Almubrad, T. Ultrastructure and 3D transmission electron tomography of collagen fibrils and proteoglycans of swollen human corneal stroma. Histol. Histopathol. 2019, 34, 91–102. [Google Scholar]
- Meek, K.M.; Newton, R.H. Organization of collagen fibrils in the corneal stroma in relation to mechanical properties and surgical practice. J. Refract. Surg. 1999, 15, 695–699. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.C.; Wilson, S.E. Descemet’s membrane development, structure, function and regeneration. Exp. Eye Res. 2020, 197, 108090. [Google Scholar] [CrossRef] [PubMed]
- Flockerzi, E.; Turner, C.; Seitz, B.; Collaborators, G.S.G.; GeKe, R.S.G. Descemet’s membrane endothelial keratoplasty is the predominant keratoplasty procedure in Germany since 2016: A report of the DOG-section cornea and its keratoplasty registry. Br. J. Ophthalmol. 2023; published online ahead of print. [Google Scholar]
- Williams, A.L.; Bohnsack, B.L. Neural crest derivatives in ocular development: Discerning the eye of the storm. Birth Defects Res. Part C Embryo Today Rev. 2015, 105, 87–95. [Google Scholar] [CrossRef]
- Gage, P.J.; Rhoades, W.; Prucka, S.K.; Hjalt, T. Fate maps of neural crest and mesoderm in the mammalian eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4200–4208. [Google Scholar] [CrossRef]
- Yoshida, S.; Shimmura, S.; Nagoshi, N.; Fukuda, K.; Matsuzaki, Y.; Okano, H.; Tsubota, K. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells 2006, 24, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Quantock, A.J.; Boote, C.; Siegler, V.; Meek, K.M. Collagen organization in the secondary chick cornea during development. Investig. Ophthalmol. Vis. Sci. 2003, 44, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V. Corneal crystallins and the development of cellular transparency. Semin. Cell Dev. Biol. 2008, 19, 82–93. [Google Scholar] [CrossRef]
- Funderburgh, M.L.; Du, Y.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005, 19, 1371–1373. [Google Scholar] [CrossRef]
- Toti, P.; Tosi, G.M.; Traversi, C.; Schurfeld, K.; Cardone, C.; Caporossi, A. CD-34 stromal expression pattern in normal and altered human corneas. Ophthalmology 2002, 109, 1167–1171. [Google Scholar] [CrossRef]
- Ramos, T.L.; Sanchez-Abarca, L.I.; Muntion, S.; Preciado, S.; Puig, N.; Lopez-Ruano, G.; Hernandez-Hernandez, A.; Redondo, A.; Ortega, R.; Rodriguez, C.; et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. CCS 2016, 14, 2. [Google Scholar] [CrossRef]
- Naylor, R.W.; McGhee, C.N.; Cowan, C.A.; Davidson, A.J.; Holm, T.M.; Sherwin, T. Derivation of Corneal Keratocyte-Like Cells from Human Induced Pluripotent Stem Cells. PLoS ONE 2016, 11, e0165464. [Google Scholar] [CrossRef] [PubMed]
- Ksander, B.R.; Kolovou, P.E.; Wilson, B.J.; Saab, K.R.; Guo, Q.; Ma, J.; McGuire, S.P.; Gregory, M.S.; Vincent, W.J.; Perez, V.L.; et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2014, 511, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Harkin, D.G.; Foyn, L.; Bray, L.J.; Sutherland, A.J.; Li, F.J.; Cronin, B.G. Concise reviews: Can mesenchymal stromal cells differentiate into corneal cells? A systematic review of published data. Stem Cells 2015, 33, 785–791. [Google Scholar] [CrossRef]
- Branch, M.J.; Hashmani, K.; Dhillon, P.; Jones, D.R.; Dua, H.S.; Hopkinson, A. Mesenchymal stem cells in the human corneal limbal stroma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5109–5116. [Google Scholar] [CrossRef] [PubMed]
- Navas, A.; Magana-Guerrero, F.S.; Dominguez-Lopez, A.; Chavez-Garcia, C.; Partido, G.; Graue-Hernandez, E.O.; Sanchez-Garcia, F.J.; Garfias, Y. Anti-Inflammatory and Anti-Fibrotic Effects of Human Amniotic Membrane Mesenchymal Stem Cells and Their Potential in Corneal Repair. Stem Cells Transl. Med. 2018, 7, 906–917. [Google Scholar] [CrossRef]
- Demirayak, B.; Yuksel, N.; Celik, O.S.; Subasi, C.; Duruksu, G.; Unal, Z.S.; Yildiz, D.K.; Karaoz, E. Effect of bone marrow and adipose tissue-derived mesenchymal stem cells on the natural course of corneal scarring after penetrating injury. Exp. Eye Res. 2016, 151, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Liu, C.Y.; Hayashi, Y.; Kao, W.W. Bone marrow mesenchymal stem cells can differentiate and assume corneal keratocyte phenotype. J. Cell. Mol. Med. 2012, 16, 1114–1124. [Google Scholar] [CrossRef]
- Komai, Y.; Ushiki, T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2244–2258. [Google Scholar]
- Mikula, E.; Winkler, M.; Juhasz, T.; Brown, D.J.; Shoa, G.; Tran, S.; Kenney, M.C.; Jester, J.V. Axial mechanical and structural characterization of keratoconus corneas. Exp. Eye Res. 2018, 175, 14–19. [Google Scholar] [CrossRef]
- Basu, S.; Hertsenberg, A.J.; Funderburgh, M.L.; Burrow, M.K.; Mann, M.M.; Du, Y.; Lathrop, K.L.; Syed-Picard, F.N.; Adams, S.M.; Birk, D.E.; et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci. Transl. Med. 2014, 6, 266ra172. [Google Scholar] [CrossRef]
- Ko, J.H.; Kim, H.J.; Jeong, H.J.; Lee, H.J.; Oh, J.Y. Mesenchymal Stem and Stromal Cells Harness Macrophage-Derived Amphiregulin to Maintain Tissue Homeostasis. Cell Rep. 2020, 30, 3806–3820.e6. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Ii, M.; Cursiefen, C.; Jackson, D.G.; Keino, H.; Tomita, M.; Van Rooijen, N.; Takenaka, H.; D’Amore, P.A.; Stein-Streilein, J.; et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Investig. 2005, 115, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Kerjaschki, D. The crucial role of macrophages in lymphangiogenesis. J. Clin. Investig. 2005, 115, 2316–2319. [Google Scholar] [CrossRef] [PubMed]
- Eslani, M.; Putra, I.; Shen, X.; Hamouie, J.; Afsharkhamseh, N.; Besharat, S.; Rosenblatt, M.I.; Dana, R.; Hematti, P.; Djalilian, A.R. Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5507–5517. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Kodati, S.; Lee, H.S.; Omoto, M.; Jin, Y.; Chauhan, S.K. Kinetics and function of mesenchymal stem cells in corneal injury. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3638–3644. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Mittal, S.K.; Foulsham, W.; Elbasiony, E.; Singhania, D.; Sahu, S.K.; Chauhan, S.K. Therapeutic efficacy of different routes of mesenchymal stem cell administration in corneal injury. Ocul. Surf. 2019, 17, 729–736. [Google Scholar] [CrossRef]
- Notara, M.; Shortt, A.J.; O’Callaghan, A.R.; Daniels, J.T. The impact of age on the physical and cellular properties of the human limbal stem cell niche. Age 2013, 35, 289–300. [Google Scholar] [CrossRef]
- Aspelund, A.; Tammela, T.; Antila, S.; Nurmi, H.; Leppanen, V.M.; Zarkada, G.; Stanczuk, L.; Francois, M.; Makinen, T.; Saharinen, P.; et al. The Schlemm’s canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J. Clin. Investig. 2014, 124, 3975–3986. [Google Scholar] [CrossRef]
- Cursiefen, C.; Schlotzer-Schrehardt, U.; Kuchle, M.; Sorokin, L.; Breiteneder-Geleff, S.; Alitalo, K.; Jackson, D. Lymphatic vessels in vascularized human corneas: Immunohistochemical investigation using LYVE-1 and podoplanin. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2127–2135. [Google Scholar]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef]
- Cursiefen, C.; Maruyama, K.; Jackson, D.G.; Streilein, J.W.; Kruse, F.E. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea 2006, 25, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Banerji, S.; Ni, J.; Wang, S.X.; Clasper, S.; Su, J.; Tammi, R.; Jones, M.; Jackson, D.G. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 1999, 144, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Wigle, J.T.; Chowdhury, K.; Gruss, P.; Oliver, G. Prox1 function is crucial for mouse lens-fibre elongation. Nat. Genet. 1999, 21, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Breiteneder-Geleff, S.; Soleiman, A.; Horvat, R.; Amann, G.; Kowalski, H.; Kerjaschki, D. Podoplanin—A specific marker for lymphatic endothelium expressed in angiosarcoma. Verh. Dtsch. Ges. Pathol. 1999, 83, 270–275. [Google Scholar] [PubMed]
- Kaipainen, A.; Korhonen, J.; Mustonen, T.; van Hinsbergh, V.W.; Fang, G.H.; Dumont, D.; Breitman, M.; Alitalo, K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. USA 1995, 92, 3566–3570. [Google Scholar] [CrossRef]
- Cursiefen, C.; Schlotzer-Schrehardt, U.; Breiteneder-Geleff, S.; Holbach, L.M. Orbital lymphangioma with positive immunohistochemistry of lymphatic endothelial markers (vascular endothelial growth factor receptor 3 and podoplanin). Graefe’s Arch. Clin. Exp. Ophthalmol. 2001, 239, 628–632. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, Q.C.C.; van Beek, J.G.M.; Kilic, E.; Verdijk, R.M. Transient Expression of Lymphatic Markers in Retrobulbar Intraconal Orbital Vasculature During Fetal Development. Investig. Ophthalmol. Vis. Sci. 2020, 61, 22. [Google Scholar] [CrossRef]
- Wong, L.L.; Lee, N.G.; Amarnani, D.; Choi, C.J.; Bielenberg, D.R.; Freitag, S.K.; D’Amore, P.A.; Kim, L.A. Orbital Angiogenesis and Lymphangiogenesis in Thyroid Eye Disease: An Analysis of Vascular Growth Factors with Clinical Correlation. Ophthalmology 2016, 123, 2028–2036. [Google Scholar] [CrossRef]
- Wang, L.Q.; Huang, Y.F.; Huang, J.X.; Yang, B.J.; Chen, B. The interactions of stromal-epithelial in a model of co-culture in the rabbit cornea. [Zhonghua Yan Ke Za Zhi] Chin. J. Ophthalmol. 2007, 43, 251–255. [Google Scholar]
- Wilson, S.E.; Liu, J.J.; Mohan, R.R. Stromal-epithelial interactions in the cornea. Prog. Retin. Eye Res. 1999, 18, 293–309. [Google Scholar] [CrossRef]
- Zieske, J.D.; Hutcheon, A.E.K.; Guo, X. Extracellular Vesicles and Cell-Cell Communication in the Cornea. Anat. Rec. 2020, 303, 1727–1734. [Google Scholar] [CrossRef]
- McKay, T.B.; Karamichos, D.; Hutcheon, A.E.K.; Guo, X.; Zieske, J.D. Corneal Epithelial-Stromal Fibroblast Constructs to Study Cell-Cell Communication In Vitro. Bioengineering 2019, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Pal-Ghosh, S.; Karpinski, B.A.; Datta Majumdar, H.; Ghosh, T.; Thomasian, J.; Brooks, S.R.; Sawaya, A.P.; Morasso, M.I.; Scholand, K.K.; de Paiva, C.S.; et al. Molecular mechanisms regulating wound repair: Evidence for paracrine signaling from corneal epithelial cells to fibroblasts and immune cells following transient epithelial cell treatment with Mitomycin C. Exp. Eye Res. 2023, 227, 109353. [Google Scholar] [CrossRef] [PubMed]
- Sotozono, C.; Kinoshita, S.; Kita, M.; Imanishi, J. Paracrine role of keratinocyte growth factor in rabbit corneal epithelial cell growth. Exp. Eye Res. 1994, 59, 385–391. [Google Scholar] [CrossRef]
- Wu, M.; Hill, L.J.; Downie, L.E.; Chinnery, H.R. Neuroimmune crosstalk in the cornea: The role of immune cells in corneal nerve maintenance during homeostasis and inflammation. Prog. Retin. Eye Res. 2022, 91, 101105. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Loughner, C.L.; Swamynathan, S.; Swamynathan, S.K. KLF4 Plays an Essential Role in Corneal Epithelial Homeostasis by Promoting Epithelial Cell Fate and Suppressing Epithelial-Mesenchymal Transition. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2785–2795. [Google Scholar] [CrossRef]
- Swamynathan, S.K.; Katz, J.P.; Kaestner, K.H.; Ashery-Padan, R.; Crawford, M.A.; Piatigorsky, J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol. Cell. Biol. 2007, 27, 182–194. [Google Scholar] [CrossRef]
- Delp, E.E.; Swamynathan, S.; Kao, W.W.; Swamynathan, S.K. Spatiotemporally Regulated Ablation of Klf4 in Adult Mouse Corneal Epithelial Cells Results in Altered Epithelial Cell Identity and Disrupted Homeostasis. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3549–3558. [Google Scholar] [CrossRef]
- Desjardins, P.; Berthiaume, R.; Couture, C.; Le-Bel, G.; Roy, V.; Gros-Louis, F.; Moulin, V.J.; Proulx, S.; Chemtob, S.; Germain, L.; et al. Impact of Exosomes Released by Different Corneal Cell Types on the Wound Healing Properties of Human Corneal Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 12201. [Google Scholar] [CrossRef]
- Fujimoto, S.; Hayashi, R.; Hara, S.; Sasamoto, Y.; Harrington, J.; Tsujikawa, M.; Nishida, K. KLF4 prevents epithelial to mesenchymal transition in human corneal epithelial cells via endogenous TGF-beta2 suppression. Regen. Ther. 2019, 11, 249–257. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Zhang, X.; Yang, S.; Lin, X.; Yang, X.; Lin, X.; Shi, J.; Wang, S.; Zhao, W.; et al. Klf4 reduces stemness phenotype, triggers mesenchymal-epithelial transition (MET)-like molecular changes, and prevents tumor progression in nasopharygeal carcinoma. Oncotarget 2017, 8, 93924–93941. [Google Scholar] [CrossRef] [PubMed]
- West-Mays, J.A.; Dwivedi, D.J. The keratocyte: Corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol. 2006, 38, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, N.; Schlunck, G.; Reinhard, T. PAX6 Expression Patterns in the Adult Human Limbal Stem Cell Niche. Cells 2023, 12, 400. [Google Scholar] [CrossRef]
- Sunny, S.S.; Lachova, J.; Dupacova, N.; Kozmik, Z. Multiple roles of Pax6 in postnatal cornea development. Dev. Biol. 2022, 491, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mort, R.L.; Bentley, A.J.; Martin, F.L.; Collinson, J.M.; Douvaras, P.; Hill, R.E.; Morley, S.D.; Fullwood, N.J.; West, J.D. Effects of aberrant Pax6 gene dosage on mouse corneal pathophysiology and corneal epithelial homeostasis. PLoS ONE 2011, 6, e28895. [Google Scholar] [CrossRef]
- Kuchalska, K.; Wawrocka, A.; Krawczynski, M.R. Novel variants in the PAX6 gene related to isolated aniridia. Congenit. Anom. 2023, 63, 109–115. [Google Scholar] [CrossRef]
- Cross, E.; Duncan-Flavell, P.J.; Howarth, R.J.; Crooks, R.O.; Thomas, N.S.; Bunyan, D.J. Screening of a large PAX6 cohort identified many novel variants and emphasises the importance of the paired and homeobox domains. Eur. J. Med. Genet. 2020, 63, 103940. [Google Scholar] [CrossRef]
- Zhang, R.; Linpeng, S.; Wei, X.; Li, H.; Huang, Y.; Guo, J.; Wu, Q.; Liang, D.; Wu, L. Novel variants in PAX6 gene caused congenital aniridia in two Chinese families. Eye 2017, 31, 956–961. [Google Scholar] [CrossRef]
- Norton, J.D. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J. Cell Sci. 2000, 113 Pt 22, 3897–3905. [Google Scholar] [CrossRef]
- Mohan, R.R.; Morgan, B.R.; Anumanthan, G.; Sharma, A.; Chaurasia, S.S.; Rieger, F.G. Characterization of Inhibitor of differentiation (Id) proteins in human cornea. Exp. Eye Res. 2016, 146, 145–153. [Google Scholar] [CrossRef]
- Tandon, A.; Sharma, A.; Rodier, J.T.; Klibanov, A.M.; Rieger, F.G.; Mohan, R.R. BMP7 gene transfer via gold nanoparticles into stroma inhibits corneal fibrosis in vivo. PLoS ONE 2013, 8, e66434. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Ku, Y.A.; Kim, S.; Chung, M.H.; Kim, Y.H.; Kim, D.H. Comparison of Corneal Epithelial Wound Healing between Topical RCI001, Solcoseryl, and Polydeoxyribonucleotide in the Murine Ocular Alkali Burn Model. Korean J. Ophthalmol. KJO 2023, 37, 236–244. [Google Scholar] [CrossRef]
- Stappenbeck, T.S.; Miyoshi, H. The role of stromal stem cells in tissue regeneration and wound repair. Science 2009, 324, 1666–1669. [Google Scholar] [CrossRef]
- Mohan, R.R.; Kempuraj, D.; D’Souza, S.; Ghosh, A. Corneal stromal repair and regeneration. Prog. Retin. Eye Res. 2022, 91, 101090. [Google Scholar] [CrossRef]
- Thill, M.; Schlagner, K.; Altenahr, S.; Ergun, S.; Faragher, R.G.; Kilic, N.; Bednarz, J.; Vohwinkel, G.; Rogiers, X.; Hossfeld, D.K.; et al. A novel population of repair cells identified in the stroma of the human cornea. Stem Cells Dev. 2007, 16, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Shiju, T.M.; Sampaio, L.P.; Martinez, V.V.; Hilgert, G.S.L.; Wilson, S.E. Transforming growth factor beta-3 localization in the corneal response to epithelial-stromal injury and effects on corneal fibroblast transition to myofibroblasts. Exp. Eye Res. 2023, 235, 109631. [Google Scholar] [CrossRef]
- Stepp, M.A.; Zhu, L.; Cranfill, R. Changes in beta 4 integrin expression and localization in vivo in response to corneal epithelial injury. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1593–1601. [Google Scholar]
- Nasser, W.; Amitai-Lange, A.; Soteriou, D.; Hanna, R.; Tiosano, B.; Fuchs, Y.; Shalom-Feuerstein, R. Corneal-Committed Cells Restore the Stem Cell Pool and Tissue Boundary following Injury. Cell Rep. 2018, 22, 323–331. [Google Scholar] [CrossRef]
- Howaldt, A.L.S.; Velmans, C.; Schultheis, A.M.; Clahsen, T.; Matthaei, M.; Kohlhase, J.; Vokuhl, C.; Büttner, R.; Netzer, C.; Demoulin, J.-B.; et al. Corneal infantile myofibromatosis caused by novel activating imatinib-responsive variants in PDGFRB. Ophthalmol. Sci. 2023; in press. [Google Scholar]
- Forbes, S.J.; Rosenthal, N. Preparing the ground for tissue regeneration: From mechanism to therapy. Nat. Med. 2014, 20, 857–869. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef]
- Wu, J.; Du, Y.; Mann, M.M.; Funderburgh, J.L.; Wagner, W.R. Corneal stromal stem cells versus corneal fibroblasts in generating structurally appropriate corneal stromal tissue. Exp. Eye Res. 2014, 120, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Qazi, Y.; Wong, G.; Monson, B.; Stringham, J.; Ambati, B.K. Corneal transparency: Genesis, maintenance and dysfunction. Brain Res. Bull. 2010, 81, 198–210. [Google Scholar] [CrossRef]
- Sriram, S.; Gibson, D.J.; Robinson, P.; Pi, L.; Tuli, S.; Lewin, A.S.; Schultz, G. Assessment of anti-scarring therapies in ex vivo organ cultured rabbit corneas. Exp. Eye Res. 2014, 125, 173–182. [Google Scholar] [CrossRef]
- Tandon, A.; Tovey, J.C.; Sharma, A.; Gupta, R.; Mohan, R.R. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr. Mol. Med. 2010, 10, 565–578. [Google Scholar] [PubMed]
- Jester, J.V.; Petroll, W.M.; Cavanagh, H.D. Corneal stromal wound healing in refractive surgery: The role of myofibroblasts. Prog. Retin. Eye Res. 1999, 18, 311–356. [Google Scholar] [CrossRef]
- Wang, J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018, 371, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Hertsenberg, A.J.; Shojaati, G.; Funderburgh, M.L.; Mann, M.M.; Du, Y.; Funderburgh, J.L. Corneal stromal stem cells reduce corneal scarring by mediating neutrophil infiltration after wounding. PLoS ONE 2017, 12, e0171712. [Google Scholar] [CrossRef]
- Gregory, A.D.; Kliment, C.R.; Metz, H.E.; Kim, K.H.; Kargl, J.; Agostini, B.A.; Crum, L.T.; Oczypok, E.A.; Oury, T.A.; Houghton, A.M. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. J. Leukoc. Biol. 2015, 98, 143–152. [Google Scholar] [CrossRef]
- Hou, Y.; Bock, F.; Hos, D.; Cursiefen, C. Lymphatic Trafficking in the Eye: Modulation of Lymphatic Trafficking to Promote Corneal Transplant Survival. Cells 2021, 10, 1661. [Google Scholar] [CrossRef]
- Mansoor, H.; Ong, H.S.; Riau, A.K.; Stanzel, T.P.; Mehta, J.S.; Yam, G.H. Current Trends and Future Perspective of Mesenchymal Stem Cells and Exosomes in Corneal Diseases. Int. J. Mol. Sci. 2019, 20, 2853. [Google Scholar] [CrossRef]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yin, J.; Hao, L.; Liu, X.; Shi, Q.; Diao, Y.; Yu, G.; Liu, L.; Chen, J.; Zhong, J. Exosomes from Human Umbilical Cord Mesenchymal Stem Cells Treat Corneal Injury via Autophagy Activation. Front. Bioeng. Biotechnol. 2022, 10, 879192. [Google Scholar] [CrossRef]
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, D.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J.L. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. Stem Cells Transl. Med. 2019, 8, 1192–1201. [Google Scholar] [CrossRef]
- Deng, S.X.; Dos Santos, A.; Gee, S. Therapeutic Potential of Extracellular Vesicles for the Treatment of Corneal Injuries and Scars. Transl. Vis. Sci. Technol. 2020, 9, 1. [Google Scholar] [CrossRef]
- Chen, T.S.; Arslan, F.; Yin, Y.; Tan, S.S.; Lai, R.C.; Choo, A.B.; Padmanabhan, J.; Lee, C.N.; de Kleijn, D.P.; Lim, S.K. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J. Transl. Med. 2011, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Donohoe, E.; Canning, A.; Moosavizadeh, S.; Buckley, F.; Brennan, M.A.; Ryan, A.E.; Ritter, T. Immunomodulatory function of licensed human bone marrow mesenchymal stromal cell-derived apoptotic bodies. Int. Immunopharmacol. 2023, 125, 111096. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef]
- Ong, H.S.; Riau, A.K.; Yam, G.H.; Yusoff, N.; Han, E.J.Y.; Goh, T.W.; Lai, R.C.; Lim, S.K.; Mehta, J.S. Mesenchymal Stem Cell Exosomes as Immunomodulatory Therapy for Corneal Scarring. Int. J. Mol. Sci. 2023, 24, 7456. [Google Scholar] [CrossRef]
- Tang, Q.; Lu, B.; He, J.; Chen, X.; Fu, Q.; Han, H.; Luo, C.; Yin, H.; Qin, Z.; Lyu, D.; et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials 2022, 280, 121320. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Izpisua Belmonte, J.C. Reprogramming development and aging: Cell differentiation as a malleable process. Curr. Opin. Cell Biol. 2012, 24, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Cheraqpour, K.; Koganti, R.; Baharnoori, S.M.; Djalilian, A.R. Concise Review: Bioengineering of Limbal Stem Cell Niche. Bioengineering 2023, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hayashida, Y.; Chen, Y.T.; Tseng, S.C. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007, 17, 26–36. [Google Scholar] [CrossRef]
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014, 32, 795–803. [Google Scholar] [CrossRef]
- Brunet, A.; Goodell, M.A.; Rando, T.A. Ageing and rejuvenation of tissue stem cells and their niches. Nat. Rev. Mol. Cell Biol. 2023, 24, 45–62. [Google Scholar] [CrossRef]
- Gonzalez, E.; Falcon-Perez, J.M. Cell-derived extracellular vesicles as a platform to identify low-invasive disease biomarkers. Expert Rev. Mol. Diagn. 2015, 15, 907–923. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, J.; Tian, F.; Cai, L.; Zhang, W.; Feng, Q.; Chang, J.; Wan, F.; Yang, Y.; Dai, B.; et al. Low-cost thermophoretic profiling of extracellular-vesicle surface proteins for the early detection and classification of cancers. Nat. Biomed. Eng. 2019, 3, 183–193. [Google Scholar] [CrossRef]
- Leszczynska, A.; Kulkarni, M.; Ljubimov, A.V.; Saghizadeh, M. Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci. Rep. 2018, 8, 15173. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Y.; Li, X.; Song, Z.; Sun, B.; Li, X.; Zhang, H. Comparison of exosomes derived from induced pluripotent stem cells and mesenchymal stem cells as therapeutic nanoparticles for treatment of corneal epithelial defects. Aging 2020, 12, 19546–19562. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volatier, T.; Cursiefen, C.; Notara, M. Current Advances in Corneal Stromal Stem Cell Biology and Therapeutic Applications. Cells 2024, 13, 163. https://doi.org/10.3390/cells13020163
Volatier T, Cursiefen C, Notara M. Current Advances in Corneal Stromal Stem Cell Biology and Therapeutic Applications. Cells. 2024; 13(2):163. https://doi.org/10.3390/cells13020163
Chicago/Turabian StyleVolatier, Thomas, Claus Cursiefen, and Maria Notara. 2024. "Current Advances in Corneal Stromal Stem Cell Biology and Therapeutic Applications" Cells 13, no. 2: 163. https://doi.org/10.3390/cells13020163
APA StyleVolatier, T., Cursiefen, C., & Notara, M. (2024). Current Advances in Corneal Stromal Stem Cell Biology and Therapeutic Applications. Cells, 13(2), 163. https://doi.org/10.3390/cells13020163






