Motile Cilia in Female and Male Reproductive Tracts and Fertility
Abstract
1. Introduction—Cilia, the Evolutionarily Conserved Structures
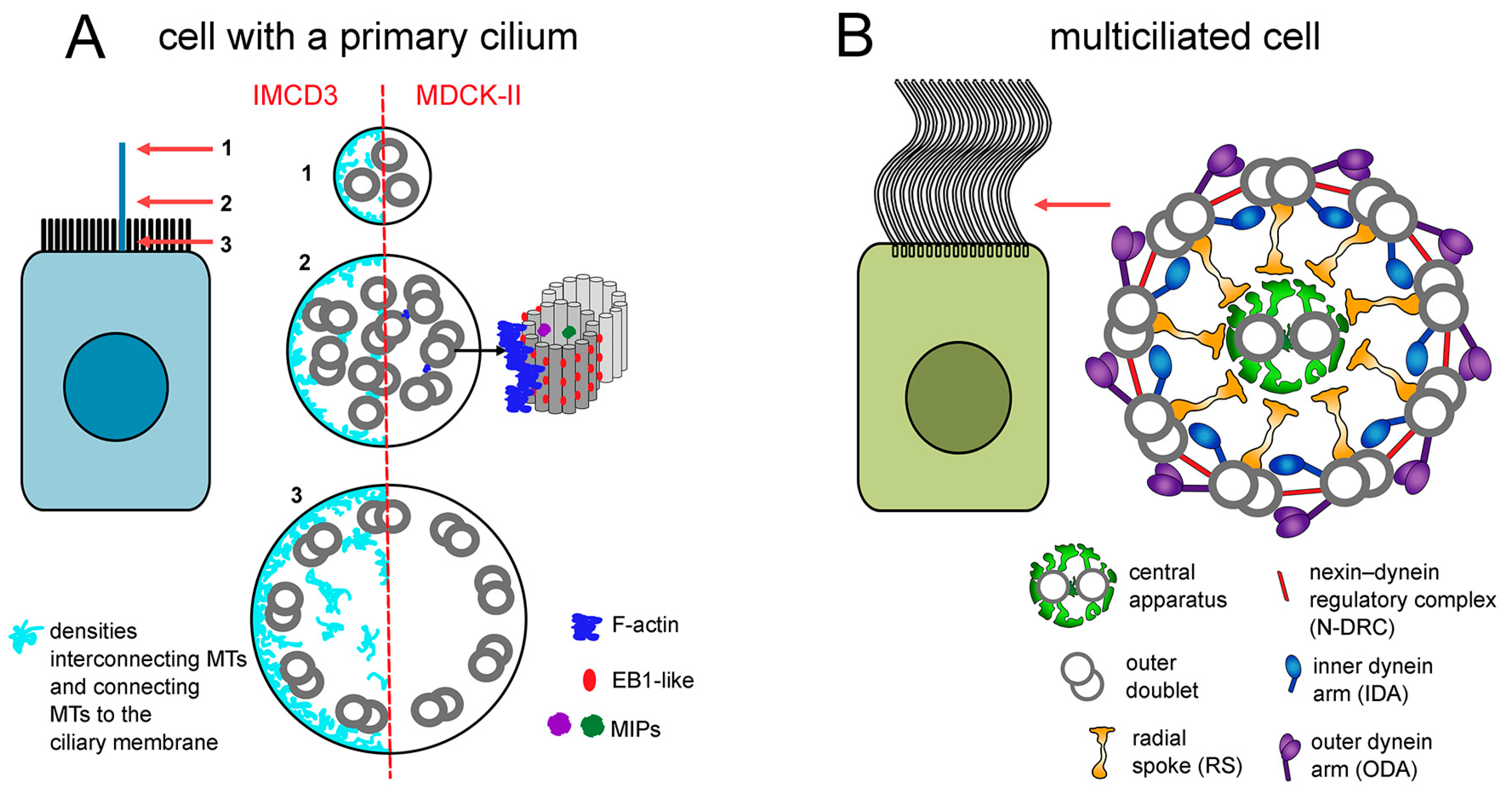
2. MCCs and Motile Cilia in Humans
3. MCCs in the Female Reproductive System
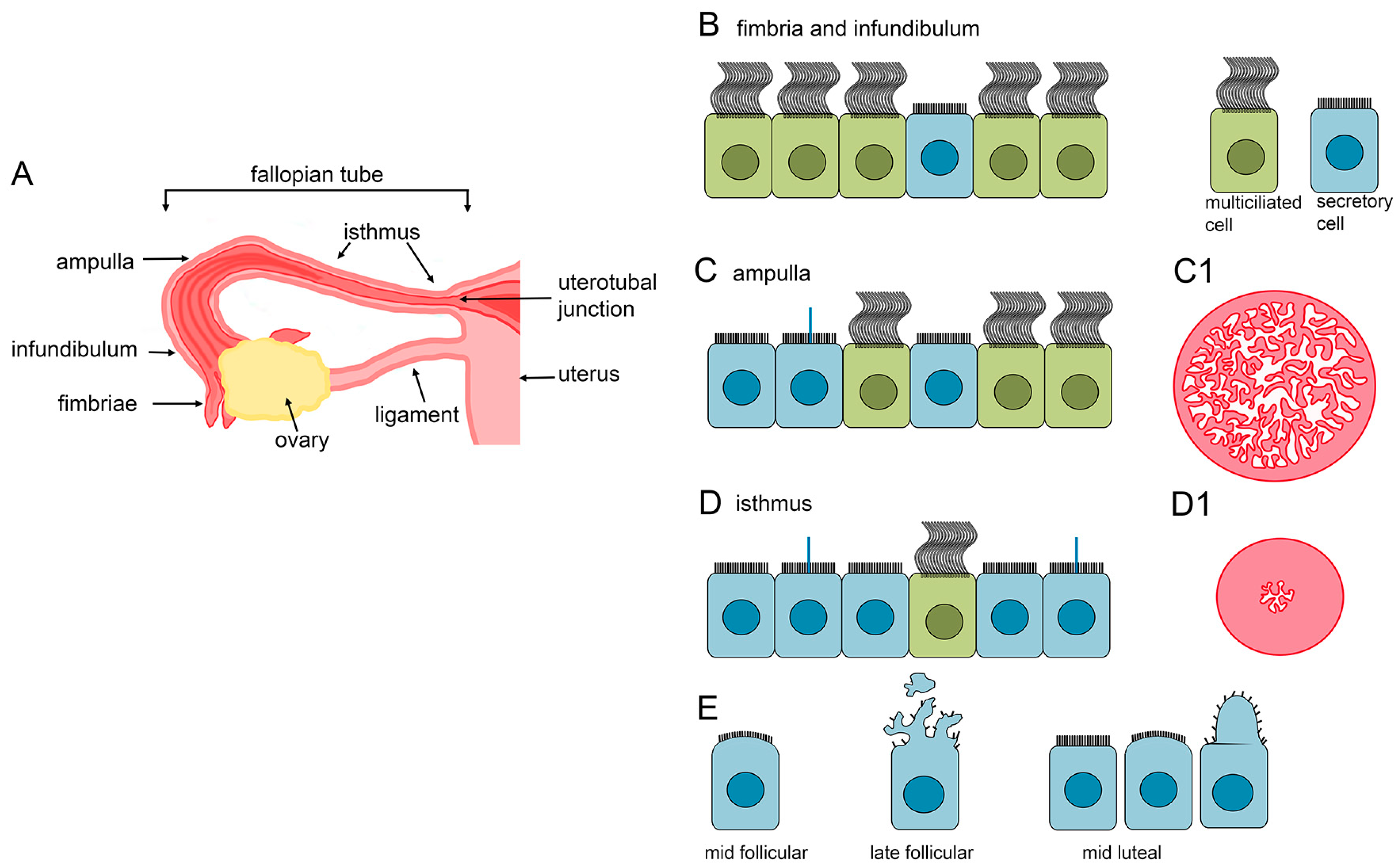
3.1. MCCs in the Fallopian Tube
3.1.1. The Fallopian Tube Morphology
3.1.2. Fallopian Tube Epithelial Cell Morphology and Structure
3.1.3. Cilia Beating Frequency
3.1.4. Hormonal Regulation of the Epithelial Cells
3.1.5. Conditions Affecting MCCs
3.1.6. Role of MCCs
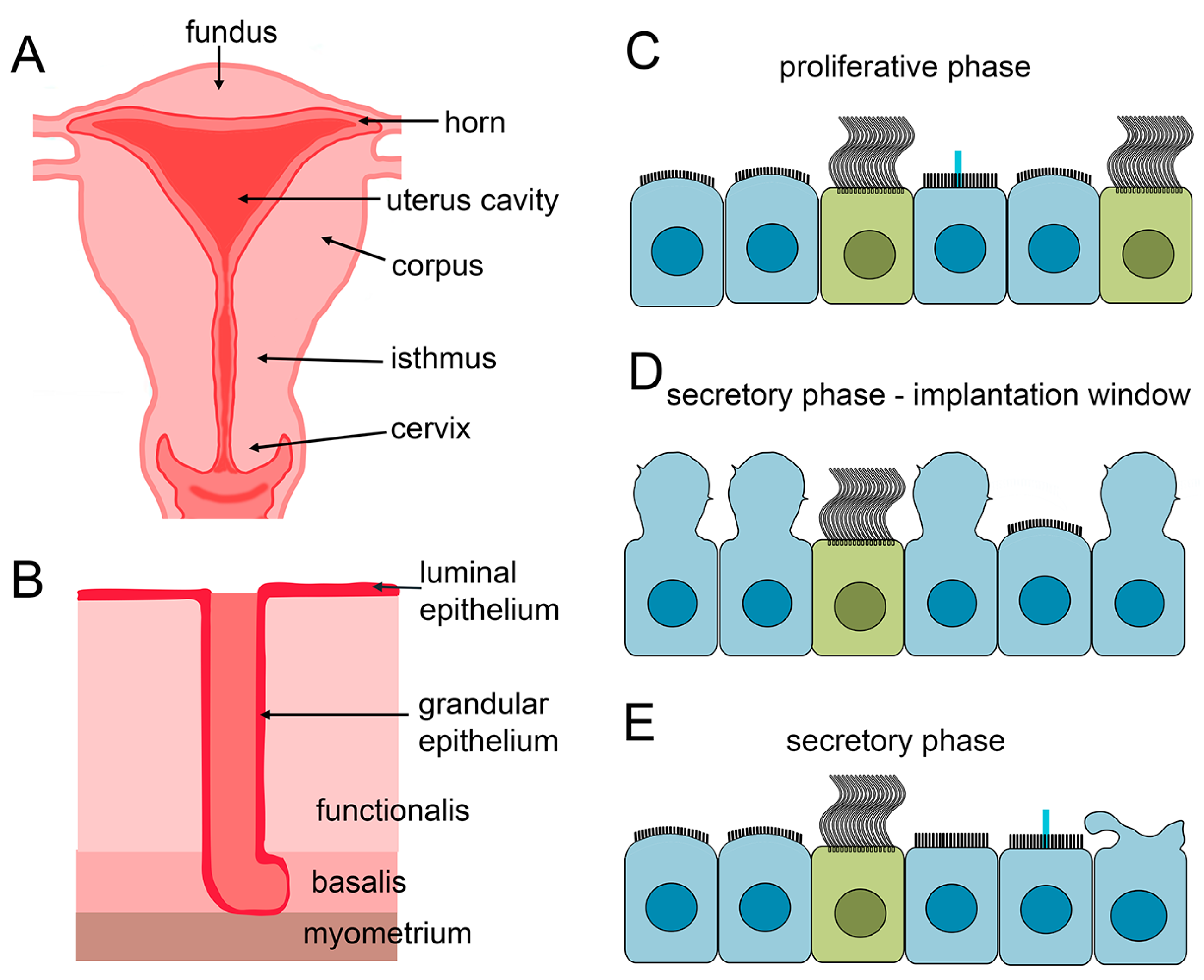
3.2. MCCs in the Endometrium of the Uterus
3.2.1. Uterus Anatomy
3.2.2. Cellular Composition of the Uterine Epithelium and Changes During the Menstrual Cycle
3.2.3. MCCs in the Different Regions of the Uterus
3.2.4. In Vitro Organoid and Assembloid Studies
3.2.5. Significance of MCCs
3.3. Cilia and Female Infertility
4. MCCs and Cilia in Efferent Ducts of the Male Reproductive System
4.1. Efferent Ductules Morphology
4.2. The Morphology of the Efferent Ductules Epithelial Cells
4.3. Role of MCCs in the Efferent Ductules
4.4. Ciliary Gene Mutations and Male Infertility
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Malley, M.A.; Leger, M.M.; Wideman, J.G.; Ruiz-Trillo, I. Concepts of the Last Eukaryotic Common Ancestor. Nat. Ecol. Evol. 2019, 3, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Santos, Z.; Azimzadeh, J.; Pereira-Leal, J.B.; Bettencourt-Dias, M. Evolution: Tracing the Origins of Centrioles, Cilia, and Flagella. J. Cell Biol. 2011, 194, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Cavalier-Smith, T. Ciliary Transition Zone Evolution and the Root of the Eukaryote Tree: Implications for Opisthokont Origin and Classification of Kingdoms Protozoa, Plantae, and Fungi. Protoplasma 2022, 259, 487–593. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.R. Evolution of Cilia. Cold Spring Harb. Perspect. Biol. 2017, 9, a028290. [Google Scholar] [CrossRef] [PubMed]
- Satir, P.; Mitchell, D.R.; Jékely, G. How Did the Cilium Evolve? Curr. Top. Dev. Biol. 2008, 85, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Derderian, C.; Canales, G.I.; Reiter, J.F. Seriously Cilia: A Tiny Organelle Illuminates Evolution, Disease and Intercellular Communication. Dev. Cell 2023, 58, 1333. [Google Scholar] [CrossRef]
- Mill, P.; Christensen, S.T.; Pedersen, L.B. Primary Cilia as Dynamic and Diverse Signalling Hubs in Development and Disease. Nat. Rev. Genet. 2023, 24, 421–441. [Google Scholar] [CrossRef]
- Jain, R.; Javidan-Nejad, C.; Alexander-Brett, J.; Horani, A.; Cabellon, M.C.; Walter, M.J.; Brody, S.L. Sensory Functions of Motile Cilia and Implication for Bronchiectasis. Front. Biosci. (Schol. Ed.) 2012, 4, 1088–1098. [Google Scholar] [CrossRef]
- Seidl, C.; Da Silva, F.; Zhang, K.; Wohlgemuth, K.; Omran, H.; Niehrs, C. Mucociliary Wnt Signaling Promotes Cilia Biogenesis and Beating. Nat. Commun. 2023, 14, 1259. [Google Scholar] [CrossRef]
- Shah, A.S.; Yehuda, B.S.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile Cilia of Human Airway Epithelia Are Chemosensory. Science 2009, 325, 1131–1134. [Google Scholar] [CrossRef]
- Mao, S.; Shah, A.S.; Moninger, T.O.; Ostedgaard, L.S.; Lu, L.; Tang, X.X.; Thornell, I.M.; Reznikov, L.R.; Ernst, S.E.; Karp, P.H.; et al. Motile Cilia of Human Airway Epithelia Contain Hedgehog Signaling Components That Mediate Noncanonical Hedgehog Signaling. Proc. Natl. Acad. Sci. USA 2018, 115, 1370–1375. [Google Scholar] [CrossRef]
- Pinskey, J.M.; Lagisetty, A.; Gui, L.; Phan, N.; Reetz, E.; Tavakoli, A.; Fu, G.; Nicastro, D. Three-Dimensional Flagella Structures from Animals’ Closest Unicellular Relatives, the Choanoflagellates. Elife 2022, 11, e78133. [Google Scholar] [CrossRef]
- Gadadhar, S.; Hirschmugl, T.; Janke, C. The Tubulin Code in Mammalian Sperm Development and Function. Semin. Cell Dev. Biol. 2023, 137, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.K.; Yost, H.J. Left–Right Development: The Roles of Nodal Cilia. Curr. Biol. 2000, 10, R149–R151. [Google Scholar] [CrossRef]
- Sasso, S.; Stibor, H.; Mittag, M.; Grossman, A.R. The Natural History of Model Organisms from Molecular Manipulation of Domesticated Chlamydomonas Reinhardtii to Survival in Nature. Elife 2018, 7, e39233. [Google Scholar] [CrossRef]
- Hagen, K.D.; McInally, S.G.; Hilton, N.D.; Dawson, S.C. Microtubule Organelles in Giardia. Adv. Parasitol. 2020, 107, 25–96. [Google Scholar] [CrossRef]
- Lyu, Q.; Li, Q.; Zhou, J.; Zhao, H. Formation and Function of Multiciliated Cells. J. Cell Biol. 2024, 223, e202307150. [Google Scholar] [CrossRef]
- Serres, C.; Escalier, D.; David, G. Ultrastructural Morphometry of the Human Sperm Flagellum with a Stereological Analysis of the Lengths of the Dense Fibres. Biol. Cell 1983, 49, 153–161. [Google Scholar] [CrossRef]
- Bjork, A.; Pitnick, S. Intensity of Sexual Selection along the Anisogamy-Isogamy Continuum. Nature 2006, 441, 742–745. [Google Scholar] [CrossRef]
- Lupold, S.; Manier, M.K.; Puniamoorthy, N.; Schoff, C.; Starmer, W.T.; Luepold, S.H.B.; Belote, J.M.; Pitnick, S. How Sexual Selection Can Drive the Evolution of Costly Sperm Ornamentation. Nature 2016, 533, 535–538. [Google Scholar] [CrossRef]
- Greenan, G.A.; Vale, R.D.; Agard, D.A. Electron Cryotomography of Intact Motile Cilia Defines the Basal Body to Axoneme Transition. J. Cell Biol. 2019, 219, e201907060. [Google Scholar] [CrossRef]
- Ishikawa, H.; Marshall, W.F. Ciliogenesis: Building the Cell’s Antenna. Nat. Rev. Mol. Cell Biol. 2011, 12, 222–234. [Google Scholar] [CrossRef]
- Yasunaga, T.; Wiegel, J.; Bergen, M.D.; Helmstädter, M.; Epting, D.; Paolini, A.; Çiçek, Ö.; Radziwill, G.; Engel, C.; Brox, T.; et al. Microridge-like Structures Anchor Motile Cilia. Nat. Commun. 2022, 13, 2056. [Google Scholar] [CrossRef]
- Streubel, J.M.S.; Pereira, G. Control of Centrosome Distal Appendages Assembly and Disassembly. Cells Dev. 2023, 174, 203839. [Google Scholar] [CrossRef]
- Blanco-Ameijeiras, J.; Lozano-Fernández, P.; Martí, E. Centrosome Maturation–in Tune with the Cell Cycle. J. Cell Sci. 2022, 135, jcs259395. [Google Scholar] [CrossRef]
- Chatzifrangkeskou, M.; Skourides, P.A. The Apical Ciliary Adhesion Complex Is Established at the Basal Foot of Motile Cilia and Depends on the Microtubule Network. Sci. Rep. 2022, 12, 19028. [Google Scholar] [CrossRef]
- Uzbekov, R.; Alieva, I. Who Are You, Subdistal Appendages of Centriole? Open Biol. 2018, 8, 180062. [Google Scholar] [CrossRef]
- Zhang, S.; Mitchell, B.J. Basal Bodies in Xenopus. Cilia 2016, 5, 2. [Google Scholar] [CrossRef]
- Soares, H.; Carmona, B.; Nolasco, S.; Melo, L.V.; Gonçalves, J. Cilia Distal Domain: Diversity in Evolutionarily Conserved Structures. Cells 2019, 8, 160. [Google Scholar] [CrossRef]
- Ma, D.; Wang, F.; Teng, J.; Huang, N.; Chen, J. Structure and Function of Distal and Subdistal Appendages of the Mother Centriole. J. Cell Sci. 2023, 136, jcs260560. [Google Scholar] [CrossRef]
- Nguyen, Q.P.H.; Liu, Z.; Albulescu, A.; Ouyang, H.; Zlock, L.; Coyaud, E.; Laurent, E.; Finkbeiner, W.; Moraes, T.J.; Raught, B.; et al. Comparative Super-Resolution Mapping of Basal Feet Reveals a Modular but Distinct Architecture in Primary and Motile Cilia. Dev. Cell 2020, 55, 209–223.e7. [Google Scholar] [CrossRef] [PubMed]
- Joukov, V.; De Nicolo, A. The Centrosome and the Primary Cilium: The Yin and Yang of a Hybrid Organelle. Cells 2019, 8, 701. [Google Scholar] [CrossRef]
- Mercey, O.; Mukherjee, S.; Guichard, P.; Hamel, V. The Molecular Architecture of the Ciliary Transition Zones. Curr. Opin. Cell Biol. 2024, 88, 102361. [Google Scholar] [CrossRef]
- Baccetti, B. Insect Sperm Cells. Adv. Insect Physiol. 1972, 9, 315–397. [Google Scholar] [CrossRef]
- Kubo, S.; Black, C.S.; Joachimiak, E.; Yang, S.K.; Legal, T.; Peri, K.; Khalifa, A.A.Z.; Ghanaeian, A.; McCafferty, C.L.; Valente-Paterno, M.; et al. Native Doublet Microtubules from Tetrahymena Thermophila Reveal the Importance of Outer Junction Proteins. Nat. Commun. 2023, 14, 2168. [Google Scholar] [CrossRef]
- Dymek, E.E.; Lin, J.; Fu, G.; Porter, M.E.; Nicastro, D.; Smith, E.F. PACRG and FAP20 Form the Inner Junction of Axonemal Doublet Microtubules and Regulate Ciliary Motility. Mol. Biol. Cell 2019, 30, 1805–1816. [Google Scholar] [CrossRef]
- Khalifa, A.A.Z.; Ichikawa, M.; Dai, D.; Kubo, S.; Black, C.S.; Peri, K.; McAlear, T.S.; Veyron, S.; Yang, S.K.; Vargas, J.; et al. The Inner Junction Complex of the Cilia Is an Interaction Hub That Involves Tubulin Post-Translational Modifications. Elife 2020, 9, e52760. [Google Scholar] [CrossRef]
- Khan, N.; Pelletier, D.; McAlear, T.S.; Croteau, N.; Veyron, S.; Bayne, A.N.; Black, C.; Ichikawa, M.; Khalifa, A.A.Z.; Chaaban, S.; et al. Crystal Structure of Human PACRG in Complex with MEIG1 Reveals Roles in Axoneme Formation and Tubulin Binding. Structure 2021, 29, 572–586.e6. [Google Scholar] [CrossRef] [PubMed]
- Shimogawa, M.M.; Wijono, A.S.; Wang, H.; Zhang, J.; Sha, J.; Szombathy, N.; Vadakkan, S.; Pelayo, P.; Jonnalagadda, K.; Wohlschlegel, J.; et al. FAP106 Is an Interaction Hub for Assembling Microtubule Inner Proteins at the Cilium Inner Junction. Nat. Commun. 2023, 14, 5225. [Google Scholar] [CrossRef]
- Bangera, M.; Dungdung, A.; Prabhu, S.; Sirajuddin, M. Doublet Microtubule Inner Junction Protein FAP20 Recruits Tubulin to the Microtubule Lattice. Structure 2023, 31, 1535–1544.e4. [Google Scholar] [CrossRef]
- Gluenz, E.; Höög, J.L.; Smith, A.E.; Dawe, H.R.; Shaw, M.K.; Gull, K. Beyond 9 + 0: Noncanonical Axoneme Structures Characterize Sensory Cilia from Protists to Humans. FASEB J. 2010, 24, 3117. [Google Scholar] [CrossRef]
- Sun, S.; Fisher, R.L.; Bowser, S.S.; Pentecost, B.T.; Sui, H. Three-Dimensional Architecture of Epithelial Primary Cilia. Proc. Natl. Acad. Sci. USA 2019, 116, 9370–9379. [Google Scholar] [CrossRef] [PubMed]
- Kiesel, P.; Alvarez Viar, G.; Tsoy, N.; Maraspini, R.; Gorilak, P.; Varga, V.; Honigmann, A.; Pigino, G. The Molecular Structure of Mammalian Primary Cilia Revealed by Cryo-Electron Tomography. Nat. Struct. Mol. Biol. 2020, 27, 1115. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, E.; Conkar, D.; Deretic, J.; Batman, U.; Frikstad, K.A.M.; Patzke, S.; Firat-Karalar, E.N. CCDC66 Regulates Primary Cilium Length and Signaling via Interactions with Transition Zone and Axonemal Proteins. J. Cell Sci. 2023, 136, jcs260327. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Zhang, R. Primary Cilia: A Closer Look at the Antenna of Cells. Curr. Biol. 2020, 30, R1494–R1496. [Google Scholar] [CrossRef] [PubMed]
- Conkar, D.; Firat-Karalar, E.N. Microtubule-Associated Proteins and Emerging Links to Primary Cilium Structure, Assembly, Maintenance, and Disassembly. FEBS J. 2021, 288, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Deretic, J.; Odabasi, E.; Firat-Karalar, E.N. The Multifaceted Roles of Microtubule-Associated Proteins in the Primary Cilium and Ciliopathies. J. Cell Sci. 2023, 136, jcs261148. [Google Scholar] [CrossRef]
- Falk, N.; Lösl, M.; Schröder, N.; Gießl, A. Specialized Cilia in Mammalian Sensory Systems. Cells 2015, 4, 500. [Google Scholar] [CrossRef]
- Odate, T.; Takeda, S.; Narita, K.; Kawahara, T. 9 + 0 and 9 + 2 Cilia Are Randomly Dispersed in the Mouse Node. Microscopy 2016, 65, 119–126. [Google Scholar] [CrossRef]
- Feistel, K.; Blum, M. Three Types of Cilia Including a Novel 9 + 4 Axoneme on the Notochordal Plate of the Rabbit Embryo. Dev. Dyn. 2006, 235, 3348–3358. [Google Scholar] [CrossRef]
- Afzelius, B.A.; Bellon, P.L.; Lanzavecchia, S. Microtubules and Their Protofilaments in the Flagellum of an Insect Spermatozoon. J. Cell Sci. 1990, 95 Pt 2, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, Z.V.; Machida, R.; Tsutsumi, K.; Reynoso-Velasco, D.; Lupetti, P.; Dallai, R. Ultrastructural Studies on Euspermatozoa and Paraspermatozoa in Mantispidae (Insecta, Neuroptera). Tissue Cell 2010, 42, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Dallai, R.; Lupetti, P.; Osella, G.; Afzelius, B.A. Giant Sperm Cells with Accessory Macrotubules in a Neuropteran Insect. Tissue Cell 2005, 37, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Osinka, A.; Poprzeczko, M.; Zielinska, M.M.; Fabczak, H.; Joachimiak, E.; Wloga, D. Ciliary Proteins: Filling the Gaps. Recent Advances in Deciphering the Protein Composition of Motile Ciliary Complexes. Cells 2019, 8, 730. [Google Scholar] [CrossRef]
- Andersen, J.S.; Vijayakumaran, A.; Godbehere, C.; Lorentzen, E.; Mennella, V.; Schou, K.B. Uncovering Structural Themes across Cilia Microtubule Inner Proteins with Implications for Human Cilia Function. Nat. Commun. 2024, 15, 2687. [Google Scholar] [CrossRef]
- Wang, X.; Fu, Y.; Beatty, W.L.; Ma, M.; Brown, A.; David Sibley, L.; Zhang, R. Cryo-EM Structure of Cortical Microtubules from Human Parasite Toxoplasma Gondii Identifies Their Microtubule Inner Proteins. Nat. Commun. 2021, 12, 3065. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.Y.; Jin, H.J.; Xia, L.; Wang, B.B.; Chen, S.R. Tektin Bundle Interacting Protein, TEKTIP1, Functions to Stabilize the Tektin Bundle and Axoneme in Mouse Sperm Flagella. Cell Mol. Life Sci. 2024, 81, 118. [Google Scholar] [CrossRef]
- Ichikawa, M.; Bui, K.H. Microtubule Inner Proteins: A Meshwork of Luminal Proteins Stabilizing the Doublet Microtubule. Bioessays 2018, 40, bies.201700209. [Google Scholar] [CrossRef]
- Tai, L.; Yin, G.; Huang, X.; Sun, F.; Zhu, Y. In-cell structural insight into the stability of sperm microtubule doublet. Cell Discov. 2023, 9, 116. [Google Scholar] [CrossRef]
- Nicastro, D.; Schwartz, C.; Pierson, J.; Gaudette, R.; Porter, M.E.; McIntosh, J.R. The Molecular Architecture of Axonemes Revealed by Cryoelectron Tomography. Science 2006, 313, 944–948. [Google Scholar] [CrossRef]
- Heuser, T.; Raytchev, M.; Krell, J.; Porter, M.E.; Nicastro, D. The Dynein Regulatory Complex Is the Nexin Link and a Major Regulatory Node in Cilia and Flagella. J. Cell Biol. 2009, 187, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Song, K.; Yanagisawa, H.A.; Fox, L.; Yagi, T.; Wirschell, M.; Hirono, M.; Kamiya, R.; Nicastro, D.; Sale, W.S. The MIA Complex Is a Conserved and Novel Dynein Regulator Essential for Normal Ciliary Motility. J. Cell Biol. 2013, 201, 263–278. [Google Scholar] [CrossRef]
- Bazan, R.; Schröfel, A.; Joachimiak, E.; Poprzeczko, M.; Pigino, G.; Wloga, D. Ccdc113/Ccdc96 Complex, a Novel Regulator of Ciliary Beating That Connects Radial Spoke 3 to Dynein g and the Nexin Link. PLoS Genet. 2021, 17, e1009388. [Google Scholar] [CrossRef] [PubMed]
- Ghanaeian, A.; Majhi, S.; McCafferty, C.L.; Nami, B.; Black, C.S.; Yang, S.K.; Legal, T.; Papoulas, O.; Janowska, M.; Valente-Paterno, M.; et al. Integrated Modeling of the Nexin-Dynein Regulatory Complex Reveals Its Regulatory Mechanism. Nat. Commun. 2023, 14, 5741. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, P.; Joachimiak, E.; Bazan, R.; Fu, G.; Poprzeczko, M.; Fabczak, H.; Nicastro, D.; Wloga, D. Ciliary Proteins Fap43 and Fap44 Interact with Each Other and Are Essential for Proper Cilia and Flagella Beating. Cell Mol. Life Sci. 2018, 75, 4479–4493. [Google Scholar] [CrossRef]
- Fu, G.; Wang, Q.; Phan, N.; Urbanska, P.; Joachimiak, E.; Lin, J.; Wloga, D.; Nicastro, D. The I1 Dynein-Associated Tether and Tether Head Complex Is a Conserved Regulator of Ciliary Motility. Mol. Biol. Cell 2018, 29, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Yanagisawa, H.; Kamiya, R.; Kikkawa, M. A Molecular Ruler Determines the Repeat Length in Eukaryotic Cilia and Flagella. Science 2014, 346, 857–860. [Google Scholar] [CrossRef]
- Lin, J.; Le, T.V.; Augspurger, K.; Tritschler, D.; Bower, R.; Fu, G.; Perrone, C.; O’Toole, E.T.; VanderWaal Mills, K.; Dymek, E.; et al. FAP57/WDR65 Targets Assembly of a Subset of Inner Arm Dyneins and Connects to Regulatory Hubs in Cilia. Mol. Biol. Cell 2019, 30, 2659–2680. [Google Scholar] [CrossRef]
- Fu, G.; Augspurger, K.; Sakizadeh, J.; Reck, J.; Bower, R.; Tritschler, D.; Gui, L.; Nicastro, D.; Porter, M.E. The MBO2/FAP58 Heterodimer Stabilizes Assembly of Inner Arm Dynein b and Reveals Axoneme Asymmetries Involved in Ciliary Waveform. Mol. Biol. Cell 2024, 35, ar72. [Google Scholar] [CrossRef]
- Samsel, Z.; Sekretarska, J.; Osinka, A.; Wloga, D.; Joachimiak, E. Central Apparatus, the Molecular Kickstarter of Ciliary and Flagellar Nanomachines. Int. J. Mol. Sci. 2021, 22, 3013. [Google Scholar] [CrossRef]
- Croft, J.T.; Zabeo, D.; Subramanian, R.; Höög, J.L. Composition, Structure and Function of the Eukaryotic Flagellum Distal Tip. Essays Biochem. 2018, 62, 815–828. [Google Scholar] [CrossRef]
- Smith, E.F.; Yang, P. The Radial Spokes and Central Apparatus: Mechano-Chemical Transducers That Regulate Flagellar Motility. Cell Motil. Cytoskelet. 2004, 57, 8–17. [Google Scholar] [CrossRef]
- Oda, T.; Yanagisawa, H.; Yagi, T.; Kikkawa, M. Mechanosignaling between Central Apparatus and Radial Spokes Controls Axonemal Dynein Activity. J. Cell Biol. 2014, 204, 807–819. [Google Scholar] [CrossRef]
- Grossman-Haham, I.; Coudray, N.; Yu, Z.; Wang, F.; Zhang, N.; Bhabha, G.; Vale, R.D. Structure of the Radial Spoke Head and Insights into Its Role in Mechanoregulation of Ciliary Beating. Nat. Struct. Mol. Biol. 2021, 28, 20–28. [Google Scholar] [CrossRef]
- Gui, M.; Wang, X.; Dutcher, S.K.; Brown, A.; Zhang, R. Ciliary Central Apparatus Structure Reveals Mechanisms of Microtubule Patterning. Nat. Struct. Mol. Biol. 2022, 29, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Wu, Z.; Wang, J.; Luo, G.; Lin, H.; Fan, Y.; Zhou, C. Hedgehog Signaling in Tissue Homeostasis, Cancers, and Targeted Therapies. Signal Transduct. Target. Ther. 2023, 8, 315. [Google Scholar] [CrossRef]
- Anvarian, Z.; Mykytyn, K.; Mukhopadhyay, S.; Pedersen, L.B.; Christensen, S.T. Cellular Signalling by Primary Cilia in Development, Organ Function and Disease. Nat. Rev. Nephrol. 2019, 15, 199–219. [Google Scholar] [CrossRef]
- Elliott, K.H.; Brugmann, S.A. Sending Mixed Signals: Cilia-Dependent Signaling during Development and Disease. Dev. Biol. 2019, 447, 28–41. [Google Scholar] [CrossRef]
- Long, X.; Chen, L.; Xiao, X.; Min, X.; Wu, Y.; Yang, Z.; Wen, X. Structure, Function, and Research Progress of Primary Cilia in Reproductive Physiology and Reproductive Diseases. Front. Cell Dev. Biol. 2024, 12, 1418928. [Google Scholar] [CrossRef]
- Gabriel, G.C.; Ganapathiraju, M.; Lo, C.W. The Role of Cilia and the Complex Genetics of Congenital Heart Disease. Annu. Rev. Genom. Hum. Genet. 2024, 25, 309–327. [Google Scholar] [CrossRef]
- Mitchison, H.M.; Valente, E.M. Motile and Non-Motile Cilia in Human Pathology: From Function to Phenotypes. J. Pathol. 2017, 241, 294–309. [Google Scholar] [CrossRef]
- Ménard, D. Morphological Studies of the Developing Human Esophageal Epithelium. Microsc. Res. Tech. 1995, 31, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.M.; Morgan, J.J. Cilia in the Human Kidney. Ultrastruct. Pathol. 1984, 6, 285–294. [Google Scholar] [CrossRef]
- Zimmermann, H.D. Cilia in the Fetal Kidney of Man. Beitr. Pathol. 1971, 143, 227–240. [Google Scholar]
- Hassan, M.O.; Subramanyan, S. Ciliated Renal Tubular Cells in Crescentic Glomerulonephritis. Ultrastruct. Pathol. 1995, 19, 201–203. [Google Scholar] [CrossRef]
- Lungarella, G.; de Santi, M.M.; Tosi, P. Ultrastructural Study of the Ciliated Cells from Renal Tubular Epithelium in Acute Progressive Glomerulonephritis. Ultrastruct. Pathol. 1984, 6, 1–7. [Google Scholar] [CrossRef]
- Eymael, J.; Willemsen, B.; Xu, J.; Mooren, F.; Steenbergen, E.; Wetzels, J.F.; Dijkman, H.; Jansen, J.; Van der Vlag, J.; Smeets, B. Motile Cilia on Kidney Proximal Tubular Epithelial Cells Are Associated With Tubular Injury and Interstitial Fibrosis. Front. Cell Dev. Biol. 2022, 10, 765887. [Google Scholar] [CrossRef]
- Marra, A.N.; Adeeb, B.D.; Chambers, B.E.; Drummond, B.E.; Ulrich, M.; Addiego, A.; Springer, M.; Poureetezadi, S.J.; Chambers, J.M.; Ronshaugen, M.; et al. Prostaglandin Signaling Regulates Renal Multiciliated Cell Specification and Maturation. Proc. Natl. Acad. Sci. USA 2019, 116, 8409–8418. [Google Scholar] [CrossRef]
- Cho, J.H.; Li, Z.A.; Zhu, L.; Muegge, B.D.; Roseman, H.F.; Lee, E.Y.; Utterback, T.; Woodhams, L.G.; Bayly, P.V.; Hughes, J.W. Islet Primary Cilia Motility Controls Insulin Secretion. Sci. Adv. 2022, 8, eabq8486. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Tanaka, Y.; Okada, Y.; Takeda, S. Nodal Flow and the Generation of Left-Right Asymmetry. Cell 2006, 125, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Corkidi, G.; Montoya, F.; González-Cota, A.L.; Hernández-Herrera, P.; Bruce, N.C.; Bloomfield-Gadêlha, H.; Darszon, A. Human Sperm Rotate with a Conserved Direction during Free Swimming in Four Dimensions. J. Cell Sci. 2023, 136, jcs261306. [Google Scholar] [CrossRef]
- Lehti, M.S.; Sironen, A. Formation and Function of Sperm Tail Structures in Association with Sperm Motility Defects. Biol. Reprod. 2017, 97, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Spassky, N.; Meunier, A. The Development and Functions of Multiciliated Epithelia. Nat. Rev. Mol. Cell Biol. 2017, 18, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, M.R.; Nanjundappa, R.; Harvey, M.N. Development of a Multiciliated Cell. Curr. Opin. Cell Biol. 2022, 77, 102105. [Google Scholar] [CrossRef]
- Liu, Z.; Nguyen, Q.P.H.; Nanjundappa, R.; Delgehyr, N.; Megherbi, A.; Doherty, R.; Thompson, J.; Jackson, C.; Albulescu, A.; Heng, Y.M.; et al. Super-Resolution Microscopy and FIB-SEM Imaging Reveal Parental Centriole-Derived, Hybrid Cilium in Mammalian Multiciliated Cells. Dev. Cell 2020, 55, 224–236.e6. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Z.; Peng, H.; Ward, S.M.; Hennig, G.W.; Zheng, H.; Yan, W. Oviductal Motile Cilia Are Essential for Oocyte Pickup but Dispensable for Sperm and Embryo Transport. Proc. Natl. Acad. Sci. USA 2021, 118, e2102940118. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, Y.; Peng, H.; Tang, C.; Hennig, G.W.; Wang, Z.; Wang, L.; Yu, T.; Klukovich, R.; Zhang, Y.; et al. Motile Cilia of the Male Reproductive System Require MiR-34/MiR-449 for Development and Function to Generate Luminal Turbulence. Proc. Natl. Acad. Sci. USA 2019, 116, 3584–3593. [Google Scholar] [CrossRef]
- Aprea, I.; Nöthe-Menchen, T.; Dougherty, G.W.; Raidt, J.; Loges, N.T.; Kaiser, T.; Wallmeier, J.; Olbrich, H.; Strünker, T.; Kliesch, S.; et al. Motility of Efferent Duct Cilia Aids Passage of Sperm Cells through the Male Reproductive System. Mol. Hum. Reprod. 2021, 27, gaab009. [Google Scholar] [CrossRef]
- Yang, H.W.; Lee, S.; Berry, B.C.; Yang, D.; Zheng, S.; Carroll, R.S.; Park, P.J.; Johnson, M.D. A Role for Mutations in AK9 and Other Genes Affecting Ependymal Cells in Idiopathic Normal Pressure Hydrocephalus. Proc. Natl. Acad. Sci. USA 2023, 120, e2300681120. [Google Scholar] [CrossRef]
- Rocca, M.S.; Piatti, G.; Michelucci, A.; Guazzo, R.; Bertini, V.; Vinanzi, C.; Caligo, M.A.; Valetto, A.; Foresta, C. A Novel Genetic Variant in DNAI2 Detected by Custom Gene Panel in a Newborn with Primary Ciliary Dyskinesia: Case Report. BMC Med. Genet. 2020, 21, 220. [Google Scholar] [CrossRef]
- Morimoto, Y.; Yoshida, S.; Kinoshita, A.; Satoh, C.; Mishima, H.; Yamaguchi, N.; Matsuda, K.; Sakaguchi, M.; Tanaka, T.; Komohara, Y.; et al. Nonsense Mutation in CFAP43 Causes Normal-Pressure Hydrocephalus with Ciliary Abnormalities. Neurology 2019, 92, E2364–E2374. [Google Scholar] [CrossRef] [PubMed]
- Raidt, J.; Loges, N.T.; Olbrich, H.; Wallmeier, J.; Pennekamp, P.; Omran, H. Primary Ciliary Dyskinesia. Presse Med. 2023, 52, 104171. [Google Scholar] [CrossRef] [PubMed]
- Rosman, J.; Contou, D.; van Nhieu, J.T.; Renaud, M.; Bottier, M.; Maitre, B.; Louis, B.; Dessap, A.M. Severe Ciliary Dyskinesia in Ventilated Patients: A Pilot Study. Am. J. Respir. Crit. Care Med. 2020, 201, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Collet, S.; Eloy, P.; Rombaux, P. Secondary Ciliary Dyskinesia in Upper Respiratory Tract. Acta Otorhinolaryngol. Belg. 2000, 54, 309–316. [Google Scholar] [PubMed]
- Jorissen, M.; Willems, T.; Van Der Schueren, B.; Verbeken, E. Secondary Ciliary Dyskinesia Is Absent after Ciliogenesis in Culture. Acta Otorhinolaryngol. Belg. 2000, 54, 333–342. [Google Scholar] [PubMed]
- Newman, L.; Chopra, J.; Dossett, C.; Shepherd, E.; Bercusson, A.; Carroll, M.; Walker, W.; Lucas, J.S.; Cheong, Y. The Impact of Primary Ciliary Dyskinesia on Female and Male Fertility: A Narrative Review. Hum. Reprod. Update 2023, 29, 347–367. [Google Scholar] [CrossRef]
- Pereira, R.; Sousa, M. Morphological and Molecular Bases of Male Infertility: A Closer Look at Sperm Flagellum. Genes 2023, 14, 383. [Google Scholar] [CrossRef]
- Sironen, A.; Shoemark, A.; Patel, M.; Loebinger, M.R.; Mitchison, H.M. Sperm Defects in Primary Ciliary Dyskinesia and Related Causes of Male Infertility. Cell Mol. Life Sci. 2020, 77, 2029–2048. [Google Scholar] [CrossRef]
- Jiao, S.Y.; Yang, Y.H.; Chen, S.R. Molecular Genetics of Infertility: Loss-of-Function Mutations in Humans and Corresponding Knockout/Mutated Mice. Hum. Reprod. Update 2021, 27, 154–189. [Google Scholar] [CrossRef]
- Holdsworth-Carson, S.J.; Menkhorst, E.; Maybin, J.A.; King, A.; Girling, J.E. Cyclic Processes in the Uterine Tubes, Endometrium, Myometrium, and Cervix: Pathways and Perturbations. Mol. Hum. Reprod. 2023, 29, gaad012. [Google Scholar] [CrossRef]
- Thiyagarajan, D.K.; Basit, H.; Jeanmonod, R. Physiology, Menstrual Cycle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wynn, R.M. Histology and Ultrastructure of the Human Endometrium. In Biology of the Uterus; Wynn, R.M., Ed.; Springer: Boston, MA, USA, 1977; pp. 341–376. [Google Scholar] [CrossRef]
- Cunha, G.R.; Robboy, S.J.; Kurita, T.; Isaacson, D.; Shen, J.; Cao, M.; Baskin, L.S. Development of the Human Female Reproductive Tract. Differentiation 2018, 103, 46. [Google Scholar] [CrossRef] [PubMed]
- Robboy, S.J.; Kurita, T.; Baskin, L.; Cunha, G.R. New Insights into Human Female Reproductive Tract Development. Differentiation 2017, 97, 9–22. [Google Scholar] [CrossRef]
- Hafez, E.S.E.; Barnhart, M.I.; Ludwig, H.; Lusher, J.; Joelsson, I.; Daniel, J.L.; Sherman, A.I.; Jordan, J.A.; Wolf, H.; Stewart, W.C.; et al. Scanning Electron Microscopy of Human Reproductive Physiology. Acta Obstet. Gynecol. Scand. Suppl. 1975, 40, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.T.; Aranda, O.L.; Matos, A.P.P.; Marchiori, E.; de Araújo, L.F.B.; Alves, H.D.L.; Machado, A.S.; Lopes, R.T.; Werner, H.; Júnior, E.A. The Human Endosalpinx: Anatomical Three-Dimensional Study and Reconstruction Using Confocal Microtomography. Pol. J. Radiol. 2019, 84, e281–e288. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.T.; Aranda, O.L.; Marchiori, E.; de Araújo, L.F.B.; Alves, H.D.L.; Lopes, R.T.; Werner, H.; Júnior, E.A. Proportional Vascularization along the Fallopian Tubes and Ovarian Fimbria: Assessment by Confocal Microtomography. Radiol. Bras. 2020, 53, 161–166. [Google Scholar] [CrossRef]
- Samberg, I.; Degani, S.; Zilberman, A.; Eibschitz, I.; Nir, I.; Scharf, M. Scanning Electron Microscopy of Human Fallopian Tube in Ectopic Pregnancy. Gynecol. Obstet. Investig. 1983, 16, 65–75. [Google Scholar] [CrossRef]
- Crow, J.; Amso, N.N.; Lewin, J.; Shaw, R.W. Morphology and Ultrastructure of Fallopian Tube Epithelium at Different Stages of the Menstrual Cycle and Menopause. Hum. Reprod. 1994, 9, 2224–2233. [Google Scholar] [CrossRef]
- Lyons, R.A.; Saridogan, E.; Djahanbakhch, O. The Reproductive Significance of Human Fallopian Tube Cilia. Hum. Reprod. Update 2006, 12, 363–372. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Kunjunni, K.T.; Varghese, S. A Comparative Study on Segmental Micro-Anatomy of the Human Fallopian Tube. Natl. J. Clin. Anat. 2021, 10, 46–50. [Google Scholar] [CrossRef]
- Seraj, H.; Nazari, M.A.; Atai, A.A.; Amanpour, S.; Azadi, M. A Review: Biomechanical Aspects of the Fallopian Tube Relevant to Its Function in Fertility. Reprod. Sci. 2024, 31, 1456–1485. [Google Scholar] [CrossRef]
- Eddy, C.A.; Pauerstein, C.J. Anatomy and physiology of the fallopian tube. Clin Obstet Gynecol. 1980, 23, 1177–1193. [Google Scholar] [CrossRef] [PubMed]
- Raveshi, M.R.; Abdul Halim, M.S.; Agnihotri, S.N.; O’Bryan, M.K.; Neild, A.; Nosrati, R. Curvature in the Reproductive Tract Alters Sperm-Surface Interactions. Nat. Commun. 2021, 12, 3446. [Google Scholar] [CrossRef] [PubMed]
- Amso, N.N.; Crow, J.; Lewin, J.; Shaw, R.W. A Comparative Morphological and Ultrastructural Study of Endometrial Gland and Fallopian Tube Epithelia at Different Stages of the Menstrual Cycle and the Menopause. Hum. Reprod. 1994, 9, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Ferenczy, A.; Richart, R.M.; Agate, F.J.; Purkerson, M.L.; Dempsey, E.W. Scanning Electron Microscopy of the Human Fallopian Tube. Science 1972, 175, 783–784. [Google Scholar] [CrossRef]
- Peters, W.M. Nature of “Basal” and “Reserve” Cells in Oviductal and Cervical Epithelium in Man. J. Clin. Pathol. 1986, 39, 306. [Google Scholar] [CrossRef]
- Ulrich, N.D.; Shen, Y.C.; Ma, Q.; Yang, K.; Hannum, D.F.; Jones, A.; Machlin, J.; Randolph, J.F.; Smith, Y.R.; Schon, S.B.; et al. Cellular Heterogeneity of Human Fallopian Tubes in Normal and Hydrosalpinx Disease States Identified Using ScRNA-Seq. Dev. Cell 2022, 57, 914–929.e7. [Google Scholar] [CrossRef]
- Patek, E.; Nilsson, L.; Johannisson, E. Scanning Electron Microscopic Study of the Human Fallopian Tube. Report I. The Proliferative and Secretory Stages. Fertil. Steril. 1972, 23, 459–465. [Google Scholar] [CrossRef]
- Hagiwara, H.; Kano, A.; Aoki, T.; Ohwada, N. Immunocytochemistry of the Striated Rootlets Associated with Solitary Cilia in Human Oviductal Secretory Cells. Histochem. Cell Biol. 2000, 114, 205–212. [Google Scholar] [CrossRef]
- Hagiwara, H.; Harada, S.; Maeda, S.; Aoki, T.; Ohwada, N.; Takata, K. Ultrastructural and Immunohistochemical Study of the Basal Apparatus of Solitary Cilia in the Human Oviduct Epithelium. J. Anat. 2002, 200, 89–96. [Google Scholar] [CrossRef]
- Raidt, J.; Werner, C.; Menchen, T.; Dougherty, G.W.; Olbrich, H.; Loges, N.T.; Schmitz, R.; Pennekamp, P.; Omran, H. Ciliary Function and Motor Protein Composition of Human Fallopian Tubes. Hum. Reprod. 2015, 30, 2871–2880. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, Q.; Yan, M.; Li, C.; Yuan, J.; Qin, G.; Zhang, J. Levonorgestrel Decreases Cilia Beat Frequency of Human Fallopian Tubes and Rat Oviducts without Changing Morphological Structure. Clin. Exp. Pharmacol. Physiol. 2015, 42, 171–178. [Google Scholar] [CrossRef]
- Critoph, F.N.; Dennis, K.J. Ciliary Activity in the Human Oviduct. Br. J. Obstet. Gynaecol. 1977, 84, 216–218. [Google Scholar] [CrossRef]
- Gaddum-Rosse, P.; Blandau, R.J.; Thiersch, J.B. Ciliary Activity in the Human and Macaca Nemestrina Oviduct. Am. J. Anat. 1973, 138, 269–275. [Google Scholar] [CrossRef]
- Ito, T. Scanning Electron Microscopic Observations on the Surface of the Human Fallopian Tube and Uterine Endometrium. Keio J. Med. 1975, 24, 159–174. [Google Scholar] [CrossRef]
- Critoph, F.N.; Dennis, K.J. The Cellular Composition of the Human Oviduct Epithelium. Br. J. Obstet. Gynaecol. 1977, 84, 219–221. [Google Scholar] [CrossRef]
- Donnez, J.; Casanas-Roux, F.; Caprasse, J.; Ferin, J.; Thomas, K. Cyclic Changes in Ciliation, Cell Height, and Mitotic Activity in Human Tubal Epithelium during Reproductive Life. Fertil. Steril. 1985, 43, 554–559. [Google Scholar] [CrossRef]
- Fadel, H.E.; Berns, D.; Zaneveld, L.J.D.; Wilbanks, G.D.; Brueschke, E.E. The Human Uterotubal Junction: A Scanning Electron Microscope Study During Different Phases of the Menstrual Cycle. Fertil. Steril. 1976, 27, 1176–1186. [Google Scholar] [CrossRef]
- Patek, E.; Nilsson, L.; Johannisson, E. Scanning Electron Microscopic Study of the Human Fallopian Tube. Report II. Fetal Life, Reproductive Life, and Postmenopause. Fertil. Steril. 1972, 23, 719–733. [Google Scholar] [CrossRef]
- Seki, K.; Rawson, J.; Eddy, C.A.; Smith, N.K.; Pauerstein, C.J. Deciliation in the Puerperal Fallopian Tube. Fertil. Steril. 1978, 29, 75–83. [Google Scholar] [CrossRef]
- Patek, E.; Nilsson, L.; Hellema, M. Scanning Electron Microscopic Study of the Human Fallopian Tube Report. IV. At Term Gestation and in the Puerperium. The Effect of a Synthetic Progestin on the Postmenopausal Tube. Fertil. Steril. 1973, 24, 832–843. [Google Scholar] [CrossRef]
- Makabe, S.; Motta, P.M.; Naguro, T.; Vizza, E.; Perrone, G.; Zichella, L. Microanatomy of the Female Reproductive Organs in Postmenopause by Scanning Electron Microscopy. Climacteric 1998, 1, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Correr, S.; Makabe, S.; Heyn, R.; Relucenti, M.; Naguro, T.; Familiari, G. Microplicae-like Structures of the Fallopian Tube in Postmenopausal Women as Shown by Electron Microscopy. Histol. Histopathol. 2006, 21, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ning, Y.; Abushahin, N.; Yuan, Z.; Wang, Y.; Wang, Y.; Yuan, B.; Cragun, J.M.; Chambers, S.K.; Hatch, K.; et al. Secretory Cell Expansion with Aging: Risk for Pelvic Serous Carcinogenesis. Gynecol. Oncol. 2013, 131, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Patek, E.; Nilsson, L. Scanning Electron Microscopic Observations on the Ciliogenesis of the Infundibulum of the Human Fetal and Adult Fallopian Tube Epithelium. Fertil. Steril. 1973, 24, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Ohwada, N.; Aoki, T.; Suzuki, T.; Takata, K. The Primary Cilia of Secretory Cells in the Human Oviduct Mucosa. Med. Mol. Morphol. 2008, 41, 193–198. [Google Scholar] [CrossRef]
- Paik, D.Y.; Janzen, D.M.; Schafenacker, A.M.; Velasco, V.S.; Shung, M.S.; Cheng, D.; Huang, J.; Witte, O.N.; Memarzadeh, S. Stem-like Epithelial Cells Are Concentrated in the Distal End of the Fallopian Tube: A Site for Injury and Serous Cancer Initiation. Stem Cells 2012, 30, 2487–2497. [Google Scholar] [CrossRef]
- Csöbönyeiová, M.; Varga, I.; Lapides, L.; Pavlíková, L.; Feitscherová, C.; Klein, M. From a Passive Conduit to Highly Dynamic Organ. What Are the Roles of Uterine Tube Epithelium in Reproduction? Physiol. Res. 2022, 71, S11–S20. [Google Scholar] [CrossRef]
- Dinh, H.Q.; Lin, X.; Abbasi, F.; Nameki, R.; Haro, M.; Olingy, C.E.; Chang, H.; Hernandez, L.; Gayther, S.A.; Wright, K.N.; et al. Single-Cell Transcriptomics Identifies Gene Expression Networks Driving Differentiation and Tumorigenesis in the Human Fallopian Tube. Cell Rep. 2021, 35, 108978. [Google Scholar] [CrossRef]
- Hu, Z.; Artibani, M.; Alsaadi, A.; Wietek, N.; Morotti, M.; Shi, T.; Zhong, Z.; Santana Gonzalez, L.; El-Sahhar, S.; Carrami, E.M.; et al. The Repertoire of Serous Ovarian Cancer Non-Genetic Heterogeneity Revealed by Single-Cell Sequencing of Normal Fallopian Tube Epithelial Cells. Cancer Cell 2020, 37, 226–242.e7. [Google Scholar] [CrossRef]
- Ghosh, A.; Syed, S.M.; Tanwar, P.S. In Vivo Genetic Cell Lineage Tracing Reveals That Oviductal Secretory Cells Self-Renew and Give Rise to Ciliated Cells. Development 2017, 144, 3031–3041. [Google Scholar] [CrossRef]
- Lyons, R.A.; Djahanbakhch, O.; Mahmood, T.; Saridogan, E.; Sattar, S.; Sheaff, M.T.; Naftalin, A.A.; Chenoy, R. Fallopian Tube Ciliary Beat Frequency in Relation to the Stage of Menstrual Cycle and Anatomical Site. Hum. Reprod. 2002, 17, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Saridogan, E.; Smutna, S.; Habib, A.M.; Djahanbakhch, O. The Effect of Ovarian Steroids on Epithelial Ciliary Beat Frequency in the Human Fallopian Tube. Hum. Reprod. 1998, 13, 2991–2994. [Google Scholar] [CrossRef] [PubMed]
- Westrom, L.; Mardh, P.A.; Von Mecklenburg, C.; Hakansson, C.H. Studies on Ciliated Epithelia of the Human Genital Tract. II. The Mucociliary Wave Pattern of Fallopian Tube Epithelium. Fertil. Steril. 1977, 28, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Paltieli, Y.; Weichselbaum, A.; Hoffman, N.; Eibschitz, I.; Kam, Z. Laser Scattering Instrument for Real Time In-Vivo Measurement of Ciliary Activity in Human Fallopian Tubes. Hum. Reprod. 1995, 10, 1638–1641. [Google Scholar] [CrossRef]
- Lyons, R.A.; Saridogan, E.; Djahanbakhch, O. The Effect of Ovarian Follicular Fluid and Peritoneal Fluid on Fallopian Tube Ciliary Beat Frequency. Hum. Reprod. 2006, 21, 52–56. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Rashedi, A.S.; Pavone, M.E.; Julie Kim, J.; Woodruff, T.K.; Burdette, J.E. Human Fallopian Tube Epithelium Co-Culture with Murine Ovarian Follicles Reveals Crosstalk in the Reproductive Cycle. Mol. Hum. Reprod. 2016, 22, 756–767. [Google Scholar] [CrossRef]
- Jackson-Bey, T.; Colina, J.; Isenberg, B.C.; Coppeta, J.; Urbanek, M.; Julie Kim, J.; Woodruff, T.K.; Burdette, J.E.; Russo, A. Exposure of Human Fallopian Tube Epithelium to Elevated Testosterone Results in Alteration of Cilia Gene Expression and Beating. Hum. Reprod. 2020, 35, 2086–2096. [Google Scholar] [CrossRef]
- Ezzati, M.; Djahanbakhch, O.; Arian, S.; Carr, B.R. Tubal Transport of Gametes and Embryos: A Review of Physiology and Pathophysiology. J. Assist. Reprod. Genet. 2014, 31, 1337–1347. [Google Scholar] [CrossRef]
- Patek, E.; Nilsson, L.; Johannisson, E.; Hellema, M.; Bout, J. Scanning Electron Microscopic Study of the Human Fallopian Tube. Report III. The Effect of Midpregnancy and of Various Steroids. Fertil. Steril. 1973, 24, 31–43. [Google Scholar] [CrossRef]
- Abdul Halim, M.S.; Dyson, J.M.; Gong, M.M.; O’Bryan, M.K.; Nosrati, R. Fallopian Tube Rheology Regulates Epithelial Cell Differentiation and Function to Enhance Cilia Formation and Coordination. Nat. Commun. 2024, 15, 7411. [Google Scholar] [CrossRef]
- Comer, M.T.; Leese, H.J.; Southgate, J. Induction of a Differentiated Ciliated Cell Phenotype in Primary Cultures of Fallopian Tube Epithelium. Hum. Reprod. 1998, 13, 3114–3120. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.J.; Forjaz, A.; Bons, J.; Bhorkar, I.; Roy, T.; Schell, D.; Queiroga, V.; Ren, K.; Kramer, D.; Huang, W.; et al. Combined Assembloid Modeling and 3D Whole-Organ Mapping Captures the Microanatomy and Function of the Human Fallopian Tube. Sci. Adv. 2024, 10, eadp6285. [Google Scholar] [CrossRef]
- Liao, S.B.; Li, H.W.R.; Ho, J.C.; Yeung, W.S.B.; Ng, E.H.Y.; Cheung, A.N.Y.; Tang, F.; O, W.S. Possible Role of Adrenomedullin in the Pathogenesis of Tubal Ectopic Pregnancy. J. Clin. Endocrinol. Metab. 2012, 97, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.R.; Liao, S.B.; Chiu, P.C.N.; Tam, W.W.; Ho, J.C.; Ng, E.H.Y.; Ho, P.C.; Yeung, W.S.B.; Tang, F.; Wai Sum, O. Expression of Adrenomedullin in Human Oviduct, Its Regulation by the Hormonal Cycle and Contact with Spermatozoa, and Its Effect on Ciliary Beat Frequency of the Oviductal Epithelium. J. Clin. Endocrinol. Metab. 2010, 95, E18–E25. [Google Scholar] [CrossRef]
- Papathanasiou, A.; Djahanbakhch, O.; Saridogan, E.; Lyons, R.A. The Effect of Interleukin-6 on Ciliary Beat Frequency in the Human Fallopian Tube. Fertil. Steril. 2008, 90, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lee, C.L.; Vijayan, M.; Yeung, W.S.B.; Ng, E.H.Y.; Wang, X.; O, W.S.; Li, R.H.W.; Zhang, Y.; Chiu, P.C.N. Adrenomedullin Insufficiency Alters Macrophage Activities in Fallopian Tube: A Pathophysiologic Explanation of Tubal Ectopic Pregnancy. Mucosal Immunol. 2020, 13, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, Y.T.; Zhu, Q.; Zhang, H.Y.; Huang, Z.; Zhang, D.; Qi, H.; Liang, G.L.; He, X.Q.; Wang, X.F.; et al. TRPV4 Is Involved in Levonorgestrel-induced Reduction in Oviduct Ciliary Beating. J. Pathol. 2019, 248, 77. [Google Scholar] [CrossRef]
- Andrade, Y.N.; Fernandes, J.; Vázquez, E.; Fernández-Fernández, J.M.; Arniges, M.; Sánchez, T.M.; Villalón, M.; Valverde, M.A. TRPV4 Channel Is Involved in the Coupling of Fluid Viscosity Changes to Epithelial Ciliary Activity. J. Cell Biol. 2005, 168, 869. [Google Scholar] [CrossRef]
- Enuka, Y.; Hanukoglu, I.; Edelheit, O.; Vaknine, H.; Hanukoglu, A. Epithelial Sodium Channels (ENaC) Are Uniformly Distributed on Motile Cilia in the Oviduct and the Respiratory Airways. Histochem. Cell Biol. 2012, 137, 339–353. [Google Scholar] [CrossRef]
- Kleyman, T.R.; Eaton, D.C. Regulating ENaC’s Gate. Am. J. Physiol. Cell Physiol. 2020, 318, C150–C162. [Google Scholar] [CrossRef]
- Hanukoglu, I.; Hanukoglu, A. Epithelial Sodium Channel (ENaC) Family: Phylogeny, Structure-Function, Tissue Distribution, and Associated Inherited Diseases. Gene 2016, 579, 95–132. [Google Scholar] [CrossRef] [PubMed]
- King, S.M.; Hilliard, T.S.; Wu, L.Y.; Jaffe, R.C.; Fazleabas, A.T.; Burdette, J.E. The Impact of Ovulation on Fallopian Tube Epithelial Cells: Evaluating Three Hypotheses Connecting Ovulation and Serous Ovarian Cancer. Endocr. Relat. Cancer 2011, 18, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Salvi, A.; Li, W.; Dipali, S.S.; Cologna, S.M.; Pavone, M.E.; Duncan, F.E.; Burdette, J.E. Follicular Fluid Aids Cell Adhesion, Spreading in an Age Independent Manner and Shows an Age-Dependent Effect on DNA Damage in Fallopian Tube Epithelial Cells. Heliyon 2024, 10, e27336. [Google Scholar] [CrossRef] [PubMed]
- Taylor Robinson, D.; Whytock, S.; Green, C.J.; Carney, F.E. Effect of Neisseria Gonorrhoeae on Human and Rabbit Oviducts. Br. J. Vener. Dis. 1974, 50, 279–288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lenz, J.D.; Dillard, J.P. Pathogenesis of Neisseria Gonorrhoeae and the Host Defense in Ascending Infections of Human Fallopian Tube. Front. Immunol. 2018, 9, 2710. [Google Scholar] [CrossRef]
- Song, R.; Walentek, P.; Sponer, N.; Klimke, A.; Lee, J.S.; Dixon, G.; Harland, R.; Wan, Y.; Lishko, P.; Lize, M.; et al. MiR-34/449 MiRNAs Are Required for Motile Ciliogenesis by Repressing Cp110. Nature 2014, 510, 115. [Google Scholar] [CrossRef]
- Wu, J.; Bao, J.; Kim, M.; Yuan, S.; Tang, C.; Zheng, H.; Mastick, G.S.; Xu, C.; Yan, W. Two MiRNA Clusters, MiR-34b/c and MiR-449, Are Essential for Normal Brain Development, Motile Ciliogenesis, and Spermatogenesis. Proc. Natl. Acad. Sci. USA 2014, 111, E2851. [Google Scholar] [CrossRef]
- Chevalier, B.; Adamiok, A.; Mercey, O.; Revinski, D.R.; Zaragosi, L.E.; Pasini, A.; Kodjabachian, L.; Barbry, P.; Marcet, B. MiR-34/449 Control Apical Actin Network Formation during Multiciliogenesis through Small GTPase Pathways. Nat. Commun. 2015, 6, 8386. [Google Scholar] [CrossRef]
- Vasquez, G.; Winston, R.M.L.; Brosens, I.A. Tubal Mucosa and Ectopic Pregnancy. Br. J. Obstet. Gynaecol. 1983, 90, 468–474. [Google Scholar] [CrossRef]
- Mahdavinezhad, F.; Gharaei, R.; Farmani, A.R.; Hashemi, F.; Kouhestani, M.; Amidi, F. The Potential Relationship Between Different Human Female Reproductive Disorders and Sperm Quality in Female Genital Tract. Reprod. Sci. 2022, 29, 695–710. [Google Scholar] [CrossRef]
- de Jonge, C. Biological Basis for Human Capacitation-Revisited. Hum. Reprod. Update 2017, 23, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Reeve, L.; Ledger, W.L.; Pacey, A.A. Does the Arg-Gly-Asp (RGD) Adhesion Sequence Play a Role in Mediating Sperm Interaction with the Human Endosalpinx? Hum. Reprod. 2003, 18, 1461–1468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, Y.Q.; Ho, P.C.; Yeung, W.S.B. Effects of Human Follicular Fluid on the Capacitation and Motility of Human Spermatozoa. Fertil. Steril. 2000, 73, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Ertan Kervancioglu, M.; Saridogan, E.; John Aitken, R.; Djahanbakhch, O. Importance of Sperm-to-Epithelial Cell Contact for the Capacitation of Human Spermatozoa in Fallopian Tube Epithelial Cell Cocultures. Fertil. Steril. 2000, 74, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Mirihagalle, S.; Hughes, J.R.; Miller, D.J. Progesterone-Induced Sperm Release from the Oviduct Sperm Reservoir. Cells 2022, 11, 1622. [Google Scholar] [CrossRef]
- Suarez, S.S.; Pacey, A.A. Sperm Transport in the Female Reproductive Tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Palma, V.; Salgado, A.M.; Villalón, M. Sperm Interaction with Human Oviductal Cells in Vitro. Hum. Reprod. 1996, 11, 1504–1509. [Google Scholar] [CrossRef]
- Miki, K.; Clapham, D.E. Rheotaxis Guides Mammalian Sperm. Curr. Biol. 2013, 23, 443–452. [Google Scholar] [CrossRef]
- Kantsler, V.; Dunkel, J.; Blayney, M.; Goldstein, R.E. Rheotaxis Facilitates Upstream Navigation of Mammalian Sperm Cells. Elife 2014, 3, e02403. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Meriano, J.; Ru, C.; Xie, S.; Luo, J.; Sun, Y. Human Sperm Rheotaxis: A Passive Physical Process. Sci. Rep. 2016, 6, 23553. [Google Scholar] [CrossRef]
- Vigil, P.; Salgado, A.M.; Cortés, M.E. Ultrastructural Interaction between Spermatozoon and Human Oviductal Cells in Vitro. J. Electron Microsc. 2012, 61, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Pacey, A.A.; Hill, C.J.; Scudamore, I.W.; Warren, M.A.; Barratt, C.L.R.; Cooke, I.D. Andrology: The Interaction in Vitro of Human Spermatozoa with Epithelial Cells from the Human Uterine (Fallopian) Tube. Human. Reproduction 1995, 10, 360–366. [Google Scholar] [CrossRef]
- Coan, M.; Vinciguerra, G.L.R.; Cesaratto, L.; Gardenal, E.; Bianchet, R.; Dassi, E.; Vecchione, A.; Baldassarre, G.; Spizzo, R.; Nicoloso, M.S. Exploring the Role of Fallopian Ciliated Cells in the Pathogenesis of High-Grade Serous Ovarian Cancer. Int. J. Mol. Sci. 2018, 19, 2512. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Wu, K.C.; Harnod, T.; Ding, D.C. Comparison of the Cost and Effect of Combined Conditioned Medium and Conventional Medium for Fallopian Tube Organoid Cultures. Cell Transplant. 2023, 32, 9636897231160216. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chu, T.Y.; Ding, D.C. Human Fallopian Tube Epithelial Cells Exhibit Stemness Features, Self-Renewal Capacity, and Wnt-Related Organoid Formation. J. Biomed. Sci. 2020, 27, 32. [Google Scholar] [CrossRef]
- Roy, A.; Matzuk, M.M. Reproductive Tract Function and Dysfunction in Women. Nat. Rev. Endocrinol. 2011, 7, 517–525. [Google Scholar] [CrossRef]
- Ameer, M.A.; Fagan, S.E.; Sosa-Stanley, J.N.; Peterson, D.C. Anatomy, Abdomen and Pelvis: Uterus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial Stem/Progenitor Cells: The First 10 Years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Cousins, F.L.; Filby, C.E.; Gargett, C.E. Endometrial Stem/Progenitor Cells-Their Role in Endometrial Repair and Regeneration. Front. Reprod. Health 2022, 3, 811537. [Google Scholar] [CrossRef]
- Hong, I.S. Endometrial Stem Cells: Orchestrating Dynamic Regeneration of Endometrium and Their Implications in Diverse Endometrial Disorders. Int. J. Biol. Sci. 2024, 20, 864–879. [Google Scholar] [CrossRef]
- Sun, B.; Cheng, X.; Wu, Q. The Endometrial Stem/Progenitor Cells and Their Niches. Stem Cell Rev. Rep. 2024, 20, 1273–1284. [Google Scholar] [CrossRef]
- Baraggino, E.; Pria, S.D.; Cuberli, C.; Bortolotti, S. Scanning Electron Microscopy of the Human Normal Endometrium. Clin. Exp. Obstet. Gynecol. 1980, 7, 66–70. [Google Scholar]
- Bentin-Ley, U.; Sjögren, A.; Nilsson, L.; Hamberger, L.; Larsen, J.F.; Horn, T. Presence of Uterine Pinopodes at the Embryo-Endometrial Interface during Human Implantation in Vitro. Hum. Reprod. 1999, 14, 515–520. [Google Scholar] [CrossRef][Green Version]
- Bartosch, C.; Lopes, J.M.; Beires, J.; Sousa, M. Human Endometrium Ultrastructure during the Implantation Window: A New Perspective of the Epithelium Cell Types. Reprod. Sci. 2011, 18, 525–539. [Google Scholar] [CrossRef]
- Jin, X.Y.; Zhao, L.J.; Luo, D.H.; Liu, L.; Dai, Y.D.; Hu, X.X.; Wang, Y.Y.; Lin, X.; Hong, F.; Li, T.C.; et al. Pinopode Score around the Time of Implantation Is Predictive of Successful Implantation Following Frozen Embryo Transfer in Hormone Replacement Cycles. Hum. Reprod. 2017, 32, 2394–2403. [Google Scholar] [CrossRef]
- Ruiz-Magaña, M.J.; Llorca, T.; Martinez-Aguilar, R.; Abadia-Molina, A.C.; Ruiz-Ruiz, C.; Olivares, E.G. Stromal Cells of the Endometrium and Decidua: In Search of a Name and an Identity†. Biol. Reprod. 2022, 107, 1166–1176. [Google Scholar] [CrossRef]
- Tempest, N.; Hill, C.J.; Maclean, A.; Marston, K.; Powell, S.G.; Al-Lamee, H.; Hapangama, D.K. Novel Microarchitecture of Human Endometrial Glands: Implications in Endometrial Regeneration and Pathologies. Hum. Reprod. Update 2022, 28, 153–171. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshihara, K.; Suda, K.; Nakaoka, H.; Yachida, N.; Ueda, H.; Sugino, K.; Mori, Y.; Yamawaki, K.; Tamura, R.; et al. Three-Dimensional Understanding of the Morphological Complexity of the Human Uterine Endometrium. iScience 2021, 24, 102258. [Google Scholar] [CrossRef]
- Garcia-Alonso, L.; Handfield, L.F.; Roberts, K.; Nikolakopoulou, K.; Fernando, R.C.; Gardner, L.; Woodhams, B.; Arutyunyan, A.; Polanski, K.; Hoo, R.; et al. Mapping the Temporal and Spatial Dynamics of the Human Endometrium in Vivo and in Vitro. Nat. Genet. 2021, 53, 1698–1711. [Google Scholar] [CrossRef]
- Wang, W.; Vilella, F.; Alama, P.; Moreno, I.; Mignardi, M.; Isakova, A.; Pan, W.; Simon, C.; Quake, S.R. Single-Cell Transcriptomic Atlas of the Human Endometrium during the Menstrual Cycle. Nat. Med. 2020, 26, 1644–1653. [Google Scholar] [CrossRef]
- Schueller, E.F. Ciliated Epithelia of the Human Uterine Mucosa. Obstet. Gynecol. 1968, 31, 215–223. [Google Scholar] [CrossRef]
- More, I.A.R.; Masterton, R.G. The Role of Oestrogen in the Control of Ciliated Cells of the Human Endometrium. J. Reprod. Fertil. 1976, 47, 19–24. [Google Scholar] [CrossRef]
- Nilsson, O.; Nygren, K.G. Scanning Electron Microscopy of Human Endometrium. Ups. J. Med. Sci. 1972, 77, 3–7. [Google Scholar] [CrossRef]
- Denholm, R.B.; More, I.A.R. Atypical Cilia of the Human Endometrial Epithelium. J. Anat. 1980, 131, 309. [Google Scholar]
- Hando, T.; Okada, D.M.; Zamboni, L. Atypical Cilia in Human Endometrium. J. Cell Biol. 1968, 39, 475–481. [Google Scholar] [CrossRef]
- Pearson-Farr, J.E.; Doherty, R.; Chatelet, D.S.; Goggin, P.; Ng, K.Y.B.; Lucas, J.S.; Cleal, J.K.; Cheong, Y.C.; Lewis, R.M. Ultrastructural Cilia Defects in Multi-Ciliated Uterine Glandular Epithelial Cells from Women with Reproductive Failure. Reproduction 2024, 167, e230173. [Google Scholar] [CrossRef]
- Satir, P.; Heuser, T.; Sale, W.S. A Structural Basis for How Motile Cilia Beat. Bioscience 2014, 64, 1073–1083. [Google Scholar] [CrossRef]
- Golinska, K.; Kink, J. Proportional Regulation of Body Form and Cortical Organelle Pattern in the Ciliate Dileptus. J. Cell Sci. 1977, 24, 11–29. [Google Scholar] [CrossRef]
- Venard, C.M.; Vasudevan, K.K.; Stearns, T. Cilium Axoneme Internalization and Degradation in Chytrid Fungi. Cytoskeleton 2020, 77, 365–378. [Google Scholar] [CrossRef]
- Wloga, D.; Camba, A.; Rogowski, K.; Manning, G.; Jerka-Dziadosz, M.; Gaertig, J. Members of the NIMA-Related Kinase Family Promote Disassembly of Cilia by Multiple Mechanisms. Mol. Biol. Cell 2006, 17, 2799–2810. [Google Scholar] [CrossRef]
- Sharma, N.; Bryant, J.; Wloga, D.; Donaldson, R.; Davis, R.C.; Jerka-Dziadosz, M.; Gaertig, J. Katanin Regulates Dynamics of Microtubules and Biogenesis of Motile Cilia. J. Cell Biol. 2007, 178, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.; Shoemark, A. Secondary Defects Detected by Transmission Electron Microscopy in Primary Ciliary Dyskinesia Diagnostics. Ultrastruct. Pathol. 2017, 41, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Castleman, W.L.; Powe, J.R.; Crawford, P.C.; Gibbs, E.P.J.; Dubovi, E.J.; Donis, R.O.; Hanshaw, D. Canine H3N8 Influenza Virus Infection in Dogs and Mice. Vet. Pathol. 2010, 47, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Ferenczy, A.; Richart, R.M.; Agate, F.J.; Purkerson, M.L.; Dempsey, E.W. Scanning Electron Microscopy of the Human Endometrial Surface Epithelium. Fertil. Steril. 1972, 23, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, P.; Nilsson, O.; Liedholm, P. Scanning Electron Microscopy of Human Preimplantation Endometrium in Normal and Clomiphene/Human Chorionic Gonadotropin-Stimulated Cycles. Fertil. Steril. 1983, 40, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Nikas, G.; Drakakis, P.; Loutradis, D.; Mara-skoufari, C.; Koumantakis, E.; Michalas, S.; Psychoyos, A. Uterine Pinopodes as Markers of the “nidation Window” in Cycling Women Receiving Exogenous Oestradiol and Progesterone. Hum. Reprod. 1995, 10, 1208–1213. [Google Scholar] [CrossRef]
- Nikas, G. Endometrial Receptivity: Changes in Cell-Surface Morphology. Semin. Reprod. Med. 2000, 18, 229–235. [Google Scholar] [CrossRef]
- Stavreus-Evers, A.; Nikas, G.; Sahlin, L.; Eriksson, H.; Landgren, B.M. Formation of Pinopodes in Human Endometrium Is Associated with the Concentrations of Progesterone and Progesterone Receptors. Fertil. Steril. 2001, 76, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Mao, D.; Liu, Y.; Chen, X.; Xu, H.; Li, T.C.; Wang, C.C. Localization of Mucin 1 in Endometrial Luminal Epithelium and Its Expression in Women with Reproductive Failure during Implantation Window. J. Mol. Histol. 2019, 50, 563–572. [Google Scholar] [CrossRef]
- Quinn, K.E.; Matson, B.C.; Wetendorf, M.; Caron, K.M. Pinopodes: Recent Advancements, Current Perspectives, and Future Directions. Mol. Cell Endocrinol. 2020, 501, 110644. [Google Scholar] [CrossRef]
- Aplin, J.D.; Ruane, P.T. Embryo-Epithelium Interactions during Implantation at a Glance. J. Cell Sci. 2017, 130, 15–22. [Google Scholar] [CrossRef]
- Lacconi, V.; Massimiani, M.; Carriero, I.; Bianco, C.; Ticconi, C.; Pavone, V.; Alteri, A.; Muzii, L.; Rago, R.; Pisaturo, V.; et al. When the Embryo Meets the Endometrium: Identifying the Features Required for Successful Embryo Implantation. Int. J. Mol. Sci. 2024, 25, 2834. [Google Scholar] [CrossRef] [PubMed]
- Demir, R.; Kayisli, U.A.; Celik-Ozenci, C.; Korgun, E.T.; Demir-Weusten, A.Y.; Arici, A. Structural Differentiation of Human Uterine Luminal and Glandular Epithelium during Early Pregnancy: An Ultrastructural and Immunohistochemical Study. Placenta 2002, 23, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Johannisson, E.; Nilsson, L. Scanning Electron Microscopic Study of the Human Endometrium. Fertil. Steril. 1972, 23, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Metzger, H. The Re-Epithelization of Endometrium after Menstrual Desquamation. Arch. Gynakol. 1976, 221, 51–60. [Google Scholar] [CrossRef]
- Ludwig, H.; Spornitz, U.M. Microarchitecture of the Human Endometrium by Scanning Electron Microscopy: Menstrual Desquamation and Remodeling. Ann. N. Y Acad. Sci. 1991, 622, 28–46. [Google Scholar] [CrossRef]
- Devesa-Peiro, A.; Sebastian-Leon, P.; Parraga-Leo, A.; Pellicer, A.; Diaz-Gimeno, P. Breaking the Ageing Paradigm in Endometrium: Endometrial Gene Expression Related to Cilia and Ageing Hallmarks in Women over 35 Years. Hum. Reprod. 2022, 37, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Loid, M.; Obukhova, D.; Kask, K.; Apostolov, A.; Meltsov, A.; Tserpelis, D.; van den Wijngaard, A.; Altmäe, S.; Yahubyan, G.; Baev, V.; et al. Aging Promotes Accumulation of Senescent and Multiciliated Cells in Human Endometrial Epithelium. Hum. Reprod. Open 2024, 2024, hoae048. [Google Scholar] [CrossRef]
- Masterton, R.; Armstrong, E.M.; More, I.A.R. The Cyclical Variation in the Percentage of Ciliated Cells in the Normal Human Endometrium. J. Reprod. Fertil. 1975, 42, 537–540. [Google Scholar] [CrossRef]
- Gould, P.R.; Barter, R.A.; Papadimitriou, J.M. An Ultrastructural, Cytochemical, and Autoradiographic Study of the Mucous Membrane of the Human Cervical Canal with Reference to Subcolumnar Basal Cells. Am. J. Pathol. 1979, 95, 1–16. [Google Scholar]
- Haider, S.; Gamperl, M.; Burkard, T.R.; Kunihs, V.; Kaindl, U.; Junttila, S.; Fiala, C.; Schmidt, K.; Mendjan, S.; Knöfler, M.; et al. Estrogen Signaling Drives Ciliogenesis in Human Endometrial Organoids. Endocrinology 2019, 160, 2282–2297. [Google Scholar] [CrossRef]
- Tian, J.; Yang, J.; Chen, T.; Yin, Y.; Li, N.; Li, Y.; Luo, X.; Dong, E.; Tan, H.; Ma, Y.; et al. Generation of Human Endometrial Assembloids with a Luminal Epithelium Using Air-Liquid Interface Culture Methods. Adv. Sci. 2023, 10, e2301868. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Guo, X.; Xu, H.; Li, Y.; Jin, B.; Zhang, X.; Shu, C.; Fan, Y.; Yu, Y.; Tian, Y.; et al. Varied Cellular Abnormalities in Thin vs. Normal Endometrium in Recurrent Implantation Failure by Single-Cell Transcriptomics. Reprod. Biol. Endocrinol. 2024, 22, 90. [Google Scholar] [CrossRef]
- Marečková, M.; Garcia-Alonso, L.; Moullet, M.; Lorenzi, V.; Petryszak, R.; Sancho-Serra, C.; Oszlanczi, A.; Icoresi Mazzeo, C.; Wong, F.C.K.; Kelava, I.; et al. An Integrated Single-Cell Reference Atlas of the Human Endometrium. Nat. Genet. 2024, 56, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
- Sarsenova, M.; Lawarde, A.; Pathare, A.D.S.; Saare, M.; Modhukur, V.; Soplepmann, P.; Terasmaa, A.; Käämbre, T.; Gemzell-Danielsson, K.; Lalitkumar, P.G.L.; et al. Endometriotic Lesions Exhibit Distinct Metabolic Signature Compared to Paired Eutopic Endometrium at the Single-Cell Level. Commun. Biol. 2024, 7, 1026. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Flynn, W.F.; Sivajothi, S.; Luo, D.; Bozal, S.B.; Davé, M.; Luciano, A.A.; Robson, P.; Luciano, D.E.; Courtois, E.T. Single-Cell Analysis of Endometriosis Reveals a Coordinated Transcriptional Programme Driving Immunotolerance and Angiogenesis across Eutopic and Ectopic Tissues. Nat. Cell Biol. 2022, 24, 1306–1318. [Google Scholar] [CrossRef]
- Vanaken, G.J.; Bassinet, L.; Boon, M.; Mani, R.; Honoré, I.; Papon, J.F.; Cuppens, H.; Jaspers, M.; Lorent, N.; Coste, A.; et al. Infertility in an Adult Cohort with Primary Ciliary Dyskinesia: Phenotype-Gene Association. Eur. Respir. J. 2017, 50, 1700314. [Google Scholar] [CrossRef]
- Schreck, L.D.; Pedersen, E.S.L.; Dexter, K.; Manion, M.; Bellu, S.; Cizeau, I.; Copeland, F.; Dexter, K.; Dixon, L.; Fernández, T.L.; et al. Infertility and Pregnancy Outcomes among Adults with Primary Ciliary Dyskinesia. Hum. Reprod. Open 2024, 2024, hoae039. [Google Scholar] [CrossRef]
- Despotes, K.A.; Zariwala, M.A.; Davis, S.D.; Ferkol, T.W. Primary Ciliary Dyskinesia: A Clinical Review. Cells 2024, 13, 974. [Google Scholar] [CrossRef]
- King, S.M. Inherently Disordered Regions of Axonemal Dynein Assembly Factors. Cytoskeleton 2023, 81, 515–528. [Google Scholar] [CrossRef]
- Lechtreck, K.F.; Delmotte, P.; Robinson, M.L.; Sanderson, M.J.; Witman, G.B. Mutations in Hydin Impair Ciliary Motility in Mice. J. Cell Biol. 2008, 180, 633–643. [Google Scholar] [CrossRef]
- Lechtreck, K.F.; Witman, G.B. Chlamydomonas Reinhardtii Hydin Is a Central Pair Protein Required for Flagellar Motility. J. Cell Biol. 2007, 176, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Park, T.J.; Kwon, T. Convergent Differentiation of Multiciliated Cells. Sci. Rep. 2023, 13, 23028. [Google Scholar] [CrossRef] [PubMed]
- Gheber, L.; Korngreen, A.; Priel, Z. Effect of Viscosity on Metachrony in Mucus Propelling Cilia. Cell Motil. Cytoskelet. 1998, 39, 9–20. [Google Scholar] [CrossRef]
- Luk, C.K.; Dulfano, M.J. Effect of PH, Viscosity and Ionic-Strength Changes on Ciliary Beating Frequency of Human Bronchial Explants. Clin. Sci. 1983, 64, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Marchini, M.; Losa, G.A.; Nava, S.; Di Nola, G.; Fedele, L. Ultrastructural Aspects of Endometrial Surface in Kartagener’s Syndrome. Fertil. Steril. 1992, 57, 461–463. [Google Scholar] [CrossRef]
- Hoque, M.; Kim, E.N.; Chen, D.; Li, F.Q.; Takemaru, K.I. Essential Roles of Efferent Duct Multicilia in Male Fertility. Cells 2022, 11, 341. [Google Scholar] [CrossRef]
- Sullivan, R.; Mieusset, R. The Human Epididymis: Its Function in Sperm Maturation. Hum. Reprod. Update 2016, 22, 574–587. [Google Scholar] [CrossRef]
- Bustos-Obregón, E.; Holstein, A.F. The Rete Testis in Man: Ultrastructural Aspects. Cell Tissue Res. 1976, 175, 1–15. [Google Scholar] [CrossRef]
- Roosen-Runge, E.C.; Holstein, A.F. The Human Rete Testis. Cell Tissue Res. 1978, 189, 409–433. [Google Scholar] [CrossRef]
- Saitoh, K.; Terada, T.; Hatakeyama, S. A Morphological Study of the Efferent Ducts of the Human Epididymis. Int. J. Androl. 1990, 13, 369–376. [Google Scholar] [CrossRef]
- Yeung, C.H.; Cooper, T.G.; Bergmann, M.; Schulze, H. Organization of Tubules in the Human Caput Epididymidis and the Ultrastructure of Their Epithelia. Am. J. Anat. 1991, 191, 261–279. [Google Scholar] [CrossRef]
- Turner, T.T. De Graaf’s Thread: The Human Epididymis. J. Androl. 2008, 29, 237–250. [Google Scholar] [CrossRef]
- Nakata, H.; Iseki, S.; Mizokami, A. Three-Dimensional Analysis of Junctions between Efferent and Epididymal Ducts in the Human Caput Epididymis. Andrology 2024, 12, 87–97. [Google Scholar] [CrossRef]
- Browne, J.A.; Leir, S.H.; Yin, S.; Harris, A. Transcriptional Networks in the Human Epididymis. Andrology 2019, 7, 741. [Google Scholar] [CrossRef] [PubMed]
- Leir, S.H.; Paranjapye, A.; Harris, A. Functional Genomics of the Human Epididymis: Further Characterization of Efferent Ducts and Model Systems by Single-Cell RNA Sequencing Analysis. Andrology 2024, 12, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Holstein, A.F. Intraepithelial Lymphocytes and Macrophages in the Human Epididymis. Cell Tissue Res. 1983, 233, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Vinay, L.; Hess, R.A.; Belleannée, C. Human Efferent Ductules and Epididymis Display Unique Cell Lineages with Motile and Primary Cilia. Andrology 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jorissen, M.; Jaspers, M. Cilia, Ciliary Movement, and Mucociliary Transport. In Nasal Physiology and Pathophysiology of Nasal Disorders; Celebi, Ö.Ö., Önerci, T.M., Eds.; Springer: Cham, Switzerlands, 2023; pp. 29–40. [Google Scholar] [CrossRef]
- Sullivan, R.; Légaré, C.; Lamontagne-Proulx, J.; Breton, S.; Soulet, D. Revisiting Structure/Functions of the Human Epididymis. Andrology 2019, 7, 748–757. [Google Scholar] [CrossRef]
- Leir, S.H.; Yin, S.; Kerschner, J.L.; Cosme, W.; Harris, A. An Atlas of Human Proximal Epididymis Reveals Cell-Specific Functions and Distinct Roles for CFTR. Life Sci. Alliance 2020, 3, e202000744. [Google Scholar] [CrossRef]
- Whitfield, M.; Thomas, L.; Bequignon, E.; Schmitt, A.; Stouvenel, L.; Montantin, G.; Tissier, S.; Duquesnoy, P.; Copin, B.; Chantot, S.; et al. Mutations in DNAH17, Encoding a Sperm-Specific Axonemal Outer Dynein Arm Heavy Chain, Cause Isolated Male Infertility Due to Asthenozoospermia. Am. J. Hum. Genet. 2019, 105, 198–212. [Google Scholar] [CrossRef]
- Amirav, I.; Wallmeier, J.; Loges, N.T.; Menchen, T.; Pennekamp, P.; Mussaffi, H.; Abitbul, R.; Avital, A.; Bentur, L.; Dougherty, G.W.; et al. Systematic Analysis of CCNO Variants in a Defined Population: Implications for Clinical Phenotype and Differential Diagnosis. Hum. Mutat. 2016, 37, 396–405. [Google Scholar] [CrossRef]
- Boon, M.; Wallmeier, J.; Ma, L.; Loges, N.T.; Jaspers, M.; Olbrich, H.; Dougherty, G.W.; Raidt, J.; Werner, C.; Amirav, I.; et al. MCIDAS Mutations Result in a Mucociliary Clearance Disorder with Reduced Generation of Multiple Motile Cilia. Nat. Commun. 2014, 5, 4418. [Google Scholar] [CrossRef]
- Henriques, A.R.; Constant, C.; Descalço, A.; Pinto, A.; Moura Nunes, J.; Sampaio, P.; Lopes, S.S.; Pereira, L.; Bandeira, T. Primary Ciliary Dyskinesia Due to CCNO Mutations-A Genotype-Phenotype Correlation Contribution. Pediatr. Pulmonol. 2021, 56, 2776–2779. [Google Scholar] [CrossRef]
- Xu, Y.; Ueda, K.; Nishikido, T.; Matsumoto, T.; Takeuchi, K. Two Japanese Pediatric Patients With Primary Ciliary Dyskinesia Caused by Loss-of-Function Variants in the CCNO Gene. Cureus 2024, 16, e58854. [Google Scholar] [CrossRef]
- Ma, C.; Wu, H.; Zhu, D.; Wang, Y.; Shen, Q.; Cheng, H.; Zhang, J.; Geng, H.; Liu, Y.; He, X.; et al. Bi-Allelic Mutations in MCIDAS and CCNO Cause Human Infertility Associated with Abnormal Gamete Transport. Clin. Genet. 2021, 100, 731–742. [Google Scholar] [CrossRef]
- Wallmeier, J.; Nielsen, K.G.; Kuehni, C.E.; Lucas, J.S.; Leigh, M.W.; Zariwala, M.A.; Omran, H. Motile Ciliopathies. Nat. Rev. Dis. Primers 2020, 6, 1–29. [Google Scholar] [CrossRef]
- Terré, B.; Lewis, M.; Gil-Gómez, G.; Han, Z.; Lu, H.; Aguilera, M.; Prats, N.; Roy, S.; Zhao, H.; Stracker, T.H. Defects in Efferent Duct Multiciliogenesis Underlie Male Infertility in GEMC1-, MCIDAS- or CCNO-Deficient Mice. Development 2019, 146, dev162628. [Google Scholar] [CrossRef]
- Danielian, P.S.; Hess, R.A.; Lees, J.A. E2f4 and E2f5 Are Essential for the Development of the Male Reproductive System. Cell Cycle 2016, 15, 250–260. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wloga, D.; Joachimiak, E.; Osinka, A.; Ahmadi, S.; Majhi, S. Motile Cilia in Female and Male Reproductive Tracts and Fertility. Cells 2024, 13, 1974. https://doi.org/10.3390/cells13231974
Wloga D, Joachimiak E, Osinka A, Ahmadi S, Majhi S. Motile Cilia in Female and Male Reproductive Tracts and Fertility. Cells. 2024; 13(23):1974. https://doi.org/10.3390/cells13231974
Chicago/Turabian StyleWloga, Dorota, Ewa Joachimiak, Anna Osinka, Salman Ahmadi, and Sumita Majhi. 2024. "Motile Cilia in Female and Male Reproductive Tracts and Fertility" Cells 13, no. 23: 1974. https://doi.org/10.3390/cells13231974
APA StyleWloga, D., Joachimiak, E., Osinka, A., Ahmadi, S., & Majhi, S. (2024). Motile Cilia in Female and Male Reproductive Tracts and Fertility. Cells, 13(23), 1974. https://doi.org/10.3390/cells13231974






