VEGF—Virus Interactions: Pathogenic Mechanisms and Therapeutic Applications
Abstract
1. Introduction
2. Pathogenic Viruses That Disrupt VEGF Levels
3. VEGF Induction in Oncogenic Viruses
4. Therapeutic Viral Capsids That Expose Peptides That Compete with VEGF
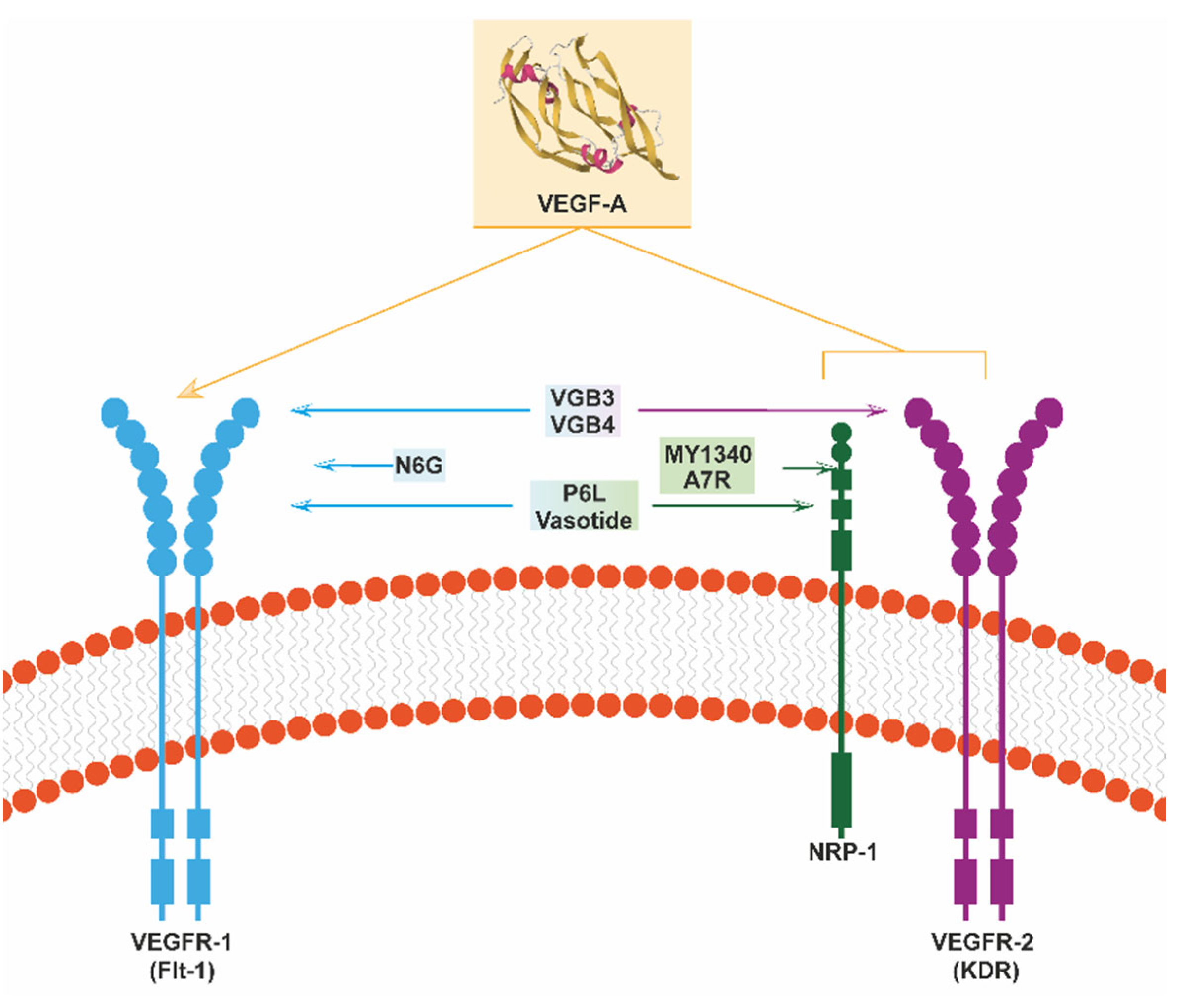
4.1. Peptides That Inhibit VEGF’s Functions: Binding Features and Biological Effects
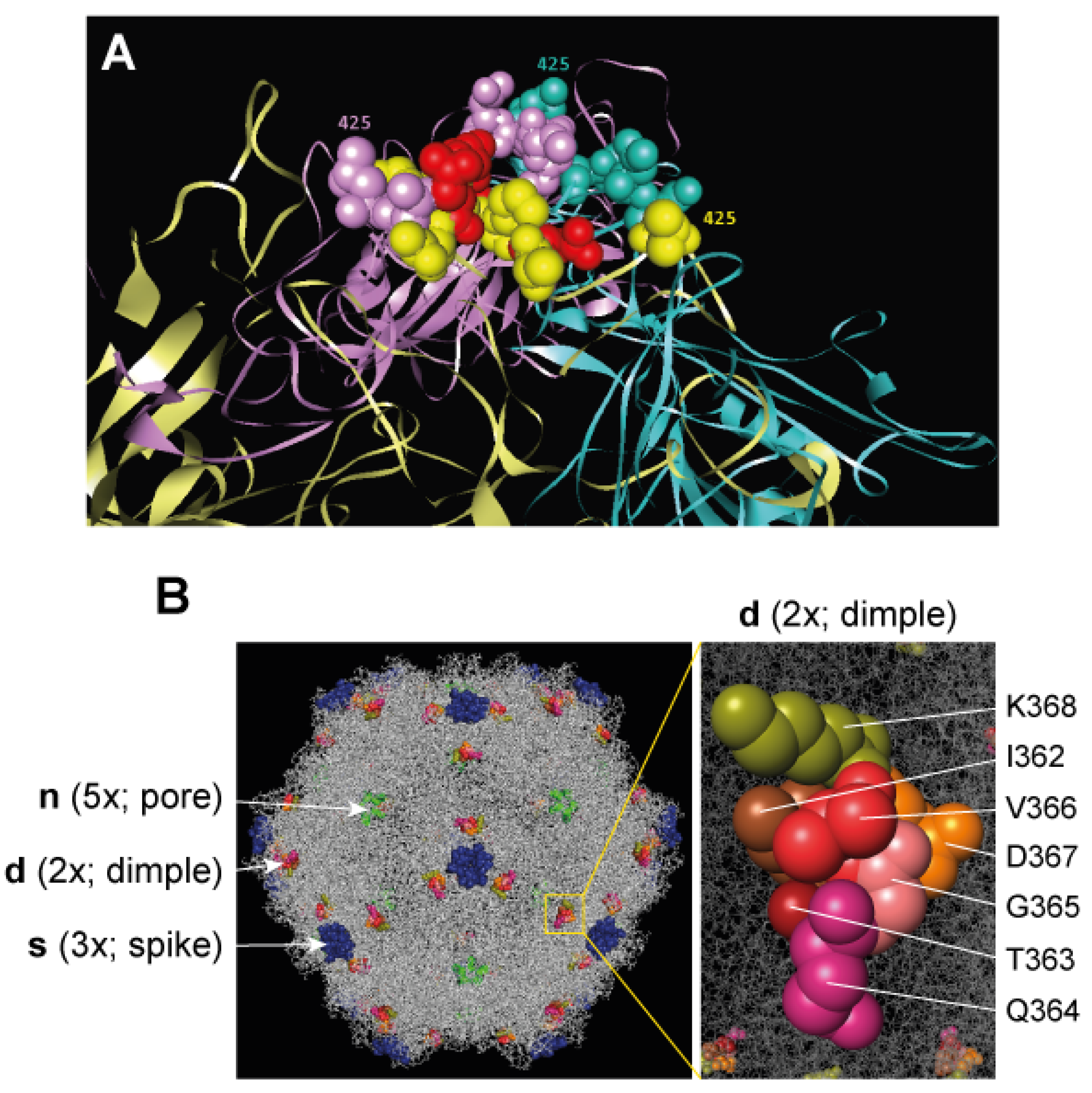
4.2. Engineering Viral Capsids with VEGF-Blocking Peptides
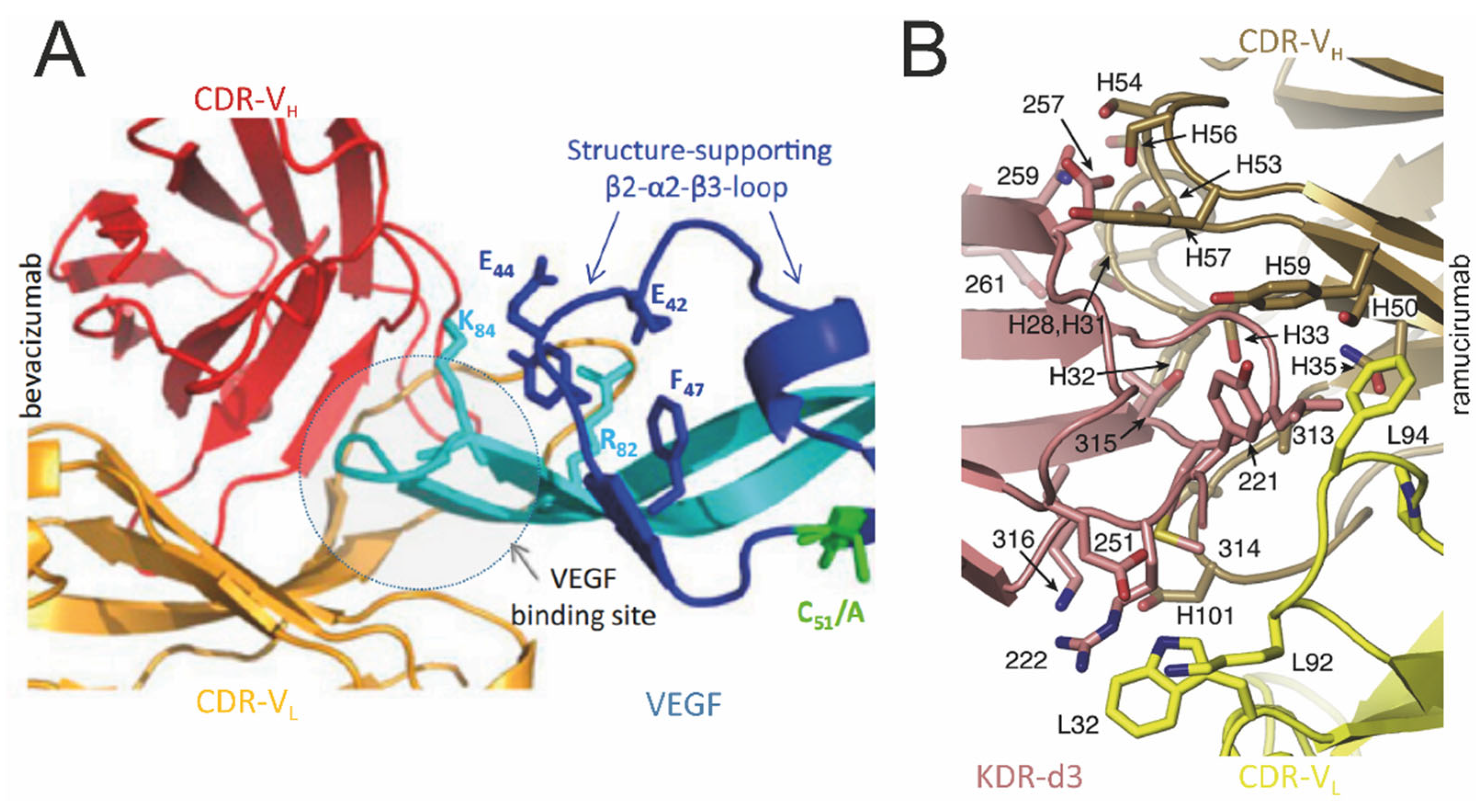
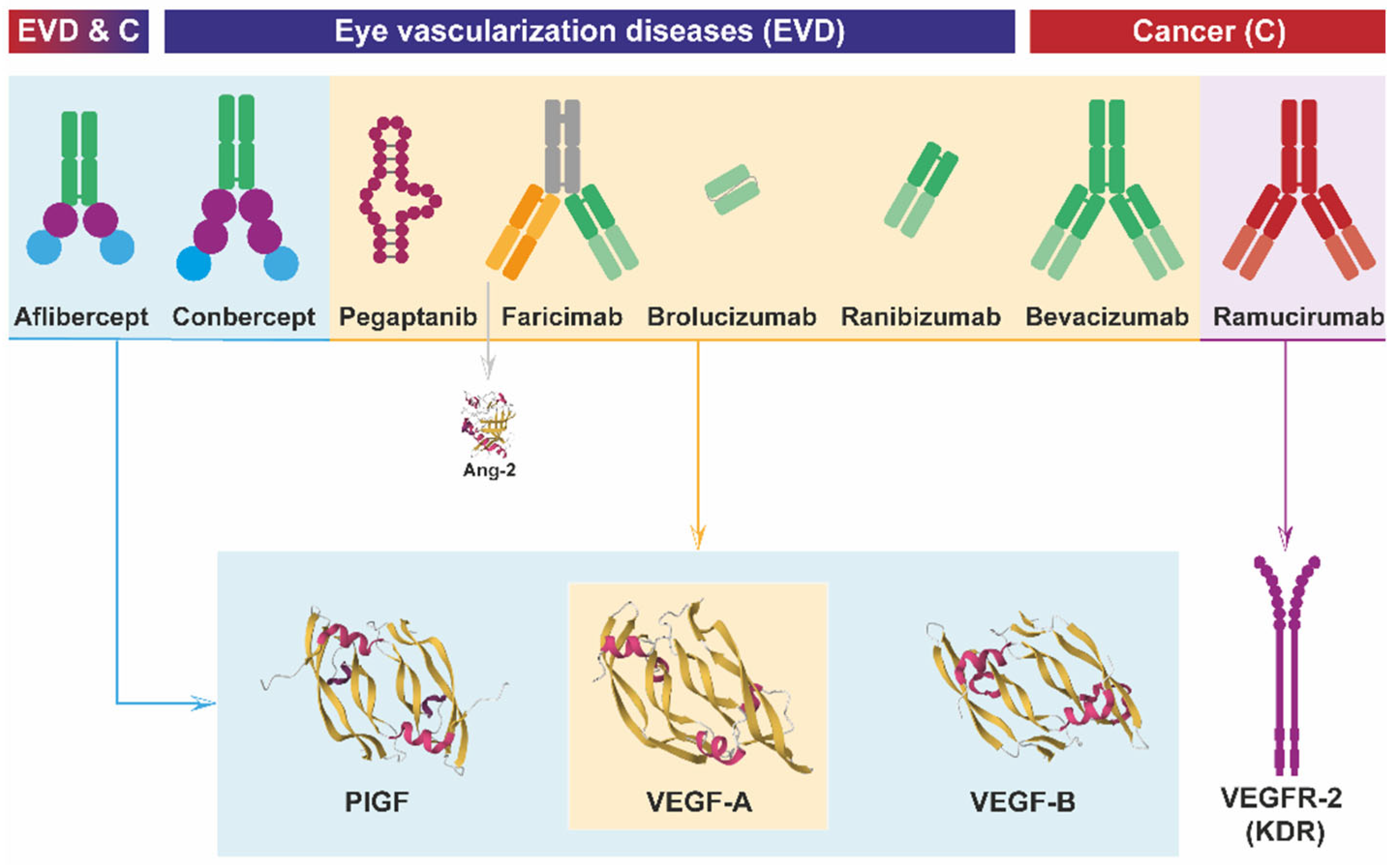
5. Anti-VEGF Antibodies: From Development to Clinical Application
6. Oncolytic Viruses as Anti-Angiogenic Agents
6.1. OV with Natural Anti-Angiogenic Properties
6.2. OV in Combined Anti-Angiogenic Therapies
6.3. OV Expressing Anti-Angiogenic Factors
7. Anti-Angiogenic AAV Vectors in Cancer Therapy
8. Therapeutic AAV Vectors Targeting the VEGF System
8.1. Pro-Angiogenic rAAV Vectors
8.2. Anti-Angiogenic rAAV Vectors
| Vector | Transgen | Assay | Results | References |
|---|---|---|---|---|
| AAV2 a | shVEGF + IGF | Rat model of lumbar disc degeneration | Reduction of disc cell death in the vertebral pulp and annulus fibrosus | [193] |
| AAV2 b | VEGF inhibitor (VID), complement inhibitor (CID) and dual inhibitor (ACVP1) | Mice models of endotoxin-induced uveitis, autoimmune uveoretinitis, and CNV | Improvement of laser induced injuries and CNV c and ACVP1 protection against ocular inflammation and neovascularization | [194] |
| AAV 2.7m8 (ADVM-022) b | Aflibercept (fusion protein made of the VEGFR1 and VEGFR2 extracellular domains, and the Fc of the human IgG1) | Single IVT d administration in non-human primates. | Long term efficacy against grade IV lesions in CNV c models. Safety and efficacy for wet and neovascular-AMD (phase 1), and for DME and diabetic retinopathy (phase 2). | [191,195,196,197] |
| AAV2, ADVM-022, AAV3b, and AAV8 b | Conbercept (KH902), recombinant protein with multiple Ig domains of VEGFR1 and VEGFR2 | IVT d administration in mice models of oxygen-induced retinopathy and laser induced CNV | Long-term efficacy. Reduction in retinal aneurysms | [192] |
| AAV5 b | Dual anti-VEGF-A miRNAs + PEDF | SR e administration in mouse model of CNV | Reduction of CNVc area and in VEGF expression. | [198] |
| AAV8 b | Anti-VEGF single-chain variable fragment | Mouse model of CNV | Long-term safe and effective effects | [180] |
| AAV8 b | VEGF Trap (nVEGFi) | SR e administration in mouse model of CNV | Increased reduction in the CNV c area, and reduction of toxicity comparing with AAV8-aflibercept | [199] |
| AAV8 b | miR-aghsRNA against VEGF | SR e administration in mouse model of CNV | Reduction of CNV c area, and no clinical signs of intra-ocular inflammation | [200] |
| AAV8 b | CRISPR-based VEGF-A suppression | SR e administration in mouse model of CNV | Partial gene disruption and partial reduction of CNV c | [201] |
9. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Risau, W.; Flamme, L. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995, 11, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms Review of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef]
- Borgström, P.; Bourdon, M.A.; Hillan, K.J.; Sriramarao, P.; Ferrara, N. Neutralizing anti-vascular endothelial growth factor antibody completely inhibits angiogenesis and growth of human prostate carcinoma micro tumors in vivo. Prostate 1998, 35, 1–10. [Google Scholar] [CrossRef]
- Benjamin, L.E.; Keshet, E. Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: Induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc. Natl. Acad. Sci. USA 1997, 94, 8761–8766. [Google Scholar] [CrossRef]
- Boehm, T.; Folkman, J.; Browder, T.; O’Reilly, M.S. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 1997, 390, 404–407. [Google Scholar] [CrossRef]
- Leung David, W.; Cachianes, G.; Kuang, W.-J.; Goeddel David, V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef]
- Keck Pamela, J.; Hauser Scott, D.; Krivi, G.; Sanzo, K.; Warren, T.; Feder, J.; Connolly, D.T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Sci. Rep. 1989, 246, 1309–1312. [Google Scholar] [CrossRef]
- Berger, D.P.; Herbstritt, L.; Dengler, W.A.; Marme, D.; Mertelsmann, R.; Fiebig, H.H. Vascular endothelial growth factor (VEGF) mRNA expression in human tumor models of different histologies. Ann. Oncol. 1995, 6, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Li, B.; Winer, J.; Armanini, M.; Gillett, N.; Phillips, H.S.; Ferrara, N. Inhibition of vascular endothelial growht factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Li, B.; Houck, K.; Winer, J.; Ferrara, N. The vascular endothelial growth factor proteins: Identification of biologically relevant regions by neutralizing monoclonal antibodies. Growth Factors 1992, 7, 53–64. [Google Scholar] [CrossRef]
- Plate, K.H.; Breier, G.; Weich, H.A.; Risau, W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 1992, 359, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer 2002, 2, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klasbrun, M. Neuropilin-1 Is Expressed by Endothelial and Tumor Cells as an Isoform-Specific Receptor for Vascular Endothelial Growth Factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef]
- Jobe, A.; Vijayan, R. Neuropilins: C-end rule peptides and their association with nociception and COVID-19. Comput. Struct. Biotechnol. J. 2021, 19, 1889–1895. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Zhang, J.; Xing, B.; Xuan, W.; Wang, H.; Huang, H.; Yang, J.; Tang, J. NRP-2 in tumor lymphangiogenesis and lymphatic metastasis. Cancer Lett. 2018, 418, 176–184. [Google Scholar] [CrossRef]
- Moutal, A.; Martin, L.F.; Boinon, L.; Gomez, K.; Ran, D.; Zhou, Y.; Stratton, H.J.; Cai, S.; Luo, S.; Gonzalez, K.B.; et al. SARS-CoV-2 spike protein co-opts VEGF-A/neuropilin-1 receptor signaling to induce analgesia. Pain 2021, 162, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Benwell, C.J.; Johnson, R.T.; Taylor, J.A.G.E.; Price, C.A.; Robinson, S.D. Endothelial VEGFR Coreceptors Neuropilin-1 and Neuropilin-2 Are Essential for Tumor Angiogenesis. Cancer Res. Commun. 2022, 2, 1626–1640. [Google Scholar] [CrossRef] [PubMed]
- Schellenburg, S.; Schulz, A.; Poitz, D.M.; Muders, M.H. Role of neuropilin-2 in the immune system. Mol. Immunol. 2017, 90, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Mishra, J.; Bodas, S.; Bhattacharya, S.; Batra, S.K.; Dutta, S.; Datta, K. Role of Neuropilin-2-mediated signaling axis in cancer progression and therapy resistance. Cancer Metastasis Rev. 2022, 41, 771–787. [Google Scholar] [CrossRef]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef]
- Hu, H.; Chen, Y.; Tan, S.; Wu, S.; Huang, Y.; Fu, S.; Luo, F.; He, J. The Research Progress of Antiangiogenic Therapy, Immune Therapy and Tumor Microenvironment. Front. Immunol. 2022, 13, 802846. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Xu, W.; Chen, J.; Yu, J.; Gamble, J.R.; McCaughan, G.W. Targeting the vasculature in hepatocellular carcinoma treatment: Starving versus normalizing blood supply. Clin. Transl. Gastroenterol. 2017, 8, e98. [Google Scholar] [CrossRef]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Patel, S.A.; Nilsson, M.B.; Le, X.; Cascone, T.; Jain, R.K.; Heymach, J.V. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin. Cancer Res. 2023, 29, 30–39. [Google Scholar] [CrossRef]
- Ferrara, N. Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev. 2010, 21, 21–26. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Mabeta, P.; Steenkamp, V. The VEGF/VEGFR Axis Revisited: Implications for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 15585. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulou, D.I.; Bala, V.M.; Zerva, E.; Pliakou, E.; Filippou, D.; Gazouli, M.; Aravantinos, G. The potential role of the combined PARP-1 and VEGF inhibition in severe SARS-CoV-2 (COVID-19) infection. J. Infect. Dev. Ctries. 2022, 16, 101–111. [Google Scholar] [CrossRef]
- Talotta, R. Impaired VEGF-A-Mediated Neurovascular Crosstalk Induced by SARS-CoV-2 Spike Protein: A Potential Hypothesis Explaining Long COVID-19 Symptoms and COVID-19 Vaccine Side Effects? Microorganisms 2022, 10, 2452. [Google Scholar] [CrossRef]
- Zeng, F.; Li, Y.; Deng, Z.; He, J.; Li, W.; Wang, L.; Lyu, T.; Li, Z.; Mei, C.; Yang, M.; et al. SARS-CoV-2 spike spurs intestinal inflammation via VEGF production in enterocytes. EMBO Mol. Med. 2022, 14, e14844. [Google Scholar] [CrossRef]
- Tsuji, M.; Kondo, M.; Sato, Y.; Miyoshi, A.; Kobayashi, F.; Arimura, K.; Yamashita, K.; Morimoto, S.; Yanagisawa, N.; Ichihara, A.; et al. Serum VEGF-A levels on admission in COVID-19 patients correlate with SP-D and neutrophils, reflecting disease severity: A prospective study. Cytokine 2024, 178, 156583. [Google Scholar] [CrossRef]
- Rauti, R.; Shahoha, M.; Leichtmann-Bardoogo, Y.; Nasser, R.; Paz, E.; Tamir, R.; Miller, V.; Babich, T.; Shaked, K.; Ehrlich, A.; et al. Effect of sars-cov-2 proteins on vascular permeability. eLife 2021, 10, e69314. [Google Scholar] [CrossRef]
- Josuttis, D.; Schwedler, C.; Heymann, G.; Gümbel, D.; Schmittner, M.D.; Kruse, M.; Hoppe, B. Vascular Endothelial Growth Factor as Potential Biomarker for COVID-19 Severity. J. Intensive Care Med. 2023, 38, 1165–1173. [Google Scholar] [CrossRef]
- Kilani, M.M.; Mohammed, K.A.; Nasreen, N.; Hardwick, J.A.; Kaplan, M.H.; Tepper, R.S.; Antony, V.B. Respiratory syncytial virus causes increased bronchial epithelial permeability. Chest 2004, 126, 186–191. [Google Scholar] [CrossRef]
- Lee, C.G.; Yoon, H.J.; Zhu, Z.; Link, H.; Wang, Z.; Gwaltney, J.; Landry, M.; Elias, J.A. Respiratory syncytial virus stimulation of vascular endothelial cell growth factor/vascular permeability factor. Am. J. Respir. Cell Mol. Biol. 2000, 23, 662–669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gazzini, S.; Cerullo, R.; Soloperto, D. VEGF as a Key Actor in Recurrent Respiratory Papillomatosis: A Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 6757–6768. [Google Scholar] [CrossRef]
- Lam, B.; Miller, J.; Kung, Y.J.; Wu, T.C.; Hung, C.F.; Roden, R.B.S.; Best, S.R. Profiling of VEGF Receptors and Immune Checkpoints in Recurrent Respiratory Papillomatosis. Laryngoscope 2024, 134, 2819–2825. [Google Scholar] [CrossRef]
- Lyttle, D.J.; Fraser, K.M.; Fleming, S.B.; Mercer, A.A.; Robinson, A.J. Homologs of vascular endothelial growth factor are encoded by the poxvirus orf virus. J. Virol. 1994, 68, 84–92. [Google Scholar] [CrossRef]
- Meyer, M.; Clauss, M.; Lepple-Wienhues, A.; Waltenberger, J.; Augustin, H.G.; Ziche, M.; Lanz, C.; Büttner, M.; Rziha, H.J.; Dehio, C. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J. 1999, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Savory, L.J.; Stacker, S.A.; Fleming, S.B.; Niven, B.E.; Mercer, A.A. Viral Vascular Endothelial Growth Factor Plays a Critical Role in Orf Virus Infection. J. Virol. 2000, 74, 10699–10706. [Google Scholar] [CrossRef] [PubMed]
- Alkharsah, K.R. VEGF upregulation in viral infections and its possible therapeutic implications. Int. J. Mol. Sci. 2018, 19, 1642. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Swanton, C.; Bernard, E.; Abbosh, C.; André, F.; Auwerx, J.; Balmain, A.; Bar-Sagi, D.; Bernards, R.; Bullman, S.; DeGregori, J.; et al. Embracing cancer complexity: Hallmarks of systemic disease. Cell 2024, 187, 1589–1616. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Yao, Y.; Guan, X.; Liu, Y.; Qi, X.; Wang, Y.; Liu, C.; Zhang, Y.; Gao, H.; et al. Avian leukosis virus subgroup J induces VEGF expression via NF-κB/PI3K-dependent IL-6 production. Oncotarget 2016, 7, 80275–80287. [Google Scholar] [CrossRef][Green Version]
- Shao, Y.Y.; Hsieh, M.S.; Wang, H.Y.; Li, Y.S.; Lin, H.; Hsu, H.W.; Huang, C.Y.; Hsu, C.H.; Cheng, A.L. Hepatitis C virus core protein potentiates proangiogenic activity of hepatocellular carcinoma cells. Oncotarget 2017, 8, 86681–86692. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abe, M.; Koga, H.; Yoshida, T.; Masuda, H.; Iwamoto, H.; Sakata, M.; Hanada, S.; Nakamura, T.; Taniguchi, E.; Kawaguchi, T.; et al. Hepatitis C virus core protein upregulates the expression of vascular endothelial growth factor via the nuclear factor-κB/hypoxia-inducible factor-1α axis under hypoxic conditions. Hepatol. Res. 2012, 42, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.G.; Na, T.Y.; Seo, H.W.; Seong, J.K.; Park, C.K.; Shin, Y.K.; Lee, M.O. Hepatitis B virus X protein induces the expression of MTA1 and HDAC1, which enhances hypoxia signaling in hepatocellular carcinoma cells. Oncogene 2008, 27, 3405–3413. [Google Scholar] [CrossRef]
- Yoo, Y.G.; Oh, S.H.; Park, E.S.; Cho, H.; Lee, N.; Park, H.; Kim, D.K.; Yu, D.Y.; Seong, J.K.; Lee, M.O. Hepatitis B virus X protein enhances transcriptional activity of hypoxia-inducible factor-1α through activation of mitogen-activated protein kinase pathway. J. Biol. Chem. 2003, 278, 39076–39084. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.J.; Lin, Y.J.; Yen, C.S.; Tsai, H.W.; Tsai, T.F.; Chang, K.Y.; Huang, W.C.; Lin, P.W.; Chiang, C.W.; Chang, T.T. Hepatitis B virus X protein upregulates mTOR signaling through IKKβ to increase cell proliferation and VEGF production in hepatocellular carcinoma. PLoS ONE 2012, 7, e41931. [Google Scholar] [CrossRef]
- Yang, J.C.; Teng, C.F.; Wu, H.C.; Tsai, H.W.; Chuang, H.C.; Tsai, T.F.; Hsu, Y.H.; Huang, W.; Wu, L.W.; Su, I.J. Enhanced expression of vascular endothelial growth factor-A in ground glass hepatocytes and its implication in hepatitis B virus hepatocarcinogenesis. Hepatology 2009, 49, 1962–1971. [Google Scholar] [CrossRef]
- Catalano, A.; Romano, M.; Martinotti, S.; Procopio, A. Enhanced expression of vascular endothelial growth factor (VEGF) plays a critical role in the tumor progression potential induced by simian virus 40 large T antigen. Oncogene 2002, 21, 2896–2900. [Google Scholar] [CrossRef][Green Version]
- Tang, X.; Zhang, Q.; Nishitani, J.; Brown, J.; Shi, S.; Le, A.D. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1α protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clin. Cancer Res. 2007, 13, 2568–2576. [Google Scholar] [CrossRef]
- Tilborghs, S.; Corthouts, J.; Verhoeven, Y.; Arias, D.; Rolfo, C.; Trinh, X.B.; van Dam, P.A. The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit. Rev. Oncol. Hematol. 2017, 120, 141–150. [Google Scholar] [CrossRef]
- Kim, S.H.; Juhnn, Y.S.; Kang, S.; Park, S.W.; Sung, M.W.; Bang, Y.J.; Song, Y.S. Human papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ERK1,2 and PI3K/Akt. Cell. Mol. Life Sci. 2006, 63, 930–938. [Google Scholar] [CrossRef]
- Li, F.; Cui, J. Human telomerase reverse transcriptase regulates vascular endothelial growth factor expression via human papillomavirus oncogene E7 in HPV-18-positive cervical cancer cells. Med. Oncol. 2015, 32, 199. [Google Scholar] [CrossRef] [PubMed]
- López-Ocejo, O.; Viloria-Petit, A.; Bequet-Romero, M.; Mukhopadhyay, D.; Rak, J.; Kerbel, R.S. Oncogenes and tumor angiogenesis: The HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene 2000, 19, 4611–4620. [Google Scholar] [CrossRef] [PubMed]
- Nayarisseri, A.; Abdalla, M.; Joshi, I.; Yadav, M.; Bhrdwaj, A.; Chopra, I.; Khan, A.; Saxena, A.; Sharma, K.; Panicker, A.; et al. Potential inhibitors of VEGFR1, VEGFR2, and VEGFR3 developed through Deep Learning for the treatment of Cervical Cancer. Sci. Rep. 2024, 14, 13251. [Google Scholar] [CrossRef]
- Wuest, T.; Zheng, M.; Efstathiou, S.; Halford, W.P.; Carr, D.J.J. The herpes simplex virus-1 transactivator infected cell protein-4 drives VEGF-A dependent Neovascularization. PLoS Pathog. 2011, 7, e1002278. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Mulik, S.; Sharma, S.; Reddy, P.B.J.; Sehrawat, S.; Rouse, B.T. Ocular neovascularization caused by HSV-1 infection results from breakdown of binding between VEGF-A and its soluble receptor. J. Immunol. 2011, 186, 3653–3665. [Google Scholar] [CrossRef]
- Stevenson, D.; Charalambous, C.; Wilson, J.B. Epstein-Barr virus latent membrane protein 1 (CAO) up-regulates VEGF and TGFα concomitant with hyperlasia, with subsequent up-regulation of p16 and MMP9. Cancer Res. 2005, 65, 8826–8835. [Google Scholar] [CrossRef]
- Murono, S.; Inoue, H.; Tanabe, T.; Joab, I.; Yoshizaki, T.; Furukawa, M.; Pagano, J.S. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc. Natl. Acad. Sci. USA 2001, 98, 6905–6910. [Google Scholar] [CrossRef]
- O’Neil, J.D.; Owen, T.J.; Wood, V.H.J.; Date, K.L.; Valentine, R.; Chukwuma, M.B.; Arrand, J.R.; Dawson, C.W.; Young, L.S. Epstein-Barr virus-encoded EBNA1 modulates the AP-1 transcription factor pathway in nasopharyngeal carcinoma cells and enhances angiogenesis in vitro. J. Gen. Virol. 2008, 89, 2833–2842. [Google Scholar] [CrossRef]
- Sakakibara, S.; Pise-Masison, C.A.; Brady, J.N.; Tosato, G. Gene Regulation and Functional Alterations Induced by Kaposi’s Sarcoma-Associated Herpesvirus-Encoded ORFK13/vFLIP in Endothelial Cells. J. Virol. 2009, 83, 2140–2153. [Google Scholar] [CrossRef]
- Aoki, Y.; Tosato, G. Role of Vascular Endothelial Growth Factor/Vascular Permeability Factor in the Pathogenesis of Kaposi’s Sarcoma-Associated Herpesvirus-Infected Primary Effusion Lymphomas. Blood 1999, 94, 4247–4254. [Google Scholar] [CrossRef]
- Wang, L.; Wakisaka, N.; Tomlinson, C.C.; DeWire, S.M.; Krall, S.; Pagano, J.S.; Damania, B. The Kaposi’s Sarcoma-Associated Herpesvirus (KSHV/HHV-8) K1 Protein Induces Expression of Angiogenic and Invasion Factors. Cancer Res. 2004, 64, 2774–2781. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Chen, S.C.; Leach, M.W.; Manfra, D.; Homey, B.; Wiekowski, M.; Sullivan, L.; Jenh, C.H.; Narula, S.K.; Chensue, S.W.; et al. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi’s sarcoma. J. Exp. Med. 2000, 191, 445–453. [Google Scholar] [CrossRef]
- Shin, Y.C.; Joo, C.H.; Gack, M.U.; Lee, H.R.; Jung, J.U. Kaposi’s sarcoma-associated herpesvirus viral IFN regulatory factor 3 stabilizes hypoxia-inducible factor-1α to induce vascular endothelial growth factor expression. Cancer Res. 2008, 68, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Vicari, D.; Foy, K.C.; Liotta, E.M.; Kaumaya, P.T.P. Engineered conformation-dependent VEGF peptide mimics are effective in inhibiting VEGF signaling pathways. J. Biol. Chem. 2011, 286, 13612–13625. [Google Scholar] [CrossRef] [PubMed]
- Giordano, R.J.; Cardó-Vila, M.; Lahdenranta, J.; Pasqualini, R.; Arap, W. Biopanning and rapid analysis of selective interactive ligands. Nat. Med. 2001, 7, 1249–1253. [Google Scholar] [CrossRef]
- Farzaneh Behelgardi, M.; Zahri, S.; Mashayekhi, F.; Mansouri, K.; Asghari, S.M. A peptide mimicking the binding sites of VEGF-A and VEGF-B inhibits VEGFR-1/-2 driven angiogenesis, tumor growth and metastasis. Sci. Rep. 2018, 8, 17924. [Google Scholar] [CrossRef]
- Farzaneh Behelgardi, M.; Zahri, S.; Gholami Shahvir, Z.; Mashayekhi, F.; Mirzanejad, L.; Asghari, S.M. Targeting signaling pathways of VEGFR1 and VEGFR2 as a potential target in the treatment of breast cancer. Mol. Biol. Rep. 2020, 47, 2061–2071. [Google Scholar] [CrossRef]
- Farzaneh Behelgardi, M.; Gholami Shahvir, Z.; Asghari, S.M. Apoptosis induction in human lung and colon cancer cells via impeding VEGF signaling pathways. Mol. Biol. Rep. 2022, 49, 3637–3647. [Google Scholar] [CrossRef]
- Sadremomtaz, A.; Ali, A.M.; Jouyandeh, F.; Balalaie, S.; Navari, R.; Broussy, S.; Mansouri, K.; Groves, M.R.; Asghari, S.M. Molecular docking, synthesis and biological evaluation of Vascular Endothelial Growth Factor (VEGF) B based peptide as antiangiogenic agent targeting the second domain of the Vascular Endothelial Growth Factor Receptor 1 (VEGFR1D2) for anticancer applicat. Signal Transduct. Target. Ther. 2020, 5, 76. [Google Scholar] [CrossRef]
- Namjoo, M.; Ghafouri, H.; Assareh, E.; Aref, A.R.; Mostafavi, E.; Hamrahi Mohsen, A.; Balalaie, S.; Broussy, S.; Asghari, S.M. A VEGFB-Based Peptidomimetic Inhibits VEGFR2-Mediated PI3K/Akt/mTOR and PLCγ/ERK Signaling and Elicits Apoptotic, Antiangiogenic, and Antitumor Activities. Pharmaceuticals 2023, 16, 906. [Google Scholar] [CrossRef]
- Michaloski, J.S.; Redondo, A.R.; Magalhães, L.S.; Cambui, C.C.; Giordano, R.J. Discovery of pan-VEGF inhibitory peptides directed to the extracellular ligand-binding domains of the VEGF receptors. Sci. Adv. 2016, 2, e1600611. [Google Scholar] [CrossRef] [PubMed]
- Giordano, R.J.; Anobom, C.D.; Cardó-Vila, M.; Kalil, J.; Valente, A.P.; Pasqualini, R.; Almeida, F.C.L.; Arap, W. Structural basis for the interaction of a vascular endothelial growth factor mimic peptide motif and its corresponding receptors. Chem. Biol. 2005, 12, 1075–1083. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giordano, R.J.; Cardó-Vila, M.; Salameh, A.; Anobom, C.D.; Zeitlin, B.D.; Hawke, D.H.; Valente, A.P.; Almeida, F.C.L.; Nör, J.E.; Sidman, R.L.; et al. From combinatorial peptide selection to drug prototype (I): Targeting the vascular endothelial growth factor receptor pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 5112–5117. [Google Scholar] [CrossRef] [PubMed]
- Sidman, R.L.; Li, J.; Lawrence, M.; Hu, W.; Musso, G.F.; Giordano, R.J.; Cardó-Vila, M.; Pasqualini, R.; Arap, W. The peptidomimetic Vasotide targets two retinal VEGF receptors and reduces pathological angiogenesis in murine and nonhuman primate models of retinal disease. Sci. Transl. Med. 2015, 7, 309ra165. [Google Scholar] [CrossRef]
- Binétruy-Tournaire, R.; Demangel, C.; Malavaud, B.; Vassy, R.; Rouyre, S.; Kraemer, M.; Ploue, J.; Derbin, C.; Perret, G.; Mazié, J.C. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J. 2000, 19, 1525–1533. [Google Scholar] [CrossRef]
- Starzec, A.; Vassy, R.; Martin, A.; Lecouvey, M.; Di Benedetto, M.; Crépin, M.; Perret, G.Y. Antiangiogenic and antitumor activities of peptide inhibiting the vascular endothelial growth factor binding to neuropilin-1. Life Sci. 2006, 79, 2370–2381. [Google Scholar] [CrossRef]
- Mo, Z.; Yu, F.; Han, S.; Yang, S.; Wu, L.; Li, P.; Jiao, S. New peptide MY1340 revert the inhibition effect of VEGF on dendritic cells differentiation and maturation via blocking VEGF-NRP-1 axis and inhibit tumor growth in vivo. Int. Immunopharmacol. 2018, 60, 132–140. [Google Scholar] [CrossRef]
- Omidfar, K.; Daneshpour, M. Advances in phage display technology for drug discovery. Expert Opin. Drug Discov. 2015, 10, 651–669. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Agbandje-McKenna, M.; Chiorini, J.A.; Mukha, D.V.; Pintel, D.J.; Qiu, J.; Soderlund-Venermo, M.; Tattersall, P.; Tijssen, P.; Gatherer, D.; et al. The family Parvoviridae. Arch. Virol. 2014, 159, 1239–1247. [Google Scholar] [CrossRef]
- Agbandje-McKenna, M.; Llamas-Saiz, A.L.; Wang, F.; Tattersall, P.; Rossmann, M.G. Functional implications of the structure of the murine parvovirus, minute virus of mice. Structure 1998, 6, 1369–1381. [Google Scholar] [CrossRef]
- Kontou, M.; Govindasamy, L.; Nam, H.-J.; Bryant, N.; Llamas-Saiz, A.L.; Foces-Foces, C.; Hernando, E.; Rubio, M.-P.; McKenna, R.; Almendral, J.M.; et al. Structural Determinants of Tissue Tropism and In Vivo Pathogenicity for the Parvovirus Minute Virus of Mice. J. Virol. 2005, 79, 10931–10943. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.; Menéndez, M.; Reguera, J.; Almendral, J.M.; Mateu, M.G. In Vitro Disassembly of a Parvovirus Capsid and Effect on Capsid Stability of Heterologous Peptide Insertions in Surface Loops. J. Biol. Chem. 2004, 279, 6517–6525. [Google Scholar] [CrossRef] [PubMed]
- Rueda, P.; Hurtado, A.; Del Barrio, M.; Luis Martínez-Torrecuadrada, J.; Kamstrup, S.; Leclerc, C.; Casal, J.I. Minor Displacements in the Insertion Site Provoke Major Differences in the Induction of Antibody Responses by Chimeric Parvovirus-like Particles. Virology 1999, 263, 89–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Büning, H.; Srivastava, A. Capsid Modifications for Targeting and Improving the Efficacy of AAV Vectors. Mol. Ther. Methods Clin. Dev. 2019, 12, 248–265. [Google Scholar] [CrossRef]
- Almendral, J.M. Assembly of simple icosahedral viruses. Subcell Biochem. 2013, 68, 307–328. [Google Scholar] [CrossRef]
- Girod, A.; Ried, M.; Wobus, C.; Lahm, H.; Leike, K.; Kleinschmidt, J.; Deléage, G.; Hallek, M. Genetic capsid modifications allow efficient re-targeting of adeno- associated virus type 2. Nat. Med. 1999, 5, 1052–1056. [Google Scholar] [CrossRef]
- Shi, W.; Arnold, G.S.; Bartlett, J.S. Insertional mutagenesis of the adeno-associated virus type 2 (AAV2) capsid gene and generation of AAV2 vectors targeted to alternative cell-surface receptors. Hum. Gene Ther. 2001, 12, 1697–1711. [Google Scholar] [CrossRef]
- Sánchez-Martínez, C.; Grueso, E.; Carroll, M.; Rommelaere, J.; Almendral, J.M. Essential role of the unordered VP2 n-terminal domain of the parvovirus MVM capsid in nuclear assembly and endosomal enlargement of the virion fivefold channel for cell entry. Virology 2012, 432, 45–56. [Google Scholar] [CrossRef]
- Grueso, E.; Sánchez-Martínez, C.; Calvo-López, T.; de Miguel, F.J.; Blanco-Menéndez, N.; Fernandez-Estevez, M.; Elizalde, M.; Sanchez, J.; Kourani, O.; Martin, D.; et al. Antiangiogenic Vascular Endothelial Growth Factor-Blocking Peptides Displayed on the Capsid of an Infectious Oncolytic Parvovirus: Assembly and Immune Interactions. J. Virol. 2019, 93, e00798-19. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.Q.; Broussy, S.; Han, B.; Fang, H. Recent advances of anti-angiogenic inhibitors targeting VEGF/VEGFR axis. Front. Pharmacol. 2023, 14, 1307860. [Google Scholar] [CrossRef]
- López-Bueno, A.; Mateu, M.G.; Almendral, J.M. High Mutant Frequency in Populations of a DNA Virus Allows Evasion from Antibody Therapy in an Immunodeficient Host. J. Virol. 2003, 77, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Calvo-López, T.; Grueso, E.; Sánchez-Martínez, C.; Almendral, J.M. Intracellular virion traffic to the endosome driven by cell type specific sialic acid receptors determines parvovirus tropism. Front. Microbiol. 2023, 13, 1063706. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular Endothelial Growth Factor. Trends Cardiovasc. Med. 1993, 3, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.S.; Yuan, H.; Matli, M.R.; Gillett, N.A.; Ferrara, N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J. Clin. Investig. 1995, 95, 1789–1797. [Google Scholar] [CrossRef]
- Asano, M.; Yukita, A.; Matsumoto, T.; Kondo, S.; Suzuki, H. Inhibition of tumor growth and metastasis by an immunoneutralizing monoclonal antibody to human vascular endothelial growth factor/vascular permeability factor121. Cancer Res. 1995, 55, 5296–52301. [Google Scholar]
- Presta, L.G.; Chen, H.; O’Connor, S.J.; Chisholm, V.; Meng, Y.G.; Krummen, L.; Winkler, M.; Ferrara, N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997, 57, 4593–4599. [Google Scholar]
- Ryan, A.M.; Eppler, D.B.; Hagler, K.E.; Bruner, R.H.; Thomford, P.J.; Hall, R.L.; Shopp, G.M.; O’Neill, C.A. Preclinical safety evaluation of rhuMAbVEGF, an antiangiogenic humanized monoclonal antibody. Toxicol. Pathol. 1999, 27, 78–86. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef]
- Shih, T.; Lindley, C. Bevacizumab: An Angiogenesis Inhibitor for the Treatment of Solid Malignancies. Clin. Ther. 2006, 28, 1779–1802. [Google Scholar] [CrossRef]
- Muller, Y.A.; Chen, Y.; Christinger, H.W.; Li, B.; Cunningham, B.C.; Lowman, H.B.; De Vos, A.M. VEGF and the Fab fragment of a humanized neutralizing antibody: Crystal structure of the complex at 2.4 Å resolution and mutational analysis of the interface. Structure 1998, 6, 1153–1167. [Google Scholar] [CrossRef]
- Wentink, M.Q.; Hackeng, T.M.; Tabruyn, S.P.; Puijk, W.C.; Schwamborn, K.; Altschuh, D.; Meloen, R.H.; Schuurman, T.; Griffioen, A.W.; Timmerman, P. Targeted vaccination against the bevacizumab binding site on VEGF using 3D-structured peptides elicits efficient antitumor activity. Proc. Natl. Acad. Sci. USA 2016, 113, 12532–12537. [Google Scholar] [CrossRef] [PubMed]
- Goedegebuure, R.S.A.; Wentink, M.Q.; van der Vliet, H.J.; Timmerman, P.; Griffioen, A.W.; de Gruijl, T.D.; Verheul, H.M.W. A Phase I Open-Label Clinical Trial Evaluating the Therapeutic Vaccine hVEGF26–104/RFASE in Patients with Advanced Solid Malignancies. Oncologist 2021, 26, e218–e229. [Google Scholar] [CrossRef] [PubMed]
- Wentink, M.Q.; Verheul, H.M.W.; Griffioen, A.W.; Schafer, K.A.; McPherson, S.; Early, R.J.; van der Vliet, H.J.; de Gruijl, T.D. A safety and immunogenicity study of immunization with hVEGF26-104/RFASE in cynomolgus monkeys. Vaccine 2018, 36, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.M.; Hurwitz, H.I. Targeted inhibition of VEGF receptor 2: An update on ramucirumab. Expert Opin. Biol. Ther. 2013, 13, 1187–1196. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; Dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Lu, D.; Jimenez, X.; Zhang, H.; Bohlen, P.; Witte, L.; Zhu, Z. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int. J. Cancer 2002, 97, 393–399. [Google Scholar] [CrossRef]
- Spratlin, J.L.; Cohen, R.B.; Eadens, M.; Gore, L.; Camidge, D.R.; Diab, S.; Leong, S.; O’Bryant, C.; Chow, L.Q.M.; Serkova, N.J.; et al. Phase I pharmacologic and biologic study of ramucirumab (imc-1121b), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J. Clin. Oncol. 2010, 28, 780–787. [Google Scholar] [CrossRef]
- Aprile, G.; Rijavec, E.; Fontanella, C.; Rihawi, K.; Grossi, F. Ramucirumab: Preclinical research and clinical development. OncoTargets Ther. 2014, 7, 1997–2006. [Google Scholar] [CrossRef]
- Franklin, M.C.; Navarro, E.C.; Wang, Y.; Patel, S.; Singh, P.; Zhang, Y.; Persaud, K.; Bari, A.; Griffith, H.; Shen, L.; et al. The structural basis for the function of two anti-VEGF receptor 2 antibodies. Structure 2011, 19, 1097–1107. [Google Scholar] [CrossRef]
- Chen, Y.; Wiesmann, C.; Fuh, G.; Li, B.; Christinger, H.W.; Mckay, P.; De Vos, A.M.; Lowman, H.B. Selection and Analysis of an Optimized Anti-VEGF Antibody: Crystal Structure of an Affinity-matured Fab in Complex with Antigen. J. Mol. Biol. 1999, 293, 865–881. [Google Scholar] [CrossRef]
- Gaudreault, J.; Gunde, T.; Floyd, H.S.; Ellis, J.; Tietz, J.; Binggeli, D.; Keller, B.; Schmidt, A.; Escher, D. Preclinical Pharmacology and Safety of ESBA1008, a Single-chain Antibody Fragment, Investigated as Potential Treatment for Age Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3025. [Google Scholar]
- Tietz, J.; Spohn, G.; Schmid, G.; Konrad, J.; Jampen, S.; Maurer, P.; Schmidt, A.; Escher, D. Affinity and Potency of RTH258 (ESBA1008), a Novel Inhibitor of Vascular Endothelial Growth Factor A for the Treatment of Retinal Disorders. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1501. [Google Scholar]
- Klein, C.; Schaefer, W.; Regula, J.T. The use of CrossMAb technology for the generation of bi- and multispecific antibodies. mAbs. 2016, 8, 1010–1020. [Google Scholar] [CrossRef]
- Fine, S.L.; Martin, D.F.; Kirkpatrick, P. Pegaptanib sodium. Nat. Rev. Drug Discov. 2005, 4, 187–188. [Google Scholar] [CrossRef]
- Bell, C.; Lynam, E.; Landfair, D.J.; Janjic, N.; Wiles, M.E. Oligonucleotide NX1838 Inhibits VEGF165-Mediated Cellular Responses In Vitro. Vitr. Cell. Dev. Biol. Anim. 1999, 35, 533–542. [Google Scholar] [CrossRef]
- Holash, J.; Davis, S.; Papadopoulos, N.; Croll, S.D.; Ho, L.; Russell, M.; Boland, P.; Leidich, R.; Hylton, D.; Burova, E.; et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. USA 2002, 99, 11393–11398. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Yan, M.; Li, H.; Yang, C.; Yu, D. Recombinant anti-vascular endothelial growth factor fusion protein efficiently suppresses choridal neovasularization in monkeys. Mol. Vis. 2008, 14, 37–49. [Google Scholar]
- Wang, Q.; Li, T.; Wu, Z.; Wu, Q.; Ke, X.; Luo, D.; Wang, H. Novel VEGF Decoy Receptor Fusion Protein Conbercept Targeting Multiple VEGF Isoforms Provide Remarkable Anti-Angiogenesis Effect In Vivo. PLoS ONE 2013, 8, e70544. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Cao, Z.; Ji, H.; Yang, X.; Iwamoto, H.; Wahlberg, E.; Länne, T.; Sun, B.; Cao, Y. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc. Natl. Acad. Sci. USA 2013, 110, 12018–12023. [Google Scholar] [CrossRef]
- Gavilondo, J.V.; Hernández-Bernal, F.; Ayala-Ávila, M.; de la Torre, A.V.; de la Torre, J.; Morera-Díaz, Y.; Bequet-Romero, M.; Sánchez, J.; Valenzuela, C.M.; Martín, Y.; et al. Specific active immunotherapy with a VEGF vaccine in patients with advanced solid tumors. Results of the CENTAURO antigen dose escalation phase I clinical trial. Vaccine 2014, 32, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Hosseini, S.A.; Astaneh, M.; Kesharwani, P.; Jaafari, M.R.; Sahebkar, A. Application of VEGF/VEGFR peptide vaccines in cancer: A systematic review of clinical trials. Crit. Rev. Oncol. Hematol. 2023, 187, 104032. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Chen, H.; Rojas, J.; Sampath, P.; Thorne, S.H. Oncolytic Vaccinia Virus Demonstrates Anti-angiogenic Effects Mediated by Targeting of VEGF. Int. J. Cancer 2014, 135, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, I.; Kaeppler, J.; Frost, S.; Seymour, L.W.; Jacobus, E.J. Attenuation of the hypoxia inducible factor pathway after oncolytic adenovirus infection coincides with decreased vessel perfusion. Cancers 2020, 12, 851. [Google Scholar] [CrossRef] [PubMed]
- Benencia, F.; Courreges, M.C.; Conejo-garcía, J.R.; Buckanovich, R.J.; Zhang, L.; Carroll, R.H.; Morgan, M.A.; Coukos, G. Oncolytic HSV Exerts Direct Antiangiogenic Activity in Ovarian Carcinoma. Hum. Gene Ther. 2005, 16, 765–778. [Google Scholar] [CrossRef]
- Breitbach, C.J.; De Silva, N.S.; Falls, T.J.; Aladl, U.; Evgin, L.; Paterson, J.; Sun, Y.Y.; Roy, D.G.; Rintoul, J.L.; Daneshmand, M.; et al. Targeting Tumor Vasculature With an Oncolytic Virus. Mol. Ther. 2011, 19, 886–894. [Google Scholar] [CrossRef]
- Bell, J.; Mcfadden, G. Viruses for Tumor Therapy. Cell Host Microbe 2014, 15, 260–265. [Google Scholar] [CrossRef]
- Eshun, F.K.; Currier, M.A.; Gillespie, R.A.; Fitzpatrick, J.L.; Baird, W.H.; Cripe, T.P. VEGF blockade decreases the tumor uptake of systemic oncolytic herpes virus but enhances therapeutic efficacy when given after virotherapy. Gene Ther. 2010, 17, 922–929. [Google Scholar] [CrossRef]
- Rhim, J.H.; Tosato, G. Targeting the Tumor Vasculature to Improve the Efficacy of Oncolytic Virus Therapy. J. Natl. Cancer Inst. 2007, 99, 1739–1741. [Google Scholar] [CrossRef][Green Version]
- Kottke, T.; Hall, G.; Pulido, J.; Diaz, R.M.; Thompson, J.; Chong, H.; Selby, P.; Coffey, M.; Pandha, H.; Chester, J.; et al. Antiangiogenic cancer therapy combined with oncolytic virotherapy leads to regression of established tumors in mice. J. Clin. Investig. 2010, 120, 1551–1560. [Google Scholar] [CrossRef]
- Jha, B.K.; Dong, B.; Nguyen, C.T.; Polyakova, I.; Silverman, R.H. Suppression of Antiviral Innate Immunity by Sunitinib Enhances Oncolytic Virotherapy. Mol. Ther. 2013, 21, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Libertini, S.; Iacuzzo, I.; Perruolo, G.; Scala, S.; Ierano, C.; Franco, R.; Hallden, G.; Portella, G. Bevacizumab Increases Viral Distribution in Human Anaplastic Thyroid Carcinoma Xenografts and Enhances the Effects of E1A-Defective Adenovirus dl 922-947. Clin. Cancer Res. 2008, 14, 6505–6514. [Google Scholar] [CrossRef] [PubMed]
- Thaci, B.; Ulasov, I.V.; Ahmed, A.U.; Ferguson, S.D.; Han, Y.; Lesniak, M.S. Anti-angiogenic therapy increases intratumoral adenovirus distribution by inducing collagen degradation. Gene Ther. 2013, 20, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Currier, M.A.; Eshun, F.K.; Sholl, A.; Chernoguz, A.; Crawford, K.; Divanovic, S.; Boon, L.; Goins, W.F.; Frischer, J.S.; Collins, M.H.; et al. VEGF blockade enables oncolytic cancer virotherapy in part by modulating intratumoral myeloid cells. Mol. Ther. 2013, 21, 1014–1023. [Google Scholar] [CrossRef]
- Tan, G.; Kasuya, H.; Sahin, T.T.; Yamamura, K.; Wu, Z.; Koide, Y.; Hotta, Y.; Shikano, T.; Yamada, S.; Kanzaki, A.; et al. Combination therapy of oncolytic herpes simplex virus HF10 and bevacizumab against experimental model of human breast carcinoma xenograft. Int. J. Biol. Sci. 2015, 136, 1718–1730. [Google Scholar] [CrossRef]
- Tomita, Y.; Kurozumi, K.; Yoo, J.Y.; Fujii, K.; Ichikawa, T.; Matsumoto, Y.; Uneda, A.; Hattori, Y.; Shimizu, T.; Otani, Y.; et al. Oncolytic herpes virus armed with vasculostatin in combination with bevacizumab abrogates glioma invasion via the CCN1 and AKT signaling pathways. Mol. Cancer Ther. 2019, 18, 1418–1429. [Google Scholar] [CrossRef]
- Hu, J.; Chen, C.; Lu, R.; Zhang, Y.; Wang, Y.; Hu, Q.; Li, W.; Wang, S.; Jing, O.; Yi, H.; et al. β -Adrenergic Receptor Inhibitor and Oncolytic Herpesvirus Combination Therapy Shows Enhanced Antitumoral and Antiangiogenic Effects on Colorectal Cancer. Front. Pharmacol. 2021, 12, 735278. [Google Scholar] [CrossRef]
- Heo, J.; Breitbach, C.J.; Moon, A.; Kim, C.W.; Patt, R.; Kim, M.K.; Lee, Y.K.; Oh, S.Y.; Woo, H.Y.; Parato, K.; et al. Sequential Therapy With JX-594, A Targeted Oncolytic Poxvirus, Followed by Sorafenib in Hepatocellular Carcinoma: Preclinical and Clinical Demonstration of Combination Efficacy. Mol. Ther. 2009, 19, 1170–1179. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, W.; Wang, J.; Gu, J.; Dang, Y.; Li, B.; Zhao, L.; Qian, C.; Qian, Q.; Liu, X. Suppression of Tumor Growth by Oncolytic Adenovirus-Mediated Delivery of an Antiangiogenic Gene, Soluble Flt-1. Mol. Ther. 2005, 11, 553–562. [Google Scholar] [CrossRef]
- Thorne, S.H.; Tam, B.Y.Y.; Kirn, D.H.; Contag, C.H.; Kuo, C.J. Selective Intratumoral Amplification of an Antiangiogenic Vector by an Oncolytic Virus Produces Enhanced Antivascular and Anti-tumor Efficacy. Mol. Ther. 2006, 13, 938–946. [Google Scholar] [CrossRef]
- Bazan-Peregrino, M.; Sainson, R.C.A.; Carlisle, R.C.; Thoma, C.; Waters, R.A.; Arvanitis, C.; Harris, A.L.; Hernandez-Alcoceba, R.; Seymour, L.W. Combining virotherapy and angiotherapy for the treatment of breast cancer. Cancer Gene Ther. 2013, 20, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.K.; Shin, H.; Oh, E.; Yoo, J.Y.; Hwang, J.K.; Shin, K.; Yu, D.C.; Yun, C.O. Potent and long-term antiangiogenic efficacy mediated by FP3-expressing oncolytic adenovirus. Int. J. Cancer 2015, 137, 2253–2269. [Google Scholar] [CrossRef] [PubMed]
- Marino, N.; Illingworth, S.; Kodialbail, P.; Patel, A.; Calderon, H.; Lear, R.; Fisher, K.D.; Champion, B.R.; Brown, A.C.N. Development of a versatile oncolytic virus platform for local intra-tumoural expression of therapeutic transgenes. PLoS ONE 2017, 12, e0177810. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Kim, J.; Kwon, Y.; Kim, E.; Kim, N.K.; Choi, H.J.; Yun, C. VEGF-specific Short Hairpin RNA—Expressing Oncolytic Adenovirus Elicits Potent Inhibition of Angiogenesis and Tumor Growth. Mol. Ther. 2007, 15, 295–302. [Google Scholar] [CrossRef]
- Kang, Y.A.; Shin, H.C.; Yoo, J.Y.; Kim, J.H.; Kim, J.S.; Yun, C.O. Novel cancer antiangiotherapy using the VEGF promoter-targeted artificial zinc-finger protein and oncolytic adenovirus. Mol. Ther. 2008, 16, 1033–1040. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, H.; Zhu, H.; Zhang, W.; Sun, D.; Han, X.; Liu, Y.; Luo, G. RCAd-LTH-shPD-L1, a double- gene recombinant oncolytic adenovirus with enhanced antitumor immunity, increases lymphocyte infiltration and reshapes the tumor microenvironment. J. Immunother. Cancer 2024, 12, e007171. [Google Scholar] [CrossRef]
- Frentzen, A.; Yu, Y.A.; Chen, N.; Zhang, Q.; Weibel, S.; Raab, V.; Szalay, A.A. Anti-VEGF single-chain antibody GLAF-1 encoded by oncolytic vaccinia virus significantly enhances antitumor therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 12915–12920. [Google Scholar] [CrossRef]
- Guse, K.; Sloniecka, M.; Diaconu, I.; Ottolino-perry, K.; Tang, N.; Ng, C.; Le Boeuf, F.; Bell, J.C.; Mccart, J.A.; Ristima, A.; et al. Antiangiogenic Arming of an Oncolytic Vaccinia Virus Enhances Antitumor Efficacy in Renal Cell Cancer Models. J. Virol. 2010, 84, 856–866. [Google Scholar] [CrossRef]
- Gholami, S.; Marano, A.; Chen, N.G.; Aguilar, R.J.; Frentzen, A.; Chen, C.; Lou, E.; Fujisawa, S.; Eveno, C.; Belin, L.; et al. A Novel Vaccinia Virus with Dual Oncolytic and Anti-angiogenic Therapeutic Effects against Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2014, 148, 489–499. [Google Scholar] [CrossRef]
- Buckel, L.; Advani, S.J.; Frentzen, A.; Zhang, Q.; Yu, Y.A.; Chen, N.G.; Ehrig, K.; Stritzker, J.; Mundt, A.J.; Szalay, A.A. Combination of fractionated irradiation with anti-VEGF expressing vaccinia virus therapy enhances tumor control by simultaneous radiosensitization of tumor associated endothelium. Int. J. Cancer 2013, 133, 2989–2999. [Google Scholar] [CrossRef]
- Huang, T.; Wang, H.; Chen, N.G.; Frentzen, A.; Minev, B.; Szalay, A.A. Expression of anti-VEGF antibody together with anti-EGFR or anti-FAP enhances tumor regression as a result of vaccinia virotherapy. Mol. Ther. Oncolytics 2015, 2, 15003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fulci, G.; Buhrman, J.S.; Stemmer-rachamimov, A.O.; Chen, J.W.; Wojtkiewicz, G.R.; Weissleder, R.; Rabkin, S.D.; Martuza, R.L. Bevacizumab With Angiostatin-armed oHSV Increases Antiangiogenesis and Decreases Bevacizumab-induced Invasion in U87 Glioma. Mol. Ther. 2012, 20, 37–45. [Google Scholar] [CrossRef]
- Lavie, M.; Struyf, S.; Stroh-Dege, A.; Rommelaere, J.; Van Damme, J.; Dinsart, C. Capacity of wild-type and chemokine-armed parvovirus H-1PV for inhibiting neo-angiogenesis. Virology 2013, 447, 221–232. [Google Scholar] [CrossRef][Green Version]
- Santiago-Ortiz, J.L.; Schaffer, D.V. Adeno-associated virus (AAV) vectors in cancer gene therapy. J. Control. Release 2016, 240, 287–301. [Google Scholar] [CrossRef]
- Hacker, U.T.; Bentler, M.; Kaniowska, D.; Morgan, M.; Büning, H. Towards Clinical Implementation of Adeno-Associated Current Status and Future Perspectives. Cancers 2020, 12, 1889. [Google Scholar] [CrossRef]
- Takei, Y.; Mizukami, H.; Saga, Y.; Yoshimura, I.; Hasumi, Y.; Takayama, T.; Kohno, T.; Matsushita, T.; Okada, T.; Kume, A.; et al. Suppression of ovarian cancer by muscle-mediated expression of soluble VEGFR-1 / Flt-1 using adeno-associated virus serotype 1-derived vector. Int. J. Cancer 2006, 120, 278–284. [Google Scholar] [CrossRef]
- Davidoff, A.M.; Nathwani, A.C.; Spurbeck, W.W.; Ng, C.Y.C.; Zhou, J.; Vanin, E.F. rAAV-mediated Long-term Liver-generated Expression of an Angiogenesis Inhibitor Can Restrict Renal Tumor Growth in Mice. Cancer Res. 2002, 62, 3077–3083. [Google Scholar]
- Streck, C.J.; Zhou, J.; Ng, C.Y.; Zhang, Y.; Nathwani, A.C.; Davidoff, A.M. Longterm Recombinant Adeno-Associated, Virus-Mediated, Liver-Generated Expression of an Angiogenesis Inhibitor Improves Survival in Mice with Disseminated Neuroblastoma. J. Am. Coll. Surg. 2004, 199, 78–86. [Google Scholar] [CrossRef]
- Mahendra, G.; Kumar, S.; Isayeva, T.; Mahasreshti, P.J.; Curiel, D.T.; Stockardt, C.R.; Grizzle, W.E.; Alapati, V.; Singh, R.; Siegal, G.P.; et al. Antiangiogenic cancer gene therapy by adeno-associated virus 2-mediated stable expression of the soluble FMS-like tyrosine kinase-1 receptor. Cancer Gene Ther. 2005, 12, 26–34. [Google Scholar] [CrossRef]
- Lu, L.; Luo, S.T.; Shi, H.S.; Li, M.; Zhang, H.L.; He, S.S.; Liu, Y.; Pan, Y.; Yang, L. AAV2-mediated gene transfer of VEGF-Trap with potent suppression of primary breast tumor growth and spontaneous pulmonary metastases by long-term expression. Oncol. Rep. 2012, 28, 1332–1338. [Google Scholar] [CrossRef]
- Li, J.; Zhu, P.; Wang, L.; Yang, L.; Zou, L.; Gao, F. Study of diffusion-weighted magnetic resonance imaging in the evaluation of the response to AAV2-VEGF-Trap neoadjuvant treatment in a triple-negative breast cancer animal model. Cancer Med. 2019, 8, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Y.; Wang, L.; Yang, L.; Zou, L.; Gao, F. Adeno-associated virus 2 mediated gene transfer of vascular endothelial growth factor Trap: A new treatment option for glioma. Cancer Biol. Ther. 2019, 20, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Harding, T.C.; Lalani, A.S.; Roberts, B.N.; Yendluri, S.; Luan, B.; Koprivnikar, K.E.; Gonzalez-edick, M.; Huan-tu, G.; Musterer, R.; Vanroey, M.J.; et al. AAV Serotype 8-Mediated Gene Delivery of a Soluble VEGF Receptor to the CNS for the Treatment of Glioblastoma. Mol. Ther. 2006, 13, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lalani, A.S.; Harding, T.C.; Gonzalez, M.; Wu, W.; Luan, B.; Tu, G.H.; Koprivnikar, K.; Vanroey, M.J.; He, Y.; et al. Inhibition of Lymphogenous Metastasis Using Adeno-Associated Virus-Mediated Gene Transfer of a Soluble VEGFR-3 Decoy Receptor. Cancer Res. 2005, 65, 6901–6909. [Google Scholar] [CrossRef] [PubMed]
- Kujala, A.; Valkonen, E.; Sallinen, H.; Tuppurainen, L.; Laakso, H.; Ylä-Herttuala, E.; Liimatainen, T.; Kujala, J.; Jokelainen, O.; Sironen, R.; et al. AAV8-mediated sVEGFR2 and sVEGFR3 gene therapy combined with chemotherapy reduces the growth and microvasculature of human ovarian cancer and prolongs the survival in mice. Front. Med. 2022, 9, 1018208. [Google Scholar] [CrossRef]
- Watanabe, M.; Boyer, J.L.; Crystal, R.G. AAVrh.10-mediated Genetic Delivery of Bevacizumab to the Pleura to Provide Local Anti-VEGF to Suppress Growth of Metastatic Lung Tumors. Gene Ther. 2010, 17, 1042–1051. [Google Scholar] [CrossRef]
- Xie, Y.; Hicks, M.J.; Kaminsky, S.M.; Moore, M.A.S.; Crystal, R.G.; Rafii, A. AAV-mediated Persistent Bevacizumab Therapy Suppresses Tumor Growth of Ovarian Cancer. Gynecol. Oncol. 2014, 135, 325–332. [Google Scholar] [CrossRef]
- Hicks, M.J.; Funato, K.; Wang, L.; Aronowitz, E.; Dyke, J.P.; Ballon, D.J.; Havlicek, D.F.; Frenk, E.Z.; De, P.B.; Chiuchiolo, M.J.; et al. Genetic Modification of Neurons to Express Bevacizumab for Local Anti-angiogenesis Treatment of Glioblastoma. Cancer Gene Ther. 2015, 22, 1–8. [Google Scholar] [CrossRef][Green Version]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene Therapy Clinical Trials Worldwide to 2017: An update. J. Control. Release 2021, 338, 610–622. [Google Scholar] [CrossRef]
- Hughes, C.P.; Flynn, N.M.J.O.; Gatherer, M.; Mcclements, M.E.; Scott, J.A.; Maclaren, R.E.; Goverdhan, S.; Glennie, M.J.; Lotery, A.J. AAV2/8 Anti-angiogenic Gene Therapy Using Single-Chain Antibodies Inhibits Murine Choroidal Neovascularization. Mol. Ther. Methods Clin. Dev. 2019, 13, 86–98. [Google Scholar] [CrossRef]
- Zhao, Z.; Anselmo, A.C.; Mitragotri, S. Viral vector-based gene therapies in the clinic. Bioeng. Transl. Med. 2021, 20, e10258. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Weigelt, C.M.; Fuchs, H.; Viollet, C.; Rust, W.; Wyatt, H.; Huber, J.; Lamla, T.; Albert, F.F.; Simon, E.; et al. Transcriptome analysis of AAV-induced retinopathy models expressing human VEGF, TNF-α, and IL-6 in murine eyes. Sci. Rep. 2022, 12, 19395. [Google Scholar] [CrossRef] [PubMed]
- Spilsbury, K.; Garrett, K.L.; Shen, W.; Constable, I.J.; Rakoczy, P.E. Overexpression of Vascular Endothelial Growth Factor (VEGF) in the Retinal Pigment Epithelium Leads to the Development of Choroidal Neovascularization Neovascularization. Am. J. Pathol. 2000, 157, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Rendahl, K.G.; Manning, W.C.; Quiroz, D.; Coyne, M.; Miller, S.S. AAV—Mediated Expression of Vascular Endothelial Growth Factor Induces Choroidal Neovascularization in Rat. Investig. Ophthalmol. Vis. Sci. 2003, 44, 781–790. [Google Scholar] [CrossRef]
- Weigelt, C.M.; Fuchs, H.; Schönberger, T.; Stierstorfer, B.; Strobel, B.; Lamla, T.; Ciossek, T.; Bakker, R.A.; Redemann, N.H. AAV-Mediated Expression of Human VEGF, TNF-α, and IL-6 Induces Retinal Pathology in Mice. Transl. Vis. Sci. Technol. 2021, 10, 15. [Google Scholar] [CrossRef]
- Liu, Y.; Long, L.; Zhang, F.; Hu, X.; Zhang, J.; Hu, C.; Wang, Y.; Xu, J. Microneedle-mediated vascular endothelial growth factor delivery promotes angiogenesis and functional recovery after stroke. J. Control. Release 2021, 338, 610–622. [Google Scholar] [CrossRef]
- Hsu, S.; Zhang, C.; Jeong, J.; Lee, S.; Mcconnell, M.; Iwakiri, Y. Enhanced meningeal lymphatic drainage ameliorates neuroinflammation and hepatic encephalopathy in cirrhotic rats. Gastroenterology 2021, 160, 1315–1329. [Google Scholar] [CrossRef]
- Simoes, L.; Boisserand, B.; Geraldo, L.H.; Bouchart, J.; El Kamouh, M.; Lee, S.; Sanganahalli, B.G.; Sapjer, M.; Zhang, S.; Lee, S.; et al. VEGF-C prophylaxis favors lymphatic drainage and modulates neuroinflammation in a stroke model. J. Exp. Med. 2024, 221, e20221983. [Google Scholar] [CrossRef]
- Miao, X.; Lin, J.; Li, A.; Gao, T.; Liu, T.; Shen, J.; Sun, Y.; Wei, J.; Bao, B.; Zheng, X. AAV-mediated VEGFA overexpression promotes angiogenesis and recovery of locomotor function following spinal cord injury via PI3K/Akt signaling. Exp. Neurol. 2024, 375, 114739. [Google Scholar] [CrossRef]
- Rezaie, E.S.; Visser, N.J.; Van Den Berg, C.; Friedrich, P.F.; Shin, A.Y.; Bishop, A.T. Vasculogenic gene therapy: No role for revitalization of structural bone allografts. J. Orthop. Res. 2023, 41, 1014–1021. [Google Scholar] [CrossRef]
- Gelfman, C.M.; Grishanin, R.; Bender, K.O.; Nguyen, A.; Greengard, J.; Sharma, P.; Nieves, J.; Kiss, S.; Gasmi, M. Comprehensive Preclinical Assessment of ADVM-022, an Intravitreal Anti-VEGF Gene Therapy for the Treatment of Neovascular AMD and Diabetic Macular Edema. J. Ocul. Pharmacol. Ther. 2021, 37, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Luo, Y.; Malachi, A.; Ko, J.; Su, Q.; Xie, J.; Tian, B.; Lin, H.; Ke, X.; Zheng, Q.; et al. Low-Dose Recombinant Adeno-Associated Virus-Mediated Inhibition of Vascular Endothelial Growth Factor Can Treat Neovascular Pathologies Without Inducing Retinal Vasculitis. Hum. Gene Ther. 2021, 32, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhao, S.; Liu, Z. Prevention of lumbar disc degeneration through co-manipulation of insulin-like growth factor 1 and vascular endothelial growth factor. Ann. Transl. Med. 2021, 9, 1572. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, P.; Verma, A.; Prasad, T.; Deng, H.; Yu, D.; Li, Q. A novel bispecific molecule delivered by recombinant AAV2 suppresses ocular inflammation and choroidal neovascularization. J. Cell Mol. Med. 2017, 21, 1555–1571. [Google Scholar] [CrossRef]
- Grishanin, R.; Vuillemenot, B.; Sharma, P.; Keravala, A.; Greengard, J.; Gelfman, C.; Blumenkrantz, M.; Lawrence, M.; Hu, W.; Kiss, S.; et al. Preclinical Evaluation of ADVM-022, a Novel Gene Therapy Approach to Treating Wet Age-Related Macular Degeneration. Mol. Ther. 2019, 27, 118–129. [Google Scholar] [CrossRef]
- Kiss, S.; Grishanin, R.; Nguyen, A.; Rosario, R.; Greengard, J.S.; Nieves, J.; Gelfman, C.M.; Gasmi, M. Analysis of Aflibercept Expression in NHPs following Intravitreal Administration of ADVM-022, a Potential Gene Therapy for nAMD. Mol. Ther. Methods Clin. Dev. 2020, 18, 345–353. [Google Scholar] [CrossRef]
- Kiss, S.; Bender, K.O.; Grishanin, R.N.; Hanna, K.M.; Nieves, J.D.; Sharma, P.; Nguyen, A.T.; Rosario, R.J.; Greengard, J.S.; Gelfman, C.M.; et al. Long-term safety evaluation of continuous intraocular delivery of aflibercept by the intravitreal gene therapy candidate ADVM-022 in nonhuman primates. Transl. Vis. Sci. Technol. 2021, 10, 34. [Google Scholar] [CrossRef]
- Askou, A.L.; Alsing, S.; Benckendorff, J.N.E.; Holmgaard, A.; Mikkelsen, J.G.; Aagaard, L.; Bek, T.; Corydon, T.J. Suppression of Choroidal Neovascularization by AAV-Based Dual-Acting Antiangiogenic Gene Therapy. Mol. Ther. Nucleic Acids 2019, 16, 38–50. [Google Scholar] [CrossRef]
- She, K.; Su, J.; Wang, Q.; Liu, Y.; Zhong, X.; Jin, X.; Zhao, Q.; Xiao, J.; Li, R.; Deng, H.; et al. Delivery of nVEGFi using AAV8 for the treatment of neovascular age-related macular degeneration. Mol. Ther. Methods Clin. Dev. 2022, 24, 210–221. [Google Scholar] [CrossRef]
- Haldrup, S.H.; Fabian-jessing, B.K.; Jakobsen, T.S.; Lindholm, A.B.; Adsersen, R.L.; Aagaard, L.; Bek, T.; Askou, A.L.; Corydon, T.J. Subretinal AAV delivery of RNAi-therapeutics targeting VEGFA reduces choroidal neovascularization in a large animal model. Mol. Ther. Methods Clin. Dev. 2024, 32, 101242. [Google Scholar] [CrossRef]
- Chung, S.H.; Sin, T.; Dang, B.; Ngo, T.; Lo, T.; Lent-schochet, D.; Meleppat, R.K.; Zawadzki, R.J.; Yiu, G. CRISPR-based VEGF suppression using paired guide RNAs for treatment of choroidal neovascularization. Mol. Ther. Nucleic Acids 2022, 28, 613–622. [Google Scholar] [CrossRef]
- Puranen, J.; Koponen, S.; Nieminen, T.; Kanerva, I.; Kokki, E.; Toivanen, P.; Urtti, A.; Ylä-Herttuala, S.; Ruponen, M. Antiangiogenic AAV2 gene therapy with a truncated form of soluble VEGFR-2 reduces the growth of choroidal neovascularization in mice after intravitreal injection. Exp. Eye Res. 2022, 224, 109237. [Google Scholar] [CrossRef]
| Name | Sequence | Binding | Features | Results | Refs |
|---|---|---|---|---|---|
| N6G | NIRRQG | VEGFR1 | Selected from a random phage display library | Less efficient binding than P6L | [75] |
| VGB4 | KQLVIKPHGQILMIRYPSSQLEM | VEGFR1 and VEGFR2 | Chimeric and overlapping peptide of VEGF-A and VEGF-B | Inhibition of tumor growth, metastasis and signaling patways | [76,77,78] |
| VGB3 | ECRPPDDGLC | VEGFR1 and VEGFR2 | Circular peptide reproducing VEGF-B | Anti-angiogenic and anti-tumor activity | [79,80] |
| PCAIWF | PCAIWF | VEGFR-1, VEGFR-2, and VEGFR-3 | Selected from a random phage display library | Inhibition of tube formation and retinal neovascularization | [81] |
| P6L | PQPRPL | VEGFR1 and NRP-1 | Overlapping peptide of VEGF-B167 and VEGF-B186 | The RPL peptide is essential for the binding | [75,82] |
| Vasotide | D(CLPRC) | VEGFR1 and NRP-1 | Cyclic peptide of retro-inverted peptidomimetic RPL from P6L | Anti-angiogenic activity in retinopathy models and inhibition of tumor growth | [83,84] |
| A7R | ATWLPPR | NRP-1 | Mimicks VEGF-A165 structure | Decreased VEGF-mediated epithelial cell proliferation and tubular formation in vivo | [85,86] |
| MY1340 | [(TKPRKHG)2-K]2-K-G | NRP-1 | VEGF c-terminal polypeptide | Anti-tumor activity in vivo | [87] |
| Oncolytic Virus | Transgen | Experimental Assay | Results | References |
|---|---|---|---|---|
| Adenovirus (ZD55) | Soluble VEGFR1 | IT * administration in xenograft subcutaneous tumor mouse model of SW620 colon cancer | Reduction in tumor growth and angiogenesis. Synergetic effect with 5-FU chemotherapy | [149] |
| Replication competent and deficient Adenovirus | Soluble VEGFR2 | IV ** injection in subcutaneous xenograft tumor mouse model of HCT 116 colon and PC-3 prostate cancer | Enhanced anti-tumor effect in coinfection treatments | [150] |
| Adenovirus (AdEHE2F) | Soluble VEGFR1 | IT * and subcutaneous administrations in murine models of ER-positive/negative breast cancer tumors. | Enhanced anti-tumor effect in ER-negative tumor | [151] |
| Adenovirus (RdB) | Soluble VEGFR3 | IT * administration in subcutaneous xenograft mouse model of lung carcinoma tumor (H460) | Improved antiangiogenic and antitumor effects | [152] |
| Adenovirus (enadenotucirev) | Anti-VEGF antibodies (NG-135) | IV ** injection in subcutaneous xenograft mouse model of A549 lung cancer tumor | Tumor burden reduction | [153] |
| Incompetent Adenovirus (Ad B7and Ad-E1) | VEGF shRNA | IT * administration in xenograft mouse model of U343 glioblastoma | Reduction in tumor growth and angiogenesis | [154] |
| Adenovirus Ad-B7-KOX | Zinc finger protein (ZFP) against VEGF promoter | IT * administration in subcutaneous xenograft mouse model of U87 human glioblastoma | Reduction in angiogenesis increasing tumor apoptosis and survival | [155] |
| Recombinant Adenovirus RCAd derived from Ad5 | Anti-VEGF antibody and PD-L1 shRNA | IT * administration in mouse models of subcutaneous xenograft and humanized immune system of U87 human glioblastoma. | Reduction of VEGF-A level and immune activation | [156] |
| Adenovirus + Vaccinia virus | Soluble VEGFR2 | IV ** injection in subcutaneous xenograft mouse model of breast cancer tumor (MDA) | Tumor remission upon AdV-VEGFR2 administration after VV | [133] |
| Replication-competent Vaccinia virus (GLV-1h68) | Single-chain (sc)anti-VEGF antibody (GLAF-1) | Single IV ** injection in subcutaneous xenograft mouse models of human tumors (DU-145 and A549) | Higher oncolytic activity than antibody monotherapy | [157] |
| Double deleted Vaccinia viruses | VEGFR-1-Ig | IT * administration in subcutaneous xenograft immunocompetent mouse model of kidney cancer (786-O). | Higher antitumor and antiangiogenic effects with reduced cytokine response | [158] |
| Vaccinia virus (GLV-1h164) | scAnti-VEGF (GLAF-2) | IT * administration in orthotopic xenograft mouse model of TNBC tumor (MDA-MB-468) | Tumor regression and anti-angiogenic effect | [159] |
| Replication-competent Vaccinia virus (GLV-1h68) | (sc)Anti-VEGF antibody (GLAF-2) and radiation | Retro-orbital injection in subcutaneous xenograft mouse model of human U87 glioblastoma | Increased viral replication and oncolysis. Anti-angiogenic effects and high radio-sensitivity | [160] |
| Replication-competent Vaccinia virus (GLV-1h68) | Anti-VEGF, anti-EGFR, and anti-FAP antibodies | Retro-orbital injection in subcutaneous xenograft mouse model of prostate tumor (DU145) | Inhibition of tumor growth and angiogenesis | [161] |
| Herpes simplex virus G47Δ | Angiostatin and bevacizumab (Avastin®) | IT * administration in orthotopic xenograft mouse model of human U87 glioblastoma | Combined treatment enhanced virus spread, tumor lysis, antiangiogenic activity and survival | [162] |
| Parvovirus H-1PV | Chemokines | IT * administration in a murine tumor model of subcutaneous implanted Kaposi sarcoma cells | Inhibition of tumor growth and VEGF expression | [163] |
| Serotype | Transgen | Assay and Experimental Model | Results | References |
|---|---|---|---|---|
| AAV1 | Soluble VEGFR1 | Skeletal muscle administration in mouse models of subcutaneous and intraperitoneal ovarian cancer | Inhibition of tumor growth and peritoneal dissemination with no adverse events | [166] |
| AAV2 | Truncated soluble VEGFR2 | Intraportal injection in orthotopic murine models of pediatric kidney tumors | Restriction of tumor development and growth | [167] |
| AAV2 | Truncated soluble VEGFR2 | Intraportal injection in murine model of metastatic neuroblastoma | Inhibition of liver metastasis and tumor vascularity with longer survival | [168] |
| AAV2 | Soluble VEGFR1 | Xenograft murine model of human ovarian tumor (SKOV3.ip1) | Tumor inhibition and increased disease-free survival | [169] |
| AAV2 | VEGF-Trap | Single intravenous injection in mouse model of breast carcinoma | Suppression of tumor growth and metastases with reduced tumor vascularization | [170] |
| AAV2 | VEGF-Trap + paclitaxel | Single intravenous injection in xenograft mouse model of triple negative breast cancer (TNBC) | Inhibition of tumor growth and angiogenesis. Synergistic effect in combination with paclitaxel | [171] |
| AAV2 | VEGF-Trap + temozolomide (TMZ) | Single intravenous injection in rat model of C6 glioma | Inhibition of tumor growth and angiogenesis. Synergistic effect combined with TMZ | [172] |
| AAV8 | Soluble VEGFR1/R2 | Intracranial administration in orthotopic mouse model of glioblastoma | Reduction in overall tumor volume and longer survival | [173] |
| AAV8 | Soluble VEGFR3-Fc | Intramuscular or intravenous administrations in xenograft metastatic murine model of human melanoma, kidney and prostate cancers | Inhibition of metastasis and lymphangiogenesis in melanoma, kidney, and prostate cancers | [174] |
| AAV8 | sVEGFR2/R3 + paclitaxel + carboplatin | Intravenous injection in xenograft mouse model of ovarian cancer | Inhibition of tumor growth and angiogenesis in combined treatments with chemotherapy | [175] |
| AAVrh.10 | Bevacizumab (Avastin®) | Intrapleural administration in mouse models of metastatic prostate carcinoma | Suppression of metastatic lung tumor growth and reduced tumor vascularization | [176] |
| AAVrh.10 | Bevacizumab (Avastin®) | Single intraperitoneal injection in mouse model of ovarian cancer | Suppression of tumor growth and angiogenesis with higher survival | [177] |
| AAVrh.10 | Bevacizumab (Avastin®) | Intracranial administration in xenograft mouse model of glioblastoma | Reduction of tumor volume and angiogenesis | [178] |
| Vector | Disease | Transgen | Assay | Results | References |
|---|---|---|---|---|---|
| AAV | Ischemic stroke | VEGF-A | Rat model of ischemic stroke | Increase functional angiogenesis and neurogenesis | [186] |
| AAV8 | Hepatic encephalopathy | VEGF-C | Cirrhotic rat model | Increase lympho-angiogenesis and disease improvement | [187] |
| AAV9 | Ischemic stroke | VEGF-C | Murine model of ischemic stroke | Increase neurogenesis and neuroprotection in pretreated animals | [188] |
| AAV9 | Spinal cord injury | VEGF-A | Murine model of spinal cord injury | Locomotor function recovery and tissue damage protection in pretreated animals | [189] |
| AAV9 | Segmental bone defects | VEGF-A +PDGF | Yucatan mini-pig model of tibial diaphyseal defect | Bone revascularization, remodeling and healing, but development of vascular tumors | [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Martínez, C.; Grueso, E.; Calvo-López, T.; Martinez-Ortega, J.; Ruiz, A.; Almendral, J.M. VEGF—Virus Interactions: Pathogenic Mechanisms and Therapeutic Applications. Cells 2024, 13, 1815. https://doi.org/10.3390/cells13211815
Sánchez-Martínez C, Grueso E, Calvo-López T, Martinez-Ortega J, Ruiz A, Almendral JM. VEGF—Virus Interactions: Pathogenic Mechanisms and Therapeutic Applications. Cells. 2024; 13(21):1815. https://doi.org/10.3390/cells13211815
Chicago/Turabian StyleSánchez-Martínez, Cristina, Esther Grueso, Tania Calvo-López, Jorge Martinez-Ortega, Ana Ruiz, and José M. Almendral. 2024. "VEGF—Virus Interactions: Pathogenic Mechanisms and Therapeutic Applications" Cells 13, no. 21: 1815. https://doi.org/10.3390/cells13211815
APA StyleSánchez-Martínez, C., Grueso, E., Calvo-López, T., Martinez-Ortega, J., Ruiz, A., & Almendral, J. M. (2024). VEGF—Virus Interactions: Pathogenic Mechanisms and Therapeutic Applications. Cells, 13(21), 1815. https://doi.org/10.3390/cells13211815







