Absence of Heme Oxygenase-1 Affects Trophoblastic Spheroid Implantation and Provokes Dysregulation of Stress and Angiogenesis Gene Expression in the Uterus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. HO-1 siRNA Transfection of Trophoblast Cells Using Electroporation
2.3. Western Blot Analysis of siRNA Transfection Efficacy
2.4. Trophoblastic Spheroid Generation and Adhesion Assay
2.5. Mice and Sample Collection
2.6. Mouse Angiogenesis RT2-Profiler™ PCR Array
2.7. Mouse Stress and Toxicity PathwayFinder™ RT2 Profiler™ PCR Array
2.8. RNA Isolation, cDNA Synthesis and Quantitative Real-Time PCR
2.9. Statistical Analysis
3. Results
3.1. HO-1 Knockdown by siRNA Impairs Implantation in an In Vitro 3D Culture Model
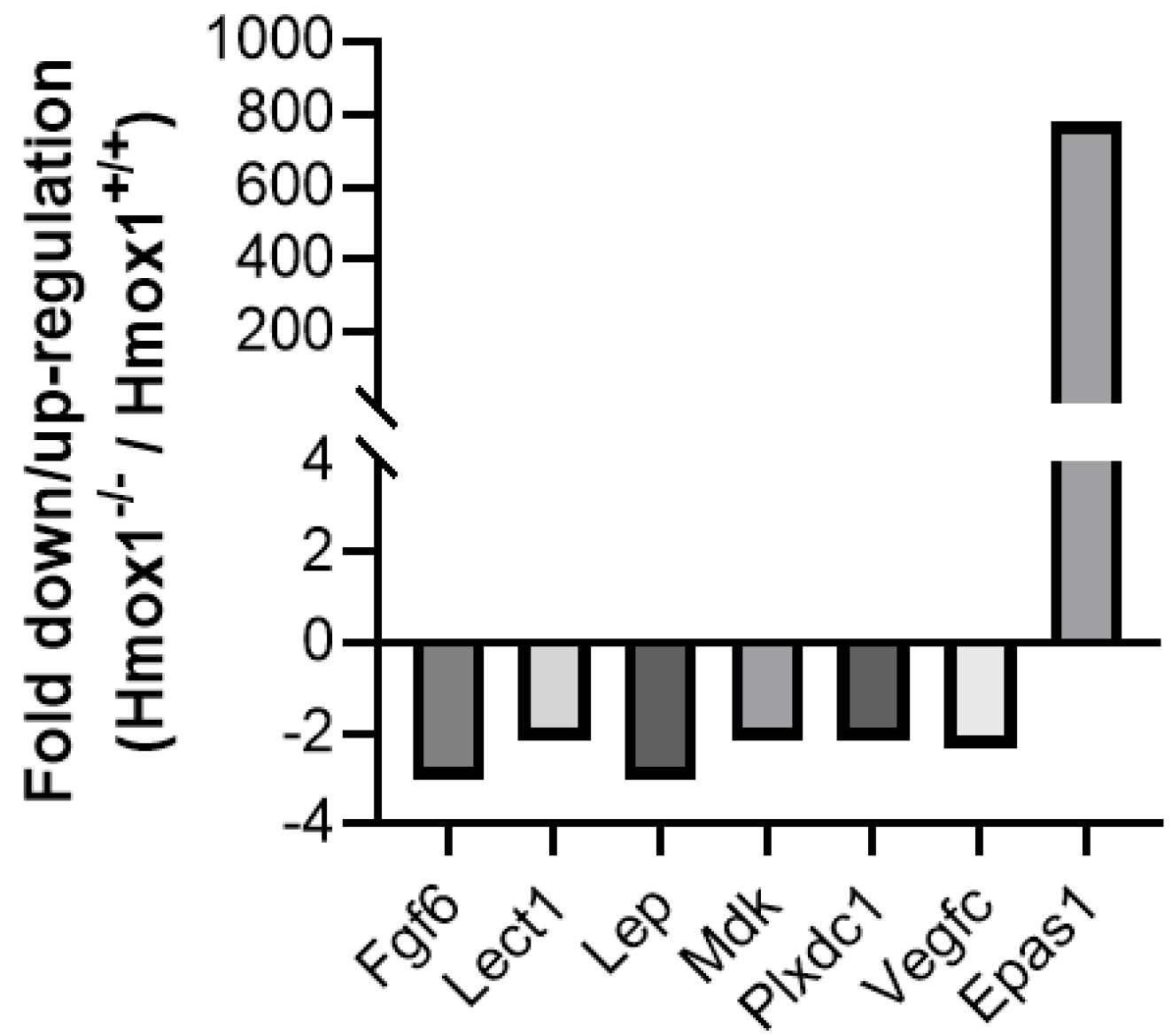
3.2. Uteruses from Hmox1−/− Females Show Altered Expression of Angiogenesis and Stress Markers
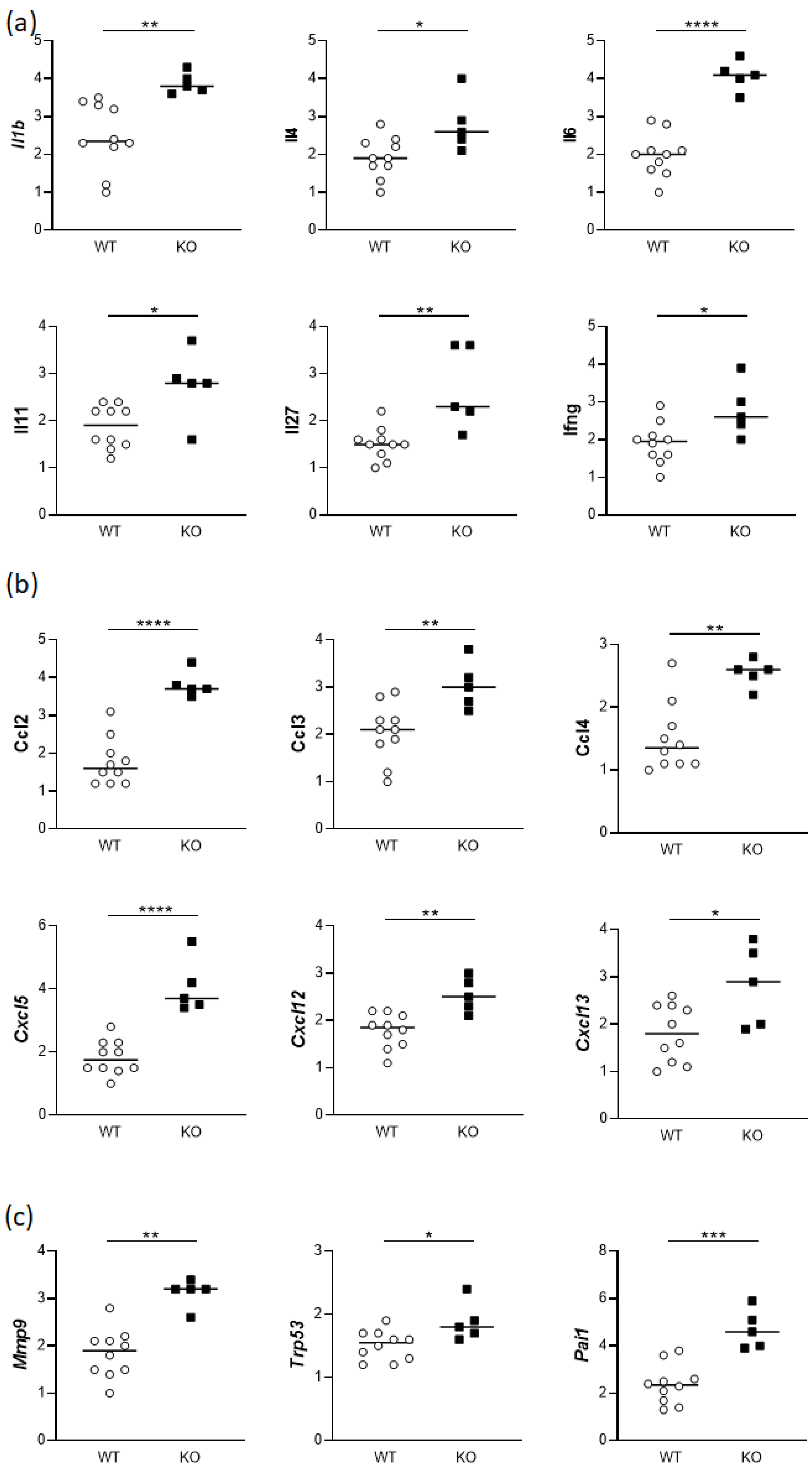
3.3. Uteruses from gd14 Hmox1−/− Females Show Altered Expression of Cytokines and Chemokines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kutty, R.K.; Kutty, G.; Rodriguez, I.R.; Chader, G.J.; Wiggert, B. Chromosomal Localization of the Human Heme Oxygenase Genes: Heme Oxygenase-1 (HMOX1) Maps to Chromosome 22q12 and Heme Oxygenase-2 (HMOX2) Maps to Chromosome 16p13.3. Genomics 1994, 20, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Choi, A.M.K. Heme Oxygenase-1: The “Emerging Molecule” Has Arrived. Am. J. Respir. Cell Mol. Biol. 2002, 27, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Costa Silva, R.C.M.; Correa, L.H.T. Heme Oxygenase 1 in Vertebrates: Friend and Foe. Cell Biochem. Biophys. 2022, 80, 97–113. [Google Scholar] [CrossRef]
- Maamoun, H.; Zachariah, M.; McVey, J.H.; Green, F.R.; Agouni, A. Heme Oxygenase (HO)-1 Induction Prevents Endoplasmic Reticulum Stress-Mediated Endothelial Cell Death and Impaired Angiogenic Capacity. Biochem. Pharmacol. 2017, 127, 46–59. [Google Scholar] [CrossRef]
- Yachie, A.; Niida, Y.; Wada, T.; Igarashi, N.; Kaneda, H.; Toma, T.; Ohta, K.; Kasahara, Y.; Koizumi, S. Oxidative Stress Causes Enhanced Endothelial Cell Injury in Human Heme Oxygenase-1 Deficiency. J. Clin. Investig. 1999, 103, 129–135. [Google Scholar] [CrossRef]
- Dirim, A.B.; Kalayci, T.; Safak, S.; Garayeva, N.; Gultekin, B.; Hurdogan, O.; Solakoglu, S.; Yazici, H.; Cefle, K.; Ozturk, S.; et al. Heme Oxygenase-1 Deficiency as an Extremely Rare Cause of AA-Type Renal Amyloidosis: Expanding the Clinical Features and Review of the Literature. Clin. Rheumatol. 2023, 42, 597–606. [Google Scholar] [CrossRef]
- Radhakrishnan, N.; Yadav, S.P.; Sachdeva, A.; Pruthi, P.K.; Sawhney, S.; Piplani, T.; Wada, T.; Yachie, A. Human Heme Oxygenase-1 Deficiency Presenting with Hemolysis, Nephritis, and Asplenia. J. Pediatr. Hematol. Oncol. 2011, 33, 74–78. [Google Scholar] [CrossRef]
- Radhakrishnan, N.; Yadav, S.P.; Sachdeva, A.; Wada, T.; Yachie, A. An Interesting Tetrad of Asplenia, Inflammation, Hemolysis, and Nephritis. Pediatr. Hematol. Oncol. 2011, 28, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Berendes, L.-S.; Westhoff, P.S.; Wittkowski, H.; Seelhöfer, A.; Varga, G.; Marquardt, T.; Park, J.H. Clinical and Molecular Analysis of a Novel Variant in Heme Oxygenase-1 Deficiency: Unraveling Its Role in Inflammation, Heme Metabolism, and Pulmonary Phenotype. Mol. Genet. Metab. Rep. 2024, 38, 101038. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S. Human Heme Oxygenase-1 Deficiency: A Lesson on Serendipity in the Discovery of the Novel Disease. Pediatr. Int. 2007, 49, 125–132. [Google Scholar] [CrossRef]
- Denschlag, D.; Marculescu, R.; Unfried, G.; Hefler, L.A.; Exner, M.; Hashemi, A.; Riener, E.-K.; Keck, C.; Tempfer, C.B.; Wagner, O. The Size of a Microsatellite Polymorphism of the Haem Oxygenase 1 Gene Is Associated with Idiopathic Recurrent Miscarriage. Mol. Human Reprod. 2004, 10, 211–214. [Google Scholar] [CrossRef]
- Kaartokallio, T.; Klemetti, M.M.; Timonen, A.; Uotila, J.; Heinonen, S.; Kajantie, E.; Kere, J.; Kivinen, K.; Pouta, A.; Lakkisto, P.; et al. Microsatellite Polymorphism in the Heme Oxygenase-1 Promoter Is Associated with Nonsevere and Late-Onset Preeclampsia. Hypertension 2014, 64, 172–177. [Google Scholar] [CrossRef]
- Kreiser, D.; Nguyen, X.; Wong, R.; Seidman, D.; Stevenson, D.; Quan, S.; Abraham, N.; Dennery, P.A. Heme Oxygenase-1 Modulates Fetal Growth in the Rat. Lab. Investig. 2002, 82, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Zhao, H.; Stevenson, D.K. A Deficiency in Haem Oxygenase-1 Induces Foetal Growth Restriction by Placental Vasculature Defects. Acta Paediatr. 2012, 101, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Langwisch, S.; Scharm, M.; Zenclussen, A.C. Using Ultrasound to Define the Time Point of Intrauterine Growth Retardation in a Mouse Model of Heme Oxygenase-1 Deficiency†. Biol. Reprod. 2020, 103, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Choubey, P.; Nandi, G.; Jain, S.; Bajaj, D.; Sharma, S.; Basu-Modak, S. Expression of Angiogenic Factors in the Placenta of Heme Oxygenase-1 Deficient Mouse Embryo. Reprod. Biol. 2023, 23, 100822. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Azuma, J.; Kalish, F.; Wong, R.J.; Stevenson, D.K. Maternal Heme Oxygenase 1 Regulates Placental Vasculature Development via Angiogenic Factors in Mice1. Biol. Reprod. 2011, 85, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Cudmore, M.; Ahmad, S.; Al-Ani, B.; Fujisawa, T.; Coxall, H.; Chudasama, K.; Devey, L.R.; Wigmore, S.J.; Abbas, A.; Hewett, P.W.; et al. Negative Regulation of Soluble Flt-1 and Soluble Endoglin Release by Heme Oxygenase-1. Circulation 2007, 115, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- El-Mousleh, T.; Casalis, P.A.; Wollenberg, I.; Zenclussen, M.L.; Volk, H.D.; Langwisch, S.; Jensen, F.; Zenclussen, A.C. Exploring the Potential of Low Doses Carbon Monoxide as Therapy in Pregnancy Complications. Med. Gas Res. 2012, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.A.; Peterson, N.; McRae, K.E.; Pudwell, J.; Tayade, C.; Smith, G.N. Carbon Monoxide Increases Utero-Placental Angiogenesis without Impacting Pregnancy Specific Adaptations in Mice. Reprod. Biol. Endocrinol. 2020, 18, 49. [Google Scholar] [CrossRef]
- Zenclussen, M.L.; Casalis, P.A.; El-Mousleh, T.; Rebelo, S.; Langwisch, S.; Linzke, N.; Volk, H.-D.; Fest, S.; Soares, M.P.; Zenclussen, A.C. Haem Oxygenase-1 Dictates Intrauterine Fetal Survival in Mice via Carbon Monoxide. J. Pathol. 2011, 225, 293–304. [Google Scholar] [CrossRef]
- Yet, S.F.; Perrella, M.A.; Layne, M.D.; Hsieh, C.M.; Maemura, K.; Kobzik, L.; Wiesel, P.; Christou, H.; Kourembanas, S.; Lee, M.E. Hypoxia Induces Severe Right Ventricular Dilatation and Infarction in Heme Oxygenase-1 Null Mice. J. Clin. Investig. 1999, 103, R23–R29. [Google Scholar] [CrossRef]
- Zenclussen, M.L.; Jensen, F.; Rebelo, S.; El-Mousleh, T.; Casalis, P.A.; Zenclussen, A.C. Heme Oxygenase-1 Expression in the Ovary Dictates a Proper Oocyte Ovulation, Fertilization, and Corpora Lutea Maintenance. Am. J. Reprod. Immunol. 2012, 67, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.H. Heme Oxygenase-1: A Therapeutic Amplification Funnel. FASEB J. 2005, 19, 1216–1219. [Google Scholar] [CrossRef]
- Poss, K.D.; Tonegawa, S. Heme Oxygenase 1 Is Required for Mammalian Iron Reutilization. Proc. Natl. Acad. Sci. USA 1997, 94, 10919–10924. [Google Scholar] [CrossRef]
- Kapturczak, M.H.; Wasserfall, C.; Brusko, T.; Campbell-Thompson, M.; Ellis, T.M.; Atkinson, M.A.; Agarwal, A. Heme Oxygenase-1 Modulates Early Inflammatory Responses: Evidence from the Heme Oxygenase-1-Deficient Mouse. Am. J. Pathol. 2004, 165, 1045–1053. [Google Scholar] [CrossRef]
- Park, H.; Cho, B.; Kim, J. Rad50 Mediates DNA Demethylation to Establish Pluripotent Reprogramming. Exp. Mol. Med. 2020, 52, 1116–1127. [Google Scholar] [CrossRef]
- Beikzadeh, M.; Latham, M.P. The Dynamic Nature of the Mre11-Rad50 DNA Break Repair Complex. Prog. Biophys. Mol. Biol. 2021, 163, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci-Da Silva, C.; Zhan, L.; Shen, J.; Kong, B.; Campbell, M.J.; Memon, N.; Hegyi, T.; Lu, L.; Guo, G.L. Effects of Total Parenteral Nutrition on Drug Metabolism Gene Expression in Mice. Acta Pharm. Sin. B 2020, 10, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Spencer, T.E. Estrogen Disruption of Neonatal Ovine Uterine Development: Effects on Gene Expression Assessed by Suppression Subtraction Hybridization. Biol. Reprod. 2005, 73, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Ansell, P.J.; Espinosa-Nicholas, C.; Curran, E.M.; Judy, B.M.; Philips, B.J.; Hannink, M.; Lubahn, D.B. In vitro and in vivo Regulation of Antioxidant Response Element-Dependent Gene Expression by Estrogens. Endocrinology 2004, 145, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Lämsä, V.; Levonen, A.-L.; Sormunen, R.; Yamamoto, M.; Hakkola, J. Heme and Heme Biosynthesis Intermediates Induce Heme Oxygenase-1 and Cytochrome P450 2A5, Enzymes with Putative Sequential Roles in Heme and Bilirubin Metabolism: Different Requirement for Transcription Factor Nuclear Factor Erythroid-Derived 2-like 2. Toxicol. Sci. 2012, 130, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Lämsä, V.; Levonen, A.-L.; Leinonen, H.; Ylä-Herttuala, S.; Yamamoto, M.; Hakkola, J. Cytochrome P450 2A5 Constitutive Expression and Induction by Heavy Metals Is Dependent on Redox-Sensitive Transcription Factor Nrf2 in Liver. Chem. Res. Toxicol. 2010, 23, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Jarvenpaa, J.; Vuoristo, J.T.; Savolainen, E.-R.; Ukkola, O.; Vaskivuo, T.; Ryynanen, M. Altered Expression of Angiogenesis-Related Placental Genes in Pre-Eclampsia Associated with Intrauterine Growth Restriction. Gynecol. Endocrinol. 2007, 23, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Montesano, R.; Vassalli, J.D.; Baird, A.; Guillemin, R.; Orci, L. Basic Fibroblast Growth Factor Induces Angiogenesis in Vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 7297–7301. [Google Scholar] [CrossRef]
- Malik, N.M.; Carter, N.D.; Murray, J.F.; Scaramuzzi, R.J.; Wilson, C.A.; Stock, M.J. Leptin Requirement for Conception, Implantation, and Gestation in the Mouse. Endocrinology 2001, 142, 5198–5202. [Google Scholar] [CrossRef]
- Zenclussen, A.C.; Blois, S.; Stumpo, R.; Olmos, S.; Arias, K.; Malan Borel, I.; Roux, M.E.; Margni, R.A. Murine Abortion Is Associated with Enhanced Interleukin-6 Levels at the Feto-Maternal Interface. Cytokine 2003, 24, 150–160. [Google Scholar] [CrossRef]
- Lockwood, C.J.; Yen, C.-F.; Basar, M.; Kayisli, U.A.; Martel, M.; Buhimschi, I.; Buhimschi, C.; Huang, S.J.; Krikun, G.; Schatz, F. Preeclampsia-Related Inflammatory Cytokines Regulate Interleukin-6 Expression in Human Decidual Cells. Am. J. Pathol. 2008, 172, 1571–1579. [Google Scholar] [CrossRef]
- Kırıcı, P.; Çağıran, F.T.; Kalı, Z.; Tanrıverdi, E.F.; Mavral, N.; Ecin, S.M. Determination of Maternal Serum Pro-Inflammatory Cytokine Changes in Intrauterine Growth Restriction. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1996–2001. [Google Scholar] [CrossRef]
- Comba, C.; Bastu, E.; Dural, O.; Yasa, C.; Keskin, G.; Ozsurmeli, M.; Buyru, F.; Serdaroglu, H. Role of Inflammatory Mediators in Patients with Recurrent Pregnancy Loss. Fertil. Steril. 2015, 104, 1467–1474.e1. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Huang, D.; Mei, Y.; Liu, Z.; Zhou, Y.; He, J.; Zhang, H.; Yin, N.; Qi, H. Identification of the Correlations between Interleukin-27 (IL-27) and Immune-Inflammatory Imbalance in Preterm Birth. Bioengineered 2021, 12, 3201–3218. [Google Scholar] [CrossRef]
- Winship, A.; Dimitriadis, E. Interleukin 11 Is Upregulated in Preeclampsia and Leads to Inflammation and Preeclampsia Features in Mice. J. Reprod. Immunol. 2018, 125, 32–38. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kim, J.H.; Kim, K.J.; Ko, E.J.; Lee, J.Y.; Ryu, C.S.; Ha, Y.H.; Kim, Y.R.; Kim, N.K. Genetic Association between Inflammatory-Related Polymorphism in STAT3, IL-1β, IL-6, TNF-α and Idiopathic Recurrent Implantation Failure. Genes 2023, 14, 1588. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Kalish, F.; Wong, R.J.; Stevenson, D.K. Infiltration of Myeloid Cells in the Pregnant Uterus Is Affected by Heme Oxygenase-1. J. Leukoc. Biol. 2017, 101, 217–226. [Google Scholar] [CrossRef]
- Palomino, D.C.T.; Marti, L.C. Chemokines and immunity. Einstein (Sao Paulo) 2015, 13, 469–473. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenclussen, M.L.; Ulrich, S.; Bauer, M.; Fink, B.; Zenclussen, A.C.; Schumacher, A.; Meyer, N. Absence of Heme Oxygenase-1 Affects Trophoblastic Spheroid Implantation and Provokes Dysregulation of Stress and Angiogenesis Gene Expression in the Uterus. Cells 2024, 13, 376. https://doi.org/10.3390/cells13050376
Zenclussen ML, Ulrich S, Bauer M, Fink B, Zenclussen AC, Schumacher A, Meyer N. Absence of Heme Oxygenase-1 Affects Trophoblastic Spheroid Implantation and Provokes Dysregulation of Stress and Angiogenesis Gene Expression in the Uterus. Cells. 2024; 13(5):376. https://doi.org/10.3390/cells13050376
Chicago/Turabian StyleZenclussen, Maria Laura, Sina Ulrich, Mario Bauer, Beate Fink, Ana Claudia Zenclussen, Anne Schumacher, and Nicole Meyer. 2024. "Absence of Heme Oxygenase-1 Affects Trophoblastic Spheroid Implantation and Provokes Dysregulation of Stress and Angiogenesis Gene Expression in the Uterus" Cells 13, no. 5: 376. https://doi.org/10.3390/cells13050376







