17β-Estradiol Stimulates Oxidative Stress Components and Thyroid Specific Genes in Porcine Thyroid Follicular Cells: Potential Differences Between Sexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Cell Culture
2.4. Cell Treatment
2.5. Cell Viability Assay
2.6. Evaluation of ROS Level
2.7. mRNA Analysis by qRT-PCR
2.8. Statistical Analysis
3. Results
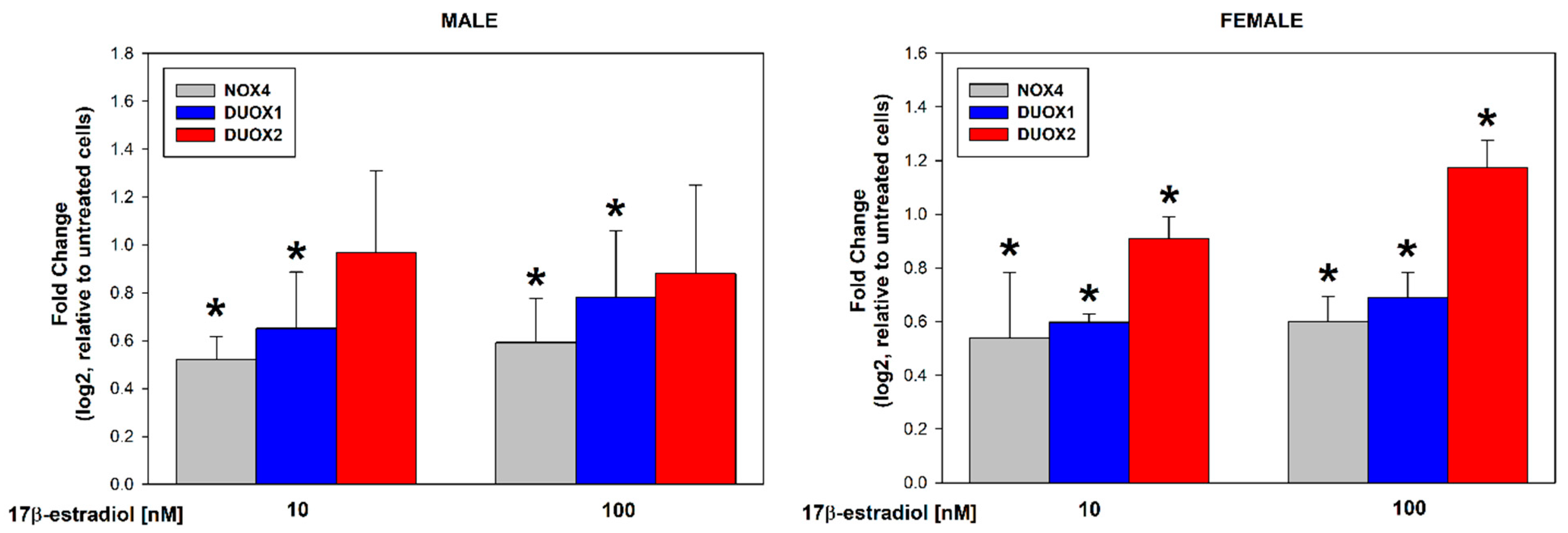
3.1. Stimulation of Thyroid NADPH Oxidases Expression in Male/Female Thyroid Cells
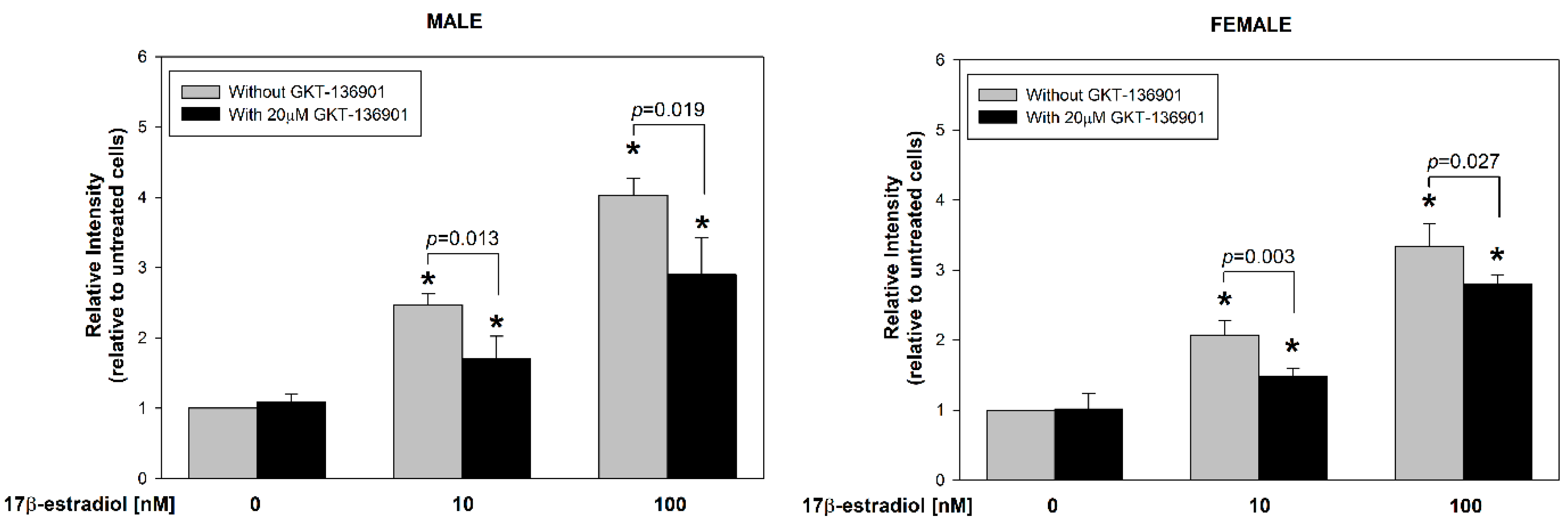
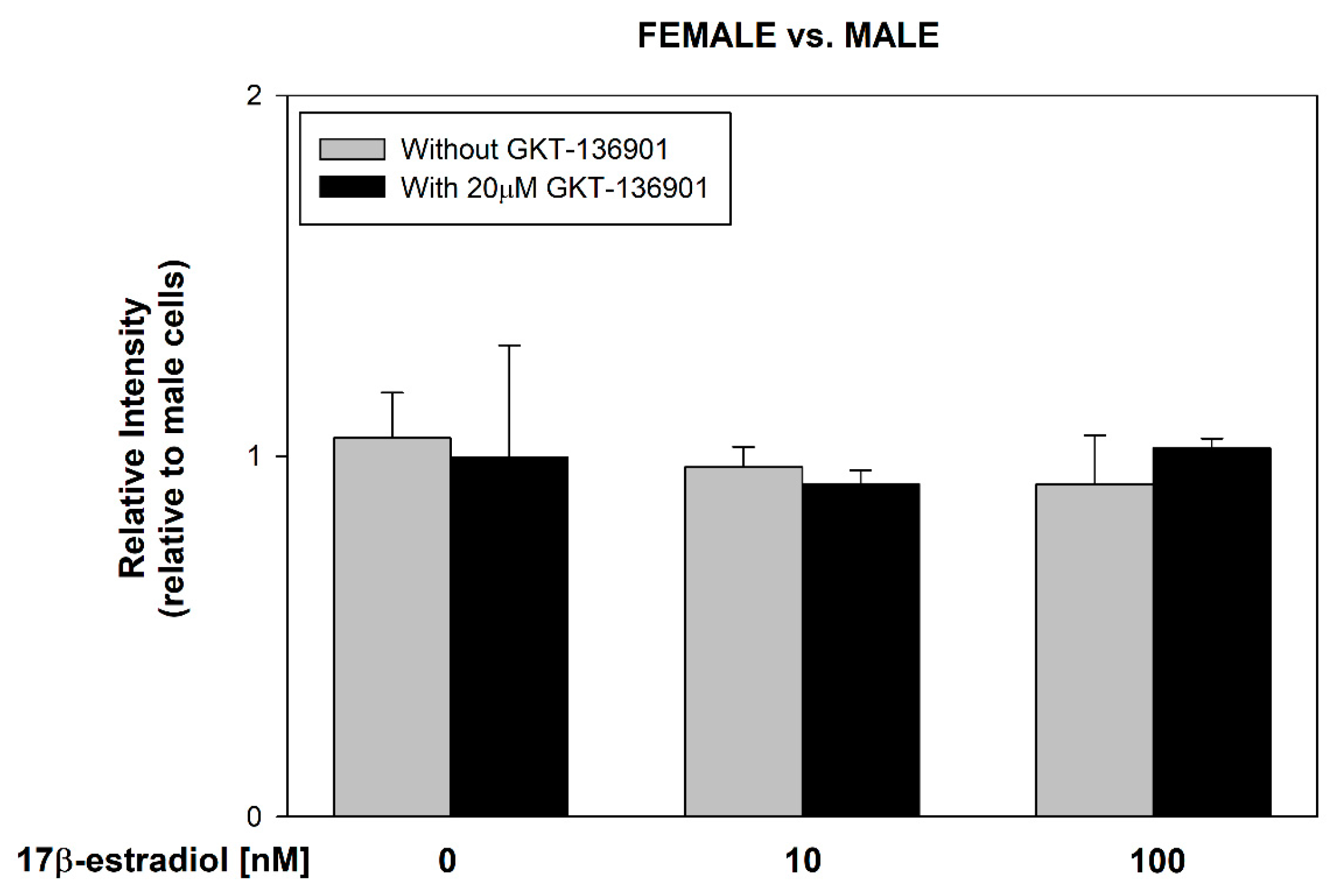
3.2. Stimulation of ROS Formation by 17β-Estradiol in Male/Female Thyroid Cells
3.3. Stimulation of Thyroid Specific Genes Expression by 17β-Estradiol in Male/Female Thyroid Cells
3.4. Stimulation of ER Stress/UPR Initiation Markers Expression by 17β-Estradiol in Male/Female Thyroid Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barakat, R.; Oakley, O.; Kim, H.; Jin, J.; Ko, C.J. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016, 49, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Banerjee, A.; Singh, D.; Thakur, G.; Kasarpalkar, N.; Gavali, S.; Gadkar, S.; Madan, T.; Mahale, S.D.; Balasinor, N.H.; et al. Estradiol: A Steroid with Multiple Facets. Horm. Metab. Res. 2018, 50, 359–374. [Google Scholar] [CrossRef]
- Angeloni, C.; Teti, G.; Barbalace, M.C.; Malaguti, M.; Falconi, M.; Hrelia, S. 17β-Estradiol enhances sulforaphane cardioprotection against oxidative stress. J. Nutr. Biochem. 2017, 42, 26–36. [Google Scholar] [CrossRef]
- Nilsen, J. Estradiol and neurodegenerative oxidative stress. Front. Neuroendocrinol. 2008, 29, 463–475. [Google Scholar] [CrossRef]
- Rynkowska, A.; Stępniak, J.; Karbownik-Lewińska, M. Fenton Reaction-Induced Oxidative Damage to Membrane Lipids and Protective Effects of 17β-Estradiol in Porcine Ovary and Thyroid Homogenates. Int. J. Environ. Res. Public Health 2020, 17, 6841. [Google Scholar] [CrossRef] [PubMed]
- Stepniak, J.; Karbownik-Lewinska, M. 17β-estradiol prevents experimentally-induced oxidative damage to membrane lipids and nuclear DNA in porcine ovary. Syst. Biol. Reprod. Med. 2016, 62, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Stępniak, J.; Koziróg, E.; Karbownik-Lewińska, M. The Protective Effect of Exogenous 17β-Estradiol against Experimentally Induced Oxidative Damage to Membrane Lipids Is Stronger in Male vs. Female Porcine Thyroids: Preliminary Results. Toxics 2023, 11, 746. [Google Scholar] [CrossRef]
- Ramesh, S.S.; Christopher, R.; Indira Devi, B.; Bhat, D.I. The vascular protective role of oestradiol: A focus on postmenopausal oestradiol deficiency and aneurysmal subarachnoid haemorrhage. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1897–1917. [Google Scholar] [CrossRef]
- Bukato, K.; Kostrzewa, T.; Gammazza, A.M.; Gorska-Ponikowska, M.; Sawicki, S. Endogenous estrogen metabolites as oxidative stress mediators and endometrial cancer biomarkers. Cell Commun. Signal. 2024, 22, 205. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, G.; Vlantis, A.; Tse, G.; van Hasselt, C. The contributions of oestrogen receptor isoforms to the development of papillary and anaplastic thyroid carcinomas. J. Pathol. 2008, 214, 425–433. [Google Scholar] [CrossRef]
- Kumar, A.; Klinge, C.M.; Goldstein, R.E. Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta. Int. J. Oncol. 2010, 36, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Derwahl, M.; Nicula, D. Estrogen and its role in thyroid cancer. Endocr. Relat. Cancer 2014, 21, T273–T283. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.Z.; Zheng, L.L.; Chen, K.H.; Wang, R.; Yi, D.D.; Jiang, C.Y.; Liu, Z.J.; Shi, X.B.; Sang, J.F. Serum sex hormones correlate with pathological features of papillary thyroid cancer. Endocrine 2024, 84, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef]
- Fortunato, R.S.; Braga, W.M.; Ortenzi, V.H.; Rodrigues, D.C.; Andrade, B.M.; Miranda-Alves, L.; Rondinelli, E.; Dupuy, C.; Ferreira, A.C.; Carvalho, D.P. Sexual dimorphism of thyroid reactive oxygen species production due to higher NADPH oxidase 4 expression in female thyroid glands. Thyroid 2013, 23, 111–119. [Google Scholar] [CrossRef]
- Stepniak, J.; Lewinski, A.; Karbownik-Lewinska, M. Sexual Dimorphism of NADPH Oxidase/H2O2 System in Rat Thyroid Cells; Effect of Exogenous 17β-Estradiol. Int. J. Mol. Sci. 2018, 19, 4063. [Google Scholar] [CrossRef] [PubMed]
- Burek, C.L.; Rose, N.R. Autoimmune thyroiditis and ROS. Autoimmun. Rev. 2008, 7, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Ameziane-El-Hassani, R.; Schlumberger, M.; Dupuy, C. NADPH oxidases: New actors in thyroid cancer? Nat. Rev. Endocrinol. 2016, 12, 485–494. [Google Scholar] [CrossRef]
- Song, Y.; Driessens, N.; Costa, M.; De Deken, X.; Detours, V.; Corvilain, B.; Maenhaut, C.; Miot, F.; Van Sande, J.; Many, M.C.; et al. Roles of hydrogen peroxide in thyroid physiology and disease. J. Clin. Endocrinol. Metab. 2007, 92, 3764–3773. [Google Scholar] [CrossRef]
- Oglio, R.; Salvarredi, L.; Rossich, L.; Copelli, S.; Pisarev, M.; Juvenal, G.; Thomasz, L. Participation of NADPH 4 oxidase in thyroid regulation. Mol. Cell. Endocrinol. 2019, 480, 65–73. [Google Scholar] [CrossRef]
- Wu, R.F.; Ma, Z.; Liu, Z.; Terada, L.S. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol. Cell. Biol. 2010, 30, 3553–3568. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Körner, H.; Wu, H.; Wei, W. Endoplasmic reticulum stress in autoimmune diseases. Immunobiology 2020, 225, 151881. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Sheng, J.; Tang, P.; Peng, X.; Zhang, R.; Zhao, X.; Hu, J.; Xu, T. The role and mechanism of NADPH oxidase in the development and progression of thyroid carcinoma. Am. J. Cancer Res. 2023, 13, 4366–4375. [Google Scholar]
- Cammarota, F.; Fiscardi, F.; Esposito, T.; de Vita, G.; Salvatore, M.; Laukkanen, M.O. Clinical relevance of thyroid cell models in redox research. Cancer Cell Int. 2015, 15, 113. [Google Scholar] [CrossRef]
- Walters, E.M.; Prather, R.S. Advancing swine models for human health and diseases. Mo. Med. 2013, 110, 212–215. [Google Scholar]
- James, B.C.; Mitchell, J.M.; Jeon, H.D.; Vasilottos, N.; Grogan, R.H.; Aschebrook-Kilfoy, B. An update in international trends in incidence rates of thyroid cancer, 1973–2007. Cancer Causes Control 2018, 29, 465–473. [Google Scholar] [CrossRef]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T4, and thyroid antibodies in the United States population (1988–1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef]
- Santin, A.P.; Furlanetto, T.W. Role of estrogen in thyroid function and growth regulation. J. Thyroid Res. 2011, 2011, 875125. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ye, Q.; Lu, H.; Wu, Z.; Chen, S.; Zheng, R. Estrogen-mediated DNMT1 and DNMT3A recruitment by EZH2 silences miR-570-3p that contributes to papillary thyroid malignancy through DPP4. Clin. Epigenet. 2024, 16, 81. [Google Scholar] [CrossRef]
- Mo, X.M.; Li, L.; Zhu, P.; Dai, Y.J.; Zhao, T.T.; Liao, L.Y.; Chen, G.G.; Liu, Z.M. Up-regulation of Hsp27 by ERα/Sp1 facilitates proliferation and confers resistance to apoptosis in human papillary thyroid cancer cells. Mol. Cell. Endocrinol. 2016, 431, 71–87. [Google Scholar] [CrossRef]
- Lv, P.P.; Tian, S.; Feng, C.; Li, J.Y.; Yu, D.Q.; Jin, L.; Shen, Y.; Yu, T.T.; Meng, Y.; Ding, G.L.; et al. Maternal High Estradiol Exposure is Associated with Elevated Thyroxine and Pax8 in Mouse Offspring. Sci. Rep. 2016, 6, 36805. [Google Scholar] [CrossRef] [PubMed]
- Kordi, F.; Khazali, H. The effect of ghrelin and estradiol on mean concentration of thyroid hormones. Int. J. Endocrinol. Metab. 2015, 13, e17988. [Google Scholar] [CrossRef] [PubMed]
- Hima, S.; Sreeja, S. Modulatory role of 17β-estradiol in the tumor microenvironment of thyroid cancer. IUBMB Life 2016, 68, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Arduc, A.; Aycicek Dogan, B.; Bilmez, S.; Imga Nasiroglu, N.; Tuna, M.M.; Isik, S.; Berker, D.; Guler, S. High prevalence of Hashimoto’s thyroiditis in patients with polycystic ovary syndrome: Does the imbalance between estradiol and progesterone play a role? Endocr. Res. 2015, 40, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Fenniche, S.; Oukabli, M.; Oubaddou, Y.; Chahdi, H.; Damiri, A.; Alghuzlan, A.; Laraqui, A.; Dakka, N.; Bakri, Y.; Dupuy, C.; et al. A Comparative Analysis of NOX4 Protein Expression in Malignant and Non-Malignant Thyroid Tumors. Curr. Issues Mol. Biol. 2023, 45, 5811–5823. [Google Scholar] [CrossRef]
- He, L.; Li, X.; Chen, Z.; Wang, W.; Wang, K.; Huang, X.; Yang, Q.; Ke, W.; Liu, J.; Zha, B. High concentration of estradiol has a negative correlation with free thyroxine during the second trimester of pregnancy. Endocr. Connect. 2022, 11, e220236. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Zhou, L.; Lei, Y.; Zhang, Y.; Huang, C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019, 25, 101047. [Google Scholar] [CrossRef]
- Dicks, N.; Gutierrez, K.; Michalak, M.; Bordignon, V.; Agellon, L.B. Endoplasmic reticulum stress, genome damage, and cancer. Front. Oncol. 2015, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef]
- Ma, D.J.; Hwang, J.S.; Noh, K.B.; Oh, S.H.; Kim, K.W.; Shin, Y.J. Role of NADPH Oxidase 4 in Corneal Endothelial Cells Is Mediated by Endoplasmic Reticulum Stress and Autophagy. Antioxidants 2023, 12, 1228. [Google Scholar] [CrossRef]
- Pedruzzi, E.; Guichard, C.; Ollivier, V.; Driss, F.; Fay, M.; Prunet, C.; Marie, J.C.; Pouzet, C.; Samadi, M.; Elbim, C.; et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell. Biol. 2004, 24, 10703–10717. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Q.; Wang, H.; Xia, S. Endoplasmic reticulum stress in T cell-mediated diseases. Scand. J. Immunol. 2023, 98, e13307. [Google Scholar] [CrossRef] [PubMed]
- Barrera, M.J.; Aguilera, S.; Castro, I.; González, S.; Carvajal, P.; Molina, C.; Hermoso, M.A.; González, M.J. Endoplasmic reticulum stress in autoimmune diseases: Can altered protein quality control and/or unfolded protein response contribute to autoimmunity? A critical review on Sjögren’s syndrome. Autoimmun. Rev. 2018, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Kuzmuk, K.N.; Schook, L.B. Pigs as a Model for Biomedical Sciences. In The Genetics of the Pig, 2nd ed.; Rothschild, M.F., Ruvinsky, A., Eds.; CAB International: Wallingford, UK, 2011; pp. 426–444. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępniak, J.; Karbownik-Lewińska, M. 17β-Estradiol Stimulates Oxidative Stress Components and Thyroid Specific Genes in Porcine Thyroid Follicular Cells: Potential Differences Between Sexes. Cells 2024, 13, 1769. https://doi.org/10.3390/cells13211769
Stępniak J, Karbownik-Lewińska M. 17β-Estradiol Stimulates Oxidative Stress Components and Thyroid Specific Genes in Porcine Thyroid Follicular Cells: Potential Differences Between Sexes. Cells. 2024; 13(21):1769. https://doi.org/10.3390/cells13211769
Chicago/Turabian StyleStępniak, Jan, and Małgorzata Karbownik-Lewińska. 2024. "17β-Estradiol Stimulates Oxidative Stress Components and Thyroid Specific Genes in Porcine Thyroid Follicular Cells: Potential Differences Between Sexes" Cells 13, no. 21: 1769. https://doi.org/10.3390/cells13211769
APA StyleStępniak, J., & Karbownik-Lewińska, M. (2024). 17β-Estradiol Stimulates Oxidative Stress Components and Thyroid Specific Genes in Porcine Thyroid Follicular Cells: Potential Differences Between Sexes. Cells, 13(21), 1769. https://doi.org/10.3390/cells13211769






