The Role of Soluble CD163 (sCD163) in Human Physiology and Pathophysiology
Abstract
:1. Introduction
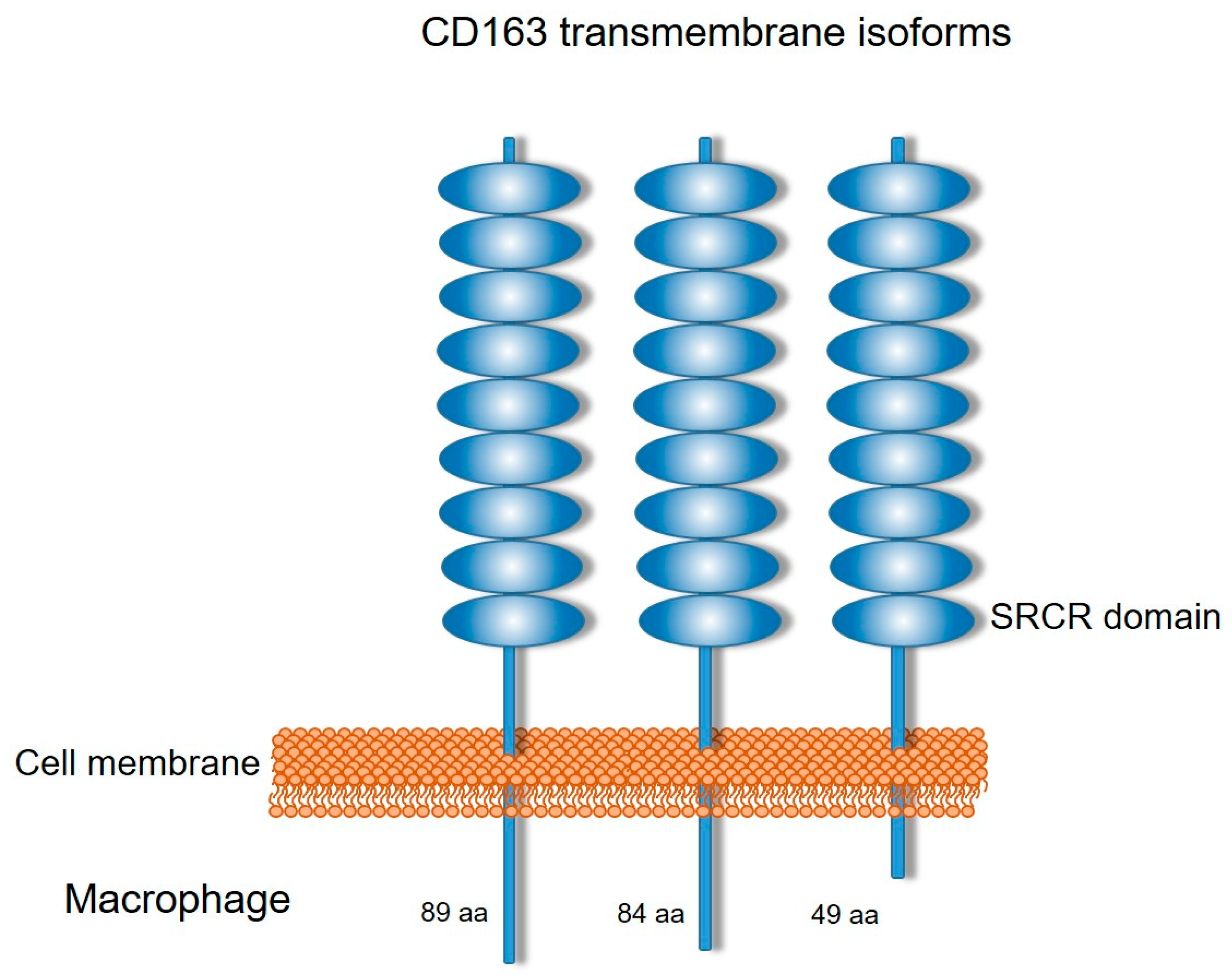
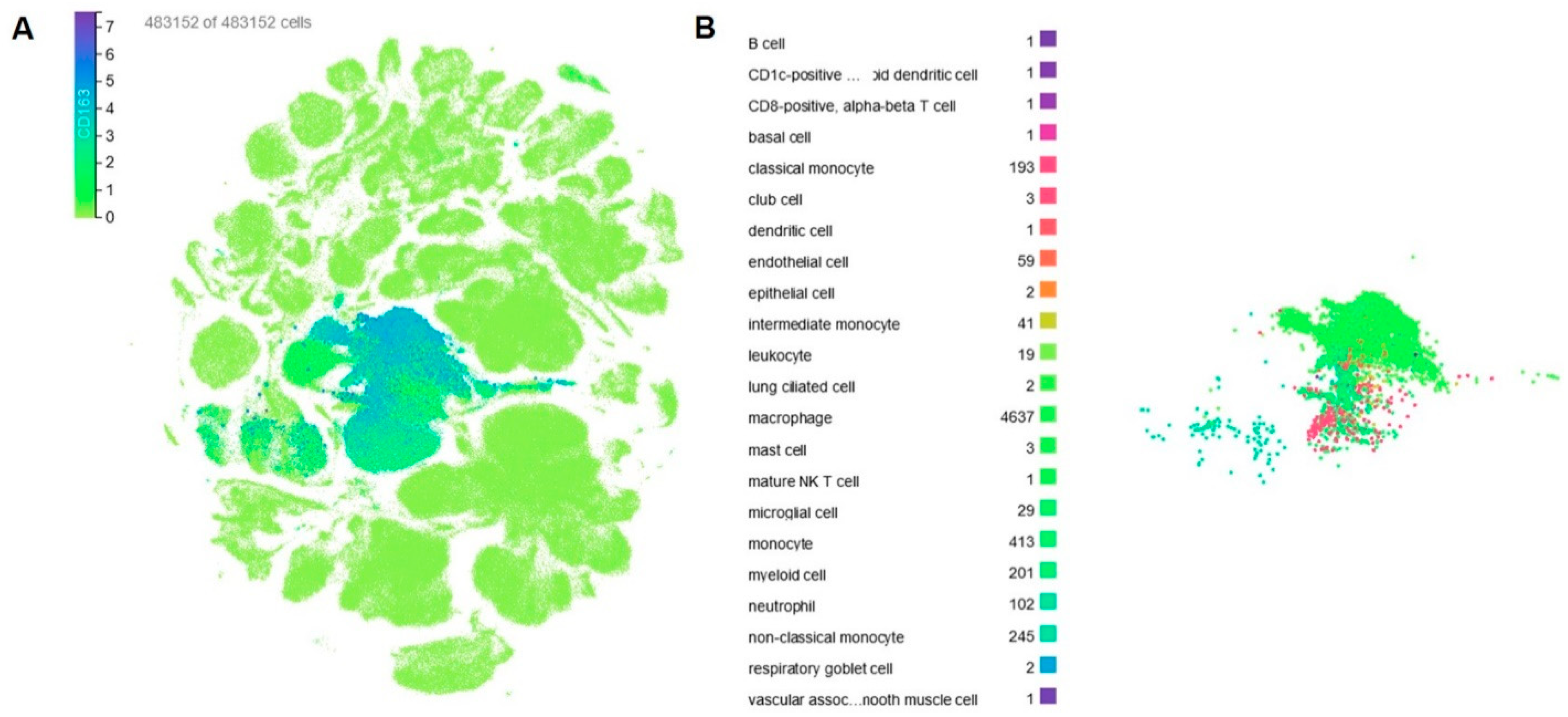
2. CD163 Expression
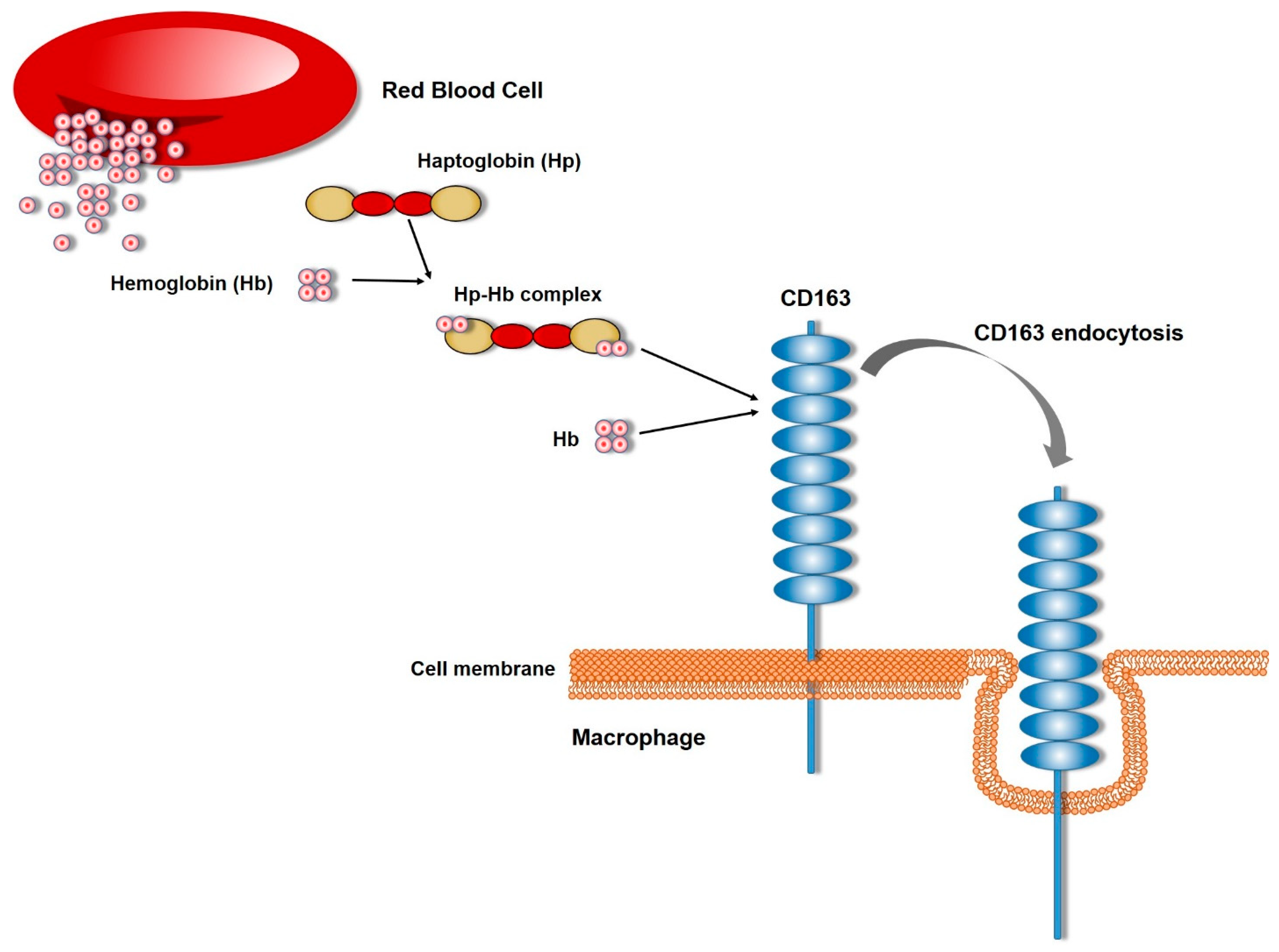
3. Role of CD163
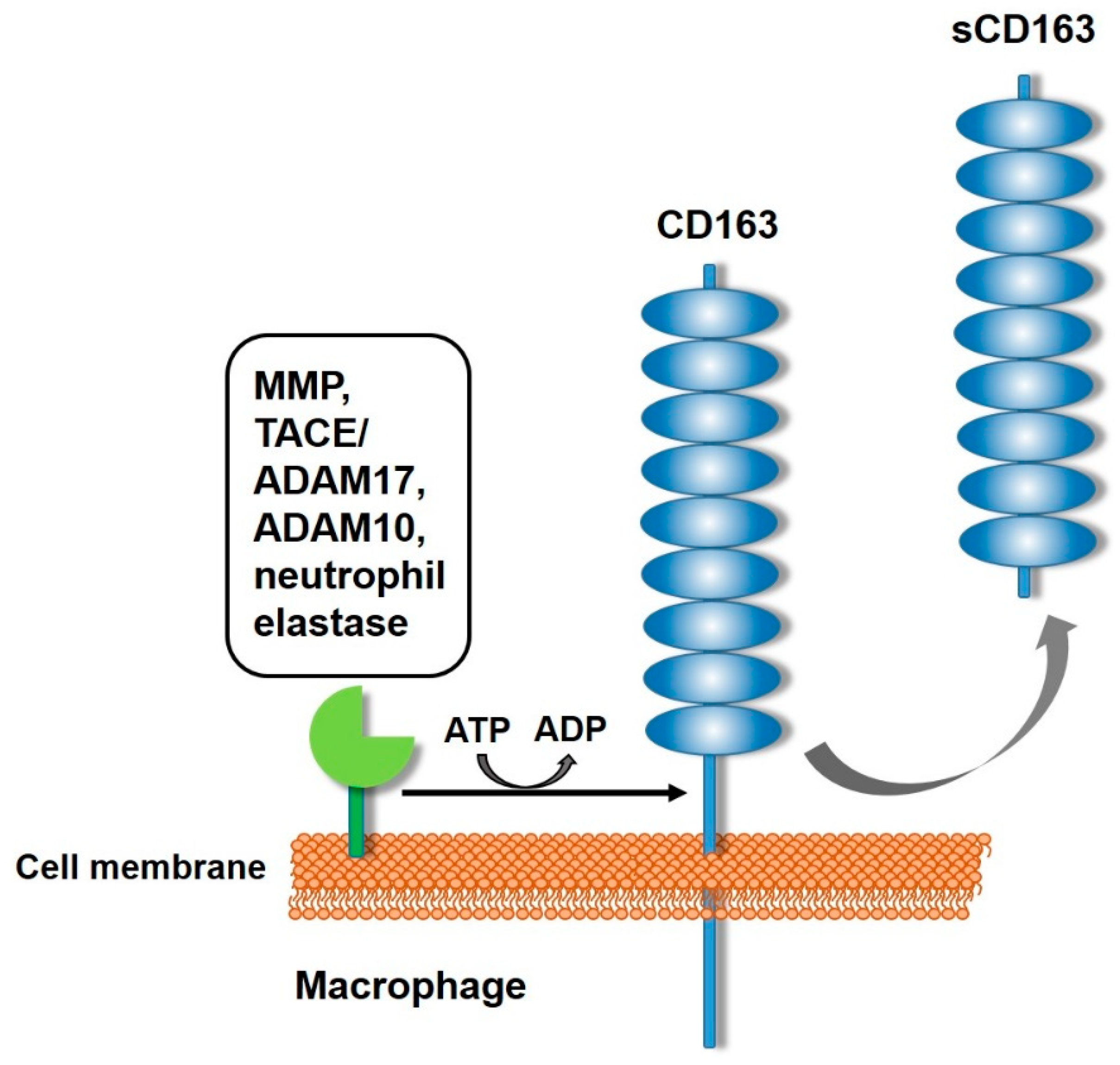
4. Generation of sCD163
5. sCD163 Activities
6. sCD163 as a Biomarker and Role in Pathological Conditions
7. sCD163 in Tumors
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Skytthe, M.K.; Graversen, J.H.; Moestrup, S.K. Targeting of CD163(+) Macrophages in Inflammatory and Malignant Diseases. Int. J. Mol. Sci. 2020, 21, 5497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zou, W.; Du, J.; Zhao, Y. The origins and homeostasis of monocytes and tissue-resident macrophages in physiological situation. J. Cell Physiol. 2018, 233, 6425–6439. [Google Scholar] [CrossRef] [PubMed]
- Alquraini, A.; El Khoury, J. Scavenger receptors. Curr. Biol. 2020, 30, R790–R795. [Google Scholar] [CrossRef] [PubMed]
- Taban, Q.; Mumtaz, P.T.; Masoodi, K.Z.; Haq, E.; Ahmad, S.M. Scavenger receptors in host defense: From functional aspects to mode of action. Cell Commun. Signal 2022, 20, 2. [Google Scholar] [CrossRef]
- Moller, H.J. Soluble CD163. Scand. J. Clin. Lab. Investig. 2012, 72, 1–13. [Google Scholar] [CrossRef]
- Gronlund, J.; Vitved, L.; Lausen, M.; Skjodt, K.; Holmskov, U. Cloning of a novel scavenger receptor cysteine-rich type I transmembrane molecule (M160) expressed by human macrophages. J. Immunol. 2000, 165, 6406–6415. [Google Scholar] [CrossRef]
- PrabhuDas, M.R.; Baldwin, C.L.; Bollyky, P.L.; Bowdish, D.M.E.; Drickamer, K.; Febbraio, M.; Herz, J.; Kobzik, L.; Krieger, M.; Loike, J.; et al. A Consensus Definitive Classification of Scavenger Receptors and Their Roles in Health and Disease. J. Immunol. 2017, 198, 3775–3789. [Google Scholar] [CrossRef]
- Calvert, J.G.; Slade, D.E.; Shields, S.L.; Jolie, R.; Mannan, R.M.; Ankenbauer, R.G.; Welch, S.K. CD163 expression confers susceptibility to porcine reproductive and respiratory syndrome viruses. J. Virol. 2007, 81, 7371–7379. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.D.; Bardot, R.; Whitworth, K.M.; Trible, B.R.; Fang, Y.; Mileham, A.; Kerrigan, M.A.; Samuel, M.S.; Prather, R.S.; Rowland, R.R.R. Replacement of Porcine CD163 Scavenger Receptor Cysteine-Rich Domain 5 with a CD163-like Homolog Confers Resistance of Pigs to Genotype 1 but Not Genotype 2 Porcine Reproductive and Respiratory Syndrome Virus. J. Virol. 2017, 91, e01521-16. [Google Scholar] [CrossRef]
- Stoian, A.M.M.; Rowland, R.R.R.; Brandariz-Nunez, A. Identification of CD163 regions that are required for porcine reproductive and respiratory syndrome virus (PRRSV) infection but not for binding to viral envelope glycoproteins. Virology 2022, 574, 71–83. [Google Scholar] [CrossRef]
- Van Gorp, H.; Van Breedam, W.; Van Doorsselaere, J.; Delputte, P.L.; Nauwynck, H.J. Identification of the CD163 protein domains involved in infection of the porcine reproductive and respiratory syndrome virus. J. Virol. 2010, 84, 3101–3105. [Google Scholar] [CrossRef] [PubMed]
- Fabriek, B.O.; van Bruggen, R.; Deng, D.M.; Ligtenberg, A.J.; Nazmi, K.; Schornagel, K.; Vloet, R.P.; Dijkstra, C.D.; van den Berg, T.K. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 2009, 113, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Law, S.K.; Micklem, K.J.; Shaw, J.M.; Zhang, X.P.; Dong, Y.; Willis, A.C.; Mason, D.Y. A new macrophage differentiation antigen which is a member of the scavenger receptor superfamily. Eur. J. Immunol. 1993, 23, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.; Buechler, C.; Langmann, T.; Schmitz, G. Genomic organization and chromosomal localization of the human CD163 (M130) gene: A member of the scavenger receptor cysteine-rich superfamily. Biochem. Biophys. Res. Commun. 1999, 260, 466–474. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Madsen, M.; Moller, H.J.; Moestrup, S.K. The macrophage scavenger receptor CD163: Endocytic properties of cytoplasmic tail variants. J. Leukoc. Biol. 2006, 79, 837–845. [Google Scholar] [CrossRef]
- Pulford, K.; Micklem, K.; McCarthy, S.; Cordell, J.; Jones, M.; Mason, D.Y. A monocyte/macrophage antigen recognized by the four antibodies GHI/61, Ber-MAC3, Ki-M8 and SM4. Immunology 1992, 75, 588–595. [Google Scholar]
- Zwadlo, G.; Voegeli, R.; Schulze Osthoff, K.; Sorg, C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp. Cell Biol. 1987, 55, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, M.M.; Tensen, C.P.; van As, J.H.; Van den Berg, T.K.; Fluitsma, D.M.; Dijkstra, C.D.; Dopp, E.A.; Droste, A.; Van Gaalen, F.A.; Sorg, C.; et al. Regulation of CD 163 on human macrophages: Cross-linking of CD163 induces signaling and activation. J. Leukoc. Biol. 1999, 66, 858–866. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, S.; Gao, Y.; Lu, Z.; Yang, Y.; Chen, J.; Li, T. Application of exosomal miRNA mediated macrophage polarization in colorectal cancer: Current progress and challenges. Oncol. Res. 2023, 32, 61–71. [Google Scholar] [CrossRef]
- Zhi, Y.; Gao, P.; Xin, X.; Li, W.; Ji, L.; Zhang, L.; Zhang, X.; Zhang, J. Clinical significance of sCD163 and its possible role in asthma (Review). Mol. Med. Rep. 2017, 15, 2931–2939. [Google Scholar] [CrossRef]
- Barros, M.H.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage polarisation: An immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, B.; Zhu, J.; Huang, W.; Han, B.; Wang, Q.; Qi, C.; Wang, M.; Liu, F. miR-106b-5p Inhibits IRF1/IFN-beta Signaling to Promote M2 Macrophage Polarization of Glioblastoma. Onco Targets Ther. 2020, 13, 7479–7492. [Google Scholar] [CrossRef] [PubMed]
- Do, T.; Tan, R.; Bennett, M.; Medvedovic, M.; Grom, A.A.; Shen, N.; Thornton, S.; Schulert, G.S. MicroRNA networks associated with active systemic juvenile idiopathic arthritis regulate CD163 expression and anti-inflammatory functions in macrophages through two distinct mechanisms. J. Leukoc. Biol. 2018, 103, 71–85. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.; Zheng, Z.; Li, C.; Mao, S.; Wu, Y.; Wang, R.; Zhang, J.; Zhang, Y.; Wang, H.; et al. Sulfatase 2 Affects Polarization of M2 Macrophages through the IL-8/JAK2/STAT3 Pathway in Bladder Cancer. Cancers 2022, 15, 131. [Google Scholar] [CrossRef]
- Poles, W.A.; Nishi, E.E.; de Oliveira, M.B.; Eugenio, A.I.P.; de Andrade, T.A.; Campos, A.; de Campos, R.R., Jr.; Vassallo, J.; Alves, A.C.; Scapulatempo Neto, C.; et al. Targeting the polarization of tumor-associated macrophages and modulating mir-155 expression might be a new approach to treat diffuse large B-cell lymphoma of the elderly. Cancer Immunol. Immunother. 2019, 68, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Tabula Sapiens, C.; Jones, R.C.; Karkanias, J.; Krasnow, M.A.; Pisco, A.O.; Quake, S.R.; Salzman, J.; Yosef, N.; Bulthaup, B.; Brown, P.; et al. The Tabula Sapiens: A multiple-organ, single-cell transcriptomic atlas of humans. Science 2022, 376, eabl4896. [Google Scholar] [CrossRef]
- Perkel, J.M. 85 million cells-and counting-at your fingertips. Nature 2024, 629, 248–249. [Google Scholar] [CrossRef]
- Abdulla, S.; Aevermann, B.; Assis, P.; Badajoz, S.; Bell, S.M.; Bezzi, E.; Cakir, B.; Chaffer, J.; Chambers, S.; Michael Cherry, J.; et al. CZ CELL×GENE Discover: A single-cell data platform for scalable exploration, analysis and modeling of aggregated data. bioRxiv 2023, 2023.10.30.563174. [Google Scholar] [CrossRef]
- Megill, C.; Martin, B.; Weaver, C.; Bell, S.; Prins, L.; Badajoz, S.; McCandless, B.; Pisco, A.O.; Kinsella, M.; Griffin, F.; et al. cellxgene: A performant, scalable exploration platform for high dimensional sparse matrices. bioRxiv 2021, 2021.04.05.438318. [Google Scholar] [CrossRef]
- Dennis, C. Haemoglobin scavenger. Nature 2001, 409, 141. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.; Moller, H.J.; Nielsen, M.J.; Jacobsen, C.; Graversen, J.H.; van den Berg, T.; Moestrup, S.K. Molecular characterization of the haptoglobin.hemoglobin receptor CD163. Ligand binding properties of the scavenger receptor cysteine-rich domain region. J. Biol. Chem. 2004, 279, 51561–51567. [Google Scholar] [CrossRef]
- Schaer, D.J.; Schaer, C.A.; Buehler, P.W.; Boykins, R.A.; Schoedon, G.; Alayash, A.I.; Schaffner, A. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood 2006, 107, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Schwartz, E.J.; West, R.B.; Warnke, R.A.; Arber, D.A.; Natkunam, Y. Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am. J. Surg. Pathol. 2005, 29, 617–624. [Google Scholar] [CrossRef]
- Xie, W.J.; Yu, H.Q.; Zhang, Y.; Liu, Q.; Meng, H.M. CD163 promotes hematoma absorption and improves neurological functions in patients with intracerebral hemorrhage. Neural Regen. Res. 2016, 11, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Sherchan, P.; Jin, P.; Huang, L.; Travis, Z.; Zhang, J.H.; Gong, Y.; Tang, J. Recombinant CCL17 Enhances Hematoma Resolution and Activation of CCR4/ERK/Nrf2/CD163 Signaling Pathway After Intracerebral Hemorrhage in Mice. Neurotherapeutics 2020, 17, 1940–1953. [Google Scholar] [CrossRef]
- Moller, H.J.; Gronbaek, H.; Schiodt, F.V.; Holland-Fischer, P.; Schilsky, M.; Munoz, S.; Hassanein, T.; Lee, W.M.; Group, U.S.A.L.F.S. Soluble CD163 from activated macrophages predicts mortality in acute liver failure. J. Hepatol. 2007, 47, 671–676. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, Y.; Luo, Y.; Zheng, J.; Gong, L.; Wang, H.; Feng, Y.; Gong, T.; Wu, D.; Wu, R.; et al. Adaptation of African swine fever virus to porcine kidney cells stably expressing CD163 and Siglec1. Front. Immunol. 2022, 13, 1015224. [Google Scholar] [CrossRef]
- Buechler, C.; Ritter, M.; Orso, E.; Langmann, T.; Klucken, J.; Schmitz, G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J. Leukoc. Biol. 2000, 67, 97–103. [Google Scholar] [CrossRef]
- Wenzel, I.; Roth, J.; Sorg, C. Identification of a novel surface molecule, RM3/1, that contributes to the adhesion of glucocorticoid-induced human monocytes to endothelial cells. Eur. J. Immunol. 1996, 26, 2758–2763. [Google Scholar] [CrossRef] [PubMed]
- Zwadlo-Klarwasser, G.; Bent, S.; Haubeck, H.D.; Sorg, C.; Schmutzler, W. Glucocorticoid-induced appearance of the macrophage subtype RM 3/1 in peripheral blood of man. Int. Arch. Allergy Appl. Immunol. 1990, 91, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Sulahian, T.H.; Hogger, P.; Wahner, A.E.; Wardwell, K.; Goulding, N.J.; Sorg, C.; Droste, A.; Stehling, M.; Wallace, P.K.; Morganelli, P.M.; et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine 2000, 12, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Schaer, D.J.; Boretti, F.S.; Schoedon, G.; Schaffner, A. Induction of the CD163-dependent haemoglobin uptake by macrophages as a novel anti-inflammatory action of glucocorticoids. Br. J. Haematol. 2002, 119, 239–243. [Google Scholar] [CrossRef]
- Philippidis, P.; Mason, J.C.; Evans, B.J.; Nadra, I.; Taylor, K.M.; Haskard, D.O.; Landis, R.C. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: Antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ. Res. 2004, 94, 119–126. [Google Scholar] [CrossRef]
- Wiley, S.R.; Winkles, J.A. TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor. Rev. 2003, 14, 241–249. [Google Scholar] [CrossRef]
- Ratajczak, W.; Atkinson, S.D.; Kelly, C. The TWEAK/Fn14/CD163 axis-implications for metabolic disease. Rev. Endocr. Metab. Disord. 2022, 23, 449–462. [Google Scholar] [CrossRef]
- Ritter, M.; Buechler, C.; Kapinsky, M.; Schmitz, G. Interaction of CD163 with the regulatory subunit of casein kinase II (CKII) and dependence of CD163 signaling on CKII and protein kinase C. Eur. J. Immunol. 2001, 31, 999–1009. [Google Scholar] [CrossRef]
- Schaer, C.A.; Schoedon, G.; Imhof, A.; Kurrer, M.O.; Schaer, D.J. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ. Res. 2006, 99, 943–950. [Google Scholar] [CrossRef]
- Van Gorp, H.; Delputte, P.L.; Nauwynck, H.J. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol. Immunol. 2010, 47, 1650–1660. [Google Scholar] [CrossRef]
- Hintz, K.A.; Rassias, A.J.; Wardwell, K.; Moss, M.L.; Morganelli, P.M.; Pioli, P.A.; Givan, A.L.; Wallace, P.K.; Yeager, M.P.; Guyre, P.M. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J. Leukoc. Biol. 2002, 72, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Etzerodt, A.; Maniecki, M.B.; Moller, K.; Moller, H.J.; Moestrup, S.K. Tumor necrosis factor alpha-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J. Leukoc. Biol. 2010, 88, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Reiss, K.; Saftig, P. The “a disintegrin and metalloprotease” (ADAM) family of sheddases: Physiological and cellular functions. Semin. Cell Dev. Biol. 2009, 20, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Onofre, G.; Kolackova, M.; Jankovicova, K.; Krejsek, J. Scavenger receptor CD163 and its biological functions. Acta Medica 2009, 52, 57–61. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Andersen, M.N.; Rittig, N.; Rodgaard-Hansen, S.; Gronbaek, H.; Moestrup, S.K.; Moller, H.J.; Etzerodt, A. The macrophage-related biomarkers sCD163 and sCD206 are released by different shedding mechanisms. J. Leukoc. Biol. 2019, 106, 1129–1138. [Google Scholar] [CrossRef]
- Kneidl, J.; Loffler, B.; Erat, M.C.; Kalinka, J.; Peters, G.; Roth, J.; Barczyk, K. Soluble CD163 promotes recognition, phagocytosis and killing of Staphylococcus aureus via binding of specific fibronectin peptides. Cell Microbiol. 2012, 14, 914–936. [Google Scholar] [CrossRef]
- Moreno, J.A.; Ortega-Gomez, A.; Delbosc, S.; Beaufort, N.; Sorbets, E.; Louedec, L.; Esposito-Farese, M.; Tubach, F.; Nicoletti, A.; Steg, P.G.; et al. In vitro and in vivo evidence for the role of elastase shedding of CD163 in human atherothrombosis. Eur. Heart J. 2012, 33, 252–263. [Google Scholar] [CrossRef]
- Subramanian, K.; Du, R.; Tan, N.S.; Ho, B.; Ding, J.L. CD163 and IgG codefend against cytotoxic hemoglobin via autocrine and paracrine mechanisms. J. Immunol. 2013, 190, 5267–5278. [Google Scholar] [CrossRef]
- Moller, H.J.; Nielsen, M.J.; Maniecki, M.B.; Madsen, M.; Moestrup, S.K. Soluble macrophage-derived CD163: A homogenous ectodomain protein with a dissociable haptoglobin-hemoglobin binding. Immunobiology 2010, 215, 406–412. [Google Scholar] [CrossRef]
- Tamara, S.; Franc, V.; Heck, A.J.R. A wealth of genotype-specific proteoforms fine-tunes hemoglobin scavenging by haptoglobin. Proc. Natl. Acad. Sci. USA 2020, 117, 15554–15564. [Google Scholar] [CrossRef]
- Kneidl, J.; Mysore, V.; Geraci, J.; Tuchscherr, L.; Loffler, B.; Holzinger, D.; Roth, J.; Barczyk-Kahlert, K. Soluble CD163 masks fibronectin-binding protein A-mediated inflammatory activation of Staphylococcus aureus infected monocytes. Cell Microbiol. 2014, 16, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Vazquez, P.A.; Bernal, L.; Paige, C.A.; Grosick, R.L.; Moracho Vilrriales, C.; Ferreira, D.W.; Ulecia-Moron, C.; Romero-Sandoval, E.A. Macrophage-specific nanotechnology-driven CD163 overexpression in human macrophages results in an M2 phenotype under inflammatory conditions. Immunobiology 2017, 222, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Akahori, H.; Karmali, V.; Polavarapu, R.; Lyle, A.N.; Weiss, D.; Shin, E.; Husain, A.; Naqvi, N.; Van Dam, R.; Habib, A.; et al. CD163 interacts with TWEAK to regulate tissue regeneration after ischaemic injury. Nat. Commun. 2015, 6, 7792. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Munoz, C.; Mendez-Barbero, N.; Svendsen, P.; Sastre, C.; Fernandez-Laso, V.; Quesada, P.; Egido, J.; Escola-Gil, J.C.; Martin-Ventura, J.L.; Moestrup, S.K.; et al. CD163 deficiency increases foam cell formation and plaque progression in atherosclerotic mice. FASEB J. 2020, 34, 14960–14976. [Google Scholar] [CrossRef] [PubMed]
- Kowal-Bielecka, O.; Bielecki, M.; Guiducci, S.; Trzcinska-Butkiewicz, B.; Michalska-Jakubus, M.; Matucci-Cerinic, M.; Brzosko, M.; Krasowska, D.; Chyczewski, L.; Kowal, K. High serum sCD163/sTWEAK ratio is associated with lower risk of digital ulcers but more severe skin disease in patients with systemic sclerosis. Arthritis Res. Ther. 2013, 15, R69. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, S.; Weeks, J.; Moreno, J.R.; He, B.; Xue, T.; Rainbolt, J.; Morita, Y.; Shu, Y.; Liu, Y.; et al. Reduced angiogenesis and delayed endochondral ossification in CD163(-/-) mice highlights a role of M2 macrophages during bone fracture repair. J. Orthop. Res. 2023, 41, 2384–2393. [Google Scholar] [CrossRef]
- Urbich, C.; Heeschen, C.; Aicher, A.; Sasaki, K.; Bruhl, T.; Farhadi, M.R.; Vajkoczy, P.; Hofmann, W.K.; Peters, C.; Pennacchio, L.A.; et al. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat. Med. 2005, 11, 206–213. [Google Scholar] [CrossRef]
- Urbich, C.; De Souza, A.I.; Rossig, L.; Yin, X.; Xing, Q.; Prokopi, M.; Drozdov, I.; Steiner, M.; Breuss, J.; Xu, Q.; et al. Proteomic characterization of human early pro-angiogenic cells. J. Mol. Cell Cardiol. 2011, 50, 333–336. [Google Scholar] [CrossRef]
- Koh, Y.W.; Park, C.S.; Yoon, D.H.; Suh, C.; Huh, J. CD163 expression was associated with angiogenesis and shortened survival in patients with uniformly treated classical Hodgkin lymphoma. PLoS ONE 2014, 9, e87066. [Google Scholar] [CrossRef]
- Park, J.Y.; Sung, J.Y.; Lee, J.; Park, Y.K.; Kim, Y.W.; Kim, G.Y.; Won, K.Y.; Lim, S.J. Polarized CD163+ tumor-associated macrophages are associated with increased angiogenesis and CXCL12 expression in gastric cancer. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 357–365. [Google Scholar] [CrossRef]
- Etzerodt, A.; Tsalkitzi, K.; Maniecki, M.; Damsky, W.; Delfini, M.; Baudoin, E.; Moulin, M.; Bosenberg, M.; Graversen, J.H.; Auphan-Anezin, N.; et al. Specific targeting of CD163(+) TAMs mobilizes inflammatory monocytes and promotes T cell-mediated tumor regression. J. Exp. Med. 2019, 216, 2394–2411. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Akahori, H.; Harari, E.; Smith, S.L.; Polavarapu, R.; Karmali, V.; Otsuka, F.; Gannon, R.L.; Braumann, R.E.; Dickinson, M.H.; et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J. Clin. Investig. 2018, 128, 1106–1124. [Google Scholar] [CrossRef]
- Rittig, N.; Svart, M.; Jessen, N.; Moller, N.; Moller, H.J.; Gronbaek, H. Macrophage activation marker sCD163 correlates with accelerated lipolysis following LPS exposure: A human-randomised clinical trial. Endocr. Connect. 2018, 7, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Gantzel, R.H.; Kjaer, M.B.; Laursen, T.L.; Kazankov, K.; George, J.; Moller, H.J.; Gronbaek, H. Macrophage Activation Markers, Soluble CD163 and Mannose Receptor, in Liver Fibrosis. Front. Med. 2020, 7, 615599. [Google Scholar] [CrossRef]
- Attia, H.; El Nagdy, M.; Abdel Halim, R.M. Preliminary Study of sCD14 and sCD163 as Predictors of Disease Severity and ICU Admission in COVID-19: Relation to Hematological Parameters, Blood Morphological Changes and Inflammatory Biomarkers. Mediterr. J. Hematol. Infect. Dis. 2023, 15, e2023046. [Google Scholar] [CrossRef]
- Aristoteli, L.P.; Moller, H.J.; Bailey, B.; Moestrup, S.K.; Kritharides, L. The monocytic lineage specific soluble CD163 is a plasma marker of coronary atherosclerosis. Atherosclerosis 2006, 184, 342–347. [Google Scholar] [CrossRef]
- Moreno, J.A.; Munoz-Garcia, B.; Martin-Ventura, J.L.; Madrigal-Matute, J.; Orbe, J.; Paramo, J.A.; Ortega, L.; Egido, J.; Blanco-Colio, L.M. The CD163-expressing macrophages recognize and internalize TWEAK: Potential consequences in atherosclerosis. Atherosclerosis 2009, 207, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Gao, P.; Li, W.; Gao, F.; Zhang, J.; Lin, H.; Zhang, J. Soluble CD163 Levels and CD163+CD14+ Monocyte/Macrophage Counts in Patients with Asthma. Iran. J. Immunol. 2018, 15, 239–245. [Google Scholar] [CrossRef]
- Matsushita, N.; Kashiwagi, M.; Wait, R.; Nagayoshi, R.; Nakamura, M.; Matsuda, T.; Hogger, P.; Guyre, P.M.; Nagase, H.; Matsuyama, T. Elevated levels of soluble CD163 in sera and fluids from rheumatoid arthritis patients and inhibition of the shedding of CD163 by TIMP-3. Clin. Exp. Immunol. 2002, 130, 156–161. [Google Scholar] [CrossRef]
- Krijgsman, D.; De Vries, N.L.; Andersen, M.N.; Skovbo, A.; Tollenaar, R.; Moller, H.J.; Hokland, M.; Kuppen, P.J.K. CD163 as a Biomarker in Colorectal Cancer: The Expression on Circulating Monocytes and Tumor-Associated Macrophages, and the Soluble Form in the Blood. Int. J. Mol. Sci. 2020, 21, 5925. [Google Scholar] [CrossRef]
- Qian, S.; Zhang, H.; Dai, H.; Ma, B.; Tian, F.; Jiang, P.; Gao, H.; Sha, X.; Sun, X. Is sCD163 a Clinical Significant Prognostic Value in Cancers? A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 585297. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhou, X.; Su, L.X.; Feng, D.; Jia, Y.H.; Xie, L.X. Clinical significance of soluble hemoglobin scavenger receptor CD163 (sCD163) in sepsis, a prospective study. PLoS ONE 2012, 7, e38400. [Google Scholar] [CrossRef]
- Hiraoka, A.; Horiike, N.; Akbar, S.M.; Michitaka, K.; Matsuyama, T.; Onji, M. Soluble CD163 in patients with liver diseases: Very high levels of soluble CD163 in patients with fulminant hepatic failure. J. Gastroenterol. 2005, 40, 52–56. [Google Scholar] [CrossRef]
- Kusi, K.A.; Gyan, B.A.; Goka, B.Q.; Dodoo, D.; Obeng-Adjei, G.; Troye-Blomberg, M.; Akanmori, B.D.; Adjimani, J.P. Levels of soluble CD163 and severity of malaria in children in Ghana. Clin. Vaccine Immunol. 2008, 15, 1456–1460. [Google Scholar] [CrossRef]
- Schaer, D.J.; Schleiffenbaum, B.; Kurrer, M.; Imhof, A.; Bachli, E.; Fehr, J.; Moller, H.J.; Moestrup, S.K.; Schaffner, A. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur. J. Haematol. 2005, 74, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Etzerodt, A.; Moestrup, S.K. CD163 and inflammation: Biological, diagnostic, and therapeutic aspects. Antioxid. Redox Signal 2013, 18, 2352–2363. [Google Scholar] [CrossRef]
- Sanchez-Torres, C.; Gomez-Puertas, P.; Gomez-del-Moral, M.; Alonso, F.; Escribano, J.M.; Ezquerra, A.; Dominguez, J. Expression of porcine CD163 on monocytes/macrophages correlates with permissiveness to African swine fever infection. Arch. Virol. 2003, 148, 2307–2323. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.J.; Wong, P.F.; AbuBakar, S.; Sam, S.S.; Shunmugarajoo, A.; Soh, Y.H.; Misbah, S.; Ab Rahman, A.K. The clinical utility of CD163 in viral diseases. Clin. Chim. Acta 2023, 541, 117243. [Google Scholar] [CrossRef]
- Marocco, R.; Carraro, A.; Zingaropoli, M.A.; Nijhawan, P.; Tortellini, E.; Guardiani, M.; Mengoni, F.; Zuccala, P.; Belvisi, V.; Kertusha, B.; et al. Role of Tocilizumab in Down Regulating sCD163 Plasmatic Levels in a Cohort of COVID-19 Patients. Front. Immunol. 2022, 13, 871592. [Google Scholar] [CrossRef]
- Parkner, T.; Sorensen, L.P.; Nielsen, A.R.; Fischer, C.P.; Bibby, B.M.; Nielsen, S.; Pedersen, B.K.; Moller, H.J. Soluble CD163: A biomarker linking macrophages and insulin resistance. Diabetologia 2012, 55, 1856–1862. [Google Scholar] [CrossRef]
- Zanni, M.V.; Burdo, T.H.; Makimura, H.; Williams, K.C.; Grinspoon, S.K. Relationship between monocyte/macrophage activation marker soluble CD163 and insulin resistance in obese and normal-weight subjects. Clin. Endocrinol. 2012, 77, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Lopez, A.; Chacon, M.R.; Bullo, M.; Maymo-Masip, E.; Martinez-Gonzalez, M.A.; Estruch, R.; Vendrell, J.; Basora, J.; Diez-Espino, J.; Covas, M.I.; et al. Serum sTWEAK concentrations and risk of developing type 2 diabetes in a high cardiovascular risk population: A nested case-control study. J. Clin. Endocrinol. Metab. 2013, 98, 3482–3490. [Google Scholar] [CrossRef] [PubMed]
- Svart, M.; Rittig, N.; Moller, N.; Moller, H.J.; Gronbaek, H. Soluble CD163 correlates with lipid metabolic adaptations in type 1 diabetes patients during ketoacidosis. J. Diabetes Investig. 2019, 10, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.C.; Hvidbjerg Gantzel, R.; Claria, J.; Trebicka, J.; Moller, H.J.; Gronbaek, H. Macrophage Activation Markers, CD163 and CD206, in Acute-on-Chronic Liver Failure. Cells 2020, 9, 1175. [Google Scholar] [CrossRef]
- Kazankov, K.; Barrera, F.; Moller, H.J.; Rosso, C.; Bugianesi, E.; David, E.; Younes, R.; Esmaili, S.; Eslam, M.; McLeod, D.; et al. The macrophage activation marker sCD163 is associated with morphological disease stages in patients with non-alcoholic fatty liver disease. Liver Int. 2016, 36, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Schonbauer, R.; Lichtenauer, M.; Paar, V.; Emich, M.; Fritzer-Szekeres, M.; Schukro, C.; Strametz-Juranek, J.; Sponder, M. Regular Training Increases sTWEAK and Its Decoy Receptor sCD163-Does Training Trigger the sTWEAK/sCD163-Axis to Induce an Anti-Inflammatory Effect? J. Clin. Med. 2020, 9, 1899. [Google Scholar] [CrossRef]
- Silva, R.L.; Santos, M.B.; Almeida, P.L.; Barros, T.S.; Magalhaes, L.; Cazzaniga, R.A.; Souza, P.R.; Luz, N.F.; Franca-Costa, J.; Borges, V.M.; et al. sCD163 levels as a biomarker of disease severity in leprosy and visceral leishmaniasis. PLoS Negl. Trop. Dis. 2017, 11, e0005486. [Google Scholar] [CrossRef]
- Kondelkova, K.; Krejsek, J.; Borska, L.; Fiala, Z.; Hamakova, K.; Andrys, C. Expression of soluble sCD163 in serum of psoriatic patients is modulated by Goeckerman therapy. Allergol. Immunopathol. 2013, 41, 158–162. [Google Scholar] [CrossRef]
- Frantz, C.; Pezet, S.; Avouac, J.; Allanore, Y. Soluble CD163 as a Potential Biomarker in Systemic Sclerosis. Dis. Markers 2018, 2018, 8509583. [Google Scholar] [CrossRef]
- Hassan, W.A.; Baraka, E.A.; Elnady, B.M.; Gouda, T.M.; Fouad, N. Serum Soluble CD163 and its association with various disease parameters in patients with systemic sclerosis. Eur. J. Rheumatol. 2016, 3, 95–100. [Google Scholar] [CrossRef]
- Lopez-Janeiro, A.; Padilla-Ansala, C.; de Andrea, C.E.; Hardisson, D.; Melero, I. Prognostic value of macrophage polarization markers in epithelial neoplasms and melanoma. A systematic review and meta-analysis. Mod. Pathol. 2020, 33, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Aiba, S. Significance of Immunosuppressive Cells as a Target for Immunotherapies in Melanoma and Non-Melanoma Skin Cancers. Biomolecules 2020, 10, 1087. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.; Brion, R.; Lintunen, M.; Kronqvist, P.; Sandholm, J.; Monkkonen, J.; Kellokumpu-Lehtinen, P.L.; Lauttia, S.; Tynninen, O.; Joensuu, H.; et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Medrek, C.; Ponten, F.; Jirstrom, K.; Leandersson, K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012, 12, 306. [Google Scholar] [CrossRef]
- Troiano, G.; Caponio, V.C.A.; Adipietro, I.; Tepedino, M.; Santoro, R.; Laino, L.; Lo Russo, L.; Cirillo, N.; Lo Muzio, L. Prognostic significance of CD68(+) and CD163(+) tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral. Oncol. 2019, 93, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Bisheshar, S.K.; van der Kamp, M.F.; de Ruiter, E.J.; Ruiter, L.N.; van der Vegt, B.; Breimer, G.E.; Willems, S.M. The prognostic role of tumor associated macrophages in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oral. Oncol. 2022, 135, 106227. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, F.; Fei, Q.; Yu, X.; Xiong, P.; Yu, X.; Dang, Y.; Hou, Z.; Lin, W.; Lin, X.; et al. Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer. J. Hematol. Oncol. 2019, 12, 124. [Google Scholar] [CrossRef]
- Stuhr, L.K.; Madsen, K.; Johansen, A.Z.; Chen, I.M.; Hansen, C.P.; Jensen, L.H.; Hansen, T.F.; Klove-Mogensen, K.; Nielsen, K.R.; Johansen, J.S. Combining sCD163 with CA 19-9 Increases the Predictiveness of Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 897. [Google Scholar] [CrossRef]
- Aggerholm-Pedersen, N.; Friis, H.N.; Baad-Hansen, T.; Moller, H.J.; Sandfeld-Paulsen, B. Macrophage Biomarkers sCD163 and sSIRPalpha in Serum Predict Mortality in Sarcoma Patients. Cancers 2023, 15, 1544. [Google Scholar] [CrossRef]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 566511. [Google Scholar] [CrossRef]
- Sumitomo, R.; Hirai, T.; Fujita, M.; Murakami, H.; Otake, Y.; Huang, C.L. M2 tumor-associated macrophages promote tumor progression in non-small-cell lung cancer. Exp. Ther. Med. 2019, 18, 4490–4498. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Q.; Zhou, X.D.; Shi, Y.; Yang, L.; Xu, S.L.; Chen, C.; Cui, Y.H.; Zhang, X.; Bian, X.W. Increased pro-angiogenic factors, infiltrating neutrophils and CD163(+) macrophages in bronchoalveolar lavage fluid from lung cancer patients. Int. Immunopharmacol. 2014, 20, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, I.; Polubiec-Kownacka, M.; Dziedzic, D.; Wolosz, D.; Rzepecki, P.; Domagala-Kulawik, J. CD163 and CCR7 as markers for macrophage polarization in lung cancer microenvironment. Cent. Eur. J. Immunol. 2019, 44, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, E.; Komohara, Y.; Shinchi, Y.; Mito, R.; Fujiwara, Y.; Ikeda, K.; Shima, T.; Shimoda, M.; Kanai, Y.; Sakagami, T.; et al. CD163-positive cancer cells are a predictor of a worse clinical course in lung adenocarcinoma. Pathol. Int. 2021, 71, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Shabo, I.; Svanvik, J. Expression of macrophage antigens by tumor cells. Adv. Exp. Med. Biol. 2011, 714, 141–150. [Google Scholar] [CrossRef]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Liu, Q.; Dou, R.; Xiong, B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer 2019, 18, 64. [Google Scholar] [CrossRef]
- Shabo, I.; Olsson, H.; Sun, X.F.; Svanvik, J. Expression of the macrophage antigen CD163 in rectal cancer cells is associated with early local recurrence and reduced survival time. Int. J. Cancer 2009, 125, 1826–1831. [Google Scholar] [CrossRef]
- Koelzer, V.H.; Canonica, K.; Dawson, H.; Sokol, L.; Karamitopoulou-Diamantis, E.; Lugli, A.; Zlobec, I. Phenotyping of tumor-associated macrophages in colorectal cancer: Impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology 2016, 5, e1106677. [Google Scholar] [CrossRef]
- Maniecki, M.B.; Etzerodt, A.; Ulhoi, B.P.; Steiniche, T.; Borre, M.; Dyrskjot, L.; Orntoft, T.F.; Moestrup, S.K.; Moller, H.J. Tumor-promoting macrophages induce the expression of the macrophage-specific receptor CD163 in malignant cells. Int. J. Cancer 2012, 131, 2320–2331. [Google Scholar] [CrossRef]
- Shabo, I.; Svanvik, J.; Lindstrom, A.; Lechertier, T.; Trabulo, S.; Hulit, J.; Sparey, T.; Pawelek, J. Roles of cell fusion, hybridization and polyploid cell formation in cancer metastasis. World J. Clin. Oncol. 2020, 11, 121–135. [Google Scholar] [CrossRef]
- Manjunath, Y.; Porciani, D.; Mitchem, J.B.; Suvilesh, K.N.; Avella, D.M.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Burke, D.H.; Li, G.; Kaifi, J.T. Tumor-Cell-Macrophage Fusion Cells as Liquid Biomarkers and Tumor Enhancers in Cancer. Int. J. Mol. Sci. 2020, 21, 1872. [Google Scholar] [CrossRef] [PubMed]
- No, J.H.; Moon, J.M.; Kim, K.; Kim, Y.B. Prognostic significance of serum soluble CD163 level in patients with epithelial ovarian cancer. Gynecol. Obstet. Investig. 2013, 75, 263–267. [Google Scholar] [CrossRef] [PubMed]
| Pathological Conditions Where Elevated sCD163 Levels Have Been Reported | |
|---|---|
| Acute Coronary Syndromes | Kidney allograft rejection |
| Acute graft-versus-host disease | Leprosis |
| Acute kidney injury | Lupus nephritis |
| Acute-on-chronic liver failure | Malaria |
| Alcoholic hepatitis | Measles |
| Asthma | Multiple sclerosis |
| Atherosclerosis | Non-alcoholic steatohepatitis |
| Atrial fibrillation | Osteoarthritis |
| Celiac disease | Proliferative Diabetic Retinopathy |
| Chronic heart failure | Psoriasis |
| Chronic viral hepatitis | Rheumatoid arthritis |
| Cirrhosis | Sarcoidosis |
| COVID-19 | Scleroderma |
| Crohn’s disease | Sepsis |
| Dengue | Sickle cell disease |
| EBV infection (Epstein–Barr virus infection) | Spondyloarthropathy |
| Gestational diabetes mellitus | Systemic lupus erythematosus |
| Hantaan virus | Systemic sclerosis |
| HBV (Hepatitis B virus), HCV (Hepatitis C virus) | Type 1 diabetes mellitus |
| Hemophagocytic lymphohistiocytosis | Type 2 diabetes mellitus |
| HIV (Immunodeficiency Virus) | Ulcerative colitis |
| Influenza | Visceral Leishmaniasis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plevriti, A.; Lamprou, M.; Mourkogianni, E.; Skoulas, N.; Giannakopoulou, M.; Sajib, M.S.; Wang, Z.; Mattheolabakis, G.; Chatzigeorgiou, A.; Marazioti, A.; et al. The Role of Soluble CD163 (sCD163) in Human Physiology and Pathophysiology. Cells 2024, 13, 1679. https://doi.org/10.3390/cells13201679
Plevriti A, Lamprou M, Mourkogianni E, Skoulas N, Giannakopoulou M, Sajib MS, Wang Z, Mattheolabakis G, Chatzigeorgiou A, Marazioti A, et al. The Role of Soluble CD163 (sCD163) in Human Physiology and Pathophysiology. Cells. 2024; 13(20):1679. https://doi.org/10.3390/cells13201679
Chicago/Turabian StylePlevriti, Andriana, Margarita Lamprou, Eleni Mourkogianni, Nikolaos Skoulas, Maria Giannakopoulou, Md Sanaullah Sajib, Zhiyong Wang, George Mattheolabakis, Antonios Chatzigeorgiou, Antonia Marazioti, and et al. 2024. "The Role of Soluble CD163 (sCD163) in Human Physiology and Pathophysiology" Cells 13, no. 20: 1679. https://doi.org/10.3390/cells13201679
APA StylePlevriti, A., Lamprou, M., Mourkogianni, E., Skoulas, N., Giannakopoulou, M., Sajib, M. S., Wang, Z., Mattheolabakis, G., Chatzigeorgiou, A., Marazioti, A., & Mikelis, C. M. (2024). The Role of Soluble CD163 (sCD163) in Human Physiology and Pathophysiology. Cells, 13(20), 1679. https://doi.org/10.3390/cells13201679








