Early-Life Respiratory Syncytial Virus (RSV) Infection Triggers Immunological Changes in Gut-Associated Lymphoid Tissues in a Sex-Dependent Manner in Adulthood
Abstract
:1. Introduction
2. Results
2.1. Early-Life RSV Infection Drives Persistent Immune Alterations in the Lungs of Adult Male Mice in the Absence of Residual Virus
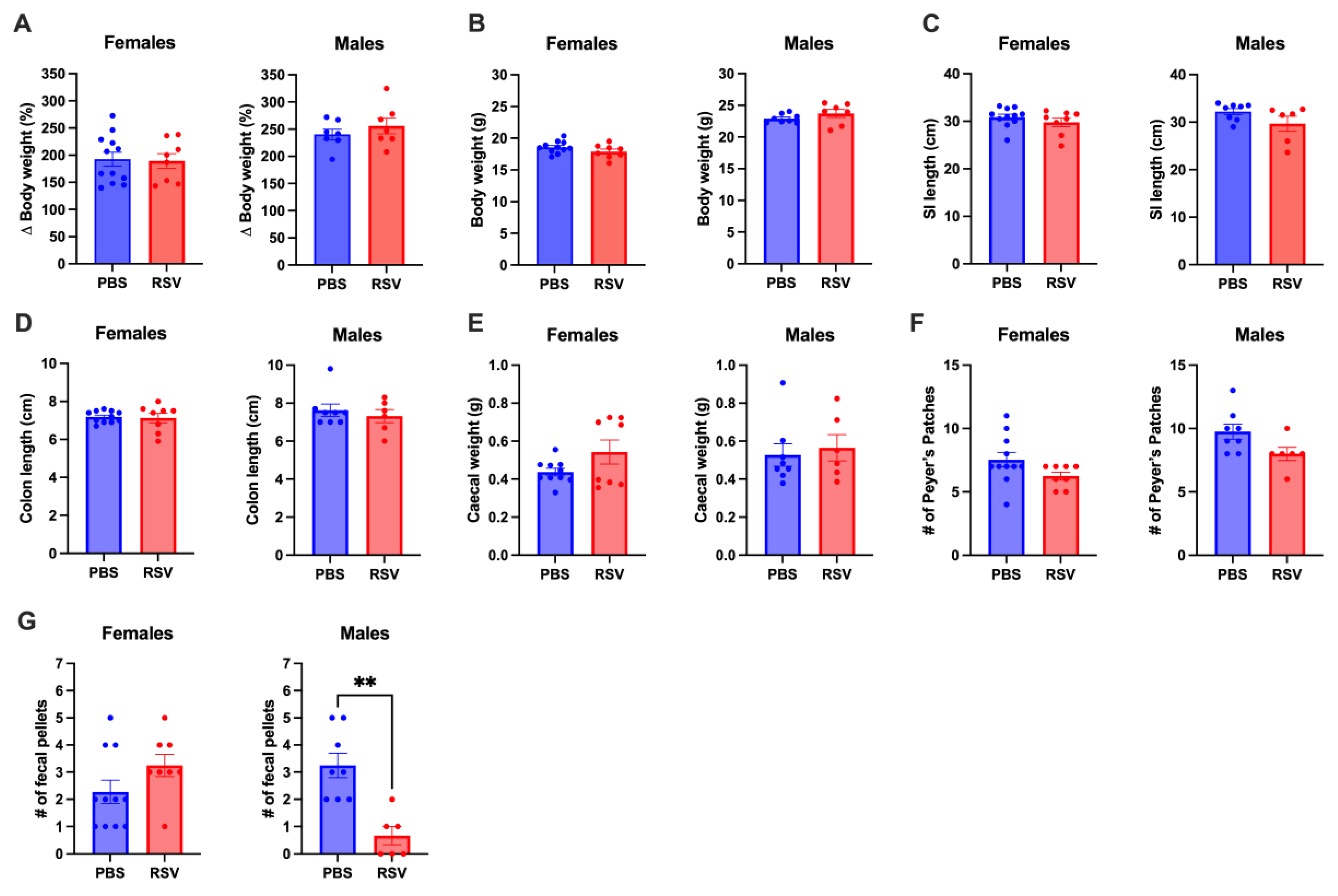
2.2. Early Life RSV Infection Does Not Affect GI Tract Anatomy
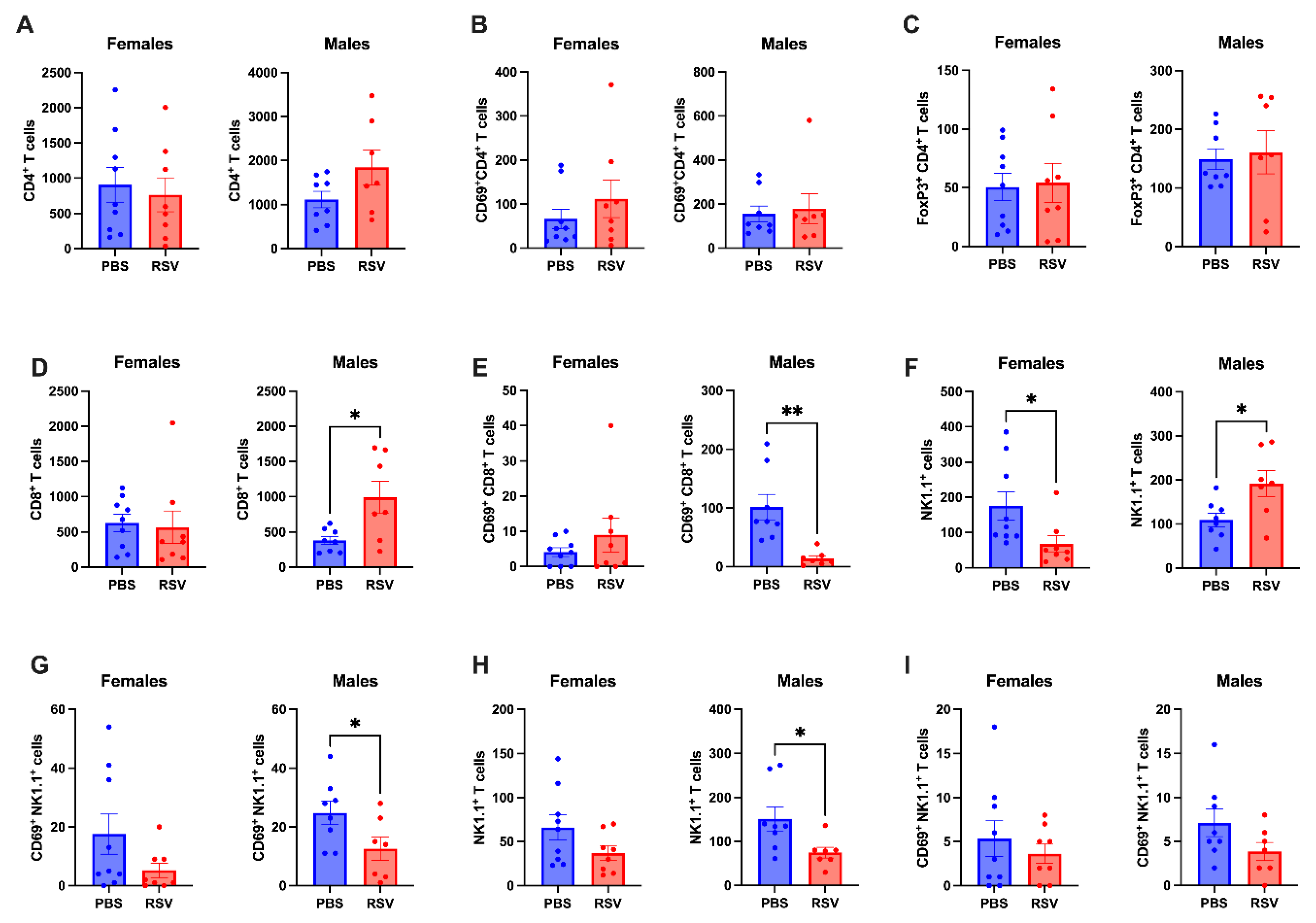
2.3. Caecal Patch NK Cells Show Differential Profiles in Male and Female Mice Following Early-Life RSV Infection
2.4. Chronic Immunological Changes in Peyer’s Patches in Male Mice Following Early Life RSV
3. Discussion
4. Materials and Methods
4.1. Animal Ethics
4.2. Respiratory Syncytial Virus (RSV)
4.3. RSV Inoculation of Mice
4.4. RNA Extraction and qPCR
4.5. Flow Cytometry
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glezen, W.P.; Taber, L.H.; Frank, A.L.; Kasel, J.A. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 1986, 140, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Malinczak, C.A.; Lukacs, N.W.; Fonseca, W. Early-Life Respiratory Syncytial Virus Infection, Trained Immunity and Subsequent Pulmonary Diseases. Viruses 2020, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Knudson, C.J.; Varga, S.M. The relationship between respiratory syncytial virus and asthma. Vet. Pathol. 2015, 52, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Hartert, T.V.; Everard, M.L.; Giezek, H.; Nelsen, L.; Mehta, A.; Patel, H.; Knorr, B.; Reiss, T.F. Predictors of asthma following severe respiratory syncytial virus (RSV) bronchiolitis in early childhood. Pediatr. Pulmonol. 2016, 51, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Malinczak, C.A.; Fonseca, W.; Rasky, A.J.; Ptaschinski, C.; Morris, S.; Ziegler, S.F.; Lukacs, N.W. Sex-associated TSLP-induced immune alterations following early-life RSV infection leads to enhanced allergic disease. Mucosal Immunol. 2019, 12, 969–979. [Google Scholar] [CrossRef]
- Anderson, E.L.; Li, Z.; Roberg, K.A.; Tisler, C.J.; DaSilva, D.F.; Pleiss, L.E.; Sullivan Dillie, K.T.; Pappas, T.E.; Gangnon, R.E.; Gern, J.E.; et al. Children who wheeze with respiratory syncytial virus (RSV) in the first year of life are more likely to have a food sensitization. J. Allergy Clin. Immunol. 2005, 115 (Suppl. 2), S170. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Yang, Y.L.; Pan, Y.Q.; He, B.S.; Zhong, T.Y. Regulatory T cells and Th1/Th2 in peripheral blood and their roles in asthmatic children. Transl. Pediatr. 2013, 2, 27–33. [Google Scholar] [CrossRef]
- Becker, Y. Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy—A review. Virus Genes 2006, 33, 235–252. [Google Scholar] [CrossRef]
- Hashimoto, K.; Durbin, J.E.; Zhou, W.; Collins, R.D.; Ho, S.B.; Kolls, J.K.; Dubin, P.J.; Sheller, J.R.; Goleniewska, K.; O’Neal, J.F.; et al. Respiratory syncytial virus infection in the absence of STAT 1 results in airway dysfunction, airway mucus, and augmented IL-17 levels. J. Allergy Clin. Immunol. 2005, 116, 550–557. [Google Scholar] [CrossRef]
- Brailey, P.M.; Lebrusant-Fernandez, M.; Barral, P. NKT cells and the regulation of intestinal immunity: A two-way street. FEBS J. 2020, 287, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Abo-Shaban, T.; Sharna, S.S.; Hosie, S.; Lee, C.Y.Q.; Balasuriya, G.K.; McKeown, S.J.; Franks, A.E.; Hill-Yardin, E.L. Issues for patchy tissues: Defining roles for gut-associated lymphoid tissue in neurodevelopment and disease. J. Neural Transm. 2023, 130, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wu, L.; Huntington, N.D.; Zhang, X. Crosstalk Between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases. Front. Immunol. 2020, 11, 282. [Google Scholar] [CrossRef]
- Ullah, H.; Arbab, S.; Tian, Y.; Liu, C.Q.; Chen, Y.; Qijie, L.; Khan, M.I.U.; Hassan, I.U.; Li, K. The gut microbiota-brain axis in neurological disorder. Front. Neurosci. 2023, 17, 1225875, From NLM PubMed-not-MEDLINE. [Google Scholar] [CrossRef]
- Liong, S.; Miles, M.A.; Mohsenipour, M.; Liong, F.; Hill-Yardin, E.L.; Selemidis, S. Influenza A virus infection during pregnancy causes immunological changes in gut-associated lymphoid tissues of offspring mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 325, G230–G238. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.A.; Liong, S.; Liong, F.; Coward-Smith, M.; Trollope, G.S.; Oseghale, O.; Erlich, J.R.; Brooks, R.D.; Logan, J.M.; Hickey, S.; et al. TLR7 promotes chronic airway disease in RSV-infected mice. Front. Immunol. 2023, 14, 1240552. [Google Scholar] [CrossRef]
- Sencio, V.; Machado, M.G.; Trottein, F. The lung-gut axis during viral respiratory infections: The impact of gut dysbiosis on secondary disease outcomes. Mucosal Immunol. 2021, 14, 296–304. [Google Scholar] [CrossRef]
- Martin, P.; Gomez, M.; Lamana, A.; Matesanz Marin, A.; Cortes, J.R.; Ramirez-Huesca, M.; Barreiro, O.; Lopez-Romero, P.; Gutierrez-Vazquez, C.; de la Fuente, H.; et al. The leukocyte activation antigen CD69 limits allergic asthma and skin contact hypersensitivity. J. Allergy Clin. Immunol. 2010, 126, 355–365, 365.e1–365.e3. [Google Scholar] [CrossRef]
- Radulovic, K.; Manta, C.; Rossini, V.; Holzmann, K.; Kestler, H.A.; Wegenka, U.M.; Nakayama, T.; Niess, J.H. CD69 regulates type I IFN-induced tolerogenic signals to mucosal CD4 T cells that attenuate their colitogenic potential. J. Immunol. 2012, 188, 2001–2013. [Google Scholar] [CrossRef]
- Hadis, U.; Wahl, B.; Schulz, O.; Hardtke-Wolenski, M.; Schippers, A.; Wagner, N.; Muller, W.; Sparwasser, T.; Forster, R.; Pabst, O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 2011, 34, 237–246. [Google Scholar] [CrossRef]
- Lin, W.; Truong, N.; Grossman, W.J.; Haribhai, D.; Williams, C.B.; Wang, J.; Martin, M.G.; Chatila, T.A. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J. Allergy Clin. Immunol. 2005, 116, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Cibrian, D.; Sanchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Stokes, K.L.; Chi, M.H.; Sakamoto, K.; Newcomb, D.C.; Currier, M.G.; Huckabee, M.M.; Lee, S.; Goleniewska, K.; Pretto, C.; Williams, J.V.; et al. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J. Virol. 2011, 85, 5782–5793. [Google Scholar] [CrossRef]
- Borgo, F.; Garbossa, S.; Riva, A.; Severgnini, M.; Luigiano, C.; Benetti, A.; Pontiroli, A.E.; Morace, G.; Borghi, E. Body Mass Index and Sex Affect Diverse Microbial Niches within the Gut. Front. Microbiol. 2018, 9, 213, From NLM PubMed-not-MEDLINE. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.N.; Siefker, D.; Vu, L.; You, D.; DeVincenzo, J.; Pierre, J.F.; Cormier, S.A. Altered gut microbiota in infants is associated with respiratory syncytial virus disease severity. BMC Microbiol. 2020, 20, 140. [Google Scholar] [CrossRef]
- Goldberg, M.R.; Mor, H.; Magid Neriya, D.; Magzal, F.; Muller, E.; Appel, M.Y.; Nachshon, L.; Borenstein, E.; Tamir, S.; Louzoun, Y.; et al. Microbial signature in IgE-mediated food allergies. Genome Med. 2020, 12, 92. [Google Scholar] [CrossRef]
- He, B.; Liu, Y.; Hoang, T.K.; Tian, X.; Taylor, C.M.; Luo, M.; Tran, D.Q.; Tatevian, N.; Rhoads, J.M. Antibiotic-modulated microbiome suppresses lethal inflammation and prolongs lifespan in Treg-deficient mice. Microbiome 2019, 7, 145. [Google Scholar] [CrossRef]
- Kawamoto, S.; Maruya, M.; Kato, L.M.; Suda, W.; Atarashi, K.; Doi, Y.; Tsutsui, Y.; Qin, H.; Honda, K.; Okada, T.; et al. Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 2014, 41, 152–165. [Google Scholar] [CrossRef]
- Liao, C.M.; Zimmer, M.I.; Shanmuganad, S.; Yu, H.T.; Cardell, S.L.; Wang, C.R. Dysregulation of CD1d-restricted type ii natural killer T cells leads to spontaneous development of colitis in mice. Gastroenterology 2012, 142, 326–334. [Google Scholar] [CrossRef]
- Liao, C.M.; Zimmer, M.I.; Wang, C.R. The functions of type I and type II natural killer T cells in inflammatory bowel diseases. Inflamm. Bowel Dis. 2013, 19, 1330–1338. [Google Scholar] [CrossRef]
- Sun, Z.; Kim, J.H.; Kim, S.H.; Kim, H.R.; Zhang, K.; Pan, Y.; Ko, M.K.; Kim, B.M.; Chu, H.; Lee, H.R.; et al. Skin-resident natural killer T cells participate in cutaneous allergic inflammation in atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 1764–1777. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, H.; Yung, J.A.; Cephus, J.Y.; Zhang, J.; Goleniewska, K.; Polosukhin, V.V.; Peebles, R.S., Jr.; Newcomb, D.C. Testosterone Decreases House Dust Mite-Induced Type 2 and IL-17A-Mediated Airway Inflammation. J. Immunol. 2018, 201, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Ursin, R.L.; Klein, S.L. Sex Differences in Respiratory Viral Pathogenesis and Treatments. Annu. Rev. Virol. 2021, 8, 393–414. [Google Scholar] [CrossRef]
- Melgaard, M.E.; Jensen, S.K.; Eliasen, A.; Pedersen, C.T.; Thorsen, J.; Mikkelsen, M.; Vahman, N.; Schoos, A.M.; Gern, J.; Brix, S.; et al. Asthma development is associated with low mucosal IL-10 during viral infections in early life. Allergy 2024. early view. [Google Scholar] [CrossRef]
- Liong, S.; Oseghale, O.; To, E.E.; Brassington, K.; Erlich, J.R.; Luong, R.; Liong, F.; Brooks, R.; Martin, C.; O’Toole, S.; et al. Influenza A virus causes maternal and fetal pathology via innate and adaptive vascular inflammation in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 24964–24973. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liong, S.; Liong, F.; Mohsenipour, M.; Hill-Yardin, E.L.; Miles, M.A.; Selemidis, S. Early-Life Respiratory Syncytial Virus (RSV) Infection Triggers Immunological Changes in Gut-Associated Lymphoid Tissues in a Sex-Dependent Manner in Adulthood. Cells 2024, 13, 1728. https://doi.org/10.3390/cells13201728
Liong S, Liong F, Mohsenipour M, Hill-Yardin EL, Miles MA, Selemidis S. Early-Life Respiratory Syncytial Virus (RSV) Infection Triggers Immunological Changes in Gut-Associated Lymphoid Tissues in a Sex-Dependent Manner in Adulthood. Cells. 2024; 13(20):1728. https://doi.org/10.3390/cells13201728
Chicago/Turabian StyleLiong, Stella, Felicia Liong, Mitra Mohsenipour, Elisa L. Hill-Yardin, Mark A. Miles, and Stavros Selemidis. 2024. "Early-Life Respiratory Syncytial Virus (RSV) Infection Triggers Immunological Changes in Gut-Associated Lymphoid Tissues in a Sex-Dependent Manner in Adulthood" Cells 13, no. 20: 1728. https://doi.org/10.3390/cells13201728
APA StyleLiong, S., Liong, F., Mohsenipour, M., Hill-Yardin, E. L., Miles, M. A., & Selemidis, S. (2024). Early-Life Respiratory Syncytial Virus (RSV) Infection Triggers Immunological Changes in Gut-Associated Lymphoid Tissues in a Sex-Dependent Manner in Adulthood. Cells, 13(20), 1728. https://doi.org/10.3390/cells13201728





