High Mobility Group Box 1 (HMGB1): Molecular Signaling and Potential Therapeutic Strategies
Abstract
1. Introduction
2. HMGB1—Molecular Structure and Functional Correlation
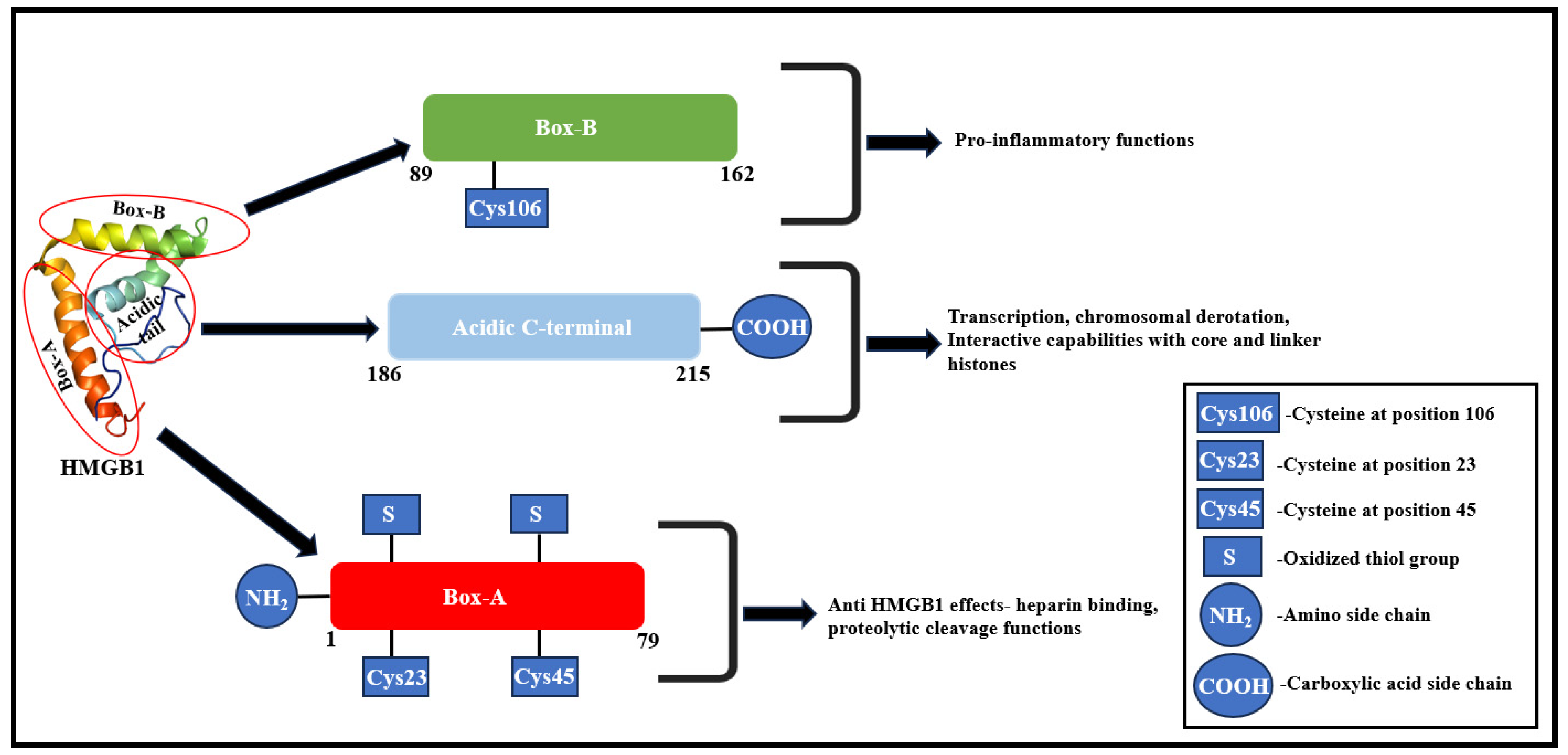
3. Pathophysiology of HMGB1 Role in the Onset of Different Diseases
3.1. HMGB1 Role in Cardiovascular Complications
3.1.1. Atherosclerosis
3.1.2. Adverse Left Ventricular Remodeling Post-Myocardial Infarction
3.2. HMGB1 Role in Renal Dysfunction
3.3. HMGB1 Role in Malignancies
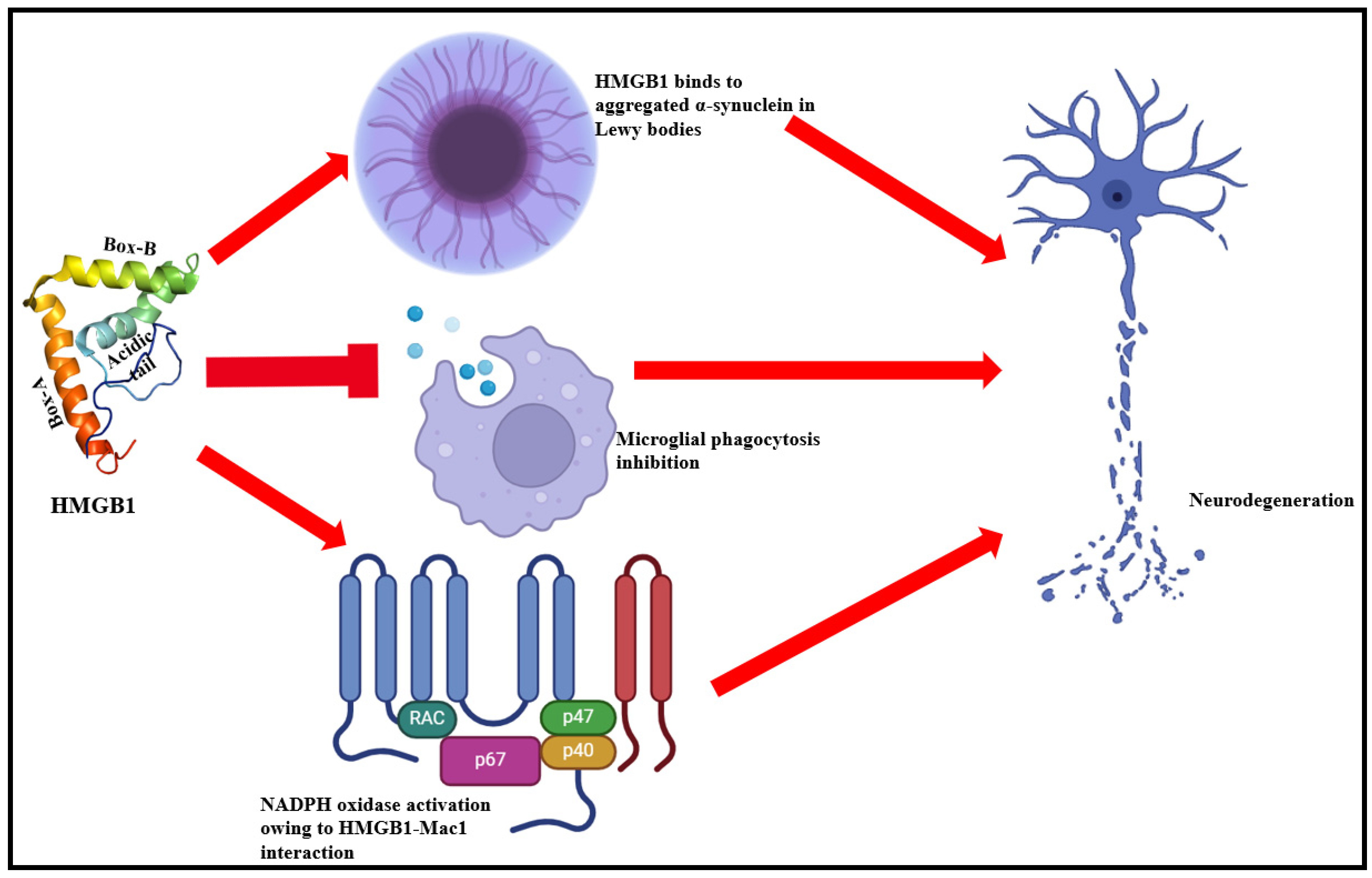
3.4. HMGB1 Role in Neurodegenerative Complications
3.5. HMGB1 Role in Autoimmune Diseases
3.5.1. Rheumatoid Arthritis (RA)
3.5.2. Systemic Lupus Erythematosus (SLE)
3.5.3. Type 1 Diabetes Mellitus (T1DM)
3.6. Metabolic Syndrome (MetS)
4. Therapeutic Perspectives
4.1. Anti-HMGB1 Antibodies
4.2. Soluble RAGE (sRAGE)
4.3. Peptides and Peptidomimetics
4.4. Small Molecule Inhibitors (SMIs)
4.4.1. SMIs Against Extracellular HMGB1 Release
4.4.2. SMIs Targeting HMGB1 Binding
5. Current Limitations
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, S.; Liu, Z.; Xu, Z.; Liu, J.; Zhang, J. High mobility group box 1 (HMGB1): A pivotal regulator of hematopoietic malignancies. J. Hematol. Oncol. 2020, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.G.; Yan, Z.; et al. HMGB1 in health and disease. Mol. Asp. Med. 2014, 40, 1–116. [Google Scholar]
- Ferrari, S.; Ronfani, L.; Calogero, S.; Bianchi, M. The mouse gene coding for high mobility group 1 protein (HMG1). J. Biol. Chem. 1994, 269, 28803–28808. [Google Scholar] [CrossRef] [PubMed]
- Gariboldi, M.; De Gregorio, L.; Ferrari, S.; Manenti, G.; Pierotti, M.A.; Bianchi, M.E.; Dragani, T.A. Mapping of the Hmg1 gene and of seven related sequences in the mouse. Mamm. Genome 1995, 6, 581–585. [Google Scholar] [CrossRef]
- Wen, L.; Huang, J.-K.; Johnson, B.H.; Gerald, R. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res. 1989, 17, 1197–1214. [Google Scholar] [CrossRef]
- Sessa, L.; Bianchi, M.E. The evolution of High Mobility Group Box (HMGB) chromatin proteins in multicellular animals. Gene 2006, 387, 133–140. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, Z.; Zeng, R.; Yao, Y. HMGB1 in kidney diseases. Life Sci. 2020, 259, 118203. [Google Scholar] [CrossRef]
- El Gazzar, M.; Yoza, B.K.; Chen, X.; Garcia, B.A.; Young, N.L.; McCall, C.E. Chromatin-specific remodeling by HMGB1 and linker histone H1 silences proinflammatory genes during endotoxin tolerance. Mol. Cell. Biol. 2009, 29, 1959–1971. [Google Scholar] [CrossRef]
- Huang, H.; Nace, G.W.; McDonald, K.-A.; Tai, S.; Klune, J.R.; Rosborough, B.R.; Ding, Q.; Loughran, P.; Zhu, X.; Beer-Stolz, D.; et al. Hepatocyte-specific high-mobility group box 1 deletion worsens the injury in liver ischemia/reperfusion: A role for intracellular high-mobility group box 1 in cellular protection. Hepatology 2014, 59, 1984–1997. [Google Scholar] [CrossRef]
- Genschel, J.; Modrich, P. Functions of MutLα, replication protein A (RPA), and HMGB1 in 5′-directed mismatch repair. J. Biol. Chem. 2009, 284, 21536–21544. [Google Scholar] [CrossRef]
- Prasad, R.; Liu, Y.; Deterding, L.J.; Poltoratsky, V.P.; Kedar, P.S.; Horton, J.K.; Kanno, S.-I.; Asagoshi, K.; Hou, E.W.; Khodyreva, S.N.; et al. HMGB1 is a cofactor in mammalian base excision repair. Mol. Cell 2007, 27, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Giavara, S.; Kosmidou, E.; Hande, M.; Bianchi, M.E.; Morgan, A.; di Fagagna, F.D.; Jackson, S.P. Yeast Nhp6A/B and mammalian Hmgb1 facilitate the maintenance of genome stability. Curr. Biol. 2005, 15, 68–72. [Google Scholar] [CrossRef]
- Andersson, U.; Yang, H.; Harris, H. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. In Seminars in Immunology; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Stumbo, A.C.; Cortez, E.; Rodrigues, C.A.; Henriques, M.D.G.M.; Porto, L.C.; Barbosa, H.S.; Carvalho, L. Mitochondrial localization of non-histone protein HMGB1 during human endothelial cell–Toxoplasma gondii infection. Cell Biol. Int. 2008, 32, 235–238. [Google Scholar] [CrossRef]
- Lee, H.; Shin, N.; Song, M.; Kang, U.-B.; Yeom, J.; Lee, C.; Ahn, Y.H.; Yoo, J.S.; Paik, Y.-K.; Kim, H. Analysis of nuclear high mobility group box 1 (HMGB1)-binding proteins in colon cancer cells: Clustering with proteins involved in secretion and extranuclear function. J. Proteome Res. 2010, 9, 4661–4670. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Livesey, K.M.; Kroemer, G.; Billiar, T.R.; Van Houten, B.; Zeh, H.J., III; Lotze, M.T. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011, 13, 701–711. [Google Scholar] [CrossRef]
- Narumi, T.; Shishido, T.; Otaki, Y.; Kadowaki, S.; Honda, Y.; Funayama, A.; Honda, S.; Hasegawa, H.; Kinoshita, D.; Yokoyama, M.; et al. High-mobility group box 1-mediated heat shock protein beta 1 expression attenuates mitochondrial dysfunction and apoptosis. J. Mol. Cell. Cardiol. 2015, 82, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Livesey, K.M.; Cheh, C.-W.; Farkas, A.; Loughran, P.; Hoppe, G.; Bianchi, M.E.; Tracey, K.J.; Zeh, H.J.; et al. Endogenous HMGB1 regulates autophagy. J. Cell Biol. 2010, 190, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-G.; Zhu, X.; Lu, R.; Messer, J.S.; Xia, Y.; Chang, E.B.; Sun, J. Intestinal epithelial HMGB1 inhibits bacterial infection via STAT3 regulation of autophagy. Autophagy 2019, 15, 1935–1953. [Google Scholar] [CrossRef]
- Kitahara, T.; Takeishi, Y.; Harada, M.; Niizeki, T.; Suzuki, S.; Sasaki, T.; Ishino, M.; Bilim, O.; Nakajima, O.; Kubota, I. High-mobility group box 1 restores cardiac function after myocardial infarction in transgenic mice. Cardiovasc. Res. 2008, 80, 40–46. [Google Scholar] [CrossRef]
- Funayama, A.; Shishido, T.; Netsu, S.; Narumi, T.; Kadowaki, S.; Takahashi, H.; Miyamoto, T.; Watanabe, T.; Woo, C.-H.; Abe, J.-I.; et al. Cardiac nuclear high mobility group box 1 prevents the development of cardiac hypertrophy and heart failure. Cardiovasc. Res. 2013, 99, 657–664. [Google Scholar] [CrossRef]
- Nakamura, Y.; Suzuki, S.; Shimizu, T.; Miyata, M.; Shishido, T.; Ikeda, K.; Saitoh, S.-I.; Kubota, I.; Takeishi, Y. High mobility group box 1 promotes angiogenesis from bone marrow-derived endothelial progenitor cells after myocardial infarction. J. Atheroscler. Thromb. 2015, 22, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.; Fernández-Justel, J.M.; Santa-María, C.; Cadoret, J.-C.; Cano-Aroca, L.; Lombraña, R.; Herranz, G.; Agresti, A.; Gómez, M. Chromatin conformation regulates the coordination between DNA replication and transcription. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Huebener, P.; Pradere, J.-P.; Hernandez, C.; Gwak, G.-Y.; Caviglia, J.M.; Mu, X.; Loike, J.D.; Jenkins, R.E.; Antoine, D.J.; Schwabe, R.F. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J. Clin. Investig. 2019, 125, 539–550. [Google Scholar] [CrossRef]
- Deng, M.; Tang, Y.; Li, W.; Wang, X.; Zhang, R.; Zhang, X.; Zhao, X.; Liu, J.; Tang, C.; Liu, Z.; et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity 2018, 49, 740–753.e7. [Google Scholar] [CrossRef]
- Ge, X.; Antoine, D.J.; Lu, Y.; Arriazu, E.; Leung, T.-M.; Klepper, A.L.; Branch, A.D.; Fiel, M.I.; Nieto, N. High mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD). J. Biol. Chem. 2014, 289, 22672–22691. [Google Scholar] [CrossRef]
- Hernandez, C.; Huebener, P.; Pradere, J.-P.; Antoine, D.J.; Friedman, R.A.; Schwabe, R.F. HMGB1 links chronic liver injury to progenitor responses and hepatocarcinogenesis. J. Clin. Investig. 2019, 128, 2436–2451. [Google Scholar] [CrossRef] [PubMed]
- Arriazu, E.; Ge, X.; Leung, T.-M.; Magdaleno, F.; Lopategi, A.; Lu, Y.; Kitamura, N.; Urtasun, R.; Theise, N.; Antoine, D.J.; et al. Signalling via the osteopontin and high mobility group box-1 axis drives the fibrogenic response to liver injury. Gut 2016, 66, 1123–1137. [Google Scholar] [CrossRef]
- Tros, M. HMGB proteins: Interactions with DNA and chromatin. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2010, 1799, 101–113. [Google Scholar]
- Li, J.; Kokkola, R.; Tabibzadeh, S.; Yang, R.; Ochani, M.; Qiang, X.; Harris, H.E.; Czura, C.J.; Wang, H.; Ulloa, L.; et al. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol. Med. 2003, 9, 37–45. [Google Scholar] [CrossRef]
- Yang, H.; Ochani, M.; Li, J.; Qiang, X.; Tanovic, M.; Harris, H.E.; Susarla, S.M.; Ulloa, L.; Wang, H.; DiRaimo, R.; et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 2004, 101, 296–301. [Google Scholar] [CrossRef]
- Ito, T.; Kawahara, K.-I.; Okamoto, K.; Yamada, S.; Yasuda, M.; Imaizumi, H.; Nawa, Y.; Meng, X.; Shrestha, B.; Hashiguchi, T.; et al. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arter. Thromb. Vasc. Biol. 2008, 28, 1825–1830. [Google Scholar] [CrossRef]
- Huttunen, H.J.; Fages, C.; Kuja-Panula, J.; Ridley, A.J.; Rauvala, H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 2002, 62, 4805–4811. [Google Scholar]
- Travers, A.A.; Thomas, J.O. Chromosomal HMG-box proteins. New Compr. Biochem. 2004, 39, 103–134. [Google Scholar]
- Thomas, J.O.; Travers, A.A. HMG1 and 2, and related ‘architectural’DNA-binding proteins. Trends Biochem. Sci. 2001, 26, 167–174. [Google Scholar] [CrossRef]
- Thomas, J.O.; Stott, K. H1 and HMGB1: Modulators of chromatin structure. Biochem. Soc. Trans. 2012, 40, 341–346. [Google Scholar] [CrossRef]
- Sheflin, L.G.; Fucile, N.W.; Spaulding, S.W. The specific interactions of HMG 1 and 2 with negatively supercoiled DNA are modulated by their acidic C-terminal domains and involve cysteine residues in their HMG 1/2 boxes. Biochemistry 1993, 32, 3238–3248. [Google Scholar] [CrossRef] [PubMed]
- Štros, M.; Štokrová, J.; Thomas, J.O. DNA looping by the HMG-box domains of HMG1 and modulation of DNA binding by the acidic C-terminal domain. Nucleic Acids Res. 1994, 22, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zeng, M.; Wang, W.; Tang, J. The HMGB1 acidic tail regulates HMGB1 DNA binding specificity by a unique mechanism. Biochem. Biophys. Res. Commun. 2007, 360, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Stros, M. DNA bending by the chromosomal protein HMG1 and its high mobility group box domains. Effect of flanking sequences. J. Biol. Chem. 1998, 273, 10355–10361. [Google Scholar] [CrossRef]
- Aizawa, S.; Nishino, H.; Saito, K.; Kimura, K.; Shirakawa, H.; Yoshida, M. Stimulation of transcription in cultured cells by high mobility group protein 1: Essential role of the acidic carboxyl-terminal region. Biochemistry 1994, 33, 14690–14695. [Google Scholar] [CrossRef]
- Ueda, T.; Chou, H.; Kawase, T.; Shirakawa, H.; Yoshida, M. Acidic C-tail of HMGB1 is required for its target binding to nucleosome linker DNA and transcription stimulation. Biochemistry 2004, 43, 9901–9908. [Google Scholar] [CrossRef] [PubMed]
- An, W.; van Holde, K.; Zlatanova, J. The non-histone chromatin protein HMG1 protects linker DNA on the side opposite to that protected by linker histones. J. Biol. Chem. 1998, 273, 26289–26291. [Google Scholar] [CrossRef] [PubMed]
- Ner, S.S.; Blank, T.; Pérez-Parallé, M.L.; Grigliatti, T.A.; Becker, P.B.; Travers, A.A. HMG-D and histone H1 interplay during chromatin assembly and early embryogenesis. J. Biol. Chem. 2001, 276, 37569–37576. [Google Scholar] [CrossRef]
- Ner, S.; Travers, A. HMG-D, the Drosophila melanogaster homologue of HMG 1 protein, is associated with early embryonic chromatin in the absence of histone H1. EMBO J. 1994, 13, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, K.; Dimitrov, S.; Reeves, R.; Wolffe, A.P. Evidence for a shared structural role for HMG1 and linker histones B4 and H1 in organizing chromatin. EMBO J. 1996, 15, 548–561. [Google Scholar] [CrossRef]
- Kwak, M.S.; Kim, H.S.; Lkhamsuren, K.; Kim, Y.H.; Gil Han, M.; Shin, J.M.; Park, I.H.; Rhee, W.J.; Lee, S.K.; Rhee, S.G.; et al. Peroxiredoxin-mediated disulfide bond formation is required for nucleocytoplasmic translocation and secretion of HMGB1 in response to inflammatory stimuli. Redox Biol. 2019, 24, 101203. [Google Scholar] [CrossRef]
- Youn, J.H.; Shin, J.-S. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J. Immunol. 2006, 177, 7889–7897. [Google Scholar] [CrossRef]
- Zhang, X.; Wheeler, D.; Tang, Y.; Guo, L.; Shapiro, R.A.; Ribar, T.J.; Means, A.R.; Billiar, T.R.; Angus, D.C.; Rosengart, M.R. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J. Immunol. 2008, 181, 5015–5023. [Google Scholar] [CrossRef]
- Richard, S.A.; Jiang, Y.; Xiang, L.H.; Zhou, S.; Wang, J.; Su, Z.; Xu, H. Post-translational modifications of high mobility group box 1 and cancer. Am. J. Transl. Res. 2017, 9, 5181–5196. [Google Scholar]
- Biorender. Create Professional Science Figures in Minutes. 2024. Available online: https://www.biorender.com/ (accessed on 21 May 2024).
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef]
- Bank, R.P.D. Solution Structure of the HMG-Box Transcription Factor 1. 2024. Available online: https://www.rcsb.org/structure/4qr9 (accessed on 21 May 2024).
- Paoletti, R.; Gotto, A.M., Jr.; Hajjar, D.P. Inflammation in atherosclerosis and implications for therapy. Circulation 2004, 109 (Suppl. 1), III-20–III-26. [Google Scholar]
- Mehra, V.C.; Ramgolam, V.S.; Bender, J.R. Cytokines and cardiovascular disease. J. Leukoc. Biol. 2005, 78, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, N.; Agrotis, A.; Tararak, E.; Antropova, Y.; Kanellakis, P.; Ilyinskaya, O.; Bobik, A. Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: Role of activated macrophages and cytokines. Cardiovasc. Pathol. 2004, 24, 2320. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, C.; Bustin, M.; Talwar, S.; Tropea, M.; Gerstenberger, E.; Shelhamer, J.H.; Suffredini, A.F. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 2003, 101, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Treutiger, C.J.; Mullins, G.E.; Johansson, A.M.; Rouhiainen, A.; Rauvala, H.M.E.; Erlandsson-Harris, H.; Andersson, U.; Yang, H.; Tracey, K.J.; Andersson, J.; et al. High mobility group 1 B-box mediates activation of human endothelium. J. Intern. Med. 2003, 254, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Mullins, G.E.; Sunden-Cullberg, J.; Johansson, A.; Rouhiainen, A.; Erlandsson-Harris, H.; Yang, H.; Tracey, K.J.; Rauvala, H.; Palmblad, J.; Andersson, J.; et al. Activation of human umbilical vein endothelial cells leads to relocation and release of high-Mobility group box chromosomal protein 1. Scand. J. Immunol. 2004, 60, 566–573. [Google Scholar] [CrossRef]
- Abeyama, K.; Stern, D.M.; Ito, Y.; Kawahara, K.I.; Yoshimoto, Y.; Tanaka, M.; Uchimura, T.; Ida, N.; Yamazaki, Y.; Yamada, S.; et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J. Clin. Investig. 2005, 115, 1267–1274. [Google Scholar] [CrossRef]
- Laszik, Z.G.; Zhou, X.J.; Ferrell, G.L.; Silva, F.G.; Esmon, C.T. Down-regulation of endothelial expression of endothelial cell protein C receptor and thrombomodulin in coronary atherosclerosis. Am. J. Pathol. 2001, 159, 797–802. [Google Scholar] [CrossRef]
- Degryse, B.; Bonaldi, T.; Scaffidi, P.; Müller, S.; Resnati, M.; Sanvito, F.; Arrigoni, G.; Bianchi, M.E. The high mobility group (Hmg) boxes of the nuclear protein Hmg1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J. Cell Biol. 2001, 152, 1197–1206. [Google Scholar] [CrossRef]
- Rouhiainen, A.; Imai, S.; Rauvala, H.; Parkkinen, J. Occurrence of amphoterin (HMG1) as an endogenous protein of human platelets that is exported to the cell surface upon platelet activation. Thromb. Haemost. 2000, 84, 1087–1094. [Google Scholar]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. New Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Bradshaw, G.; Gutierrez, A.; Miyake, J.H.; Davis, K.R.; Li, A.C.; Glass, C.K.; Curtiss, L.K.; Davis, R.A. Facilitated replacement of Kupffer cells expressing a paraoxonase-1 transgene is essential for ameliorating atherosclerosis in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 11029–11034. [Google Scholar] [CrossRef] [PubMed]
- Cipollone, F.; Iezzi, A.; Fazia, M.; Zucchelli, M.; Pini, B.; Cuccurullo, C.; De Cesare, D.; De Blasis, G.; Muraro, R.; Bei, R.; et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: Role of glycemic control. Circulation 2003, 108, 1070–1077. [Google Scholar] [CrossRef]
- Park, L.; Raman, K.G.; Lee, K.J.; Lu, Y.; Ferran, L.J., Jr.; Chow, W.S.; Stern, D.; Schmidt, A.M. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med. 1998, 4, 1025–1031. [Google Scholar] [CrossRef]
- Bucciarelli, L.G.; Wendt, T.; Qu, W.; Lu, Y.; Lalla, E.; Rong, L.L.; Goova, M.T.; Moser, B.; Kislinger, T.; Lee, D.C.; et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E–null mice. Circulation 2002, 106, 2827–2835. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Pathophysiology of myocardial infarction. Compr. Physiol. 2011, 5, 1841–1875. [Google Scholar]
- Thankam, F.G.; Agrawal, D.K. Infarct zone: A novel platform for exosome trade in cardiac tissue regeneration. J. Cardiovasc. Transl. Res. 2020, 13, 686–701. [Google Scholar] [CrossRef]
- Foglio, E.; Pellegrini, L.; Russo, M.A.; Limana, F. HMGB1-mediated activation of the inflammatory-reparative response following myocardial infarction. Cells 2022, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Anzai, A.; Ko, S.; Fukuda, K. Immune and inflammatory networks in myocardial infarction: Current research and its potential implications for the clinic. Int. J. Mol. Sci. 2022, 23, 5214. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Anzai, T.; Naito, K.; Miyasho, T.; Okamoto, M.; Yokota, H.; Yamada, S.; Maekawa, Y.; Takahashi, T.; Yoshikawa, T.; et al. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc. Res. 2009, 81, 565–573. [Google Scholar] [CrossRef]
- Kuveljic, J.; Djordjevic, A.; Zivotic, I.; Dekleva, M.; Kolakovic, A.; Zivkovic, M.; Stankovic, A.; Djuric, T. Expression of HMGB1, TGF-β1, BIRC3, ADAM17, CDKN1A, and FTO in Relation to Left Ventricular Remodeling in Patients Six Months after the First Myocardial Infarction: A Prospective Study. Genes 2024, 15, 1296. [Google Scholar] [CrossRef]
- He, X.; Du, T.; Long, T.; Liao, X.; Dong, Y.; Huang, Z.-P. Signaling cascades in the failing heart and emerging therapeutic strategies. Signal Transduct. Target. Ther. 2022, 7, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Poston, J.T.; Koyner, J.L. Sepsis associated acute kidney injury. BMJ 2019, 364, k4891. [Google Scholar] [CrossRef] [PubMed]
- Czura, C.J.; Yang, H.; Amella, C.A.; Tracey, K.J. HMGB1 in the immunology of sepsis (not septic shock) and arthritis. Adv. Immunol. 2004, 84, 181–200. [Google Scholar]
- Wang, H.; Yang, H.; Tracey, K.J. Extracellular role of HMGB1 in inflammation and sepsis. J. Intern. Med. 2004, 255, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kawahara, K.; Nakamura, T.; Yamada, S.; Nakamura, T.; Abeyama, K.; Hashiguchi, T.; Maruyama, I. High-mobility group box 1 protein promotes development of microvascular thrombosis in rats. J. Thromb. Haemost. 2007, 5, 109–116. [Google Scholar] [CrossRef]
- Good, D.W.; George, T.; Watts, B.A., III. High-mobility group box 1 inhibits HCO3−absorption in medullary thick ascending limb through a basolateral receptor for advanced glycation end products pathway. Am. J. Physiol. Ren. Physiol. 2015, 309, F720–F730. [Google Scholar] [CrossRef]
- Zheng, S.; Pan, Y.; Wang, C.; Liu, Y.; Shi, M.; Ding, G. HMGB1 turns renal tubular epithelial cells into inflammatory promoters by interacting with TLR4 during sepsis. J. Interf. Cytokine Res. 2016, 36, 9–19. [Google Scholar] [CrossRef]
- Bruchfeld, A.; Qureshi, A.R.; Lindholm, B.; Barany, P.; Yang, L.; Stenvinkel, P.; Tracey, K.J. High mobility group box protein-1 correlates with renal function in chronic kidney disease (CKD). Mol. Med. 2008, 14, 109–115. [Google Scholar] [CrossRef]
- Zhu, N.; Yuan, W.; Zhou, Y.; Liu, J.; Bao, J.; Hao, J.; Miao, W. High mobility group box protein-1 correlates with microinflammatory state and nutritional status in continuous ambulatory peritoneal dialysis patients. J. Artif. Organs 2011, 14, 125–132. [Google Scholar] [CrossRef]
- Jin, X.; Rong, S.; Yuan, W.; Gu, L.; Jia, J.; Wang, L.; Yu, H.; Zhuge, Y. High mobility group box 1 promotes aortic calcification in chronic kidney disease via the Wnt/β-catenin pathway. Front. Physiol. 2018, 9, 665. [Google Scholar] [CrossRef]
- Lynch, J.; Nolan, S.; Slattery, C.; Feighery, R.; Ryan, M.P.; McMorrow, T. High-mobility group box protein 1: A novel mediator of inflammatory-induced renal epithelial-mesenchymal transition. Am. J. Nephrol. 2010, 32, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Li, C.; Ran, R.; Chen, S.-Y. Surfactant protein A deficiency exacerbates renal interstitial fibrosis following obstructive injury in mice. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guo, Y.; Fu, H.; Hu, S.; Pan, J.; Wang, Y.; Cheng, J.; Song, J.; Yu, Q.; Zhang, S.; et al. Chop deficiency prevents UUO-induced renal fibrosis by attenuating fibrotic signals originated from Hmgb1/TLR4/NFκB/IL-1β signaling. Cell Death Dis. 2015, 6, e1847. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Eltrich, N.; Lichtnekert, J.; Anders, H.-J.; Vielhauer, V. The NLRP3/ASC inflammasome promotes T-cell-dependent immune complex glomerulonephritis by canonical and noncanonical mechanisms. Kidney Int. 2014, 86, 965–978. [Google Scholar] [CrossRef]
- Tachibana, S.; Iyoda, M.; Matsumoto, K.; Wada, Y.; Suzuki, T.; Iseri, K.; Kanazawa, N.; Shibata, T. Recombinant human soluble thrombomodulin attenuates anti-glomerular basement membrane glomerulonephritis in Wistar–Kyoto rats through anti-inflammatory effects. Nephrol. Dial. Transplant. 2019, 34, 774–782. [Google Scholar] [CrossRef]
- Anders, H.-J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef]
- Chen, X.; Ma, J.; Kwan, T.; Stribos, E.G.D.; Messchendorp, A.L.; Loh, Y.W.; Wang, X.; Paul, M.; Cunningham, E.C.; Habib, M.; et al. Blockade of HMGB1 attenuates diabetic nephropathy in mice. Sci. Rep. 2018, 8, 8319. [Google Scholar] [CrossRef]
- Iwata, Y.; Furuichi, K.; Sakai, N.; Yamauchi, H.; Shinozaki, Y.; Zhou, H.; Kurokawa, Y.; Toyama, T.; Kitajima, S.; Okumura, T.; et al. Dendritic cells contribute to autoimmune kidney injury in MRL-Faslpr mice. J. Rheumatol. 2009, 36, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Burbano, C.; Gómez-Puerta, J.A.; Muñoz-Vahos, C.; Vanegas-García, A.; Rojas, M.; Vásquez, G.; Castaño, D. HMGB1+ microparticles present in urine are hallmarks of nephritis in patients with systemic lupus erythematosus. Eur. J. Immunol. 2019, 49, 323–335. [Google Scholar] [CrossRef]
- Fogo, A.B.; Lusco, M.A.; Najafian, B.; Alpers, C.E. AJKD Atlas of Renal Pathology: Minimal Mesangial and Mesangial Proliferative Lupus Nephritis (ISN/RPS Class I and II). Am. J. Kidney Dis. 2017, 70, e7–e8. [Google Scholar] [CrossRef]
- Feng, X.; Hao, J.; Liu, Q.; Yang, L.; Lv, X.; Zhang, Y.; Xing, L.; Xu, N.; Liu, S. HMGB1 mediates IFN-γ-induced cell proliferation in MMC cells through regulation of cyclin D1/CDK4/p16 pathway. J. Cell. Biochem. 2012, 113, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.J.; Liu, S.X.; Wu, C.; Kang, P.P.; Liu, Q.J.; Hao, J.; Li, H.B.; Li, F.; Zhang, Y.J.; Fu, X.H.; et al. The PTEN/PI3K/Akt signaling pathway mediates HMGB1-induced cell proliferation by regulating the NF-κB/cyclin D1 pathway in mouse mesangial cells. Am. J. Physiol. Cell Physiol. 2014, 306, C1119–C1128. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wu, C.; Yang, M.; Liu, Q.; Li, H.; Liu, J.; Zhang, Y.; Hao, Y.; Kang, L.; Zhang, Y.; et al. Role of PI3K/Akt signal pathway on proliferation of mesangial cell induced by HMGB1. Tissue Cell 2016, 48, 121–125. [Google Scholar] [CrossRef]
- Feng, X.; Yang, R.; Tian, Y.; Miao, X.; Guo, H.; Gao, F.; Yang, L.; Zhao, S.; Zhang, W.; Liu, J.; et al. HMGB1 protein promotes glomerular mesangial matrix deposition via TLR2 in lupus nephritis. J. Cell. Physiol. 2020, 235, 5111–5119. [Google Scholar] [CrossRef]
- Zhong, H.; Li, X.; Zhou, S.; Jiang, P.; Liu, X.; Ouyang, M.; Nie, Y.; Chen, X.; Zhang, L.; Liu, Y.; et al. Interplay between RAGE and TLR4 regulates HMGB1-induced inflammation by promoting cell surface expression of RAGE and TLR4. J. Immunol. 2020, 205, 767–775. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting inflammation driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Huttunen, H.J.; Rauvala, H. Amphoterin as an extracellular regulator of cell motility: From discovery to disease. J. Intern. Med. 2004, 255, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Sappington, P.L.; Yang, R.; Yang, H.; Tracey, K.J.; Delude, R.L.; Fink, M.P. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology 2002, 123, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Banerjee, S.; Palmer, A.; Zheng, G.; Chen, A.; Bosland, M.C.; Kajdacsy-Balla, A.; Kalyanasundaram, R.; Munirathinam, G. HMGB1 in hormone-related cancer: A potential therapeutic target. Horm. Cancer 2014, 5, 127–139. [Google Scholar] [CrossRef]
- Pomi, F.L.; Borgia, F.; Custurone, P.; Vaccaro, M.; Pioggia, G.; Gangemi, S. Role of HMGB1 in cutaneous melanoma: State of the art. Int. J. Mol. Sci. 2022, 23, 9327. [Google Scholar] [CrossRef]
- Lu, H.; Zhu, M.; Qu, L.; Shao, H.; Zhang, R.; Li, Y. Oncogenic role of HMGB1 as an alarming in robust prediction of immunotherapy response in colorectal cancer. Cancers 2022, 14, 4875. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Peng, G.; Huang, H.; Liu, F.; Kong, D.P.; Dong, K.Q.; Dai, L.H.; Zhou, Z.; Wang, K.J.; Yang, J.; et al. Blocking the feedback loop between neuroendocrine differentiation and macrophages improves the therapeutic effects of enzalutamide (MDV3100) on prostate cancer. Clin. Cancer Res. 2018, 24, 708–723. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, C.; Sevko, A.; Jiang, H.; Lichtenberger, R.; Reith, M.; Tarnanidis, K.; Holland-Letz, T.; Umansky, L.; Beckhove, P.; Sucker, A.; et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin. Cancer Res. 2015, 21, 5453–5459. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Chang, Y.; Liang, X.; Cardinal, J.S.; Huang, H.; Thorne, S.H.; Monga, S.P.; Geller, D.A.; Lotze, M.T.; Tsung, A. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology 2012, 55, 1863–1875. [Google Scholar] [CrossRef]
- Shafat, M.S.; Gnaneswaran, B.; Bowles, K.M.; Rushworth, S.A. The bone marrow microenvironment—Home of the leukemic blasts. Blood Rev. 2017, 31, 277–286. [Google Scholar] [CrossRef]
- Tian, X.; Shen, H.; Li, Z.; Wang, T.; Wang, S. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J. Hematol. Oncol. 2019, 12, 84. [Google Scholar] [CrossRef]
- Palumbo, G.A.; Parrinello, N.L.; Giallongo, C.; D’amico, E.; Zanghì, A.; Puglisi, F.; Conticello, C.; Chiarenza, A.; Tibullo, D.; Di Raimondo, F.; et al. Monocytic myeloid derived suppressor cells in hematological malignancies. Int. J. Mol. Sci. 2019, 20, 5459. [Google Scholar] [CrossRef]
- Parker, K.H.; Sinha, P.; Horn, L.A.; Clements, V.K.; Yang, H.; Li, J.; Tracey, K.J.; Ostrand-Rosenberg, S. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res. 2014, 74, 5723–5733. [Google Scholar] [CrossRef]
- Son, M.; Porat, A.; He, M.; Suurmond, J.; Santiago-Schwarz, F.; Andersson, U.; Coleman, T.R.; Volpe, B.T.; Tracey, K.J.; Al-Abed, Y.; et al. C1q and HMGB1 reciprocally regulate human macrophage polarization. Blood 2016, 128, 2218–2228. [Google Scholar] [CrossRef]
- Jia, L.; Clear, A.; Liu, F.-T.; Matthews, J.; Uddin, N.; McCarthy, A.; Hoxha, E.; Durance, C.; Iqbal, S.; Gribben, J.G. Extracellular HMGB1 promotes differentiation of nurse-like cells in chronic lymphocytic leukemia. Blood 2014, 123, 1709–1719. [Google Scholar] [CrossRef]
- Senda, N.; Miyagaki, T.; Kamijo, H.; Nakajima, R.; Oka, T.; Takahashi, N.; Suga, H.; Yoshizaki, A.; Asano, Y.; Sugaya, M.; et al. Increased HMGB1 levels in lesional skin and sera in patients with cutaneous T-cell lymphoma. Eur. J. Dermatol. 2018, 28, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Chou, D.K.; Zhang, J.; Smith, F.I.; McCaffery, P.; Jungalwala, F.B. Developmental expression of receptor for advanced glycation end products (RAGE), amphoterin and sulfoglucuronyl (HNK-1) carbohydrate in mouse cerebellum and their role in neurite outgrowth and cell migration. J. Neurochem. 2004, 90, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, H.; Setoguchi, T.; Yone, K.; Souda, M.; Yoshida, H.; Kawahara, K.-I.; Maruyama, I.; Komiya, S. High mobility group box 1 is upregulated after spinal cord injury and is associated with neuronal cell apoptosis. Spine 2010, 35, 1109–1115. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, K.; Li, J.; Xu, N.; Gong, G.; Wang, G.; Yu, Y.; Dong, H.; Xiong, L. Beneficial effects of hydrogen gas against spinal cord ischemia–reperfusion injury in rabbits. Brain Res. 2011, 1378, 125–136. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, Q.; Zhou, Y.; Gou, X.; Hou, L.; Chen, S.; Zhu, Z.; Xiong, L. Ethyl pyruvate attenuates spinal cord ischemic injury with a wide therapeutic window through inhibiting high-mobility group box 1 release in rabbits. Anesthesiology 2009, 110, 1279–1286. [Google Scholar] [CrossRef]
- Shibasaki, M.; Sasaki, M.; Miura, M.; Mizukoshi, K.; Ueno, H.; Hashimoto, S.; Tanaka, Y.; Amaya, F. Induction of high mobility group box-1 in dorsal root ganglion contributes to pain hypersensitivity after peripheral nerve injury. Pain 2010, 149, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-B.; Choi, J.S.; Yu, Y.-M.; Nam, K.; Piao, C.-S.; Kim, S.-W.; Lee, M.-H.; Han, P.-L.; Park, J.-S.; Lee, J.-K. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J. Neurosci. 2006, 26, 6413–6421. [Google Scholar] [CrossRef]
- Gao, H.-M.; Zhou, H.; Zhang, F.; Wilson, B.C.; Kam, W.; Hong, J.-S. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J. Neurosci. 2011, 31, 1081–1092. [Google Scholar] [CrossRef]
- Mazarati, A.; Maroso, M.; Iori, V.; Vezzani, A.; Carli, M. High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and receptor for advanced glycation end products. Exp. Neurol. 2011, 232, 143–148. [Google Scholar] [CrossRef]
- Takata, K.; Kitamura, Y.; Kakimura, J.-I.; Shibagaki, K.; Tsuchiya, D.; Taniguchi, T.; Smith, M.A.; Perry, G.; Shimohama, S. Role of high mobility group protein-1 (HMG1) in amyloid-β homeostasis. Biochem. Biophys. Res. Commun. 2003, 301, 699–703. [Google Scholar] [CrossRef]
- Lindersson, E.K.; Højrup, P.; Gai, W.P.; Locker, D.; Martin, D.; Jensen, P.H. α-Synuclein filaments bind the transcriptional regulator HMGB-1. Neuroreport 2004, 15, 2735–2739. [Google Scholar] [PubMed]
- Andersson, Å.; Covacu, R.; Sunnemark, D.; Danilov, A.I.; Bianco, A.D.; Khademi, M.; Wallström, E.; Lobell, A.; Brundin, L.; Lassmann, H.; et al. Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J. Leukoc. Biol. 2008, 84, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Casula, M.; Iyer, A.; Spliet, W.; Anink, J.; Steentjes, K.; Sta, M.; Troost, D.; Aronica, E. Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience 2011, 179, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Torikai, M.; Kuwazuru, A.; Tanaka, M.; Horai, N.; Fukuda, T.; Yamada, S.; Nagayama, S.; Hashiguchi, K.; Sunahara, N.; et al. Extracellular high mobility group box chromosomal protein 1 is a coupling factor for hypoxia and inflammation in arthritis. Arthritis Rheum. 2008, 58, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- Kokkola, R.; Sundberg, E.; Ulfgren, A.; Palmblad, K.; Li, J.; Wang, H.; Ulloa, L.; Yang, H.; Yan, X.; Furie, R.; et al. High mobility group box chromosomal protein 1: A novel proinflammatory mediator in synovitis. Arthritis Rheum. 2002, 46, 2598–2603. [Google Scholar] [CrossRef]
- Goldstein, R.S.; Bruchfeld, A.; Yang, L.; Qureshi, A.R.; Gallowitsch-Puerta, M.; Patel, N.B.; Huston, B.J.; Chavan, S.; Rosas-Ballina, M.; Gregersen, P.K.; et al. Cholinergic anti-inflammatory pathway activity and high mobility group box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol. Med. 2007, 13, 210–215. [Google Scholar] [CrossRef]
- Taniguchi, N.; Kawahara, K.; Yone, K.; Hashiguchi, T.; Yamakuchi, M.; Goto, M.; Inoue, K.; Yamada, S.; Ijiri, K.; Matsunaga, S.; et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003, 48, 971–981. [Google Scholar] [CrossRef]
- Huang, Q.; Ma, Y.; Adebayo, A.; Pope, R.M. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007, 56, 2192–2201. [Google Scholar] [CrossRef]
- Andersson, U.; Harris, H.E. The role of HMGB1 in the pathogenesis of rheumatic disease. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2010, 1799, 141–148. [Google Scholar] [CrossRef]
- Mouri, F.; Tsukada, J.; Mizobe, T.; Higashi, T.; Yoshida, Y.; Minami, Y.; Izumi, H.; Kominato, Y.; Kohno, K.; Tanaka, Y. Intracellular HMGB1 transactivates the human IL1B gene promoter through association with an Ets transcription factor PU.1. Eur. J. Haematol. 2008, 80, 10–19. [Google Scholar] [CrossRef]
- Palmblad, K.; Sundberg, E.; Diez, M.; Söderling, R.; Aveberger, A.-C.; Andersson, U.; Harris, H.E. Morphological characterization of intra-articular HMGB1 expression during the course of collagen-induced arthritis. Arthritis Res. Ther. 2007, 9, R35. [Google Scholar] [CrossRef] [PubMed]
- Yamoah, K.; Brebene, A.; Baliram, R.; Inagaki, K.; Dolios, G.; Arabi, A.; Majeed, R.; Amano, H.; Wang, R.; Yanagisawa, R.; et al. High-mobility group box proteins modulate tumor necrosis factor-α expression in osteoclastogenesis via a novel deoxyribonucleic acid sequence. Mol. Endocrinol. 2008, 22, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Han, J.-Y.; Xi, C.-X.; Xie, J.-X.; Feng, X.; Wang, C.-Y.; Mei, L.; Xiong, W.-C. HMGB1 regulates RANKL-induced osteoclastogenesis in a manner dependent on RAGE. J. Bone Miner. Res. 2008, 23, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Pisetsky, D.S. Expression of high mobility group protein 1 in the sera of patients and mice with systemic lupus erythematosus. Ann. Rheum. Dis. 2008, 67, 727–728. [Google Scholar] [CrossRef]
- Ma, C.Y.; Jiao, Y.L.; Zhang, J.; Yang, Q.R.; Zhang, Z.F.; Shen, Y.J.; Chen, Z.J.; Zhao, Y.R. Elevated plasma level of HMGB1 is associated with disease activity and combined alterations with IFN-alpha and TNF-alpha in systemic lupus erythematosus. Rheumatol. Int. 2012, 32, 395–402. [Google Scholar] [CrossRef]
- Ardoin, S.P.; Pisetsky, D.S. Developments in the scientific understanding of lupus. Arthritis Res. Ther. 2008, 10, 218. [Google Scholar] [CrossRef]
- Popovic, K.; Ek, M.; Espinosa, A.; Padyukov, L.; Harris, H.E.; Wahren-Herlenius, M.; Nyberg, F. Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum. 2005, 52, 3639–3645. [Google Scholar] [CrossRef]
- Barkauskaite, V.; Ek, M.; Popovic, K.; Harris, H.; Wahren-Herlenius, M.; Nyberg, F. Translocation of the novel cytokine HMGB1 to the cytoplasm and extracellular space coincides with the peak of clinical activity in experimentally UV-induced lesions of cutaneous lupus erythematosus. Lupus 2007, 16, 794–802. [Google Scholar] [CrossRef]
- Urbonaviciute, V.; Furnrohr, B.G.; Meister, S.; Munoz, L.; Heyder, P.; De Marchis, F.; Bianchi, M.E.; Kirschning, C.; Wagner, H.; Manfredi, A.A.; et al. Induction of inflammatory and immune responses by HMGB1–nucleosome complexes: Implications for the pathogenesis of SLE. J. Exp. Med. 2008, 205, 3007–3018. [Google Scholar] [CrossRef]
- Lee, Y.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Association between toll-like receptor polymorphisms and systemic lupus erythematosus: A meta-analysis update. Lupus 2016, 25, 593–601. [Google Scholar] [CrossRef]
- Komatsuda, A.; Wakui, H.; Iwamoto, K.; Ozawa, M.; Togashi, M.; Masai, R.; Maki, N.; Hatakeyama, T.; Sawada, K. Up-regulated expression of Toll-like receptors mRNAs in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin. Exp. Immunol. 2008, 152, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, Y.; Dai, J.; Medzhitov, R.; Freudenberg, M.A.; Zhang, P.L.; Li, Z. TLR4 up-regulation at protein or gene level is pathogenic for lupus-like autoimmune disease. J. Immunol. 2006, 177, 6880–6888. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Gorycka, A.; Wajda, A.; Stypinska, B.; Walczuk, E.; Rzeszotarska, E.; Walczyk, M.; Haladyj, E.; Romanowska-Prochnicka, K.; Felis-Giemza, A.; Lewandowska, A.; et al. Variety of endosomal TLRs and Interferons (IFN-α, IFN-β, IFN-γ) expression profiles in patients with SLE, SSc and MCTD. Clin. Exp. Immunol. 2021, 204, 49–63. [Google Scholar] [CrossRef]
- Kimura, J.; Ichii, O.; Miyazono, K.; Nakamura, T.; Horino, T.; Otsuka-Kanazawa, S.; Kon, Y. Overexpression of Toll-like receptor 8 correlates with the progression of podocyte injury in murine autoimmune glomerulonephritis. Sci. Rep. 2014, 4, 7290. [Google Scholar] [CrossRef]
- Christensen, S.R.; Shupe, J.; Nickerson, K.; Kashgarian, M.; Flavell, R.A.; Shlomchik, M.J. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 2006, 25, 417–428. [Google Scholar] [CrossRef]
- Leibler, C.; John, S.; Elsner, R.A.; Thomas, K.B.; Smita, S.; Joachim, S.; Levack, R.C.; Callahan, D.J.; Gordon, R.A.; Bastacky, S.; et al. Genetic dissection of TLR9 reveals complex regulatory and cryptic proinflammatory roles in mouse lupus. Nat. Immunol. 2022, 23, 1457–1469. [Google Scholar] [CrossRef]
- Han, J.; Zhong, J.; Wei, W.; Wang, Y.; Huang, Y.; Yang, P.; Purohit, S.; Dong, Z.; Wang, M.-H.; She, J.-X.; et al. Extracellular high-Mobility group box 1 acts as an innate immune mediator to enhance autoimmune progression and diabetes onset in NOD mice. Diabetes 2008, 57, 2118–2127. [Google Scholar] [CrossRef]
- Wu, H.; Li, R.; Wei, Z.-H.; Zhang, X.-L.; Chen, J.-Z.; Dai, Q.; Xie, J.; Xu, B. Diabetes-induced oxidative stress in endothelial progenitor cells may be sustained by a positive feedback loop involving high mobility group box-1. Oxidative Med. Cell. Longev. 2016, 2016, 1943918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhong, J.; Yang, P.; Gong, F.; Wang, C.-Y. HMGB1, an innate alarmin, in the pathogenesis of type 1 diabetes. Int. J. Clin. Exp. Pathol. 2010, 3, 24. [Google Scholar]
- Zhang, J.; Chen, L.; Wang, F.; Zou, Y.; Li, J.; Luo, J.; Khan, F.; Sun, F.; Li, Y.; Liu, J.; et al. Extracellular HMGB1 exacerbates autoimmune progression and recurrence of type 1 diabetes by impairing regulatory T cell stability. Diabetologia 2020, 63, 987–1001. [Google Scholar] [CrossRef]
- Devaraj, S.; Dasu, M.R.; Rockwood, J.; Winter, W.; Griffen, S.C.; Jialal, I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: Further evidence of a proinflammatory state. J. Clin. Endocrinol. Metab. 2008, 93, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Major, A.M.; Devaraj, S. Global toll-like receptor 4 knockout results in decreased renal inflammation, fibrosis and podocytopathy. J. Diabetes its Complicat. 2014, 28, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Frisardi, V.; Matrone, C.; Street, M.E. Metabolic syndrome and autophagy: Focus on HMGB1 protein. Front. Cell Dev. Biol. 2021, 9, 654913. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D. Assessing and managing the metabolic syndrome in children and adolescents. Nutrients 2019, 11, 1788. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Song, D.; Baek, J.-H.; Kim, K.; Kim, J.; Song, T.-J.; Lee, H.S.; Choi, D.; Kim, Y.D.; Nam, H.S.; et al. Poor long-term outcomes in stroke patients with asymptomatic coronary artery disease in heart CT. Atherosclerosis 2017, 265, 7–13. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, H.; Su, S.; Harshfield, G.; Sullivan, J.; Webb, C.; Blumenthal, J.A.; Wang, X.; Huang, Y.; Treiber, F.A.; et al. High-mobility group box-1 is associated with obesity, inflammation, and subclinical cardiovascular risk among young adults: A longitudinal cohort study. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2776–2784. [Google Scholar] [CrossRef]
- Foglio, E.; Pellegrini, L.; Germani, A.; Russo, M.A.; Limana, F. HMGB1-mediated apoptosis and autophagy in ischemic heart diseases. Vasc. Biol. 2019, 1, H89–H96. [Google Scholar] [CrossRef]
- Giacobbe, A.; Granese, R.; Grasso, R.; Salpietro, V.; Corrado, F.; Giorgianni, G.; Foti, G.; Amadore, D.; Triolo, O.; Giunta, L.; et al. Association between maternal serum high mobility group box 1 levels and pregnancy complicated by gestational diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 414–418. [Google Scholar] [CrossRef]
- Wang, H.; Qu, H.; Deng, H. Plasma HMGB-1 levels in subjects with obesity and type 2 diabetes: A cross-sectional study in China. PLoS ONE 2015, 10, e0136564. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamakuchi, M.; Biswas, K.K.; Aryal, B.; Yamada, S.; Hashiguchi, T.; Maruyama, I. HMGB1 is secreted by 3T3-L1 adipocytes through JNK signaling and the secretion is partially inhibited by adiponectin. Obesity 2016, 24, 1913–1921. [Google Scholar] [CrossRef]
- Montanini, L.; Cirillo, F.; Smerieri, A.; Pisi, G.; Giardino, I.; d’Apolito, M.; Spaggiari, C.; Bernasconi, S.; Amarri, S.; Street, M.E. HMGB1 is increased by CFTR loss of function, is lowered by insulin, and increases in vivo at onset of CFRD. J. Clin. Endocrinol. Metab. 2016, 101, 1274–1281. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cirillo, F.; Catellani, C.; Lazzeroni, P.; Sartori, C.; Nicoli, A.; Amarri, S.; La Sala, G.B.; Street, M.E. MiRNAs regulating insulin sensitivity are dysregulated in polycystic ovary syndrome (PCOS) ovaries and are associated with markers of inflammation and insulin sensitivity. Front. Endocrinol. 2019, 10, 879. [Google Scholar] [CrossRef]
- Jialal, I.; Rajamani, U.; Adams-Huet, B.; Kaur, H. Circulating pathogen associated molecular pattern—Binding proteins and High Mobility Group Box protein 1 in nascent metabolic syndrome: Implications for cellular Toll-like receptor activity. Atherosclerosis 2014, 236, 182–187. [Google Scholar] [CrossRef]
- Arrigo, T.; Chirico, V.; Salpietro, V.; Munafò, C.; Ferraù, V.; Gitto, E.; Lacquaniti, A.; Salpietro, C. High-mobility group protein B1: A new biomarker of metabolic syndrome in obese children. Eur. J. Endocrinol. 2013, 168, 631–638. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suda, K.; Kitagawa, Y.; Ozawa, S.; Saikawa, Y.; Ueda, M.; Ebina, M.; Yamada, S.; Hashimoto, S.; Fukata, S.; Abraham, E.; et al. Anti-high-mobility group box chromosomal protein 1 antibodies improve survival of rats with sepsis. World J. Surg. 2006, 30, 1755–1762. [Google Scholar] [CrossRef]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef]
- Sawa, H.; Ueda, T.; Takeyama, Y.; Yasuda, T.; Shinzeki, M.; Nakajima, T.; Kuroda, Y. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J. Gastroenterol. 2006, 12, 7666–7670. [Google Scholar] [CrossRef] [PubMed]
- Kokkola, R.; Li, J.; Sundberg, E.; Aveberger, A.; Palmblad, K.; Yang, H.; Tracey, K.J.; Andersson, U.; Harris, H.E. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003, 48, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Hikiba, Y.; Shibata, W.; Ohmae, T.; Yanai, A.; Ogura, K.; Yamada, S.; Omata, M. Essential roles of high-mobility group box 1 in the development of murine colitis and colitis-associated cancer. Biochem. Biophys. Res. Commun. 2007, 360, 394–400. [Google Scholar] [CrossRef]
- Yang, R.; Harada, T.; Mollen, K.P.; Prince, J.M.; Levy, R.M.; Englert, J.A.; Gallowitsch-Puerta, M.; Yang, L.; Yang, H.; Tracey, K.J.; et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol. Med. 2006, 12, 105–114. [Google Scholar] [CrossRef]
- Hamada, N.; Maeyama, T.; Kawaguchi, T.; Yoshimi, M.; Fukumoto, J.; Yamada, M.; Yamada, S.; Kuwano, K.; Nakanishi, Y. The role of high mobility group box1 in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2008, 39, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Tsung, A.; Sahai, R.; Tanaka, H.; Nakao, A.; Fink, M.P.; Lotze, M.T.; Yang, H.; Li, J.; Tracey, K.J.; Geller, D.A.; et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005, 201, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Otoshi, K.-I.; Kikuchi, S.-I.; Kato, K.; Sekiguchi, M.; Konno, S.-I. Anti-HMGB1 neutralization antibody improves pain-related behavior induced by application of autologous nucleus pulposus onto nerve roots in rats. Spine 2011, 36, E692–E698. [Google Scholar] [CrossRef]
- Matsuoka, N.; Itoh, T.; Watarai, H.; Sekine-Kondo, E.; Nagata, N.; Okamoto, K.; Mera, T.; Yamamoto, H.; Yamada, S.; Maruyama, I.; et al. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J. Clin. Investig. 2010, 120, 735–743. [Google Scholar] [CrossRef]
- Stills, H.F. Polyclonal antibody production. In The Laboratory Rabbit, Guinea Pig, Hamster, and OTHER Rodents; Elsevier: Amsterdam, The Netherlands, 2012; pp. 259–274. [Google Scholar]
- Vaisman-Mentesh, A.; Gutierrez-Gonzalez, M.; DeKosky, B.J.; Wine, Y. The molecular mechanisms that underlie the immune biology of anti-drug antibody formation following treatment with monoclonal antibodies. Front. Immunol. 2020, 11, 01951. [Google Scholar] [CrossRef] [PubMed]
- Pecetta, S.; Finco, O.; Seubert, A. Quantum leap of monoclonal antibody (mAb) discovery and development in the COVID-19 era. Semin. Immunol. 2020, 50, 101427. [Google Scholar] [CrossRef]
- Qin, S.; Wang, H.; Yuan, R.; Li, H.; Ochani, M.; Ochani, K.; Rosas-Ballina, M.; Czura, C.J.; Huston, J.M.; Miller, E.; et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 2006, 203, 1637–1642. [Google Scholar] [CrossRef]
- Gao, Q.; Ma, L.L.; Gao, X.; Yan, W.; Williams, P.; Yin, D.P. TLR4 mediates early graft failure after intraportal islet transplantation. Am. J. Transplant. 2010, 10, 1588–1596. [Google Scholar] [CrossRef]
- Schieberck, H.; Lundbäck, P.; Palmblad, K. Monoclonal anti-HMGB1 antibody protection in two experimental arthritis models. Mol Med. 2011, 17, 1039–1044. [Google Scholar] [CrossRef]
- Liu, K.; Mori, S.; Takahashi, H.K.; Tomono, Y.; Wake, H.; Kanke, T.; Sato, Y.; Hiraga, N.; Adachi, N.; Yoshino, T.; et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007, 21, 3904–3916. [Google Scholar] [CrossRef]
- Nakamura, Y.; Morioka, N.; Abe, H.; Zhang, F.F.; Hisaoka-Nakashima, K.; Liu, K.; Nishibori, M.; Nakata, Y. Neuropathic pain in rats with a partial sciatic nerve ligation is alleviated by intravenous injection of monoclonal antibody to high mobility group box-1. PLoS ONE 2013, 8, e73640. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Liu, K.; Agari, T.; Yasuhara, T.; Morimoto, J.; Okazaki, M.; Takeuchi, H.; Toyoshima, A.; Sasada, S.; Shinko, A.; et al. Anti-high mobility group box 1 antibody exerts neuroprotection in a rat model of Parkinson’s disease. Exp. Neurol. 2016, 275, 220–231. [Google Scholar]
- Palumbo, R.; De Marchis, F.; Pusterla, T.; Conti, A.; Alessio, M.; Bianchi, M.E. Src family kinases are necessary for cell migration induced by extracellular HMGB1. J. Leukoc. Biol. 2009, 86, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, M.; Raucci, A.; Muñoz, L.M.; Livoti, E.; Celona, B.; Venereau, E.; Apuzzo, T.; De Marchis, F.; Pedotti, M.; Bachi, A.; et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J. Exp. Med. 2012, 209, 551–563. [Google Scholar] [CrossRef]
- Sitia, G.; Iannacone, M.; Aiolfi, R.; Isogawa, M.; van Rooijen, N.; Scozzesi, C.; Bianchi, M.E.; von Andrian, U.H.; Chisari, F.V.; Guidotti, L.G. Kupffer cells hasten resolution of liver immunopathology in mouse models of viral hepatitis. PLOS Pathog. 2011, 7, e1002061. [Google Scholar] [CrossRef]
- Kanellakis, P.; Agrotis, A.; Kyaw, T.S.; Koulis, C.; Ahrens, I.; Mori, S.; Takahashi, H.K.; Liu, K.; Peter, K.; Nishibori, M.; et al. High-mobility group box protein 1 neutralization reduces development of diet-induced atherosclerosis in apolipoprotein E–deficient mice. Arter. Thromb. Vasc. Biol. 2011, 31, 313–319. [Google Scholar] [CrossRef]
- Jube, S.; Rivera, Z.S.; Bianchi, M.E.; Powers, A.; Wang, E.; Pagano, I.; Pass, H.I.; Gaudino, G.; Carbone, M.; Yang, H. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012, 72, 3290–3301. [Google Scholar] [CrossRef]
- Zhang, J.; Takahashi, H.K.; Liu, K.; Wake, H.; Liu, R.; Maruo, T.; Date, I.; Yoshino, T.; Ohtsuka, A.; Mori, S.; et al. Anti-high mobility group box-1 monoclonal antibody protects the blood–brain barrier from ischemia-induced disruption in rats. Stroke 2011, 42, 1420–1428. [Google Scholar] [CrossRef]
- Raucci, A.; Cugusi, S.; Antonelli, A.; Barabino, S.M.; Monti, L.; Bierhaus, A.; Reiss, K.; Saftig, P.; Bianchi, M.E. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 2008, 22, 3716–3727. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, H.; Yamamoto, Y.; Sakurai, S.; Petrova, R.G.; Abedin, M.J.; Li, H.; Yasui, K.; Takeuchi, M.; Makita, Z.; Takasawa, S.; et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003, 370, 1097–1109. [Google Scholar] [CrossRef]
- Pilzweger, C.; Holdenrieder, S. Circulating HMGB1 and RAGE as clinical biomarkers in malignant and autoimmune diseases. Diagnostics 2015, 5, 219–253. [Google Scholar] [CrossRef]
- Cataldegirmen, G.; Zeng, S.; Feirt, N.; Ippagunta, N.; Dun, H.; Qu, W.; Lu, Y.; Rong, L.L.; Hofmann, M.A.; Kislinger, T.; et al. Rage limits regeneration after massive liver injury by coordinated suppression of TNF-α and NF-κB. J. Exp. Med. 2005, 201, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Raman, K.G.; Sappington, P.L.; Yang, R.; Levy, R.M.; Prince, J.M.; Liu, S.; Watkins, S.K.; Schmidt, A.M.; Billiar, T.R.; Fink, M.P. The role of RAGE in the pathogenesis of intestinal barrier dysfunction after hemorrhagic shock. Am. J. Physiol. Liver Physiol. 2006, 291, G556–G565. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wendt, T.M.; Tanji, N.; Guo, J.; Kislinger, T.R.; Qu, W.; Lu, Y.; Bucciarelli, L.G.; Rong, L.L.; Moser, B.; Markowitz, G.S.; et al. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am. J. Pathol. 2003, 162, 1123–1137. [Google Scholar] [CrossRef] [PubMed]
- Goova, M.T.; Li, J.; Kislinger, T.; Qu, W.; Lu, Y.; Bucciarelli, L.G.; Nowygrod, S.; Wolf, B.M.; Caliste, X.; Yan, S.F.; et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am. J. Pathol. 2001, 159, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Blood, D.C.; del Toro, G.; Canet, A.; Lee, D.C.; Qu, W.; Tanji, N.; Lu, Y.; Lalla, E.; Fu, C.; et al. Blockade of RAGE–amphoterin signalling suppresses tumour growth and metastases. Nature 2000, 405, 354–360. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Ju, Z.; Ragab, A.A.; Lundbäck, P.; Long, W.; Valdés-Ferrer, S.I.; He, M.; Pribis, J.P.; Li, J.; et al. MD-2 is required for disulfide HMGB1---Dependent TLR4 signaling. J. Exp. Med. 2015, 212, 5–14. [Google Scholar] [CrossRef]
- Yang, H.; Lundbäck, P.; Ottosson, L.; Erlandsson-Harris, H.; Venereau, E.; Bianchi, M.E.; Al-Abed, Y.; Andersson, U.; Tracey, K.J. Redox modifications of cysteine residues regulate the cytokine activity of HMGB1. Mol. Med. 2021, 27, 58. [Google Scholar] [CrossRef]
- Cai, J.; Yuan, H.; Wang, Q.; Yang, H.; Al-Abed, Y.; Hua, Z.; Wang, J.; Chen, D.; Wu, J.; Lu, B.; et al. HMGB1-driven inflammation and intimal hyperplasia after arterial injury involves cell-specific actions mediated by TLR4. Arter. Thromb. Vasc. Biol. 2015, 35, 2579–2593. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tajima, Y. HMGB1 is a promising therapeutic target for acute liver failure. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 673–682. [Google Scholar] [CrossRef]
- Davé, S.H.; Tilstra, J.S.; Matsuoka, K.; Li, F.; DeMarco, R.A.; Beer-Stolz, D.; Sepulveda, A.R.; Fink, M.P.; Lotze, M.T.; Plevy, S.E. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J. Leukoc. Biol. 2009, 86, 633–643. [Google Scholar] [CrossRef]
- Yang, R.; Shaufl, A.L.; Killeen, M.E.; Fink, M.P. Ethyl pyruvate ameliorates liver injury secondary to severe acute pancreatitis. J. Surg. Res. 2009, 153, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, H.; Zhao, J.; Pan, H.; Mao, L. Beneficial effects of ethyl pyruvate through inhibiting high-mobility group box 1 expression and TLR4/NF-B pathway after traumatic brain injury in the rat. Mediat. Inflamm. 2011, 2011, 807142. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Sarkar, A.; Walle, L.V.; Vitari, A.C.; Amer, A.O.; Wewers, M.D.; Tracey, K.J.; Kanneganti, T.-D.; Dixit, V.M. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 2010, 185, 4385–4392. [Google Scholar] [CrossRef] [PubMed]
- Killeen, M.E.; Englert, J.A.; Stolz, D.B.; Song, M.; Han, Y.; Delude, R.L.; Kellum, J.A.; Fink, M.P. The phase 2 enzyme inducers ethacrynic acid, DL-sulforaphane, and oltipraz inhibit lipopolysaccharide-induced high-mobility group box 1 secretion by RAW 264.7 cells. J. Pharmacol. Exp. Ther. 2006, 316, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gorasiya, S.; Antoine, D.J.; Sitapara, R.A.; Wu, W.; Sharma, L.; Yang, H.; Ashby, C.R., Jr.; Vasudevan, D.; Zur, M.; et al. The compromise of macrophage functions by hyperoxia is attenuated by ethacrynic acid via inhibition of NF-κB–mediated release of high-mobility group box-1. Am. J. Respir. Cell Mol. Biol. 2015, 52, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nam, Y.; Koo, J.Y.; Lim, D.; Park, J.; Ock, J.; Kim, J.; Suk, K.; Park, S.B. A small molecule binding HMGB1 and HMGB2 inhibits microglia-mediated neuroinflammation. Nat. Chem. Biol. 2014, 10, 1055–1060. [Google Scholar] [CrossRef]
- Iachettini, S.; Ciccarone, F.; Maresca, C.; Angelo, C.D.; Petti, E.; Di Vito, S.; Ciriolo, M.R.; Zizza, P.; Biroccio, A. The telomeric protein TERF2/TRF2 impairs HMGB1-driven autophagy. Autophagy 2023, 19, 1479–1490. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kwak, M.S.; Shin, J.M.; Hayuningtyas, R.A.; Choi, J.E.; Shin, J.-S. Inflachromene inhibits autophagy through modulation of Beclin 1 activity. J. Cell Sci. 2018, 131, jcs211201. [Google Scholar] [CrossRef]
- Yin, X.-Y.; Tang, X.-H.; Wang, S.-X.; Zhao, Y.-C.; Jia, M.; Yang, J.-J.; Ji, M.-H.; Shen, J.-C. HMGB1 mediates synaptic loss and cognitive impairment in an animal model of sepsis-associated encephalopathy. J. Neuroinflamm. 2023, 20, 69. [Google Scholar] [CrossRef]
- Teng, S.; Zhu, Z.; Wu, C.; He, Y.; Zhou, S. Inflachromene inhibits intimal hyperplasia through the HMGB1/2-regulated TLR4-NF-κB pathway. Int. Immunopharmacol. 2023, 119, 110198. [Google Scholar] [CrossRef]
- Musumeci, D.; Roviello, G.N.; Montesarchio, D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol. Ther. 2014, 141, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ashok, M.; Li, J.; Yang, H.; Sama, A.E.; Wang, H. A major ingredient of green tea rescues mice from lethal sepsis partly by inhibiting HMGB1. PLoS ONE 2007, 2, e1153. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Xiao, W.; Zhang, H.; Lotze, M.T.; Wang, H.; Xiao, X. Quercetin prevents LPS-induced high-mobility group box 1 release and proinflammatory function. Am. J. Respir. Cell Mol. Biol. 2009, 41, 651–660. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.-K.; Bae, J.W.; Bae, J.-S. Inhibitory effects of lycopene on HMGB1-mediated pro-inflammatory responses in both cellular and animal models. Food Chem. Toxicol. 2012, 50, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, S.; Iwasaka, H.; Hagiwara, S.; Noguchi, T. Gabexate mesilate inhibits the expression of HMGB1 in lipopolysaccharide-induced acute lung injury. J. Surg. Res. 2011, 165, 142–150. [Google Scholar] [CrossRef]
- Hagiwara, S.; Iwasaka, H.; Togo, K.; Noguchi, T. A neutrophil elastase inhibitor, sivelestat, reduces lung injury following endotoxin-induced shock in rats by inhibiting HMGB1. Inflammation 2008, 31, 227–234. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Liu, L.; Yang, R.; Cui, L.; Li, M. Atorvastatin protects rat brains against permanent focal ischemia and downregulates HMGB1, HMGB1 receptors (RAGE and TLR4), NF-κB expression. Neurosci. Lett. 2010, 471, 152–156. [Google Scholar] [CrossRef]
- Liu, M.; Yu, Y.; Jiang, H.; Zhang, L.; Zhang, P.-P.; Yu, P.; Jia, J.-G.; Chen, R.-Z.; Zou, Y.-Z.; Ge, J.-B. Simvastatin suppresses vascular inflammation and atherosclerosis in ApoE−/− mice by downregulating the HMGB1-RAGE axis. Acta Pharmacol. Sin. 2013, 34, 830–836. [Google Scholar] [CrossRef]
- C. 2024. Available online: https://www.scientific-computing.com/press-releases/chembiodraw-140 (accessed on 21 May 2024).
- Sakamoto, R.; Okano, M.; Takena, H.; Ohtsuki, K. Inhibitory effect of glycyrrhizin on the phosphorylation and DNA-binding abilities of high mobility group proteins 1 and 2 in vitro. Biol. Pharm. Bull. 2001, 24, 906–911. [Google Scholar] [CrossRef]
- Mollica, L.; De Marchis, F.; Spitaleri, A.; Dallacosta, C.; Pennacchini, D.; Zamai, M.; Agresti, A.; Trisciuoglio, L.; Musco, G.; Bianchi, M.E. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem. Biol. 2007, 14, 431–441. [Google Scholar] [CrossRef]
- Vergoten, G.; Bailly, C. Analysis of glycyrrhizin binding to protein HMGB1. Med. Drug Discov. 2020, 7, 100058. [Google Scholar] [CrossRef]
- Singh, G.B.; Zhang, Y.; Boini, K.M.; Koka, S. High mobility group box 1 mediates TMAO-induced endothelial dysfunction. Int. J. Mol. Sci. 2019, 20, 3570. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Xiang, L.; Yuan, L.; Hu, L.; Wu, W.; Cai, L.; Yin, L.; Dong, H. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS ONE 2014, 9, e89450. [Google Scholar] [CrossRef] [PubMed]
- Ogiku, M.; Kono, H.; Hara, M.; Tsuchiya, M.; Fujii, H. Glycyrrhizin prevents liver injury by inhibition of high-mobility group box 1 production by kupffer cells after ischemia-reperfusion in rats. J. Pharmacol. Exp. Ther. 2011, 339, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Mollica, L.; Curioni, A.; Andreoni, W.; Bianchi, M.E.; Musco, G. The binding domain of the HMGB1 inhibitor carbenoxolone: Theory and experiment. Chem. Phys. Lett. 2008, 456, 236–242. [Google Scholar] [CrossRef]
- Mollica, L.; Morra, G.; Colombo, G.; Musco, G. HMGB1–carbenoxolone interactions: Dynamics insights from combined nuclear magnetic resonance and molecular dynamics. Chem. Asian J. 2011, 6, 1171–1180. [Google Scholar] [CrossRef]
- Du, D.; Yan, J.; Ren, J.; Lv, H.; Li, Y.; Xu, S.; Wang, Y.; Ma, S.; Qu, J.; Tang, W.; et al. Synthesis, biological evaluation, and molecular modeling of glycyrrhizin derivatives as potent high-mobility group box-1 inhibitors with anti-heart-failure activity in vivo. J. Med. Chem. 2012, 56, 97–108. [Google Scholar] [CrossRef]
- Li, W.; Sama, A.; Wang, H.; Sama, A.E. Role of HMGB1 in cardiovascular diseases. Curr. Opin. Pharmacol. 2006, 6, 130–135. [Google Scholar] [CrossRef]
- Zhu, K.; Fan, R.; Cao, Y.; Yang, W.; Zhang, Z.; Zhou, Q.; Ren, J.; Shi, X.; Gao, Y.; Guo, X. Glycyrrhizin attenuates myocardial ischemia reperfusion injury by suppressing Inflammation, oxidative stress, and ferroptosis via the HMGB1-TLR4-GPX4 pathway. Exp. Cell Res. 2024, 435, 113912. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, B.; Peng, W.; Xu, Z. Protective effect of glycyrrhizin on coronary microembolization-induced myocardial dysfunction in rats. Pharmacol. Res. Perspect. 2021, 9, e00714. [Google Scholar] [CrossRef]
- Choi, H.W.; Tian, M.; Song, F.; Venereau, E.; Preti, A.; Park, S.-W.; Hamilton, K.; Swapna, G.V.T.; Manohar, M.; Moreau, M.; et al. Aspirin’s active metabolite salicylic acid targets high mobility group box 1 to modulate inflammatory responses. Mol. Med. 2015, 21, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Gerö, D.; Szoleczky, P.; Módis, K.; Pribis, J.P.; Al-Abed, Y.; Yang, H.; Chevan, S.; Billiar, T.R.; Tracey, K.J.; Szabo, C. Identification of pharmacological modulators of HMGB1-induced inflammatory response by cell-based screening. PLoS ONE 2013, 8, e65994. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davaanyam, D.; Lee, H.; Seol, S.-I.; Oh, S.-A.; Kim, S.-W.; Lee, J.-K. HMGB1 induces hepcidin upregulation in astrocytes and causes an acute iron surge and subsequent ferroptosis in the postischemic brain. Exp. Mol. Med. 2023, 55, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Yin, J.; Zhang, Z.; Xiang, H.; Wang, J.; Zhu, D.; Xu, X.; Cao, Y. Destruction in maternal-fetal interface of URSA patients via the increase of the HMGB1-RAGE/TLR2/TLR4-NF-κB signaling pathway. Life Sci. 2020, 250, 117543. [Google Scholar] [CrossRef]
- Yang, H.; Pellegrini, L.; Napolitano, A.; Giorgi, C.; Jube, S.; Preti, A.; Jennings, C.J.; De Marchis, F.; Flores, E.G.; Larson, D.; et al. Aspirin delays mesothelioma growth by inhibiting HMGB1-mediated tumor progression. Cell Death Dis. 2015, 6, e1786. [Google Scholar] [CrossRef]
- Yang, H.; Andersson, U.; Brines, M. Neurons are a primary driver of inflammation via release of HMGB1. Cells 2021, 10, 2791. [Google Scholar] [CrossRef]
- Yang, H.; Zeng, Q.; Silverman, H.A.; Gunasekaran, M.; George, S.J.; Devarajan, A.; Addorisio, M.E.; Li, J.; Tsaava, T.; Shah, V.; et al. HMGB1 released from nociceptors mediates inflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2102034118. [Google Scholar] [CrossRef]
- Behl, T.; Sharma, E.; Sehgal, A.; Kaur, I.; Kumar, A.; Arora, R.; Pal, G.; Kakkar, M.; Kumar, R.; Bungau, S. Expatiating the molecular approaches of HMGB1 in diabetes mellitus: Highlighting signalling pathways via RAGE and TLRs. Mol. Biol. Rep. 2021, 48, 1869–1881. [Google Scholar] [CrossRef]
- Wang, D.; Liu, K.; Fukuyasu, Y.; Teshigawara, K.; Fu, L.; Wake, H.; Ohtsuka, A.; Nishibori, M. HMGB1 translocation in neurons after ischemic insult: Subcellular localization in mitochondria and peroxisomes. Cells 2020, 9, 643. [Google Scholar] [CrossRef]
- Xue, J.; Suarez, J.S.; Minaai, M.; Li, S.; Gaudino, G.; Pass, H.I.; Carbone, M.; Yang, H. HMGB1 as a therapeutic target in disease. J. Cell. Physiol. 2021, 236, 3406–3419. [Google Scholar] [CrossRef]
- Ferrara, M.; Chialli, G.; Ferreira, L.M.; Ruggieri, E.; Careccia, G.; Preti, A.; Piccirillo, R.; Bianchi, M.E.; Sitia, G.; Venereau, E. Oxidation of HMGB1 is a dynamically regulated process in physiological and pathological conditions. Front. Immunol. 2020, 11, 1122. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, S.; Marei, O.; Elalfy, O.; Zaben, M. Neurogenesis after traumatic brain injury—The complex role of HMGB1 and neuroinflammation. Neuropharmacology 2021, 183, 108400. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, G.; Artinger, M.; Locati, M.; Perez, L.; Legler, D.F.; Bianchi, M.E.; Rüegg, C.; Thelen, M.; Marchese, A.; Rocchi, M.B.; et al. β-Arrestin1 and β-Arrestin2 Are Required to Support the Activity of the CXCL12/HMGB1 Heterocomplex on CXCR4. Front. Immunol. 2020, 11, 550824. [Google Scholar] [CrossRef] [PubMed]
- Kazama, H.; Ricci, J.E.; Herndon, J.M.; Hoppe, G.; Green, D.R.; Ferguson, T.A. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 2008, 29, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Zeh, H.J.; Rubartelli, A.; Sparvero, L.J.; Amoscato, A.A.; Washburn, N.R.; DeVera, M.E.; Liang, X.; Tör, M.; Billiar, T. The grateful dead: Damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol. Rev. 2007, 220, 60–81. [Google Scholar] [CrossRef]
- Volchuk, A.; Ye, A.; Chi, L.; Steinberg, B.E.; Goldenberg, N.M. Indirect regulation of HMGB1 release by gasdermin D. Nat. Commun. 2020, 11, 4561. [Google Scholar] [CrossRef]
- Li, W.; Deng, M.; Loughran, P.A.; Yang, M.; Lin, M.; Yang, C.; Gao, W.; Jin, S.; Li, S.; Cai, J.; et al. LPS induces active HMGB1 release from hepatocytes into exosomes through the coordinated activities of TLR4 and caspase-11/GSDMD signaling. Front. Immunol. 2020, 11, 229. [Google Scholar] [CrossRef]
- Kwak, M.S.; Choi, S.; Kim, J.; Lee, H.; Park, I.H.; Oh, J.; Mai, D.N.; Cho, N.H.; Nam, K.T.; Shin, J.S. SARS-CoV-2 Infection Induces HMGB1 Secretion Through Post-Translational Modification and PANoptosis. Immune Netw. 2022, 23, e26. [Google Scholar] [CrossRef]
- Kwak, M.S.; Kim, H.S.; Lee, B.; Kim, Y.H.; Son, M.; Shin, J.-S. Immunological significance of HMGB1 post-translational modification and redox biology. Front. Immunol. 2020, 11, 1189. [Google Scholar] [CrossRef]
- Feng, W.; Wang, J.; Yan, X.; Zhang, Q.; Chai, L.; Wang, Q.; Shi, W.; Chen, Y.; Liu, J.; Qu, Z.; et al. ERK/Drp1-dependent mitochondrial fission contributes to HMGB1-induced autophagy in pulmonary arterial hypertension. Cell Prolif. 2021, 54, e13048. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Qiu, Y.; Shi, Y.; Cai, J.; Wang, B.; Wei, X.; Ke, Q.; Sui, X.; Wang, Y.; et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J. Hematol. Oncol. 2018, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Wu, Y.-T.; Yu, T.-H.; Wei, Y.-H. (Eds.) Mitochondria in mesenchymal stem cell biology and cell therapy: From cellular differentiation to mitochondrial transfer. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Ito, H.; Fujita, K.; Tagawa, K.; Chen, X.; Homma, H.; Sasabe, T.; Shimizu, J.; Shimizu, S.; Tamura, T.; Muramatsu, S.; et al. HMGB1 facilitates repair of mitochondrial DNA damage and extends the lifespan of mutant ataxin-1 knock-in mice. EMBO Mol. Med. 2015, 7, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.-W.; Ko, A.-R.; Kang, T.-C. Mitochondrial translocation of high mobility group box 1 facilitates LIM kinase 2-mediated programmed necrotic neuronal death. Front. Cell. Neurosci. 2016, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Cao, L.; Yang, X.; Zhao, X.; Liu, X.; Han, Y.; Xue, Y.; Jiang, H.; Chi, Z. Role of mitochondrial fission in neuronal injury in pilocarpine-induced epileptic rats. Neuroscience 2013, 245, 157–165. [Google Scholar] [CrossRef] [PubMed]
- IvanoviĆ-BurmazoviĆ, I. Reactivity of manganese superoxide dismutase mimics toward superoxide and nitric oxide: Selectivity versus cross-reactivity. Adv. Inorg. Chem. 2012, 64, 53–95. [Google Scholar]
- Urbonaviciute, V.; Meister, S.; Fürnrohr, B.G.; Frey, B.; Gückel, E.; Schett, G.; Herrmann, M.; Voll, R.E. Oxidation of the alarmin high-mobility group box 1 protein (HMGB1) during apoptosis: Brief Definite Report. Autoimmunity 2009, 42, 305–307. [Google Scholar] [CrossRef]
- Guo, Y.; Dai, W.; Zheng, Y.; Qiao, W.; Chen, W.; Peng, L.; Zhou, H.; Zhao, T.; Liu, H.; Zheng, F.; et al. Mechanism and regulation of microglia polarization in intracerebral hemorrhage. Molecules 2022, 27, 7080. [Google Scholar] [CrossRef]
- Sun, Z.; Nyanzu, M.; Yang, S.; Zhu, X.; Wang, K.; Ru, J.; Yu, E.; Zhang, H.; Wang, Z.; Shen, J.; et al. VX765 attenuates pyroptosis and HMGB1/TLR4/NF-κB pathways to improve functional outcomes in TBI mice. Oxidative Med. Cell. Longev. 2020, 2020, 7879629. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, L.; Wang, Q.; Cao, J.-H.; Chen, Y.; Wang, W.; Zhu, B.-D.; Wei, Z.-H.; Li, R.; Li, C.-Y.; et al. Elevated branched-chain amino acid promotes atherosclerosis progression by enhancing mitochondrial-to-nuclear H2O2-disulfide HMGB1 in macrophages. Redox Biol. 2023, 62, 102696. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Datta, S.; Rahman, M.A.; Koka, S.; Boini, K.M. High Mobility Group Box 1 (HMGB1): Molecular Signaling and Potential Therapeutic Strategies. Cells 2024, 13, 1946. https://doi.org/10.3390/cells13231946
Datta S, Rahman MA, Koka S, Boini KM. High Mobility Group Box 1 (HMGB1): Molecular Signaling and Potential Therapeutic Strategies. Cells. 2024; 13(23):1946. https://doi.org/10.3390/cells13231946
Chicago/Turabian StyleDatta, Sayantap, Mohammad Atiqur Rahman, Saisudha Koka, and Krishna M. Boini. 2024. "High Mobility Group Box 1 (HMGB1): Molecular Signaling and Potential Therapeutic Strategies" Cells 13, no. 23: 1946. https://doi.org/10.3390/cells13231946
APA StyleDatta, S., Rahman, M. A., Koka, S., & Boini, K. M. (2024). High Mobility Group Box 1 (HMGB1): Molecular Signaling and Potential Therapeutic Strategies. Cells, 13(23), 1946. https://doi.org/10.3390/cells13231946






