Post-Anesthesia Cognitive Dysfunction in Mice Is Associated with an Age-Related Increase in Neuronal Intracellular [Ca2+]—Neuroprotective Effect of Reducing Intracellular [Ca2+]: In Vivo and In Vitro Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Model
2.2. Anesthesia, Surgical Procedure, and In Vivo Measurements of Intracellular Ca2+ Concentration
2.3. Ca2+-Selective Microelectrodes
2.4. Preparation of Primary Dissociated Hippocampal Neurons
2.5. Recording of Resting Membrane Potential and [Ca2+] In Vivo and In Vitro
2.6. Exposure to Isoflurane In Vitro
2.7. Measurement of Reactive Oxygen Species
2.8. Measurements of Calpain Activity
2.9. Lactate Dehydrogenase Assay
2.10. Morris Water Maze Test
2.11. Solutions
2.12. Statistical Analysis
3. Results
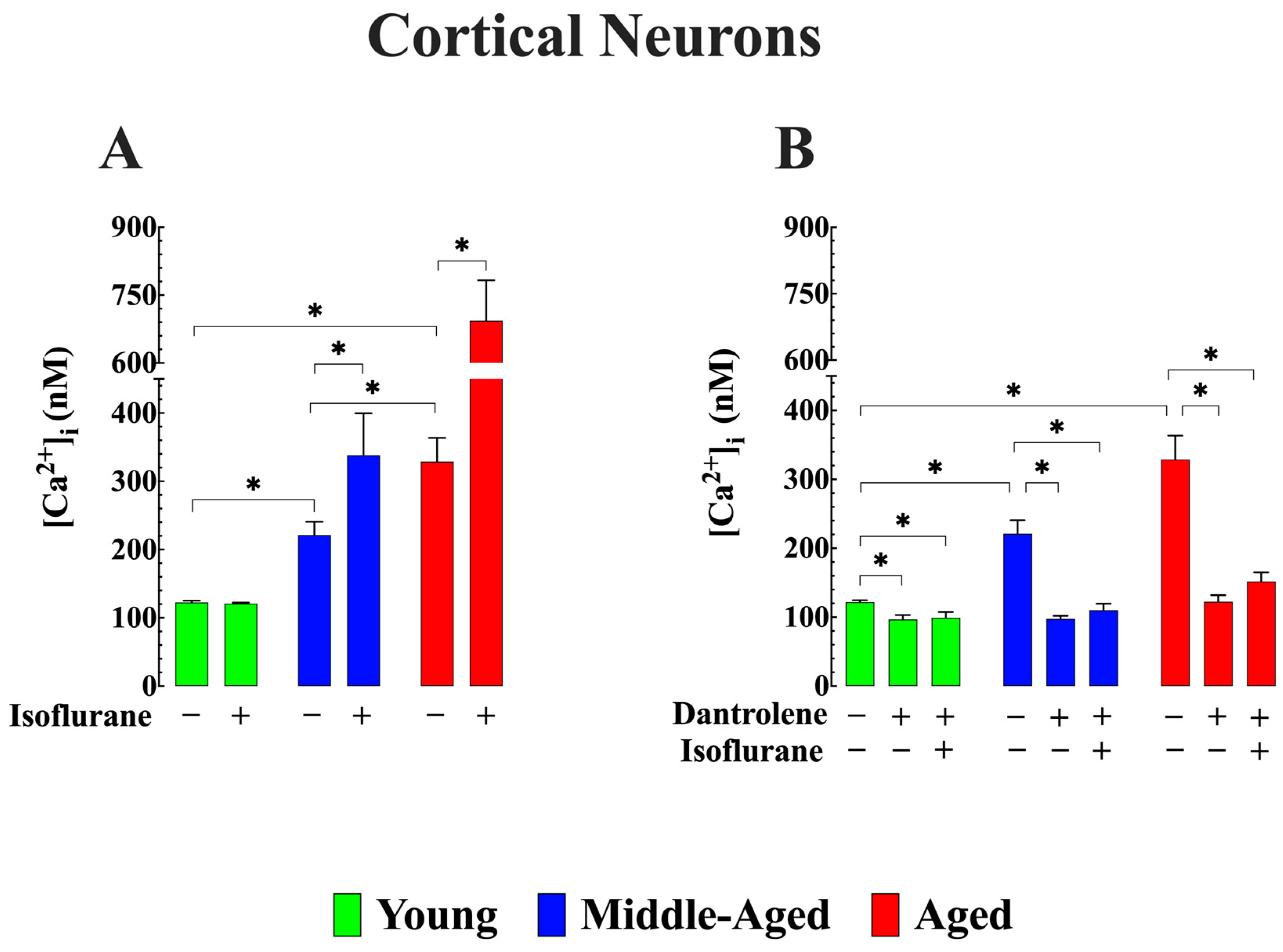
3.1. Effect of Isoflurane on [Ca2+]i in Cortical Neurons (In Vivo)
3.2. Reduction of Abnormal [Ca2+]i Averts Additional Elevation Induced by Isoflurane in Aged Cortical Neurons (In Vivo)
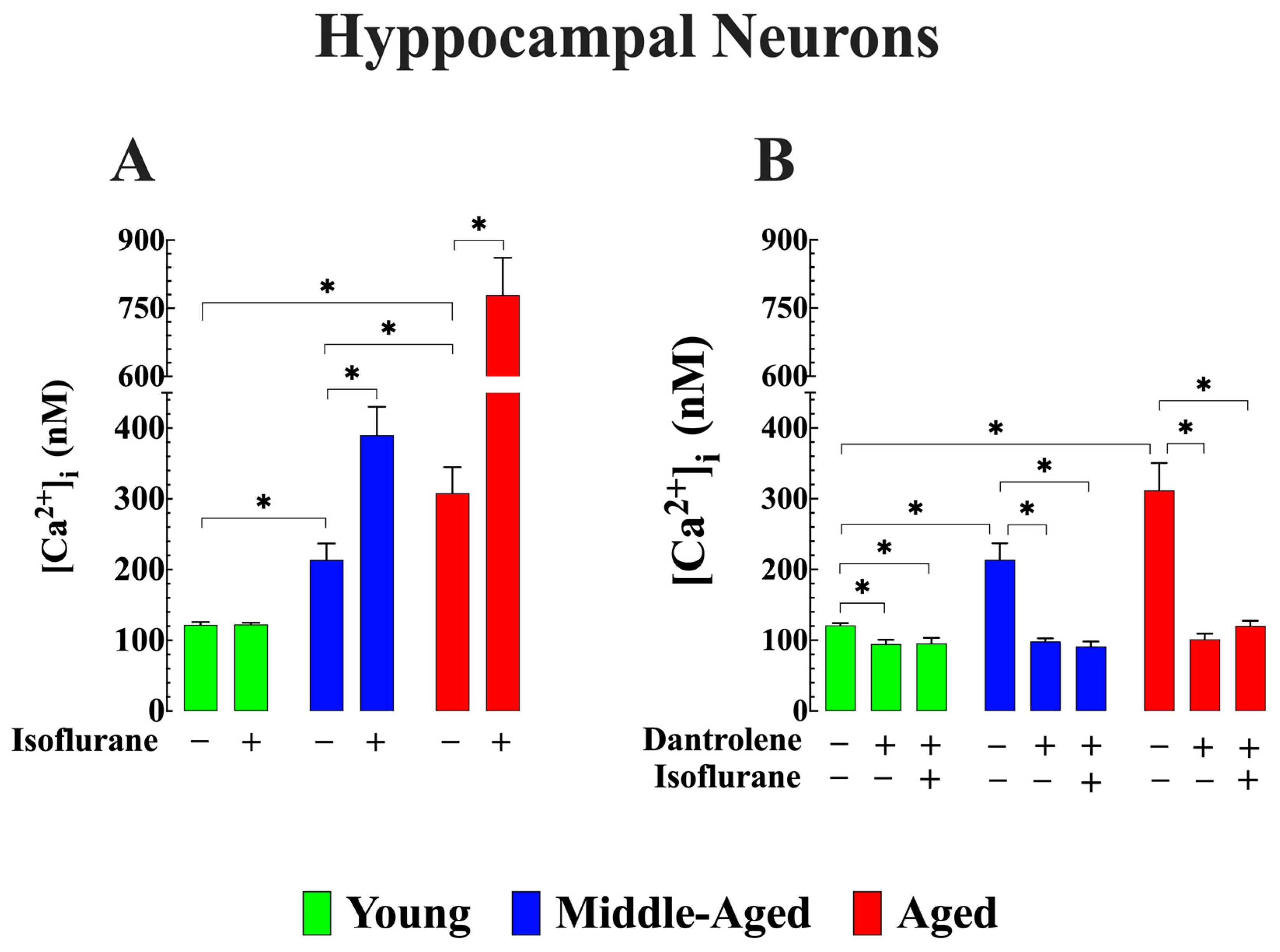
3.3. Cytoprotective Effects of Lowering [Ca2+]i on Isoflurane-Induced Elevation of [Ca2+]i in Aged Hippocampal Neurons (In Vitro)
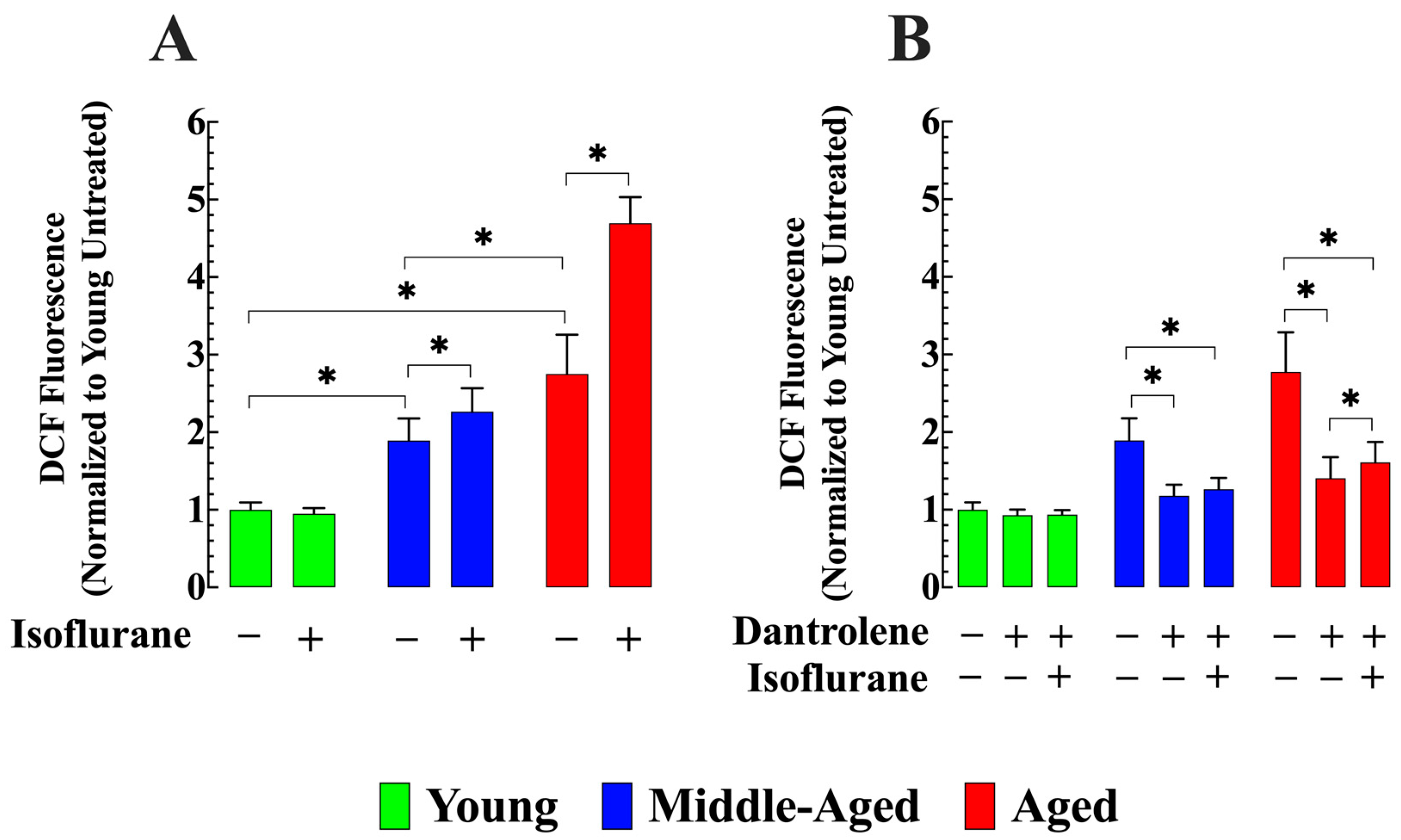
3.4. Dantrolene Reverses the Elevated Production of ROS and Prevents Its Increase by Isoflurane in Aged Neurons
3.5. Dantrolene Diminish Calpain Activity and Prevents Isoflurane-Induced Elevation in Older Neurons
3.6. Dantrolene Attenuates LDH Leak and Inhibits Isoflurane-Induced Elevation in Aging Neurons
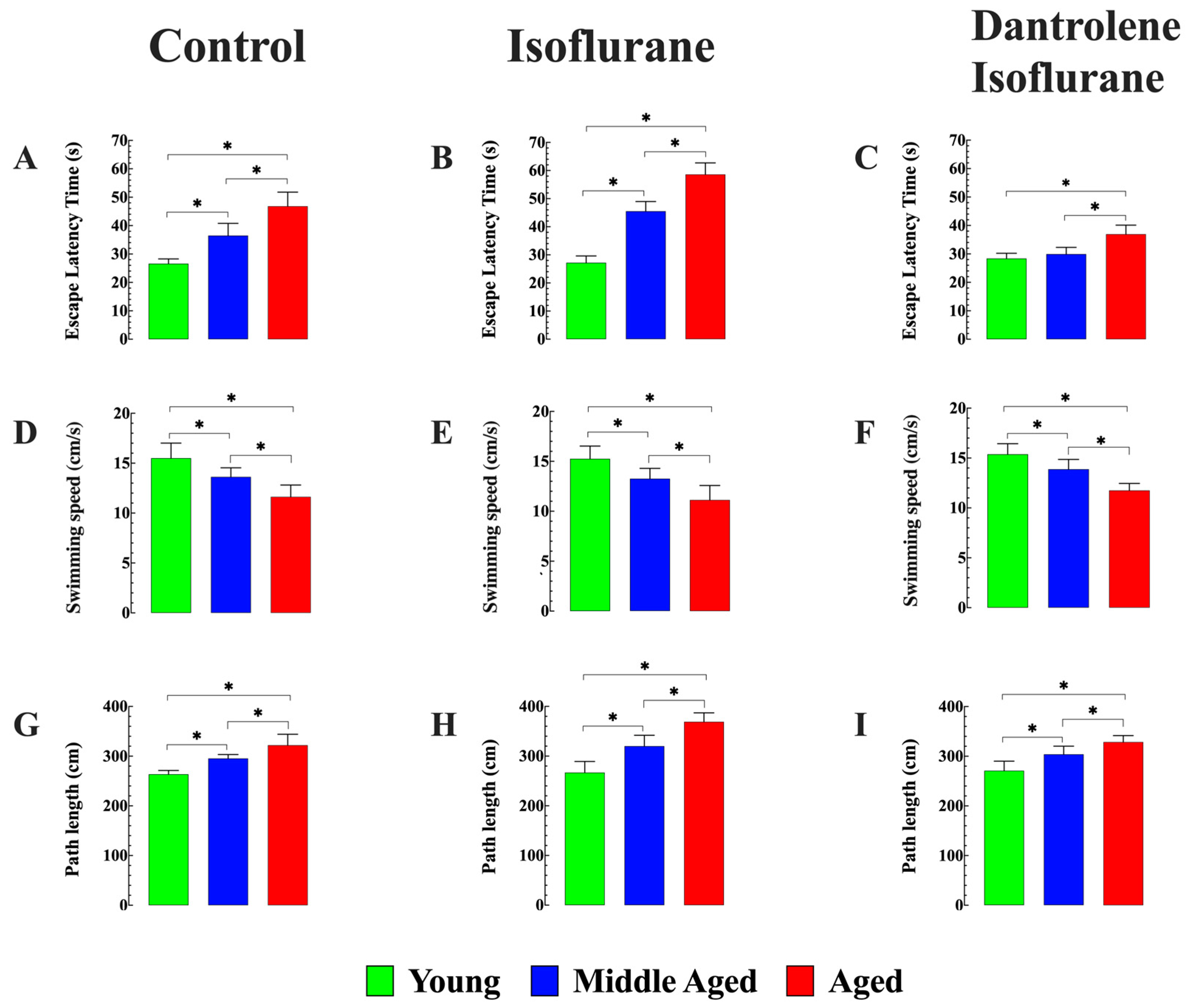
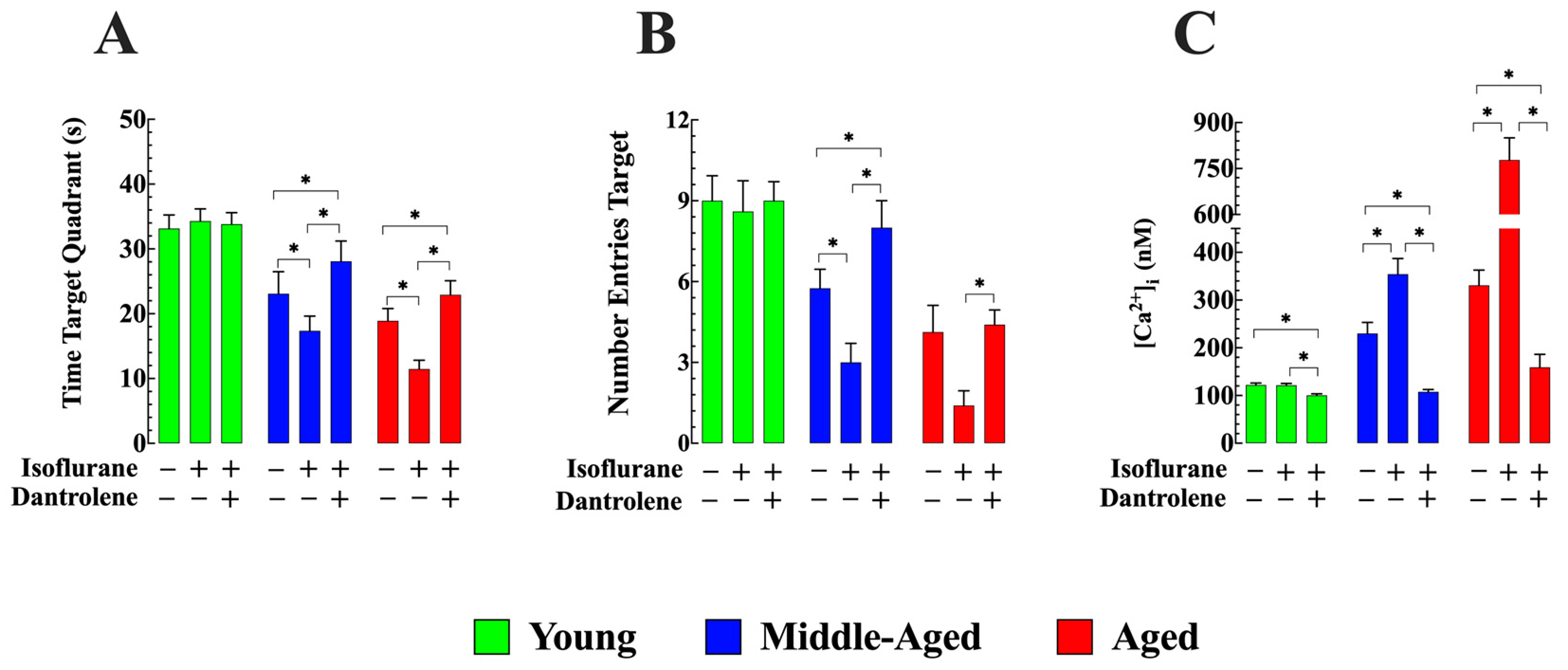
3.7. Dantrolene Enhances Cognitive Function and Prevents Isoflurane-Induced Further Decline in Aged Mice
4. Discussion
- It reaffirmed our previous discovery that middle-aged and aged WT C57BL6/J mice exhibit chronically elevated [Ca2+]i in cortical neurons in vivo and hippocampal pyramidal neurons in vitro compared to young mice [26].
- In vivo and in vitro exposure to isoflurane led to a significant increase in [Ca2+]i in middle-aged and aged cortical and hippocampal neurons but did not affect neuronal [Ca2+]i in young mice. However, dantrolene treatment reduced the basal level of [Ca2+]i across all age groups and prevented or reduced the elevation of [Ca2+]i associated with exposure to isoflurane in middle-aged and aged neurons.
- Middle-aged and aged hippocampal pyramidal neurons had higher ROS production, elevated calpain activity, and increased LDH leak compared to young neurons. These age-related changes were mitigated by dantrolene treatment. The administration of isoflurane further increased all three parameters over base levels, but pretreatment with dantrolene prevented or attenuated the effects of isoflurane.
- The elevated [Ca2+]i observed in middle-aged and aged animals was associated with age-related cognitive deficits evaluated using MWM. Isoflurane further aggravated cognitive deficits in aged mice. As seen with the increase of [Ca2+]i dantrolene pretreatment prevented or alleviated the isoflurane-induced performance decline. The ability of dantrolene to prevent isoflurane-induced performance decline was associated with reduced neuronal [Ca2+]i.
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bedford, P.D. Adverse cerebral effects of anaesthesia on old people. Lancet 1955, 269, 259–263. [Google Scholar] [CrossRef]
- Sauer, A.M.; Kalkman, C.; van Dijk, D. Postoperative cognitive decline. J. Anesth. 2009, 23, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Kotekar, N.; Shenkar, A.; Nagaraj, R. Postoperative cognitive dysfunction—Current preventive strategies. Clin. Interv. Aging 2018, 13, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, J.A.; Iqbal, Z.; Gandhi, S.D.; Patterson, K.M.; Hyde, T.F.; Reddy, D.M.; Hudetz, A.G.; Warltier, D.C. Postoperative cognitive dysfunction in older patients with a history of alcohol abuse. Anesthesiology 2007, 106, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Lin, C.C.; Chen, K.B.; Kuo, Y.C.; Li, C.Y.; Chung, C.J. Increased risk of dementia in people with previous exposure to general anesthesia: A nationwide population-based case-control study. Alzheimers Dement. 2014, 10, 196–204. [Google Scholar] [CrossRef]
- van den Berg, E.; Kloppenborg, R.P.; Kessels, R.P.; Kappelle, L.J.; Biessels, G.J. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochim. Biophys. Acta 2009, 1792, 470–481. [Google Scholar] [CrossRef]
- Lynch, M.A. Long-term potentiation and memory. Physiol. Rev. 2004, 84, 87–136. [Google Scholar] [CrossRef]
- Needham, M.J.; Webb, C.E.; Bryden, D.C. Postoperative cognitive dysfunction and dementia: What we need to know and do. Br. J. Anaesth. 2017, 119, i115–i125. [Google Scholar] [CrossRef]
- Belrose, J.C.; Noppens, R.R. Anesthesiology and cognitive impairment: A narrative review of current clinical literature. BMC Anesthesiol. 2019, 19, 241. [Google Scholar] [CrossRef]
- Jevtovic-Todorovic, V.; Hartman, R.E.; Izumi, Y.; Benshoff, N.D.; Dikranian, K.; Zorumski, C.F.; Olney, J.W.; Wozniak, D.F. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 2003, 23, 876–882. [Google Scholar] [CrossRef]
- Evered, L.A.; Silbert, B.S. Postoperative Cognitive Dysfunction and Noncardiac Surgery. Anesth. Analg. 2018, 127, 496–505. [Google Scholar] [CrossRef]
- Dilmen, O.K.; Meco, B.C.; Evered, L.A.; Radtke, F.M. Postoperative neurocognitive disorders: A clinical guide. J. Clin. Anesth. 2024, 92, 111320. [Google Scholar] [CrossRef]
- Mandal, P.K.; Schifilliti, D.; Mafrica, F.; Fodale, V. Inhaled anesthesia and cognitive performance. Drugs Today 2009, 45, 47–54. [Google Scholar] [CrossRef]
- Wise-Faberowski, L.; Zhang, H.; Ing, R.; Pearlstein, R.D.; Warner, D.S. Isoflurane-induced neuronal degeneration: An evaluation in organotypic hippocampal slice cultures. Anesth. Analg. 2005, 101, 651–657. [Google Scholar] [CrossRef]
- Xie, Z.; Dong, Y.; Maeda, U.; Alfille, P.; Culley, D.J.; Crosby, G.; Tanzi, R.E. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology 2006, 104, 988–994. [Google Scholar] [CrossRef]
- Zhang, G.; Dong, Y.; Zhang, B.; Ichinose, F.; Wu, X.; Culley, D.J.; Crosby, G.; Tanzi, R.E.; Xie, Z. Isoflurane-induced caspase-3 activation is dependent on cytosolic calcium and can be attenuated by memantine. J. Neurosci. 2008, 28, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Kvolik, S.; Glavas-Obrovac, L.; Bares, V.; Karner, I. Effects of inhalation anesthetics halothane, sevoflurane, and isoflurane on human cell lines. Life Sci. 2005, 77, 2369–2383. [Google Scholar] [CrossRef]
- Li, Z.; Ni, C.; Xia, C.; Jaw, J.; Wang, Y.; Cao, Y.; Xu, M.; Guo, X. Calcineurin/nuclear factor-kappaB signaling mediates isoflurane-induced hippocampal neuroinflammation and subsequent cognitive impairment in aged rats. Mol. Med. Rep. 2017, 15, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lu, Y.; Dong, Y.; Zhang, G.; Zhang, Y.; Xu, Z.; Culley, D.J.; Crosby, G.; Marcantonio, E.R.; Tanzi, R.E.; et al. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-alpha, IL-6, and IL-1beta. Neurobiol. Aging 2012, 33, 1364–1378. [Google Scholar] [CrossRef]
- Narahashi, T.; Aistrup, G.L.; Lindstrom, J.M.; Marszalec, W.; Nagata, K.; Wang, F.; Yeh, J.Z. Ion channel modulation as the basis for general anesthesia. Toxicol. Lett. 1998, 100–101, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Liang, G.; Yang, H.; Wang, Q.; Hawkins, B.; Madesh, M.; Wang, S.; Eckenhoff, R.G. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology 2008, 108, 251–260. [Google Scholar] [CrossRef]
- Wei, H.; Xie, Z. Anesthesia, calcium homeostasis and Alzheimer’s disease. Curr. Alzheimer Res. 2009, 6, 30–35. [Google Scholar] [CrossRef]
- Yang, H.; Liang, G.; Hawkins, B.J.; Madesh, M.; Pierwola, A.; Wei, H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology 2008, 109, 243–250. [Google Scholar] [CrossRef]
- Xu, Z.; Qian, B. Sevoflurane anesthesia-mediated oxidative stress and cognitive impairment in hippocampal neurons of old rats can be ameliorated by expression of brain derived neurotrophic factor. Neurosci. Lett. 2020, 721, 134785. [Google Scholar] [CrossRef]
- Ge, H.W.; Hu, W.W.; Ma, L.L.; Kong, F.J. Endoplasmic reticulum stress pathway mediates isoflurane-induced neuroapoptosis and cognitive impairments in aged rats. Physiol. Behav. 2015, 151, 16–23. [Google Scholar] [CrossRef]
- Uryash, A.; Flores, V.; Adams, J.A.; Allen, P.D.; Lopez, J.R. Memory and Learning Deficits Are Associated with Ca2+ Dyshomeostasis in Normal Aging. Front. Aging Neurosci. 2020, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Eltit, J.M.; Ding, X.; Pessah, I.N.; Allen, P.D.; Lopez, J.R. Nonspecific sarcolemmal cation channels are critical for the pathogenesis of malignant hyperthermia. FASEB J. 2013, 27, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.R.; Alamo, L.; Caputo, C.; DiPolo, R.; Vergara, S. Determination of ionic calcium in frog skeletal muscle fibers. Biophys. J. 1983, 43, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Leon, J.; Sakumi, K.; Castillo, E.; Sheng, Z.; Oka, S.; Nakabeppu, Y. 8-Oxoguanine accumulation in mitochondrial DNA causes mitochondrial dysfunction and impairs neuritogenesis in cultured adult mouse cortical neurons under oxidative conditions. Sci. Rep. 2016, 6, 22086. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.; Yang, Y.S.; Rah, J.C.; Choi, J.H. Different resting membrane potentials in posterior parietal cortex and prefrontal cortex in the view of recurrent synaptic strengths and neural network dynamics. Front. Cell Neurosci. 2023, 17, 1153970. [Google Scholar] [CrossRef]
- Hu, W.; Bean, B.P. Differential Control of Axonal and Somatic Resting Potential by Voltage-Dependent Conductances in Cortical Layer 5 Pyramidal Neurons. Neuron 2018, 97, 1315–1326 e1313. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Jennings, J. Response of astrocytes to gamma-aminobutyric acid in the neonatal rat optic nerve. Neurosci. Lett. 1994, 168, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xu, G.; Wang, W.; Enyeart, J.J.; Zhou, M. Dual patch voltage clamp study of low membrane resistance astrocytes in situ. Mol. Brain 2014, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Robin, G.; Lopez, J.R.; Espinal, G.M.; Hulsizer, S.; Hagerman, P.J.; Pessah, I.N. Calcium dysregulation and Cdk5-ATM pathway involved in a mouse model of fragile X-associated tremor/ataxia syndrome. Hum. Mol. Genet. 2017, 26, 2649–2666. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.R.; Uryash, A.; Kolster, J.; Esteve, E.; Zhang, R.; Adams, J.A. Enhancing Endogenous Nitric Oxide by Whole Body Periodic Acceleration Elicits Neuroprotective Effects in Dystrophic Neurons. Mol. Neurobiol. 2018, 55, 8680–8694. [Google Scholar] [CrossRef] [PubMed]
- Ubezio, P.; Civoli, F. Flow cytometric detection of hydrogen peroxide production induced by doxorubicin in cancer cells. Free Radic. Biol. Med. 1994, 16, 509–516. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, Y.C.; Chin, Y.T.; Wang, C.C.; Hwang, L.L.; Yang, L.Y.; Lu, D.Y. 2, 3, 5, 4′-tetrahydroxystilbene-2-O-beta-D-glucoside protects against neuronal cell death and traumatic brain injury-induced pathophysiology. Aging 2022, 14, 2607–2627. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Culley, D.J.; Baxter, M.; Yukhananov, R.; Crosby, G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth. Analg. 2003, 96, 1004–1009. [Google Scholar] [CrossRef]
- Culley, D.J.; Baxter, M.G.; Crosby, C.A.; Yukhananov, R.; Crosby, G. Impaired acquisition of spatial memory 2 weeks after isoflurane and isoflurane-nitrous oxide anesthesia in aged rats. Anesth. Analg. 2004, 99, 1393–1397. [Google Scholar] [CrossRef]
- Shaughnessy, M.R.; Hofmeister, E.H. A systematic review of sevoflurane and isoflurane minimum alveolar concentration in domestic cats. Vet. Anaesth. Analg. 2014, 41, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.R.; Allen, P.; Alamo, L.; Ryan, J.F.; Jones, D.E.; Sreter, F. Dantrolene prevents the malignant hyperthermic syndrome by reducing free intracellular calcium concentration in skeletal muscle of susceptible swine. Cell Calcium 1987, 8, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.R.; Medina, P.; Alamo, L. Dantrolene sodium is able to reduce the resting ionic [Ca2+]i in muscle from humans with malignant hyperthermia. Muscle Nerve 1987, 10, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, S.; Zhu, T.; Ou, M.; Zhang, D.; Sun, H.; Liu, J.; Chen, X.; Hemmings, H.C.; Zhou, C. Isoflurane Suppresses Hippocampal High-frequency Ripples by Differentially Modulating Pyramidal Neurons and Interneurons in Mice. Anesthesiology 2021, 135, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Follmann, M.; Hessler, G.; Kleemann, H.W.; Hachtel, S.; Fuchs, B.; Weissmann, N.; Linz, W.; Schmidt, T.; Lohn, M.; et al. Discovery and pharmacological characterization of a novel potent inhibitor of diacylglycerol-sensitive TRPC cation channels. Br. J. Pharmacol. 2015, 172, 3650–3660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Z.; Wang, H.; Dong, Y.; Shi, H.N.; Culley, D.J.; Crosby, G.; Marcantonio, E.R.; Tanzi, R.E.; Xie, Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann. Neurol. 2012, 71, 687–698. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, S.C.; Lei, M.; Wang, Z.; Xiong, K. Regulatory role of calpain in neuronal death. Neural Regen. Res. 2018, 13, 556–562. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Zhu, Y. MiR-342-5p protects neurons from cerebral ischemia induced-apoptosis through regulation of Akt/NF-kappaB pathways by targeting CCAR2. J. Stroke Cerebrovasc. Dis. 2023, 32, 106901. [Google Scholar] [CrossRef]
- de Fiebre, N.C.; Sumien, N.; Forster, M.J.; de Fiebre, C.M. Spatial learning and psychomotor performance of C57BL/6 mice: Age sensitivity and reliability of individual differences. Age 2006, 28, 235–253. [Google Scholar] [CrossRef]
- Xie, Z.; Tanzi, R.E. Alzheimer’s disease and post-operative cognitive dysfunction. Exp. Gerontol. 2006, 41, 346–359. [Google Scholar] [CrossRef]
- Wang, C.M.; Chen, W.C.; Zhang, Y.; Lin, S.; He, H.F. Update on the Mechanism and Treatment of Sevoflurane-Induced Postoperative Cognitive Dysfunction. Front. Aging Neurosci. 2021, 13, 702231. [Google Scholar] [CrossRef]
- Brini, M.; Cali, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef]
- Metwally, E.; Zhao, G.; Zhang, Y.Q. The calcium-dependent protease calpain in neuronal remodeling and neurodegeneration. Trends Neurosci. 2021, 44, 741–752. [Google Scholar] [CrossRef]
- Bano, D.; Nicotera, P. Ca2+ signals and neuronal death in brain ischemia. Stroke 2007, 38, 674–676. [Google Scholar] [CrossRef]
- Biessels, G.; Gispen, W.H. The calcium hypothesis of brain aging and neurodegenerative disorders: Significance in diabetic neuropathy. Life Sci. 1996, 59, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.R.; Lyckman, A.; Oddo, S.; Laferla, F.M.; Querfurth, H.W.; Shtifman, A. Increased intraneuronal resting [Ca2+] in adult Alzheimer’s disease mice. J. Neurochem. 2008, 105, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.G.; Chapman, D.C. Dantrolene sodium in the treatment of malignant hypertension. S. Afr. Med. J. 1982, 62, 503–504. [Google Scholar] [PubMed]
- Lopez, J.R.; Gerardi, A.; Lopez, M.J.; Allen, P.D. Effects of dantrolene on myoplasmic free [Ca2+] measured in vivo in patients susceptible to malignant hyperthermia. Anesthesiology 1992, 76, 711–719. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yano, M.; Suetomi, T.; Ono, M.; Tateishi, H.; Mochizuki, M.; Xu, X.; Uchinoumi, H.; Okuda, S.; Yamamoto, T.; et al. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J. Am. Coll. Cardiol. 2009, 53, 1993–2005. [Google Scholar] [CrossRef]
- Helland, L.A.; Lopez, J.R.; Taylor, S.R.; Trube, G.; Wanek, L.A. Effects of calcium “antagonists” on vertebrate skeletal muscle cells. Ann. N. Y. Acad. Sci. 1988, 522, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Cherednichenko, G.; Ward, C.W.; Feng, W.; Cabrales, E.; Michaelson, L.; Samso, M.; Lopez, J.R.; Allen, P.D.; Pessah, I.N. Enhanced excitation-coupled calcium entry in myotubes expressing malignant hyperthermia mutation R163C is attenuated by dantrolene. Mol. Pharmacol. 2008, 73, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, A.; Schousboe, A. Dantrolene prevents glutamate cytotoxicity and Ca2+ release from intracellular stores in cultured cerebral cortical neurons. J. Neurochem. 1991, 56, 1075–1078. [Google Scholar] [CrossRef]
- Hayashi, T.; Kagaya, A.; Motohashi, N.; Yamawaki, S. Possible mechanism of dantrolene stabilization of cultured neuroblastoma cell plasma membranes. J. Neurochem. 1994, 63, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, J.; Lvovskaya, S.; Herndon, E.; Supnet, C.; Bezprozvanny, I. Dantrolene is neuroprotective in Huntington’s disease transgenic mouse model. Mol. Neurodegener. 2011, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Liou, B.; Peng, Y.; Li, R.; Inskeep, V.; Zhang, W.; Quinn, B.; Dasgupta, N.; Blackwood, R.; Setchell, K.D.; Fleming, S.; et al. Modulating ryanodine receptors with dantrolene attenuates neuronopathic phenotype in Gaucher disease mice. Hum. Mol. Genet. 2016, 25, 5126–5141. [Google Scholar] [CrossRef] [PubMed]
- Muehlschlegel, S.; Sims, J.R. Dantrolene: Mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit Care 2009, 10, 103–115. [Google Scholar] [CrossRef]
- Wuis, E.W.; Rijntjes, N.V.; Van der Kleijn, E. Whole-body autoradiography of 14C-dantrolene in the marmoset monkey. Pharmacol. Toxicol. 1989, 64, 156–158. [Google Scholar] [CrossRef]
- Enokizono, J.; Kusuhara, H.; Ose, A.; Schinkel, A.H.; Sugiyama, Y. Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug Metab. Dispos. 2008, 36, 995–1002. [Google Scholar] [CrossRef]
- Patti, F.; Maccagnano, C.; Panico, A.M.; Giammona, G.; Rampello, L.; Reggio, A.; Di Giorgio, R.M.; Nicoletti, F. Effects of dantrolene sodium on gabaergic activity in spinal cord, corpus striatum, substantia nigra and cerebral cortex in rat. Acta Neurol. 1981, 3, 384–388. [Google Scholar]
- Wei, H.; Perry, D.C. Dantrolene is cytoprotective in two models of neuronal cell death. J. Neurochem. 1996, 67, 2390–2398. [Google Scholar] [CrossRef] [PubMed]
- Oules, B.; Del Prete, D.; Greco, B.; Zhang, X.; Lauritzen, I.; Sevalle, J.; Moreno, S.; Paterlini-Brechot, P.; Trebak, M.; Checler, F.; et al. Ryanodine receptor blockade reduces amyloid-beta load and memory impairments in Tg2576 mouse model of Alzheimer disease. J. Neurosci. 2012, 32, 11820–11834. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Y.; Yu, S.; Wang, Y.; Meng, Q.; Liang, G.; Eckenhoff, M.F.; Wei, H. Intranasal administration of dantrolene increased brain concentration and duration. PLoS ONE 2020, 15, e0229156. [Google Scholar] [CrossRef] [PubMed]
- Utili, R.; Boitnott, J.K.; Zimmerman, H.J. Dantrolene-associated hepatic injury. Incidence and character. Gastroenterology 1977, 72, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Tonnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Peng, T.I.; Jou, M.J. Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wei, C.L.; Zhang, W.R.; Cheng, H.P.; Liu, J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol. Sin. 2006, 27, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Mijares, A.; Allen, P.D.; Lopez, J.R. Senescence Is Associated With Elevated Intracellular Resting [Ca2+] in Mice Skeletal Muscle Fibers. An in vivo Study. Front. Physiol. 2020, 11, 601189. [Google Scholar] [CrossRef]
- Weber, J.J.; Anger, S.C.; Pereira Sena, P.; Incebacak Eltemur, R.D.; Huridou, C.; Fath, F.; Gross, C.; Casadei, N.; Riess, O.; Nguyen, H.P. Calpains as novel players in the molecular pathogenesis of spinocerebellar ataxia type 17. Cell Mol. Life Sci. 2022, 79, 262. [Google Scholar] [CrossRef]
- Nixon, R.A. The calpains in aging and aging-related diseases. Ageing Res. Rev. 2003, 2, 407–418. [Google Scholar] [CrossRef]
- Qiu, L.L.; Pan, W.; Luo, D.; Zhang, G.F.; Zhou, Z.Q.; Sun, X.Y.; Yang, J.J.; Ji, M.H. Dysregulation of BDNF/TrkB signaling mediated by NMDAR/Ca2+/calpain might contribute to postoperative cognitive dysfunction in aging mice. J. Neuroinflamm. 2020, 17, 23. [Google Scholar] [CrossRef]
- Fischer, D.R.; Sun, X.; Williams, A.B.; Gang, G.; Pritts, T.A.; James, J.H.; Molloy, M.; Fischer, J.E.; Paul, R.J.; Hasselgren, P.O. Dantrolene reduces serum TNFalpha and corticosterone levels and muscle calcium, calpain gene expression, and protein breakdown in septic rats. Shock 2001, 15, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Muramatsu, R.; Maedera, N.; Tsunematsu, H.; Hamaguchi, M.; Koyama, Y.; Kuroda, M.; Ono, K.; Sawada, M.; Yamashita, T. Extracellular Lactate Dehydrogenase A Release from Damaged Neurons Drives Central Nervous System Angiogenesis. EBioMedicine 2018, 27, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Liang, G.; Yang, H. Isoflurane preconditioning inhibited isoflurane-induced neurotoxicity. Neurosci. Lett. 2007, 425, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liang, G.; Chen, Q.; Joseph, D.J.; Meng, Q.; Eckenhoff, R.G.; Eckenhoff, M.F.; Wei, H. Anesthetic-induced neurodegeneration mediated via inositol 1,4,5-trisphosphate receptors. J. Pharmacol. Exp. Ther. 2010, 333, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, M.; Zhen, Y.; Yue, Y.; Sherman, J.; Zheng, H.; Li, S.; Tanzi, R.E.; Marcantonio, E.R.; Xie, Z. The effects of isoflurane and desflurane on cognitive function in humans. Anesth. Analg. 2012, 114, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zuo, Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology 2011, 61, 1354–1359. [Google Scholar] [CrossRef]
- Zuo, C.L.; Wang, C.M.; Liu, J.; Shen, T.; Zhou, J.P.; Hao, X.R.; Pan, Y.Z.; Liu, H.C.; Lian, Q.Q.; Lin, H. Isoflurane anesthesia in aged mice and effects of A1 adenosine receptors on cognitive impairment. CNS Neurosci. Ther. 2018, 24, 212–221. [Google Scholar] [CrossRef]
- Liang, L.; Wei, H. Dantrolene, a treatment for Alzheimer disease? Alzheimer Dis. Assoc. Disord. 2015, 29, 1–5. [Google Scholar] [CrossRef]
- Harrison, F.E.; Hosseini, A.H.; McDonald, M.P. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav. Brain Res. 2009, 198, 247–251. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uryash, A.; Mijares, A.; Lopez, C.E.; Adams, J.A.; Allen, P.D.; Lopez, J.R. Post-Anesthesia Cognitive Dysfunction in Mice Is Associated with an Age-Related Increase in Neuronal Intracellular [Ca2+]—Neuroprotective Effect of Reducing Intracellular [Ca2+]: In Vivo and In Vitro Studies. Cells 2024, 13, 264. https://doi.org/10.3390/cells13030264
Uryash A, Mijares A, Lopez CE, Adams JA, Allen PD, Lopez JR. Post-Anesthesia Cognitive Dysfunction in Mice Is Associated with an Age-Related Increase in Neuronal Intracellular [Ca2+]—Neuroprotective Effect of Reducing Intracellular [Ca2+]: In Vivo and In Vitro Studies. Cells. 2024; 13(3):264. https://doi.org/10.3390/cells13030264
Chicago/Turabian StyleUryash, Arkady, Alfredo Mijares, Carlos E. Lopez, Jose A. Adams, Paul D. Allen, and Jose R. Lopez. 2024. "Post-Anesthesia Cognitive Dysfunction in Mice Is Associated with an Age-Related Increase in Neuronal Intracellular [Ca2+]—Neuroprotective Effect of Reducing Intracellular [Ca2+]: In Vivo and In Vitro Studies" Cells 13, no. 3: 264. https://doi.org/10.3390/cells13030264
APA StyleUryash, A., Mijares, A., Lopez, C. E., Adams, J. A., Allen, P. D., & Lopez, J. R. (2024). Post-Anesthesia Cognitive Dysfunction in Mice Is Associated with an Age-Related Increase in Neuronal Intracellular [Ca2+]—Neuroprotective Effect of Reducing Intracellular [Ca2+]: In Vivo and In Vitro Studies. Cells, 13(3), 264. https://doi.org/10.3390/cells13030264







