The Immunopathology of Pulmonary Rejection after Murine Lung Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Orthotopic Murine Lung Transplant Model
2.2. Study Design
2.3. In Vivo Microcomputed Tomography
2.4. Isolation of Pulmonary Cells
2.5. Flow Cytometry of Pulmonary Cells
2.6. Histology
2.7. Statistical Analysis
3. Results
3.1. Survival after Murine Orthotopic Single-Lung Transplantation
3.2. Alteration in Lung Density after Allograft Transplantation
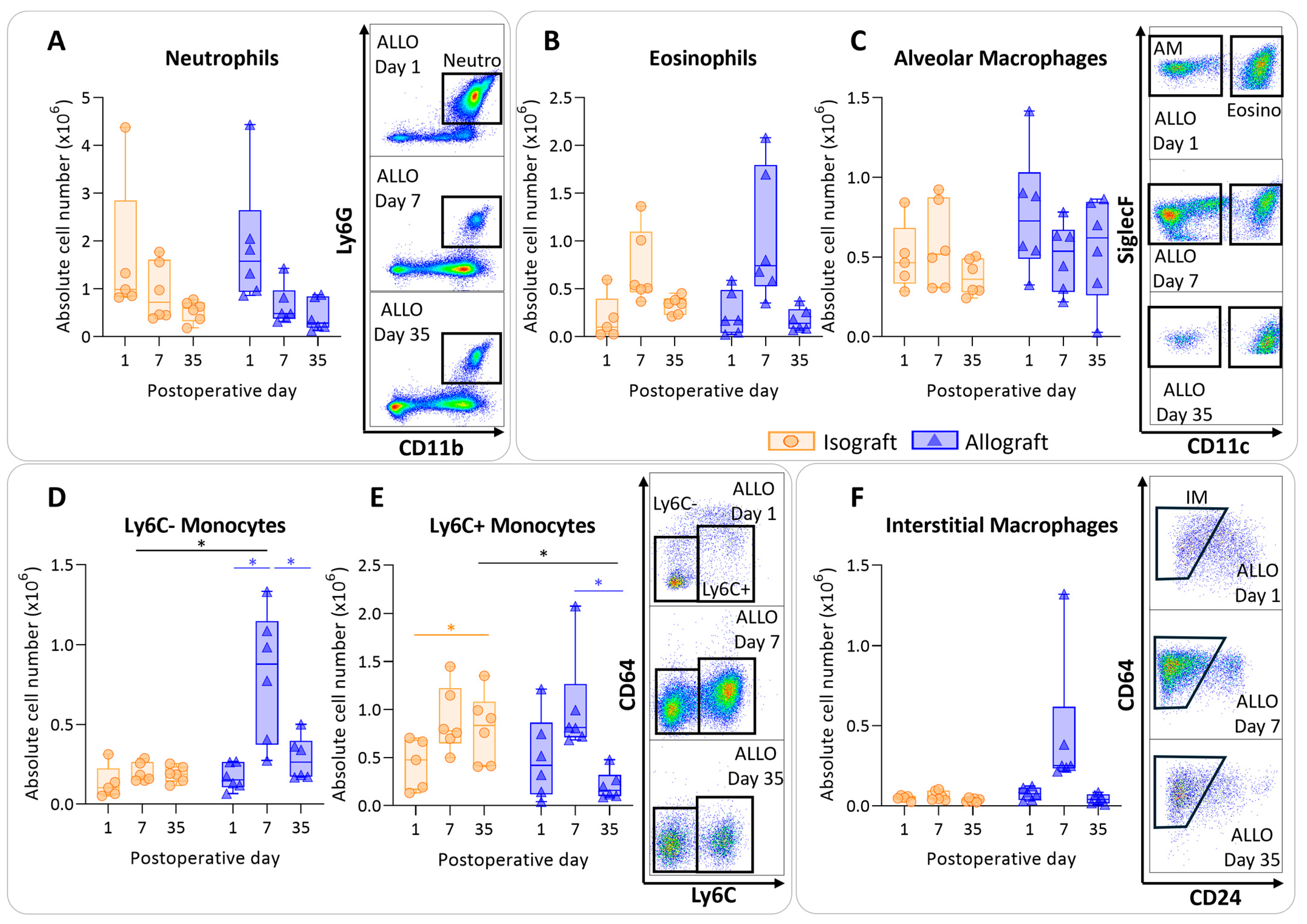
3.3. Pulmonary Myeloid Cells after Murine Lung Transplantation
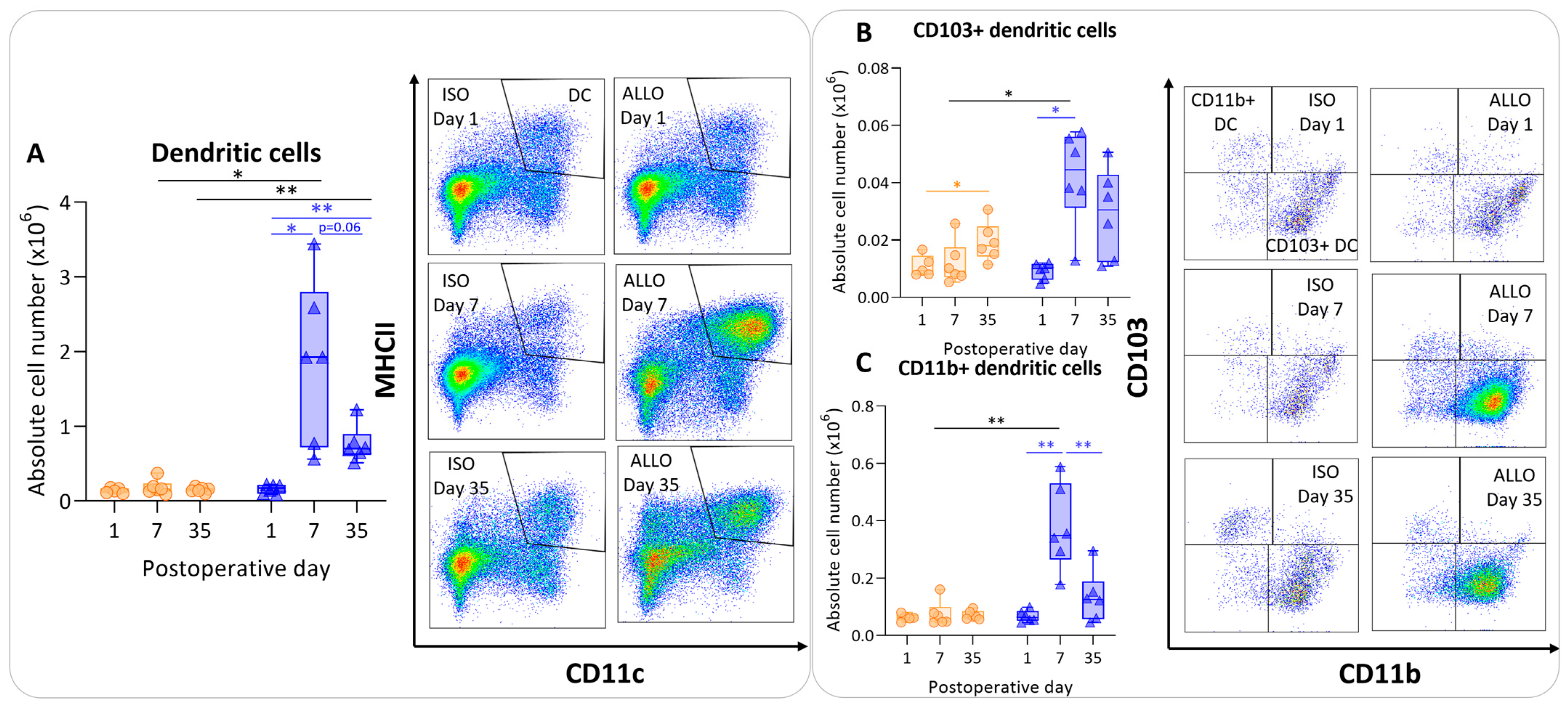
3.4. Antigen-Presenting Cells after Murine Lung Transplantation
3.5. Pulmonary Lymphoid Cells after Murine Lung Transplantation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Mark, S.C.; Hoek, R.A.S.; Hellemons, M.E. Developments in lung transplantation over the past decade. Eur. Respir. Rev. 2020, 29, 190132. [Google Scholar] [CrossRef]
- Sayegh, M.H.; Carpenter, C.B. Transplantation 50 years later—Progress, challenges, and promises. N. Engl. J. Med. 2004, 351, 2761–6276. [Google Scholar] [CrossRef]
- Verleden, G.M.; Glanville, A.R.; Lease, E.D.; Fisher, A.J.; Calabrese, F.; Corris, P.A.; Ensor, C.R.; Gottlieb, J.; Hachem, R.R.; Lama, V.; et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria and approaches to treatment. A consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transpl. 2019, 38, 493–503. [Google Scholar] [CrossRef]
- Abraham, A.S.; Singh, M.; Abraham, M.S.; Ahuja, S. Epidemiology and Long-Term Outcomes in Thoracic Transplantation. J. Cardiovasc. Dev. Dis. 2023, 10, 397. [Google Scholar] [CrossRef]
- Bos, S.; Milross, L.; Filby, A.J.; Vos, R.; Fisher, A.J. Immune processes in the pathogenesis of chronic lung allograft dysfunction: Identifying the missing pieces of the puzzle. Eur. Respir. Rev. 2022, 31, 220060. [Google Scholar] [CrossRef] [PubMed]
- Kloc, M.; Ghobrial, R.M. Chronic allograft rejection: A significant hurdle to transplant success. Burn Trauma 2014, 2, 2321–3868. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.N.; Plumb, T.J.; Crowley, S.D.; Coffman, T.M. Effector mechanisms in transplant rejection. Immunol. Rev. 2003, 196, 51–64. [Google Scholar] [CrossRef]
- Tilney, N.L.; Whitley, W.D.; Diamond, J.R.; Kupiec-Weglinski, J.W.; Adams, D.H. Chronic rejection—An undefined conundrum. Transplantation 1991, 52, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Hachem, R.R. The role of the immune system in lung transplantation: Towards improved long-term results. J. Thorac. Dis. 2019, 11 (Suppl. 14), S1721. [Google Scholar] [CrossRef]
- Royer, P.J.; Olivera-Botello, G.; Koutsokera, A.; Aubert, J.D.; Bernasconi, E.; Tissot, A.; Pison, C.; Nicod, L.; Boissel, J.-P.; Magnan, A. Chronic lung allograft dysfunction: A systematic review of mechanisms. Transplantation 2016, 100, 1803–1814. [Google Scholar] [CrossRef]
- Shah, R.J.; Diamond, J.M. Update in chronic lung allograft dysfunction. Clin. Chest Med. 2017, 38, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Lama, V.N.; Belperio, J.A.; Christie, J.D.; El-Chemaly, S.; Fishbein, M.C.; Gelman, A.E.; Hancock, W.W.; Keshavjee, S.; Kreisel, D.; Laubach, V.E.; et al. Models of lung transplant research: A consensus statement from the National Heart, Lung, and Blood Institute workshop. JCI Insight 2017, 2, e93121. [Google Scholar] [CrossRef] [PubMed]
- Jungraithmayr, W.M.; Korom, S.; Hillinger, S.; Weder, W. A mouse model of orthotopic, single-lung transplantation. J. Thorac. Cardiovasc. Surg. 2009, 137, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.N.; Chen, B.; Liu, X.; Kurie, J.M.; Kim, J. A method for orthotopic transplantation of lung cancer in mice. Hedgehog Signal. Methods Protoc. 2022, 2374, 231–242. [Google Scholar]
- Berghen, N.; Dekoster, K.; Marien, E.; Dabin, J.; Hillen, A.; Wouters, J.; Deferme, J.; Vosselman, T.; Tiest, E.; Lox, M.; et al. Radiosafe micro-computed tomography for longitudinal evaluation of murine disease models. Sci. Rep. 2019, 9, 17598. [Google Scholar] [CrossRef] [PubMed]
- Vande Velde, G.; Poelmans, J.; De Langhe, E.; Hillen, A.; Vanoirbeek, J.; Himmelreich, U.; Lories, R.J. Longitudinal micro-CT provides biomarkers of lung disease that can be used to assess the effect of therapy in preclinical mouse models, and reveal compensatory changes in lung volume. Dis. Model Mech. 2016, 9, 91–98. [Google Scholar] [PubMed]
- Pollenus, E.; Pham, T.T.; Vandermosten, L.; Possemiers, H.; Knoops, S.; Opdenakker, G.; Van den Steen, P.E. CCR2 is dispensable for disease resolution but required for the restoration of leukocyte homeostasis upon experimental malaria-associated acute respiratory distress syndrome. Front. Immunol. 2021, 11, 628643. [Google Scholar] [CrossRef]
- Laubach, V.E.; Sharma, A.K. Mechanisms of lung ischemia-reperfusion injury. Curr. Opin. Organ Transplant. 2016, 21, 246. [Google Scholar] [CrossRef]
- Neurohr, C.; Huppmann, P.; Samweber, B.; Leuschner, S.; Zimmermann, G.; Leuchte, H.; Baumgartner, R.; Hatz, R.; Frey, L.; Ueberfuhr, P.; et al. Prognostic value of bronchoalveolar lavage neutrophilia in stable lung transplant recipients. J. Heart Lung Transplant. 2009, 28, 468–474. [Google Scholar] [CrossRef]
- Corris, P.A.; Christie, J.D. Update in transplantation 2006. Am. J. Respir. Crit. Care Med. 2007, 175, 432–435. [Google Scholar] [CrossRef]
- Bharat, A.; Kuo, E.; Steward, N.; Aloush, A.; Hachem, R.; Trulock, E.P.; Patterson, G.A.; Meyers, B.F.; Mohanakumar, T. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann. Thorac. Surg. 2008, 86, 189–197. [Google Scholar] [CrossRef]
- Kawashima, M.; Juvet, S.C. The role of innate immunity in the long-term outcome of lung transplantation. Ann. Transl. Med. 2020, 8, 412. [Google Scholar] [CrossRef]
- Verleden, S.E.; Ruttens, D.; Vandermeulen, E.; van Raemdonck, D.E.; Vanaudenaerde, B.M.; Verleden, G.M.; Vos, R. Elevated bronchoalveolar lavage eosinophilia correlates with poor outcome after lung transplantation. Transplantation 2014, 97, 83–89. [Google Scholar] [CrossRef]
- Darley, D.R.; Ma, J.; Huszti, E.; Fiset, P.; Levy, L.; Hwang, D.M.; Pal, P.; Klement, W.; Zamel, R.; Keshavjee, S.; et al. Eosinophils in Transbronchial Biopsies: A Predictor of Chronic Lung Allograft Dysfunction and Reduced Survival After Lung Transplantation. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2021, 34, 62–75. [Google Scholar]
- Vandermeulen, E.; Lammertyn, E.; Verleden, S.E.; Ruttens, D.; Bellon, H.; Ricciardi, M.; Somers, J.; Bracke, K.R.; Van Den Eynde, K.; Tousseyn, T.; et al. Immunological diversity in phenotypes of chronic lung allograft dysfunction: A comprehensive immunohistochemical analysis. Transpl. Int. 2017, 30, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Lam, C.; Oliver, J.; Oishi, H.; Teskey, G.; Beber, S.; Boonstra, K.; Umaña, J.M.; Buhari, H.; Joe, B.; et al. Donor Batf3 inhibits murine lung allograft rejection and airway fibrosis. Mucosal. Immunol. 2023, 16, 104–120. [Google Scholar] [CrossRef]
- Ward, C.; Whitford, H.; Snell, G.; Bao, H.; Zheng, L.; Reid, D.; Williams, T.J.; Walters, E.H. Bronchoalveolar lavage macrophage and lymphocyte phenotypes in lung transplant recipients. J. Heart Lung Transplant. 2001, 20, 1064–1074. [Google Scholar] [CrossRef]
- Bos, S.; Filby, A.J.; Vos, R.; Fisher, A.J. Effector immune cells in chronic lung allograft dysfunction: A systematic review. Immunology 2022, 166, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, D.R.; Lanier, L.L.; Greenland, J.R. Natural killer cells in lung transplantation. Thorax 2019, 74, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Martinu, T.; Pavlisko, E.N.; Chen, D.F.; Palmer, S.M. Acute allograft rejection: Cellular and humoral processes. Clin. Chest Med. 2011, 32, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Evers, A.; Atanasova, S.; Fuchs-Moll, G.; Petri, K.; Wilker, S.; Zakrzewicz, A.; Hirschburger, M.; Padberg, W.; Grau, V. Adaptive and innate immune responses in a rat orthotopic lung transplant model of chronic lung allograft dysfunction. Transpl. Int. 2015, 28, 95–107. [Google Scholar] [CrossRef]
- Yamada, Y.; Brüstle, K.; Jungraithmayr, W. T helper cell subsets in experimental lung allograft rejection. J. Surg. Res. 2019, 233, 74–81. [Google Scholar] [CrossRef]
- Misumi, K.; Wheeler, D.S.; Aoki, Y.; Combs, M.P.; Braeuer, R.R.; Higashikubo, R.; Li, W.; Kreisel, D.; Vittal, R.; Myers, J.; et al. Humoral immune responses mediate the development of a restrictive phenotype of chronic lung allograft dysfunction. JCI Insight 2020, 5, e136533. [Google Scholar] [CrossRef]
- Smirnova, N.F.; Conlon, T.M.; Morrone, C.; Dorfmuller, P.; Humbert, M.; Stathopoulos, G.T.; Umkehrer, S.; Pfeiffer, F.; Yildirim, A.Ö.; Eickelberg, O. Inhibition of B cell–dependent lymphoid follicle formation prevents lymphocytic bronchiolitis after lung transplantation. JCI Insight 2019, 4, e123971. [Google Scholar] [CrossRef]
- Matsumoto, H.; Suzuki, H.; Yamanaka, T.; Kaiho, T.; Hata, A.; Inage, T.; Ito, T.; Kamata, T.; Tanaka, K.; Sakairi, Y.; et al. Anti-CD20 Antibody and Calcineurin Inhibitor Combination Therapy Effectively Suppresses Antibody-Mediated Rejection in Murine Orthotopic Lung Transplantation. Life 2023, 13, 2042. [Google Scholar] [CrossRef]
- Colvin, R.B.; Hirohashi, T.; Farris, A.B.; Minnei, F.; Collins, A.B.; Smith, R.N. Emerging role of B cells in chronic allograft dysfunction. Kidney Int. 2010, 78, S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Girnita, A.L.; Lee, T.M.; McCurry, K.R.; Baldwin, W.M., III; Yousem, S.A.; Detrick, B.; Pilewski, J.; Toyoda, Y.; Jelinek, L.; Lomago, J.; et al. Anti-human leukocyte antigen antibodies, vascular C4d deposition and increased soluble c4d in broncho-alveolar lavage of lung allografts. Transplantation 2008, 86, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fueyo, A.; Markmann, J.F. Immune exhaustion and transplantation. Am. J. Transplant. 2016, 16, 1953–1957. [Google Scholar] [CrossRef]
- Tissot, A.; Danger, R.; Claustre, J.; Magnan, A.; Brouard, S. Early Identification of Chronic Lung Allograft Dysfunction: The Need of Biomarkers. Front. Immunol. 2019, 10, 1681. [Google Scholar] [CrossRef]
- Koutsokera, A.; Royer, P.J.; Fritz, A.; Benden, C.; Tissot, A.; Aubert, J.D.; Antonietti, J.P.; Botturi-Cavaillès, K.; Magnan, A.; Pison, C.; et al. Risk factors for chronic lung allograft dysfunction (CLAD) in the SysCLAD cohort. Eur. Respir. J. 2015, 46, PA1800. [Google Scholar]
- Venado, A.; Kukreja, J.; Greenland, J.R. Chronic lung allograft dysfunction. Thorac. Surg. Clin. 2022, 32, 231–242. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaes, J.; Pollenus, E.; Hooft, C.; Liu, H.; Aelbrecht, C.; Cambier, S.; Jin, X.; Van Slambrouck, J.; Beeckmans, H.; Kerckhof, P.; et al. The Immunopathology of Pulmonary Rejection after Murine Lung Transplantation. Cells 2024, 13, 241. https://doi.org/10.3390/cells13030241
Kaes J, Pollenus E, Hooft C, Liu H, Aelbrecht C, Cambier S, Jin X, Van Slambrouck J, Beeckmans H, Kerckhof P, et al. The Immunopathology of Pulmonary Rejection after Murine Lung Transplantation. Cells. 2024; 13(3):241. https://doi.org/10.3390/cells13030241
Chicago/Turabian StyleKaes, Janne, Emilie Pollenus, Charlotte Hooft, Hengshuo Liu, Celine Aelbrecht, Seppe Cambier, Xin Jin, Jan Van Slambrouck, Hanne Beeckmans, Pieterjan Kerckhof, and et al. 2024. "The Immunopathology of Pulmonary Rejection after Murine Lung Transplantation" Cells 13, no. 3: 241. https://doi.org/10.3390/cells13030241
APA StyleKaes, J., Pollenus, E., Hooft, C., Liu, H., Aelbrecht, C., Cambier, S., Jin, X., Van Slambrouck, J., Beeckmans, H., Kerckhof, P., Velde, G. V., Van Raemdonck, D., Yildirim, A. Ö., Van den Steen, P. E., Vos, R., Ceulemans, L. J., & Vanaudenaerde, B. M. (2024). The Immunopathology of Pulmonary Rejection after Murine Lung Transplantation. Cells, 13(3), 241. https://doi.org/10.3390/cells13030241





