Sox17 and Other SoxF-Family Proteins Play Key Roles in the Hematopoiesis of Mouse Embryos
Abstract
:1. Introduction
2. Hematopoiesis in Mice
3. Phenotypes in Transcription Factors SoxF Family Genes-Deficient Mice
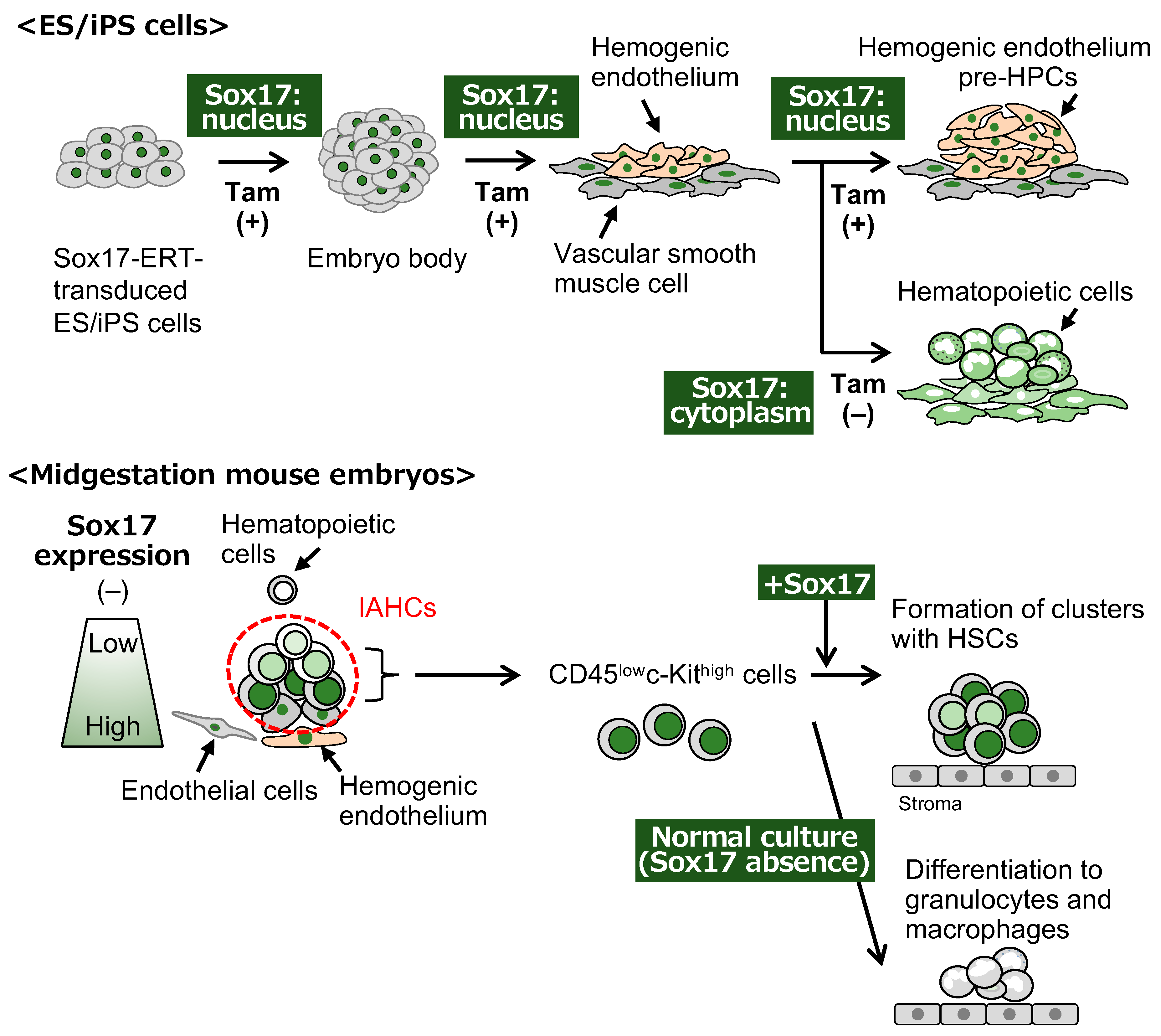
4. Function of SoxF Proteins in Early Embryonic and Hematopoietic Development in the Mouse Embryo
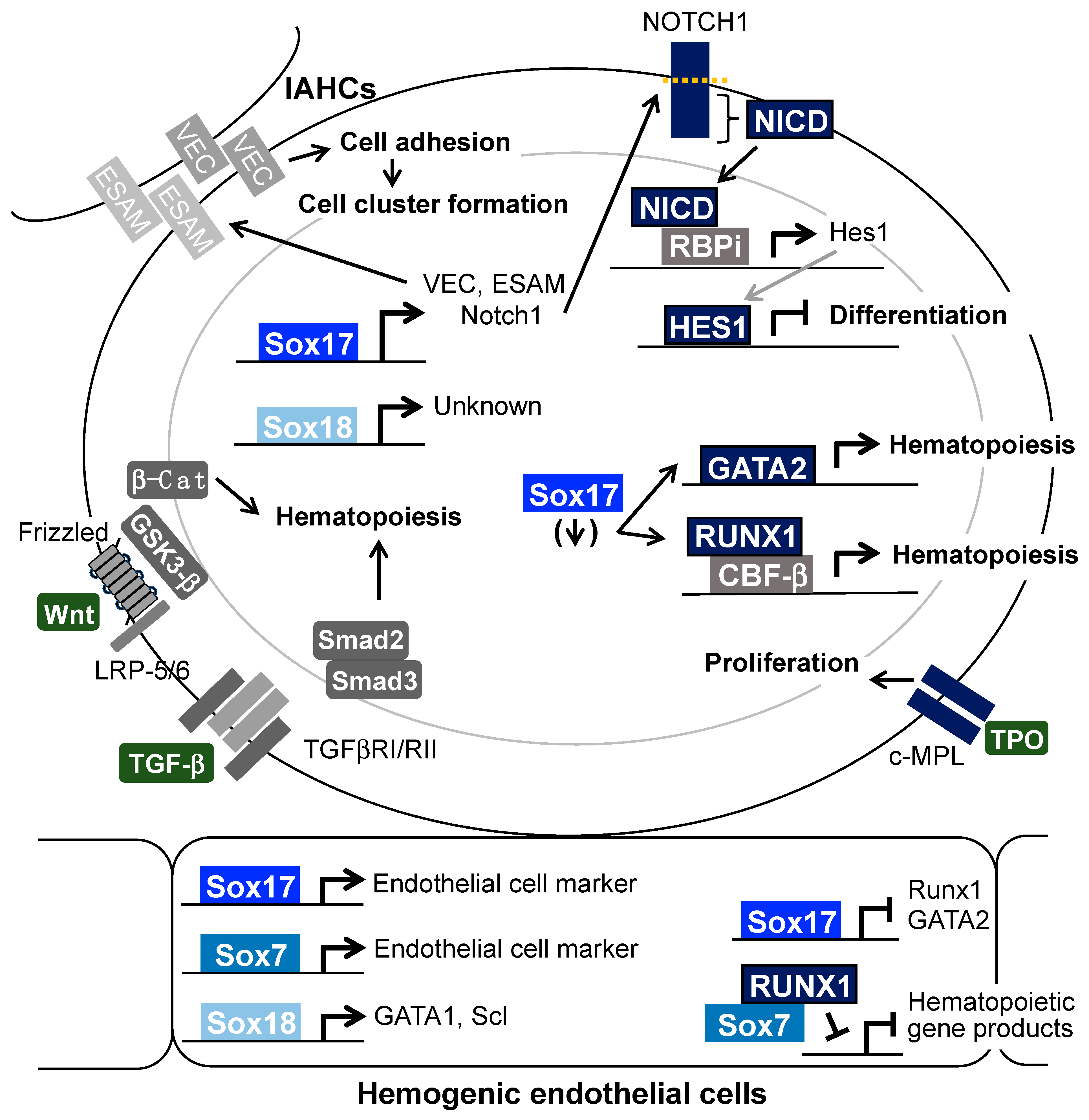
5. Relationship Between SoxF Proteins and Signal Molecules in Midgestation Hematopoiesis in Mice
5.1. Notch
5.2. Runx1 and GATA2
5.3. Adherent Molecules
5.4. The Wnt Signaling Pathway
5.5. The TGF-β Signaling Pathway
5.6. Thrombopoietin/c-Mpl
6. Role of SoxF Family Members in Hematopoiesis of the Fetal Liver and Adult BM
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cumano, A.; Ferraz, J.C.; Klaine, M.; Di Santo, J.P.; Godin, I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity 2001, 15, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Porayette, P.; Glosson, N.L.; Conway, S.J.; Carlesso, N.; Cardoso, A.A.; Kaplan, M.H.; Yoder, M.C. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood 2012, 119, 5706–5714. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Gomez Perdiguero, E.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.W.; Pollard, J.W.; et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2021, 336, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Böiers, C.; Carrelha, J.; Lutteropp, M.; Luc, S.; Green, J.C.A.; Azzoni, E.; Woll, P.S.; Mead, A.J.; Hultquist, A.; Swiers, G.; et al. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell 2013, 13, 535–548. [Google Scholar] [CrossRef]
- Dzierzak, E.; Speck, N.A. Of lineage and legacy: The development of mammalian hematopoietic stem cells. Nat. Immunol. 2008, 9, 129–136. [Google Scholar] [CrossRef]
- Medvinsky, A.; Rybtsov, S.; Taoudi, S. Embryonic origin of the adult hematopoietic system: Advances and questions. Development 2011, 138, 1017–1031. [Google Scholar] [CrossRef]
- Yoder, M.C. Inducing definitive hematopoiesis in a dish. Nat. Biotechnol. 2014, 32, 539–541. [Google Scholar] [CrossRef]
- Okuda, T.; van Deursen, J.; Hiebert, S.W.; Grosveld, G.; Downing, J.R. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 1996, 84, 321–330. [Google Scholar] [CrossRef]
- Cai, Z.; de Bruijn, M.; Ma, X.; Dortland, B.; Luteijn, T.; Downing, R.J.; Dzierzak, E. Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo. Immunity 2000, 13, 423–431. [Google Scholar] [CrossRef]
- Robert-Moreno, A.; Guiu, J.; Ruiz-Herguido, C.; López, M.E.; Inglés-Esteve, J.; Riera, L.; Tipping, A.; Enver, T.; Dzierzak, E.; Gridley, T.; et al. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J. 2008, 27, 1886–1895. [Google Scholar] [CrossRef]
- Swiers, G.; de Bruijn, M.; Speck, N.A. Hematopoietic stem cell emergence in the conceptus and the role of Runx1. Int. J. Dev. Biol. 2010, 54, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Drevon, C.; Jaffredo, T. Cell interactions and cell signaling during hematopoietic development. Exp. Cell Res. 2014, 329, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Menegatti, S.; de Kruijf, M.; Garcia-Alegria, E.; Lacaud, G.; Kouskoff, V. Transcriptional control of blood cell emergence. FEBS Lett. 2019, 593, 3304–3315. [Google Scholar] [CrossRef] [PubMed]
- Omatsu, Y.; Nagasawa, T. The critical and specific transcriptional regulator of the microenvironmental niche for hematopoietic stem and progenitor cells. Curr. Opin. Hematol. 2015, 22, 330–336. [Google Scholar] [CrossRef]
- Frenette, P.S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 2019, 20, 303–320. [Google Scholar]
- Cheshier, S.H.; Morrison, S.J.; Liao, X.; Weissman, I.L. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 1999, 96, 3120–3125. [Google Scholar] [CrossRef]
- Arai, F.; Hirao, A.; Ohmura, M.; Sato, H.; Matsuoka, S.; Takubo, K.; Ito, K.; Koh, G.Y.; Suda, T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004, 118, 149–161. [Google Scholar] [CrossRef]
- Karpova, D.; Rettig, M.P.; DiPersio, J.F. Mobilized peripheral blood: An updated perspective. F1000Research 2019, 8, 2125. [Google Scholar] [CrossRef]
- Goodell, M.A.; Brose, K.; Paradis, G.; Conner, A.S.; Mulligan, R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996, 183, 1797–1806. [Google Scholar] [CrossRef]
- Osawa, M.; Hanada, K.; Hamada, H.; Nakauchi, H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 1996, 273, 242–245. [Google Scholar] [CrossRef]
- Spangrude, G.J.; Heimfeld, S.; Weissman, I.L. Purification and characterization of mouse hematopoietic stem cells. Science 1988, 241, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Spangrude, G.J.; Brooks, D.M. Mouse strain variability in the expression of the hematopoietic stem cell antigen Ly-6A/E by bone marrow cells. Blood 1933, 82, 3327–3332. [Google Scholar] [CrossRef]
- Spangrude, G.J.; Brooks, D.M.; Tumas, D.B. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: In vivo expansion of stem cell phenotype but not function. Blood 1955, 85, 1006–1016. [Google Scholar] [CrossRef]
- Ogawa, M.; Matsuzaki, Y.; Nishikawa, S.; Hayashi, S.; Kunisada, T.; Sudo, T.; Kina, T.; Nakauchi, H.; Nishikawa, S. Expression and function of c-kit in hemopoietic progenitor cells. J. Exp. Med. 1991, 174, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Nakauchi, H.; Nagayoshi, K.; Nishikawa, S.; Miura, Y.; Suda, T. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood 1992, 80, 3044–3050. [Google Scholar] [CrossRef]
- Kiel, M.J.; Yilmaz, O.H.; Iwashita, T.; Yilmaz, O.H.; Terhorst, C.; Morrison, S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005, 121, 1109–1121. [Google Scholar] [CrossRef]
- Shin, J.Y.; Hu, W.; Naramura, M.; Park, C.Y. High c-Kit expression identifies hematopoietic stem cells with impaired self-renewal and megakaryocytic bias. J. Exp. Med. 2014, 211, 217–231. [Google Scholar] [CrossRef]
- Chen, J.; Feng, X.; Desierto, M.J.; Keyvanfar, K.; Young, N.S. IFN-γ-mediated hematopoietic cell destruction in murine models of immune-mediated bone marrow failure. Blood 2015, 126, 2621–2631. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, C.; Teng, Y.; Jiang, R.; Huang, X.; Liu, S.; Wan, J.; Broxmeyer, H.E.; Guo, B. Phorbol ester induced ex vivo expansion of rigorously-defined phenotypic but not unctional human cord blood hematopoietic stem cells: A cautionary tale demonstrating that phenotype does not always recapitulate stem cell function. Leukemia 2019, 33, 2962–2966. [Google Scholar] [CrossRef]
- Silver, L.; Palis, J. Initiation of murine embryonic erythropoiesis: A spatial analysis. Blood 1997, 89, 1154–1164. [Google Scholar] [CrossRef]
- Ciau-Uitz, A.; Monteiro, R.; Kirmizitas, A.; Patient, R. Developmental hematopoiesis: Ontogeny, genetic programming and conservation. Exp. Hematol. 2014, 42, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Palis, J.; Robertson, S.; Kennedy, M.; Wall, C.; Keller, G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 1999, 126, 5073–5084. [Google Scholar] [CrossRef] [PubMed]
- Frame, J.M.; Fegan, K.H.; Conway, S.J.; McGrath, K.E.; Palis, J. Definitive Hematopoiesis in the Yolk Sac Emerges from Wnt-Responsive Hemogenic Endothelium Independently of Circulation and Arterial Identity. Stem Cells 2016, 34, 431–444. [Google Scholar] [CrossRef]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shelley, W.C.; Seo, W.; Vemula, S.; Lin, Y.; Liu, Y.; Kapur, R.; Taniuchi, I.; Yoshimoto, M. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfβ for their development. Proc. Natl. Acad. Sci. USA 2014, 111, 12151–12156. [Google Scholar] [CrossRef]
- Kauts, M.L.; Vink, C.S.; Dzierzak, E. Hematopoietic (stem) cell development—How divergent are the roads taken? FEBS Lett. 2016, 590, 3975–3986. [Google Scholar] [CrossRef]
- Zovein, A.C.; Hofmann, J.J.; Lynch, M.; French, W.J.; Turlo, K.A.; Yang, Y.; Becker, M.S.; Zanetta, L.; Dejana, E.; Gasson, J.C.; et al. Fate Tracing Reveals the Endothelial Origin of Hematopoietic Stem Cells. Cell Stem Cell 2008, 3, 625–636. [Google Scholar] [CrossRef]
- Chen, M.J.; Yokomizo, T.; Zeigler, B.M.; Dzierzak, E.; Speck, N.A. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 2009, 457, 887–891. [Google Scholar] [CrossRef]
- Yokomizo, T.; Dzierzak, E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development 2010, 137, 3651–3661. [Google Scholar] [CrossRef]
- Yamashita, J.; Itoh, H.; Hirashima, M.; Ogawa, M.; Nishikawa, S.; Yurugi, T.; Naito, M.; Nakao, K.; Nishikawa, S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 2000, 408, 92–96. [Google Scholar] [CrossRef]
- Lancrin, C.; Sroczynska, P.; Stephenson, C.; Allen, T.; Kouskoff, V.; Lacaud, G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 2009, 457, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Minehata, K.; Sakimura, K.; Nakano, T.; Hara, T. In vitro generation of HSC-like cells from murine ESCs/iPSCs by enforced expression of LIM-homeobox transcription factor Lhx2. Blood 2011, 117, 3748–3758. [Google Scholar] [CrossRef] [PubMed]
- Kyba, M.; Perlingeiro, R.C.R.; Daley, G.Q. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell 2002, 109, 29–37. [Google Scholar] [CrossRef]
- Dahl, L.; Richter, K.; Hägglund, A.-C.; Carlsson, L. Lhx2 expression promotes self-renewal of a distinct multipotential hematopoietic progenitor cell in embryonic stem cell-derived embryoid bodies. PLoS ONE 2008, 3, e2025. [Google Scholar] [CrossRef]
- Sugimura, R.; Jha, D.K.; Han, A.; Soria-Valles, C.; da Rocha, E.L.; Lu, Y.-F.; Goettel, J.A.; Serrao, E.; Rowe, R.G.; Malleshaiah, M.; et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature 2012, 545, 432–438. [Google Scholar] [CrossRef]
- Ng, E.S.; Sarila, G.; Li, J.Y.; Edirisinghe, H.S.; Saxena, R.; Sun, S.; Bruveris, F.F.; Labonne, T.; Sleebs, N.; Maytum, A.; et al. Long-term engrafting multilineage hematopoietic cells differentiated from human induced pluripotent stem cells. Nat. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Taoudi, S.; Gonneau, C.; Moore, K.; Sheridan, J.M.; Blackburn, C.C.; Taylor, E.; Medvinsky, A. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell 2008, 3, 99–108. [Google Scholar] [CrossRef]
- de Pater, E.; Kaimakis, P.; Vink, C.S.; Yokomizo, T.; Yamada-Inagawa, T.; van der Linden, R.; Kartalaei, P.S.; Camper, S.A.; Speck, N.; Dzierzak, E. Gata2 is required for HSC generation and survival. J. Exp. Med. 2013, 210, 2843–2850. [Google Scholar] [CrossRef]
- Yokomizo, T.; Watanabe, N.; Umemoto, T.; Matsuo, J.; Harai, R.; Kihara, Y.; Nakamura, E.; Tada, N.; Sato, T.; Takaku, T.; et al. Hlf marks the developmental pathway for hematopoietic stem cells but not for erythro-myeloid progenitors. J. Exp. Med. 2019, 216, 1599–1614. [Google Scholar] [CrossRef]
- Taoudi, S.; Morrison, A.M.; Inoue, H.; Gribi, R.; Ure, J.; Medvinsky, A. Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. Development 2005, 132, 4179–4191. [Google Scholar] [CrossRef]
- Nobuhisa, I.; Yamasaki, S.; Ramadan, A.; Taga, T. CD45lowc-Kithighcells have hematopoietic properties in the mouse aorta-gonad-mesonephros region. Exp. Cell Res. 2012, 318, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Rybtsov, S.; Batsivari, A.; Bilotkach, K.; Paruzina, D.; Senserrich, J.; Nerushev, O.; Medvinsky, A. Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43(-) embryonic precursor. Stem Cell Rep. 2014, 3, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Rybtsov, S.; Sobiesiak, M.; Taoudi, S.; Souilhol, C.; Senserrich, J.; Liakhovitskaia, A.; Ivanovs, A.; Frampton, J.; Zhao, S.; Medvinsky, A. Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J. Exp. Med. 2011, 208, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, K.E.; Gekas, C.; Wang, Y.; Lux, C.T.; Francis, C.S.; Chan, D.N.; Conway, S.; Orkin, S.H.; Yoder, M.C.; Mikkola, H. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell 2008, 2, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Dzierzak, E.; Robin, C. Placenta as a source of hematopoietic stem cells. Trends Mol. Med. 2010, 16, 361–367. [Google Scholar] [CrossRef]
- Ramadan, A.; Nobuhisa, I.; Yamasak, S.; Nakagata, N.; Taga, T. Cells with hematopoietic activity in the mouse placenta reside in side population. Genes Cells 2010, 15, 982–994. [Google Scholar] [CrossRef]
- Gao, X.; Xu, C.; Asada, N.; Frenette, P.S. The hematopoietic stem cell niche: From embryo to adult. Development 2018, 145, dev139691. [Google Scholar] [CrossRef]
- Uchida, N.; Dykstra, B.; Lyons, K.; Leung, F.; Kristiansen, M.; Eaves, C. ABC transporter activities of murine hematopoietic stem cells vary according to their developmental and activation status. Blood 2004, 103, 4487–4495. [Google Scholar] [CrossRef]
- Bowie, M.B.; McKnight, K.D.; Kent, D.G.; McCaffrey, L.; Hoodless, P.A.; Eaves, C.J. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J. Clin. Investig. 2006, 116, 2808–2816. [Google Scholar] [CrossRef]
- Samokhvalov, I.M.; Samokhvalova, N.I.; Nishikawa, S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature 2007, 446, 1056–1061. [Google Scholar] [CrossRef]
- Bowles, J.; Schepers, G.; Koopman, P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 2000, 227, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Julian, L.M.; McDonald, A.C.; Stanford, W.L. Direct reprogramming with SOX factors: Masters of cell fate. Curr. Opin. Genet. Dev. 2017, 46, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Harley, V.R.; Pontiggia, A.; Goodfellow, P.N.; Lovell-Badge, R.; Bianchi, M.E. SRY, like HMG1, recognizes sharp angles in DNA. EMBO J. 1992, 11, 4497–4506. [Google Scholar] [CrossRef] [PubMed]
- Harley, V.R.; Jackson, D.I.; Hextall, P.J.; Hawkins, J.R.; Berkovitz, G.D.; Sockanathan, S.; Lovell-Badge, R.; Goodfellow, P.N. DNA binding activity of recombinant SRY from normal males and XY females. Science 1992, 255, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Lilly, A.J.; Lacaud, G.; Kouskoff, V. SOXF transcription factors in cardiovascular development. Semin. Cell Dev. Biol. 2017, 63, 50–57. [Google Scholar] [CrossRef]
- Wat, M.J.; Beck, T.F.; Hernández-García, A.; Yu, Z.; Veenma, D.; Garcia, M.; Holder, A.M.; Wat, J.J.; Chen, Y.; Mohila, C.A.; et al. Mouse model reveals the role of SOX7 in the development of congenital diaphragmatic hernia associated with recurrent deletions of 8p23.1. Hum. Mol. Genet. 2012, 21, 4115–4125. [Google Scholar] [CrossRef]
- Kanai-Azuma, M.; Kanai, Y.; Gad, J.M.; Tajima, Y.; Taya, C.; Kurohmaru, M.; Sanai, Y.; Yonekawa, H.; Yazaki, K.; Tam, P.P.L.; et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 2002, 129, 2367–2379. [Google Scholar] [CrossRef]
- Kim, I.; Saunders, T.L.; Morrison, S.J. Sox17 Dependence Distinguishes the Transcriptional Regulation of Fetal from Adult Hematopoietic Stem Cells. Cell 2007, 130, 470–483. [Google Scholar] [CrossRef]
- Uemura, M.; Ozawa, A.; Nagata, T.; Kurasawa, K.; Tsunekawa, N.; Nobuhisa, I.; Taga, T.; Hara, K.; Kudo, A.; Kawakami, H.; et al. Sox17 haploinsufficiency results in perinatal biliary atresia and hepatitis in C57BL/6 background mice. Development 2013, 140, 639–648. [Google Scholar] [CrossRef]
- Uemura, M.; Higashi, M.; Pattarapanawan, M.; Takami, S.; Ichikawa, N.; Higashiyama, H.; Furukawa, T.; Fujishiro, J.; Fukumura, Y.; Yao, T.; et al. Gallbladder wall abnormality in biliary atresia of mouse Sox17 (+/−) neonates and human infants. Dis. Model. Mech. 2020, 13, dmm042119. [Google Scholar] [CrossRef]
- Higashiyama, H.; Ozawa, A.; Sumitomo, H.; Uemura, M.; Fujino, K.; Igarashi, H.; Imaimatsu, K.; Tsunekawa, N.; Hirate, Y.; Kurohmaru, M.; et al. Embryonic cholecystitis and defective gallbladder contraction in the Sox17-haploinsufficient mouse model of biliary atresia. Development 2017, 144, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Hirate, Y.; Suzuki, H.; Kawasumi, M.; Takase, H.M.; Igarashi, H.; Naquet, P.; Kanai, Y.; Kanai-Azuma, M. Mouse Sox17 haploinsufficiency leads to female subfertility due to impaired implantation. Sci. Rep. 2016, 6, 24171. [Google Scholar] [CrossRef] [PubMed]
- Saba, R.; Kitajima, K.; Rainbow, L.; Engert, S.; Uemura, M.; Ishida, H.; Kokkinopoulos, I.; Shintani, Y.; Miyagawa, S.; Kanai, Y.; et al. Endocardium differentiation through Sox17 expression in endocardium precursor cells regulates heart development in mice. Sci. Rep. 2019, 9, 11953. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.; Natale, J.; Chew, L.J.; Belachew, S.; Cheng, Y.; Aguirre, A.; Lytle, J.; Nait-Oumesmar, B.; Kerninon, C.; Kanai-Azuma, M.; et al. Identification of Sox17 as a transcription factor that regulates oligodendrocyte development. J. Neurosci. 2006, 26, 9722–9735. [Google Scholar] [CrossRef]
- Chew, L.J.; Shen, W.; Ming, X.; Senatorov, V.V.J.; Chen, H.L.; Cheng, Y.; Hong, E.; Knoblach, S.; Gallo, V. SRY-box containing gene 17 regulates the Wnt/β-catenin signaling pathway in oligodendrocyte progenitor cells. J. Neurosci. 2011, 31, 13921–13935. [Google Scholar] [CrossRef]
- Chew, L.J.; Ming, X.; McEllin, B.; Dupree, J.; Hong, E.; Catron, M.; Fauveau, M.; Nait-Oumesmar, B.; Gallo, V. Sox17 Regulates a Program of Oligodendrocyte Progenitor Cell Expansion and Differentiation during Development and Repair. Cell Rep. 2019, 29, 3173–3186. [Google Scholar] [CrossRef]
- Pennisi, D.; Gardner, J.; Chambers, D.; Hosking, B.; Peters, J.; Muscat, G.; Abbott, C.; Koopman, P. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat. Genet. 2000, 24, 434–437. [Google Scholar] [CrossRef]
- François, M.; Caprini, A.; Hosking, B.; Orsenigo, F.; Wilhelm, D.; Browne, C.; Paavonen, K.; Karnezis, T.; Shayan, R.; Downes, M.; et al. Sox18 induces development of the lymphatic vasculature in mice. Nature 2008, 456, 643–647. [Google Scholar] [CrossRef]
- Villani, R.; Hodgson, S.; Legrand, J.; Greaney, J.; Wong, H.Y.; Pichol-Thievend, C.; Adolphe, C.; Wainwight, B.; Francois, M.; Khosrotehrani, K. Dominant-negative Sox18 function inhibits dermal papilla maturation and differentiation in all murine hair types. Development 2017, 144, 1887–1895. [Google Scholar] [CrossRef]
- Geng, Q.; Deng, H.; Fu, J.; Cui, F. SOX18 exerts tumor-suppressive functions in papillary thyroid carcinoma through inhibition of Wnt/β-catenin signaling. Exp. Cell Res. 2020, 396, 112249. [Google Scholar] [CrossRef]
- Hosking, B.; François, M.; Wilhelm, D.; Orsenigo, F.; Caprini, A.; Svingen, T.; Tutt, D.; Davidson, T.; Browne, C.; Dejana, E.; et al. Sox7 and Sox17 are strain-specific modifiers of the lymphangiogenic defects caused by Sox18 dysfunction in mice. Development 2009, 136, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Higashijima, Y.; Kanki, Y. Molecular mechanistic insights: The emerging role of SOXF transcription factors in tumorigenesis and development. Semin. Cancer Biol. 2020, 67, 39–48. [Google Scholar] [CrossRef]
- Shimoda, M.; Kanai-Azuma, M.; Hara, K.; Miyazaki, S.; Kanai, Y.; Monden, M.; Miyazaki, J. Sox17 plays a substantial role in late-stage differentiation of the extraembryonic endoderm in vitro. J. Cell Sci. 2007, 120, 3859–3869. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, I.; Barkey, W.; Schwarzfischer, M.; Theis, F.J.; Lickert, H. The Sox17-mCherry fusion mouse line allows visualization of endoderm and vascular endothelial development. Genesis 2012, 50, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Artus, J.; Piliszek, A.; Hadjantonakis, A.K. The primitive endoderm lineage of the mouse blastocyst: Sequential transcription factor activation and regulation of differentiation by Sox17. Dev. Biol. 2011, 350, 393–404. [Google Scholar] [CrossRef]
- Jauch, R.; Aksoy, I.; Hutchins, A.P.; Ng, C.K.L.; Tian, X.F.; Chen, J.; Palasingam, P.; Robson, P.; Stanton, L.W.; Kolatkar, P.R. Conversion of Sox17 into a pluripotency reprogramming factor by reengineering its association with Oct4 on DNA. Stem Cells 2011, 29, 940–995. [Google Scholar] [CrossRef]
- Aksoy, I.; Jauch, R.; Chen, J.; Dyla, M.; Divakar, U.; Bogu, G.K.; Teo, R.; Leng Ng, C.K.; Herath, W.; Lili, S.; et al. Stanton LW Oct4 switches partnering from Sox2 to Sox17 to reinterpret the enhancer code and specify endoderm. EMBO J. 2013, 32, 938–953. [Google Scholar] [CrossRef]
- Kinoshita, M.; Shimosato, D.; Yamane, M.; Niwa, H. Sox7 is dispensable for primitive endoderm differentiation from mouse ES cells. BMC Dev. Biol. 2015, 15, 37. [Google Scholar] [CrossRef]
- Gandillet, A.; Serrano, A.G.; Pearson, S.; Lie-A-Ling, M.; Lacaud, G.; Kouskoff, V. Sox7-sustained expression alters the balance between proliferation and differentiation of hematopoietic progenitors at the onset of blood specification. Blood 2009, 114, 4813–4822. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Hara, K.; Kanai-Azuma, M.; Matsui, T.; Miura, Y.; Tsunekawa, N.; Kurohmaru, M.; Saijoh, Y.; Koopman, P.; Kanai, Y. Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochem. Biophys. Res. Commun. 2007, 360, 539–544. [Google Scholar] [CrossRef]
- Zhou, Y.; Williams, J.; Smallwood, P.M.; Nathans, J. Sox7, Sox17, and Sox18 Cooperatively Regulate Vascular Development in the Mouse Retina. PLoS ONE 2015, 10, e0143650. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Marsboom, G.; Jambusaria, A.; Xiong, S.; Toth, P.T.; Benevolenskaya, E.V.; Rehman, J.; Malik, A.B. Sox17 is required for endothelial regeneration following inflammation-induced vascular injury. Nat. Commun. 2019, 10, 2126. [Google Scholar] [CrossRef] [PubMed]
- Irion, S.; Clarke, R.L.; Luche, H.; Kim, I.; Morrison, S.J.; Fehling, H.J.; Keller, G.M. Temporal specification of blood progenitors from mouse embryonic stem cells and induced pluripotent stem cells. Development 2010, 137, 2829–2839. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.L.; Yzaguirre, A.D.; Yashiro-Ohtani, Y.; Bondue, A.; Blanpain, C.; Pear, W.S.; Speck, N.A.; Keller, G. The expression of Sox17 identifies and regulates haemogenic endothelium. Nat. Cell Biol. 2013, 15, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Nakajima-Takagi, Y.; Osawa, M.; Oshima, M.; Takagi, H.; Miyagi, S.; Endoh, M.; Endo, T.A.; Takayama, N.; Eto, K.; Toyoda, T.; et al. Role of SOX17 in hematopoietic development from human embryonic stem cells. Blood 2013, 121, 447–458. [Google Scholar] [CrossRef]
- Lizama, C.O.; Hawkins, J.S.; Schmitt, C.E.; Bos, F.L.; Zape, J.P.; Cautivo, K.M.; Borges-Pinto, H.; Rhyner, A.M.; Yu, H.; Donohoe, M.E.; et al. Repression of arterial genes in hemogenic endothelium is sufficient for haematopoietic fate acquisition. Nat. Commun. 2015, 6, 7739. [Google Scholar] [CrossRef]
- Saito, K.; Nobuhisa, I.; Harada, K.; Takahashi, S.; Anani, M.; Lickert, H.; Kanai-Azuma, M.; Kanai, Y.; Taga, T. Maintenance of hematopoietic stem and progenitor cells in fetal intra-aortic hematopoietic clusters by the Sox17-Notch1-Hes1 axis. Exp. Cell Res. 2018, 365, 145–155. [Google Scholar] [CrossRef]
- Nobuhisa, I.; Osawa, M.; Uemura, M.; Kishikawa, Y.; Anani, M.; Harada, K.; Takagi, H.; Saito, K.; Kanai-Azuma, M.; Kanai, Y.; et al. Sox17-Mediated Maintenance of Fetal Intra-Aortic Hematopoietic Cell Clusters. Mol. Cell. Biol. 2014, 34, 1976–1990. [Google Scholar] [CrossRef]
- Lilly, A.J.; Costa, G.; Largeot, A.; Fadlullah, M.Z.H.; Lie-A-Ling, M.; Lacaud, G.; Kouskoff, V. Interplay between SOX7 and RUNX1 regulates hemogenic endothelial fate in the yolk sac. Development 2016, 143, 4341–4351. [Google Scholar] [CrossRef]
- Costa, G.; Mazan, A.; Gandillet, A.; Pearson, S.; Lacaud, G.; Kouskoff, V. SOX7 regulates the expression of VE-cadherin in the haemogenic endothelium at the onset of haematopoietic development. Development 2012, 139, 1587–1598. [Google Scholar] [CrossRef]
- Serrano, A.G.; Gandillet, A.; Pearson, S.; Lacaud, G.; Kouskoff, V. Contrasting effects of Sox17- and Sox18-sustained expression at the onset of blood specification. Blood 2010, 115, 3895–3898. [Google Scholar] [CrossRef] [PubMed]
- Nafria, M.; Bonifer, C.; Stanley, E.G.; Ng, E.S.; Elefanty, A.G. Protocol for the Generation of Definitive Hematopoietic Progenitors from Human Pluripotent Stem Cells. STAR Protoc. 2020, 1, 100130. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Uenishi, G.; Park, M.A.; Liu, P.; Suknuntha, K.; Raymond, M.; Choi, Y.J.; Thomson, J.A.; Ong, I.M.; Slukvin, I.I. SOX17 integrates HOXA and arterial programs in hemogenic endothelium to drive definitive lympho-myeloid hematopoiesis. Cell Rep. 2021, 34, 108758. [Google Scholar] [CrossRef] [PubMed]
- Gama-Norton, L.; Ferrando, E.; Ruiz-Herguido, C.; Liu, Z.; Guiu, J.; Islam, A.B.; Lee, S.U.; Yan, M.; Guidos, C.J.; López-Bigas, N.; et al. Notch signal strength controls cell fate in the haemogenic endothelium. Nat. Commun. 2015, 6, 8510. [Google Scholar] [CrossRef] [PubMed]
- Hadland, B.K.; Varnum-Finney, B.; Poulos, M.G.; Moon, R.T.; Butler, J.M.; Rafii, S.; Bernstein, I.D. Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros-derived hematopoietic stem cells. J. Clin. Investig. 2015, 125, 2032–2045. [Google Scholar] [CrossRef]
- Guiu, J.; Shimizu, R.; D’Altri, T.; Fraser, S.T.; Hatakeyama, J.; Bresnick, E.H.; Kageyama, R.; Dzierzak, E.; Yamamoto, M.; Espinosa, L.; et al. Hes repressors are essential regulators of hematopoietic stem cell development downstream of Notch signaling. J. Exp. Med. 2013, 210, 71–84. [Google Scholar] [CrossRef]
- Ma, Z.; Xu, J.; Wu, L.; Wang, J.; Lin, Q.; Chowdhury, F.A.; Mazumder, M.H.H.; Hu, G.; Li, X.; Du, W. Hes1 deficiency causes hematopoietic stem cell exhaustion. Stem Cells 2020, 38, 756–768. [Google Scholar] [CrossRef]
- Porcheri, C.; Golan, O.; Calero-Nieto, F.J.; Thambyrajah, R.; Ruiz-Herguido, C.; Wang, X.; Catto, F.; Guillén, Y.; Sinha, R.; González, J.; et al. Notch ligand Dll4 impairs cell recruitment to aortic clusters and limits blood stem cell generation. EMBO J. 2020, 39, e104270. [Google Scholar] [CrossRef]
- Tang, Y.; Bai, H.; Urs, S.; Wang, Z.; Liaw, L. Notch1 activation in embryonic VE-cadherin populations selectively blocks hematopoietic stem cell generation and fetal liver hematopoiesis. Transgenic Res. 2013, 22, 403–410. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, B.; Zhang, F.; Wang, Y.; Shen, B.; Liu, Y.; Guo, Y.; Fan, Y.; Qiu, J. Sox7 is involved in antibody-dependent endothelial cell activation and renal allograft injury via the Jagged1-Notch1 pathway. Exp. Cell Res. 2019, 375, 20–27. [Google Scholar] [CrossRef]
- Jiang, T.; Li, Z.; Zhao, D.; Hui, B.; Zheng, Z. SOX18 enhances the proliferation and migration of airway smooth muscle cells induced by tumor necrosis factor-α via the regulation of Notch1 signaling. Int. Immunopharmacol. 2021, 96, 107746. [Google Scholar] [CrossRef] [PubMed]
- Lie-A-Ling, M.; Marinopoulou, E.; Lilly, A.J.; Challinor, M.; Patel, R.; Lancrin, C.; Kouskoff, V.; Lacaud, G. Regulation of RUNX1 dosage is crucial for efficient blood formation from hemogenic endothelium. Development 2018, 145, dev149419. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Nobuhisa, I.; Saito, K.; Gerel, M.; Itabashi, A.; Harada, K.; Osawa, M.; Endo, T.A.; Iwama, A.; Taga, T. Sox17-mediated expression of adherent molecules is required for the maintenance of undifferentiated hematopoietic cluster formation in midgestation mouse embryos. Differentiation 2020, 115, 53–61. [Google Scholar] [CrossRef]
- Yokota, T.; Oritani, K.; Butz, S.; Kokame, K.; Kincade, P.W.; Miyata, T.; Vestweber, D.; Kanakura, Y. The endothelial antigen ESAM marks primitive hematopoietic progenitors throughout life in mice. Blood 2009, 113, 2914–2923. [Google Scholar] [CrossRef]
- Sudo, T.; Yokota, T.; Oritani, K.; Satoh, Y.; Sugiyama, T.; Ishida, T.; Shibayama, H.; Ezoe, S.; Fujita, N.; Tanaka, H.; et al. The endothelial antigen ESAM monitors hematopoietic stem cell status between quiescence and self-renewal. J. Immunol. 2012, 189, 200–210. [Google Scholar] [CrossRef]
- Ueda, T.; Yokota, T.; Okuzaki, D.; Uno, Y.; Mashimo, T.; Kubota, Y.; Sudo, T.; Ishibashi, T.; Shingai, Y.; Doi, Y.; et al. Endothelial Cell-Selective Adhesion Molecule Contributes to the Development of Definitive Hematopoiesis in the Fetal Liver. Stem Cell Rep. 2019, 13, 992–1005. [Google Scholar] [CrossRef]
- Tan, D.S.; Holzner, M.; Weng, M.; Srivastava, Y.; Jauch, R. SOX17 in cellular reprogramming and cancer. Semin. Cancer Biol. 2020, 67, 65–73. [Google Scholar] [CrossRef]
- Trowbridge, J.J.; Guezguez, B.; Moon, R.T.; Bhatia, M. Wnt3a activates dormant c-Kit(-) bone marrow-derived cells with short-term multilineage hematopoietic reconstitution capacity. Stem Cells 2010, 28, 1379–1389. [Google Scholar] [CrossRef]
- Reya, T.; Duncan, A.W.; Ailles, L.; Domen, J.; Scherer, D.C.; Willert, K.; Hintz, L.; Nusse, R.; Weissman, I.L. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 2003, 423, 409–414. [Google Scholar] [CrossRef]
- Kitajima, K.; Nakajima, M.; Kanokoda, M.; Kyba, M.; Dandapat, A.; Tolar, J.; Saito, M.K.; Toyoda, M.; Umezawa, A.; Hara, T. GSK3β inhibition activates the CDX/HOX pathway and promotes hemogenic endothelial progenitor differentiation from human pluripotent stem cells. Exp. Hematol. 2016, 44, 10–68. [Google Scholar] [CrossRef]
- Ng, E.S.; Azzola, L.; Bruveris, F.F.; Calvanese, V.; Phipson, B.; Vlahos, K.; Hirst, C.; Jokubaitis, V.J.; Yu, Q.C.; Maksimovic, J.; et al. Differentiation of human embryonic stem cells to HOXA(+) hemogenic vasculature that resembles the aorta-gonad-mesonephros. Nat. Biotechnol. 2016, 34, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Galat, Y.; Dambaeva, S.; Elcheva, I.; Khanolkar, A.; Beaman, K.; Iannaccone, P.M.; Galat, V. Cytokine-free directed differentiation of human pluripotent stem cells efficiently produces hemogenic endothelium with lymphoid potential. Stem Cell Res. Ther. 2017, 8, 67. [Google Scholar] [CrossRef]
- Ruiz-Herguido, C.; Guiu, J.; D’Altri, T.; Inglés-Esteve, J.; Dzierzak, E.; Espinosa, L.; Bigas, A. Hematopoietic stem cell development requires transient Wnt/β-catenin activity. J. Exp. Med. 2012, 209, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Vargel, Ö.; Zhang, Y.; Kosim, K.; Ganter, K.; Foehr, S.; Mardenborough, Y.; Shvartsman, M.; Enright, A.J.; Krijgsveld, J.; Lancrin, C. Activation of the TGFβ pathway impairs endothelial to haematopoietic transition. Sci. Rep. 2016, 6, 21518. [Google Scholar] [CrossRef]
- Wang, C.; Tang, X.; Sun, X.; Miao, Z.; Lv, Y.; Yang, Y.; Zhang, H.; Zhang, P.; Liu, Y.; Du, L.; et al. TGFβ inhibition enhances the generation of hematopoietic progenitors from human ES cell-derived hemogenic endothelial cells using a stepwise strategy. Cell Res. 2012, 22, 194–207. [Google Scholar] [CrossRef]
- Lempereur, A.; Canto, P.Y.; Richard, C.; Martin, S.; Thalgott, J.; Raymond, K.; Lebrin, F.; Drevon, C.; Jaffredo, T. The TGFβ pathway is a key player for the endothelial-to-hematopoietic transition in the embryonic aorta. Dev. Biol. 2018, 434, 292–303. [Google Scholar] [CrossRef]
- Lange, A.W.; Keiser, A.R.; Wells, J.M.; Zorn, A.M.; Whitsett, J.A. Sox17 promotes cell cycle progression and inhibits TGF-beta/Smad3 signaling to initiate progenitor cell behavior in the respiratory epithelium. PLoS ONE 2009, 4, e5711. [Google Scholar] [CrossRef]
- Howell, E.D.; Yzaguirre, A.D.; Gao, P.; Lis, R.; He, B.; Lakadamyali, M.; Rafii, S.; Tan, K.; Speck, N.A. Efficient hemogenic endothelial cell specification by RUNX1 is dependent on baseline chromatin accessibility of RUNX1-regulated TGFβ target genes. Genes Dev. 2021, 35, 1475–1489. [Google Scholar] [CrossRef]
- Thambyrajah, R.; Fadlullah, M.Z.H.; Proffitt, M.; Patel, R.; Cowley, S.M.; Kuskoff, V.; Lacaud, G. HDAC1 and HDAC2 Modulate TGF-β Signaling during Endothelial-to-Hematopoietic Transition. Stem Cell Rep. 2018, 10, 1369–1383. [Google Scholar] [CrossRef]
- Fox, N.; Priestley, G.; Papayannopoulou, T.; Kaushansky, K. Thrombopoietin expands hematopoietic stem cells after transplantation. J. Clin. Investig. 2002, 110, 389–394. [Google Scholar] [CrossRef]
- Wilkinson, A.C.; Ishida, R.; Kikuchi, M.; Sudo, K.; Morita, M.; Crisostomo, R.V.; Yamamoto, R.; Loh, K.M.; Nakamura, Y.; Watanabe, M.; et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature 2019, 571, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Petit-Cocault, L.; Volle-Challier, C.; Fleury, M.; Péault, B.; Souyri, M. Dual role of Mpl receptor during the establishment of definitive hematopoiesis. Development 2007, 134, 3031–3040. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Nobuhisa, I.; Anani, M.; Saito, K.; Taga, T. Thrombopoietin contributes to the formation and the maintenance of hematopoietic progenitor-containing cell clusters in the aorta-gonad-mesonephros region. Cytokine 2017, 95, 35–42. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Kim, I.; Lim, M.S.; Morrison, S.J. Sox17 expression confers self-renewal potential and fetal stem cell characteristics upon adult hematopoietic progenitors. Genes Dev. 2011, 25, 1613–1627. [Google Scholar] [CrossRef]
- Corada, M.; Orsenigo, F.; Morini, M.F.; Pitulescu, M.E.; Bhat, G.; Nyqvist, D.; Breviario, F.; Conti, V.; Briot, A.; Iruela-Arispe, M.L.; et al. Sox17 is indispensable for acquisition and maintenance of arterial identity. Nat. Commun. 2013, 4, 2609. [Google Scholar] [CrossRef]
- Chhabra, A.; Mikkola, H.K. Return to youth with Sox17. Genes Dev. 2011, 25, 1557–1562. [Google Scholar] [CrossRef]
- Cuvertino, S.; Lacaud, G.; Kouskoff, V. SOX7-enforced expression promotes the expansion of adult blood progenitors and blocks B-cell development. Open Biol. 2016, 6, 160070. [Google Scholar] [CrossRef]
- Anani, M.; Nobuhisa, I.; Osawa, M.; Iwama, A.; Harada, K.; Saito, K.; Taga, T. Sox17 as a candidate regulator of myeloid restricted differentiation potential. Dev. Growth Differ. 2014, 56, 469–479. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobuhisa, I.; Melig, G.; Taga, T. Sox17 and Other SoxF-Family Proteins Play Key Roles in the Hematopoiesis of Mouse Embryos. Cells 2024, 13, 1840. https://doi.org/10.3390/cells13221840
Nobuhisa I, Melig G, Taga T. Sox17 and Other SoxF-Family Proteins Play Key Roles in the Hematopoiesis of Mouse Embryos. Cells. 2024; 13(22):1840. https://doi.org/10.3390/cells13221840
Chicago/Turabian StyleNobuhisa, Ikuo, Gerel Melig, and Tetsuya Taga. 2024. "Sox17 and Other SoxF-Family Proteins Play Key Roles in the Hematopoiesis of Mouse Embryos" Cells 13, no. 22: 1840. https://doi.org/10.3390/cells13221840
APA StyleNobuhisa, I., Melig, G., & Taga, T. (2024). Sox17 and Other SoxF-Family Proteins Play Key Roles in the Hematopoiesis of Mouse Embryos. Cells, 13(22), 1840. https://doi.org/10.3390/cells13221840





