Heparan Sulfate Modulation Affects Breast Cancer Cell Adhesion and Transmigration across In Vitro Blood–Brain Barrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
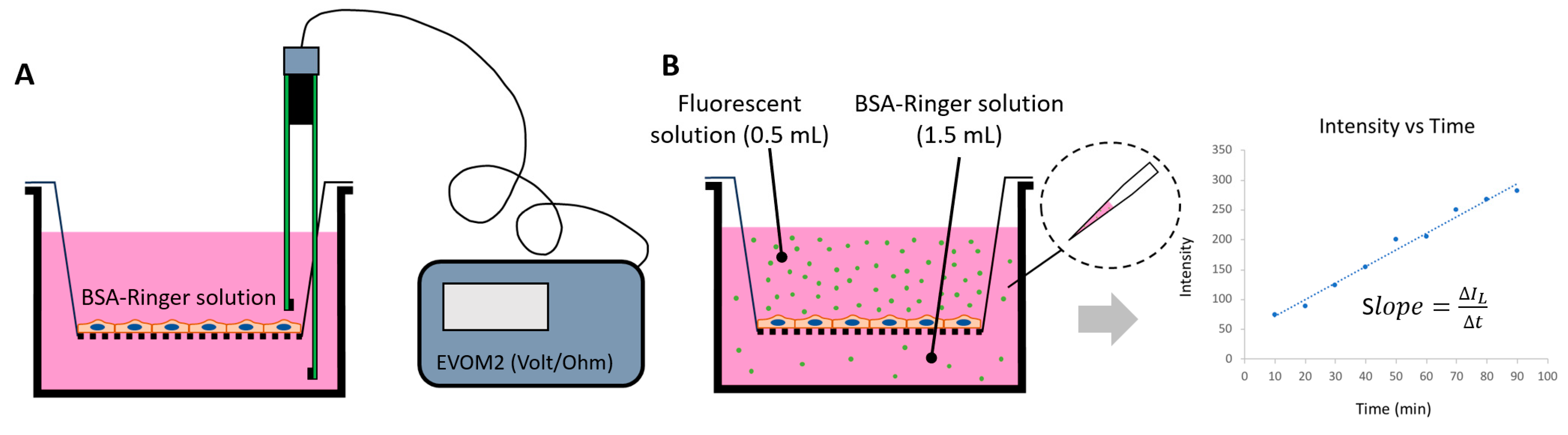
2.2. Generation of In Vitro BBBs
2.3. Quantification of Heparan Sulfate (HS) at In Vitro BBB and MB231
2.4. Modulation of HS of In Vitro BBB and MB231 by Various Agents
2.5. Determination of Solute Permeability (P) of In Vitro BBB
2.6. Quantification of MB231 Cell Adhesion to the In Vitro BBB Generated on a Glass-Bottom Dish
2.7. Quantification of MB231 Cell Transmigration across the In Vitro BBB Generated on a Transwell Filter
2.8. Statistical Analysis
3. Results
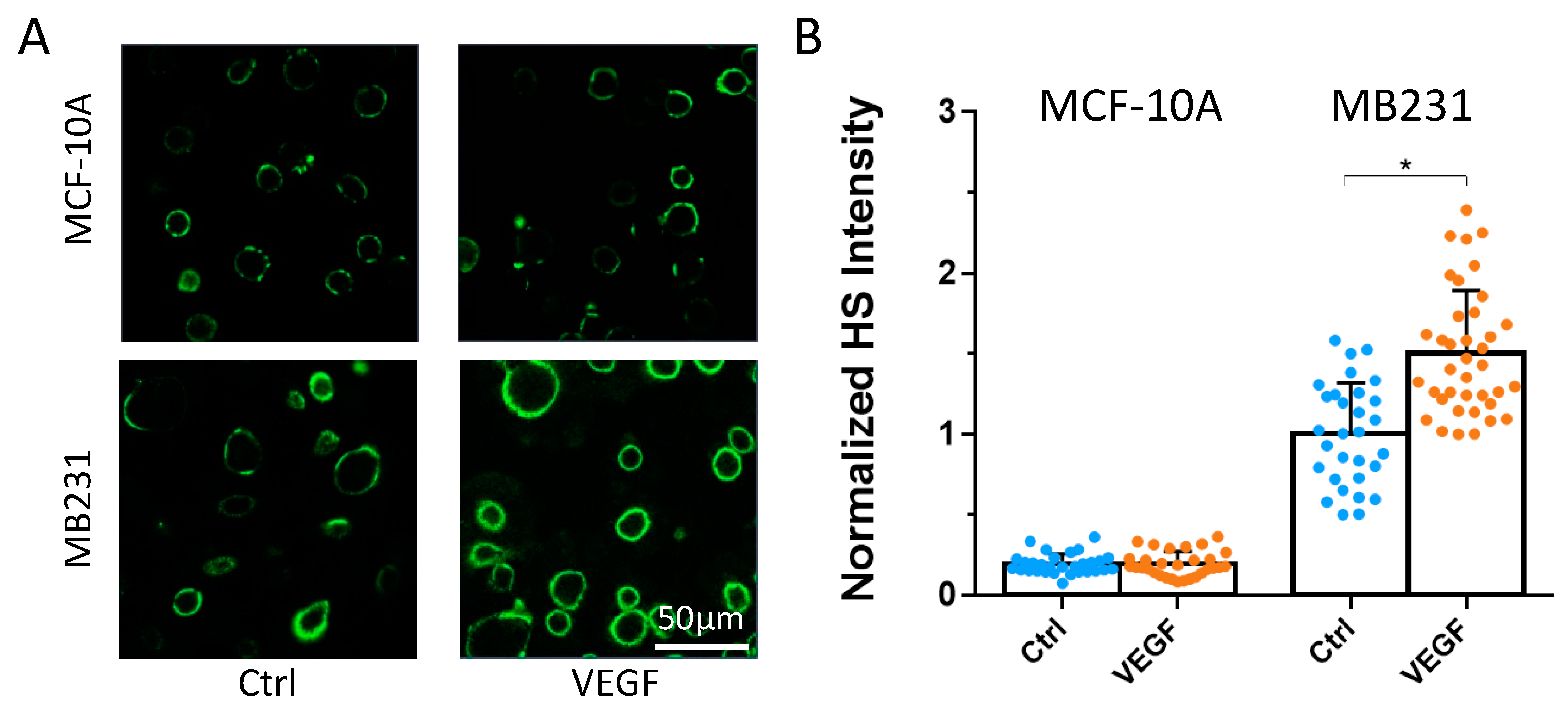
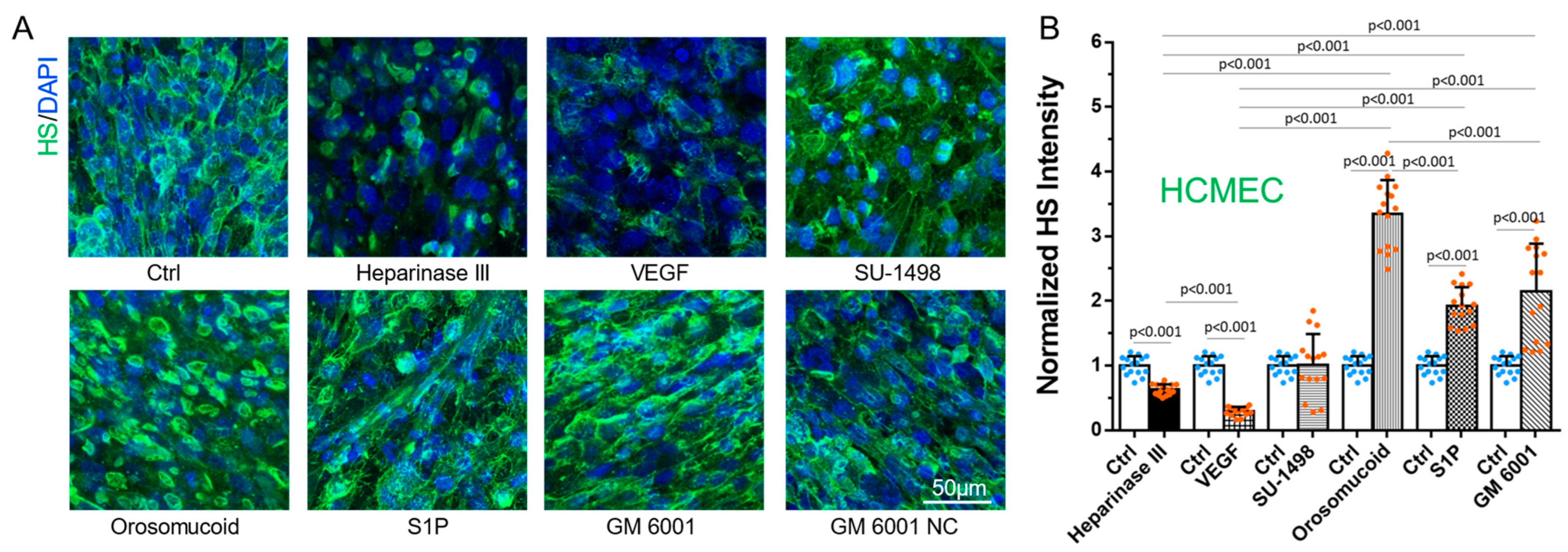
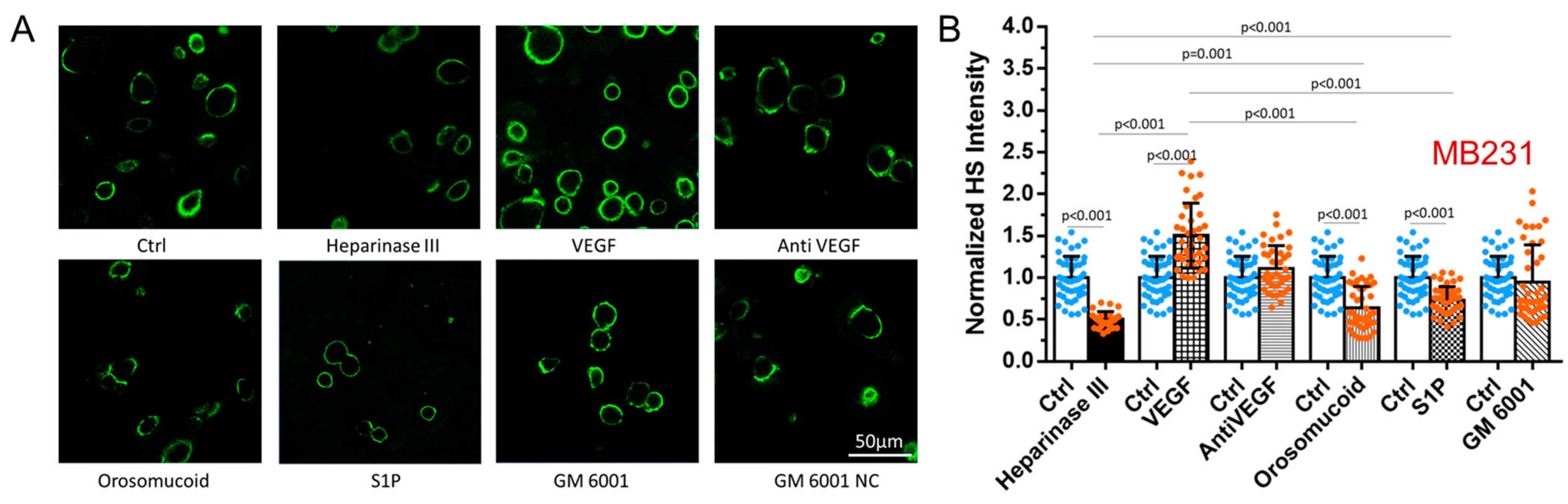
3.1. Differential Effects of VEGF, Orosomucoid, and S1P on the HS of In Vitro BBB and MB231
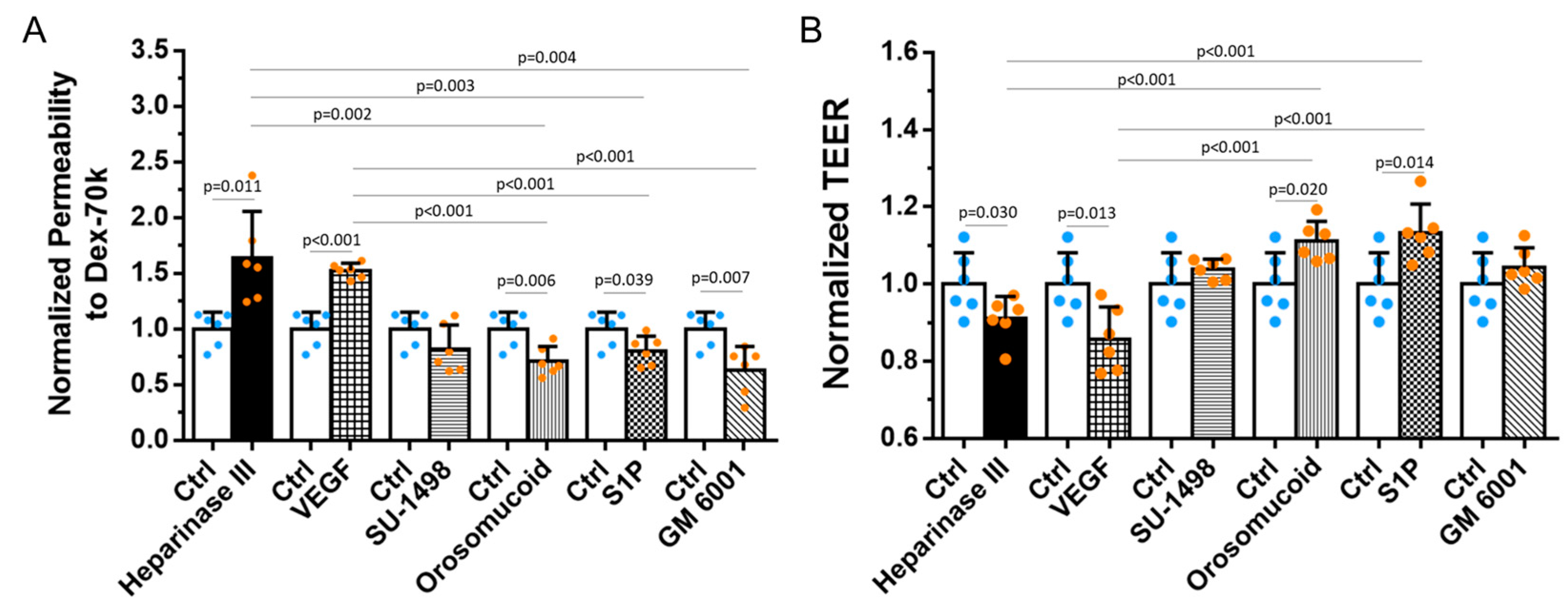
3.2. Effects of HS Modulation on the Barrier Functions of In Vitro BBB
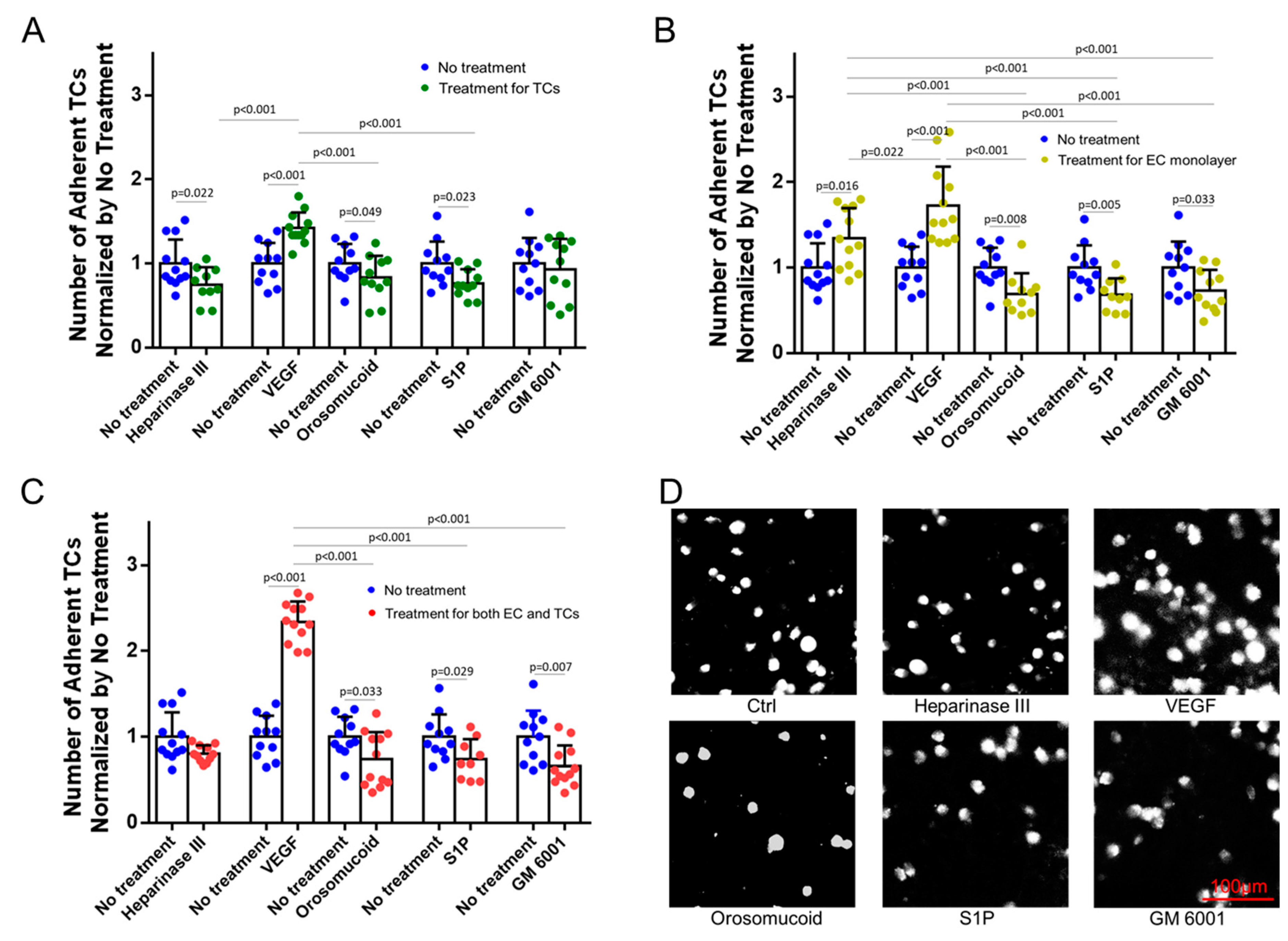
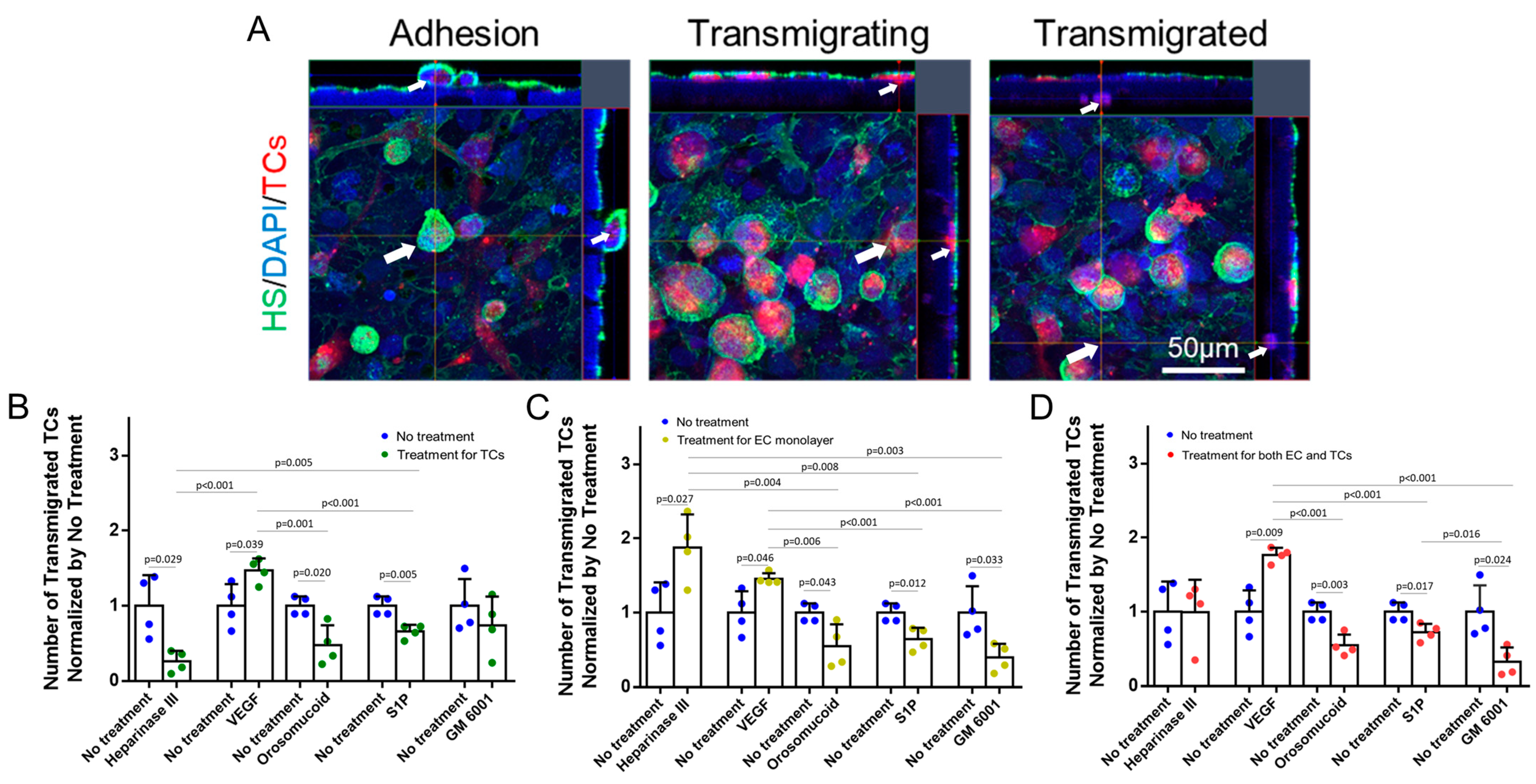
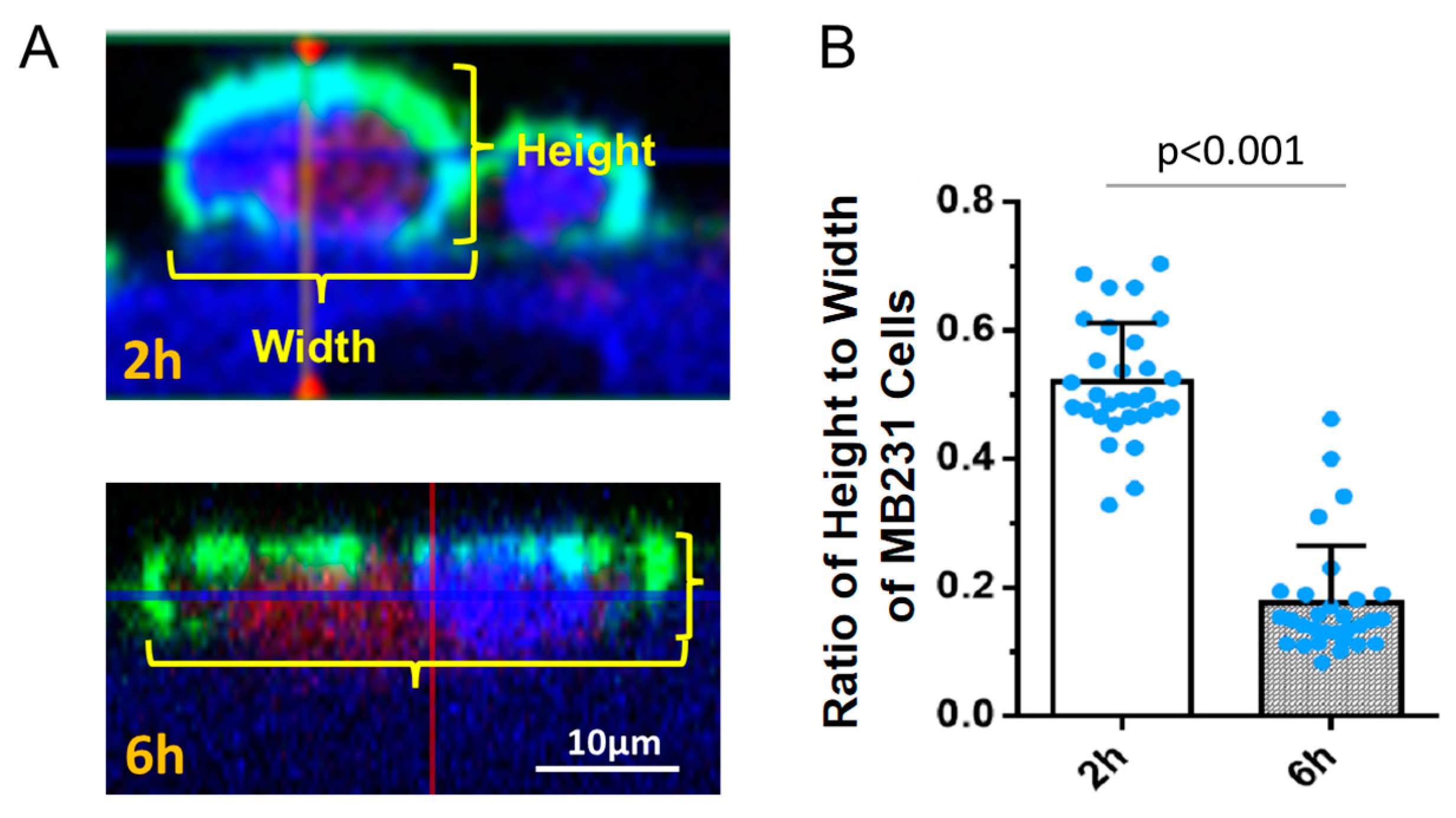
3.3. Effects of HS Modulation on MB231 Adhesion to and Transmigration across In Vitro BBB
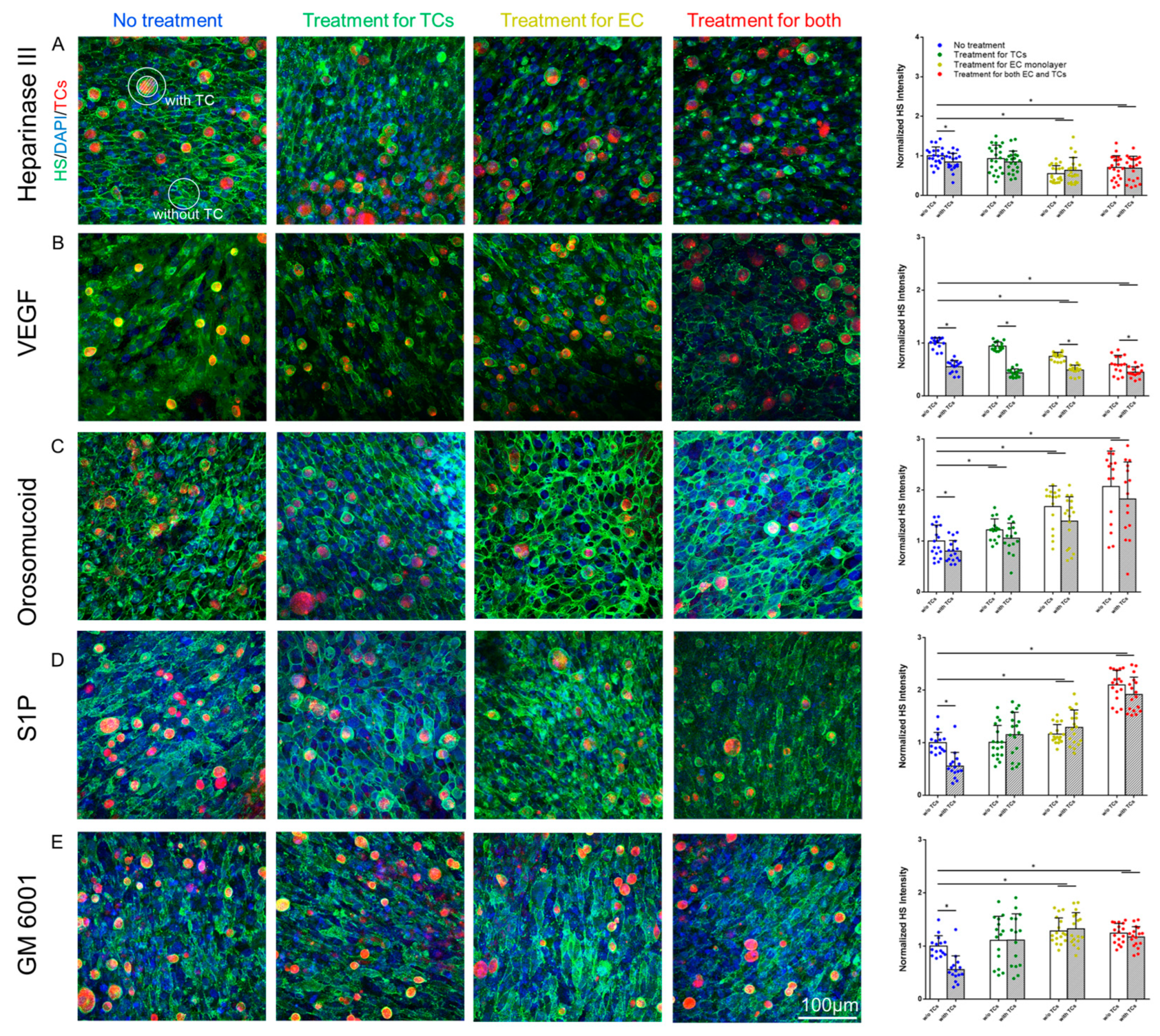
3.4. Effects of MB231 Adhesion and Various Pretreatments on HS of In Vitro BBB
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Gassmann, P.; Haier, J. The tumor cell-host organ interface in the early onset of metastatic organ colonisation. Clin. Exp. Metastasis 2008, 25, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Naumov, G.N.; Varghese, H.J.; Nadkarni, K.V.; MacDonald, I.C.; Groom, A.C. Critical steps in hematogenous metastasis: An overview. Surg. Oncol. Clin. N. Am. 2001, 10, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Bendas, G.; Borsig, L. Cancer cell adhesion and metastasis: Selectins, integrins, and the inhibitory potential of heparins. Int. J. Cell Biol. 2012, 2012, 676731. [Google Scholar] [CrossRef] [PubMed]
- Brenner, W.; Langer, P.; Oesch, F.; Edgell, C.J.; Wieser, R.J. Tumor cell—Endothelium adhesion in an artificial venule. Anal. Biochem. 1995, 225, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Whisler, J.A.; Jeon, J.S.; Kamm, R.D. Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr. Biol. 2013, 5, 1262–1271. [Google Scholar] [CrossRef]
- Eichler, A.F.; Chung, E.; Kodack, D.P.; Loeffler, J.S.; Fukumura, D.; Jain, R.K. The biology of brain metastases-translation to new therapies. Nat. Rev. Clin. Oncol. 2011, 8, 344–356. [Google Scholar] [CrossRef]
- Glinskii, O.V.; Li, F.; Wilson, L.S.; Barnes, S.; Rittenhouse-Olson, K.; Barchi, J.J., Jr.; Pienta, K.J.; Glinsky, V.V. Endothelial integrin α3β1 stabilizes carbohydrate-mediated tumor/endothelial cell adhesion and induces macromolecular signaling complex formation at the endothelial cell membrane. Oncotarget 2014, 5, 1382–1389. [Google Scholar] [CrossRef]
- Kienast, Y.; von Baumgarten, L.; Fuhrmann, M.; Klinkert, W.E.; Goldbrunner, R.; Herms, J.; Winkler, F. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 2010, 16, 116–122. [Google Scholar] [CrossRef]
- Kuperwasser, C.; Dessain, S.; Bierbaum, B.E.; Garnet, D.; Sperandio, K.; Gauvin, G.P.; Naber, S.P.; Weinberg, R.A.; Rosenblatt, M. A mouse model of human breast cancer metastasis to human bone. Cancer Res. 2005, 65, 6130–6138. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, D.; Strilic, B.; Sivaraj, K.K.; Wettschureck, N.; Offermanns, S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 2013, 24, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.L.; Huot, J.; Auger, F.A. Mechanisms by which E-selectin regulates diapedesis of colon cancer cells under flow conditions. Cancer Res. 2008, 68, 5167–5176. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflug. Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Tarbell, J.M.; Cancel, L.M. The glycocalyx and its significance in human medicine. J. Intern. Med. 2016, 280, 97–113. [Google Scholar] [CrossRef]
- Schmidt, E.P.; Yang, Y.; Janssen, W.J.; Gandjeva, A.; Perez, M.J.; Barthel, L.; Zemans, R.L.; Bowman, J.C.; Koyanagi, D.E.; Yunt, Z.X.; et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012, 18, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, K.B.; Arkill, K.P.; Neal, C.R.; Harper, S.J.; Foster, R.R.; Satchell, S.C.; Bates, D.O.; Salmon, A.H.J. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J. Physiol. 2017, 595, 5015–5035. [Google Scholar] [CrossRef]
- Buffone, A.; Weaver, V.M. Don’t sugarcoat it: How glycocalyx composition influences cancer progression. J. Cell Biol. 2020, 219, e201910070. [Google Scholar] [CrossRef]
- Jin, J.; Fang, F.; Gao, W.; Chen, H.; Wen, J.; Wen, X.; Chen, J. The Structure and Function of the Glycocalyx and Its Connection With Blood-Brain Barrier. Front. Cell. Neurosci. 2021, 15, 739699. [Google Scholar] [CrossRef]
- Foote, C.A.; Soares, R.N.; Ramirez-Perez, F.I.; Ghiarone, T.; Aroor, A.; Manrique-Acevedo, C.; Padilla, J.; Martinez-Lemus, L. Endothelial Glycocalyx. Compr. Physiol. 2022, 12, 3781–3811. [Google Scholar] [CrossRef]
- Van Teeffelen, J.W.; Brands, J.; Stroes, E.S.; Vink, H. Endothelial glycocalyx: Sweet shield of blood vessels. Trends Cardiovasc. Med. 2007, 17, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef] [PubMed]
- Curry, F.E.; Adamson, R.H. Endothelial Glycocalyx: Permeability Barrier and Mechanosensor. Ann. Biomed. Eng. 2012, 40, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Brouns, S.L.N.; Provenzale, I.; van Geffen, J.P.; van der Meijden, P.E.J.; Heemskerk, J.W.M. Localized endothelial-based control of platelet aggregation and coagulation under flow: A proof-of-principle vessel-on-a-chip study. J. Thromb. Haemost. 2020, 18, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.H.; Murphy, H.A.; George, E.M. The glycocalyx: A central regulator of vascular function. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2021, 320, R508–R518. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Fan, J.; Zeng, M.; Zhang, L.; Fu, B.M. Adhesion of malignant mammary tumor cells MDA-MB-231 to microvessel wall increases microvascular permeability via degradation of endothelial surface glycocalyx. J. Appl. Physiol. 2012, 113, 1141–1153. [Google Scholar] [CrossRef]
- Fan, J.; Fu, B.M. Quantification of Malignant Breast Cancer Cell MDA-MB-231 Transmigration Across Brain and Lung Microvascular Endothelium. Ann. Biomed. Eng. 2016, 44, 2189–2201. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, M.; Fu, B.M. Sphingosine-1-phosphate reduces adhesion of malignant mammary tumor cells MDA-MB-231 to microvessel walls by protecting endothelial surface glycocalyx. Cell. Mol. Biol. 2017, 63, 16–22. [Google Scholar] [CrossRef]
- Bates, D.O. Vascular endothelial growth factors and vascular permeability. Cardiovasc. Res. 2010, 87, 262–271. [Google Scholar] [CrossRef]
- Shen, S.; Fan, J.; Cai, B.; Lv, Y.; Zeng, M.; Hao, Y.; Giancotti, F.G.; Fu, B.M. Vascular endothelial growth factor enhances cancer cell adhesion to microvascular endothelium in vivo. Exp. Physiol. 2010, 95, 369–379. [Google Scholar] [CrossRef]
- Lee, T.H.; Avraham, H.K.; Jiang, S.; Avraham, S. Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J. Biol. Chem. 2003, 278, 5277–5284. [Google Scholar] [CrossRef] [PubMed]
- Bacac, M.; Stamenkovic, I. Metastatic cancer cell. Annu. Rev. Pathol. 2008, 3, 221–247. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Cai, B.; Zeng, M.; Hao, Y.; Giancotti, F.G.; Fu, B.M. Integrin β4 signaling promotes mammary tumor cell adhesion to brain microvascular endothelium by inducing ErbB2-mediated secretion of VEGF. Ann. Biomed. Eng. 2011, 39, 2223–2241. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.M.; Yang, J.; Cai, B.; Fan, J.; Zhang, L.; Zeng, M. Reinforcing endothelial junctions prevents microvessel permeability increase and tumor cell adhesion in microvessels in vivo. Sci. Rep. 2015, 5, 15697. [Google Scholar] [CrossRef]
- Mensah, S.A.; Nersesyan, A.A.; Harding, I.C.; Lee, C.I.; Tan, X.; Banerjee, S.; Niedre, M.; Torchilin, V.P.; Ebong, E.E. Flow-regulated endothelial glycocalyx determines metastatic cancer cell activity. FASEB J. 2020, 34, 6166–6184. [Google Scholar] [CrossRef]
- Kang, H.; Wu, Q.; Sun, A.; Liu, X.; Fan, Y.; Deng, X. Cancer Cell Glycocalyx and Its Significance in Cancer Progression. Int. J. Mol. Sci. 2018, 19, 2484. [Google Scholar] [CrossRef]
- Zeng, Y.; Qiu, Y.; Jiang, W.; Fu, B.M. Glycocalyx Acts as a Central Player in the Development of Tumor Microenvironment by Extracellular Vesicles for Angiogenesis and Metastasis. Cancers 2022, 14, 5415. [Google Scholar] [CrossRef]
- Paszek, M.J.; DuFort, C.C.; Rossier, O.; Bainer, R.; Mouw, J.K.; Godula, K.; Hudak, J.E.; Lakins, J.N.; Wijekoon, A.C.; Cassereau, L.; et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 2014, 511, 319–325. [Google Scholar] [CrossRef]
- Kanyo, N.; Kovacs, K.D.; Saftics, A.; Szekacs, I.; Peter, B.; Santa-Maria, A.R.; Walter, F.R.; Dér, A.; Deli, M.A.; Horvath, R. Glycocalyx regulates the strength and kinetics of cancer cell adhesion revealed by biophysical models based on high resolution label-free optical data. Sci. Rep. 2020, 10, 22422. [Google Scholar] [CrossRef]
- Möckl, L.; Pedram, K.; Roy, A.R.; Krishnan, V.; Gustavsson, A.K.; Dorigo, O.; Bertozzi, C.R.; Moerner, W.E. Quantitative Super-Resolution Microscopy of the Mammalian Glycocalyx. Dev. Cell 2019, 50, 57–72.e56. [Google Scholar] [CrossRef]
- Weyers, A.; Yang, B.; Yoon, D.S.; Park, J.H.; Zhang, F.; Lee, K.B.; Linhardt, R.J. A structural analysis of glycosaminoglycans from lethal and nonlethal breast cancer tissues: Toward a novel class of theragnostics for personalized medicine in oncology? Omics 2012, 16, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Woods, E.C.; Kai, F.; Barnes, J.M.; Pedram, K.; Pickup, M.W.; Hollander, M.J.; Weaver, V.M.; Bertozzi, C.R. A bulky glycocalyx fosters metastasis formation by promoting G1 cell cycle progression. eLife 2017, 6, e25752. [Google Scholar] [CrossRef] [PubMed]
- Qazi, H.; Shi, Z.D.; Song, J.W.; Cancel, L.M.; Huang, P.; Zeng, Y.; Roberge, S.; Munn, L.L.; Tarbell, J.M. Heparan sulfate proteoglycans mediate renal carcinoma metastasis. Int. J. Cancer 2016, 139, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.R.; Buffone Jr, A.; Hammer, D.A. T lymphocytes migrate upstream after completing the leukocyte adhesion cascade. Cell Adhes. Migr. 2019, 13, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Buffone, A., Jr.; Anderson, N.R.; Hammer, D.A. Migration against the direction of flow is LFA-1-dependent in human hematopoietic stem and progenitor cells. J. Cell Sci. 2018, 131, jcs205575. [Google Scholar] [CrossRef]
- Büll, C.; Stoel, M.A.; den Brok, M.H.; Adema, G.J. Sialic acids sweeten a tumor’s life. Cancer Res. 2014, 74, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Woods, E.C.; Vukojicic, P.; Bertozzi, C.R. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2016, 113, 10304–10309. [Google Scholar] [CrossRef]
- Mitchell, M.J.; King, M.R. Physical biology in cancer. 3. The role of cell glycocalyx in vascular transport of circulating tumor cells. Am. J. Physiol. Cell Physiol. 2014, 306, C89–C97. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Y.; Fu, B.M. Differential effects of vascular endothelial growth factor on glycocalyx of endothelial and tumor cells and potential targets for tumor metastasis. APL Bioeng. 2022, 6, 016101. [Google Scholar] [CrossRef]
- Zeng, Y.; Ebong, E.E.; Fu, B.M.; Tarbell, J.M. The structural stability of the endothelial glycocalyx after enzymatic removal of glycosaminoglycans. PLoS ONE 2012, 7, e43168. [Google Scholar] [CrossRef]
- Fu, B.M. Quantification of In Vitro Blood-Brain Barrier Permeability. Methods Mol. Biol. 2022, 2375, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Waters, M.; Andrews, A.; Honarmandi, P.; Ebong, E.E.; Rizzo, V.; Tarbell, J.M. Fluid shear stress induces the clustering of heparan sulfate via mobility of glypican-1 in lipid rafts. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H811–H820. [Google Scholar] [CrossRef] [PubMed]
- Paszek, M.J.; Boettiger, D.; Weaver, V.M.; Hammer, D.A. Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLoS Comput. Biol. 2009, 5, e1000604. [Google Scholar] [CrossRef] [PubMed]
- Perrot-Applanat, M.; Di Benedetto, M. Autocrine functions of VEGF in breast tumor cells: Adhesion, survival, migration and invasion. Cell Adhes. Migr. 2012, 6, 547–553. [Google Scholar] [CrossRef]
- Sanderson, R.D. Heparan sulfate proteoglycans in invasion and metastasis. Semin. Cell Dev. Biol. 2001, 12, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Couchman, J.R.; Chen, L.; Woods, A. Syndecans and cell adhesion. Int. Rev. Cytol. 2001, 207, 113–150. [Google Scholar] [CrossRef]
- Blackhall, F.H.; Merry, C.L.; Davies, E.J.; Jayson, G.C. Heparan sulfate proteoglycans and cancer. Br. J. Cancer 2001, 85, 1094–1098. [Google Scholar] [CrossRef]
- Bernfield, M.; Götte, M.; Park, P.W.; Reizes, O.; Fitzgerald, M.L.; Lincecum, J.; Zako, M. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 1999, 68, 729–777. [Google Scholar] [CrossRef]
- Lim, H.C.; Multhaupt, H.A.; Couchman, J.R. Cell surface heparan sulfate proteoglycans control adhesion and invasion of breast carcinoma cells. Mol. Cancer 2015, 14, 15. [Google Scholar] [CrossRef]
- Ma, Y.-Q.; Geng, J.-G. Heparan Sulfate-Like Proteoglycans Mediate Adhesion of Human Malignant Melanoma A375 Cells to P-Selectin Under Flow1. J. Immunol. 2000, 165, 558–565. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Numa, F.; Suminami, Y.; Murakami, A.; Murakami, T.; Kato, H. Altered proliferative and metastatic potential associated with increased expression of syndecan-1. Tumor Biol. 1998, 19, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Lee, G.Y.; Ong, C.N.; Lim, C.T. AFM indentation study of breast cancer cells. Biochem. Biophys. Res. Commun. 2008, 374, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, J.E.; Pinney, E. Quantitation of specific binding of orosomucoid to cultured microvascular endothelium: Role in capillary permeability. Am. J. Physiol. 1992, 263 Pt 2, H48–H55. [Google Scholar] [CrossRef]
- Yuan, W.; Li, G.; Zeng, M.; Fu, B.M. Modulation of the blood-brain barrier permeability by plasma glycoprotein orosomucoid. Microvasc. Res. 2010, 80, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Nishi, K.; Kikuchi, M.; Kadowaki, D.; Tokutomi, Y.; Tokutomi, N.; Nishi, K.; Suenaga, A.; Otagiri, M. α1-Acid glycoprotein suppresses rat acute inflammatory paw edema through the inhibition of neutrophils activation and prostaglandin E2 generation. Biol. Pharm. Bull. 2007, 30, 1226–1230. [Google Scholar] [CrossRef]
- Kremer, J.M.; Wilting, J.; Janssen, L.H. Drug binding to human alpha-1-acid glycoprotein in health and disease. Pharmacol. Rev. 1988, 40, 1–47. [Google Scholar]
- Haraldsson, B.; Rippe, B. Orosomucoid as one of the serum components contributing to normal capillary permselectivity in rat skeletal muscle. Acta Physiol. Scand. 1987, 129, 127–135. [Google Scholar] [CrossRef]
- Takabe, K.; Paugh, S.W.; Milstien, S.; Spiegel, S. “Inside-out” signaling of sphingosine-1-phosphate: Therapeutic targets. Pharmacol. Rev. 2008, 60, 181–195. [Google Scholar] [CrossRef]
- Hänel, P.; Andréani, P.; Gräler, M.H. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007, 21, 1202–1209. [Google Scholar] [CrossRef]
- Bode, C.; Sensken, S.C.; Peest, U.; Beutel, G.; Thol, F.; Levkau, B.; Li, Z.; Bittman, R.; Huang, T.; Tölle, M.; et al. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J. Cell. Biochem. 2010, 109, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Curry, F.E.; Clark, J.F.; Adamson, R.H. Erythrocyte-derived sphingosine-1-phosphate stabilizes basal hydraulic conductivity and solute permeability in rat microvessels. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H825–H834. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Adamson, R.H.; Curry, F.R.; Tarbell, J.M. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H363–H372. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Dinavahi, S.S.; Feng, Q.; Gowda, R.; Ramisetti, S.; Xia, X.; LaPenna, K.B.; Chirasani, V.R.; Cho, S.H.; Hafenstein, S.L.; et al. Activating Sphingosine-1-phospahte signaling in endothelial cells increases myosin light chain phosphorylation to decrease endothelial permeability thereby inhibiting cancer metastasis. Cancer Lett. 2021, 506, 107–119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Shteyman, D.B.; Hachem, Z.; Ulay, A.A.; Fan, J.; Fu, B.M. Heparan Sulfate Modulation Affects Breast Cancer Cell Adhesion and Transmigration across In Vitro Blood–Brain Barrier. Cells 2024, 13, 190. https://doi.org/10.3390/cells13020190
Li Y, Shteyman DB, Hachem Z, Ulay AA, Fan J, Fu BM. Heparan Sulfate Modulation Affects Breast Cancer Cell Adhesion and Transmigration across In Vitro Blood–Brain Barrier. Cells. 2024; 13(2):190. https://doi.org/10.3390/cells13020190
Chicago/Turabian StyleLi, Yunfei, David B. Shteyman, Zeina Hachem, Afaf A. Ulay, Jie Fan, and Bingmei M. Fu. 2024. "Heparan Sulfate Modulation Affects Breast Cancer Cell Adhesion and Transmigration across In Vitro Blood–Brain Barrier" Cells 13, no. 2: 190. https://doi.org/10.3390/cells13020190






