Heavy Metals in Umbilical Cord Blood: Effects on Epigenetics and Child Development
Abstract
:1. Introduction
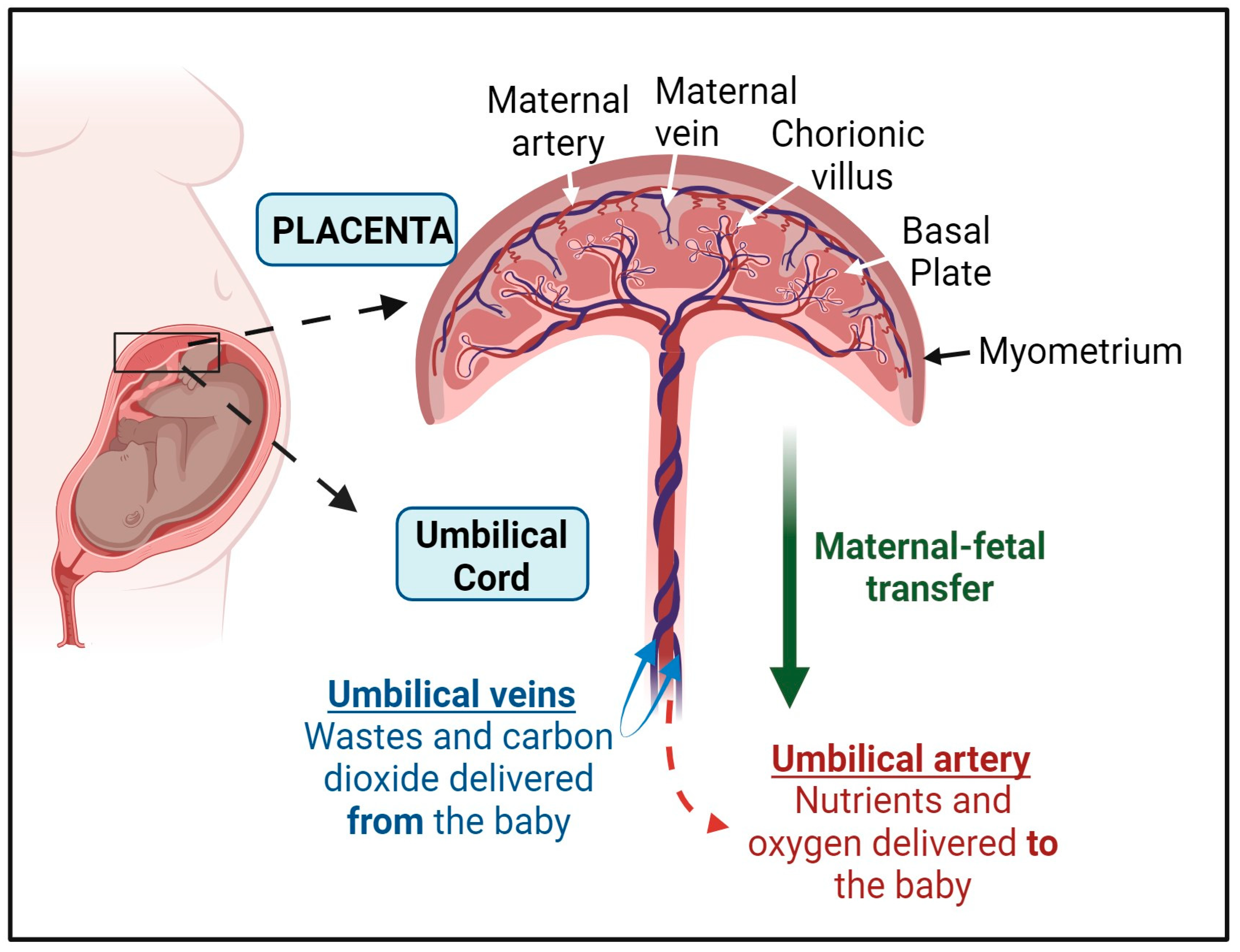
2. The Structure of the Placenta and the Umbilical Cord
3. The Placenta as a Gatekeeper of Maternal and Fetal Exposures
4. Heavy Metal Exposures and Umbilical Cord Blood DNA Methylation
4.1. Arsenic Exposure
4.2. Mercury Exposure
4.3. Mercury and Arsenic Co-Exposure
4.4. Cadmium Exposure
4.5. Lead Exposure
4.6. Exposure to Mixtures of Heavy Metals
5. Short-Term and Long-Term Health Outcomes of Fetal Exposures to Heavy Metals in Relation DNA Methylation
6. Discussion
7. Future Studies
- British Anti-Lewisite (BAL) is one of the earliest chelators developed, which was initially used during World War II for treating poisoning from arsenic and other heavy metals. BAL works by forming stable complexes with metals, allowing for their removal from the body.
- Dimercaptopropane-1-sulfonate (DMPS) is another important chelator that has been used effectively for mercury and lead poisoning. Its water-soluble properties facilitate its use in clinical settings, enabling the rapid mobilization of heavy metals for excretion.
- Meso-2,3-dimercaptosuccinic acid (DMSA) is widely recognized for its efficacy in treating lead poisoning, especially in children. DMSA is a more favorable option due to its low toxicity and ability to penetrate biological membranes effectively, promoting the elimination of lead and other metals while minimizing adverse effects.
- Sodium 2,3-monoisoamyl DMSA (MiADMSA) and monomethyl DMSA (MmDMSA) are derivatives of DMSA that have been developed to enhance chelation efficiency and reduce toxicity. These modifications aim to optimize the chelation process while maintaining safety.
- Monocyclohexyl DMSA (MchDMSA) is another variant designed to improve the pharmacokinetic profile of DMSA, further facilitating heavy metal removal from the body.
- Calcium disodium ethylenediamine tetra-acetic acid (CaNa2EDTA) is a well-established chelator used primarily for lead poisoning. EDTA binds to lead ions and other metals, enabling their renal excretion. The use of calcium disodium EDTA is particularly advantageous in pediatric populations due to its safety profile.
- Calcium trisodium diethylenetriaminepentaacetate is another chelator similar to EDTA that is used for various heavy metals, including lead and cadmium. It offers similar benefits in terms of efficacy and safety.
- D-penicillamine is an alternative chelating agent originally derived from penicillin, which is commonly used to treat copper overload in conditions like Wilson’s disease. It also has applications in the removal of lead and mercury, though its use can be limited by side effects.
- Tetraethylenetetramine (TETA), also known as trientine, serves as a chelator primarily for copper and is often utilized in the management of Wilson’s disease.
- Nitrilotriacetic acid (NTA) is a synthetic chelating agent that has been studied for its ability to bind various metals and has applications in both industrial and clinical settings.
- Deferoxamine (DFO) is a crucial chelator specifically for iron overload conditions, such as thalassemia and hemochromatosis. It forms complexes with excess iron, facilitating its elimination from the body and reducing the risk of iron-induced toxicity.
- Deferiprone (L1) is another iron chelator that has been effective in managing iron overload. It is particularly useful in patients who do not respond well to DFO and can help maintain lower iron levels in the body.
- Vitamin E (α-tocopherol) is a lipid-soluble antioxidant that plays a critical role in protecting cell membranes from oxidative damage. It works by scavenging lipid peroxyl radicals, thereby inhibiting lipid peroxidation, a process that can lead to cell membrane breakdown, organelle dysfunction, and cell death. In the context of heavy metal toxicity, vitamin E has been shown to mitigate oxidative damage in organs such as the liver, kidneys, and brain. For instance, studies have demonstrated that vitamin E supplementation can reduce lipid peroxidation and improve antioxidant enzyme activities in animals exposed to cadmium, suggesting its protective role against Cd-induced hepatotoxicity and nephrotoxicity. Moreover, vitamin E may reduce lead-induced neurotoxicity by preventing oxidative damage to neural cells, offering protection against cognitive and developmental impairments associated with early-life lead exposure.
- Vitamin C (ascorbic acid), a water-soluble antioxidant, also plays a crucial role in combating oxidative stress induced by heavy metal exposure. It acts as a direct scavenger of ROS, neutralizing free radicals before they can cause cellular harm. Vitamin C also enhances the activity of other antioxidants, including vitamin E, by regenerating its active form after it has neutralized free radicals. Research suggests that vitamin C can alleviate the toxic effects of heavy metals like lead and mercury by reducing ROS levels, protecting against DNA damage, and enhancing the excretion of these metals from the body. In individuals exposed to lead, for example, vitamin C has been shown to reduce blood lead levels and improve antioxidant status, making it a valuable component of chelation therapy. Additionally, vitamin C can ameliorate mercury-induced oxidative damage in the kidneys and liver by strengthening endogenous antioxidant defenses.
- Astaxanthin, a naturally occurring carotenoid found in marine organisms such as microalgae, salmon, and shrimp, has emerged as a powerful antioxidant with potential therapeutic benefits in heavy metal toxicity. Astaxanthin is known for its superior free radical scavenging capabilities, which are significantly stronger than those of vitamins E and C. Its unique molecular structure allows it to span cell membranes and protect both the lipid bilayer and aqueous compartments from oxidative damage. In studies examining the protective effects of astaxanthin against cadmium and lead toxicity, it has been shown to reduce oxidative stress markers, enhance the activity of antioxidant enzymes, and improve mitochondrial function. Additionally, astaxanthin’s anti-inflammatory properties further contribute to its protective effects by mitigating the inflammatory responses triggered by heavy metal exposure, which are often driven by oxidative stress.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chaudhuri, S.N.; Butala, S.J.; Ball, R.W.; Braniff, C.T.; Rocky, C. Mountain Biomonitoring, Pilot study for utilization of dried blood spots for screening of lead, mercury and cadmium in newborns. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 298–316. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.W.; Ng, R.R.G.; Chang, T.Y.; Lim, C.H.F.; Zheng, R.T.; Ma, W.; Chua, M.C.; Chan, J.K.Y.; Yap, F.K.P.; Loy, S.L. Preliminary assessment of the Healthy Early Life Moments (HELMS) webinars in empowering Developmental Origins of Health and Disease (DOHaD) concept among healthcare professionals—A pragmatic serial cross-sectional study. J. Perinat. Med. 2024, 52, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Akhabir, L.; Stringer, R.; Desai, D.; Mandhane, P.J.; Azad, M.B.; Moraes, T.J.; Subbarao, P.; Turvey, S.E.; Pare, G.; Anand, S.S.; et al. DNA methylation changes in cord blood and the developmental origins of health and disease—A systematic review and replication study. BMC Genom. 2022, 23, 221. [Google Scholar] [CrossRef] [PubMed]
- Safi-Stibler, S.; Gabory, A. Epigenetics and the Developmental Origins of Health and Disease: Parental environment signalling to the epigenome, critical time windows and sculpting the adult phenotype. Semin. Cell Dev. Biol. 2020, 97, 172–180. [Google Scholar] [CrossRef]
- Briana, D.D.; Malamitsi-Puchner, A. Perinatal biomarkers implying ‘Developmental Origins of Health and Disease’ consequences in intrauterine growth restriction. Acta Paediatr. 2020, 109, 1317–1322. [Google Scholar] [CrossRef]
- Rodger, E.J.; Chatterjee, A. The epigenomic basis of common diseases. Clin. Epigenetics 2017, 9, 5. [Google Scholar] [CrossRef]
- Simeoni, U.; Armengaud, J.B.; Siddeek, B.; Tolsa, J.F. Perinatal Origins of Adult Disease. Neonatology 2018, 113, 393–399. [Google Scholar] [CrossRef]
- Ghazi, T.; Naidoo, P.; Naidoo, R.N.; Chuturgoon, A.A. Prenatal Air Pollution Exposure and Placental DNA Methylation Changes: Implications on Fetal Development and Future Disease Susceptibility. Cells 2021, 10, 3025. [Google Scholar] [CrossRef]
- Lapehn, S.; Paquette, A.G. The Placental Epigenome as a Molecular Link Between Prenatal Exposures and Fetal Health Outcomes Through the DOHaD Hypothesis. Curr. Environ. Health Rep. 2022, 9, 490–501. [Google Scholar] [CrossRef]
- Mortillo, M.; Marsit, C.J. Select Early-Life Environmental Exposures and DNA Methylation in the Placenta. Curr. Environ. Health Rep. 2023, 10, 22–34. [Google Scholar] [CrossRef]
- Vlahos, A.; Mansell, T.; Saffery, R.; Novakovic, B. Human placental methylome in the interplay of adverse placental health, environmental exposure, and pregnancy outcome. PLoS Genet. 2019, 15, e1008236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, Z.; Lin, M.; Zhu, L.; Xu, L.; Wang, X. Deciphering the Epigenetic Landscape: Placental Development and Its Role in Pregnancy Outcomes. Stem Cell Rev. Rep. 2024, 20, 996–1014. [Google Scholar] [CrossRef] [PubMed]
- Maltepe, E.; Bakardjiev, A.I.; Fisher, S.J. The placenta: Transcriptional, epigenetic, and physiological integration during development. J. Clin. Investig. 2010, 120, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Persutte, W.H.; Hobbins, J. Single umbilical artery: A clinical enigma in modern prenatal diagnosis. Ultrasound Obstet. Gynecol. 1995, 6, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.A. Anatomy and embryology of umbilicus in newborns: A review and clinical correlations. Front. Med. 2016, 10, 271–277. [Google Scholar] [CrossRef]
- Lewis, K.; Spirnak, P.W. Umbilical Vein Catheterization; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Moshiri, M.; Zaidi, S.F.; Robinson, T.J.; Bhargava, P.; Siebert, J.R.; Dubinsky, T.J.; Katz, D.S. Comprehensive imaging review of abnormalities of the umbilical cord. Radiographics 2014, 34, 179–196. [Google Scholar] [CrossRef]
- Spurway, J.; Logan, P.; Pak, S. The development, structure and blood flow within the umbilical cord with particular reference to the venous system. Australas. J. Ultrasound Med. 2012, 15, 97–102. [Google Scholar] [CrossRef]
- Kim, D.W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.D.; Borlongan, C.V. Wharton’s jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. [Google Scholar] [CrossRef]
- Lyons, F.G.; Mattei, T.A. Sources, Identification, and Clinical Implications of Heterogeneity in Human Umbilical Cord Stem Cells. Adv. Exp. Med. Biol. 2019, 1169, 243–256. [Google Scholar]
- Nanaev, A.K.; Kohnen, G.; Milovanov, A.P.; Domogatsky, S.P.; Kaufmann, P. Stromal differentiation and architecture of the human umbilical cord. Placenta 1997, 18, 53–64. [Google Scholar] [CrossRef]
- Can, A.; Karahuseyinoglu, S. Concise review: Human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells 2007, 25, 2886–2895. [Google Scholar] [CrossRef] [PubMed]
- Parry, E.W. Some electron microscope observations on the mesenchymal structures of full-term umbilical cord. J. Anat. 1970, 107, 505–518. [Google Scholar] [PubMed]
- Lim, I.J.; Phan, T.T. Epithelial and mesenchymal stem cells from the umbilical cord lining membrane. Cell Transplant. 2014, 23, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Ruetze, M.; Gallinat, S.; Lim, I.J.; Chow, E.; Phan, T.T.; Staeb, F.; Wenck, H.; Deppert, W.; Knott, A. Common features of umbilical cord epithelial cells and epidermal keratinocytes. J. Dermatol. Sci. 2008, 50, 227–231. [Google Scholar] [CrossRef]
- Devi, S.; Bongale, A.M.; Tefera, M.A.; Dixit, P.; Bhanap, P. Fresh Umbilical Cord Blood-A Source of Multipotent Stem Cells, Collection, Banking, Cryopreservation, and Ethical Concerns. Life 2023, 13, 1794. [Google Scholar] [CrossRef]
- Ballen, K. Challenges in umbilical cord blood stem cell banking for stem cell reviews and reports. Stem Cell Rev. Rep. 2010, 6, 8–14. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Short, B.; Brouard, N.; Occhiodoro-Scott, T.; Ramakrishnan, A.; Simmons, P.J. Mesenchymal stem cells. Arch. Med. Res. 2003, 34, 565–571. [Google Scholar] [CrossRef]
- Kucia, M.; Halasa, M.; Wysoczynski, M.; Baskiewicz-Masiuk, M.; Moldenhawer, S.; Zuba-Surma, E.; Czajka, R.; Wojakowski, W.; Machalinski, B.; Ratajczak, M.Z. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: Preliminary report. Leukemia 2007, 21, 297–303. [Google Scholar] [CrossRef]
- McGuckin, C.P.; Forraz, N.; Baradez, M.O.; Navran, S.; Zhao, J.; Urban, R.; Tilton, R.; Denner, L. Production of stem cells with embryonic characteristics from human umbilical cord blood. Cell Prolif. 2005, 38, 245–255. [Google Scholar] [CrossRef]
- Stefanska, K.; Ozegowska, K.; Hutchings, G.; Popis, M.; Moncrieff, L.; Dompe, C.; Janowicz, K.; Pienkowski, W.; Gutaj, P.; Shibli, J.A.; et al. Human Wharton’s Jelly-Cellular Specificity, Stemness Potency, Animal Models, and Current Application in Human Clinical Trials. J. Clin. Med. 2020, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.T. Biobanking and Regenerative Medicine: An Overview. J. Clin. Med. 2018, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D. Epigenetics and metabolism in 2011: Epigenetics, the life-course and metabolic disease. Nat Rev Endocrinol 2011, 8, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.; Sutrave, P.; Gascoigne, E.; Givens, M.B.; Fry, R.C.; Manuck, T.A. Exposure to toxic metals and per- and polyfluoroalkyl substances and the risk of preeclampsia and preterm birth in the United States: A review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100308. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar]
- Chung, J.Y.; Yu, S.D.; Hong, Y.S. Environmental source of arsenic exposure. J. Prev. Med. Public Health 2014, 47, 253–257. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef]
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium Pollution in European Water, Sources, Health Risk, and Remediation Strategies: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, C.H.; An, M.J.; Shin, G.S.; Lee, H.M.; Kim, J.Y.; Hwang, J.Y.; Lee, J.H.; Kim, J.W. Exposure to mercury induced early apoptotic signals in human placental BeWo cells through alteration of cell cycle regulation. Mol. Cell. Toxicol. 2020, 16, 419–429. [Google Scholar] [CrossRef]
- Luyten, L.J.; Saenen, N.D.; Janssen, B.G.; Vrijens, K.; Plusquin, M.; Roels, H.A.; Debacq-Chainiaux, F.; Nawrot, T.S. Air pollution and the fetal origin of disease: A systematic review of the molecular signatures of air pollution exposure in human placenta. Environ. Res. 2018, 166, 310–323. [Google Scholar] [CrossRef]
- Rager, J.E.; Bangma, J.; Carberry, C.; Chao, A.; Grossman, J.; Lu, K.; Manuck, T.A.; Sobus, J.R.; Szilagyi, J.; Fry, R.C. Review of the environmental prenatal exposome and its relationship to maternal and fetal health. Reprod. Toxicol. 2020, 98, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, X.F.; Cullen, W.R.; Weinfeld, M.; Le, X.C. Arsenic binding to proteins. Chem. Rev. 2013, 113, 7769–7792. [Google Scholar] [CrossRef] [PubMed]
- Milton, A.H.; Hussain, S.; Akter, S.; Rahman, M.; Mouly, T.A.; Mitchell, K. A Review of the Effects of Chronic Arsenic Exposure on Adverse Pregnancy Outcomes. Int. J. Environ. Res. Public Health 2017, 14, 556. [Google Scholar] [CrossRef]
- Shirai, S.; Suzuki, Y.; Yoshinaga, J.; Mizumoto, Y. Maternal exposure to low-level heavy metals during pregnancy and birth size. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2010, 45, 1468–1474. [Google Scholar] [CrossRef]
- Zhu, M.; Fitzgerald, E.F.; Gelberg, K.H.; Lin, S.; Druschel, C.M. Maternal low-level lead exposure and fetal growth. Environ. Health Perspect. 2010, 118, 1471–1475. [Google Scholar] [CrossRef]
- Bellinger, D.C. Very low lead exposures and children’s neurodevelopment. Curr. Opin. Pediatr. 2008, 20, 172–177. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; McCarty, K.M.; Steckling, N.; Lettmeier, B. Mercury exposure and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 2010, 40, 186–215. [Google Scholar] [CrossRef]
- Tolins, M.; Ruchirawat, M.; Landrigan, P. The developmental neurotoxicity of arsenic: Cognitive and behavioral consequences of early life exposure. Ann. Glob. Health 2014, 80, 303–314. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Gao, Y.; Zhang, Y.; Wang, C.; Zhou, Y.; Hu, Y.; Shi, R.; Tian, Y. Effects of prenatal exposure to cadmium on neurodevelopment of infants in Shandong, China. Environ. Pollut. 2016, 211, 67–73. [Google Scholar] [CrossRef]
- Lin, C.M.; Doyle, P.; Wang, D.; Hwang, Y.H.; Chen, P.C. Does prenatal cadmium exposure affect fetal and child growth? Occup. Environ. Med. 2011, 68, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A. Toxicity and Fate of Heavy Metals with Particular Reference to Developing Foetus. Adv. Life Sci. 2012, 2, 29–38. [Google Scholar] [CrossRef]
- Meir, A.Y.; Huang, W.; Cao, T.; Hong, X.; Wang, G.; Pearson, C.; Adams, W.G.; Wang, X.; Liang, L. Umbilical cord DNA methylation is associated with body mass index trajectories from birth to adolescence. eBioMedicine 2023, 91, 104550. [Google Scholar]
- Kile, M.L.; Houseman, E.A.; Baccarelli, A.A.; Quamruzzaman, Q.; Rahman, M.; Mostofa, G.; Cardenas, A.; Wright, R.O.; Christiani, D.C. Effect of prenatal arsenic exposure on DNA methylation and leukocyte subpopulations in cord blood. Epigenetics 2014, 9, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.L.; Jima, D.; Sharp, G.C.; McCullough, L.E.; Park, S.S.; Gowdy, K.M.; Skaar, D.; Cowley, M.; Maguire, R.L.; Fuemmeler, B.; et al. Maternal pre-pregnancy obesity, offspring cord blood DNA methylation, and offspring cardiometabolic health in early childhood: An epigenome-wide association study. Epigenetics 2019, 14, 325–340. [Google Scholar] [CrossRef]
- Morales, E.; Groom, A.; Lawlor, D.A.; Relton, C.L. DNA methylation signatures in cord blood associated with maternal gestational weight gain: Results from the ALSPAC cohort. BMC Res. Notes 2014, 7, 278. [Google Scholar] [CrossRef]
- Nemoda, Z.; Massart, R.; Suderman, M.; Hallett, M.; Li, T.; Coote, M.; Cody, N.; Sun, Z.S.; Soares, C.N.; Turecki, G.; et al. Maternal depression is associated with DNA methylation changes in cord blood T lymphocytes and adult hippocampi. Transl. Psychiatry 2015, 5, e545. [Google Scholar] [CrossRef]
- Xu, R.; Hong, X.; Zhang, B.; Huang, W.; Hou, W.; Wang, G.; Wang, X.; Igusa, T.; Liang, L.; Ji, H. DNA methylation mediates the effect of maternal smoking on offspring birthweight: A birth cohort study of multi-ethnic US mother-newborn pairs. Clin. Epigenetics 2021, 13, 47. [Google Scholar] [CrossRef]
- Kresovich, J.K.; Zheng, Y.; Cardenas, A.; Joyce, B.T.; Rifas-Shiman, S.L.; Oken, E.; Gillman, M.W.; Hivert, M.F.; Baccarelli, A.A.; Hou, L. Cord blood DNA methylation and adiposity measures in early and mid-childhood. Clin. Epigenetics 2017, 9, 86. [Google Scholar] [CrossRef]
- Relton, C.L.; Groom, A.; St Pourcain, B.; Sayers, A.E.; Swan, D.C.; Embleton, N.D.; Pearce, M.S.; Ring, S.M.; Northstone, K.; Tobias, J.H.; et al. DNA methylation patterns in cord blood DNA and body size in childhood. PLoS ONE 2012, 7, e31821. [Google Scholar] [CrossRef]
- Vehmeijer, F.O.L.; Kupers, L.K.; Sharp, G.C.; Salas, L.A.; Lent, S.; Jima, D.D.; Tindula, G.; Reese, S.; Qi, C.; Gruzieva, O.; et al. DNA methylation and body mass index from birth to adolescence: Meta-analyses of epigenome-wide association studies. Genome Med. 2020, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Funk, W.E.; McGee, J.K.; Olshan, A.F.; Ghio, A.J. Quantification of arsenic, lead, mercury and cadmium in newborn dried blood spots. Biomarkers 2013, 18, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Weyde, K.V.F.; Olsen, A.K.; Duale, N.; Kamstra, J.H.; Skogheim, T.S.; Caspersen, I.H.; Engel, S.M.; Biele, G.; Xia, Y.; Meltzer, H.M.; et al. Gestational blood levels of toxic metal and essential element mixtures and associations with global DNA methylation in pregnant women and their infants. Sci. Total Environ. 2021, 787, 147621. [Google Scholar] [CrossRef]
- Pilsner, J.R.; Hu, H.; Ettinger, A.; Sanchez, B.N.; Wright, R.O.; Cantonwine, D.; Lazarus, A.; Lamadrid-Figueroa, H.; Mercado-Garcia, A.; Tellez-Rojo, M.M.; et al. Influence of prenatal lead exposure on genomic methylation of cord blood DNA. Environ. Health Perspect. 2009, 117, 1466–1471. [Google Scholar] [CrossRef]
- Herrera-Moreno, J.F.; Estrada-Gutierrez, G.; Wu, H.; Bloomquist, T.R.; Rosa, M.J.; Just, A.C.; Lamadrid-Figueroa, H.; Tellez-Rojo, M.M.; Wright, R.O.; Baccarelli, A.A. Prenatal lead exposure, telomere length in cord blood, and DNA methylation age in the PROGRESS prenatal cohort. Environ. Res. 2022, 205, 112577. [Google Scholar] [CrossRef]
- Cardenas, A.; Rifas-Shiman, S.L.; Godderis, L.; Duca, R.C.; Navas-Acien, A.; Litonjua, A.A.; DeMeo, D.L.; Brennan, K.J.; Amarasiriwardena, C.J.; Hivert, M.F.; et al. Prenatal Exposure to Mercury: Associations with Global DNA Methylation and Hydroxymethylation in Cord Blood and in Childhood. Environ. Health Perspect. 2017, 125, 087022. [Google Scholar] [CrossRef]
- Leung, Y.K.; Ouyang, B.; Niu, L.; Xie, C.; Ying, J.; Medvedovic, M.; Chen, A.; Weihe, P.; Valvi, D.; Grandjean, P.; et al. Identification of sex-specific DNA methylation changes driven by specific chemicals in cord blood in a Faroese birth cohort. Epigenetics 2018, 13, 290–300. [Google Scholar] [CrossRef]
- Taeubert, M.J.; de Prado-Bert, P.; Geurtsen, M.L.; Mancano, G.; Vermeulen, M.J.; Reiss, I.K.M.; Caramaschi, D.; Sunyer, J.; Sharp, G.C.; Julvez, J.; et al. Maternal iron status in early pregnancy and DNA methylation in offspring: An epigenome-wide meta-analysis. Clin. Epigenetics 2022, 14, 59. [Google Scholar] [CrossRef]
- Brodkin, E.; Copes, R.; Mattman, A.; Kennedy, J.; Kling, R.; Yassi, A. Lead and mercury exposures: Interpretation and action. Can. Med. Assoc. J. 2007, 176, 59–63. [Google Scholar] [CrossRef]
- Murcia, M.; Ballester, F.; Enning, A.M.; Iniguez, C.; Valvi, D.; Basterrechea, M.; Rebagliato, M.; Vioque, J.; Maruri, M.; Tardon, A.; et al. Prenatal mercury exposure and birth outcomes. Environ. Res. 2016, 151, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M. Effects of arsenic on maternal and fetal health. Annu. Rev. Nutr. 2009, 29, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Ullah, Z.; Rashid, A.; Ghani, J.; Talib, M.A.; Shahab, A.; Lun, L. Arsenic Contamination, Water Toxicity, Source Apportionment, and Potential Health Risk in Groundwater of Jhelum Basin, Punjab, Pakistan. Biol. Trace Elem. Res. 2023, 201, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Hamadani, J.D.; Tofail, F.; Nermell, B.; Gardner, R.; Shiraji, S.; Bottai, M.; Arifeen, S.E.; Huda, S.N.; Vahter, M. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: A population-based cohort study. Int. J. Epidemiol. 2011, 40, 1593–1604. [Google Scholar] [CrossRef]
- Liaw, J.; Marshall, G.; Yuan, Y.; Ferreccio, C.; Steinmaus, C.; Smith, A.H. Increased childhood liver cancer mortality and arsenic in drinking water in northern Chile. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Vahter, M.; Ekstrom, E.C.; Persson, L.A. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environ. Health Perspect. 2011, 119, 719–724. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, J.; Benbrahim-Tallaa, L.; Ward, J.M.; Logsdon, D.; Diwan, B.A.; Waalkes, M.P. Aberrant DNA methylation and gene expression in livers of newborn mice transplacentally exposed to a hepatocarcinogenic dose of inorganic arsenic. Toxicology 2007, 236, 7–15. [Google Scholar] [CrossRef]
- Yorifuji, T.; Tsuda, T.; Grandjean, P. Unusual cancer excess after neonatal arsenic exposure from contaminated milk powder. J. Natl. Cancer Inst. 2010, 102, 360–361. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Banerjee, M.; Giri, A.K. Role of genomic instability in arsenic-induced carcinogenicity. A review. Environ. Int. 2013, 53, 29–40. [Google Scholar] [CrossRef]
- Hossain, M.B.; Vahter, M.; Concha, G.; Broberg, K. Environmental arsenic exposure and DNA methylation of the tumor suppressor gene p16 and the DNA repair gene MLH1: Effect of arsenic metabolism and genotype. Metallomics 2012, 4, 1167–1175. [Google Scholar] [CrossRef]
- Broberg, K.; Ahmed, S.; Engstrom, K.; Hossain, M.B.; Mlakar, S.J.; Bottai, M.; Grander, M.; Raqib, R.; Vahter, M. Arsenic exposure in early pregnancy alters genome-wide DNA methylation in cord blood, particularly in boys. J. Dev. Orig. Health Dis. 2014, 5, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa-Jotaki, S.; Sakurai, K.; Eguchi, A.; Tanabe, H.; Watanabe, M.; Mori, C. Association between mercury in cord serum and sex-specific DNA methylation in cord tissues. J. Dev. Orig. Health Dis. 2021, 12, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Lamborg, C.H.; Hammerschmidt, C.R.; Bowman, K.L.; Swarr, G.J.; Munson, K.M.; Ohnemus, D.C.; Lam, P.J.; Heimburger, L.E.; Rijkenberg, M.J.; Saito, M.A. A global ocean inventory of anthropogenic mercury based on water column measurements. Nature 2014, 512, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Choi, A.L.; Karagas, M.R.; Marien, K.; Rheinberger, C.M.; Schoeny, R.; Sunderland, E.; Korrick, S. Which fish should I eat? Perspectives influencing fish consumption choices. Environ. Health Perspect. 2012, 120, 790–798. [Google Scholar] [CrossRef]
- Vahter, M.; Akesson, A.; Lind, B.; Bjors, U.; Schutz, A.; Berglund, M. Longitudinal study of methylmercury and inorganic mercury in blood and urine of pregnant and lactating women, as well as in umbilical cord blood. Environ. Res. 2000, 84, 186–194. [Google Scholar] [CrossRef]
- St-Pierre, M.V.; Serrano, M.A.; Macias, R.I.; Dubs, U.; Hoechli, M.; Lauper, U.; Meier, P.J.; Marin, J.J. Expression of members of the multidrug resistance protein family in human term placenta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1495–R1503. [Google Scholar] [CrossRef]
- Stern, A.H.; Smith, A.E. An assessment of the cord blood:maternal blood methylmercury ratio: Implications for risk assessment. Environ. Health Perspect. 2003, 111, 1465–1470. [Google Scholar] [CrossRef]
- Evseenko, D.A.; Paxton, J.W.; Keelan, J.A. ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1357–R1365. [Google Scholar] [CrossRef]
- Jauniaux, E.; Gulbis, B.; Burton, G.J. The human first trimester gestational sac limits rather than facilitates oxygen transfer to the foetus—A review. Placenta 2003, 24 (Suppl. A), S86–S93. [Google Scholar] [CrossRef]
- Boucher, O.; Muckle, G.; Jacobson, J.L.; Carter, R.C.; Kaplan-Estrin, M.; Ayotte, P.; Dewailly, E.; Jacobson, S.W. Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: Results from the Environmental Contaminants and Child Development Study in Nunavik. Environ. Health Perspect. 2014, 122, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Ramos, R.; Lopez-Espinosa, M.J.; Diez, S.; Vioque, J.; Ballester, F.; Fernandez, M.F. Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environ. Res. 2010, 110, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Weihe, P.; White, R.F.; Debes, F.; Araki, S.; Yokoyama, K.; Murata, K.; Sorensen, N.; Dahl, R.; Jorgensen, P.J. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997, 19, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Thurston, S.W.; Bellinger, D.C.; Amarasiriwardena, C.; Korrick, S.A. Prenatal exposure to mercury and fish consumption during pregnancy and attention-deficit/hyperactivity disorder-related behavior in children. Arch. Pediatr. Adolesc. Med. 2012, 166, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, A.; Koestler, D.C.; Houseman, E.A.; Jackson, B.P.; Kile, M.L.; Karagas, M.R.; Marsit, C.J. Differential DNA methylation in umbilical cord blood of infants exposed to mercury and arsenic in utero. Epigenetics 2015, 10, 508–515. [Google Scholar] [CrossRef]
- Bakulski, K.M.; Lee, H.; Feinberg, J.I.; Wells, E.M.; Brown, S.; Herbstman, J.B.; Witter, F.R.; Halden, R.U.; Caldwell, K.; Mortensen, M.E.; et al. Prenatal mercury concentration is associated with changes in DNA methylation at TCEANC2 in newborns. Int. J. Epidemiol. 2015, 44, 1249–1262. [Google Scholar] [CrossRef]
- Lozano, M.; Yousefi, P.; Broberg, K.; Soler-Blasco, R.; Miyashita, C.; Pesce, G.; Kim, W.J.; Rahman, M.; Bakulski, K.M.; Haug, L.S.; et al. DNA methylation changes associated with prenatal mercury exposure: A meta-analysis of prospective cohort studies from PACE consortium. Environ. Res. 2022, 204, 112093. [Google Scholar] [CrossRef]
- Cardenas, A.; Rifas-Shiman, S.L.; Agha, G.; Hivert, M.F.; Litonjua, A.A.; DeMeo, D.L.; Lin, X.; Amarasiriwardena, C.J.; Oken, E.; Gillman, M.W.; et al. Persistent DNA methylation changes associated with prenatal mercury exposure and cognitive performance during childhood. Sci. Rep. 2017, 7, 288. [Google Scholar] [CrossRef]
- Nyanza, E.C.; Dewey, D.; Manyama, M.; Martin, J.W.; Hatfield, J.; Bernier, F.P. Maternal exposure to arsenic and mercury and associated risk of adverse birth outcomes in small-scale gold mining communities in Northern Tanzania. Environ. Int. 2020, 137, 105450. [Google Scholar] [CrossRef]
- ATSDR. Priority List of Hazardous Substances.Online 2011. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/3378161 (accessed on 24 October 2024).
- ATSDR. Toxicological Profile for Cadmium; Centers for Diseases Control: Atlanta, GA, USA, 2008. [Google Scholar]
- Arita, A.; Costa, M. Epigenetics in metal carcinogenesis: Nickel, arsenic, chromium and cadmium. Metallomics 2009, 1, 222–228. [Google Scholar] [CrossRef]
- Waalkes, M.P. Cadmium carcinogenesis in review. J. Inorg. Biochem. 2000, 79, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Bonithon-Kopp, C.; Huel, G.; Moreau, T.; Wendling, R. Prenatal exposure to lead and cadmium and psychomotor development of the child at 6 years. Neurobehav. Toxicol. Teratol. 1986, 8, 307–310. [Google Scholar] [PubMed]
- Llanos, M.N.; Ronco, A.M. Fetal growth restriction is related to placental levels of cadmium, lead and arsenic but not with antioxidant activities. Reprod. Toxicol. 2009, 27, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Tawara, K.; Honda, R.; Nakagawa, H.; Tanebe, K.; Saito, S. Relationship between newborn size and mother’s blood cadmium levels, Toyama, Japan. Arch. Environ. Health 2004, 59, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Ronco, A.M.; Arguello, G.; Munoz, L.; Gras, N.; Llanos, M. Metals content in placentas from moderate cigarette consumers: Correlation with newborn birth weight. Biometals 2005, 18, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Salpietro, C.D.; Gangemi, S.; Minciullo, P.L.; Briuglia, S.; Merlino, M.V.; Stelitano, A.; Cristani, M.; Trombetta, D.; Saija, A. Cadmium concentration in maternal and cord blood and infant birth weight: A study on healthy non-smoking women. J. Perinat. Med. 2002, 30, 395–399. [Google Scholar] [CrossRef]
- Tian, L.L.; Zhao, Y.C.; Wang, X.C.; Gu, J.L.; Sun, Z.J.; Zhang, Y.L.; Wang, J.X. Effects of gestational cadmium exposure on pregnancy outcome and development in the offspring at age 4.5 years. Biol. Trace Elem. Res. 2009, 132, 51–59. [Google Scholar] [CrossRef]
- World Health Organization. Preventing Disease Through Healthy Environments: Exposure to Cadmium: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Fagioni, M.; D’Amici, G.M.; Timperio, A.M.; Zolla, L. Proteomic analysis of multiprotein complexes in the thylakoid membrane upon cadmium treatment. J. Proteome Res. 2009, 8, 310–326. [Google Scholar] [CrossRef]
- Galano, E.; Arciello, A.; Piccoli, R.; Monti, D.M.; Amoresano, A. A proteomic approach to investigate the effects of cadmium and lead on human primary renal cells. Metallomics 2014, 6, 587–597. [Google Scholar] [CrossRef]
- Vilahur, N.; Vahter, M.; Broberg, K. The Epigenetic Effects of Prenatal Cadmium Exposure. Curr. Environ. Health Rep. 2015, 2, 195–203. [Google Scholar] [CrossRef]
- Sanders, A.P.; Smeester, L.; Rojas, D.; DeBussycher, T.; Wu, M.C.; Wright, F.A.; Zhou, Y.H.; Laine, J.E.; Rager, J.E.; Swamy, G.K.; et al. Cadmium exposure and the epigenome: Exposure-associated patterns of DNA methylation in leukocytes from mother-baby pairs. Epigenetics 2014, 9, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Kim, E.; Won, S.; Kim, W.J. Association between prenatal cadmium exposure and cord blood DNA methylation. Environ. Res. 2022, 212, 113268. [Google Scholar] [CrossRef] [PubMed]
- Kippler, M.; Engstrom, K.; Mlakar, S.J.; Bottai, M.; Ahmed, S.; Hossain, M.B.; Raqib, R.; Vahter, M.; Broberg, K. Sex-specific effects of early life cadmium exposure on DNA methylation and implications for birth weight. Epigenetics 2013, 8, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Igra, A.M.; Hellberg, A.; Engstrom, K.; Raqib, R.; Rahman, A.; Vahter, M.; Kippler, M.; Broberg, K. Maternal exposure to cadmium during pregnancy is associated with changes in DNA methylation that are persistent at 9 years of age. Environ. Int. 2022, 163, 107188. [Google Scholar] [CrossRef] [PubMed]
- Cowley, M.; Skaar, D.A.; Jima, D.D.; Maguire, R.L.; Hudson, K.M.; Park, S.S.; Sorrow, P.; Hoyo, C. Effects of Cadmium Exposure on DNA Methylation at Imprinting Control Regions and Genome-Wide in Mothers and Newborn Children. Environ. Health Perspect. 2018, 126, 037003. [Google Scholar] [CrossRef]
- Koh, E.J.; Yu, S.Y.; Kim, S.H.; Lee, J.S.; Hwang, S.Y. Prenatal Exposure to Heavy Metals Affects Gestational Age by Altering DNA Methylation Patterns. Nanomaterials 2021, 11, 2871. [Google Scholar] [CrossRef]
- Barltrop, D. Lead poisoning in childhood. Postgrad. Med. J. 1968, 44, 537–542. [Google Scholar] [CrossRef]
- Goyer, R.A. Transplacental transport of lead. Environ. Health Perspect. 1990, 89, 101–105. [Google Scholar] [CrossRef]
- Marshall, A.T.; McConnell, R.; Lanphear, B.P.; Thompson, W.K.; Herting, M.M.; Sowell, E.R. Risk of lead exposure, subcortical brain structure, and cognition in a large cohort of 9- to 10-year-old children. PLoS ONE 2021, 16, e0258469. [Google Scholar] [CrossRef]
- Borja-Aburto, V.H.; Hertz-Picciotto, I.; Lopez, R.M.; Farias, P.; Rios, C.; Blanco, J. Blood lead levels measured prospectively and risk of spontaneous abortion. Am. J. Epidemiol. 1999, 150, 590–597. [Google Scholar] [CrossRef]
- Andrews, K.W.; Savitz, D.A.; Hertz-Picciotto, I. Prenatal lead exposure in relation to gestational age and birth weight: A review of epidemiologic studies. Am. J. Ind. Med. 1994, 26, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Jelliffe-Pawlowski, L.L.; Miles, S.Q.; Courtney, J.G.; Materna, B.; Charlton, V. Effect of magnitude and timing of maternal pregnancy blood lead (Pb) levels on birth outcomes. J. Perinatol. 2006, 26, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.; Leviton, A.; Rabinowitz, M.; Allred, E.; Needleman, H.; Schoenbaum, S. Weight gain and maturity in fetuses exposed to low levels of lead. Environ. Res. 1991, 54, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cossio, T.; Peterson, K.E.; Sanin, L.H.; Fishbein, E.; Palazuelos, E.; Aro, A.; Hernandez-Avila, M.; Hu, H. Decrease in birth weight in relation to maternal bone-lead burden. Pediatrics 1997, 100, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Stampfer, M.J.; Manson, J.E.; Rosner, B.; Hankinson, S.E.; Colditz, G.A.; Willett, W.C.; Hennekens, C.H. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ 1997, 315, 396–400. [Google Scholar] [CrossRef]
- Sen, A.; Heredia, N.; Senut, M.C.; Land, S.; Hollocher, K.; Lu, X.; Dereski, M.O.; Ruden, D.M. Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci. Rep. 2015, 5, 14466. [Google Scholar] [CrossRef]
- Sen, A.; Cingolani, P.; Senut, M.C.; Land, S.; Mercado-Garcia, A.; Tellez-Rojo, M.M.; Baccarelli, A.A.; Wright, R.O.; Ruden, D.M. Lead exposure induces changes in 5-hydroxymethylcytosine clusters in CpG islands in human embryonic stem cells and umbilical cord blood. Epigenetics 2015, 10, 607–621. [Google Scholar] [CrossRef]
- Okamoto, Y.; Iwai-Shimada, M.; Nakai, K.; Tatsuta, N.; Mori, Y.; Aoki, A.; Kojima, N.; Takada, T.; Satoh, H.; Jinno, H. Global DNA Methylation in Cord Blood as a Biomarker for Prenatal Lead and Antimony Exposures. Toxics 2022, 10, 157. [Google Scholar] [CrossRef]
- Zeng, Z.; Huo, X.; Zhang, Y.; Hylkema, M.N.; Wu, Y.; Xu, X. Differential DNA methylation in newborns with maternal exposure to heavy metals from an e-waste recycling area. Environ. Res. 2019, 171, 536–545. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, C.; Li, W.D.; Xu, X.D.; Cui, F.P.; Chen, P.P.; Deng, Y.L.; Miao, Y.; Luo, Q.; Zeng, J.Y.; et al. Individual and mixtures of metal exposures in associations with biomarkers of oxidative stress and global DNA methylation among pregnant women. Chemosphere 2022, 293, 133662. [Google Scholar] [CrossRef] [PubMed]
- Bozack, A.K.; Rifas-Shiman, S.L.; Coull, B.A.; Baccarelli, A.A.; Wright, R.O.; Amarasiriwardena, C.; Gold, D.R.; Oken, E.; Hivert, M.F.; Cardenas, A. Prenatal metal exposure, cord blood DNA methylation and persistence in childhood: An epigenome-wide association study of 12 metals. Clin. Epigenetics 2021, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Montes-Castro, N.; Alvarado-Cruz, I.; Torres-Sanchez, L.; Garcia-Aguiar, I.; Barrera-Hernandez, A.; Escamilla-Nunez, C.; Del Razo, L.M.; Quintanilla-Vega, B. Prenatal exposure to metals modified DNA methylation and the expression of antioxidant- and DNA defense-related genes in newborns in an urban area. J. Trace Elem. Med. Biol. 2019, 55, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, G.; Hattis, D.; Sonawane, B. Incorporating pharmacokinetic differences between children and adults in assessing children’s risks to environmental toxicants. Toxicol. Appl. Pharmacol. 2004, 198, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Ha, E.H.; Kim, B.N.; Ha, M.; Kim, Y.; Park, H.; Hong, Y.C.; Kim, K.N. Associations of prenatal and early childhood mercury exposure with autistic behaviors at 5years of age: The Mothers and Children’s Environmental Health (MOCEH) study. Sci. Total Environ. 2017, 605–606, 251–257. [Google Scholar] [CrossRef]
- Tan, S.W.; Meiller, J.C.; Mahaffey, K.R. The endocrine effects of mercury in humans and wildlife. Crit. Rev. Toxicol. 2009, 39, 228–269. [Google Scholar] [CrossRef]
- Nisevic, J.R.; Prpic, I.; Kolic, I.; Bazdaric, K.; Tratnik, J.S.; Prpic, I.S.; Mazej, D.; Spiric, Z.; Barbone, F.; Horvat, M. Combined prenatal exposure to mercury and LCPUFA on newborn’s brain measures and neurodevelopment at the age of 18 months. Environ. Res. 2019, 178, 108682. [Google Scholar] [CrossRef]
- Wu, S.; Hivert, M.F.; Cardenas, A.; Zhong, J.; Rifas-Shiman, S.L.; Agha, G.; Colicino, E.; Just, A.C.; Amarasiriwardena, C.; Lin, X.; et al. Exposure to Low Levels of Lead in Utero and Umbilical Cord Blood DNA Methylation in Project Viva: An Epigenome-Wide Association Study. Environ. Health Perspect. 2017, 125, 087019. [Google Scholar] [CrossRef]
- Mio, H.; Kagami, N.; Yokokawa, S.; Kawai, H.; Nakagawa, S.; Takeuchi, K.; Sekine, S.; Hiraoka, A. Isolation and characterization of a cDNA for human mouse, and rat full-length stem cell growth factor, a new member of C-type lectin superfamily. Biochem. Biophys. Res. Commun. 1998, 249, 124–130. [Google Scholar] [CrossRef]
- Hiraoka, A.; Yano, K.-I.; Kagami, N.; Takeshige, K.; Mio, H.; Anazawa, H.; Sugimoto, S. Stem cell growth factor: In situ hybridization analysis on the gene expression, molecular characterization and in vitro proliferative activity of a recombinant preparation on primitive hematopoietic progenitor cells. Hematol. J. 2001, 2, 307–315. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Gao, D.; Jing, J.; Hu, Q. Prenatal and postnatal lead exposure and cognitive development of infants followed over the first three years of life: A prospective birth study in the Pearl River Delta region, China. Neurotoxicology 2014, 44, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Virgolini, M.B.; Rossi-George, A.; Weston, D.; Cory-Slechta, D.A. Influence of low level maternal Pb exposure and prenatal stress on offspring stress challenge responsivity. Neurotoxicology 2008, 29, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Tamayo-Ortiz, M.; Tang, L.; Sanchez, B.N.; Cantoral, A.; Meeker, J.D.; Dolinoy, D.C.; Roberts, E.F.; Martinez-Mier, E.A.; Lamadrid-Figueroa, H.; et al. Early Life Exposure in Mexico to ENvironmental Toxicants (ELEMENT) Project. BMJ Open 2019, 9, e030427. [Google Scholar] [CrossRef] [PubMed]
- Gulson, B.L.; Jameson, C.W.; Mahaffey, K.R.; Mizon, K.J.; Korsch, M.J.; Vimpani, G. Pregnancy increases mobilization of lead from maternal skeleton. J. Lab. Clin. Med. 1997, 130, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Lagerkvist, B.J.; Ekesrydh, S.; Englyst, V.; Nordberg, G.F.; Soderberg, H.A.; Wiklund, D.E. Increased blood lead and decreased calcium levels during pregnancy: A prospective study of Swedish women living near a smelter. Am. J. Public Health 1996, 86, 1247–1252. [Google Scholar] [CrossRef]
- Barker, D.J.; Gluckman, P.D.; Godfrey, K.M.; Harding, J.E.; Owens, J.A.; Robinson, J.S. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, C.; Jin, J.; Li, W.; Liang, J.; Zhou, S.; Weng, Z.; Zhou, Y.; Liao, X.; Gu, A. Early-life exposure to lead changes cardiac development and compromises long-term cardiac function. Sci. Total Environ. 2023, 904, 166667. [Google Scholar] [CrossRef]
- Vidal, A.C.; Semenova, V.; Darrah, T.; Vengosh, A.; Huang, Z.; King, K.; Nye, M.D.; Fry, R.; Skaar, D.; Maguire, R.; et al. Maternal cadmium, iron and zinc levels, DNA methylation and birth weight. BMC Pharmacol. Toxicol. 2015, 16, 20. [Google Scholar] [CrossRef]
- Li, L.; Keverne, E.B.; Aparicio, S.A.; Ishino, F.; Barton, S.C.; Surani, M.A. Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science 1999, 284, 330–333. [Google Scholar] [CrossRef]
- Reeves, P.G.; Chaney, R.L. Marginal nutritional status of zinc, iron, and calcium increases cadmium retention in the duodenum and other organs of rats fed rice-based diets. Environ. Res. 2004, 96, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, M.M.; Moniuszko-Jakoniuk, J. Interactions between cadmium and zinc in the organism. Food Chem. Toxicol. 2001, 39, 967–980. [Google Scholar] [CrossRef] [PubMed]
- States, J.C.; Singh, A.V.; Knudsen, T.B.; Rouchka, E.C.; Ngalame, N.O.; Arteel, G.E.; Piao, Y.; Ko, M.S. Prenatal arsenic exposure alters gene expression in the adult liver to a proinflammatory state contributing to accelerated atherosclerosis. PLoS ONE 2012, 7, e38713. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Sharrett, A.R.; Silbergeld, E.K.; Schwartz, B.S.; Nachman, K.E.; Burke, T.A.; Guallar, E. Arsenic exposure and cardiovascular disease: A systematic review of the epidemiologic evidence. Am. J. Epidemiol. 2005, 162, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.T.; Chou, H.J.; Han, B.C.; Huang, S.Y. Lifelong inorganic arsenic compounds consumption affected blood pressure in rats. Food Chem. Toxicol. 2007, 45, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Santra, A.; Lahiri, S.; Mazumder, D.N.G. Implications of oxidative stress and hepatic cytokine (TNF-alpha and IL-6) response in the pathogenesis of hepatic collagenesis in chronic arsenic toxicity. Toxicol. Appl. Pharmacol. 2005, 204, 18–26. [Google Scholar] [CrossRef]
- Hanson, M.A.; Skinner, M.K. Developmental origins of epigenetic transgenerational inheritance. Environ. Epigenet 2016, 2, dvw002. [Google Scholar] [CrossRef]
- Boskovic, A.; Rando, O.J. Transgenerational Epigenetic Inheritance. Annu. Rev. Genet. 2018, 52, 21–41. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Maamar, M.B.; Skinner, M.K. Environmentally Induced Epigenetic Transgenerational Inheritance and the Weismann Barrier: The Dawn of Neo-Lamarckian Theory. J. Dev. Biol. 2020, 8, 28. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenet 2018, 4, dvy016. [Google Scholar] [CrossRef]
- Vaiserman, A.M.; Koliada, A.K.; Jirtle, R.L. Non-genomic transmission of longevity between generations: Potential mechanisms and evidence across species. Epigenetics Chromatin 2017, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Senut, M.C.; Sen, A.; Cingolani, P.; Shaik, A.; Land, S.J.; Ruden, D.M. Lead exposure disrupts global DNA methylation in human embryonic stem cells and alters their neuronal differentiation. Toxicol. Sci. 2014, 139, 142–161. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, K.N.; Krafft, K.M.; Bornschein, R.L.; Hammond, P.B.; Berger, O.; Succop, P.A.; Bier, M. Low-level fetal lead exposure effect on neurobehavioral development in early infancy. Pediatrics 1987, 80, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Whitworth, R.H.; Baloh, R.W.; Staehling, N.W.; Barthel, W.F.; Rosenblum, B.F. Neuropsychological dysfunction in children with chronic low-level lead absorption. Lancet 1975, 1, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Needleman, H.L.; Gunnoe, C.; Leviton, A.; Reed, R.; Peresie, H.; Maher, C.; Barrett, P. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N. Engl. J. Med. 1979, 300, 689–695. [Google Scholar] [CrossRef]
- Adinolfi, M. The development of the human blood-CSF-brain barrier. Dev. Med. Child. Neurol. 1985, 27, 532–537. [Google Scholar] [CrossRef]
- Andersen, H.R.; Nielsen, J.B.; Grandjean, P. Toxicologic evidence of developmental neurotoxicity of environmental chemicals. Toxicology 2000, 144, 121–127. [Google Scholar] [CrossRef]
- Sakamoto, M.; Kubota, M.; Liu, X.J.; Murata, K.; Nakai, K.; Satoh, H. Maternal and fetal mercury and n-3 polyunsaturated fatty acids as a risk and benefit of fish consumption to fetus. Environ. Sci. Technol. 2004, 38, 3860–3863. [Google Scholar] [CrossRef]
- National Research Council. Toxicological Eff Ects of Methylmercury; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Grosse, S.D.; Matte, T.D.; Schwartz, J.; Jackson, R.J. Economic gains resulting from the reduction in children’s exposure to lead in the United States. Environ. Health Perspect. 2002, 110, 563–569. [Google Scholar] [CrossRef]
- Trasande, L.; Landrigan, P.J.; Schechter, C. Public health and economic consequences of methyl mercury toxicity to the developing brain. Environ. Health Perspect. 2005, 113, 590–596. [Google Scholar] [CrossRef] [PubMed]
- U. S. Environmental Protection Agency. Chemical Hazard Data Availability Study: What Do We Really Know About the Safety of High Production Volume Chemicals? Office of Pollution Prevention and Toxics: Washington, DC, USA, 1998. [Google Scholar]
- Horsthemke, B.; Wagstaff, J. Mechanisms of imprinting of the Prader-Willi/Angelman region. Am. J. Med. Genet. A 2008, 146A, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Ceccatelli, S.; Dare, E.; Moors, M. Methylmercury-induced neurotoxicity and apoptosis. Chem. Biol. Interact. 2010, 188, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Aschner, M.; Syversen, T. Glutathione modulation influences methyl mercury induced neurotoxicity in primary cell cultures of neurons and astrocytes. Neurotoxicology 2006, 27, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Rocha, J.B.; Aschner, M. Mechanisms of methylmercury-induced neurotoxicity: Evidence from experimental studies. Life Sci. 2011, 89, 555–563. [Google Scholar] [CrossRef]
- Koga, M.; Serritella, A.V.; Messmer, M.M.; Hayashi-Takagi, A.; Hester, L.D.; Snyder, S.H.; Sawa, A.; Sedlak, T.W. Glutathione is a physiologic reservoir of neuronal glutamate. Biochem. Biophys. Res. Commun. 2011, 409, 596–602. [Google Scholar] [CrossRef]
- Horsthemke, B. A critical view on transgenerational epigenetic inheritance in humans. Nat. Commun. 2018, 9, 2973. [Google Scholar] [CrossRef]
- Pyatha, S.; Kim, H.; Lee, D.; Kim, K. Co-exposure to lead, mercury, and cadmium induces neurobehavioral impairments in mice by interfering with dopaminergic and serotonergic neurotransmission in the striatum. Front. Public Health 2023, 11, 1265864. [Google Scholar] [CrossRef]
- Hudson, K.M.; Belcher, S.M.; Cowley, M. Maternal cadmium exposure in the mouse leads to increased heart weight at birth and programs susceptibility to hypertension in adulthood. Sci. Rep. 2019, 9, 13553. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Navas-Acien, A.; Menke, A.; Crainiceanu, C.M.; Pastor-Barriuso, R.; Guallar, E. Cadmium exposure and all-cause and cardiovascular mortality in the U. S. Gen. Popul. Environ. Health Perspect. 2012, 120, 1017–1022. [Google Scholar] [CrossRef]
- Wobus, A.M.; Loser, P. Present state and future perspectives of using pluripotent stem cells in toxicology research. Arch. Toxicol. 2011, 85, 79–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Lan, Y.F.; Rehman, K.; Jiang, Y.H.; Maimaitiyiming, Y.; Zhu, D.Y.; Naranmandura, H. Effect of arsenic compounds on the in vitro differentiation of mouse embryonic stem cells into cardiomyocytes. Chem. Res. Toxicol. 2015, 28, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Farrell, K.; Lee, M.Y.; Kothapalli, C.R. Sensitivity of neural stem cell survival, differentiation and neurite outgrowth within 3D hydrogels to environmental heavy metals. Toxicol. Lett. 2016, 242, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Engstrom, A.K.; Xia, Z. Cadmium impairs the survival and proliferation of cultured adult subventricular neural stem cells through activation of the JNK and p38 MAP kinases. Toxicology 2017, 380, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Gadhia, S.R.; Calabro, A.R.; Barile, F.A. Trace metals alter DNA repair and histone modification pathways concurrently in mouse embryonic stem cells. Toxicol. Lett. 2012, 212, 169–179. [Google Scholar] [CrossRef]
- Koyama, H.; Kamogashira, T.; Yamasoba, T. Heavy Metal Exposure: Molecular Pathways, Clinical Implications, and Protective Strategies. Antioxidants 2024, 13, 76. [Google Scholar] [CrossRef]
- Sikora, E.; Bielak-Zmijewska, A.; Dudkowska, M.; Krzystyniak, A.; Mosieniak, G.; Wesierska, M.; Wlodarczyk, J. Cellular Senescence in Brain Aging. Front. Aging Neurosci. 2021, 13, 646924. [Google Scholar] [CrossRef]
- Vielee, S.T.; Wise, J.P., Jr. Among Gerontogens, Heavy Metals Are a Class of Their Own: A Review of the Evidence for Cellular Senescence. Brain Sci. 2023, 13, 500. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, P.; Ling, X.; Zhou, L. Advancements in therapeutic drugs targeting of senescence. Ther. Adv. Chronic Dis. 2020, 11, 2040622320964125. [Google Scholar] [CrossRef]
- Flora, S.J.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef]
- Abu-El-Zahab, H.S.H.; Hamza, R.Z.; Montaser, M.M.; El-Mahdi, M.M.; Al-Harthi, W.A. Antioxidant, antiapoptotic, antigenotoxic, and hepatic ameliorative effects of L-carnitine and selenium on cadmium-induced hepatotoxicity and alterations in liver cell structure in male mice. Ecotoxicol. Environ. Saf. 2019, 173, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]

| Heavy Metal | Country | Phenotypic Outcome | Tissue Methylation Measured | Reference |
|---|---|---|---|---|
| Arsenic (As) | Norway | No association observed between maternal As levels and 5 mC in pregnant mothers or newborns | Cord blood and maternal whole blood | [65] |
| Lead (Pb) | Mexico | No associations were reported between cord blood Pb and cord blood genomic DNA methylation | Leukocyte DNA from umbilical cord blood samples | [66] |
| No association of prenatal Pb exposures with telomere length and DNA-methylation-based predictors of age in cord blood | Whole cord blood | [67] | ||
| Mercury (Hg) | USA | No association reported between prenatal maternal RBC-Hg and 5-methylcytosine (%-5mC) | Whole cord blood | [68] |
| Faroe Islands, Denmark | Most of differentially methylated CpG sites (214 sites) were not associated with MeHg | Total cord blood | [69] | |
| Iron | Several European countries | Cord blood serum ferritin concentrations were not associated with cord blood DNA methylation levels at three identified CpGs: two CpGs (cg02806645 and cg06322988) in PRR23A and one CpG (cg04468817) in PRSS22 | Whole cord blood | [70] |
| Heavy Metal | Country | Key Attributes | Key Epigenetic Outcome | Reference |
| Arsenic (As) | Bangladesh | Cancer-related genes | Decrease in DNA methylation in boys | [82] |
| Bangladesh | DNA methylation in cord blood adjusting for leukocyte-tagged differentially methylated regions | Significantly associated with leukocyte subpopulations, specifically CD+4 and CD+8 populations | [55] | |
| Mercury (Hg) | Japan | HDHD1 gene that encodes pseudouridine-5′-phosphatase (PUDP) | DNA methylation within HDHD1 increased with Hg concentration | [83] |
| Korea | Hg levels were measured in cord blood to elucidate the association between prenatal and early childhood Hg exposure and autistic behaviors in preschool-age children | Hg concentrations at late pregnancy, in cord blood, and at 2 and 3 years of age were associated with autistic behaviors at 5 years of age | [139] | |
| Croatia and Italy | Hg from fish consumption during pregnancy on newborn’s brain development and child neurodevelopment using UCB | Morphological changes in the brains of newborns were detected on Hg exposure. The width of the frontal gyrus was greater in the exposed group and the length of the cerebellum narrower than in the unexposed group | [141] | |
| USA | Global 5-hydroxymethylcytosine (%-5hmC) and 5-methylcytosine(%-5mC) DNA content in blood in preterm birth cohort | Hg exposure was associated with lower global %-5hmC DNA content in cord blood | [68] | |
| Spain, Korea, USA, Japan, UK, Norway, Greece | Differential DNAm at cg24184221 in MED31 (gene involved in lipid metabolism and RNA Pol II function) in relation to prenatal MeHg exposure | Hypo/hypermethylation of several genes involved in growth and cell cycle processes during fetal development | [98] | |
| USA | DNA methylation changes in a genomic region of the Paraoxonase 1 (PON1) gene | Higher DNA methylation levels of the PON1 region were associated with lower cognitive test scores in early childhood for both sexes | [99] | |
| As and Hg co-exposure | USA | There is a high proportion of loci located in CpG islands and in south shore regions and all these loci were hypermethylated | A decrease in the proportion of monocytes and an increase in B-cell population in female infants | [96] |
| Tanzania | Genes affecting placental function, oxidative stress, and fetal growth | As and Hg exposure was related to an increased incidence of spontaneous abortion, stillbirth/preterm birth, etc. | [100] | |
| Heavy Metal | Country | Gene/Element | Epigenetic Outcome | Reference |
| Cadmium (Cd) | USA | Genes that encode for proteins that control transcriptional regulation and apoptosis | Differentially methylated genes showed hypermethylation | [115] |
| Korea | Two differentially methylated CpG sites, cg05537752 and cg24904393 | Fetus susceptible to Cd-induced epigenetic modifications (trimester specific) | [116] | |
| Bangladesh | CpG sites that were positively associated with Cd were inversely correlated with birth weight | In girls, methylation changes in genes related to organ development, morphology, and mineralization of bone; in boys it was cell death-related genes | [117] | |
| Bangladesh | Six differentially methylated CpG sites (DMPs) in the children’s PBMCs were associated with gestational Cd exposure and 11 DMPs with the children’s long-term Cd exposure | Gestational Cd exposure leads to hypomethylation of DMRs and adverse outcomes in children | [118] | |
| USA | In newborn cord blood and maternal blood, 641 and 1,945 Cd-associated DMRs were identified, respectively | The top three functional categories for genes affected were body mass index (BMI), blood pressure, and body weight | [119] | |
| Korea | A decrease in gestational age was observed by DNA methylation at a specific CpG site, cg21010642 | The CpG site was annotated to a gene involved in early embryonic development, thus causing preterm birth | [120] | |
| USA | Elevated maternal Cd levels were associated with higher DNA methylation at the DMR regulating PEG3, and less consistently at IGF2/H19 and MEG3 | Elevated maternal blood Cd levels were associated with lower birth weight | [153] | |
| Lead | USA | Mothers with high neonatal blood Pb levels correlate with altered DNA methylation at 564 loci in their children’s neonatal blood | Memory loss and loss of cognitive function | [131] |
| Mexico | Several 5hmC and 5mC clusters as potential candidates for sex-specific epigenetic biomarkers for prenatal Pb exposure | Genes for neurodevelopment | [166] | |
| Mexico | An inverse dose–response relationship was observed of patella Pb with UCB methylation | The epigenome of the developing fetus is influenced by maternal cumulative Pb burdens, leading to disease susceptibility throughout life course | [66] | |
| USA | Pb concentrations in RBCs from prenatal maternal blood samples were measured and genome-wide methylation levels at 482,397 CpG loci in UCB were analyzed | In utero Pb exposure gave rise to altered methylation levels at CpGs located within or near important regulatory genes of hematopoietic and nervous functions (i.e., CLEC11A and DNHD1) | [142] | |
| Heavy metal co-exposure | Japan | Prenatal exposure—As, Cd, Hg, Pb, antimony (Sb)—with global DNA methylation in UCB DNA were determined | Significant positive correlations were observed among Pb levels, maternal age, and hmC content; consistent positive associations between Pb and Sb levels and mC and hmC content were observed; these are biomarkers for future disease risks | [133] |
| China | Pb, Cd, Mn, and Cr on neonatal DNA methylation patterns | Genes involved in neurodevelopment were affected | [134] | |
| China | A panel of 16 metals and 3 oxidative stress biomarkers were measured; Alu and LINE-in cord blood were analyzed | Positive associations were observed between As, Cd, Tl, Ba, Ni, V, Co, Zn, Cu, Se, and Mo and at least one oxidative stress biomarker | [135] | |
| USA | Prenatal metal exposure was associated with DNAm, including DMRs annotated to genes involved in neurodevelopment | [136] | ||
| Mexico | The authors investigated the impact of prenatal metal exposure (As, Cu, Hg, Mn, Mo, Pb, Se, and Zn) on DNA methylation and mRNA expression in 181 umbilical cord blood samples | DNA methylation is affected by prenatal metal exposure, which could cause alterations in the expression of repair genes, leading to lower capacity for DNA damage repair in newborns | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, S.; Ruden, D.M. Heavy Metals in Umbilical Cord Blood: Effects on Epigenetics and Child Development. Cells 2024, 13, 1775. https://doi.org/10.3390/cells13211775
Dutta S, Ruden DM. Heavy Metals in Umbilical Cord Blood: Effects on Epigenetics and Child Development. Cells. 2024; 13(21):1775. https://doi.org/10.3390/cells13211775
Chicago/Turabian StyleDutta, Sudipta, and Douglas M. Ruden. 2024. "Heavy Metals in Umbilical Cord Blood: Effects on Epigenetics and Child Development" Cells 13, no. 21: 1775. https://doi.org/10.3390/cells13211775
APA StyleDutta, S., & Ruden, D. M. (2024). Heavy Metals in Umbilical Cord Blood: Effects on Epigenetics and Child Development. Cells, 13(21), 1775. https://doi.org/10.3390/cells13211775








