Deep-Learning-Based Analysis Reveals a Social Behavior Deficit in Mice Exposed Prenatally to Nicotine
Abstract
:1. Introduction
2. Materials and Methods
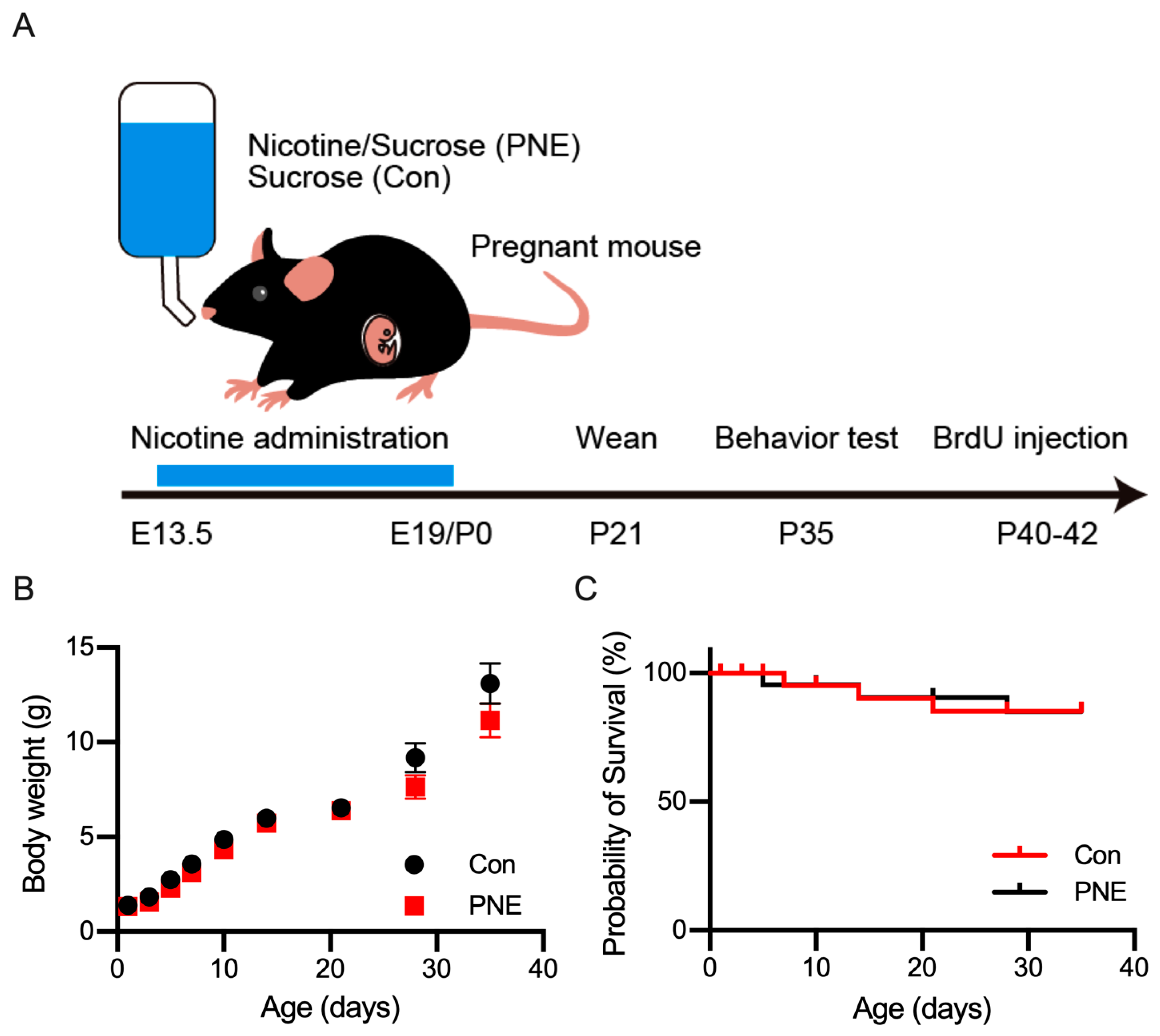
2.1. Animal Care and Administration of Nicotine to Pregnant Mice
2.2. Animal Behavioral Tests
2.2.1. Habituation
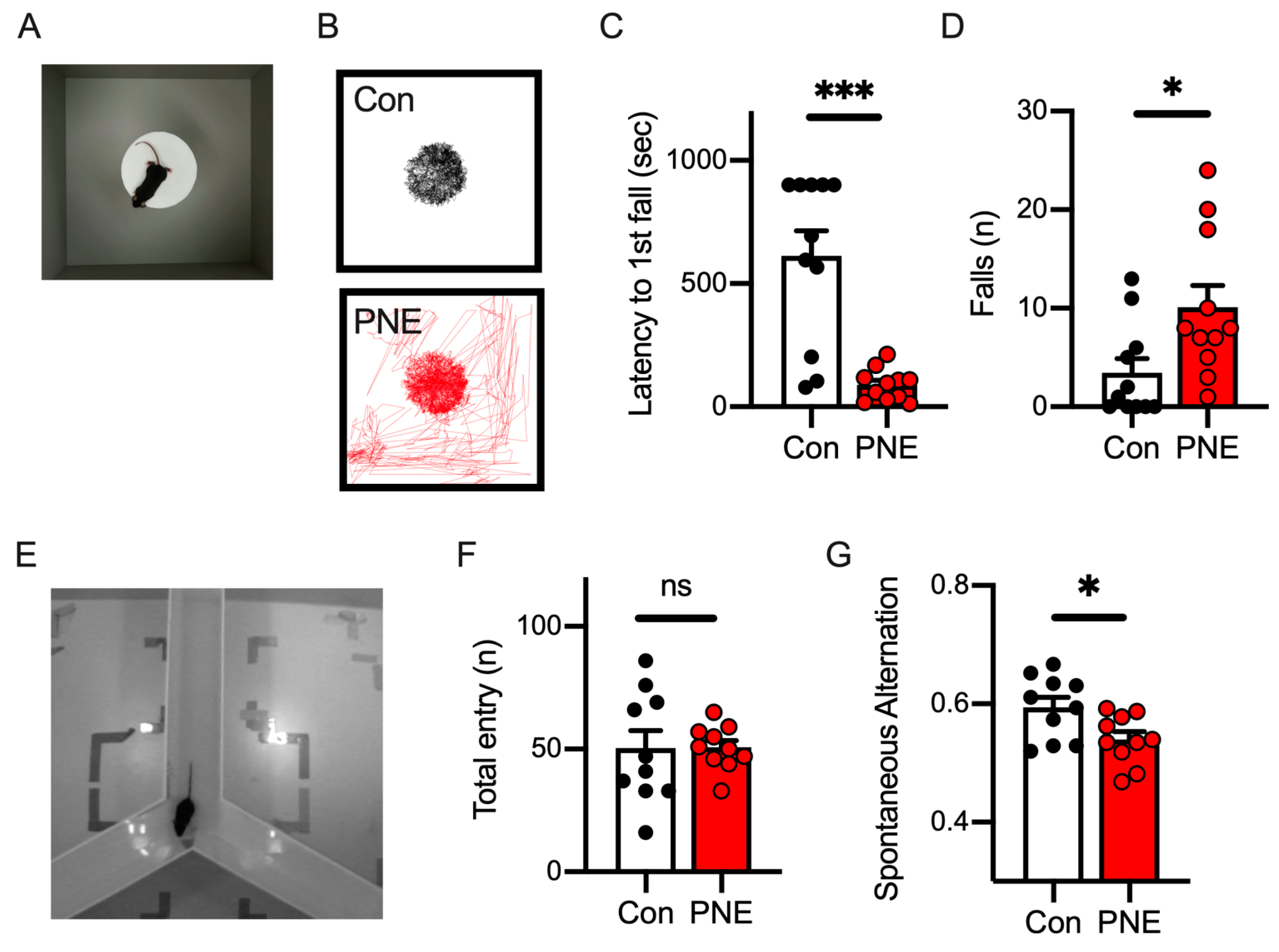
2.2.2. Cliff Avoidance Reaction (CAR) Test
2.2.3. Y-Maze Test
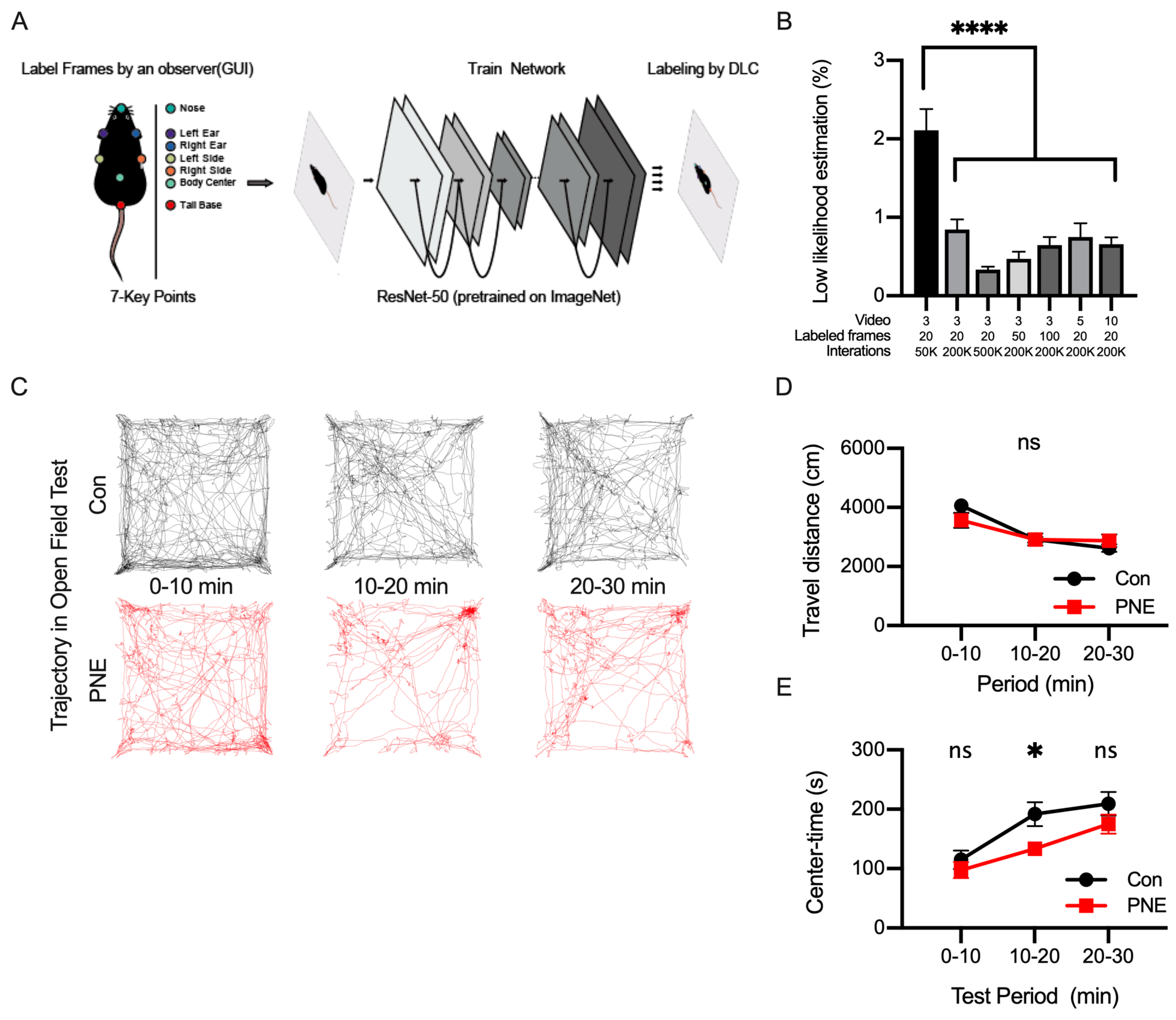
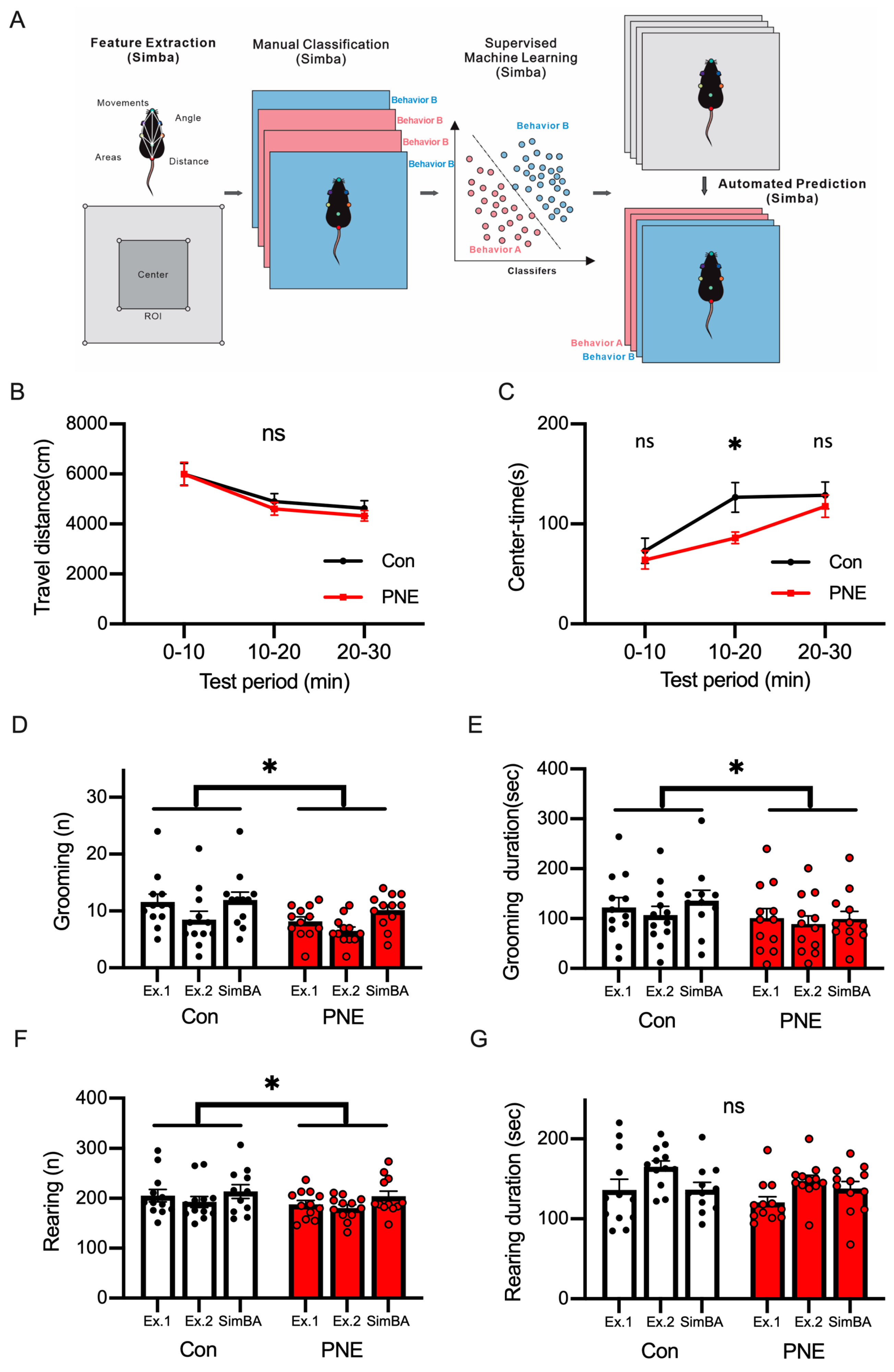
2.2.4. Open-Field Test
2.2.5. Juvenile Interaction Test
2.3. Network Construction and Evaluation for Behavioral Analysis Using DeepLabCut
2.4. Supervised Behavioral Classification Using SimBA
2.5. BrdU Injection and Histological Analysis
2.6. Manual Analysis of Behaviors and Statistical Analyses
3. Results
3.1. Prenatal Nicotine Exposure Mice Exhibited Impulsive Behavior and a Deficit in Working Memory
3.2. Pose Estimation of a Single Mouse in the Open-Field Test by Using DeepLabCut
3.3. Deep-Learning-Based Classification of Behaviors in a Single Animal by Using SimBA
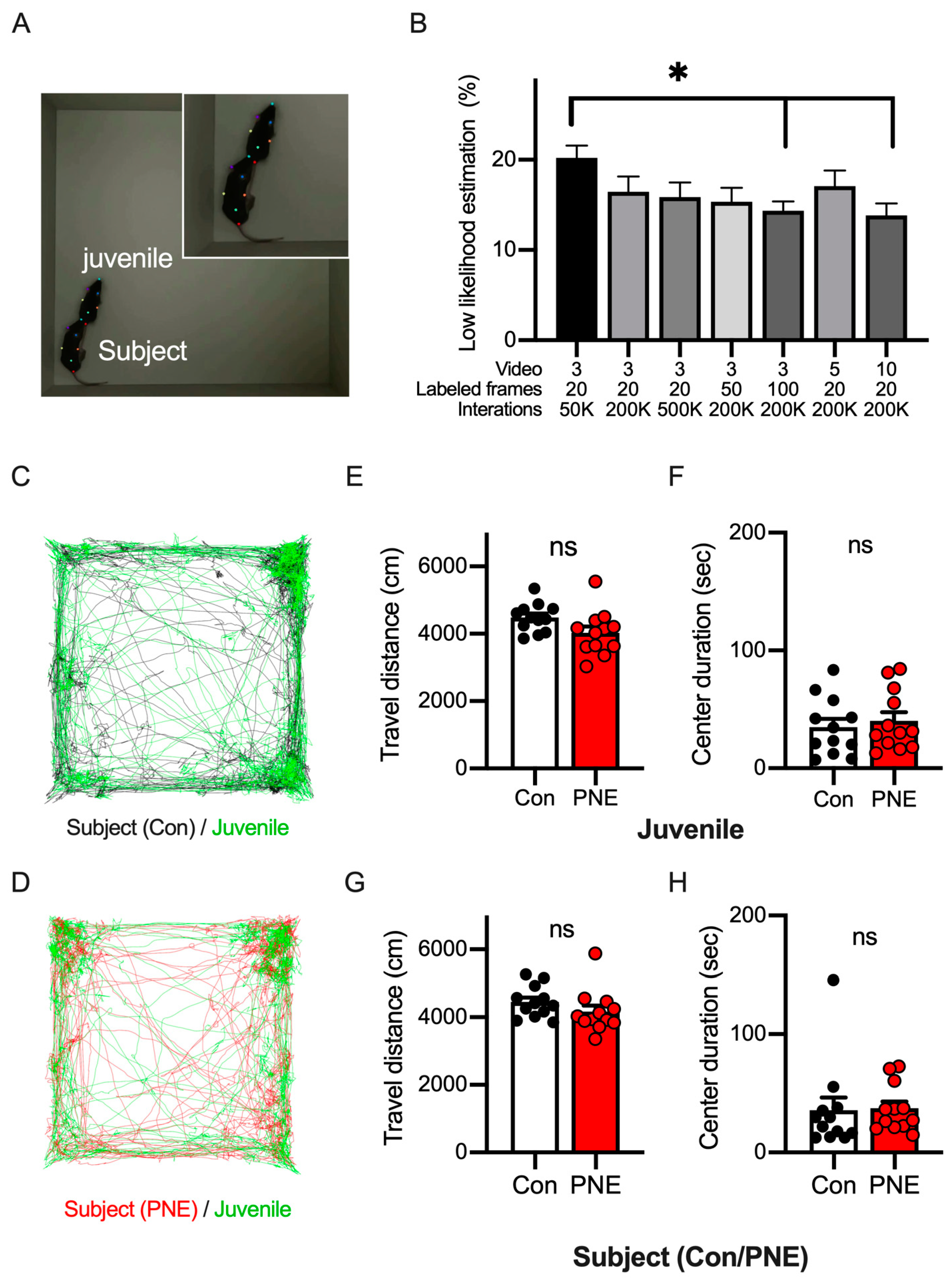
3.4. Pose Estimation of Two C57BL/6J Mice by Using DeepLabCut Requires Training Data from Diverse Videos and Frames
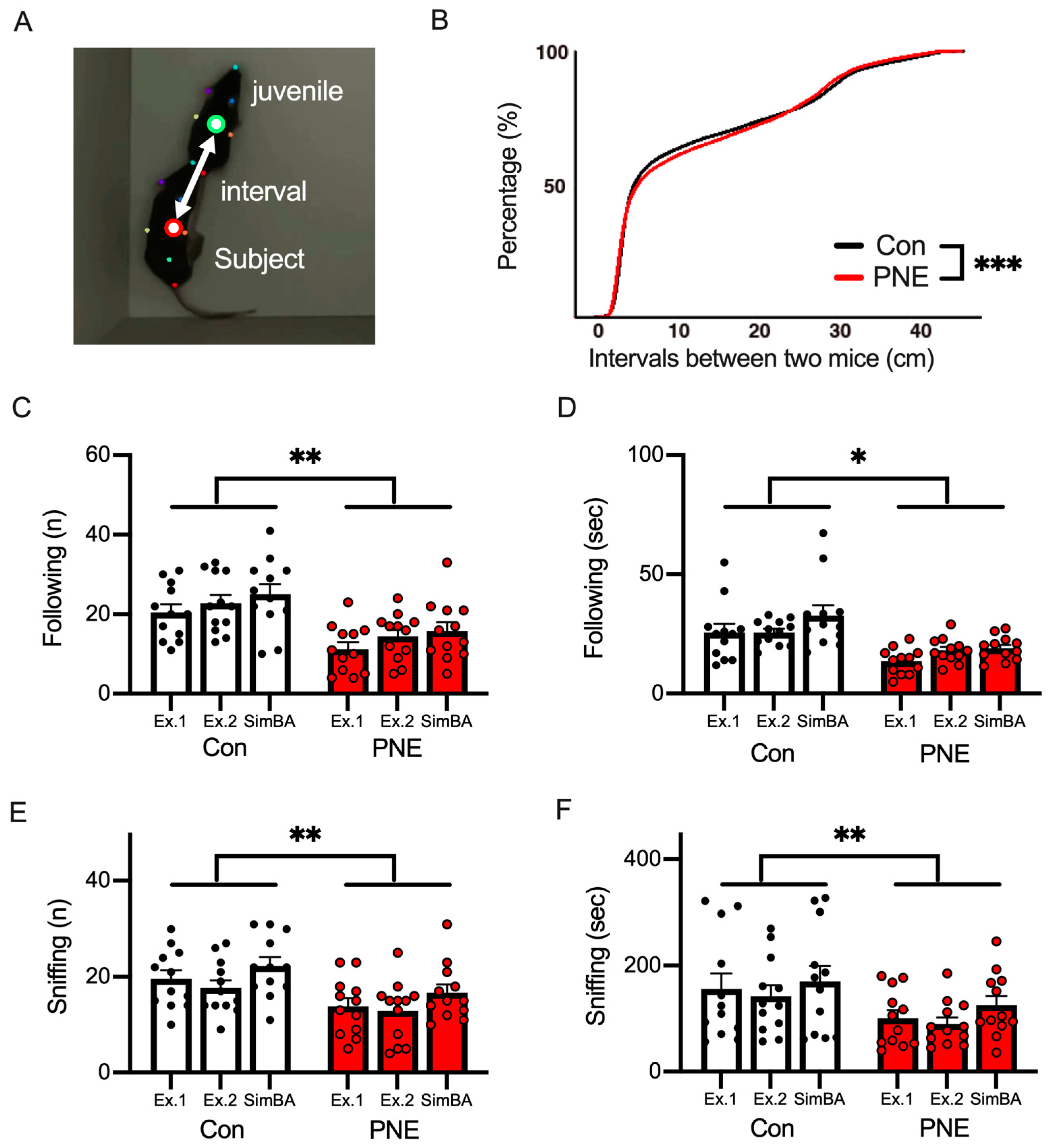
3.5. Social Behavior Was Altered by Prenatal Exposure to Nicotine in Mice
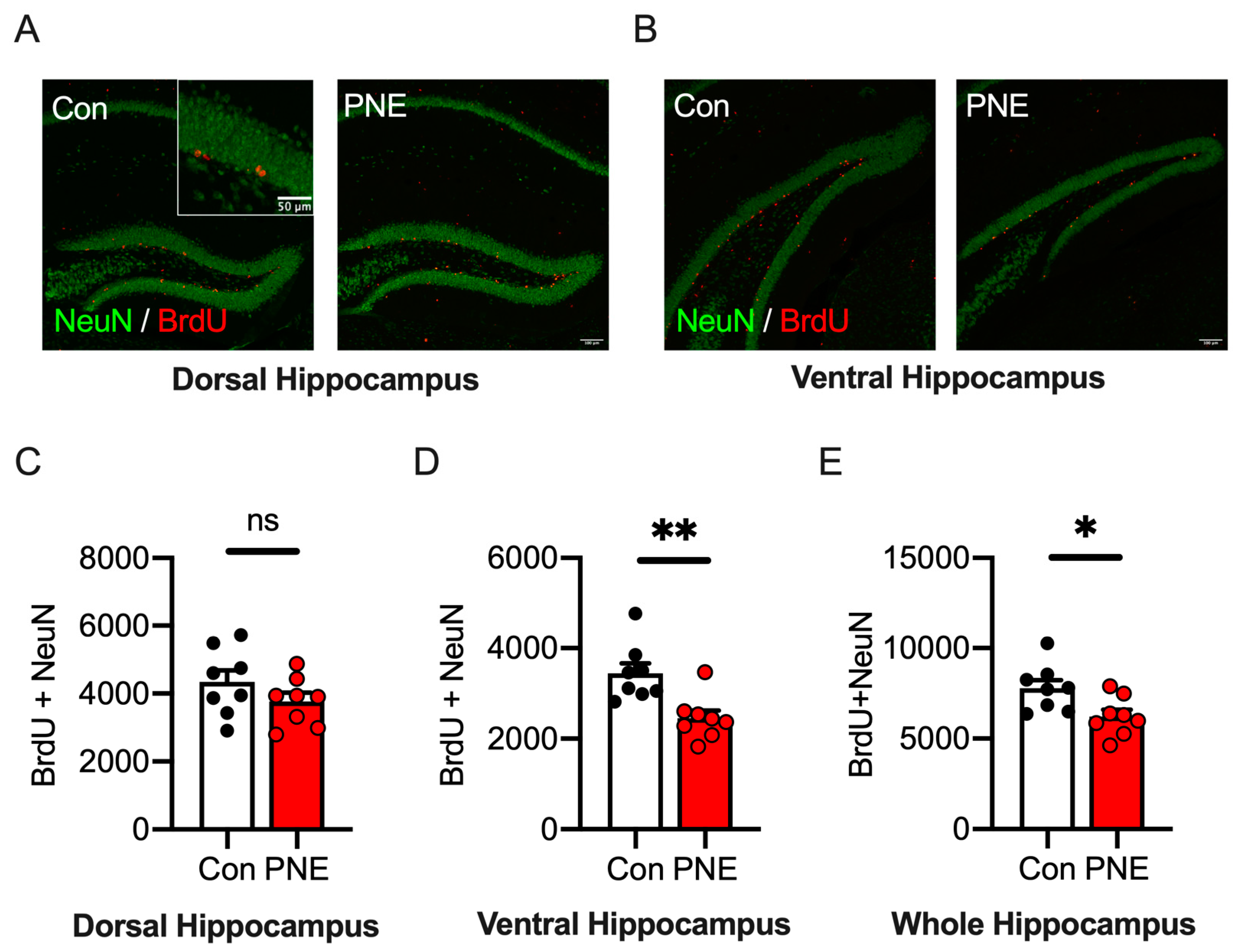
3.6. Adult Hippocampal Neurogenesis in the Ventral Area of the Hippocampus Is Decreased in PNE Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Office of the Surgeon General (US); Office on Smoking and Health (US). The Health Consequences of Smoking: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2004.
- Wickström, R. Effects of nicotine during pregnancy: Human and experimental evidence. Curr. Neuropharmacol. 2007, 5, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.; Stein, Z.A.; Susser, M.; Warburton, D. Smoking: A risk factor for spontaneous abortion. N. Engl. J. Med. 1977, 297, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Haglund, B.; Cnattingius, S. Cigarette smoking as a risk factor for sudden infant death syndrome: A population-based study. Am. J. Public Health 1990, 80, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.C.; Carvalheira, A.P.P.; Ferrari, A.P.; Malta, M.B.; de Barros Leite Carvalhaes, M.A.; de Lima Parada, C.M.G. Smoking during pregnancy and harm reduction in birth weight: A cross-sectional study. BMC Pregnancy Childbirth 2018, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Milberger, S.; Biederman, J.; Faraone, S.V.; Chen, L.; Jones, J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am. J. Psychiatry 1996, 153, 1138–1142. [Google Scholar] [CrossRef]
- Langley, K.; Heron, J.; Smith, G.D.; Thapar, A. Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: Testing for intrauterine effects. Am. J. Epidemiol. 2012, 176, 261–268. [Google Scholar] [CrossRef]
- Zhu, J.L.; Olsen, J.; Liew, Z.; Li, J.; Niclasen, J.; Obel, C. Parental smoking during pregnancy and ADHD in children: The Danish national birth cohort. Pediatrics 2014, 134, e382–e388. [Google Scholar] [CrossRef]
- Gustavson, K.; Ystrom, E.; Stoltenberg, C.; Susser, E.; Surén, P.; Magnus, P.; Knudsen, G.P.; Smith, G.D.; Langley, K.; Rutter, M.; et al. Smoking in Pregnancy and Child ADHD. Pediatrics 2017, 139, e20162509. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, A.M.; McKee, S.A.; Picciotto, M.R. Maternal smoking and autism spectrum disorder: Meta-analysis with population smoking metrics as moderators. Sci. Rep. 2017, 7, 4315. [Google Scholar] [CrossRef]
- Caramaschi, D.; Taylor, A.E.; Richmond, R.C.; Havdahl, K.A.; Golding, J.; Relton, C.L.; Munafò, M.R.; Davey Smith, G.; Rai, D. Maternal smoking during pregnancy and autism: Using causal inference methods in a birth cohort study. Transl. Psychiatry 2018, 8, 262. [Google Scholar] [CrossRef]
- von Ehrenstein, O.S.; Cui, X.; Yan, Q.; Aralis, H.; Ritz, B. Maternal Prenatal Smoking and Autism Spectrum Disorder in Offspring: A California Statewide Cohort and Sibling Study. Am. J. Epidemiol. 2021, 190, 728–737. [Google Scholar] [CrossRef]
- Lin, I.H.; Yang, L.; Dalley, J.W.; Tsai, T.H. Trans-placental transfer of nicotine: Modulation by organic cation transporters. Biomed. Pharmacother. 2022, 145, 112489. [Google Scholar] [CrossRef] [PubMed]
- Hellström-Lindahl, E.; Nordberg, A. Smoking during pregnancy: A way to transfer the addiction to the next generation? Respiration 2002, 69, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Moretti, M.; Gaimarri, A.; Zanardi, A.; Clementi, F.; Zoli, M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem. Pharmacol. 2007, 74, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Pistillo, F.; Gotti, C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 2015, 96, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Ajarem, J.S.; Ahmad, M. Prenatal nicotine exposure modifies behavior of mice through early development. Pharmacol. Biochem. Behav. 1998, 59, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Pauly, J.R.; Sparks, J.A.; Hauser, K.F.; Pauly, T.H. In utero nicotine exposure causes persistent, gender-dependant changes in locomotor activity and sensitivity to nicotine in C57Bl/6 mice. Int. J. Dev. Neurosci. 2004, 22, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lee, K.P.; Spencer, T.J.; Biederman, J.; Bhide, P.G. Transgenerational transmission of hyperactivity in a mouse model of ADHD. J. Neurosci. 2014, 34, 2768–2773. [Google Scholar] [CrossRef]
- Alkam, T.; Mamiya, T.; Kimura, N.; Yoshida, A.; Kihara, D.; Tsunoda, Y.; Aoyama, Y.; Hiramatsu, M.; Kim, H.C.; Nabeshima, T. Prenatal nicotine exposure decreases the release of dopamine in the medial frontal cortex and induces atomoxetine-responsive neurobehavioral deficits in mice. Psychopharmacology 2017, 234, 1853–1869. [Google Scholar] [CrossRef]
- Kantak, K.M. Rodent models of attention-deficit hyperactivity disorder: An updated framework for model validation and therapeutic drug discovery. Pharmacol. Biochem. Behav. 2022, 216, 173378. [Google Scholar] [CrossRef]
- Shacka, J.J.; Fennell, O.B.; Robinson, S.E. Prenatal nicotine sex-dependently alters agonist-induced locomotion and stereotypy. Neurotoxicol. Teratol. 1997, 19, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.D.; Chen, W.J. Gender-related response in open-field activity following developmental nicotine exposure in rats. Pharmacol. Biochem. Behav. 2004, 78, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Mathis, A.; Mamidanna, P.; Cury, K.M.; Abe, T.; Murthy, V.N.; Mathis, M.W.; Bethge, M. DeepLabCut: Markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 2018, 21, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.D.; Tabris, N.; Matsliah, A.; Turner, D.M.; Li, J.; Ravindranath, S.; Papadoyannis, E.S.; Normand, E.; Deutsch, D.S.; Wang, Z.Y.; et al. SLEAP: A deep learning system for multi-animal pose tracking. Nat. Methods 2022, 19, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.R.; Goodwin, N.L.; Choong, J.J.; Hwang, S.; Wright, H.R.; Norville, Z.C.; Tong, X.; Lin, D.; Bentzley, B.S.; Eshel, N.; et al. Simple Behavioral Analysis (SimBA)—An open source toolkit for computer classification of complex social behaviors in experimental animals. bioRxiv 2020. [Google Scholar] [CrossRef]
- Nath, T.; Mathis, A.; Chen, A.C.; Patel, A.; Bethge, M.; Mathis, M.W. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 2019, 14, 2152–2176. [Google Scholar] [CrossRef] [PubMed]
- Bothe, G.W.; Bolivar, V.J.; Vedder, M.J.; Geistfeld, J.G. Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp. Med. 2005, 55, 326–334. [Google Scholar] [PubMed]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Nonneman, R.J.; Segall, S.K.; Andrade, G.M.; Crawley, J.N.; Magnuson, T.R. Social approach and repetitive behavior in eleven inbred mouse strains. Behav. Brain Res. 2008, 191, 118–129. [Google Scholar] [CrossRef]
- Tabuchi, K.; Blundell, J.; Etherton, M.R.; Hammer, R.E.; Liu, X.; Powell, C.M.; Südhof, T.C. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 2007, 318, 71–76. [Google Scholar] [CrossRef]
- Badawi, M.; Mori, T.; Kurihara, T.; Yoshizawa, T.; Nohara, K.; Kouyama-Suzuki, E.; Yanagawa, T.; Shirai, Y.; Tabuchi, K. Risperidone Mitigates Enhanced Excitatory Neuronal Function and Repetitive Behavior Caused by an ASD-Associated Mutation of SIK1. Front. Mol. Neurosci. 2021, 14, 706494. [Google Scholar] [CrossRef]
- Wagner, G.C.; Reuhl, K.R.; Cheh, M.; McRae, P.; Halladay, A.K. A new neurobehavioral model of autism in mice: Pre- and postnatal exposure to sodium valproate. J. Autism Dev. Disord. 2006, 36, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, T.; Kulangara, K.; Antoniello, K.; Markram, H. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc. Natl. Acad. Sci. USA 2007, 104, 13501–13506. [Google Scholar] [CrossRef]
- Zhang, R.; Cai, Y.; Xiao, R.; Zhong, H.; Li, X.; Guo, L.; Xu, H.; Fan, X. Human amniotic epithelial cell transplantation promotes neurogenesis and ameliorates social deficits in BTBR mice. Stem Cell Res. Ther. 2019, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Gioia, R.; Seri, T.; Diamanti, T.; Fimmanò, S.; Vitale, M.; Ahlenius, H.; Kokaia, Z.; Tirone, F.; Micheli, L.; Biagioni, S.; et al. Adult hippocampal neurogenesis and social behavioural deficits in the R451C Neuroligin3 mouse model of autism are reverted by the antidepressant fluoxetine. J. Neurochem. 2023, 165, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, J.; Gong, H.; Liu, T.; Li, X.; Fan, X. Implication of Hippocampal Neurogenesis in Autism Spectrum Disorder: Pathogenesis and Therapeutic Implications. Curr. Neuropharmacol. 2023, 21, 2266–2282. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Kasem, E.A.; Suzuki-Kouyama, E.; Cao, X.; Li, X.; Kurihara, T.; Uemura, T.; Yanagawa, T.; Tabuchi, K. Deficiency of calcium/calmodulin-dependent serine protein kinase disrupts the excitatory-inhibitory balance of synapses by down-regulating GluN2B. Mol. Psychiatry 2019, 24, 1079–1092. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Furuyashiki, T.; Yamada, K.; Nagai, T.; Bito, H.; Tanaka, Y.; Kitaoka, S.; Ushikubi, F.; Nabeshima, T.; Narumiya, S. Prostaglandin E receptor EP1 controls impulsive behavior under stress. Proc. Natl. Acad. Sci. USA 2005, 102, 16066–16071. [Google Scholar] [CrossRef]
- Sarnyai, Z.; Sibille, E.L.; Pavlides, C.; Fenster, R.J.; McEwen, B.S.; Toth, M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 14731–14736. [Google Scholar] [CrossRef]
- Winslow, J.T. Mouse social recognition and preference. Curr. Protoc. Neurosci. 2003, 22, 8.16.1–8.16.16. [Google Scholar] [CrossRef]
- Gunaydin, L.A.; Grosenick, L.; Finkelstein, J.C.; Kauvar, I.V.; Fenno, L.E.; Adhikari, A.; Lammel, S.; Mirzabekov, J.J.; Airan, R.D.; Zalocusky, K.A.; et al. Natural neural projection dynamics underlying social behavior. Cell 2014, 157, 1535–1551. [Google Scholar] [CrossRef]
- Pang, B.; Mori, T.; Badawi, M.; Zhou, M.; Guo, Q.; Suzuki-Kouyama, E.; Yanagawa, T.; Shirai, Y.; Tabuchi, K. An Epilepsy-Associated Mutation of Salt-Inducible Kinase 1 Increases the Susceptibility to Epileptic Seizures and Interferes with Adrenocorticotropic Hormone Therapy for Infantile Spasms in Mice. Int. J. Mol. Sci. 2022, 23, 7927. [Google Scholar] [CrossRef]
- Mori, T.; Morimoto, K. Rabies virus glycoprotein variants display different patterns in rabies monosynaptic tracing. Front. Neuroanat. 2014, 7, 47. [Google Scholar] [CrossRef]
- Kinjo, T.; Ito, M.; Seki, T.; Fukuhara, T.; Bolati, K.; Arai, H.; Suzuki, T. Prenatal exposure to valproic acid is associated with altered neurocognitive function and neurogenesis in the dentate gyrus of male offspring rats. Brain Res. 2019, 1723, 146403. [Google Scholar] [CrossRef]
- Strange, B.A.; Witter, M.P.; Lein, E.S.; Moser, E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014, 15, 655–669. [Google Scholar] [CrossRef]
- O’Leary, O.F.; Cryan, J.F. A ventral view on antidepressant action: Roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends Pharmacol. Sci. 2014, 35, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Spencer, T.J.; Biederman, J.; Bhide, P.G. Attention and working memory deficits in a perinatal nicotine exposure mouse model. PLoS ONE 2018, 13, e0198064. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Briggs, S.J.; Christopher, N.C.; Rose, J.E. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol. Teratol. 1993, 15, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Fan, F.; McCarthy, D.M.; Zhang, L.; Cannon, E.N.; Spencer, T.J.; Biederman, J.; Bhide, P.G. A prenatal nicotine exposure mouse model of methylphenidate responsive ADHD-associated cognitive phenotypes. Int. J. Dev. Neurosci. 2017, 58, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Ilott, N.; Brolese, G.; Bizarro, L.; Asherson, P.J.; Stolerman, I.P. Prenatal exposure to nicotine impairs performance of the 5-choice serial reaction time task in adult rats. Neuropsychopharmacology 2011, 36, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Yang, M.; Lord, C.; Crawley, J.N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010, 11, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sham, P.C.; Owen, M.J.; He, L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Hum. Mol. Genet. 2006, 15, 2276–2284. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Larsson, H. Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 2019, 24, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Pavăl, D.; Micluția, I.V. The Dopamine Hypothesis of Autism Spectrum Disorder Revisited: Current Status and Future Prospects. Dev. Neurosci. 2021, 43, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Pavăl, D. The dopamine hypothesis of autism spectrum disorder: A comprehensive analysis of the evidence. Int. Rev. Neurobiol. 2023, 173, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Giros, B.; Jaber, M.; Jones, S.R.; Wightman, R.M.; Caron, M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996, 379, 606–612. [Google Scholar] [CrossRef]
- Bouchatta, O.; Manouze, H.; Ba-M’Hamed, S.; Landry, M.; Bennis, M. Neonatal 6-OHDA Lesion Model in Mouse Induces Cognitive Dysfunctions of Attention-Deficit/Hyperactivity Disorder (ADHD) during Young Age. Front. Behav. Neurosci. 2020, 14, 27. [Google Scholar] [CrossRef]
- Mergy, M.A.; Gowrishankar, R.; Gresch, P.J.; Gantz, S.C.; Williams, J.; Davis, G.L.; Wheeler, C.A.; Stanwood, G.D.; Hahn, M.K.; Blakely, R.D. The rare DAT coding variant Val559 perturbs DA neuron function, changes behavior, and alters in vivo responses to psychostimulants. Proc. Natl. Acad. Sci. USA 2014, 111, E4779–E4788. [Google Scholar] [CrossRef]
- Contreras, D.; Piña, R.; Carvallo, C.; Godoy, F.; Ugarte, G.; Zeise, M.; Rozas, C.; Morales, B. Methylphenidate Restores Behavioral and Neuroplasticity Impairments in the Prenatal Nicotine Exposure Mouse Model of ADHD: Evidence for Involvement of AMPA Receptor Subunit Composition and Synaptic Spine Morphology in the Hippocampus. Int. J. Mol. Sci. 2022, 23, 7099. [Google Scholar] [CrossRef]
- Román, V.; Adham, N.; Foley, A.G.; Hanratty, L.; Farkas, B.; Lendvai, B.; Kiss, B. Cariprazine alleviates core behavioral deficits in the prenatal valproic acid exposure model of autism spectrum disorder. Psychopharmacology 2021, 238, 2381–2392. [Google Scholar] [CrossRef]
- Lauer, J.; Zhou, M.; Ye, S.; Menegas, W.; Schneider, S.; Nath, T.; Rahman, M.M.; Di Santo, V.; Soberanes, D.; Feng, G.; et al. Multi-animal pose estimation, identification and tracking with DeepLabCut. Nat. Methods 2022, 19, 496–504. [Google Scholar] [CrossRef]
- Karashchuk, P.; Rupp, K.L.; Dickinson, E.S.; Walling-Bell, S.; Sanders, E.; Azim, E.; Brunton, B.W.; Tuthill, J.C. Anipose: A toolkit for robust markerless 3D pose estimation. Cell Rep. 2021, 36, 109730. [Google Scholar] [CrossRef] [PubMed]
- Cope, E.C.; Briones, B.A.; Brockett, A.T.; Martinez, S.; Vigneron, P.A.; Opendak, M.; Wang, S.S.; Gould, E. Immature Neurons and Radial Glia, But Not Astrocytes or Microglia, Are Altered in Adult Cntnap2 and Shank3 Mice, Models of Autism. eNeuro 2016, 3, ENEURO.0196-16.2016. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, D.T.; O’Neill, S.M.; Narayan, S.; Tiwari, A.; Arnold, E.; Samaroo, H.D.; Du, F.; Ring, R.H.; Campbell, B.; Pletcher, M.; et al. Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis. Mol. Autism 2011, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Watanabe, T. Distinct Frontoparietal Brain Dynamics Underlying the Co-Occurrence of Autism and ADHD. eNeuro 2023, 10, ENEURO.0146-23.2023. [Google Scholar] [CrossRef]
- Sanz-Cervera, P.; Pastor-Cerezuela, G.; González-Sala, F.; Tárraga-Mínguez, R.; Fernández-Andrés, M.I. Sensory Processing in Children with Autism Spectrum Disorder and/or Attention Deficit Hyperactivity Disorder in the Home and Classroom Contexts. Front. Psychol. 2017, 8, 1772. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Qiu, W.; Ohashi, N.; Sun, L.; Wronski, M.-L.; Kouyama-Suzuki, E.; Shirai, Y.; Yanagawa, T.; Mori, T.; Tabuchi, K. Deep-Learning-Based Analysis Reveals a Social Behavior Deficit in Mice Exposed Prenatally to Nicotine. Cells 2024, 13, 275. https://doi.org/10.3390/cells13030275
Zhou M, Qiu W, Ohashi N, Sun L, Wronski M-L, Kouyama-Suzuki E, Shirai Y, Yanagawa T, Mori T, Tabuchi K. Deep-Learning-Based Analysis Reveals a Social Behavior Deficit in Mice Exposed Prenatally to Nicotine. Cells. 2024; 13(3):275. https://doi.org/10.3390/cells13030275
Chicago/Turabian StyleZhou, Mengyun, Wen Qiu, Nobuhiko Ohashi, Lihao Sun, Marie-Louis Wronski, Emi Kouyama-Suzuki, Yoshinori Shirai, Toru Yanagawa, Takuma Mori, and Katsuhiko Tabuchi. 2024. "Deep-Learning-Based Analysis Reveals a Social Behavior Deficit in Mice Exposed Prenatally to Nicotine" Cells 13, no. 3: 275. https://doi.org/10.3390/cells13030275
APA StyleZhou, M., Qiu, W., Ohashi, N., Sun, L., Wronski, M.-L., Kouyama-Suzuki, E., Shirai, Y., Yanagawa, T., Mori, T., & Tabuchi, K. (2024). Deep-Learning-Based Analysis Reveals a Social Behavior Deficit in Mice Exposed Prenatally to Nicotine. Cells, 13(3), 275. https://doi.org/10.3390/cells13030275





