P. falciparum Invasion and Erythrocyte Aging
Abstract
:1. Introduction: The Malaria Burden
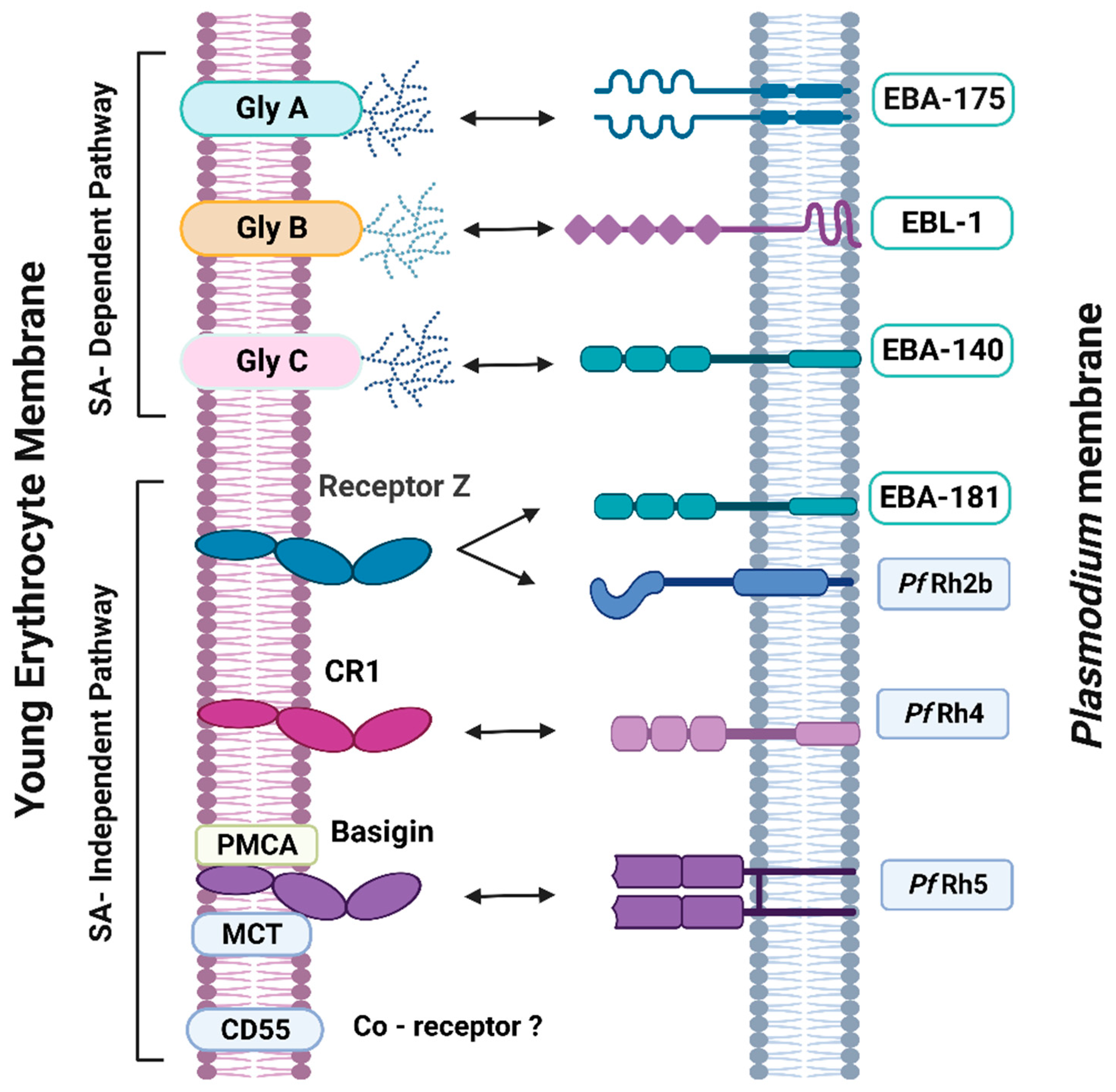
2. Malarial Erythrocyte Receptors and Plasmodium Ligands
3. Erythrocyte Age-Related Markers
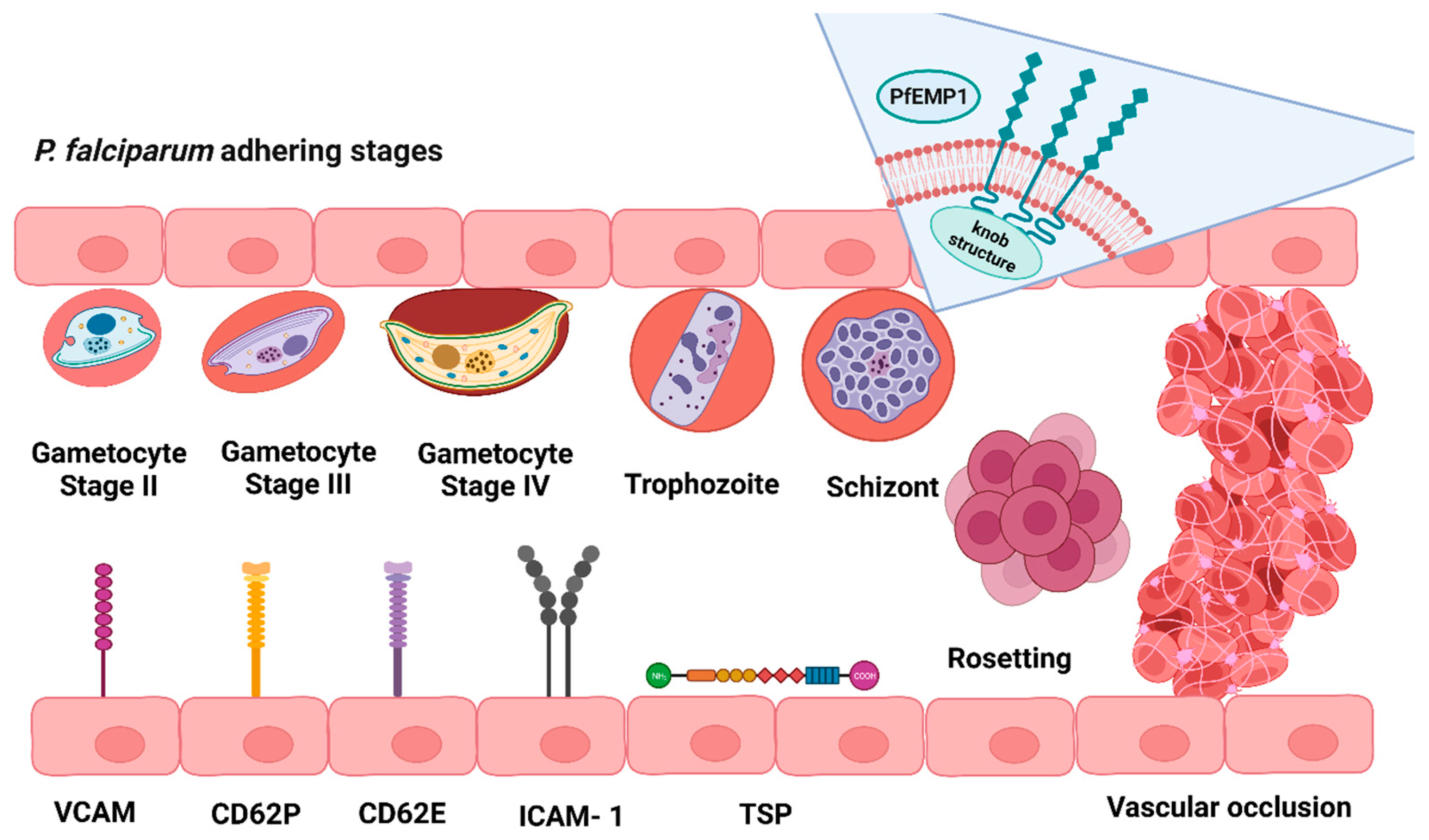
4. Effect of Plasmodium spp. Invasion on Mechanical and Molecular Erythrocyte Properties
5. Changes in Plasmodium Invasion Strategies during Erythrocyte Senescence
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World malaria report 2022; World Health Organization: Geneva, Switzerland, 2022; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Leal Filho, W.; May, J.; May, M.; Nagy, G.J. Climate change and malaria: Some recent trends of malaria incidence rates and average annual temperature in selected sub-Saharan African countries from 2000 to 2018. Malar. J. 2023, 22, 248. [Google Scholar] [CrossRef] [PubMed]
- Wellems, T.E.; Plowe, C.V. Chloroquine-Resistant Malaria. J. Infect. Dis. 2001, 184, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Gomez, G.M.; D’Arrigo, G.; Sanchez, C.P.; Berger, F.; Wade, R.C.; Lanzer, M. PfCRT mutations conferring piperaquine resistance in falciparum malaria shape the kinetics of quinoline drug binding and transport. PLoS Pathog. 2023, 19, e1011436. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.S.; Dhingra, S.K.; Mok, S.; Yeo, T.; Wicht, K.J.; Kümpornsin, K.; Takala-Harrison, S.; Witkowski, B.; Fairhurst, R.M.; Ariey, F.; et al. Emerging Southeast Asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat. Commun. 2018, 9, 3314. [Google Scholar] [CrossRef] [PubMed]
- Veiga, M.I.; Dhingra, S.K.; Henrich, P.P.; Straimer, J.; Gnädig, N.; Uhlemann, A.C.; Martin, R.E.; Lehane, A.M.; Fidock, D.A. Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies. Nat. Commun. 2016, 7, 11553. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, N.; Fall, B.; Pascual, A.; Fall, M.; Baret, E.; Camara, C.; Nakoulima, A.; Diatta, B.; Fall, K.B.; Mbaye, P.S.; et al. Role of Pfmdr1 in in vitro Plasmodium falciparum susceptibility to chloroquine, quinine, monodesethylamodiaquine, mefloquine, lumefantrine, and dihydroartemisinin. Antimicrob. Agents Chemother. 2014, 58, 7032–7040. [Google Scholar] [CrossRef]
- Alvarez, E.; Lavandera, J.L.; Vanderwall, D.E.; Green, D.V.; Kumar, V.; Hasan, S.; Brown, J.R.; Peishoff, C.E.; Cardon, L.R.; Garcia-Bustos, J.F.; et al. Thousands of chemical starting points for antimalarial lead identification. Nature 2010, 465, 305–310. [Google Scholar] [CrossRef]
- Spiegel, H.; Boes, A.; Kastilan, R.; Kapelski, S.; Edgue, G.; Beiss, V.; Chubodova, I.; Scheuermayer, M.; Pradel, G.; Schillberg, S.; et al. The stage-specific in vitro efficacy of a malaria antigen cocktail provides valuable insights into the development of effective multi-stage vaccines. Biotechnol. J. 2015, 10, 1651–1659. [Google Scholar] [CrossRef]
- Vijayan, A.; Chitnis, C.E. Development of Blood Stage Malaria Vaccines. Methods Mol. Biol. 2019, 2013, 199–218. [Google Scholar] [CrossRef]
- Molina-Franky, J.; Patarroyo, M.E.; Kalkum, M.; Patarroyo, M.A. The Cellular and Molecular Interaction Between Erythrocytes and Plasmodium falciparum Merozoites. Front. Cell. Infect. Microbiol. 2022, 12, 816574. [Google Scholar] [CrossRef]
- Dluzewski, A.R.; Mitchell, G.H.; Fryer, P.R.; Griffiths, S.; Wilson, R.J.; Gratzer, W.B. Origins of the parasitophorous vacuole membrane of the malaria parasite, Plasmodium falciparum, in human red blood cells. J. Cell Sci. 1992, 102(Pt3), 527–532. [Google Scholar] [CrossRef] [PubMed]
- Cowman, A.F.; Tonkin, C.J.; Tham, W.H.; Duraisingh, M.T. The Molecular Basis of Erythrocyte Invasion by Malaria Parasites. Cell Host Microbe 2017, 22, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.H.; Hadley, T.J.; McGinniss, M.H.; Klotz, F.W.; Miller, L.H. Invasion of erythrocytes by Plasmodium falciparum malaria parasites: Evidence for receptor heterogeneity and two receptors. Blood 1986, 67, 1519–1521. [Google Scholar] [CrossRef] [PubMed]
- Sim, B.K.; Chitnis, C.E.; Wasniowska, K.; Hadley, T.J.; Miller, L.H. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 1994, 264, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Marinkovic, M.; Russo, C.; McKnight, C.J.; Coetzer, T.L.; Chishti, A.H. Identification of a specific region of Plasmodium falciparum EBL-1 that binds to host receptor glycophorin B and inhibits merozoite invasion in human red blood cells. Mol. Biochem. Parasitol. 2012, 183, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.H.; Malpede, B.M.; Batchelor, J.D.; Tolia, N.H. Crystal and Solution Structures of Plasmodium falciparum Erythrocyte-binding Antigen 140 Reveal Determinants of Receptor Specificity during Erythrocyte Invasion. J. Biol. Chem. 2012, 287, P36830–P36836. [Google Scholar] [CrossRef]
- Lobo, C.A.; Rodriguez, M.; Reid, M.; Lustigman, S. Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl). Blood 2003, 101, 4628–4631. [Google Scholar] [CrossRef]
- Daniels, G. Functional aspects of red cell antigens. Blood Rev. 1999, 13, 14–35. [Google Scholar] [CrossRef]
- Lopez, G.H.; Wei, L.; Ji, Y.; Condon, J.A.; Luo, G.; Hyland, C.A.; Flower, R.L. GYP*Kip, a novel GYP(B-A-B) hybrid allele, encoding the MNS48 (KIPP) antigen. Transfusion 2016, 56, 539–541. [Google Scholar] [CrossRef]
- Hollox, E.J.; Louzada, S. Genetic variation of glycophorins and infectious disease. Immunogenetics 2023, 75, 201–206. [Google Scholar] [CrossRef]
- Miller, L.H.; Haynes, J.D.; McAuliffe, F.M.; Shiroishi, T.; Durocher, J.R.; McGinniss, M.H. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. J. Exp. Med. 1977, 146, 277–281. [Google Scholar] [CrossRef]
- Ochola-Oyier, L.I.; Wamae, K.; Omedo, I.; Ogola, C.; Matharu, A.; Musabyimana, J.P.; Njogu, F.K.; Marsh, K. Few Plasmodium falciparum merozoite ligand and erythrocyte receptor pairs show evidence of balancing selection. Infect. Genet. Evol. 2019, 69, 235–245. [Google Scholar] [CrossRef]
- Mayer, D.C.; Cofie, J.; Jiang, L.; Hartl, D.L.; Tracy, E.; Kabat, J.; Mendoza, L.H.; Miller, L.H. Glycophorin B is the erythrocyte receptor of Plasmodium falciparum erythrocyte-binding ligand, EBL-1. Proc. Natl. Acad. Sci. USA 2009, 106, 5348–5352. [Google Scholar] [CrossRef]
- Jaskiewicz, E.; Jodłowska, M.; Kaczmarek, R.; Zerka, A. Erythrocyte glycophorins as receptors for Plasmodium merozoites. Parasit. Vectors 2019, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Dankwa, S.; Chaand, M.; Kanjee, U.; Jiang, R.H.Y.; Nobre, L.V.; Goldberg, J.M.; Bei, A.K.; Moechtar, M.A.; Grüring, C.; Ahouidi, A.D.; et al. Genetic Evidence for Erythrocyte Receptor Glycophorin B Expression Levels Defining a Dominant Plasmodium falciparum Invasion Pathway into Human Erythrocytes. Infect. Immun. 2017, 85, e00074-17. [Google Scholar] [CrossRef] [PubMed]
- Duraisingh, M.T.; Maier, A.G.; Triglia, T.; Cowman, A.F. Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 2003, 100, 4796–4801. [Google Scholar] [CrossRef] [PubMed]
- Tham, W.H.; Wilson, D.W.; Reiling, L.; Chen, L.; Beeson, J.G.; Cowman, A.F. Antibodies to reticulocyte binding protein-like homologue 4 inhibit invasion of Plasmodium falciparum into human erythrocytes. Infect. Immun. 2009, 77, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Gaur, D.; Singh, S.; Jiang, L.; Diouf, A.; Miller, L.H. Recombinant Plasmodium falciparum reticulocyte homology protein 4 binds to erythrocytes and blocks invasion. Proc. Natl. Acad. Sci. USA 2007, 104, 17789–17794. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, J.; Simpson, K.M.; Triglia, T.; Plouffe, D.; Tonkin, C.J.; Duraisingh, M.T.; Maier, A.G.; Winzeler, E.A.; Cowman, A.F. Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. Science 2005, 309, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Spadafora, C.; Awandare, G.A.; Kopydlowski, K.M.; Czege, J.; Moch, J.K.; Finberg, R.W.; Tsokos, G.C.; Stoute, J.A. Complement receptor 1 is a sialic acid-independent erythrocyte receptor of Plasmodium falciparum. PLoS Pathog. 2010, 6, e1000968. [Google Scholar] [CrossRef]
- Awandare, G.A.; Spadafora, C.; Moch, J.K.; Dutta, S.; Haynes, J.D.; Stoute, J.A. Plasmodium falciparum field isolates use complement receptor 1 (CR1) as a receptor for invasion of erythrocytes. Mol. Biochem. Parasitol. 2011, 177, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Lustigman, S.; Montero, E.; Oksov, Y.; Lobo, C.A. PfRH5: A novel reticulocyte-binding family homolog of Plasmodium falciparum that binds to the erythrocyte, and an investigation of its receptor. PLoS ONE 2008, 3, e3300. [Google Scholar] [CrossRef]
- Wanaguru, M.; Liu, W.; Hahn, B.H.; Rayner, J.C.; Wright, G.J. RH5-Basigin interaction plays a major role in the host tropism of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2013, 110, 20735–20740. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, A.; Constantin, C.F.; Hirschi, S.; Henrich, S.; Bildl, W.; Fakler, B.; Draper, S.J.; Schulte, U.; Higgins, M.K. Erythrocyte invasion-neutralising antibodies prevent Plasmodium falciparum RH5 from binding to basigin-containing membrane protein complexes. Elife 2023, 5, e83681. [Google Scholar] [CrossRef] [PubMed]
- Egan, E.S.; Jiang, R.H.; Moechtar, M.A.; Barteneva, N.S.; Weekes, M.P.; Nobre, L.V.; Gygi, S.P.; Paulo, J.A.; Frantzreb, C.; Tani, Y.; et al. A forward genetic screen identifies erythrocyte CD55 as essential for Plasmodium falciparum invasion. Science 2015, 348, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Diédhiou, C.K.; Moussa, R.A.; Bei, A.K.; Daniels, R.; Papa Mze, N.; Ndiaye, D.; Faye, N.; Wirth, D.; Amambua-Ngwa, A.; Mboup, S.; et al. Temporal changes in Plasmodium falciparum reticulocyte binding protein homolog 2b (PfRh2b) in Senegal and The Gambia. Malar. J. 2019, 18, 239. [Google Scholar] [CrossRef] [PubMed]
- Stoute, J.A. Complement-regulatory proteins in severe malaria: Too little or too much of a good thing? Trends Parasitol. 2005, 21, 218–223, Erratum in: Trends Parasitol. 2005, 21, 358. [Google Scholar] [CrossRef] [PubMed]
- Eggleton, P.; Tenner, A.J.; Reid, K.B. C1q receptors. Clin. Exp. Immunol. 2012, 120, 406–412. [Google Scholar] [CrossRef]
- Kosoy, R.; Ransom, M.; Chen, H.; Marconi, M.; Macciardi, F.; Glorioso, N.; Gregersen, P.K.; Cusi, D.; Seldin, M.F. Evidence for malaria selection of a CR1 haplotype in Sardinia. Genes Immun. 2011, 12, 582–588. [Google Scholar] [CrossRef]
- Tham, W.H.; Wilson, D.W.; Lopaticki, S.; Schmidt, C.Q.; Tetteh-Quarcoo, P.B.; Barlow, P.N.; Richard, D.; Corbin, J.E.; Beeson, J.G.; Cowman, A.F. Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand. Proc. Natl. Acad. Sci. USA 2010, 107, 17327–17332. [Google Scholar] [CrossRef]
- Muramatsu, T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J. Biochem. 2016, 159, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Supper, V.; Schiller, H.B.; Paster, W.; Forster, F.; Boulègue, C.; Mitulovic, G.; Leksa, V.; Ohradanova-Repic, A.; Machacek, C.; Schatzlmaier, P.; et al. Association of CD147 and Calcium Exporter PMCA4 Uncouples IL-2 Expression from Early TCR Signaling. J. Immunol. 2016, 196, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Bedu-Addo, G.; Meese, S.; Mockenhaupt, F.P. An ATP2B4 polymorphism protects against malaria in pregnancy. J. Infect. Dis. 2013, 207, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Mariga, S.T.; Kolko, M.; Gjedde, A.; Bergersen, L.H. Lactate transport and receptor actions in cerebral malaria. Front. Neurosci. 2014, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Ndila, C.M.; Uyoga, S.; Macharia, A.W.; Nyutu, G.; Peshu, N.; Ojal, J.; Shebe, M.; Awuondo, K.O.; Mturi, N.; Tsofa, B.; et al. MalariaGEN Consortium. Human candidate gene polymorphisms and risk of severe malaria in children in Kilifi, Kenya: A case-control association study. Lancet Haematol. 2018, 5, e333–e345. [Google Scholar] [CrossRef]
- Wright, K.E.; Hjerrild, K.A.; Bartlett, J.; Douglas, A.D.; Jin, J.; Brown, R.E.; Illingworth, J.J.; Ashfield, R.; Clemmensen, S.B.; de Jongh, W.A.; et al. Structure of malaria invasion protein RH5 with erythrocyte basigin and blocking antibodies. Nature 2014, 515, 427–430. [Google Scholar] [CrossRef]
- Crosnier, C.; Bustamante, L.Y.; Bartholdson, S.J.; Bei, A.K.; Theron, M.; Uchikawa, M.; Mboup, S.; Ndir, O.; Kwiatkowski, D.P.; Duraisingh, M.T.; et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 2011, 480, 534–537. [Google Scholar] [CrossRef]
- Storry, J.R.; Reid, M.E.; Yazer, M.H. The Cromer blood group system: A review. Immunohematology 2010, 26, 109–118. [Google Scholar] [CrossRef]
- Coyne, C.B.; Bergelson, J.M. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 2006, 124, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Sahar, T.; Reddy, K.S.; Bharadwaj, M.; Pandey, A.K.; Singh, S.; Chitnis, C.E.; Gaur, D. Plasmodium falciparum reticulocyte binding-like homologue protein 2 (PfRH2) is a key adhesive molecule involved in erythrocyte invasion. PLoS ONE 2011, 6, e17102. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Uboldi, A.D.; Marapana, D.; Czabotar, P.E.; Epp, C.; Bujard, H.; Taylor, N.L.; Perugini, M.A.; Hodder, A.N.; Cowman, A.F. The merozoite surface protein 1 complex is a platform for binding to human erythrocytes by Plasmodium falciparum. J. Biol. Chem. 2014, 289, 25655–25669. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.A.; Dhawan, S.; Singh, S.; Singh, B.; Gupta, P.; Pandey, A.; Mohmmed, A.; Gaur, D.; Chitnis, C.E. A thrombospondin structural repeat containing rhoptry protein from Plasmodium falciparum mediates erythrocyte invasion. Cell. Microbiol. 2013, 15, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Hayton, K.; Dumoulin, P.; Henschen, B.; Liu, A.; Papakrivos, J.; Wellems, T.E. Various PfRH5 polymorphisms can support Plasmodium falciparum invasion into the erythrocytes of owl monkeys and rats. Mol. Biochem. Parasitol. 2013, 187, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Salinas, N.D.; Tolia, N.H. Red cell receptors as access points for malaria infection. Curr. Opin. Hematol. 2016, 23, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Mock, D.M.; Matthews, N.I.; Zhu, S.; Strauss, R.G.; Schmidt, R.L.; Nalbant, D.; Cress, G.A.; Widness, J.A. Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities. Transfusion 2011, 51, 1047–1057. [Google Scholar] [CrossRef]
- Thiagarajan, P.; Parker, C.J.; Prchal, J.T. How Do Red Blood Cells Die? Front. Physiol. 2021, 12, 655393. [Google Scholar] [CrossRef]
- Antonelou, M.H.; Kriebardis, A.G.; Papassideri, I.S. Aging and death signalling in mature red cells: From basic science to transfusion practice. Blood Transfus. 2010, 8 (Suppl. S3), s39–s47. [Google Scholar] [CrossRef]
- Lutz, H.U.; Bogdanova, A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front. Physiol. 2013, 4, 387. [Google Scholar] [CrossRef]
- Waugh, S.M.; Walder, J.A.; Low, P.S. Partial characterization of the copolymerization reaction of erythrocyte membrane band 3 with hemichromes. Biochemistry 1987, 26, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Badior, K.; Casey, J.R. Large conformational dynamics in Band 3 protein: Significance for erythrocyte senescence signalling, Biochim. Biophys. Acta BBA Biomembr. 2021, 1863, 183678. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.M. Aging of cell membrane molecules leads to appearance of an aging antigen and removal of senescent cells. Gerontology 1985, 31, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H.U. Naturally occurring autoantibodies in mediating clearance of senescent red blood cells. Adv. Exp. Med. Biol. 2012, 750, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.M. Mechanism of removal of senescent cells by human macrophages in situ. Proc. Natl. Acad. Sci. USA 1975, 72, 3521–3525. [Google Scholar] [CrossRef] [PubMed]
- Safeukui, I.; Buffet, P.A.; Deplaine, G.; Perrot, S.; Brousse, V.; Ndour, A.; Nguyen, M.; Mercereau-Puijalon, O.; David, P.H.; Milon, G.; et al. Quantitative assessment of sensing and sequestration of spherocytic erythrocytes by the human spleen. Blood 2012, 120, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Satchwell, T.J.; Toye, A.M. Band 3, an essential red blood cell hub of activity. Haematologica 2021, 106, 2792–2793. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H.U.; Bussolino, F.; Flepp, R.; Fasler, S.; Stammler, P.; Kazatchkine, M.D.; Arese, P. Naturally occurring anti-band-3 antibodies and complement together mediate phagocytosis of oxidatively stressed human erythrocytes. Proc. Natl. Acad. Sci. USA 1987, 84, 7368–7372. [Google Scholar] [CrossRef] [PubMed]
- Almukadi, H.; Schwake, C.; Kaiser, M.M.; Mayer, D.C.G.; Schiemer, J.; Baldwin, M.R.; Hegde, S.; Lu, Y.; Hanada, T.; Chishti, A.H. Human erythrocyte band 3 is a host receptor for Plasmodium falciparum glutamic acid-rich protein. Blood 2019, 133, 470–480. [Google Scholar] [CrossRef]
- Lu, J.; Chu, R.; Yin, Y.; Yu, H.; Xu, Q.; Yang, B.; Sun, Y.; Song, J.; Wang, Q.; Xu, J.; et al. Glycosylphosphatidylinositol-anchored micronemal antigen (GAMA) interacts with the band 3 receptor to promote erythrocyte invasion by malaria parasites. J. Biol. Chem. 2022, 298, 101765. [Google Scholar] [CrossRef]
- Fishelson, Z.; Marikovsky, Y. Reduced CR1 expression on aged human erythrocytes: Immuno-electron microscopic and functional analysis. Mech. Ageing Dev. 1993, 72, 25–35. [Google Scholar] [CrossRef]
- Ripoche, J.; Sim, R.B. Loss of complement receptor type 1 (CR1) on ageing of erythrocytes. Studies of proteolytic release of the receptor. Biochem. J. 1986, 235, 815–821. [Google Scholar] [CrossRef]
- Pascual, M.; Lutz, H.U.; Steiger, G.; Stammler, P.; Schifferli, J.A. Release of vesicles enriched in complement receptor 1 from human erythrocytes. J. Immunol. 1993, 151, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Blanc, L.; De Gassart, A.; Géminard, C.; Bette-Bobillo, P.; Vidal, M. Exosome release by reticulocytes—An integral part of the red blood cell differentiation system. Blood Cells Mol. Dis. 2005, 35, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Willekens, F.L.A.; Werre, J.M.; Groenen-Döpp, Y.A.M.; Roerdinkholder-Stoelwinder, B.; de Pauw, B.; Bosman, G.J.C.G.M. Erythrocyte vesiculation: A self-protective mechanism? Br. J. Haematol. 2008, 141, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2020, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.; Coronado, L.M.; Garrido, A.C.; Durant-Archibald, A.A.; Spadafora, C. Volatile organic compounds associated with Plasmodium falciparum infection in vitro. Parasit. Vectors 2017, 10, 215. [Google Scholar] [CrossRef]
- Trautsch, C.; Tannert, C.; Maretzki, D. Disproportional loss of membrane constituents in the course of erythrocyte aging. Acta Biol. Medica Ger. 1981, 40, 743–746. [Google Scholar]
- Miyahara, K.; Spiro, M.J. Nonuniform loss of membrane glycoconjugates during in vivo aging of human erythrocytes: Studies of normal and diabetic red cell saccharides. Arch. Biochem. Biophys. 1984, 232, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, R.L.; Healey, G.; Patton, K.A.; Veale, M.F. Red blood cell age determines the impact of storage and leukocyte burden on cell adhesion molecules, glycophorin A and the release of annexin V. Transfus. Apher. Sci. 2006, 34, 15–23. [Google Scholar] [CrossRef]
- Biasini, G.M.; Botrè, F.; de la Torre, X.; Donati, F. Age-Markers on the Red Blood Cell Surface and Erythrocyte Microparticles may Constitute a Multi-parametric Strategy for Detection of Autologous Blood Transfusion. Sports Med. Open 2023, 9, 113. [Google Scholar] [CrossRef]
- Lux, S.E. 4th. Anatomy of the red cell membrane skeleton: Unanswered questions. Blood 2016, 127, 187–199. [Google Scholar] [CrossRef]
- Mohandas, N.; Blanc, L. Parasite hijacks red cell membrane proteins. Blood 2023, 142, 1942–1944. [Google Scholar] [CrossRef]
- Baro, B.; Kim, C.Y.; Lin, C.; Kongsomboonvech, A.K.; Tetard, M.; Peterson, N.A.; Salinas, N.D.; Tolia, N.H.; Egan, E.S. Plasmodium falciparum exploits CD44 as a coreceptor for erythrocyte invasion. Blood 2023, 142, 2016–2028. [Google Scholar] [CrossRef]
- Morrissey, M.A.; Kern, N.; Vale, R.D. CD47 Ligation Repositions the Inhibitory Receptor SIRPA to Suppress Integrin Activation and Phagocytosis. Immunity 2020, 53, 290–302.e6, Erratum in: Immunity 2023, 56, 2172. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Jamieson, C.H.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Balduini, C.; Balduini, C.L.; Ascari, E. Membrane glycopeptides from old and young human erythrocytes. Biochem. J. 1974, 140, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Gattegno, L.; Bladier, D.; Garnier, M.; Cornillot, P. Changes in carbohydrate content of surface membranes of human erythrocytes during aging. Carbohydr. Res. 1976, 52, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; Tuo, W.W.; Wang, D.; Kang, L.L.; Chen, X.Y.; Luo, M. Restoring the youth of aged red blood cells and extending their lifespan in circulation by remodeling membrane sialic acid. J. Cell. Mol. Med. 2016, 20, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.P.; Cesar, C.L.; Barjas-Castro, M. de L. Electrical properties of the red blood cell membrane and immunohematological investigation. Rev. Bras. Hematol. Hemoter. 2011, 33, 297–301. [Google Scholar] [CrossRef]
- Ayi, K.; Lu, Z.; Serghides, L.; Ho, J.M.; Finney, C.; Wang, J.C.Y.; Liles, W.C.; Kain, K.C. CD47-SIRPα Interactions Regulate Macrophage Uptake of Plasmodium falciparum-Infected Erythrocytes and Clearance of Malaria in vivo. Infect. Immun. 2016, 84, 2002–2011. [Google Scholar] [CrossRef]
- Coste, I.; Gauchat, J.F.; Wilson, A.; Izui, S.; Jeannin, P.; Delneste, Y.; MacDonald, H.R.; Bonnefoy, J.Y.; Renno, T. Unavailability of CD147 leads to selective erythrocyte trapping in the spleen. Blood 2001, 97, 3984–3988. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Singh, A. Splenectomy Modulates the Erythrocyte Turnover and Basigin (CD147) Expression in Mice. Indian J. Hematol. Blood Transfus. 2020, 36, 711–718. [Google Scholar] [CrossRef]
- Seki, M.; Arashiki, N.; Takakuwa, Y.; Nitta, K.; Nakamura, F. Reduction in flippase activity contributes to surface presentation of phosphatidylserine in human senescent erythrocytes. J. Cell. Mol. Med. 2020, 24, 13991–14000. [Google Scholar] [CrossRef]
- Bernhardt, I.; Nguyen, D.B.; Wesseling, M.C.; Kaestner, L. Intracellular Ca2+ Concentration and Phosphatidylserine Exposure in Healthy Human Erythrocytes in Dependence on in vivo Cell Age. Front. Physiol. 2020, 10, 1629. [Google Scholar] [CrossRef]
- Arese, P.; Turrini, F.; Schwarzer, E. Band 3/complement-mediated recognition and removal of normally senescent and pathological human erythrocytes. Cell. Physiol. Biochem. 2005, 16, 133–146. [Google Scholar] [CrossRef]
- Gao, Y.; Lv, L.; Liu, S.; Ma, G.; Su, Y. Elevated levels of thrombin-generating microparticles in stored red blood cells. Vox Sang. 2013, 105, 11–17. [Google Scholar] [CrossRef]
- Greenwalt, T.J. Autologous and aged blood donors. JAMA 1987, 257, 1220–1221. [Google Scholar] [CrossRef] [PubMed]
- Kriebardis, A.G.; Antonelou, M.H.; Stamoulis, K.E.; Economou-Petersen, E.; Margaritis, L.H.; Papassideri, I.S. RBC-derived vesicles during storage: Ultrastructure, protein composition, oxidation, and signaling components. Transfusion 2008, 48, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Koshiar, R.L.; Somajo, S.; Norström, E.; Dahlbäck, B. Erythrocyte-derived microparticles supporting activated protein C-mediated regulation of blood coagulation. PLoS ONE 2014, 9, e104200. [Google Scholar]
- Bosman, G.J.; Cluitmans, J.C.; Groenen, Y.A.; Werre, J.M.; Willekens, F.L.; Novotný, V.M. Susceptibility to hyperosmotic stress-induced phosphatidylserine exposure increases during red blood cell storage. Transfusion 2011, 51, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Koshkaryev, A.; Livshits, L.; Pajic-Lijakovic, I.; Gural, A.; Barshtein, G.; Yedgar, S. Non-oxidative band-3 clustering agents cause the externalization of phosphatidylserine on erythrocyte surfaces by a calcium-independent mechanism. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183231. [Google Scholar] [CrossRef] [PubMed]
- Livshits, L.; Barshtein, G.; Arbell, D.; Gural, A.; Levin, C.; Guizouarn, H. Do We Store Packed Red Blood Cells under “Quasi-Diabetic” Conditions? Biomolecules 2021, 11, 992. [Google Scholar] [CrossRef]
- Rand, R.P. Mechanical properties of the red cell membrane: II. Viscoelastic breakdown of the membrane. Biophys. J. 1964, 4, 303–316. [Google Scholar] [CrossRef]
- Kozlov, M.M.; Lerche, D.; Meier, W. RBC membrane instability for large pipette deformation. A theoretical approach. Biorheology 1988, 25, 843–856. [Google Scholar] [CrossRef]
- Gueguen, M.; Bidet, J.M.; Durand, F.; Driss, F.; Joffre, A.; Genetet, B. Filtration pressure and red blood cell deformability: Evaluation of a new device: Erythrometre. Biorheol. Suppl. 1984, 1, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Islamzada, E.; Matthews, K.; Guo, Q.; Santoso, A.T.; Duffy, S.P.; Scott, M.D.; Ma, H. Deformability based sorting of stored red blood cells reveals donor-dependent aging curves. Lab. Chip 2020, 20, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Dulinska, I.; Targosz, M.; Strojny, W.; Lekka, M.; Czuba, P.; Balwierz, W.; Szymonski, M. Stiffness of normal and pathological erythrocytes studied by means of atomic force microscopy. J. Biochem. Biophys. Methods 2006, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Meiselman, H.J. Determination of red blood cell shape recovery time constant in a Couette system by the analysis of light reflectance and ektacytometry. Biorheology 1996, 33, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, M.; Ince, H.Y.; Yalcin, O.; Ajdžanović, V.; Spasojević, I.; Meiselman, H.J.; Baskurt, O.K. The effect of alcohols on red blood cell mechanical properties and membrane fluidity depends on their molecular size. PLoS ONE 2013, 8, e76579. [Google Scholar] [CrossRef] [PubMed]
- Brandão, M.M.; Fontes, A.; Barjas-Castro, M.L.; Barbosa, L.C.; Costa, F.F.; Cesar, C.L.; Saad, S.T. Optical tweezers for measuring red blood cell elasticity: Application to the study of drug response in sickle cell disease. Eur. J. Haematol. 2003, 70, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Smart, T.; Nobre-Cardoso, J.; Richards, C.; Bhatnagar, R.; Tufail, A.; Shima, D.H.; Jones, P.; Pavesio, C. Assessment of red blood cell deformability in type 2 diabetes mellitus and diabetic retinopathy by dual optical tweezers stretching technique. Sci. Rep. 2016, 6, 15873. [Google Scholar] [CrossRef]
- Rabai, M.; Meiselman, H.J.; Wenby, R.B.; Detterich, J.A.; Feinberg, J. Analysis of light scattering by red blood cells in ektacytometry using global pattern fitting. Biorheology 2012, 49, 317–328. [Google Scholar] [CrossRef]
- Barshtein, G.; Gural, A.; Arbell, D.; Barkan, R.; Livshits, L.; Pajic-Lijakovic, I.; Yedgar, S. Red Blood Cell Deformability Is Expressed by a Set of Interrelated Membrane Proteins. Int. J. Mol. Sci. 2023, 24, 12755. [Google Scholar] [CrossRef]
- Sherman, I.W.; Eda, S.; Winograd, E. Erythrocyte aging and malaria. Cell. Mol. Biol. 2004, 50, 159–169. [Google Scholar] [PubMed]
- Duez, J.; Holleran, J.P.; Ndour, P.A.; Pionneau, C.; Diakité, S.; Roussel, C.; Dussiot, M.; Amireault, P.; Avery, V.M.; Buffet, P.A. Mechanical clearance of red blood cells by the human spleen: Potential therapeutic applications of a biomimetic RBC filtration method. Transfus. Clin. Biol. 2015, 22, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Spatz, J.; Mills, J.P.; Micoulet, A.; Dao, M.; Lim, C.T.; Beil, M.; Seufferlein, T. Reprint of: Connections between single-cell biomechanics and human disease states: Gastrointestinal cancer and malaria. Acta Biomater. 2015, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Omodeo-Salè, F.; Motti, A.; Basilico, N.; Parapini, S.; Olliaro, P.; Taramelli, D. Accelerated senescence of human erythrocytes cultured with Plasmodium falciparum. Blood 2003, 102, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Salinas, N.D.; Paing, M.M.; Adhikari, J.; Gross, M.L.; Tolia, N. Moderately Neutralizing Epitopes in Nonfunctional Regions Dominate the Antibody Response to Plasmodium falciparum EBA-140. Infect. Immun. 2019, 87, e00716-18. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.; Matuschewski, K.; Maier, A.G. Of membranes and malaria: Phospholipid asymmetry in Plasmodium falciparum-infected red blood cells. Cell. Mol. Life Sci. 2021, 78, 4545–4561. [Google Scholar] [CrossRef] [PubMed]
- Ahiya, A.I.; Bhatnagar, S.; Morrisey, J.M.; Beck, J.R.; Vaidya, A.B. Dramatic Consequences of Reducing Erythrocyte Membrane Cholesterol on Plasmodium falciparum. Microbiol. Spectr. 2022, 10, e0015822. [Google Scholar] [CrossRef] [PubMed]
- Turrini, F.; Arese, P.; Yuan, J.; Low, P.S. Clustering of integral membrane proteins of the human erythrocyte membrane stimulates autologous IgG binding, complement deposition, and phagocytosis. J. Biol. Chem. 1991, 266, 23611–23617. [Google Scholar] [CrossRef]
- Turrini, F.; Mannu, F.; Arese, P.; Yuan, J.; Low, P.S. Characterization of the autologous antibodies that opsonize erythrocytes with clustered integral membrane proteins. Blood 1993, 81, 3146–3152. [Google Scholar] [CrossRef]
- Turrini, F.; Mannu, F.; Cappadoro, M.; Ulliers, D.; Giribaldi, G.; Arese, P. Binding of naturally occurring antibodies to oxidatively and nonoxidatively modified erythrocyte band 3. Biochim. Biophys. Acta 1994, 1190, 297–303. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Kager, P.A.; Vreeken, J.; White, N.J. Abnormal blood flow and red blood cell deformability in severe malaria. Parasitol. Today 2000, 16, 228–232. [Google Scholar] [CrossRef]
- Paul, A.; Pallavi, R.; Tatu, U.S.; Natarajan, V. The bystander effect in optically trapped red blood cells due to Plasmodium falciparum infection. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 220–223. [Google Scholar] [CrossRef]
- Barber, B.E.; Russell, B.; Grigg, M.J.; Zhang, R.; William, T.; Amir, A.; Lau, Y.L.; Chatfield, M.D.; Dondorp, A.M.; Anstey, N.M.; et al. Reduced red blood cell deformability in Plasmodium knowlesi malaria. Blood Adv. 2018, 2, 433–443. [Google Scholar] [CrossRef]
- Drenckhahn, D. Removal of Old and Abnormal Red Blood Cells from Circulation: Mechanical and Immunologic Mechanisms. In Blood Cells, Rheology, and Aging; Platt, D., Ed.; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar] [CrossRef]
- Hosseini, S.M.; and Feng, J.J. How malaria parasites reduce the deformability of infected red blood cells. Biophys. J. 2012, 103, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aingaran, M.; Zhang, R.; Law, S.K.; Peng, Z.; Undisz, A.; Meyer, E.; Diez-Silva, M.; Burke, T.A.; Spielmann, T.; Lim, C.T.; et al. Host cell deformability is linked to transmission in the human malaria parasite Plasmodium falciparum. Cell. Microbiol. 2012, 14, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, M.; Niang, M.; Deplaine, G.; Perrot, S.; Bischoff, E.; Ndour, P.A.; Silvestrini, F.; Khattab, A.; Milon, G.; David, P.H.; et al. A switch in infected erythrocyte deformability at the maturation and blood circulation of Plasmodium falciparum transmission stages. Blood 2012, 119, e172–e180. [Google Scholar] [CrossRef] [PubMed]
- Neveu, G.; Lavazec, C. Erythrocyte Membrane Makeover by Plasmodium falciparum Gametocytes. Front. Microbiol. 2019, 10, 2652. [Google Scholar] [CrossRef] [PubMed]
- Metwally, N.G.; Tilly, A.K.; Lubiana, P.; Roth, L.K.; Dörpinghaus, M.; Lorenzen, S.; Schuldt, K.; Witt, S.; Bachmann, A.; Tidow, H.; et al. Characterisation of Plasmodium falciparum populations selected on the human endothelial receptors P-selectin, E-selectin, CD9 and CD151. Sci. Rep. 2017, 7, 4069. [Google Scholar] [CrossRef] [PubMed]
- Depond, M.; Henry, B.; Buffet, P.; Ndour, P.A. Methods to Investigate the Deformability of RBC During Malaria. Front. Physiol. 2020, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, M.; Elphinstone, R.E.; Conroy, A.L.; Kain, K.C. Contrasting pediatric and adult cerebral malaria: The role of the endothelial barrier. Virulence 2013, 4, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Stoute, J.A. Complement receptor 1 and malaria. Cell. Microbiol. 2011, 13, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.H.; Usami, S.; Chien, S. Alteration in the rheologic properties of Plasmodium knowlesi-infected red cells. A possible mechanism for capillary obstruction. J. Clin. Investig. 1971, 50, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, C.J.; Graham, A.L.; Huijben, S.; Barclay, V.C.; Long, G.H.; Grenfell, B.T.; Read, A.F.; Bjørnstad, O.N. Partitioning regulatory mechanisms of within-host malaria dynamics using the effective propagation number. Science 2011, 333, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Eraky Mohamed, T.; Abd El-Rahman Ahmed, I.; Shazly Mostafa, H.; Abdelrahman Mohamed, M. Mechanics of deformation of malaria-infected red blood cells. Mech. Res. Commun. 2021, 113, 103666. [Google Scholar] [CrossRef]
- Hawking, F.; Wilson, M.E.; Gammage, K. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 1971, 65, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Joice, R.; Nilsson, S.K.; Montgomery, J.; Dankwa, S.; Egan, E.; Morahan, B.; Seydel, K.B.; Bertuccini, L.; Alano, P.; Williamson, K.C.; et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med. 2014, 6, 244re5. [Google Scholar] [CrossRef]
- Bachmann, A.; Esser, C.; Petter, M.; Predehl, S.; von Kalckreuth, V.; Schmiedel, S.; Bruchhaus, I.; Tannich, E. Absence of erythrocyte sequestration and lack of multicopy gene family expression in Plasmodium falciparum from a splenectomized malaria patient. PLoS ONE 2009, 4, e7459. [Google Scholar] [CrossRef]
- Meibalan, E.; Marti, M. Biology of Malaria Transmission. Cold Spring Harb. Perspect. Med. 2017, 7, a025452. [Google Scholar] [CrossRef]
- Glenister, F.K.; Coppel, R.L.; Cowman, A.F.; Mohandas, N.; Cooke, B.M. Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood 2002, 99, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.P.; Diez-Silva, M.; Quinn, D.J.; Dao, M.; Lang, M.J.; Tan, K.S.; Lim, C.T.; Milon, G.; David, P.H.; Mercereau-Puijalon, O.; et al. Effect of plasmodial RESA protein on deformability of human red blood cells harboring Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2007, 104, 9213–9217. [Google Scholar] [CrossRef] [PubMed]
- Kaviratne, M.; Khan, S.M.; Jarra, W.; Preiser, P.R. Small variant STEVOR antigen is uniquely located within Maurer’s clefts in Plasmodium falciparum-infected red blood cells. Eukaryot. Cell 2002, 1, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Sam-Yellowe, T.Y.; Florens, L.; Johnson, J.R.; Wang, T.; Drazba, J.A.; Le Roch, K.G.; Zhou, Y.; Batalov, S.; Carucci, D.J.; Winzeler, E.A.; et al. A Plasmodium gene family encoding Maurer’s cleft membrane proteins: Structural properties and expression profiling. Genome Res. 2004, 14, 1052–1059. [Google Scholar] [CrossRef]
- Lavazec, C.; Sanyal, S.; Templeton, T.J. Hypervariability within the Rifin, Stevor and Pfmc-2TM superfamilies in Plasmodium falciparum. Nucleic Acids Res. 2006, 34, 6696–6707. [Google Scholar] [CrossRef]
- Blythe, J.E.; Yam, X.Y.; Kuss, C.; Bozdech, Z.; Holder, A.A.; Marsh, K.; Langhorne, J.; Preiser, P.R. Plasmodium falciparum STEVOR proteins are highly expressed in patient isolates and located in the surface membranes of infected red blood cells and the apical tips of merozoites. Infect. Immun. 2008, 76, 3329–3336. [Google Scholar] [CrossRef]
- Kaur, J.; Hora, R. ‘2TM proteins’: An antigenically diverse superfamily with variable functions and export pathways. PeerJ 2018, 6, e4757. [Google Scholar] [CrossRef]
- Sanyal, S.; Egee, S.; Bouyer, G.; Perrot, S.; Safeukui, I.; Bischoff, E.; Buffet, P.; Deitsch, K.W.; Mercereau-Puijalon, O.; David, P.H.; et al. Plasmodium falciparum STEVOR proteins impact erythrocyte mechanical properties. Blood 2012, 119, e1–e8. [Google Scholar] [CrossRef]
- McRobert, L.; Preiser, P.; Sharp, S.; Jarra, W.; Kaviratne, M.; Taylor, M.C.; Renia, L.; Sutherland, C.J. Distinct trafficking and localization of STEVOR proteins in three stages of the Plasmodium falciparum life cycle. Infect. Immun. 2004, 72, 6597–6602. [Google Scholar] [CrossRef] [PubMed]
- Sharp, S.; Lavstsen, T.; Fivelman, Q.L.; Saeed, M.; McRobert, L.; Templeton, T.J.; Jensen, A.T.; Baker, D.A.; Theander, T.G.; Sutherland, C.J. Programmed transcription of the var gene family, but not of stevor, in Plasmodium falciparum gametocytes. Eukaryot. Cell 2006, 5, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Petter, M.; Haeggström, M.; Khattab, A.; Fernandez, V.; Klinkert, M.Q.; Wahlgren, M. Variant proteins of the Plasmodium falciparum RIFIN family show distinct subcellular localization and developmental expression patterns. Mol. Biochem. Parasitol. 2007, 156, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.P.; Le Roch, K.G.; Sarr, O.; Ndiaye, D.; Lukens, A.; Zhou, Y.; Ndir, O.; Mboup, S.; Sultan, A.; Winzeler, E.A.; et al. . In vivo transcriptome of Plasmodium falciparum reveals overexpression of transcripts that encode surface proteins. J. Infect. Dis. 2005, 191, 1196–1203. [Google Scholar] [CrossRef]
- Huang, Y.X.; Wu, Z.J.; Mehrishi, J.; Huang, B.T.; Chen, X.Y.; Zheng, X.J.; Liu, W.J.; Luo, M. Human red blood cell aging: Correlative changes in surface charge and cell properties. J. Cell. Mol. Med. 2011, 15, 2634–2642. [Google Scholar] [CrossRef]
- Leong, Y.W.; Russell, B.; Malleret, B.; Rénia, L. Erythrocyte tropism of malarial parasites: The reticulocyte appeal. Front. Microbiol. 2022, 13, 1022828. [Google Scholar] [CrossRef] [PubMed]
- Kerlin, D.H.; Gatton, M.L. Preferential invasion by Plasmodium merozoites and the self-regulation of parasite burden. PLoS ONE 2013, 8, e57434. [Google Scholar] [CrossRef]
- Amir, A.; Russell, B.; Liew, J.; Moon, R.W.; Fong, M.Y.; Vythilingam, I.; Subramaniam, V.; Snounou, G.; Lau, Y.L. Invasion characteristics of a Plasmodium knowlesi line newly isolated from a human. Sci. Rep. 2016, 6, 24623. [Google Scholar] [CrossRef]
- McQueen, P.G.; McKenzie, F.E. Age-structured red blood cell susceptibility and the dynamics of malaria infections. Proc. Natl. Acad. Sci. USA 2004, 101, 9161–9166. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, F.E.; Jeffery, G.M.; Collins, W.E. Plasmodium malariae blood-stage dynamics. J. Parasitol. 2001, 87, 626–637. [Google Scholar] [CrossRef]
- Abate, A.; Bouyssou, I.; Mabilotte, S.; Doderer-Lang, C.; Dembele, L.; Menard, D.; Golassa, L. Vivax malaria in Duffy-negative patients shows invariably low asexual parasitaemia: Implication towards malaria control in Ethiopia. Malar. J. 2022, 21, 230. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Mathias, J.L.S.; Albuquerque, S.R.L.; Almeida, A.C.G.; Dantas, A.C.; Anselmo, F.C.; Lima, E.S.; Lacerda, M.V.G.; Nogueira, P.A.; Ramasawmy, R.; et al. Duffy blood system and G6PD genetic variants in vivax malaria patients from Manaus, Amazonas, Brazil. Malar. J. 2022, 21, 144. [Google Scholar] [CrossRef]
- Woolley, I.J.; Wood, E.M.; Sramkoski, R.M.; Zimmerman, P.A.; Miller, J.P.; Kazura, J.W. Expression of Duffy antigen receptor for chemokines during reticulocyte maturation: Using a CD71 flow cytometric technique to identify reticulocytes. Immunohematology 2005, 21, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Moras, M.; Lefevre, S.D.; Ostuni, M.A. From Erythroblasts to Mature Red Blood Cells: Organelle Clearance in Mammals. Front. Physiol. 2017, 8, 1076. [Google Scholar] [CrossRef] [PubMed]
- Coban, C.; Lee, M.S.J.; Ishii, K.J. Tissue-specific immunopathology during malaria infection. Nat. Rev. Immunol. 2018, 18, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Butcher, G.A.; Mitchell, G.H.; Cohen, S. Mechanism of host specificity in malarial infection. Nature 1973, 244, 40–42. [Google Scholar] [CrossRef]
- Swann, A.I. The relationship of erythrocyte age and parasitization with Plasmodium gallinaceum in chickens. Can. J. Comp. Med. 1974, 38, 391–397. [Google Scholar]
- Ben Mamoun, C.; Prigge, S.T.; Vial, H. Targeting the Lipid Metabolic Pathways for the Treatment of Malaria. Drug Dev. Res. 2010, 71, 44–55. [Google Scholar] [CrossRef]
- Ancelin, M.L.; Parant, M.; Thuet, M.J.; Philippot, J.R.; Vial, H.J. Increased permeability to choline in simian erythrocytes after Plasmodium knowlesi infection. Biochem. J. 1991, 273 Pt 3, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Ramaprasad, A.; Burda, P.C.; Calvani, E.; Sait, A.J.; Palma-Duran, S.A.; Withers-Martinez, C.; Hackett, F.; Macrae, J.; Collinson, L.; Gilberger, T.W.; et al. A choline-releasing glycerophosphodiesterase essential for phosphatidylcholine biosynthesis and blood stage development in the malaria parasite. eLife 2022, 11, e82207. [Google Scholar] [CrossRef]
- Kirk, K.; Poli de Figueiredo, C.E.; Elford, B.C.; Ellory, J.C. Effect of cell age on erythrocyte choline transport: Implications for the increased choline permeability of malaria-infected erythrocytes. Biochem. J. 1992, 283, 617–619. [Google Scholar] [CrossRef]
- Griffiths, R.E.; Kupzig, S.; Cogan, N.; Mankelow, T.J.; Betin, V.M.S.; Trakarnsanga, K.; Massey, E.J.; Lane, J.D.; Parsons, S.F.; Anstee, D.J. Maturing reticulocytes internalize plasma membrane in glycophorin A–containing vesicles that fuse with autophagosomes before exocytosis. Blood 2012, 119, 6296–6306. [Google Scholar] [CrossRef]
- Malleret, B.; Xu, F.; Mohandas, N.; Suwanarusk, R.; Chu, C.; Leite, J.A.; Low, K.; Turner, C.; Sriprawat, K.; Zhang, R.; et al. Significant biochemical, biophysical and metabolic diversity in circulating human cord blood reticulocytes. PLoS ONE 2013, 8, e76062. [Google Scholar] [CrossRef] [PubMed]
- Aniweh, Y.; Gao, X.; Hao, P.; Meng, W.; Lai, S.K.; Gunalan, K.; Chu, T.T.; Sinha, A.; Lescar, J.; Chandramohanadas, R.; et al. P. falciparum RH5-Basigin interaction induces changes in the cytoskeleton of the host RBC. Cell. Microbiol. 2017, 19, e12747. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-Rosa, M.F.; Tayler, N.M.; Dorta, D.; Coronado, L.M.; Spadafora, C. P. falciparum Invasion and Erythrocyte Aging. Cells 2024, 13, 334. https://doi.org/10.3390/cells13040334
Alves-Rosa MF, Tayler NM, Dorta D, Coronado LM, Spadafora C. P. falciparum Invasion and Erythrocyte Aging. Cells. 2024; 13(4):334. https://doi.org/10.3390/cells13040334
Chicago/Turabian StyleAlves-Rosa, María Fernanda, Nicole M. Tayler, Doriana Dorta, Lorena M. Coronado, and Carmenza Spadafora. 2024. "P. falciparum Invasion and Erythrocyte Aging" Cells 13, no. 4: 334. https://doi.org/10.3390/cells13040334





