Islet Transplantation: Current Limitations and Challenges for Successful Outcomes
Abstract
1. Introduction
2. Quality of Islet Preparation for Transplantation: Where Do We Stand?
2.1. Importance of the ECM for Isolation Effectiveness and β-Cell Function
2.2. Ischemia: The Dark Side of Islet Isolation Success
3. ER Stress Has Side Effects on Pancreatic β-Cells
4. Mitochondrial Dysfunction in Pancreatic β-Cells Leads to Impaired Insulin Secretion and Apoptosis
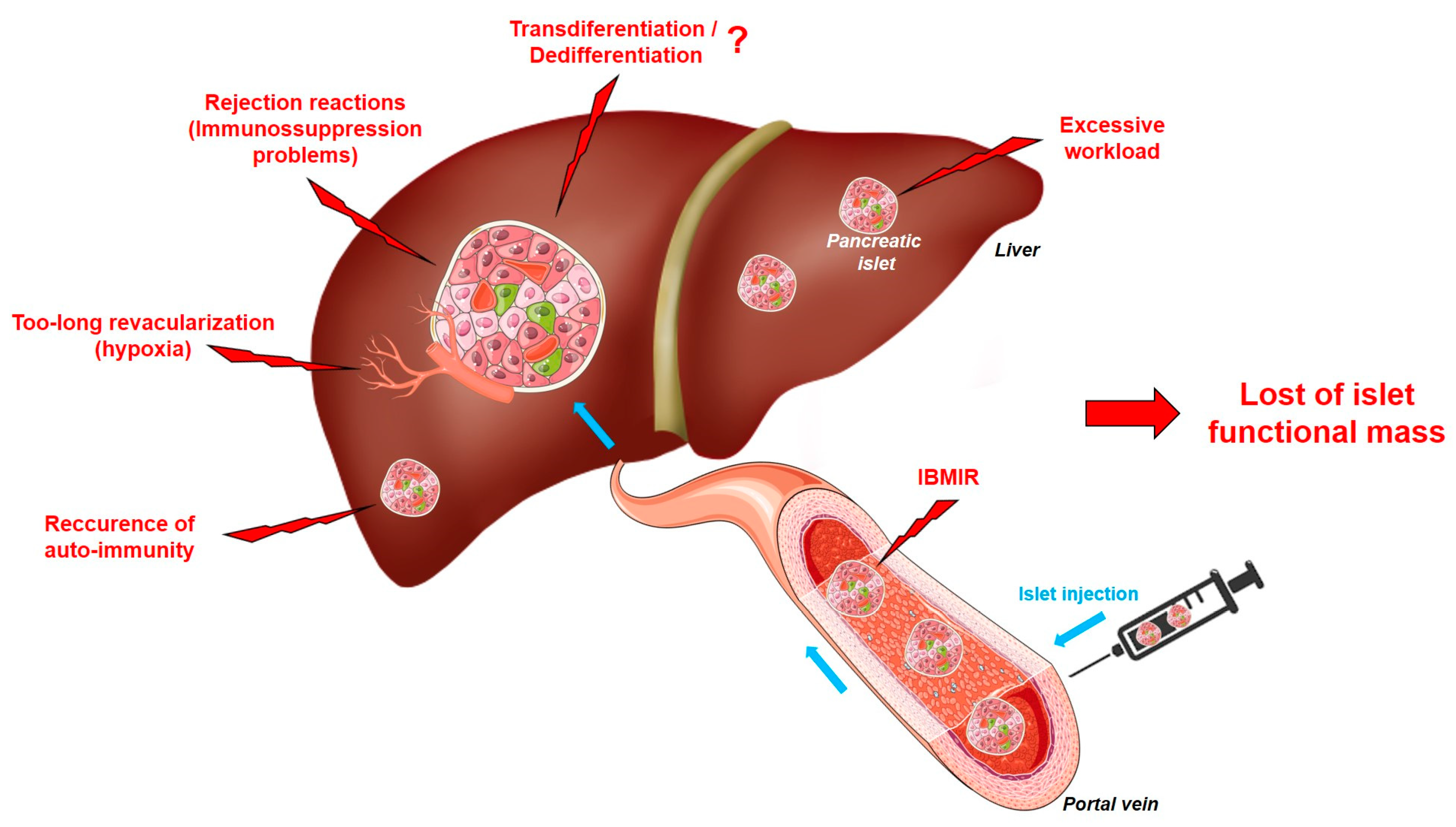
5. The Limits of Islet Transplantation: Where Do We Stand?
5.1. Islet Transplantation and Immunosuppression
5.2. IBMIR
5.3. Islet Revascularization
5.4. Insulin Is Needed After Transplantation to Protect Islets from Their New Environment
5.5. Could the Efficacy of Islet Transplantation Be Affected by Cellular Transdifferentiation/Dedifferentiation?
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ilonen, J.; Lempainen, J.; Veijola, R. The Heterogeneous Pathogenesis of Type 1 Diabetes Mellitus. Nat. Rev. Endocrinol. 2019, 15, 635–650. [Google Scholar] [CrossRef]
- Lablanche, S.; Borot, S.; Wojtusciszyn, A.; Skaare, K.; Penfornis, A.; Malvezzi, P.; Badet, L.; Thivolet, C.; Morelon, E.; Buron, F.; et al. Ten-Year Outcomes of Islet Transplantation in Patients with Type 1 Diabetes: Data from the Swiss-French GRAGIL Network. Am. J. Transplant. 2021, 21, 3725–3733. [Google Scholar] [CrossRef] [PubMed]
- Rickels, M.R.; Eggerman, T.L.; Bayman, L.; Qidwai, J.C.; Alejandro, R.; Bridges, N.D.; Hering, B.J.; Markmann, J.F.; Senior, P.A.; Hunsicker, L.G.; et al. Long-Term Outcomes with Islet-Alone and Islet-After-Kidney Transplantation for Type 1 Diabetes in the Clinical Islet Transplantation Consortium: The CIT-08 Study. Diabetes Care 2022, 45, 2967–2975. [Google Scholar] [CrossRef] [PubMed]
- Chetboun, M.; Drumez, E.; Ballou, C.; Maanaoui, M.; Payne, E.; Barton, F.; Kerr-Conte, J.; Vantyghem, M.-C.; Piemonti, L.; Rickels, M.R.; et al. Association Between Primary Graft Function and 5-Year Outcomes of Islet Allogeneic Transplantation in Type 1 Diabetes: A Retrospective, Multicentre, Observational Cohort Study in 1210 Patients from the Collaborative Islet Transplant Registry. Lancet Diabetes Endocrinol. 2023, 11, 391–401. [Google Scholar] [CrossRef]
- Shapiro, A.M.J.; Pokrywczynska, M.; Ricordi, C. Clinical Pancreatic Islet Transplantation. Nat. Rev. Endocrinol. 2017, 13, 268–277. [Google Scholar] [CrossRef]
- Fiorina, P.; Folli, F.; Zerbini, G.; Maffi, P.; Gremizzi, C.; Di Carlo, V.; Socci, C.; Bertuzzi, F.; Kashgarian, M.; Secchi, A. Islet Transplantation Is Associated with Improvement of Renal Function Among Uremic Patients with Type I Diabetes Mellitus and Kidney Transplants. J. Am. Soc. Nephrol. 2003, 14, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Maanaoui, M.; Lenain, R.; Foucher, Y.; Buron, F.; Blancho, G.; Antoine, C.; Caillard, S.; Kessler, L.; Le Quintrec, M.; Villard, O.; et al. Islet-After-Kidney Transplantation Versus Kidney Alone in Kidney Transplant Recipients with Type 1 Diabetes (KAIAK): A Population-Based Target Trial Emulation in France. Lancet Diabetes Endocrinol. 2024, 12, 716–724. [Google Scholar] [CrossRef]
- Mailliez, A.; Ternynck, C.; Jannin, A.; Lemaître, M.; Chevalier, B.; Le Mapihan, K.; Defrance, F.; Mackowiak, M.-A.; Rollin, A.; Mehdi, M.; et al. Cognitive Outcome After Islet Transplantation. Transplant. Direct 2023, 9, e1493. [Google Scholar] [CrossRef]
- Merani, S.; Shapiro, A.M.J. Current Status of Pancreatic Islet Transplantation. Clin. Sci. 2006, 110, 611–625. [Google Scholar] [CrossRef]
- Vantyghem, M.-C.; de Koning, E.J.P.; Pattou, F.; Rickels, M.R. Advances in β-Cell Replacement Therapy for the Treatment of Type 1 Diabetes. Lancet 2019, 394, 1274–1285. [Google Scholar] [CrossRef]
- Forbes, S.; Flatt, A.J.; Bennett, D.; Crookston, R.; Pimkova, M.; Birtles, L.; Pernet, A.; Wood, R.C.; Burling, K.; Barker, P.; et al. The Impact of Islet Mass, Number of Transplants, and Time Between Transplants on Graft Function in a National Islet Transplant Program. Am. J. Transplant. 2022, 22, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Rickels, M.R.; Stock, P.G.; de Koning, E.J.P.; Piemonti, L.; Pratschke, J.; Alejandro, R.; Bellin, M.D.; Berney, T.; Choudhary, P.; Johnson, P.R.; et al. Defining Outcomes for β-Cell Replacement Therapy in the Treatment of Diabetes: A Consensus Report on the Igls Criteria from the IPITA/EPITA Opinion Leaders Workshop. Transpl. Int. 2018, 31, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Marfil-Garza, B.A.; Shapiro, A.M.J.; Kin, T. Clinical Islet Transplantation: Current Progress and New Frontiers. J. Hepatobiliary Pancreat. Sci. 2021, 28, 243–254. [Google Scholar] [CrossRef]
- Naziruddin, B.; Iwahashi, S.; Kanak, M.A.; Takita, M.; Itoh, T.; Levy, M.F. Evidence for Instant Blood-Mediated Inflammatory Reaction in Clinical Autologous Islet Transplantation. Am. J. Transplant. 2014, 14, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Miki, A.; Ricordi, C.; Sakuma, Y.; Yamamoto, T.; Misawa, R.; Mita, A.; Molano, R.D.; Vaziri, N.D.; Pileggi, A.; Ichii, H. Divergent Antioxidant Capacity of Human Islet Cell Subsets: A Potential Cause of Beta-Cell Vulnerability in Diabetes and Islet Transplantation. PLoS ONE 2018, 13, e0196570. [Google Scholar] [CrossRef]
- Marfil-Garza, B.A.; Imes, S.; Verhoeff, K.; Hefler, J.; Lam, A.; Dajani, K.; Anderson, B.; O’Gorman, D.; Kin, T.; Bigam, D.; et al. Pancreatic Islet Transplantation in Type 1 Diabetes: 20-Year Experience from a Single-Centre Cohort in Canada. Lancet Diabetes Endocrinol. 2022, 10, 519–532. [Google Scholar] [CrossRef]
- Nalbach, L.; Roma, L.P.; Schmitt, B.M.; Becker, V.; Körbel, C.; Wrublewsky, S.; Pack, M.; Später, T.; Metzger, W.; Menger, M.M.; et al. Improvement of Islet Transplantation by the Fusion of Islet Cells with Functional Blood Vessels. EMBO Mol. Med. 2021, 13, e12616. [Google Scholar] [CrossRef]
- Porter, J.M.; Guerassimoff, L.; Castiello, F.R.; Charette, A.; Tabrizian, M. INGAP-Peptide Variants as a Novel Therapy for Type 1 Diabetes: Effect on Human Islet Insulin Secretion and Gene Expression. Pharmaceutics 2022, 14, 1833. [Google Scholar] [CrossRef]
- Montanari, E.; Gonelle-Gispert, C.; Seebach, J.D.; Knoll, M.F.; Bottino, R.; Bühler, L.H. Immunological Aspects of Allogeneic Pancreatic Islet Transplantation: A Comparison Between Mouse and Human. Transpl. Int. 2019, 32, 903–912. [Google Scholar] [CrossRef]
- Kale, A.; Rogers, N.M. No Time to Die-How Islets Meet Their Demise in Transplantation. Cells 2023, 12, 796. [Google Scholar] [CrossRef]
- Meier, R.P.H.; Meyer, J.; Muller, Y.D.; Szot, G.L.; Bédat, B.; Andres, A.; Massé, N.; Lablanche, S.; Puppa, G.; Bosco, D.; et al. Pancreas Collagen Digestion During Islet of Langerhans Isolation—A Prospective Study. Transpl. Int. 2020, 33, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, D.; Brandhorst, H.; Acreman, S.; Johnson, P.R.V. The Ischaemic Preconditioning Paradox and Its Implications for Islet Isolation from Heart-Beating and Non Heart-Beating Donors. Sci. Rep. 2022, 12, 19321. [Google Scholar] [CrossRef] [PubMed]
- Arous, C.; Wehrle-Haller, B. Role and Impact of the Extracellular Matrix on Integrin-Mediated Pancreatic β-Cell Functions. Biol. Cell 2017, 109, 223–237. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, M.; Xu, W.; Zhang, Y.; Pan, L.; Wang, L.; Wang, F.; Lu, Y. The Collagen Matrix Regulates the Survival and Function of Pancreatic Islets. Endocrine 2024, 83, 537–547. [Google Scholar] [CrossRef]
- Llacua, L.A.; Faas, M.M.; de Vos, P. Extracellular Matrix Molecules and Their Potential Contribution to the Function of Transplanted Pancreatic Islets. Diabetologia 2018, 61, 1261–1272. [Google Scholar] [CrossRef]
- Arous, C.; Mizgier, M.L.; Rickenbach, K.; Pinget, M.; Bouzakri, K.; Wehrle-Haller, B. Integrin and Autocrine IGF2 Pathways Control Fasting Insulin Secretion in β-Cells. J. Biol. Chem. 2020, 295, 16510–16528. [Google Scholar] [CrossRef]
- Barillaro, M.; Schuurman, M.; Wang, R. β1-Integrin-A Key Player in Controlling Pancreatic Beta-Cell Insulin Secretion via Interplay with SNARE Proteins. Endocrinology 2022, 164, bqac179. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, D.C.; Gonelle-Gispert, C.; Furukawa, M.; Halban, P.A.; Pessin, J.E. Glucose-Stimulated Insulin Secretion Is Coupled to the Interaction of Actin with the t-SNARE (Target Membrane Soluble N-Ethylmaleimide-Sensitive Factor Attachment Protein Receptor Protein) Complex. Mol. Endocrinol. 2003, 17, 732–742. [Google Scholar] [CrossRef]
- Gibly, R.F.; Graham, J.G.; Luo, X.; Lowe, W.L.; Hering, B.J.; Shea, L.D. Advancing Islet Transplantation: From Engraftment to the Immune Response. Diabetologia 2011, 54, 2494–2505. [Google Scholar] [CrossRef]
- Doherty, D.T.; Khambalia, H.A.; van Dellen, D.; Jennings, R.E.; Piper Hanley, K. Unlocking the Post-Transplant Microenvironment for Successful Islet Function and Survival. Front. Endocrinol. 2023, 14, 1250126. [Google Scholar] [CrossRef]
- Santini-González, J.; Simonovich, J.A.; Castro-Gutiérrez, R.; González-Vargas, Y.; Abuid, N.J.; Stabler, C.L.; Russ, H.A.; Phelps, E.A. In Vitro Generation of Peri-Islet Basement Membrane-like Structures. Biomaterials 2021, 273, 120808. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, D.; Brandhorst, H.; Lee Layland, S.; Acreman, S.; Schenke-Layland, K.; Johnson, P.R.V. Basement Membrane Proteins Improve Human Islet Survival in Hypoxia: Implications for Islet Inflammation. Acta Biomater. 2022, 137, 92–102. [Google Scholar] [CrossRef]
- Brandhorst, D.; Brandhorst, H.; Acreman, S.; Johnson, P.R.V. Perlecan: An Islet Basement Membrane Protein with Protective Anti-Inflammatory Characteristics. Bioengineering 2024, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.E.M.; Marinho, T.S.; Martins, F.F.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Treatment with Semaglutide, a GLP-1 Receptor Agonist, Improves Extracellular Matrix Remodeling in the Pancreatic Islet of Diet-Induced Obese Mice. Life Sci. 2023, 319, 121502. [Google Scholar] [CrossRef] [PubMed]
- Langlois, A.; Dumond, A.; Vion, J.; Pinget, M.; Bouzakri, K. Crosstalk Communications Between Islets Cells and Insulin Target Tissue: The Hidden Face of Iceberg. Front. Endocrinol. 2022, 13, 836344. [Google Scholar] [CrossRef]
- Daniel, B.; Livne, A.; Cohen, G.; Kahremany, S.; Sasson, S. Endothelial Cell–Derived Triosephosphate Isomerase Attenuates Insulin Secretion From Pancreatic Beta Cells of Male Rats. Endocrinology 2021, 162, bqaa234. [Google Scholar] [CrossRef]
- Hellman, B.; Idahl, L.A.; Danielsson, A. Adenosine Triphosphate Levels of Mammalian Pancreatic B Cells After Stimulation with Glucose and Hypoglycemic Sulfonylureas. Diabetes 1969, 18, 509–516. [Google Scholar] [CrossRef]
- Dionne, K.E.; Colton, C.K.; Yarmush, M.L. Effect of Hypoxia on Insulin Secretion by Isolated Rat and Canine Islets of Langerhans. Diabetes 1993, 42, 12–21. [Google Scholar] [CrossRef]
- Komatsu, H.; Kang, D.; Medrano, L.; Barriga, A.; Mendez, D.; Rawson, J.; Omori, K.; Ferreri, K.; Tai, Y.-C.; Kandeel, F.; et al. Isolated Human Islets Require Hyperoxia to Maintain Islet Mass, Metabolism, and Function. Biochem. Biophys. Res. Commun. 2016, 470, 534–538. [Google Scholar] [CrossRef]
- Kaddis, J.S.; Danobeitia, J.S.; Niland, J.C.; Stiller, T.; Fernandez, L.A. Multicenter Analysis of Novel and Established Variables Associated with Successful Human Islet Isolation Outcomes. Am. J. Transplant. 2010, 10, 646–656. [Google Scholar] [CrossRef]
- Friberg, A.S.; Lundgren, T.; Malm, H.; Felldin, M.; Nilsson, B.; Jenssen, T.; Kyllönen, L.; Tufveson, G.; Tibell, A.; Korsgren, O. Transplanted Functional Islet Mass: Donor, Islet Preparation, and Recipient Factors Influence Early Graft Function in Islet-After-Kidney Patients. Transplantation 2012, 93, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Danielson, K.K.; Ropski, A.; Harvat, T.; Barbaro, B.; Paushter, D.; Qi, M.; Oberholzer, J. Systematic Analysis of Donor and Isolation Factor’s Impact on Human Islet Yield and Size Distribution. Cell Transplant. 2013, 22, 2323–2333. [Google Scholar] [CrossRef]
- Reich, D.J.; Mulligan, D.C.; Abt, P.L.; Pruett, T.L.; Abecassis, M.M.I.; D’Alessandro, A.; Pomfret, E.A.; Freeman, R.B.; Markmann, J.F.; Hanto, D.W.; et al. ASTS Recommended Practice Guidelines for Controlled Donation After Cardiac Death Organ Procurement and Transplantation. Am. J. Transplant. 2009, 9, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Corlett, M.P.; Scharp, D.W. The Effect of Pancreatic Warm Ischemia on Islet Isolation in Rats and Dogs. J. Surg. Res. 1988, 45, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Wassmer, C.-H.; Lebreton, F.; Bellofatto, K.; Perez, L.; Cottet-Dumoulin, D.; Andres, A.; Bosco, D.; Berney, T.; Othenin-Girard, V.; Martinez De Tejada, B.; et al. Bio-Engineering of Pre-Vascularized Islet Organoids for the Treatment of Type 1 Diabetes. Transpl. Int. 2021, 35, 10214. [Google Scholar] [CrossRef]
- Berney, T.; Boffa, C.; Augustine, T.; Badet, L.; de Koning, E.; Pratschke, J.; Socci, C.; Friend, P. Utilization of Organs from Donors After Circulatory Death for Vascularized Pancreas and Islet of Langerhans Transplantation: Recommendations from an Expert Group. Transpl. Int. 2016, 29, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.F.; Liu, A.W.; Peters, M.J.; Willard, J.R.; Rabbani, Z.; Bartholomew, E.C.; Ottley, A.; Hull, R.L. Markers of Islet Endothelial Dysfunction Occur in Male B6.BKS(D)-Leprdb/J Mice and May Contribute to Reduced Insulin Release. Endocrinology 2017, 158, 293–303. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef]
- Vivot, K.; Langlois, A.; Bietiger, W.; Dal, S.; Seyfritz, E.; Pinget, M.; Jeandidier, N.; Maillard, E.; Gies, J.-P.; Sigrist, S. Pro-Inflammatory and Pro-Oxidant Status of Pancreatic Islet In Vitro Is Controlled by TLR-4 and HO-1 Pathways. PLoS ONE 2014, 9, e107656. [Google Scholar] [CrossRef]
- Cardozo, A.K.; Ortis, F.; Storling, J.; Feng, Y.-M.; Rasschaert, J.; Tonnesen, M.; Van Eylen, F.; Mandrup-Poulsen, T.; Herchuelz, A.; Eizirik, D.L. Cytokines Downregulate the Sarcoendoplasmic Reticulum Pump Ca2+ ATPase 2b and Deplete Endoplasmic Reticulum Ca2+, Leading to Induction of Endoplasmic Reticulum Stress in Pancreatic Beta-Cells. Diabetes 2005, 54, 452–461. [Google Scholar] [CrossRef]
- Fischer, F.; Hamann, A.; Osiewacz, H.D. Mitochondrial Quality Control: An Integrated Network of Pathways. Trends Biochem. Sci. 2012, 37, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, K.; Nishiwaki, C.; Nakamichi, Y.; Yamashita, S.-I.; Kanki, T.; Ohara-Imaizumi, M. Imeglimin Mitigates the Accumulation of Dysfunctional Mitochondria to Restore Insulin Secretion and Suppress Apoptosis of Pancreatic β-Cells from db/db Mice. Sci. Rep. 2024, 14, 6178. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-Cells in Type 1 and Type 2 Diabetes Mellitus: Different Pathways to Failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Perkins, H.T.; Allan, V. Intertwined and Finely Balanced: Endoplasmic Reticulum Morphology, Dynamics, Function, and Diseases. Cells 2021, 10, 2341. [Google Scholar] [CrossRef]
- Kotler, J.L.M.; Street, T.O. Mechanisms of Protein Quality Control in the Endoplasmic Reticulum by a Coordinated Hsp40-Hsp70-Hsp90 System. Annu. Rev. Biophys. 2023, 52, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Diane, A.; Allouch, A.; Mu-U-Min, R.B.A.; Al-Siddiqi, H.H. Endoplasmic Reticulum Stress in Pancreatic β-Cell Dysfunctionality and Diabetes Mellitus: A Promising Target for Generation of Functional hPSC-Derived β-Cells In Vitro. Front. Endocrinol. 2024, 15, 1386471. [Google Scholar] [CrossRef]
- Cnop, M.; Toivonen, S.; Igoillo-Esteve, M.; Salpea, P. Endoplasmic Reticulum Stress and eIF2α Phosphorylation: The Achilles Heel of Pancreatic β Cells. Mol. Metab. 2017, 6, 1024–1039. [Google Scholar] [CrossRef]
- Yong, J.; Johnson, J.D.; Arvan, P.; Han, J.; Kaufman, R.J. Therapeutic Opportunities for Pancreatic β-Cell ER Stress in Diabetes Mellitus. Nat. Rev. Endocrinol. 2021, 17, 455–467. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J. Endoplasmic Reticulum (ER) Stress and Its Role in Pancreatic β-Cell Dysfunction and Senescence in Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 4843. [Google Scholar] [CrossRef]
- Feliziani, C.; Fernandez, M.; Quassollo, G.; Holstein, D.; Bairo, S.M.; Paton, J.C.; Paton, A.W.; de Batista, J.; Lechleiter, J.D.; Bollo, M. Ca2+ Signalling System Initiated by Endoplasmic Reticulum Stress Stimulates PERK Activation. Cell Calcium 2022, 106, 102622. [Google Scholar] [CrossRef]
- Merrins, M.J.; Corkey, B.E.; Kibbey, R.G.; Prentki, M. Metabolic Cycles and Signals for Insulin Secretion. Cell Metab. 2022, 34, 947–968. [Google Scholar] [CrossRef] [PubMed]
- Scheuner, D.; Vander Mierde, D.; Song, B.; Flamez, D.; Creemers, J.W.M.; Tsukamoto, K.; Ribick, M.; Schuit, F.C.; Kaufman, R.J. Control of mRNA Translation Preserves Endoplasmic Reticulum Function in Beta Cells and Maintains Glucose Homeostasis. Nat. Med. 2005, 11, 757–764. [Google Scholar] [CrossRef]
- Morikawa, S.; Urano, F. The Role of ER Stress in Diabetes: Exploring Pathological Mechanisms Using Wolfram Syndrome. Int. J. Mol. Sci. 2022, 24, 230. [Google Scholar] [CrossRef]
- McCall, M.; Shapiro, A.M.J. Update on Islet Transplantation. Cold Spring Harb. Perspect. Med. 2012, 2, a007823. [Google Scholar] [CrossRef] [PubMed]
- Plemper, R.K.; Böhmler, S.; Bordallo, J.; Sommer, T.; Wolf, D.H. Mutant Analysis Links the Translocon and BiP to Retrograde Protein Transport for ER Degradation. Nature 1997, 388, 891–895. [Google Scholar] [CrossRef]
- Brodsky, J.L.; Werner, E.D.; Dubas, M.E.; Goeckeler, J.L.; Kruse, K.B.; McCracken, A.A. The Requirement for Molecular Chaperones During Endoplasmic Reticulum-Associated Protein Degradation Demonstrates That Protein Export and Import Are Mechanistically Distinct. J. Biol. Chem. 1999, 274, 3453–3460. [Google Scholar] [CrossRef]
- Omori, K.; Kobayashi, E.; Rawson, J.; Takahashi, M.; Mullen, Y. Mechanisms of Islet Damage Mediated by Pancreas Cold Ischemia/Rewarming. Cryobiology 2016, 73, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.A.; Sidarala, V.; Kaufman, B.A.; Soleimanpour, S.A. Mitochondrial Metabolism and Dynamics in Pancreatic Beta Cell Glucose Sensing. Biochem. J. 2023, 480, 773–789. [Google Scholar] [CrossRef]
- Maechler, P.; Wollheim, C.B. Mitochondrial Function in Normal and Diabetic Beta-Cells. Nature 2001, 414, 807–812. [Google Scholar] [CrossRef]
- Cantley, J.; Davenport, A.; Vetterli, L.; Nemes, N.J.; Whitworth, P.T.; Boslem, E.; Thai, L.M.; Mellett, N.; Meikle, P.J.; Hoehn, K.L.; et al. Disruption of Beta Cell Acetyl-CoA Carboxylase-1 in Mice Impairs Insulin Secretion and Beta Cell Mass. Diabetologia 2019, 62, 99–111. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Rorsman, P. ATP-Sensitive K+ Channels: A Link Between B-Cell Metabolism and Insulin Secretion. Biochem. Soc. Trans. 1990, 18, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, K.; Yamashita, S.-I.; Akimoto, Y.; Nishiwaki, C.; Nakamichi, Y.; Udagawa, H.; Abe, M.; Sakimura, K.; Kanki, T.; Ohara-Imaizumi, M. A New Beta Cell-Specific Mitophagy Reporter Mouse Shows That Metabolic Stress Leads to Accumulation of Dysfunctional Mitochondria Despite Increased Mitophagy. Diabetologia 2023, 66, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Wiederkehr, A.; Szanda, G.; Akhmedov, D.; Mataki, C.; Heizmann, C.W.; Schoonjans, K.; Pozzan, T.; Spät, A.; Wollheim, C.B. Mitochondrial Matrix Calcium Is an Activating Signal for Hormone Secretion. Cell Metab. 2011, 13, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, E.; Haythorne, E.; Dickerson, M.T.; Lopez-Noriega, L.; Pullen, T.J.; da Silva Xavier, G.; Davis, S.P.X.; Martinez-Sanchez, A.; Semplici, F.; Rizzuto, R.; et al. The Pore-Forming Subunit MCU of the Mitochondrial Ca2+ Uniporter Is Required for Normal Glucose-Stimulated Insulin Secretion In Vitro and In Vivo in Mice. Diabetologia 2020, 63, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Kabra, U.D.; Jastroch, M. Mitochondrial Dynamics and Insulin Secretion. Int. J. Mol. Sci. 2023, 24, 13782. [Google Scholar] [CrossRef]
- Reinhardt, F.; Schultz, J.; Waterstradt, R.; Baltrusch, S. Drp1 Guarding of the Mitochondrial Network Is Important for Glucose-Stimulated Insulin Secretion in Pancreatic Beta Cells. Biochem. Biophys. Res. Commun. 2016, 474, 646–651. [Google Scholar] [CrossRef]
- Nan, J.; Lee, J.S.; Moon, J.H.; Lee, S.-A.; Park, Y.J.; Lee, D.-S.; Chung, S.S.; Park, K.S. SENP2 Regulates Mitochondrial Function and Insulin Secretion in Pancreatic β Cells. Exp. Mol. Med. 2022, 54, 72–80. [Google Scholar] [CrossRef]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef]
- Las, G.; Oliveira, M.F.; Shirihai, O.S. Emerging Roles of β-Cell Mitochondria in Type-2-Diabetes. Mol. Aspects Med. 2020, 71, 100843. [Google Scholar] [CrossRef]
- Li, J.; Inoue, R.; Togashi, Y.; Okuyama, T.; Satoh, A.; Kyohara, M.; Nishiyama, K.; Tsuno, T.; Miyashita, D.; Kin, T.; et al. Imeglimin Ameliorates β-Cell Apoptosis by Modulating the Endoplasmic Reticulum Homeostasis Pathway. Diabetes 2022, 71, 424–439. [Google Scholar] [CrossRef]
- Vial, G.; Chauvin, M.-A.; Bendridi, N.; Durand, A.; Meugnier, E.; Madec, A.-M.; Bernoud-Hubac, N.; Pais de Barros, J.-P.; Fontaine, É.; Acquaviva, C.; et al. Imeglimin Normalizes Glucose Tolerance and Insulin Sensitivity and Improves Mitochondrial Function in Liver of a High-Fat, High-Sucrose Diet Mice Model. Diabetes 2015, 64, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Dubourg, J.; Fouqueray, P.; Quinslot, D.; Grouin, J.-M.; Kaku, K. Long-Term Safety and Efficacy of Imeglimin as Monotherapy or in Combination with Existing Antidiabetic Agents in Japanese Patients with Type 2 Diabetes (TIMES 2): A 52-Week, Open-Label, Multicentre Phase 3 Trial. Diabetes Obes. Metab. 2022, 24, 609–619. [Google Scholar] [CrossRef]
- Sanada, J.; Obata, A.; Fushimi, Y.; Kimura, T.; Shimoda, M.; Ikeda, T.; Nogami, Y.; Obata, Y.; Yamasaki, Y.; Nakanishi, S.; et al. Imeglimin Exerts Favorable Effects on Pancreatic β-Cells by Improving Morphology in Mitochondria and Increasing the Number of Insulin Granules. Sci. Rep. 2022, 12, 13220. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Ono, M.; Tsuno, T.; Inoue, R.; Fukunaka, A.; Okuyama, T.; Kyohara, M.; Togashi, Y.; Fukushima, S.; Atsumi, T.; et al. Protective Effects of Imeglimin and Metformin Combination Therapy on β-Cells in db/db Male Mice. Endocrinology 2023, 164, bqad095. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Kredo-Russo, S.; Mandelbaum, A.D.; Ness, A.; Alon, I.; Lennox, K.A.; Behlke, M.A.; Hornstein, E. Pancreas-Enriched miRNA Refines Endocrine Cell Differentiation. Development 2012, 139, 3021–3031. [Google Scholar] [CrossRef]
- Latreille, M.; Herrmanns, K.; Renwick, N.; Tuschl, T.; Malecki, M.T.; McCarthy, M.I.; Owen, K.R.; Rülicke, T.; Stoffel, M. miR-375 Gene Dosage in Pancreatic β-Cells: Implications for Regulation of β-Cell Mass and Biomarker Development. J. Mol. Med. 2015, 93, 1159–1169. [Google Scholar] [CrossRef]
- Poy, M.N.; Eliasson, L.; Krutzfeldt, J.; Kuwajima, S.; Ma, X.; Macdonald, P.E.; Pfeffer, S.; Tuschl, T.; Rajewsky, N.; Rorsman, P.; et al. A Pancreatic Islet-Specific microRNA Regulates Insulin Secretion. Nature 2004, 432, 226–230. [Google Scholar] [CrossRef]
- Sims, E.K.; Lakhter, A.J.; Anderson-Baucum, E.; Kono, T.; Tong, X.; Evans-Molina, C. MicroRNA 21 Targets BCL2 mRNA to Increase Apoptosis in Rat and Human Beta Cells. Diabetologia 2017, 60, 1057–1065. [Google Scholar] [CrossRef]
- Krishnan, P.; Branco, R.C.S.; Weaver, S.A.; Chang, G.; Lee, C.-C.; Syed, F.; Evans-Molina, C. miR-146a-5p Mediates Inflammation-Induced β Cell Mitochondrial Dysfunction and Apoptosis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Landstra, C.P.; Nijhoff, M.F.; Roelen, D.L.; de Vries, A.P.J.; de Koning, E.J.P. Diagnosis and Treatment of Allograft Rejection in Islet Transplantation. Am. J. Transplant. 2023, 23, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Monti, P.; Vignali, D.; Piemonti, L. Monitoring Inflammation, Humoral and Cell-Mediated Immunity in Pancreas and Islet Transplants. Curr. Diabetes Rev. 2015, 11, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, A.E.; Donate-Correa, J.; Rovira, J.; Cuesto, G.; Luis-Ravelo, D.; Fernandes, M.X.; Acevedo-Arozena, A.; Diekmann, F.; Acebes, A.; Torres, A.; et al. Inhibition of the mTOR Pathway: A New Mechanism of β Cell Toxicity Induced by Tacrolimus. Am. J. Transplant. 2019, 19, 3240–3249. [Google Scholar] [CrossRef] [PubMed]
- Perrier, Q.; Cottet-Rouselle, C.; de-Beaumont, M.; Noble, J.; Lablanche, S. From the Clinical to the Bench: Exploring the Insulin Modulation Effects of Tacrolimus and Belatacept. Cell Transplant. 2024, 33, 09636897241246577. [Google Scholar] [CrossRef] [PubMed]
- Terrec, F.; Jouve, T.; Naciri-Bennani, H.; Benhamou, P.-Y.; Malvezzi, P.; Janbon, B.; Giovannini, D.; Rostaing, L.; Noble, J. Late Conversion From Calcineurin Inhibitors to Belatacept in Kidney-Transplant Recipients Has a Significant Beneficial Impact on Glycemic Parameters. Transplant. Direct 2020, 6, e517. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.R.N.; Poggioli, R.; Ambut, J.; Bozkurt, N.C.; Alvarez, A.M.; Padilla, N.; Vendrame, F.; Ricordi, C.; Baidal, D.A.; Alejandro, R. Impact of GAD65 and IA2 Autoantibodies on Islet Allograft Survival. Front. Clin. Diabetes Healthc. 2023, 4, 1269758. [Google Scholar] [CrossRef]
- Alibashe-Ahmed, M.; Brioudes, E.; Reith, W.; Bosco, D.; Berney, T. Toll-like Receptor 4 Inhibition Prevents Autoimmune Diabetes in NOD Mice. Sci. Rep. 2019, 9, 19350. [Google Scholar] [CrossRef]
- Hull, C.M.; Peakman, M.; Tree, T.I.M. Regulatory T Cell Dysfunction in Type 1 Diabetes: What’s Broken and How Can We Fix It? Diabetologia 2017, 60, 1839–1850. [Google Scholar] [CrossRef]
- Wu, X.; Chen, P.-I.; Whitener, R.L.; MacDougall, M.S.; Coykendall, V.M.N.; Yan, H.; Kim, Y.B.; Harper, W.; Pathak, S.; Iliopoulou, B.P.; et al. CD39 Delineates Chimeric Antigen Receptor Regulatory T Cell Subsets with Distinct Cytotoxic & Regulatory Functions Against Human Islets. Front. Immunol. 2024, 15, 1415102. [Google Scholar] [CrossRef]
- Cabello-Kindelan, C.; Mackey, S.; Sands, A.; Rodriguez, J.; Vazquez, C.; Pugliese, A.; Bayer, A.L. Immunomodulation Followed by Antigen-Specific Treg Infusion Controls Islet Autoimmunity. Diabetes 2020, 69, 215–227. [Google Scholar] [CrossRef]
- Ludwig, B.; Reichel, A.; Steffen, A.; Zimerman, B.; Schally, A.V.; Block, N.L.; Colton, C.K.; Ludwig, S.; Kersting, S.; Bonifacio, E.; et al. Transplantation of Human Islets Without Immunosuppression. Proc. Natl. Acad. Sci. USA 2013, 110, 19054–19058. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, P.-O.; Espes, D.; Sedigh, A.; Rotem, A.; Zimerman, B.; Grinberg, H.; Goldman, T.; Barkai, U.; Avni, Y.; Westermark, G.T.; et al. Transplantation of Macroencapsulated Human Islets Within the Bioartificial Pancreas βAir to Patients with Type 1 Diabetes Mellitus. Am. J. Transplant. 2018, 18, 1735–1744. [Google Scholar] [CrossRef]
- Berney, T.; Wassmer, C.H.; Lebreton, F.; Bellofatto, K.; Fonseca, L.M.; Bignard, J.; Hanna, R.; Peloso, A.; Berishvili, E. From Islet of Langerhans Transplantation to the Bioartificial Pancreas. Presse Med. 2022, 51, 104139. [Google Scholar] [CrossRef]
- Iacovacci, V.; Ricotti, L.; Menciassi, A.; Dario, P. The Bioartificial Pancreas (BAP): Biological, Chemical and Engineering Challenges. Biochem. Pharmacol. 2016, 100, 12–27. [Google Scholar] [CrossRef]
- Tunbridge, M.J.; Luo, X.; Thomson, A.W. Negative Vaccination Strategies for Promotion of Transplant Tolerance. Transplantation 2024, 108, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Lefaucheur, C. Antibody-Mediated Rejection of Solid-Organ Allografts. N. Engl. J. Med. 2018, 379, 1150–1160. [Google Scholar] [CrossRef]
- Haas, M.; Loupy, A.; Lefaucheur, C.; Roufosse, C.; Glotz, D.; Seron, D.; Nankivell, B.J.; Halloran, P.F.; Colvin, R.B.; Akalin, E.; et al. The Banff 2017 Kidney Meeting Report: Revised Diagnostic Criteria for Chronic Active T Cell-Mediated Rejection, Antibody-Mediated Rejection, and Prospects for Integrative Endpoints for Next-Generation Clinical Trials. Am. J. Transplant. 2018, 18, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Berney, T.; Mamin, A.; James Shapiro, A.M.; Ritz-Laser, B.; Brulhart, M.-C.; Toso, C.; Demuylder-Mischler, S.; Armanet, M.; Baertschiger, R.; Wojtusciszyn, A.; et al. Detection of Insulin mRNA in the Peripheral Blood After Human Islet Transplantion Predicts Deterioration of Metabolic Control. Am. J. Transplant. 2006, 6, 1704–1711. [Google Scholar] [CrossRef][Green Version]
- Kanak, M.A.; Takita, M.; Shahbazov, R.; Lawrence, M.C.; Chung, W.Y.; Dennison, A.R.; Levy, M.F.; Naziruddin, B. Evaluation of MicroRNA375 as a Novel Biomarker for Graft Damage in Clinical Islet Transplantation. Transplantation 2015, 99, 1568–1573. [Google Scholar] [CrossRef]
- Gala-Lopez, B.L.; Neiman, D.; Kin, T.; O’Gorman, D.; Pepper, A.R.; Malcolm, A.J.; Pianzin, S.; Senior, P.A.; Campbell, P.; Glaser, B.; et al. Beta Cell Death by Cell-Free DNA and Outcome After Clinical Islet Transplantation. Transplantation 2018, 102, 978–985. [Google Scholar] [CrossRef]
- Caldara, R.; Tomajer, V.; Monti, P.; Sordi, V.; Citro, A.; Chimienti, R.; Gremizzi, C.; Catarinella, D.; Tentori, S.; Paloschi, V.; et al. Allo Beta Cell Transplantation: Specific Features, Unanswered Questions, and Immunological Challenge. Front. Immunol. 2023, 14, 1323439. [Google Scholar] [CrossRef] [PubMed]
- Mattke, J.; Vasu, S.; Darden, C.M.; Kumano, K.; Lawrence, M.C.; Naziruddin, B. Role of Exosomes in Islet Transplantation. Front. Endocrinol. 2021, 12, 681600. [Google Scholar] [CrossRef] [PubMed]
- Turan, A.; Tarique, M.; Zhang, L.; Kazmi, S.; Ulker, V.; Tedla, M.G.; Badal, D.; Yolcu, E.S.; Shirwan, H. Engineering Pancreatic Islets to Transiently Codisplay on Their Surface Thrombomodulin and CD47 Immunomodulatory Proteins as a Means of Mitigating Instant Blood-Mediated Inflammatory Reaction Following Intraportal Transplantation. J. Immunol. 2024, 212, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B.; Ekdahl, K.N.; Korsgren, O. Control of Instant Blood-Mediated Inflammatory Reaction to Improve Islets of Langerhans Engraftment. Curr. Opin. Organ Transplant. 2011, 16, 620–626. [Google Scholar] [CrossRef]
- Hårdstedt, M.; Lindblom, S.; Karlsson-Parra, A.; Nilsson, B.; Korsgren, O. Characterization of Innate Immunity in an Extended Whole Blood Model of Human Islet Allotransplantation. Cell Transplant. 2016, 25, 503–515. [Google Scholar] [CrossRef]
- Johansson, H.; Goto, M.; Dufrane, D.; Siegbahn, A.; Elgue, G.; Gianello, P.; Korsgren, O.; Nilsson, B. Low Molecular Weight Dextran Sulfate: A Strong Candidate Drug to Block IBMIR in Clinical Islet Transplantation. Am. J. Transplant. 2006, 6, 305–312. [Google Scholar] [CrossRef]
- Kanak, M.A.; Takita, M.; Itoh, T.; SoRelle, J.A.; Murali, S.; Kunnathodi, F.; Shahbazov, R.; Lawrence, M.C.; Levy, M.F.; Naziruddin, B. Alleviation of Instant Blood-Mediated Inflammatory Reaction in Autologous Conditions Through Treatment of Human Islets with NF-κB Inhibitors. Transplantation 2014, 98, 578–584. [Google Scholar] [CrossRef]
- Hering, B.J.; Kandaswamy, R.; Ansite, J.D.; Eckman, P.M.; Nakano, M.; Sawada, T.; Matsumoto, I.; Ihm, S.-H.; Zhang, H.-J.; Parkey, J.; et al. Single-Donor, Marginal-Dose Islet Transplantation in Patients with Type 1 Diabetes. JAMA 2005, 293, 830–835. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Z.; Gou, W.; Adams, D.B.; Cui, W.; Morgan, K.A.; Strange, C.; Wang, H. α-1 Antitrypsin Enhances Islet Engraftment by Suppression of Instant Blood-Mediated Inflammatory Reaction. Diabetes 2017, 66, 970–980. [Google Scholar] [CrossRef]
- Turan, A.; Zhang, L.; Tarique, M.; Ulker, V.; Arguc, F.N.; Badal, D.; Yolcu, E.S.; Shirwan, H. Engineering Pancreatic Islets with a Novel Form of Thrombomodulin Protein to Overcome Early Graft Loss Triggered by Instant Blood-Mediated Inflammatory Reaction. Am. J. Transplant. 2023, 23, 619–628. [Google Scholar] [CrossRef]
- Pepper, A.R.; Gala-Lopez, B.; Ziff, O.; Shapiro, A.M.J. Revascularization of Transplanted Pancreatic Islets and Role of the Transplantation Site. Clin. Dev. Immunol. 2013, 2013, 352315. [Google Scholar] [CrossRef] [PubMed]
- Jansson, L.; Carlsson, P.-O. Graft Vascular Function After Transplantation of Pancreatic Islets. Diabetologia 2002, 45, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Wittig, C.; Laschke, M.W.; Scheuer, C.; Menger, M.D. Incorporation of Bone Marrow Cells in Pancreatic Pseudoislets Improves Posttransplant Vascularization and Endocrine Function. PLoS ONE 2013, 8, e69975. [Google Scholar] [CrossRef][Green Version]
- Aamodt, K.I.; Powers, A.C. Signals in the Pancreatic Islet Microenvironment Influence β-Cell Proliferation. Diabetes Obes. Metab. 2017, 19, 124–136. [Google Scholar] [CrossRef]
- Nikolova, G.; Strilic, B.; Lammert, E. The Vascular Niche and Its Basement Membrane. Trends Cell Biol. 2007, 17, 19–25. [Google Scholar] [CrossRef]
- Figliolini, F.; Cantaluppi, V.; De Lena, M.; Beltramo, S.; Romagnoli, R.; Salizzoni, M.; Melzi, R.; Nano, R.; Piemonti, L.; Tetta, C.; et al. Isolation, Characterization and Potential Role in Beta Cell-Endothelium Cross-Talk of Extracellular Vesicles Released from Human Pancreatic Islets. PLoS ONE 2014, 9, e102521. [Google Scholar] [CrossRef] [PubMed]
- Kado, T.; Tomimaru, Y.; Kobayashi, S.; Harada, A.; Sasaki, K.; Iwagami, Y.; Yamada, D.; Noda, T.; Takahashi, H.; Kita, S.; et al. Skeletal Myoblast Cells Enhance the Function of Transplanted Islets in Diabetic Mice. J. Diabetes Res. 2024, 2024, 5574968. [Google Scholar] [CrossRef]
- Reinert, R.B.; Brissova, M.; Shostak, A.; Pan, F.C.; Poffenberger, G.; Cai, Q.; Hundemer, G.L.; Kantz, J.; Thompson, C.S.; Dai, C.; et al. Vascular Endothelial Growth Factor-a and Islet Vascularization Are Necessary in Developing, but Not Adult, Pancreatic Islets. Diabetes 2013, 62, 4154–4164. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wan, J.; Xu, Y.; Huang, Y.; Wang, D.; Zhu, D.; Chen, Q.; Lu, Y.; Guo, Q. Endothelial Cells Promote Pseudo-islet Function Through BTC-EGFR-JAK/STAT Signaling Pathways. Ann. Biomed. Eng. 2024, 52, 2610–2626. [Google Scholar] [CrossRef]
- Piemonti, L. Islet Transplantation. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Walker, S.; Appari, M.; Forbes, S. Considerations and Challenges of Islet Transplantation and Future Therapies on the Horizon. Am. J. Physiol. Endocrinol. Metab. 2022, 322, E109–E117. [Google Scholar] [CrossRef]
- Shapey, I.M.; Summers, A.; Yiannoullou, P.; Khambalia, H.; Fullwood, C.; Hanley, N.A.; Casey, J.; Forbes, S.; Rosenthal, M.; Johnson, P.R.; et al. Donor Insulin Use Predicts Beta-Cell Function After Islet Transplantation. Diabetes Obes. Metab. 2020, 22, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Mazzucato, C.; Bonner-Weir, S. Pancreatic β Cell Regeneration as a Possible Therapy for Diabetes. Cell Metab. 2018, 27, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, C. Dedifferentiation: Inspiration for Devising Engineering Strategies for Regenerative Medicine. NPJ Regen. Med. 2020, 5, 14. [Google Scholar] [CrossRef]
- Ilegems, E.; Berggren, P.-O. The Eye as a Transplantation Site to Monitor Pancreatic Islet Cell Plasticity. Front. Endocrinol. 2021, 12, 652853. [Google Scholar] [CrossRef]
- Furuyama, K.; Chera, S.; van Gurp, L.; Oropeza, D.; Ghila, L.; Damond, N.; Vethe, H.; Paulo, J.A.; Joosten, A.M.; Berney, T.; et al. Diabetes Relief in Mice by Glucose-Sensing Insulin-Secreting Human α-Cells. Nature 2019, 567, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Remedi, M.S.; Emfinger, C. Pancreatic β-Cell Identity In Diabetes. Diabetes Obes. Metab. 2016, 18, 110–116. [Google Scholar] [CrossRef]
- Son, J.; Accili, D. Reversing Pancreatic β-Cell Dedifferentiation in the Treatment of Type 2 Diabetes. Exp. Mol. Med. 2023, 55, 1652–1658. [Google Scholar] [CrossRef]
- Oger, F.; Bourouh, C.; Friano, M.E.; Courty, E.; Rolland, L.; Gromada, X.; Moreno, M.; Carney, C.; Rabhi, N.; Durand, E.; et al. β-Cell-Specific E2f1 Deficiency Impairs Glucose Homeostasis, β-Cell Identity, and Insulin Secretion. Diabetes 2023, 72, 1112–1126. [Google Scholar] [CrossRef]
- De Jesus, D.F.; Kulkarni, R.N. Epigenetic Modifiers of Islet Function and Mass. Trends Endocrinol. Metab. 2014, 25, 628–636. [Google Scholar] [CrossRef]
- Bornaque, F.; Delannoy, C.P.; Courty, E.; Rabhi, N.; Carney, C.; Rolland, L.; Moreno, M.; Gromada, X.; Bourouh, C.; Petit, P.; et al. Glucose Regulates m6A Methylation of RNA in Pancreatic Islets. Cells 2022, 11, 291. [Google Scholar] [CrossRef]
- Rabhi, N.; Hannou, S.A.; Froguel, P.; Annicotte, J.-S. Cofactors As Metabolic Sensors Driving Cell Adaptation in Physiology and Disease. Front. Endocrinol. 2017, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Monroe, J.; Koutmou, K.S. A Molecular-Level Perspective on the Frequency, Distribution, and Consequences of Messenger RNA Modifications. Wiley Interdiscip. Rev. RNA 2020, 11, e1586. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, A.L.G.; Nassif, N.T.; O’Brien, B.A.; Simpson, A.M. Pancreatic Transdifferentiation Using β-Cell Transcription Factors for Type 1 Diabetes Treatment. Cells 2022, 11, 2145. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langlois, A.; Pinget, M.; Kessler, L.; Bouzakri, K. Islet Transplantation: Current Limitations and Challenges for Successful Outcomes. Cells 2024, 13, 1783. https://doi.org/10.3390/cells13211783
Langlois A, Pinget M, Kessler L, Bouzakri K. Islet Transplantation: Current Limitations and Challenges for Successful Outcomes. Cells. 2024; 13(21):1783. https://doi.org/10.3390/cells13211783
Chicago/Turabian StyleLanglois, Allan, Michel Pinget, Laurence Kessler, and Karim Bouzakri. 2024. "Islet Transplantation: Current Limitations and Challenges for Successful Outcomes" Cells 13, no. 21: 1783. https://doi.org/10.3390/cells13211783
APA StyleLanglois, A., Pinget, M., Kessler, L., & Bouzakri, K. (2024). Islet Transplantation: Current Limitations and Challenges for Successful Outcomes. Cells, 13(21), 1783. https://doi.org/10.3390/cells13211783





