Abstract
Aging populations worldwide are placing age-related diseases at the forefront of the research agenda. The therapeutic potential of natural substances, especially propolis and its components, has led to these products being promising agents for alleviating several cellular and molecular-level changes associated with age-related diseases. With this in mind, scientists have introduced a contextual framework to guide future aging research, called the hallmarks of aging. This framework encompasses various mechanisms including genomic instability, epigenetic changes, mitochondrial dysfunction, inflammation, impaired nutrient sensing, and altered intercellular communication. Propolis, with its rich array of bioactive compounds, functions as a potent functional food, modulating metabolism, gut microbiota, inflammation, and immune response, offering significant health benefits. Studies emphasize propolis’ properties, such as antitumor, cardioprotective, and neuroprotective effects, as well as its ability to mitigate inflammation, oxidative stress, DNA damage, and pathogenic gut bacteria growth. This article underscores current scientific evidence supporting propolis’ role in controlling molecular and cellular characteristics linked to aging and its hallmarks, hypothesizing its potential in geroscience research. The aim is to discover novel therapeutic strategies to improve health and quality of life in older individuals, addressing existing deficits and perspectives in this research area.
1. Introduction
It is estimated that the global population of people over the age of 64 years will more than double, increasing from 761 million in 2021 to 1.6 billion by 2050 [1]. The segment comprising individuals who are 80 years and beyond is experiencing a more accelerated rate of expansion. When it comes to acknowledging the significance of aging societies, apprehension tends to be the prevailing reaction. Expressions such as “grey tsunami” or “silver tsunami” insinuate that a larger population of older individuals will impose a burden on societies, especially considering the limited success of global endeavors in extending the period of healthy life [2]. At present, medical interventions primarily increase lifespan by prolonging the duration of morbidity [3]. An interesting aspect is that, despite the upward trend in average human life expectancy, there has been no corresponding rise in the maximum lifespan. Moreover, there is a considerable diversity in aging rates within a given species. Senescence is genetically rooted, but it is possible to modulate a species’ genetically determined lifespan by manipulating genes or adjusting dietary habits [4,5,6]. Taking queen honey bees as a case in point, their lifespan is typically around ten times more extensive than that of worker bees, despite possessing identical genetic material [7]. In this setting, multiple studies consistently provide undeniable proof of the foundational relevance of gene–diet interactions in molding individual disparities in health outcomes and longevity [7,8,9,10,11].
Aging is a broad concept that refers to the gradual decline in functionality that is commonly observed in most living organisms as they grow older [12,13]. It is outlined as a weakened response to stress, an amplification of homeostatic instability, and a heightened susceptibility to age-related diseases. Aging coincides with conditions like type 2 diabetes, cancer, and cardiovascular and neurodegenerative diseases, among many others [14].
Significant progress in research has provided critical knowledge about the processes and intrinsic molecular and cellular factors behind the biology of aging [15,16,17,18,19,20,21]. Referred to as hallmarks of aging, many factors implicated in the aging process have been identified, involving persistent inflammation, mitochondrial dysfunction, genomic instability, telomere shortening, epigenetic modifications, impaired protein homeostasis, compromised autophagy, dysregulated nutrient sensing, disrupted intercellular communication, and imbalance of gut microbiota, among other emerging factors [22]. Although aging and age-related diseases present clinically distinct characteristics, research findings uphold support for the concept that both arise as a result of distinctive combinations of changes within a limited set of fundamental biological processes [23]. While the hallmarks of aging manifest in all individuals, the precise interplay among these mechanisms and their collective contribution or causal relationships in the broader aging process and the emergence of age-related diseases are not fully understood. Nevertheless, these hallmarks may present a more holistic insight into both aging itself and age-related diseases, paving the way for research in interventions and therapies to enhance health in older individuals [24]. Incorporating environmental interventions, particularly nutrition, for the purpose of regulating the fundamental biological pillars of aging and age-related diseases has ignited scientific attention and propelled research efforts in the biomedical sphere. Propolis, together with its bioactive constituents, represents an emerging bioresource with prospects in the nutraceutical and pharmaceutical fields [25,26,27,28,29,30]. In light of this, propolis is acknowledged for its potential to serve as a foundation for the formulation of innovative pharmaceuticals.
This article is centered on recent scientific evidence that endorses propolis and its bioactive components’ capacity to regulate the primary cellular and molecular traits linked to aging (Figure 1). These characteristics of aging are also relevant to age-related diseases. We posit that the existing pool of evidence positions these bee-derived substances as promising contenders in the field of geroscience research, with the goal of discovering novel therapeutic strategies to improve health and quality of life in old age. We also tackle the shortcomings and prospects in this area of investigation.
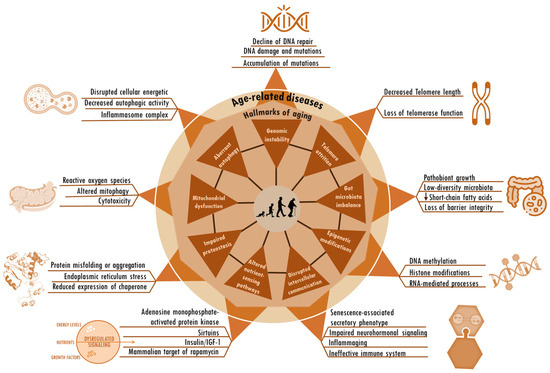
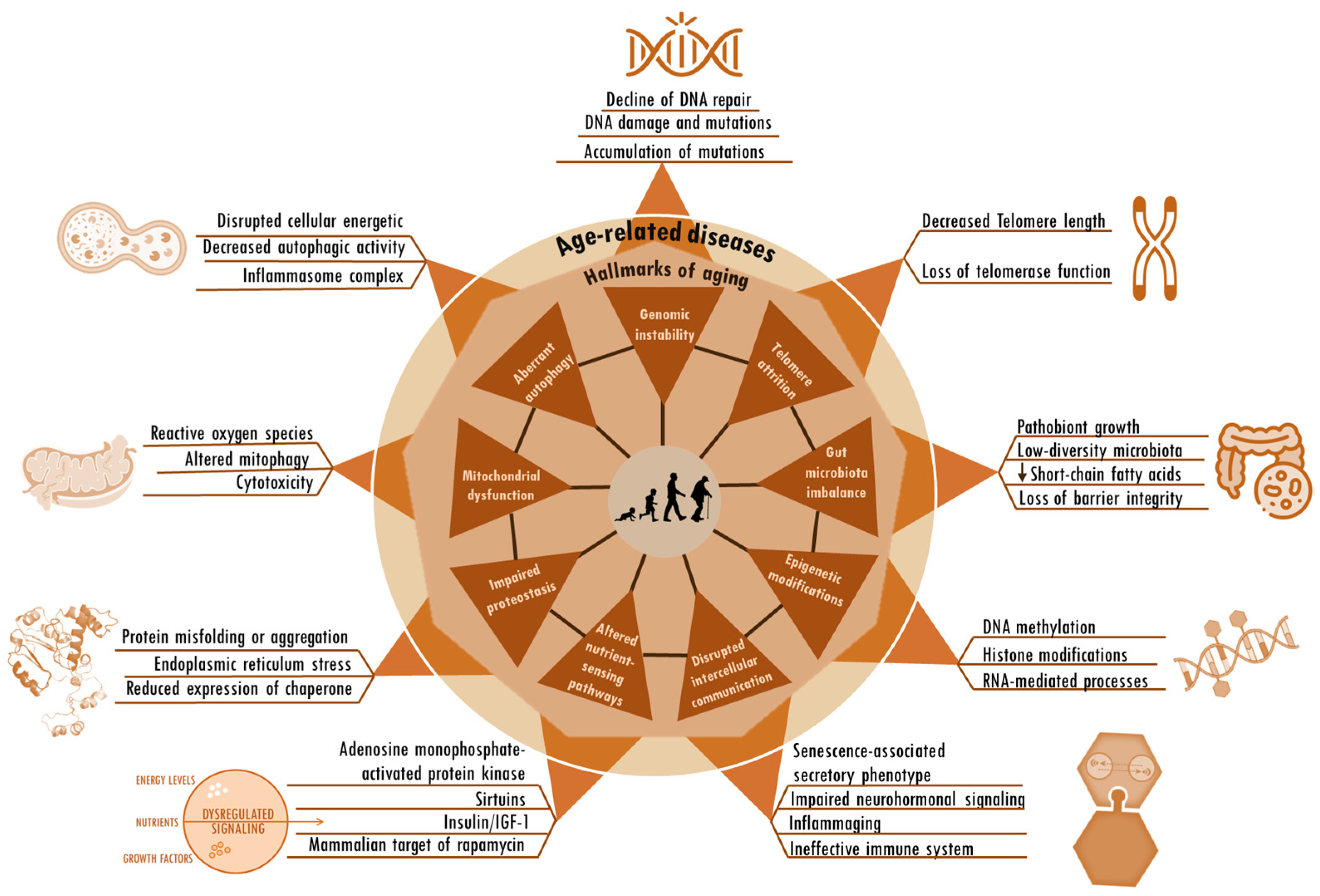
Figure 1.
Hallmarks of aging and age-related alterations. The hallmarks of aging, including genomic instability, telomere attrition, aberrant autophagy, gut microbiota imbalance, mitochondrial dysfunction, epigenetic modifications, altered nutrient-sensing pathways, impaired protein homeostasis, and disrupted intercellular communication, are deeply interconnected. These same factors seem to be root cause contributors to age-related diseases. The particular factors and sequences that trigger the shift from the normal changes of aging to the onset of age-related diseases remain unknown.
2. Propolis
Bees produce propolis, a resinous substance composed of plant elements, and apply it in stratified layers within hives to seal cracks, protect against microorganisms, regulate temperature, and encapsulate deceased intruders to prevent contamination of the colonies [31,32]. A propolis-enriched environment in bee colonies has been observed to impact bacterial populations and contribute to the stability of the bee microbiota [33]. Produced by several cataloged species, the composition of propolis varies according to geography, bee species, and local flora. Despite the potential variation among its more than 800 compounds, the samples show similarities [34]. Moreover, propolis is rich in vital vitamins, including A, D, and those from the B complex, as well as essential minerals such as calcium, magnesium, potassium, manganese, zinc, and iron. Additionally, it comprises specific fatty acids, enzymes originating from bee saliva, polysaccharides, and sugars that are frequently identified in propolis [35,36]. In the realm of organic constituents, noteworthy elements include terpenes and steroids, as well as sugars and amino acids, along with various flavonoids. Notable among the flavonoids are caffeic, cinnamic, p-coumaric, and chicoric acids, as well as quercetin, pinocembrin, baicalin, galangin, and chrysin, owing to their significant antiglycation [37,38,39], cardioprotective [40,41,42,43,44], neuroprotective [45,46,47,48,49,50], cytostatic [51,52,53], radioprotective [54,55,56,57], antimicrobial [58,59,60,61,62,63], anticancer [64,65,66,67,68,69,70], antioxidant [71,72,73,74,75,76], immunomodulatory [77,78,79,80,81,82,83], antidiabetic [84,85,86,87,88,89], and anti-inflammatory [90,91,92,93,94,95] activities and influence on gut health [80,85,96,97,98] and lipid metabolism [99,100,101,102,103,104] (Figure 2). Nevertheless, the precise mechanisms through which propolis’s bioactive constituents operate remain incompletely understood. Studies tie their effects to the mitigation of mitochondrial dysfunction, oxidative stress, inflammation, and the scavenging of free radicals. Under normal physiological circumstances, reactive oxygen species serve as cellular signaling entities involved in processes such as cell growth, adhesion, differentiation, senescence, and apoptosis. However, heightened production of oxidants is indicative of progressing inflammation [105]. Ongoing oxidative stress and inflammation are common denominators in various pathologies, including cancer, cardiovascular diseases, diabetes, neurological disorders, psychiatric illnesses, kidney diseases, lung diseases, and aging [106,107]. Elevated levels of oxidative stress, quantifiable through biomarkers, have been observed in elderly individuals or those with unhealthy lifestyles, characterized by the consumption of unwholesome diets, tobacco use, alcohol consumption, lack of physical exercise, and genetic predisposition [106,107]. The concerted action of antioxidants in ameliorating the deleterious effects of oxidative stress is achieved through the activities of antioxidant enzymes and vitamins C and E, as well as flavonoids, such as those found in propolis [108,109]. Numerous studies substantiate the potential roles and effectiveness of propolis and its compounds in counteracting the detrimental effects associated with various acute and chronic diseases [25,26,27,28,110,111,112]. The therapeutic abilities of propolis and its active components in pharmacological applications and medicine have been affirmed. Ongoing research is also rooted in a more in-depth understanding of its biological activities.
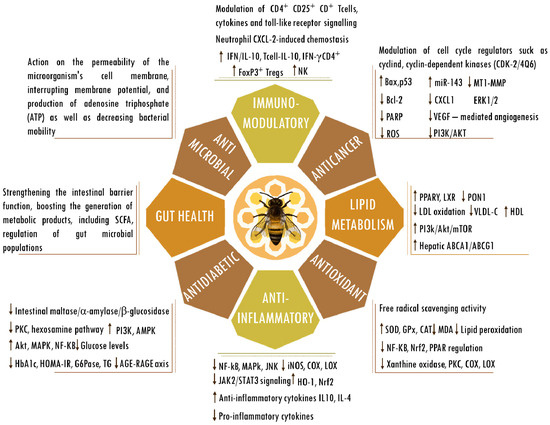
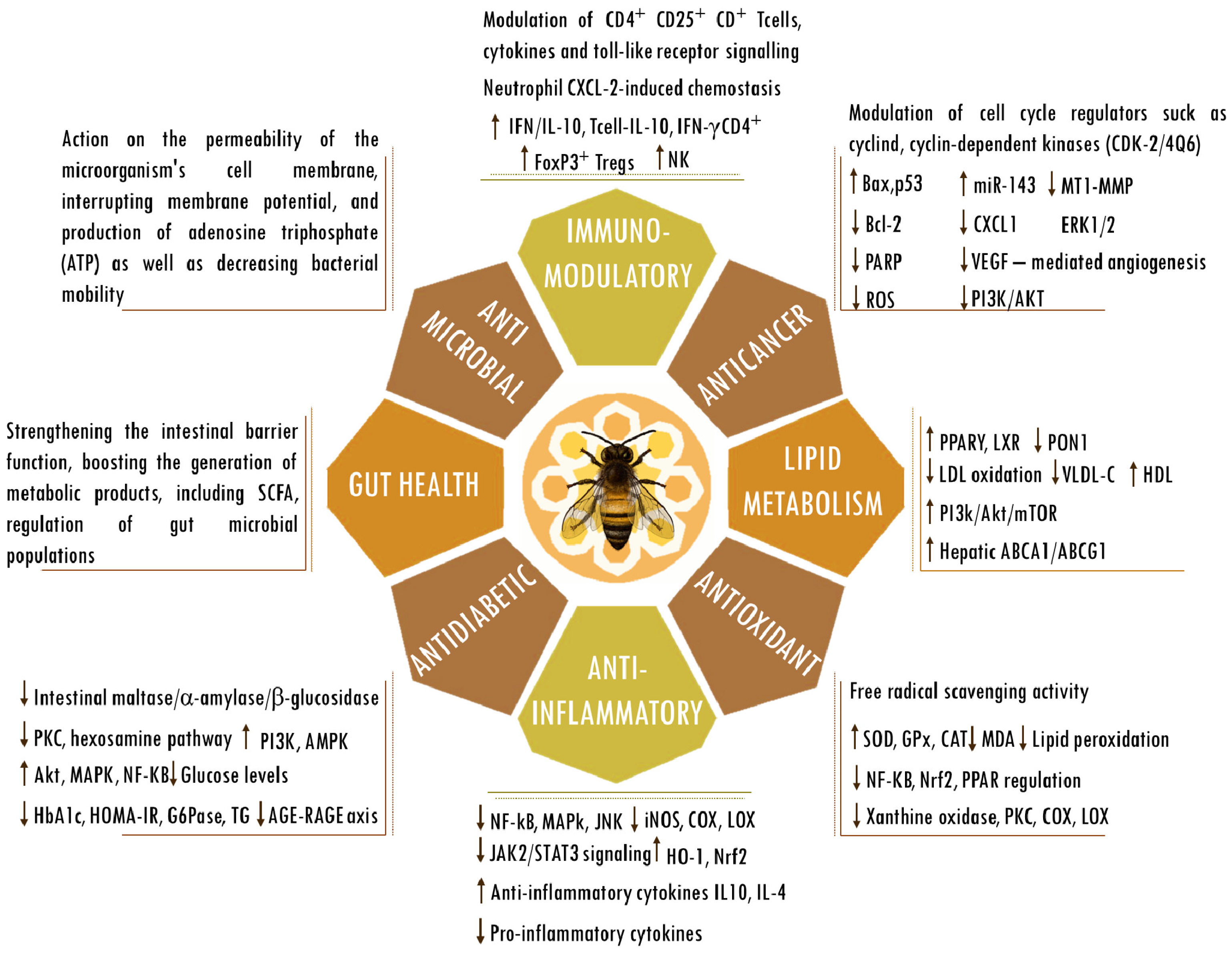
Figure 2.
Biological activities of propolis. Abbreviations: ABCA1, ATP-binding cassette subfamily A member 1; ABCG1, ATP-binding cassette (ABC) subfamily G member 1; AGEs, advanced glycation end products; Bax, bcl-2-like protein 4; Bcl-2, B cell lymphoma 2; CAT, catalase; CD, cluster of differentiation; COX; cyclooxygenase; CXCL-2, chemokine (C-X-C motif) ligand 2; ERK, extracellular signal-regulated kinase; FOXP3, forkhead box P3; G6Pase, glucose-6-phosphatase; GPx, glutathione peroxidase; HbA1C, hemoglobin A1C; HDL, high-density lipoprotein; HO-1, heme oxygenase-1; HOMA-IR, homeostasis model assessment-estimated insulin resistance; iNOS, inducible nitrogen oxide synthase; IFN, interferon; IL, interleukin; JAK2/STAT3, Janus kinase 2/signal transducer and activator of transcription 3; JNK, Jun kinase; LDL, low-density lipoprotein; LOX; lipoxygenase; LXRs, liver X receptors; MDA, malondialdehyde; mTOR, mammalian target of rapamycin; MT1-MMP, membrane type 1-matrix metalloproteinase; miR143, microRNA 143; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NK, natural killer cells; Nrf2, nuclear factor erythroid 2-related factor 2; PPARγ, peroxisome proliferator-activated receptor gamma; PARP, poly (ADP-ribose) polymerase; p53, tumor protein p53; PI3K/AKT, phosphatidylinositol 3-kinase/ protein kinase B; PKC, protein kinase C; PON1, paraoxonase-1; RAGE, receptor for AGEs; regulatory T cells (Tregs); ROS, reactive oxygen species; SCFAs, short-chain fatty acids; SOD, superoxide dismutase; TG, triglyceride; VEGF, vascular endothelial growth factor; VLDL-c, very-low-density lipoprotein cholesterol. The up and down arrows indicate an increase or reduction in the mentioned components.
3. Genomic Stability
A stable genome is crucial for cells to reliably pass on genetic information. Accumulating genome damage and somatic mutations that induce genome instability stand out as the primary mechanisms driving aging [113]. The aging process is associated with an observed escalation in the frequency of damages to DNA (i.e., single- and double-strand breaks, crosslinks, modified bases, oxidative-induced, and depurination or depyrimidination of sugars) and mutations (i.e., insertions, deletions, or substitutions), which contribute to age-related genomic instability [114]. Cell cycle stress, alterations in gene expression, and modifications in gene regulation are outcomes of genomic instability. A broad spectrum of alterations occurs at the molecular level, including the overexpression of proinflammatory genes and inflammatory adipocytokines, impaired metabolism of glycans, glucose, and fatty acids, increases in collagen crosslinking, abnormal insulin-IGF1, mitochondrial dysfunction, disturbance of germline genetic heterogeneity, dysbiosis, and dysregulation of signaling pathways such as FoxO, heat shock proteins, NF-Κb, and mTOR signaling [14,115]. Actually, genomic instability is functionally intertwined with every hallmark of aging [116]. In the end, this might account for cellular degeneration and functional decline that occur with age. The ultimate results of genomic instability include aging and the manifestation of age-related diseases.
Genomic instability is pivotal in both the initiation and progression of cancer [117]. For instance, a visible manifestation of aging-related DNA damage is sun-damaged skin. The formation of ultraviolet light (UV)-induced DNA mutations is a crucial contributing factor to the elevated risk of cancer development associated with age [118]. Studies have shown that apigenin, an active component present in propolis, plays a crucial role in safeguarding skin keratinocytes from ultraviolet B-induced DNA damage and oxidative stress [119]. This protective effect is achieved through the activation of nucleotide excision repair genes, facilitation of cyclobutane ring repair, inhibition of ROS production, and downregulation of NF-κB and MAPK signaling pathways. The protective properties of propolis extracts are evident in human experimental in vitro skin models, where they effectively inhibit UV-induced photodamage by mitigating the occurrence of DNA strand breaks and enhancing cell viability [120]. For instance, propolis is acknowledged as a powerful inhibitor of tyrosinase, a pivotal enzyme in the melanogenesis pathway [121]. Given that UV irradiation can trigger oxidative stress leading to DNA damage and pigmentation disorders, Stavropoulou et al. examined various samples of Greek propolis. Their evaluation involved assessing free radical scavenging, anticollagenase, and antityrosinase activities to determine the antioxidant and anti-aging attributes of propolis. The study results underscored that samples featuring higher levels of flavonoids showcased superior antioxidant activity and noteworthy collagenase inhibition. In contrast, samples rich in terpenoids showed heightened antityrosinase activity [122]. Compelling evidence supports the roles of propolis and its polyphenolic components in combating cancer by countering events such as elevated DNA damage, mutations, strand breaks, chromosomal instability, deregulated DNA repair machinery, impaired cell cycle checkpoints, and dysregulated homologous recombination [123]. Recent research indicates a notable escalation in age-related hepatocellular carcinoma among older individuals [124]. Cigarette smoke-derived carcinogens such as 4-aminobiphenyl (ABP) contribute to the development of hepatocellular carcinoma in humans. The enzyme cytochrome P450 family 2 subfamily E member 1 (CYP2E1), present across mammalian species, can trigger procarcinogens and convert certain specific drugs into cytotoxic products [125]. According to research findings, propolis treatment can inhibit the conversion of the tobacco carcinogen 4-aminobiphenyl (4-ABP) into its metabolite, N-hydroxy-ABP, by inhibiting CYP2E1 expression [126]. This results in reduced production of reactive oxygen species (ROS) and genotoxicity, serving as a protective measure against cancer development. The induction of mutation, cell death, malformation, and cancer is a well-known consequence of ionizing radiation on both somatic and germ cells. Flavonoids are important components of propolis that have neuroprotective effects against brain damage caused by ionizing radiation, attributed in part to their ability to scavenge free radicals and stabilize the DNA double-helix structure [127]. A primary mechanism widely documented to counteract toxic molecules is the removal of reactive oxygen species (ROS) by antioxidants, preventing their interaction with other molecules, including DNA [118]. Studies have confirmed the powerful antioxidant capacity of propolis and its components. The SH-SY5Y cell line is frequently employed as an in vitro experimental model for investigating Parkinson’s disease. A study reported that pretreatment of SH-SY5Y cells with Brazilian green propolis decreases H2O2-generated ROS derived from mitochondria along with a reduction in the signal intensity of 8-oxo-2′-deoxyguanosine (8-oxo-dG lesion is highly mutagenic) [128].
The issue of fertility preservation has gained significant attention in the context of cancer treatment. In a mouse study, in addition to restoring testicular testosterone levels, Indian propolis also alleviated testicular toxicity caused by mitomycin-C by decreasing DNA damage and enhancing antioxidant activity [129]. In alignment with this, a study examining the genotoxicity, cytotoxicity, and clonogenic death of Chinese hamster ovary cells exposed to gamma radiation indicated the potential utility of propolis in mitigating the adverse effects caused by ionizing radiation [130]. It has been observed that apigenin becomes integrated into the nuclear matrix of prostate epithelial cells. Its binding to nucleic acid bases within the matrix is believed to underlie its antioxidant and chemopreventive activities [131].
Iron’s impact on the aging process is frequently underestimated. Despite its importance for living organisms, its reactivity poses a potential risk [132]. With aging, there is a buildup of iron that correlates with the onset of many age-related diseases. Through its strong iron-binding ability and high lipophilicity, caffeic acid phenethyl ester (CAPE), a constituent of propolis, offers protection against cellular DNA damage caused by iron [133]. Studies have demonstrated a correlation between the antioxidant effects of Portuguese propolis extracts and their ability to prevent DNA damage induced by Fe2+ [134].
Diabetes emerges as a key health issue affecting the elderly population. The literature findings suggest that the use of propolis and epigallocatechin gallate results in a significant increase in the survival rate of diabetic mice [135]. Moreover, the administration of this dietary supplementation yields a notable reduction in lipid peroxidation levels in the kidney, liver, and brain tissue, along with reduced DNA damage in the peripheral lymphocytes of diabetic animals [135].
4. Telomere Length
Unique genomic segments called telomeres reside at the extremities of linear chromosomes [136]. Their primary function is to ensure chromosome integrity and genome stability, offering protection against harm and deterioration [137]. Without a telomere maintenance mechanism, telomeres in adult somatic cells undergo a reduction in length after each round of mitotic cell division, ultimately resulting in cellular senescence and aging [138]. The length of telomeres, a complex hereditary characteristic, imposes a significant constraint on telomere function and is intricately tied to the aging process and age-related diseases [139]. Lifestyle stress and environmental factors, such as diet, can influence telomere length [140]. The results of research involving human subjects showed that prolonged and frequent intake of bee products, including propolis, is linked to telomere length [141]. In Nasir et al.’s (2015) study, which involved comparing beekeepers consuming bee products with non-beekeeper control subjects abstaining from bee products and beekeeping-related activities, it was observed that annual bee product consumption correlated with an average increase of 0.258 kbp in telomere length [141]. The results also revealed that a greater daily frequency of bee product intake was associated with an average increment of 2.66 kbp in telomere length [141]. It has been suggested that telomere length might benefit positively from an antioxidant-rich diet, as telomeres are known to be prone to shortening due to ROS-induced damage, as well as being particularly vulnerable to the prevalent lesion 8-oxo-guanine and its accumulation [142].
Quercetin is a major flavonoid compound found in propolis that has gained significant recognition for its antioxidant and anti-inflammatory properties, along with its potential anticancer and antitumor effects [143]. For instance, targeting human telomeric G-quadruplex DNA represents one of the mechanisms through which this propolis component demonstrates its anticancer activity [144]. These findings propose quercetin as a strong contender for telomere targeting and as a potent anticancer agent. Additionally, quercetin acts as a suppressor of telomerase, an enzyme responsible for the maintenance of telomere integrity and length [145,146,147]. Telomerase overexpression, a characteristic feature in almost all human cancers, plays a key role in sustaining telomere length and contributes to abnormal cell proliferation and immortalization [148]. Caffeic acid phenethyl ester (CAPE), another potent bioactive compound found in propolis, also exhibits antitumoral and antioxidant properties, as well as cytotoxic and apoptotic effects. The induction of apoptosis by CAPE has been linked to its ability to induce the activity of the catalytic subunit of telomerase, known as human telomerase reverse transcriptase [149]. In parallel, by specifically targeting leukemia cells derived from leukemia patients, Manisa propolis effectively reduces the expression of human telomerase reverse transcriptase, with chrysin identified as a key constituent responsible for this effect [150,151]. Telomerase activation in cancer has been attributed to several mechanisms, including the involvement of oncogenes like Wnt, which serve as transcriptional regulators of telomerase [148]. The ability of Aydın Turkish propolis to modulate the expression of microRNAs in glioblastoma and brain cancer stem cells underscores its anticancer effect, with the WNT and NOTCH pathways being implicated [152].
5. Gut Microbiota
The microbiota is the distinctive combination of living microorganisms existing in a particular environment, for instance, the gut microbiota, while the microbiome encompasses all the microbial genomes within that habitat [153]. In addition to providing a nutrient-rich environment to the microbiota, the ecosystem resulting from the intricate symbiotic relationship between the host and the gut microbiota influences several key functions within the mammalian host, encompassing homeostasis, nutritional responses, metabolism, and immunity [154]. The mounting evidence indicates that disruptions in host–microbe symbiosis can cause compromised gut permeability, allowing gut microbiome-derived metabolites to reach the systemic circulation, triggering an inflammatory state, oxidative stress, and systemic immune responses [155]. Microbiota disruptions can negatively impact body processes and significantly increase the risk of developing diseases such as cancer, neurodegenerative diseases, and heart disease [156]. Evidence points to the gut microbiota’s active involvement in the age-related regulation of host gut inflammation and barrier permeability [157].
A rise in microbiota diversity is observed during the transition from childhood to adulthood, followed by a decline in individuals aged over 65 [156,158]. Starting in middle/late adulthood, intestinal microbiomes undergo a transformation towards greater uniqueness as people age [159]. In contrast, this deviation does not occur in less healthy subjects, and the unique measure of low gut microbiota is an indicator of increased mortality risk in a 4-year follow-up period [159]. Generally, the microbiota of older individuals is marked by reduced diversity, a prevalence of phyla Bacteroidetes over Firmicutes, an upsurge in opportunistic enteropathogens, and a decline in short-chain fatty acid (SCFA)-producing bacteria [160]. Cohort investigations focusing on the elderly reveal a greater prevalence of core genera, namely Bacteroides, Parabacteroides, and Alistipes, in individuals aged 65 years and older in comparison to younger, healthy counterparts [161]. Other research findings indicate a reduction in anaerobic species like Bifidobacteria, coupled with a rise in Lactobacillaceae and Enterobacteriaceae, including Escherichia coli, among the elderly [162]. Alongside a reduction in microbial diversity, shifts in the composition of gut commensals involve a prominent increase in opportunistic and potentially proinflammatory commensal microbes, accompanied by a decrease in beneficial bacteria, including those from the Verrucomicrobia phylum [163]. This results in compromised integrity of the intestinal epithelium barrier, fostering increased gut permeability and subsequent systemic inflammation, thereby escalating the risk of developing age-related diseases and premature death [160].
The research indicates that the host’s long-term life history, including dietary habits, can have a substantial effect on the microbiome at later life stages [164]. In a study involving elderly Japanese subjects, researchers examined the correlation between fecal quercetin metabolism, intestinal microbiota composition, and dietary intake. The results indicated that making changes to one’s diet can bring about alterations in the gut microbiome and, in turn, affect polyphenol metabolism, thereby potentially benefiting the health of the elderly [165]. Studies have uncovered compelling evidence suggesting that quercetin, a flavonoid ubiquitously present in propolis, has a pronounced influence on the gut milieu, with consequential effects on the regulation of intestinal microbiota [166]. In mice subjected to a high-fat diet, quercetin restored gut microbiota balance and the associated endotoxemia triggered by Toll-like receptor 4 (TLR-4)-NF-κB signaling pathway activation [167]. Additionally, this propolis constituent suppressed inflammasome response and endoplasmic reticulum stress pathway activation, leading to the reversal of gut–liver axis disturbances [167]. Similar outcomes were observed in a study where propolis supplementation was found to have a stabilizing effect on the intestinal microbiota profile, suppress the TLR4 pathway and proinflammatory mediators in muscle, and decrease blood levels of lipopolysaccharide (LPS) induced by a high-fat diet in rodents [168]. Toll-like receptor 4 (TLR4) signaling is a critical step in the inflammatory cascade induced by LPSs. Immune cells recognize LPSs through TLR4, which undergoes a conformational change that triggers the recruitment of intracellular adaptor proteins, such as MyD88 (myeloid differentiation primary response 88), initiating downstream signaling by involving other proteins. Eventually, this signaling cascade activates the transcription factor NF-κB, which results in the production and release of proinflammatory chemokines and cytokines, like tumor necrosis factor-alpha (TNF-α) and interleukins (IL-1, IL-6, etc.).
The interplay between gut microbiota and the development of obesity, metabolic syndrome, and type 2 diabetes has been the subject of various studies, with a particular focus on changes in the regulation of glucose and insulin sensitivity. SCFAs arise from anaerobic bacterial fermentation in the gastrointestinal tract. They influence the metabolism of lipids and carbohydrates, primarily through the inhibition of glycolysis and by promoting either lipogenesis or gluconeogenesis [169,170]. Maintaining a proper equilibrium between lipogenesis and the oxidative degradation of fatty acids, SCFAs also impact glucose metabolism systemically [170]. SCFAs regulate pathways governing mechanisms, encompassing receptors, synthesis, hormones, and perception, as they target different body organs. Functioning as strong secretagogues for glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), they amplify the sense of satiety through the gut–brain axis. As a result, this may lead to an indirect reduction in appetite and subsequent food consumption [171]. Through their interaction with the free fatty acid receptors FFA2 and FFA3, SCFAs effectively prevent nuclear factor kappa B activation, leading to the suppression of tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) release [80]. This inhibition is crucial as persistent inflammation can induce insulin resistance in bodily organs, ultimately impairing glucose tolerance. In diabetic rats, propolis treatment led to improvements in the tight junctions and gap junctions in the intestinal epithelium, repairing intestinal mucosal damage [85]. Beyond the elevation of SCFA levels, the study also indicated propolis’ hypoglycemic effects and its positive influence on gut microbial assemblages, restoring the intestinal microbiota microecology of diabetic rats. In line, studies have revealed that propolis administration effectively counteracts dysbiosis, leading to an increase in SCFA production in feces and a decrease in the expression of inflammation-related genes (TNFα and IL-6) in the liver and muscle [80]. These effects provided protection against sarcopenic obesity in Db/Db mice, a model of type II diabetes and obesity. Sarcopenia refers to the natural loss of muscle mass, strength, and muscle function that occurs as a result of aging [172]. Both sarcopenia and cachexia are incapacitating conditions characterized by muscle deficiency, leading to compromised individual function and physical performance.
As the population ages, it is likely that the prevalence of cancer cachexia will increase in the coming years. Experimental data indicate that cancer development causes significant shifts in the intestinal microbiome’s composition, stimulating the release of inflammatory mediators and reactive oxygen species and contributing to the onset of cachexia [173]. Through the modulation of essential factors linked to sarcopenia, such as microbiota dysregulation, inflammation, metabolic disturbances, and oxidative stress, propolis and its active components appear to hold promise in mitigating muscular decline [174]. However, research into their effects on skeletal muscle loss and weakness is still in its early stages.
Osteoarthritis takes precedence as the most prevalent arthritis type in older adults, and it ranks high among the common causes of physical disability in adults. The identification of intestinal microbiota DNA in the synovium of individuals with osteoarthritis piques interest [175]. In light of the connections between gut microbiota imbalances and the development and progression of osteoarthritis, there is ongoing research into the feasibility of using gut microbiota as a therapeutic target for its treatment [175]. In an osteoarthritis rat model, a study highlighted the positive impact of quercetin treatment on mitigating intestinal flora disorder and restoring fecal metabolite abnormalities [166]. Multiple studies have been undertaken to investigate the influence of propolis and its key constituents on gut microbiota in both health and disease settings [53,85,97,98,154,176,177,178,179,180,181,182,183,184,185]. Although early research suggests that propolis may help optimize gut health, more comprehensive studies are needed before clinical recommendations can be made.
6. Autophagy
Via the lysosome degradation pathway, autophagy serves as a critical mechanism for maintaining cellular homeostasis through the clearance of intracellular surplus or damaged molecules and organelles [186]. Autophagy enables cells to dispose of and recycle cellular waste, improving cell health and survival during times of stress or nutrient deficiency. It helps the cell to remove dysfunctional or damaged organelles, proteins, and other cellular components, promoting cellular rejuvenation, stress adaptation, and overall health. Autophagy is of utmost importance in multiple physiological processes, comprising development, immunity, and disease prevention (e.g., cancer, neurodegenerative disorders, and infections). Consequently, its abnormal regulation is implicated in aging and a diverse array of human diseases [187]. The growing body of evidence strongly suggests that autophagic activity decreases as individuals age, heightening their susceptibility to age-related diseases [188]. Moreover, autophagy functions as a negative modulator of inflammasomes. There is a bidirectional relationship between inflammatory mediators and autophagy, as each can regulate the other’s activity.
The study of Hsieh et al. points out that Taiwanese green propolis suppresses the NLRP3 inflammasome through autophagy stimulation, leading to a reduction in gouty arthritis inflammation [189]. In mouse macrophages, Brazilian green propolis extract is involved in lowering IL-1β secretion while simultaneously inhibiting caspase-1 activation and NLRP3 inflammasome [190]. The nucleotide-binding oligomerization domain (NOD)-like receptor family of proteins (NLRPs), such as NLRP3 and NLRC4, is recognized for orchestrating the primary innate immune response to cellular stress [191]. In the presence of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), both NLRP3 and NLRC4 inflammasomes can trigger inflammation and cellular death [192,193]. Once activated, these sensor molecules initiate the formation of the inflammasome complex, which in turn activates caspase-1, an enzyme involved in the production of proinflammatory cytokines such as interleukin-1 beta (IL-1β) and interleukin-18 (IL-18) [194]. Thus, deficiency of autophagy can result in excessive activation of the inflammasome, leading to pathological hyperinflammation. Arias et al. highlighted the beneficial effects of propolis treatment by reverting osteoarthritis-like biological changes induced by IL-1β stimulation in the chondrocytes of rabbits [195]. This bee-derived product reduced the expression of proteins associated with autophagy, such as microtubule-associated protein 1 light chain 3 alpha (LC3) and autophagy-related 5 (ATG5), as well as AKT1, and nitric oxide production. The RAC-alpha serine/threonine-protein kinase (AKT) serves as an upstream controller of autophagy [196]. Autophagy can be modulated by AKT through mammalian target of rapamycin complex 1 (mTORC1), and while AKT is generally known to inhibit autophagy, certain natural components have been observed to induce autophagy alongside an elevation in AKT phosphorylation [195]. Another investigation by Arias and the research team showcased that propolis provides protection to osteoarthritic chondrocytes by modulating microRNAs that govern autophagy proteins, including AKT1, ATG5, and LC3 [197]. Additionally, Arias et al. (2023) also conducted an in vivo study on knee articular cartilage in senescent rats, revealing that propolis treatment ameliorated osteoarthritis severity, improved the structural integrity of cartilage, and increased chondrocyte density [198]. In their work, Wang et al. (2023) detailed the pinocembrin protective effects on cartilaginous endplate chondrocytes [199]. These authors underscored the importance of pinocembrin’s action, specifically through Nrf-2-mediated activation of mitochondrial autophagy and inhibition of ferroptosis in preventing ROS-induced degeneration and calcification of cartilaginous endplate chondrocytes in a model of intervertebral disc degeneration. The nuclear factor erythroid 2-related factor 2 (Nrf2) governs the transcription of numerous genes related to inflammation, redox balance, detoxification, and metabolism [200]. Through its regulatory function, Nrf2 enhances the expression of several detoxifying antioxidant molecules, such as heme-oxygenase-1 (HO-1), NADPH, glutathione S-transferase, and quinone oxidoreductase.
Oxidized low-density lipoprotein (Ox-LDL) plays a critical role in the development of atherosclerosis, an age-related condition, where advancing age acts as an independent risk factor for its progression. Brazilian green propolis exhibits the capability to suppress apoptosis and autophagy triggered by Ox-LDL damage in human umbilical vein endothelial cells (HUVECs) through the PI3K/Akt/mTOR-mediated Nrf2/HO-1 pathway [201]. In another experiment involving HUVECs subjected to lipopolysaccharide, Chinese propolis showed positive anti-inflammatory effects by inhibiting autophagy and the MAPK/NF-κB signaling pathway [202]. The well-established understanding is that multiple molecules, including proinflammatory mediators, redox-sensitive protein kinases, and phosphatases, have the ability to trigger NF-κB signaling and promote ROS generation. As a result, elevated ROS levels serve as a feedback mechanism to further activate NF-κB signaling. Each of these factors can notably trigger the activation of the inflammasome. Additionally, diminished autophagic activity may cause a loss of regulatory control over the NF-κB complex, resulting in its degradation through selective autophagy [203]. Aging-related impairment in autophagy’s clearance function fosters a permissive milieu for the direct or indirect activation of NF-κB signaling, giving rise to an age-associated proinflammatory phenotype that relies on inflammasome activation.
Over the past years, widespread documentation has highlighted the anti-inflammatory and antitumor activities of propolis and its active ingredients [28,204]. Autophagy’s crucial involvement in the favorable outcomes of propolis, particularly targeting the proliferation of cancerous cells within an inflammatory milieu, has been brought to light. Research has provided evidence that propolis, along with its key component caffeic acid phenethyl ester (CAPE), effectively suppresses breast cancer cell proliferation by modulating the TLR4 signaling pathway, leading to the activation of apoptosis and autophagy mechanisms [205]. Propolis and CAPE have the ability to hinder the TLR4 signaling pathway by inhibiting key molecules such as MyD88, TLR4, IRAK4, and TRIF, affecting both the MyD88-dependent and MyD88-independent signaling pathways. The consequential reduction in NF-κB activation and subsequent cytokine release mediated through the TLR4/NF-κB signaling pathway triggers autophagy, contributing to the alleviation of inflammation. Transforming growth factor-β1 (TGF-β1) is known to prompt autophagy in many different cellular systems [206]. TGF-β is renowned for its role in activating the canonical Smad signaling pathway. In line with this reasoning, a study unveiled that through the inhibition of the TGF-β1/Smad3 pathway and the concurrent activation of autophagy, CAPE plays a crucial role in attenuating liver fibrosis [207].
7. Mitochondria
Mitochondria are among the early cellular structures affected when cells face detrimental external factors or bioactive agents. Subsequently, they might incompletely reduce molecular oxygen, giving rise to superoxide radicals and becoming the primary source of reactive oxygen species within the cell. Studies have highlighted the importance of propolis extracts as essential scavengers of intramitochondrial ROS, effectively reducing superoxide levels in a concentration-dependent manner [208]. Research yields credible data supporting the implication of oxidants originating from mitochondria and mitochondrial oxidative injury in amyotrophic lateral sclerosis-associated mutant Cu/Zn superoxide dismutase (SOD1) [209,210]. In alignment with this reasoning, evidence has been presented showing that both kaempferol and kaempferide, two flavonoids existing in Brazilian green propolis, possess the capability to restrain mutant SOD1-triggered superoxide production within mitochondria [211]. This underscores the beneficial impact of propolis active ingredients in counteracting neurotoxicity induced by mutant SOD1 in a cellular model.
The β-amyloid peptide-induced toxicity accumulation in the brain contributes to pathogenesis in Alzheimer’s disease. An investigation showed that pinocembrin’s protective effects against Aβ-induced toxicity involve improvements in mitochondrial membrane potential and the suppression of mitochondrial ROS production. It also plays a role in restoring Bcl-2 and cytochrome c levels, while deactivating caspase 3 and caspase 9 [212].
A substantial body of evidence underlines the central role of mitochondrial dysfunction in Parkinson’s disease pathological processes [213,214]. Research indicates that pinocembrin’s aptitude to shield SH-SY5Y cells from Aβ-induced neurotoxicity involves both activating Nrf2/HO-1 pathways and halting mitochondria-dependent apoptosis [215]. The neurotoxin MPP+ operates by inducing the opening of the mitochondrial permeability transition pore, resulting in the release of cytochrome c, with crucial implications for cellular damage and death. Pretreatment of SH-SY5Y neuroblastoma cells with pinocembrin effectively counteracts MPP+-induced mitochondrial malfunctions, encompassing reduced membrane potential, decreased Bcl-2/Bax ratio, and the liberation of cytochrome c [216]. These effects collectively contribute to the maintenance of neuronal viability. In addition to its role in attenuating dopaminergic loss in the MPTP rodent model of Parkinson’s disease, oral administration of CAPE also has the ability to inhibit toxicity induced by the neurotoxin MPP+ in vitro [217]. Furthermore, it directly suppresses the liberation of cytochrome c and apoptosis-inducing factors from mitochondria caused by neurotoxins, underscoring the potent neuroprotective effects of CAPE.
Cerebral ischemia stands as a prominent contributor to global disability and mortality rates. Age stands as a nonmodifiable risk element for cerebral ischemia [218]. The level of adenosine triphosphate (ATP) within cells has been identified as a significant factor in influencing the extent of damage resulting from cerebral ischemia/reperfusion. The process of oxidative phosphorylation in mitochondria contributes to the production of roughly 80–90% of cellular ATP. Through experimentation centered on rat cerebellar neuronal–glial cell cultures exposed to ischemia, researchers found that the application of polyethylene glycol aqueous extract of propolis yielded a noteworthy increase in neuronal viability. This improvement was coupled with a decrease in mitochondrial superoxide levels [219]. Additionally, the extract notably shielded the cultures against hypoxia-induced elevation of inflammatory cytokines like TNF-α, IL-1β, and IL-6 and effectively countered the reduction in both baseline mitochondrial and ATP-coupled respiration rates, resulting in a significant augmentation of the overall mitochondrial respiratory capacity. Another study highlighted that pinocembrin’s protective effects on brain mitochondria are instrumental in enhancing cognitive function in rats exposed to chronic cerebral hypoperfusion. This positive outcome stemmed from the improvement in mitochondrial complex I activity, membrane potential status, alleviation of mitochondrial swelling, and correction of cytochrome oxidase deficiencies [220].
Both heart and liver mitochondria undergo atypical biochemical modifications upon the introduction of antineoplastic agents doxorubicin or vinblastine [221]. These changes include a significant increase in the production of malondialdehyde and anion superoxide, as well as the activation of swelling processes and a worsening of respiratory control. Nevertheless, providing rats with propolis extract prior to antineoplastic agent injections shielded the heart and liver tissues from mitochondrial stress [221]. Studies have suggested that apoptosis stands out as the primary process by which propolis demonstrates its antitumor effects [152,222,223,224]. In the course of apoptosis, the mitochondrial membrane potential is compromised by the establishment of permeability transition pores. These pores arise from the influence of distinct stimuli, initiating intracellular signaling cascades that prompt the diminishment of mitochondrial membrane potential. Propolis has the ability to trigger apoptosis by influencing the Bcl-2/Bax-mediated modulation of mitochondrial membrane potential [225]. The effects of Brazilian green propolis on human lung cancer A549 cells were investigated, revealing its ability to induce apoptosis through a mechanism involving the downregulation of the antiapoptotic gene Bcl-XL and the upregulation of the pro-apoptotic genes Bax and Noxa [226]. The net effect of these changes is the disruption of mitochondrial membrane potential. Research revealed that employing a combination of propolis, thermal cycling-hyperthermia, and low-intensity ultrasound can restrain the growth of pancreatic cancer cell line PANC-1 via ROS-modulated mitochondrial apoptosis [227]. The findings also underscore that mitochondrial dysfunction is regulated through the MAPK pathway, culminating in apoptosis via enhanced poly (ADP-ribose) polymerase (PARP) cleavage. Docetaxel is used extensively in advanced prostate cancer chemotherapy, but a substantial number of patients develop resistance to this treatment. In this context, a study has described the positive effects resulting from the combined treatment of docetaxel with CAPE, leading to apoptosis and metabolism interference in docetaxel-resistant prostate cancer cells [228]. This approach led to a decrease in protein levels of Bcl-2, AKT2, c-Myc, apoptosis and caspase activation inhibitor (AVEN), and pyruvate kinase M2 (PKM2), alongside an increase in protein concentration of Bax, caspase 3, cytochrome c, glucose-6-phosphate dehydrogenase (G6PD), and acylglycerol kinase (AGK).
A research effort involving isolated rat liver mitochondria unveiled that the glycolic extract sourced from Baccharis dracunculifolia, the primary botanical origin utilized by honeybees for crafting Brazilian green propolis, displayed noteworthy capabilities in scavenging 1,1-diphenyl-2-picrylhydrazyl radicals and superoxide anions, along with the ability to chelate Fe2+ ions [229]. The treatment also resulted in a decline of basal H2O2 generation and suppressed the mitochondrial production of ROS triggered by Fe2+ or t-BuOOH, one of the most active lipid hydroperoxides in oxidation processes. Additionally, it effectively prevented lipid oxidation within mitochondrial membranes, preserved protein thiol groups, and prevented GSH oxidation. These outcomes underscore the robust antioxidant prowess of this extract, safeguarding liver mitochondria from oxidative harm, and potentially playing a role in the antioxidant and hepatoprotective properties associated with green propolis.
8. Nutrient-Sensing Pathways
Cell viability relies on the continuous regulation of energy demands in accordance with nutrient availability. Through the recognition and responsive modulation of fuel substrates such as carbohydrates, lipids, amino acids, and vitamins, the cell can effectively adapt to varying nutrient signals [230]. Nutrient-sensing pathways have evolved to manage cellular energy levels, maintain metabolic stability, and oversee a variety of biological functions. These pathways undergo a loss of regulation and a reduction in their overall effectiveness as individuals age [231]. Nutrient-sensing signaling pathways establish a connection between dietary choices to the aging process. In this regard, calorie restriction has emerged as the most effective approach for promoting a longer, healthier lifespan by regulating these signaling networks across various model organisms [232]. In fact, current findings from longevity research indicate a smaller genetic contribution and a much greater influence of environmental factors than previously assumed [233].
The signaling mechanisms that participate in the detection and interpretation of nutrient and energy levels, encompassing pathways such as insulin and insulin-like growth factor (IGF-1) signaling, as well as those involving mammalian target of rapamycin (mTOR), adenosine monophosphate-activated protein kinase (AMPK), and sirtuins, can provide the molecular groundwork for understanding how lifestyle factors are linked to the process of aging [234].
Insulin/IGF-1 signaling (IIS) nutrient sensing emerged as the first pathway proven to control aging and age-related diseases in organisms [235]. The IIS pathway is essential in managing glucose uptake and glycogen synthesis. It initiates the phosphoinositide 3-kinase (PI3K)/Akt pathway. This intracellular signaling route modulates proliferation, survival, cell growth, and angiogenesis. IIS exhibits regulatory control over FOXO (forkhead box O) transcription factors. When activated, these factors enhance cellular stress resistance, playing a role in the broader context of aging and longevity. Inhibiting FOXO activity via Akt-mediated phosphorylation is a mechanism through which IIS influences processes such as apoptosis and oxidative stress response [236]. The IIS and the PI3K/AKT/mTOR pathways are intricately linked, and their crosstalk is crucial for regulating processes such as protein synthesis, cell growth, and metabolism and maintaining cellular homeostasis [237].
The mTOR plays a crucial role in regulating aging and age-related diseases through its involvement in two separate complexes, known as mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) [238]. Functioning as a controller of protein synthesis, mTORC1 operates by phosphorylating its primary targets, 4E-BP (eukaryotic initiation factor 4E binding proteins) and S6K1 (p70 S6 kinase 1) [239]. Under calorie-restricted conditions, mTOR is downregulated, facilitating an augmentation of catabolic activity. In contrast, in the presence of abundant nutrients, mTORC1 becomes active, stimulating anabolic pathways, including protein, nucleotide, and lipid biosynthesis, while simultaneously hindering processes that govern protein and organelle turnover, such as autophagy and lysosome biogenesis [240]. An excess of nutritional amino acids is tied to aging and diseases. Studies have pointed out that the suppression of mTORC1 by rapamycin can mimic the outcomes of dietary restriction, including the reversal of aging-related phenotype and the promotion of longevity [241,242].
Functioning as an energy sensor, AMPK adjusts cellular metabolism in alignment with fluctuations in energy availability. Through the inhibition of anabolic pathways and the promotion of catabolic processes, AMPK assists cells in coping with energy stress [243]. AMPK also has the ability to suppress proinflammatory signaling pathways, including NF-κB. Moreover, AMPK interacts with sirtuins, particularly SIRT1, establishing a regulatory network that influences how cells respond to stress and energy levels [244]. As NAD(+)-dependent histone deacetylases, sirtuins exhibit a pronounced sensitivity to cellular energy status. Their influence extends to the modulation of cellular metabolism, involving energy production, DNA repair, oxidative stress, glucose homeostasis, and lipid metabolism [245,246]. The extensively researched SIRT1 engages with transcription factors including p53, FOXO, and PGC-1α, which play roles in cellular stress response, apoptosis, and mitochondrial biogenesis. The nutrient-sensing pathways (IIS, mTOR, AMPK, sirtuins) collectively act as key nodes that significantly influence aging and age-related diseases.
Evidence suggests that propolis and its bioactive constituents can influence nutrient-sensing pathways, prompting an examination of their potential therapeutic benefits across different health domains and in addressing age-associated diseases. A study showed that chrysin’s beneficial effects on diabetic nephropathy involve the modulation of lipid metabolism through the activation of AMPK in HFD/STZ-induced diabetic mice [247]. In a parallel manner, a 4-week regimen of chrysin treatment in rats successfully reversed glucose elevation, inflammation, and the decline in serum insulin levels associated with aging, indicating that chrysin helps preserve islet function [248]. The research conducted by Oriquat et al. revealed that chrysin exhibited notable effects in mitigating weight gain and rectifying hyperglycemia, insulin resistance, and dyslipidemia while simultaneously improving adipocytokine levels in rats with metabolic disturbances induced by a high-fat diet [249]. Moreover, the authors provided evidence that chrysin regulates hepatic signaling by activating the AMPK pathway and inhibiting the mTOR and lipogenic pathways, resulting in anti-obesity and antisteatotic effects [249]. The prevalence of nonalcoholic fatty liver disease (NAFLD) is 39% among individuals aged 40 to 50 and exceeds 40% in those aged 70 and above [250]. The pathological manifestations of NAFLD span from simple steatosis to the more intricate necroinflammatory fibrosing disorder recognized as steatohepatitis. Research findings indicate that flavonoids found in propolis possess the ability to alleviate steatohepatitis in HepG2 cells [251]. The hepatoprotective effects of flavonoids entail boosting AMPK activation, mTOR-NF-κBp65 interaction, and PTEN levels. In addition, the results pointed out that naringenin, specifically, developed a stable complex with AMPK, indicating its potential as a viable candidate for NAFLD treatment [251].
In the research conducted by Cardinault and their team, it was suggested that the polyphenols in propolis prompted the transactivation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), resulting in the amelioration of glucose homeostasis, elevated production of antioxidant molecules, and protection against oxidative stress in diabesity in high-fat-fed mice [252,253]. Another study demonstrated that propolis lowered fat accumulation in the adipose tissues of rats on a high-fat diet by suppressing peroxisome proliferator-activated receptor γ (PPARγ), which plays a role in regulating genes associated with adipocyte differentiation [254]. A research study highlighted the favorable outcomes of Iranian propolis supplementation for individuals with type 2 diabetes mellitus, showing declines in postprandial blood glucose, serum insulin, insulin resistance, and inflammatory cytokines while also indicating elevations in HDL levels [102]. In a study involving nondiabetic volunteers with obesity and insulin resistance, the supplementation of standardized poplar propolis extract powder for a period of three months positively affected insulin homeostasis through its impact on both insulin resistance and secretion [255]. According to this study, propolis may act preventively on the physiopathological mechanisms of type 2 diabetes, potentially hindering the disease’s development.
It has been shown that galangin and pinocembrin contained in propolis promise to alleviate insulin resistance in HepG2 cells by modulating the protein kinase-B (Akt)/mTOR signaling pathway. These substances showcase their efficacy by distinctly boosting the phosphorylation levels of human insulin receptor (IR), Akt, and glycogen synthase kinase-3 beta (GSK3β) while notably reducing the phosphorylation of insulin receptor substrate (IRS) [256]. Another study reported that the potential of Brazilian propolis to prevent hyperglycemia lies in its ability to promote the translocation of insulin-sensitive glucose transporter (GLUT) 4 and enhance glucose uptake [257]. Additionally, it activates the phosphorylation of PI3K and AMPK in skeletal muscle.
Extensive research has delved into chrysin’s capacity to restore insulin sensitivity and its effects on diabetes [248]. As an illustration, chrysin’s positive impact on glycolipid metabolism has been documented by its capacity to activate AMPK, thereby regulating the PI3K/AKT signaling pathway. This effect was observed in studies using high-fat diet/streptozotocin (STZ)-induced C57BL/6J mice [258].
The expression of LOX-1, the receptor for oxidized low-density lipoprotein (ox-LDL), is heightened in diseases such as atherosclerosis, hypertension, diabetes, and others, facilitating the production of ROS. Chinese popular propolis, as evidenced by Chang et al.’s study, was found to alleviate injury in endothelial cells induced by oxidized low-density lipoprotein. This protective mechanism encompassed the activation of the PI3K/Akt/mTOR signaling pathway, inhibition of LOX-1/p38 MAPK, suppression of ROS production, and preservation of mitochondrial membrane potential to hinder apoptosis and autophagy [259]. These findings present a novel perspective for understanding the mechanisms that contribute to the protective actions of propolis against endothelial apoptosis. In a rat model of diabetes-induced atherosclerosis initiated by HFD/STZ administration, quercetin exhibits the potential to improve atherosclerotic pathophysiology by stimulating AMP activation. This crucial regulatory protein in glycolipid metabolism functions by elevating downstream SIRT1 protein levels and diminishing NF-κB, thereby mitigating inflammatory and oxidative stress responses [260]. Studies have provided evidence suggesting quercetin as a potential therapeutic agent for mitigating the damage induced by myocardial ischemia through significant upregulation of SIRT1, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), and Bcl-2 proteins, accompanied by a reduction in Bax protein expression [261].
Research has also underscored quercetin’s capacity to mitigate mitochondrial dysfunction and promote biogenesis in osteoarthritis-afflicted rats by increasing the expression levels of SIRT1, PGC-1α, NRF1, NFR2, TFAM, and phospho-AMPKα, suggesting its action through the AMPK/SIRT1 pathway [262,263]. Taken together, studies have suggested quercetin’s capacity to serve as a promising therapeutic option to tackle age-related diseases through its engagement with SIRT1 pathways [264,265,266]. Current research findings have indicated that quercetin exerts protective roles in age-associated diseases by activating SIRT1, resulting in the coordination of a broad spectrum of cellular processes linked to the aging phenomenon. These processes encompass oxidative stress through SIRT1/Keap1/Nrf2/HO-1 along with PI3K/Akt/GSK-3β pathways, inflammatory reaction via SIRT1/NF-κB signaling, mitochondrial status via SIRT1/PGC1α/eIF2α/ATF4/CHOP, and autophagy through the SIRT1/FOXO pathways [266]. Through their research on prostate cancer cells, Tseng et al. discovered that CAPE treatment effectively suppressed PI3K-Akt signaling, a pathway often hyperactive in cancers and fostering tumor progression. These results underscore CAPE’s potential as an inhibitor targeting the PI3K/Akt pathway, presenting novel avenues for cancer treatment research [267]. Havermann et al. showed that CAPE enhances resistance to thermal stress and extends Caenorhabditis elegans’s lifespan through the regulation of the DAF-16 signaling pathway [268]. The authors detailed that CAPE induces the nuclear translocation of DAF-16, a critical step for the extension of the nematode’s lifespan. In Caenorhabditis elegans, DAF-16 is the unique ortholog representing the FOXO proteins family. It stands as the key downstream target and primary effector in the DAF-2/insulin-like signaling pathway [269]. In this line of research, a significant lifespan-extending effect in Caenorhabditis elegans has been observed through the administration of Okinawa propolis, a resinous mixture produced by honeybees [8]. Taira et al. found that Okinawa propolis, in addition to promoting nematode’s longevity, demonstrated an antimelanogenic effect in melanoma cells and anticancer properties against lung cancer cells. All these effects of propolis were attributed to the inhibition of the oncogenic/aging/melanogenic p21-activated kinase (PAK1) [8].
9. Intercellular Communication
Intercellular communication is an integrative hallmark of aging, involving the exchange of signals between cells through various mechanisms, including direct cell-to-cell contact, secretion of signaling molecules (such as hormones, growth factors, cytokines, and neurotransmitters), and extracellular vesicles (such as exosomes) [270]. During aging processes, cells secrete a range of proinflammatory cytokines, growth factors, and matrix remodeling enzymes as part of the senescence-associated secretory phenotype (SASP) [270]. Studies have established that these factors can prompt neighboring cells to enter a senescent state, potentially accelerating aging and playing a role in the onset of various age-associated diseases [271].
As individuals grow older, the signaling network of chemical messages throughout the body gives rise to a continual, low-level systemic inflammation, coupled with the ongoing activation of the innate immune system. This phenomenon, termed ‘inflammaging’, represents a smoldering proinflammatory phenotype that hastens the aging process and is linked to the development of numerous age-related diseases [23,272]. Several distinct factors converge to induce inflammaging, and one of them is the SASP, which emerges as a direct outcome of another signature of aging—cellular senescence.
Immunosenescence is a term that summarizes the amplification of the aging phenotype, characterized by changes in immune system function. These shifts involve disturbances in T cell populations, a decline in the ability to respond to antigens, a reduced capacity to clear defective host cells, and the lingering presence of low-grade inflammation [273]. The recognition of disturbed intercellular communication as a contributor to aging is growing. In particular, impaired neurohormonal signaling and disrupted inflammatory pathways, in conjunction with an ineffective immune system, have been implicated in this context [234]. Researchers are pursuing approaches to adjust dysregulated intercellular communication pathways in an experimental setting. For instance, administration of anti-inflammatory and anti-oxidant agents, dietary restriction, and the modification of the gut environment seem important in this vein.
In this context, Gao et al. showed that Brazilian green propolis supplementation counteracts age-associated immune changes in aged mice [274]. This is evidenced by increased serum IgG levels, improved specific antibody response, and enhanced phagocytic ability.
Zhu et al. (2018) conducted a study revealing that Mini-Mental State Examination (MMSE) scores of elderly individuals residing in high-altitude areas declined, suggesting mild cognitive impairment over a 24-month period [275]. This decline was associated with an elevation in serum inflammation markers. Interestingly, individuals with prolonged consumption of Brazilian green propolis exhibited a significant decrease in systemic inflammation and demonstrated improved MMSE scores [275]. Other research indicated that Brazilian green propolis treatment in an animal model of Alzheimer’s disease demonstrated efficacy in reducing the elevation of IL-6 inflammatory cytokine levels in plasma, concurrently dampening the immune response in glial cells [49].
The research conducted by Ali et al. documented the protective effects of a standardized pomegranate and propolis blend in Parkinsonian rats. The study indicated that, apart from alleviating motor and cognitive deficits, the treatment increased striatal concentrations of dopamine, norepinephrine, and serotonin. It also suppressed lipid peroxidation, enhanced total antioxidant capacity, restored SOD, and concomitantly reduced caspase-3 as well as inflammatory markers IL-1β, TNF-α, and iNOs [276].
The administration of a drug combination consisting of quercetin and dasatinib, a tyrosine kinase inhibitor, effectively counteracts age-related increases in senescence and inflammation within the adipose tissue of old mice [277]. This treatment not only reduces the age-related elevation of senescence-associated β-galactosidase but also diminishes the expression of aging-related genes such as p16 and p21. Moreover, the cocktail attenuates the upregulation of proinflammatory SASP genes, including mcp1, tnf-α, il-1α, il-1β, il-6, cxcl2, and cxcl10 while concurrently suppressing the infiltration of T cells and macrophages in perigonadal white adipose tissue [277]. Research findings indicate that quercetin enhances cell-to-cell communication by promoting the expression of gap junction proteins, which play a crucial role in facilitating communication between adjacent cells [278]. Through the upregulation of connexin43, quercetin effectively inhibits the proliferation of metastatic human breast tumor cell lines [278].
10. Proteostasis
Research conducted in recent decades has brought to light the cell’s remarkable capacity to uphold proteostasis in the face of diverse and demanding circumstances. The significance of this capability becomes especially apparent as we gain a deeper comprehension of the fact that a disruption in this process is a root cause of numerous age-related conditions. In mammalian cells, protein homeostasis, also referred to as proteostasis, is a complex process responsible for maintaining the proteome’s stability through the management of protein synthesis, folding, trafficking, post-translational modifications, and degradation. An intricate and adaptable proteostasis network oversees these functions with the involvement of diverse molecular chaperones, along with the ubiquitin–proteasome and lysosomal–autophagy systems. Their interplay preserves the integrity and operational efficiency of the cellular proteome while reducing the likelihood of misfolding or aggregation, which serve as prominent indicators of age-related proteinopathies [279,280]. Scientific investigations have suggested that low doses of CAPE can produce favorable effects in addressing disorders marked by protein aggregation through the activation of hypoxia-inducible factor-1α (HIF-1α) and the suppression of stress-induced protein aggregation [281]. The endoplasmic reticulum is pivotal for maintaining protein quality through the initiation of the unfolded protein response (UPR) [282]. In eukaryotic systems, glucose-regulating protein 78 (GRP78), a chaperone protein within the heat shock protein family (HSPA5), assumes the central role in regulating the UPR that takes place within the endoplasmic reticulum’s lumen [283]. Hirata et al.’s research (2021) uncovered the ability of artepillin C to suppress both neuronal cell death related to ER stress and protein aggregation. Its effectiveness was shown through the inhibition of tunicamycin-induced protein aggregation in the hippocampal cell line HT22 and the spontaneous protein aggregation of mutant canine superoxide dismutase 1, akin to human amyotrophic lateral sclerosis, in Neuro2a cells [284]. The study indicated that artepillin C has the ability to inhibit the protein kinase R-like endoplasmic reticulum kinase (PERK) and inositol-requiring enzyme 1 (IRE1) branches within the ER stress pathway [284]. Artepillin C also had a significant impact on reducing the levels of growth arrest and DNA damage-inducible gene 153 (GADD153) and spliced X-box binding protein 1 (XBP1) induced by tunicamycin. Hirata et al.’s study implied that artepillin C displays chemical chaperone-like properties. The PERK, IRE1, and activating transcription factor 6 (ATF6), positioned in the ER membrane, serve as the protein sensor activators directing UPR regulation [285]. Under physiological conditions, the binding immunoglobulin protein (BiP), also known as GRP78, represses the activity of these sensors, binding them. The accumulation of unfolded/misfolded proteins in the ER results in the release of BiP from stress sensors. Once released, each protein initiates a different regulatory mechanism that increases protein-folding capacity and reduces protein load on the ER. Activation of PERK results in the inhibition of the eukaryotic initiation factor 2α (eIF2), leading to a global inhibition of protein synthesis [286]. PERK enables the translation of GADD153, inducing cell death in response to ER stress. In turn, the cytosolic portion of IRE1 contains a kinase and an endoribonuclease domain, both of which are required for the unconventional splicing of X-box binding protein 1 (XBP1) messenger RNA (mRNA). The spliced XBP1 mRNA encodes a functional transcription factor that translocates to the nucleus, regulating the expression of genes involved in protein folding, folding quality control, ER-associated degradation (ERAD), and lipid biosynthesis. Research has revealed that the ethanol extract of propolis is proficient in preventing the activation of the endoplasmic reticulum stress signaling pathway triggered by oxidized low-density lipoprotein or tunicamycin. This inhibition encompasses the phosphorylation of double-stranded RNA-activated PERK and eIF2α, together with the elevation of GRP78 and CHOP, a pro-apoptotic molecule [287]. In their 2017 study, Tomiyama et al. showed that pretreating human SH-SY5Y neuroblastoma cells with CAPE can trigger endoplasmic reticulum (ER) stress and subsequent autophagy, playing a role in cell survival following cytotoxic injury caused by the neurotoxin 6-hydroxydopamine (6-OHDA) [288]. The authors posited that autophagy represents a mechanism implicated in the protective effects of ER preconditioning. Emerging findings indicate that the initiation of the UPR in Parkinson’s disease critically relies on the activation of the chaperone GRP78/binding immunoglobulin protein (BIP). This highlights that neuroprotective substances that can modulate the expression of GRP78/BiP have the potential to regulate endoplasmic reticulum stress in experimental models of this neurodegenerative condition [289]. Current research has presented findings on the ability of polyphenol-based nanosheets of propolis to regulate protein aggregates implicated in neurodegenerative conditions. A study has provided evidence of the polyphenolic fraction of propolis nanosheets binding to α-synuclein fibrils while also showcasing substantial dose-dependent inhibition of α-synuclein aggregation by these nanosheets [290].
Chrysin exerts its pro-death effects on prostate cancer cells by inducing mitochondrial-mediated apoptosis and endoplasmic reticulum stress, the latter driven by the activation of UPR proteins like GRP78, PERK, and eIF2α [291]. The disruption of mortalin-p53 complexes is one of the mechanisms underlying CAPE’s anticancer and antimetastasis properties [292]. Mortalin is an hsp70 chaperone abundantly found on the surface of cancer cells. To harness this knowledge, scientists employed a specialized cell-internalizing antimortalin antibody to engineer mortalin-targeting CAPE nanoparticles. These innovative nanoparticles demonstrated heightened selective anticancer efficacy in both in vitro and in vivo evaluations, indicating their potential as a potent nanomedicine for combating cancer [293]. Akin to CAPE, artepillin C also induces the activation of p53’s tumor suppressor by disrupting its binding with mortalin [294]. Evidence supports CAPE’s capacity to hinder E6-associated protein (E6AP), thereby disrupting the interaction between p53 and the E3 ubiquitin ligase E6AP. As a result, the ubiquitination of p53 is prevented, underscoring CAPE’s anticervical cancer properties [295].
11. Epigenetics
Epigenetic regulation is the process of governing gene expression while keeping the actual genetic code unchanged. Epigenetic markers function as genetic regulators, dictating which segments of DNA are accessible for transcription [296]. The primary epigenetic changes involve DNA methylation, modification of histone proteins, and RNA-mediated processes that impact gene expression. These changes are heritable and often reversible [297]. They are significant contributors to variations in gene expression patterns among different tissues and can also exert influence over gene expression across different age groups. Epigenetic changes are susceptible to environmental influences, functioning as a liaison between gene expression and the environment [298]. Hence, environmental factors like diet or medications can govern epigenetic adjustments, providing a means for the environment to dictate an organism’s genetic constitution. As an organism progresses through its life, the scale, kind, and distribution of epigenetic adjustments shift, causing variations in gene expression that may have adverse effects on the organism. Such epigenetic changes have the potential to disrupt cellular function and foster diseases [299,300]. An increasing number of epigenetic modifications have been documented in the control of critical genes relevant to aging and its associated diseases, such as cancer. In this context, substances that possess epigenetic modulation capabilities, particularly inhibitors of histone deacetylases and DNA methyltransferases, have garnered expanded interest in the domains of aging and cancer research.
It is widely recognized that, apart from genetic mutations, epigenetic changes also accrue as people age, potentially contributing to the process of carcinogenesis. As an example, telomerase and p16INK4a (p16, cyclin-dependent kinase inhibitor 2A) are representative epigenetic targets that feature in both aging and cancer scenarios [301]. In this context, the focus is on substances able to change epigenetic processes. Naturally derived substances such as propolis and its components have the potential to influence or suppress epigenetic modifiers. A case in point is quercetin, a dietary polyphenol acknowledged as an agent capable of modulating epigenetic processes [301]. It is established that T24 human bladder carcinoma cells display hypermethylation of genes associated with cell cycle regulation, particularly p16INK4a and RASSF1A. Quercetin-induced demethylation of these genes restores their transcriptional function, suggesting their role in impeding cell growth and promoting apoptosis in T24 cells after exposure to quercetin [123].
Tumorigenesis is marked by the critical involvement of activated NF-κBp65 (RelA) and aberrant histone deacetylase (HDAC) activity [302]. The use of HDACs is on the rise as a developing strategy to impede the NF-κB pathway, with their modulation of NF-κB activity centered on the RelA/p65 subunit [303,304]. In the investigation conducted by Zheng et al. (2014) using human esophageal cancer cell line EC-9706, it was found that quercetin treatment resulted in the inhibition of RelA/p65 activity, downregulation of HDAC1, RelA, and cyclin D1, and upregulation of caspase-3 and p16INK4α [305]. The literature indicates that the regulation of COX-2 expression upon exposure to proinflammatory mediators involves transcriptional activation of NF-κB, CCAAT/enhancer binding protein (C/EBP), and CREB-2 [306]. In line, studies have also provided insights into how quercetin regulates COX-2, a pivotal mediator involved in inflammation and tumorigenesis. The work of Xiao et al. revealed a marked inhibition of COX-2 mRNA and COX-2 promoter activation in breast cancer cells upon treatment with quercetin [307]. Additionally, they outlined quercetin’s substantial inhibition of key drivers involved in COX-2 transcriptional activation (NF-κB, CREB2, and C/EBPβ), thereby impeding the recruitment of the coactivator p300 histone acetyl transferase to the COX-2 promoter [307]. These findings hint at quercetin’s potential application in the treatment of COX-2-associated diseases.
Studies have shown that quercetin exerts control over the expression of diverse chromatin-modifying factors and reduces the activity of class I/II histone deacetylases, DNA methyltransferases 1, and histone methyltransferases in human cervical cancer (HeLa) cells; the extent of the modulation varies with the dose [308]. In addition, this intervention yielded reduced overall DNA methylation levels, while tested tumor suppressor genes exhibited a significant dose-dependent reduction in promoter methylation, leading to the reinstatement of their expression [308]. The influence of propolis and its bioactive components, with a particular emphasis on quercetin, in shaping microRNA expression presents an engaging avenue for regulating inflammation, differentiation, proliferation, apoptosis, and immune responses [197,309,310,311,312,313].
The phenomenon of postovulatory aging is acknowledged for its detrimental impact on both oocyte quality and the development of ensuing embryos, representing a major impediment to the success of human assisted reproductive technology. Despite this, the options for counteracting the deterioration of aged oocytes are limited in scope. Experimental data have pointed out that quercetin can postpone the postovulatory aging of mice oocytes. The treatment involving this propolis component mitigated the age-related irregularities in the way spindles were organized and mitochondria were distributed, all the while preserving SIRT expression and histone methylation [314]. Nonetheless, our insight into the role of epigenetic mechanisms in these strategies for addressing aging is in its early stages [300].
12. Shortcomings and Perspectives
Available data suggest that characteristics of aging serve as primary catalysts behind aging-associated diseases. The significance of aging hallmarks becomes more pronounced when considered as an interlinked network rather than isolated biological processes. Propolis’s complex chemical composition equips it with a spectrum of biological attributes and multifaceted pharmacological effects. Propolis exhibits the capability to simultaneously interact with multiple biological targets, inducing pleiotropic effects. Therefore, grasping the mechanisms of propolis action poses a significant challenge, requiring the identification of primary targets and an understanding of the interconnected networks of diverse signaling pathways. Currently, computational frameworks empower the integration of extensive and diverse sets of biological data. Artificial intelligence algorithms excel in the analysis of integrated data, pinpointing patterns and relationships. Additionally, these algorithms can appraise molecular structures and evaluate binding affinities and pharmacological properties. By modeling the relationships between drug candidates (propolis and its bioactive compounds), targets, and pathways, researchers can gain insights into the multifaceted mechanisms of drug action. Moreover, computational tools are essential to systems biology approaches, which model the behavior of entire biological systems. These models enable the simulation of propolis’s impact on signaling pathways and cellular processes, offering a comprehensive outlook on its mechanism of action in the context of aging and age-related diseases.
Although propolis and its components hold promise as potential interventions that can address these fundamental facets governing both aging and age-related diseases, this research topic is in its infancy, and considerable work is still needed to gain a comprehensive grasp of their mechanisms of operation. Attention is drawn to the imperative of standardizing this bee-derived product while implementing rigorous quality control measures. Certain researchers have already taken steps in this direction, particularly in relation to Brazilian green propolis [315] and propolis of the poplar variety [316,317,318]. It is only when such standardization is achieved that this natural product can truly serve as a viable adjunctive therapy with internationally recognized quality control standards.
While evidence has emerged from preclinical studies, more rigorously designed clinical trials involving humans are essential to determine the efficacy and safety of propolis in addressing age-related diseases and the aging process. For a comprehensive assessment of the mechanisms of action and biological effects of propolis and its constituents, studies should cover different life stages. It is of great significance to note that older adults are frequently overlooked in research endeavors. The assessment of the long-term safety of propolis supplementation, especially in older individuals, is vital to verify the absence of adverse effects linked to its extended use. There is a need to explore potential interactions between propolis and frequently prescribed medications, particularly for individuals dealing with various health conditions. Moreover, efforts should be intensified to encourage multicenter collaborations.
It is of utmost importance to determine the scope of benefits of propolis and its compounds and optimize their therapeutic capabilities. The refinement of delivery strategies and chemical adjustments, resulting in the development of more powerful compounds with superior pharmacodynamic and pharmacokinetic profiles, holds the potential to advance the utilization of these natural bee-derived products as effective interventions for humans. In this scenario, the work by Takahashi et al. showed that the esterization of bioactive compounds from propolis with the water-soluble 1,2,3-triazolyl alcohol resulted in a 30-fold augmentation of artepillin C’s anti-oncogenic P21-activated kinase1 (PAK1) activity, and CAPE exhibited a remarkable 140-fold enhancement [319]. This led to noteworthy improvements in both anticancer activities and cell permeability of these propolis compounds. Moreover, the research of Vasileva et al. uncovered the potential anti-aging effects of the natural deep eutectic extracts (NADES) of propolis in mutant S. cerevisiae yeast strains, which manifest premature aging features [320]. NADES are formed by primary metabolites derived from plants. Their distinctive features, including biodegradability and biocompatibility, position them as alternative options in various concepts and applications that traditionally employ organic solvents. In addition to their nontoxic attributes, NADES have the potential to amplify the biological activities of the extracted compounds [321].
Taken together, the continuous growth of nanomedicine and food nanotechnology, along with the advancements in bioinformatics and artificial intelligence technologies over the last few years, has opened up opportunities for the establishment of new approaches for dietary assessment and the exploration of physiological outcomes [322,323,324,325]. Additionally, there is an expectation that the incorporation of cutting-edge methodological approaches, including multi-omics strategies like transcriptomics, metabolomics, proteomics, genomics, epigenomics, and microbiomics, will propel advancements in this area.
Author Contributions
Conceptualization, C.S.; investigation, C.S. and V.G.; writing—original draft preparation, C.S., V.G., J.F., F.S. and F.F.; writing—review and editing, C.S., V.G., J.F. and F.F.; visualization, C.S.; supervision, C.S.; project administration, C.S.; funding acquisition, C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the São Paulo Research Foundation (FAPESP), grant number 2020/12791-6, Conselho Nacional de Desenvolvimento Científico e Tecnológico, grant number 304268/2020-8, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES), Finance Code 001.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Social Report of the United Nations. Leaving No One behind in an Ageing World; U.S. Department of Economic and Social Affairs: New York, NY, USA, 2023.
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.L.; Keller, H.H.; Freund-Levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D.; et al. Nutritional Considerations for Healthy Aging and Reduction in Age-Related Chronic Disease. Adv. Nutr. 2017, 8, 17–26. [Google Scholar] [CrossRef]
- Crimmins, E. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, D.S.; Lee, H.Y.; Abozaid, L.S.; Min, K.J. Mechanisms of Lifespan Regulation by Calorie Restriction and Intermittent Fasting in Model Organisms. Nutrients 2022, 12, 1194. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Pilling, L.C.; Ferrucci, L. The genetics of human ageing. Nat. Rev. Genet. 2020, 21, 88–101. [Google Scholar] [CrossRef]
- Jabalameli, M.R.; Zhang, Z.D. Unravelling genetic components of longevity. Nat. Aging 2022, 2, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Ricigliano, V.A.; Mott, B.M.; Copeland, D.C.; Floyd, A.S.; Maes, P. The queen’s gut refines with age: Longevity phenotypes in a social insect model. Microbiome 2018, 6, 108. [Google Scholar] [CrossRef]
- Taira, N.; Nguyen, B.C.; Be Tu, P.T.; Tawata, S. Effect of Okinawa Propolis on PAK1 Activity, Caenorhabditis elegans Longevity, Melanogenesis, and Growth of Cancer Cells. J. Agric. Food Chem. 2016, 64, 5484–5489. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, V.; Rijo-Ferreira, F.; Izumo, M.; Xu, P.; Wight-Carter, M.; Green, C.B.; Takahashi, J.S. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science 2022, 376, 1192–1202. [Google Scholar] [CrossRef]
- Spadaro, O.; Youm, Y.; Shchukina, I.; Ryu, S.; Sidorov, S.; Ravussin, A.; Nguyen, K.; Aladyeva, E.; Predeus, A.N.; Smith, S.R.; et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science 2022, 375, 671–677. [Google Scholar] [CrossRef]
- Qi, L. Nutrition for precision health: The time is now. Obesity 2022, 30, 1335–1344. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef]
- De la Fuente, M.; Miquel, J. An update of the oxidation-inflammation theory of aging: The involvement of the immune system in oxi-inflamm-aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevLity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef]
- Toda, I.M.; Ceprián, N.; Díaz-Del Cerro, E.; De la Fuente, M. The Role of Immune Cells in Oxi-Inflamm-Aging. Cells 2021, 10, 2974. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef]
- Lu, Y.R.; Tian, X.; Sinclair, D.A. The Information Theory of Aging. Nat. Aging 2023, 3, 1486–1499. [Google Scholar] [CrossRef]
- Baechle, J.J.; Chen, N.; Makhijani, P.; Winer, S.; Furman, D.; Winer, D.A. Chronic Inflammation and the Hallmarks of Aging. Mol. Metab. 2023, 74, 101755. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef]
- Schmauck-Medina, T.; Molière, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Perera, C.O.; Tandean, S. Can Propolis Be a Useful Adjuvant in Brain and Neurological Disorders and Injuries? A Systematic Scoping Review of the Latest Experimental Evidence. Biomedicines 2021, 9, 1227. [Google Scholar] [CrossRef]
- Zulhendri, F.; Ravalia, M.; Kripal, K.; Chandrasekaran, K.; Fearnley, J.; Perera, C.O. Propolis in Metabolic Syndrome and Its Associated Chronic Diseases: A Narrative Review. Antioxidants 2021, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Francisco, R.; Saraiva, A.; Francisco, S.; Carrascosa, C.; Raposo, A. The Cardiovascular Therapeutic Potential of Propolis—A Comprehensive Review. Biology 2021, 10, 27. [Google Scholar] [CrossRef]
- Forma, E.; Bryś, M. Anticancer Activity of Propolis and Its Compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef]
- Zulhendri, F.; Lesmana, R.; Tandean, S.; Christoper, A.; Chandrasekaran, K.; Irsyam, I.; Suwantika, A.A.; Abdulah, R.; Wathoni, N. Recent Update on the Anti-Inflammatory Activities of Propolis. Molecules 2022, 27, 8473. [Google Scholar] [CrossRef]
- Nazari-Bonab, H.; Jamilian, P.; Radkhah, N.; Zarezadeh, M.; Ebrahimi-Mameghani, M. The Effect of Propolis Supplementation in Improving Antioxidant Status: A Systematic Review and Meta-Analysis of Controlled Clinical Trials. Phytother. Res. 2023, 37, 3712–3723. [Google Scholar] [CrossRef]
- Weis, W.A.; Ripari, N.; Conte, F.L.; Honorio, M.S.; Sartori, A.A.; Matucci, R.H.; Sforcin, J.M. An Overview about Apitherapy and Its Clinical Applications. Phytomed. Plus 2022, 2, 100239. [Google Scholar] [CrossRef]
- Bobiş, O. Plants: Sources of Diversity in Propolis Properties. Plants 2022, 11, 2298. [Google Scholar] [CrossRef] [PubMed]
- Saelao, P.; Borba, R.S.; Ricigliano, V.; Spivak, M.; Simone-Finstrom, M. Honeybee Microbiome Is Stabilized in the Presence of Propolis. Biol. Lett. 2020, 16, 20200003. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.; Bankova, V.; Viljoen, A.M. Propolis: Chemical Diversity and Challenges in Quality Control. Proc. Phytochem. Soc. Eur. 2022, 21, 1887–1911. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.L. Biological Activity of Bee Propolis in Health and Disease. Asian Pac. J. Cancer Prev. 2006, 7, 22–31. [Google Scholar] [PubMed]
- Rivera-Yañez, N.; Rivera-Yañez, C.R.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Méndez-Cruz, A.R.; Nieto-Yañez, O. Biomedical Properties of Propolis on Diverse Chronic Diseases and Its Potential Applications and Health Benefits. Nutrients 2020, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.A.; Valentim, I.B.; Camatari, F.O.S.; de Almeida, A.M.M.; Goulart, H.F.; Ferro, J.N.S.; Barreto, E.O.; Cavalcanti, B.C.; Bottoli, C.B.G.; Goulart, M.O.F. Polyphenol profile by UHPLC-MS/MS, anti-glycation, antioxidant and cytotoxic activities of several samples of propolis from the northeastern semi-arid region of Brazil. Pharm. Biol. 2017, 55, 1884–1893. [Google Scholar] [CrossRef]
- Kazemi, F.; Divsalar, A.; Saboury, A.A.; Seyedarabi, A. Propolis nanoparticles prevent structural changes in human hemoglobin during glycation and fructation. Colloids Surf. B 2019, 177, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Egawa, T.; Ohno, Y.; Yokoyama, S.; Yokokawa, T.; Tsuda, S.; Goto, K.; Hayashi, T. The Protective Effect of Brazilian Propolis against Glycation Stress in Mouse Skeletal Muscle. Foods 2019, 8, 439. [Google Scholar] [CrossRef]
- Goncalves, V.C.; Pinheiro, D.J.L.L.; de la Rosa, T.; de Almeida, A.G.; Scorza, F.A.; Scorza, C.A. Propolis as A Potential Disease-Modifying Strategy in Parkinson’s Disease: Cardioprotective and Neuroprotective Effects in the 6-OHDA Rat Model. Nutrients 2020, 12, 1551. [Google Scholar] [CrossRef]
- Barary, M.; Hosseinzadeh, R.; Kazemi, S.; Liang, J.J.; Mansoori, R.; Sio, T.T.; Hosseini, M.; Moghadamnia, A.A. The effect of propolis on 5-fluorouracil-induced cardiac toxicity in rats. Sci. Rep. 2022, 12, 8661. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, R.; Tang, Q.; Chen, J.Y.; Wang, Y.; Sui, D.J.; Liu, A.D. Flavonoid Extract from Propolis Provides Cardioprotection following Myocardial Infarction by Activating PPAR-γ. Evid.-Based Complement. Altern. Med. 2022, 2022, 1333545. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, V.C.; Silva da Fonsêca, V.; de Paula Faria, D.; Izidoro, M.A.; Berretta, A.A.; de Almeida, A.G.; Fonseca, F.L.A.; Scorza, F.A.; Scorza, C.A. Propolis induces cardiac metabolism changes in 6-hydroxydopamine animal model: A dietary intervention as a potential cardioprotective approach in Parkinson’s disease. Front. Pharmacol. 2022, 13, 1013703. [Google Scholar] [CrossRef]
- Maddahi, M.; Nattagh-Eshtivani, E.; Jokar, M.; Barati, M.; Tabesh, H.; Safarian, M.; Khosravi, M. The effect of propolis supplementation on cardiovascular risk factors in women with rheumatoid arthritis: A double-blind, placebo-controlled randomized clinical trial. Phytother. Res. 2023, 37, 5424–5434. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zheng, L.; Xu, L.; Tu, J.; Gu, X. Pinocembrin mitigates depressive-like behaviors induced by chronic unpredictable mild stress through ameliorating neuroinflammation and apoptosis. Mol. Med. 2020, 26, 53. [Google Scholar] [CrossRef] [PubMed]
- Palaz, M.N.; Akcay, E. The Impact of Propolis Factor Caffeic Acid Phenethyl-Ester on the Cerebral Vasospasm and Early Brain Damage in the Experimentally Induced Subarachnoid Hemorrhage on Rats. World Neurosurg. 2020, 138, e736–e742. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Apitherapy for Parkinson’s Disease: A Focus on the Effects of Propolis and Royal Jelly. Oxidative Med. Cell. Longev. 2020, 2020, 1727142. [Google Scholar] [CrossRef]
- Köseoğlu Toksoy, C.; Sarıtaş, Z.K.; Türk Börü, Ü.; Zeytin Demiral, G.; Görücü, F.; Bülbül, A.; Demirel, H.H.; Koç, Y. Investigation of the protective effect of anzer propolis in cerebral ischemia-reperfusion injury. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8004–8012. [Google Scholar]
- Ito, T.; Degawa, T.; Okumura, N. Brazilian green propolis prevents Alzheimer’s disease-like cognitive impairment induced by amyloid beta in mice. BMC Complement. Med. Ther. 2023, 23, 416. [Google Scholar] [CrossRef]
- Mamashli, F.; Meratan, A.A.; Ghasemi, A.; Obeidi, N.; Salmani, B.; Atarod, D.; Pirhaghi, M.; Moosavi-Movahedi, F.; Mohammad-Zaheri, M.; Shahsavani, M.B.; et al. Neuroprotective Effect of Propolis Polyphenol-Based Nanosheets in Cellular and Animal Models of Rotenone-Induced Parkinson’s Disease. ACS Chem. Neurosci. 2023, 14, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, H.; Kletsas, D.; Melliou, E.; Chinou, I. Antiproliferative activity of Greek propolis. J. Med. Food 2010, 13, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Nandakumar, N.; Palaniswami, R.; Nishigaki, Y.; Nishigaki, I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur. J. Med. Chem. 2014, 86, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Wang, Y.X.; Yu, N.Q.; Hu, D.; Wang, X.Y.; Chen, X.G.; Liao, Y.W.; Yao, J.; Wang, H.; He, L.; et al. Brazilian Green Propolis Extract Synergizes with Protoporphyrin IX-mediated Photodynamic Therapy via Enhancement of Intracellular Accumulation of Protoporphyrin IX and Attenuation of NF-κB and COX-2. Molecules 2017, 22, 732. [Google Scholar] [CrossRef] [PubMed]
- Benković, V.; Knezević, A.H.; Dikić, D.; Lisicić, D.; Orsolić, N.; Basić, I.; Kopjar, N. Radioprotective effects of quercetin and ethanolic extract of propolis in gamma-irradiated mice. Arh. Hig. Rada Toksikol. 2009, 60, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, C.O.; Aliyazicioglu, Y.; Demir, S.; Turan, I.; Bahat, Z.; Misir, S.; Deger, O. Evaluation of the radioprotective effect of Turkish propolis on foreskin fibroblast cells. J. Cancer Res. Ther. 2016, 12, 990–994. [Google Scholar]
- Avci, G.G.; Erdim, I.; Ozmen, Z.C.; Gevrek, F.; Colak, S.; Demirsoy, M.S.; Bozkurt, H. The effect of systemic application of propolis on tongue damage and oral mucositis in rats exposed to radiation. Eur. Arch. Otorhinolaryngol. 2022, 279, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, B.; Melero, A.; Montoro, A.; San Onofre, N.; Soriano, J.M. Radioprotective Effects from Propolis: A Review. Molecules 2023, 28, 5842. [Google Scholar] [CrossRef] [PubMed]
- Aytekin, A.A.; Tanrıverdi, S.T.; Köse, F.A.; Kart, D.; Eroğlu, İ.; Özer, Ö. Propolis loaded liposomes: Evaluation of antimicrobial and antioxidant activities. J. Liposome Res. 2020, 30, 107–116. [Google Scholar] [CrossRef]
- Navarro-Pérez, M.L.; Vadillo-Rodríguez, V.; Fernández-Babiano, I.; Pérez-Giraldo, C.; Fernández-Calderón, M.C. Antimicrobial activity of a novel Spanish propolis against planktonic and sessile oral Streptococcus spp. Sci. Rep. 2021, 11, 23860. [Google Scholar] [CrossRef]
- Sampaio, G.A.M.; Lacerda-Santos, R.; Cavalcanti, Y.W.; Vieira, G.H.A.; Nonaka, C.F.W.; Alves, P.M. Antimicrobial properties, mechanics, and fluoride release of ionomeric cements modified by red propolis. Angle Orthod. 2021, 91, 522–527. [Google Scholar] [CrossRef]
- Silva, T.S.; Silva, J.M.B.; Braun, G.H.; Mejia, J.A.A.; Capatinta, G.V.C.; Santos, M.F.C.; Tanimoto, M.H.; Bastos, J.K.; Parreira, R.L.T.; Orenha, R.P.; et al. Green and Red Brazilian Propolis: Antimicrobial Potential and Anti-Virulence against ATCC and Clinically Isolated Multidrug-Resistant Bacteria. Chem. Biodivers. 2021, 18, e2100307. [Google Scholar]
- Assis, M.A.S.; Ramos, L.P.; Hasna, A.A.; de Queiroz, T.S.; Pereira, T.C.; Lima, P.M.N.; Berretta, A.A.; Marcucci, M.C.; Carvalho, C.A.T.; Oliveira, L.D. Antimicrobial and Antibiofilm Effect of Brazilian Green Propolis Aqueous Extract against Dental Anaerobic Bacteria. Molecules 2022, 27, 8128. [Google Scholar] [CrossRef]
- Widelski, J.; Okińczyc, P.; Suśniak, K.; Malm, A.; Paluch, E.; Sakipov, A.; Zhumashova, G.; Ibadullayeva, G.; Sakipova, Z.; Korona-Glowniak, I. Phytochemical Profile and Antimicrobial Potential of Propolis Samples from Kazakhstan. Molecules 2023, 28, 2984. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, M.F.; Yılmaz, E.; Timirci-Kahraman, Ö.; Saygılı, N.; Kısakesen, H.İ.; Gazioğlu, S.; Gören, A.C.; Eronat, A.P.; Ceviz, A.B.; Öztürk, T.; et al. Different propolis samples, phenolic content, and breast cancer cell lines: Variable cytotoxicity ranging from ineffective to potent. IUBMB Life 2019, 71, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Frión-Herrera, Y.; Gabbia, D.; Scaffidi, M.; Zagni, L.; Cuesta-Rubio, O.; De Martin, S.; Carrara, M. Cuban brown propolis interferes in the crosstalk between colorectal cancer cells and M2 macrophages. Nutrients 2020, 12, 2040. [Google Scholar] [CrossRef] [PubMed]
- Misir, S.; Aliyazicioglu, Y.; Demir, S.; Turan, I.; Hepokur, C. Effect of Turkish Propolis on miRNA Expression, Cell Cycle, and Apoptosis in Human Breast Cancer (MCF-7) Cells. Nutr. Cancer 2020, 72, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.M.; Donia, T.; Abu-Khudir, R.; Ramadan, H.; Ali, E.M.M.; Mohamed, T.M. Propolis Potentiates Methotrexate Anticancer Mechanism and Reduces its Toxic Effects. Nutr. Cancer 2020, 72, 460–480. [Google Scholar] [CrossRef]
- Alanazi, S.; Alenzi, N.; Alenazi, F.; Tabassum, H.; Watson, D. Chemical characterization of Saudi propolis and its antiparasitic and anticancer properties. Sci. Rep. 2021, 11, 5390. [Google Scholar] [CrossRef] [PubMed]
- AlDreini, S.; Fatfat, Z.; Abou Ibrahim, N.; Fatfat, M.; Gali-Muhtasib, H.; Khalife, H. Thymoquinone enhances the antioxidant and anticancer activity of Lebanese propolis. World J. Clin. Oncol. 2023, 14, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.H.; Liu, C.Y.; Chang, K.W.; Lai, C.L.; Chang, S.C.; Huang, C.J. Propolis Has an Anticancer Effect on Early Stage Colorectal Cancer by Affecting Epithelial Differentiation and Gut Immunity in the Tumor Microenvironment. Nutrients 2023, 15, 4494. [Google Scholar] [CrossRef]
- Chi, Y.; Luo, L.; Cui, M.; Hao, Y.; Liu, T.; Huang, X.; Guo, X. Chemical Composition and Antioxidant Activity of Essential Oil of Chinese Propolis. Chem. Biodivers. 2020, 17, e1900489. [Google Scholar] [CrossRef]
- Alaribe, C.S.; Esposito, T.; Sansone, F.; Sunday, A.; Pagano, I.; Piccinelli, A.L.; Celano, R.; Cuesta Rubio, O.; Coker, H.A.; Nabavi, S.M.; et al. Nigerian propolis: Chemical composition, antioxidant activity and α-amylase and α-glucosidase inhibition. Nat. Prod. Res. 2021, 35, 3095–3099. [Google Scholar] [CrossRef]
- Boufadi, M.Y.; Soubhye, J.; Van Antwerpen, P. Anti-inflammatory, antioxidant effects, and bioaccessibility of Tigzirt propolis. J. Food Biochem. 2021, 45, e13663. [Google Scholar] [CrossRef]
- Salehi, A.; Hosseini, S.M.; Kazemi, S. Antioxidant and Anticarcinogenic Potentials of Propolis for Dimethylhydrazine-Induced Colorectal Cancer in Wistar Rats. Biomed Res. Int. 2022, 2022, 8497562. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Trusheva, B.; Chimshirova, R.; Antonova, D.; Gechovska, K.; Thanh, L.N.; Lien, N.T.P.; Phuong, D.T.L.; Bankova, V. Chemical Profile and Antioxidant Capacity of Propolis from Tetragonula, Lepidotrigona, Lisotrigona and Homotrigona Stingless Bee Species in Vietnam. Molecules 2022, 27, 7834. [Google Scholar] [CrossRef] [PubMed]
- Arung, E.T.; Syafrizal; Kusuma, I.W.; Paramita, S.; Amen, Y.; Kim, Y.U.; Naibaho, N.M.; Ramadhan, R.; Ariyanta, H.A.; Fatriasari, W.; et al. Antioxidant, anti-inflammatory and anti-acne activities of stingless bee (Tetragonula biroi) propolis. Fitoterapia 2023, 164, 105375. [Google Scholar] [CrossRef] [PubMed]
- Bachiega, T.F.; Orsatti, C.L.; Pagliarone, A.C.; Sforcin, J.M. The effects of propolis and its isolated compounds on cytokine production by murine macrophages. Phytother. Res. 2012, 26, 1308–1313. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological properties and therapeutic applications of propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.A.; Rosalen, P.L.; Dias, N.A.; Gomes, B.J.N.; Blosfeld-Lopes, L.; Ikegaki, M.; de Alencar, S.M.; Burger, E. Brazilian Red Propolis shows antifungal and immunomodulatory activities against Paracoccidioides brasiliensis. J. Ethnopharmacol. 2021, 277, 114181. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hamaguchi, M.; Bamba, R.; Nakajima, H.; Yoshimura, Y.; Kimura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; et al. Brazilian Green Propolis Improves Gut Microbiota Dysbiosis and Protects against Sarcopenic Obesity. J. Cachexia Sarcopenia Muscle 2022, 13, 3028–3047. [Google Scholar] [CrossRef]
- Al-Kahtani, S.N.; Alaqil, A.A.; Abbas, A.O. Modulation of Antioxidant Defense, Immune Response, and Growth Performance by Inclusion of Propolis and Bee Pollen into Broiler Diets. Animals 2022, 12, 1658. [Google Scholar] [CrossRef]
- Rebouças-Silva, J.; Amorim, N.A.; Jesus-Santos, F.H.; de Lima, J.A.; Lima, J.B.; Berretta, A.A.; Borges, V.M. Leishmanicidal and immunomodulatory properties of Brazilian green propolis extract (EPP-AF®) and a gel formulation in a pre-clinical model. Front. Pharmacol. 2023, 14, 1013376. [Google Scholar] [CrossRef]
- Abdel-Maksoud, E.M.; Daha, A.A.E.F.; Taha, N.M.; Lebda, M.A.; Sadek, K.M.; Alshahrani, M.Y.; Ahmed, A.E.; Shukry, M.; Fadl, S.E.; Elfeky, M. Effects of ginger extract and/or propolis extract on immune system parameters of vaccinated broilers. Poult. Sci. 2023, 102, 102903. [Google Scholar] [CrossRef]
- Usman, U.Z.; Bakar, A.B.A.; Mohamed, M. Propolis improves pregnancy outcomes and placental oxidative stress status in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2018, 18, 324. [Google Scholar] [CrossRef]
- Xue, M.; Liu, Y.; Xu, H.; Zhou, Z.; Ma, Y.; Sun, T.; Liu, M.; Zhang, H.; Liang, H. Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats. Biomed. Pharmacother. 2019, 118, 109393. [Google Scholar] [CrossRef] [PubMed]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Aboulghazi, A.; Ferreira-Santos, P.; Genisheva, Z.; Teixeira, J.A.; Lyoussi, B. Effect of antioxidant-rich propolis and bee pollen extracts against D-glucose induced type 2 diabetes in rats. Food Res. Int. 2020, 138 Pt B, 109802. [Google Scholar] [CrossRef]
- Taleb, R.A.; Djebli, N.; Chenini, H.; Sahin, H.; Kolayli, S. In vivo and in vitro anti-diabetic activity of ethanolic propolis extract. J. Food Biochem. 2020, 44, e13267. [Google Scholar]
- Salrian, A.A.; Behzadi, A.; Oloumi, M.M.; Farajli Abbasi, M.; Delshad, S.; Moghadaszadeh, M. Amplification of Wound Healing by Propolis and Honey Ointment in Healthy and Diabetic Rat Models; Histopathological and Morphometric Findings. Arch. Razi Inst. 2022, 77, 1673–1681. [Google Scholar] [PubMed]
- Ochoa-Morales, P.D.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; Patiño-Laguna, A.D.J. Anti-hyperglycemic effects of propolis or metformin in type 2 Diabetes Mellitus.International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Int. J. Vitam. Nutr. Res. 2023, 93, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.M.; Alves, A.V.F.; Queiroz, L.A.; Lima, B.S.; Filho, R.N.P.; Araújo, A.A.S.; de Albuquerque Júnior, R.L.C.; Cardoso, J.C. The photoprotective and anti-inflammatory activity of red propolis extract in rats. J. Photochem. Photobiol. B Biol. 2018, 180, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Lee, D.Y.; Kim, E.H. Anti-inflammatory and anti-oxidative effect of Korean propolis on Helicobacter pylori-induced gastric damage in vitro. J. Microbiol. 2020, 58, 878–885. [Google Scholar] [CrossRef]
- Sokeng, S.D.; Talla, E.; Sakava, P.; Fokam Tagne, M.A.; Henoumont, C.; Sophie, L.; Mbafor, J.T.; Tchuenguem Fohouo, F.N. Anti-Inflammatory and Analgesic Effect of Arachic Acid Ethyl Ester Isolated from Propolis. Biomed Res. Int. 2020, 2020, 8797284. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Hosoya, T.; Yoshizumi, K.; Sato, H.; Kumazawa, S. Phytochemical and anti-inflammatory properties of Senegalese propolis and isolated compounds. Fitoterapia 2021, 151, 104861. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Valencia, D.; Ortega-García, J.; Carvajal-Millan, E.; Díaz-Ríos, J.C.; Mendez-Pfeiffer, P.; Soto-Bracamontes, C.M.; Garibay-Escobar, A.; Alday, E.; Velazquez, C. Anti-Inflammatory Potential of Seasonal Sonoran Propolis Extracts and Some of Their Main Constituents. Molecules 2023, 28, 4496. [Google Scholar] [CrossRef]
- Valverde, T.M.; Soares, B.N.G.S.; Nascimento, A.M.D.; Andrade, Â.L.; Sousa, L.R.D.; Vieira, P.M.A.; Santos, V.R.; Seibert, J.B.; Almeida, T.C.S.; Rodrigues, C.F.; et al. Anti-Inflammatory, Antimicrobial, Antioxidant and Photoprotective Investigation of Red Propolis Extract as Sunscreen Formulation in Polawax Cream. Int. J. Mol. Sci. 2023, 24, 5112. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.A.C.; Braga, D.C.A.; Moura, S.A.L.; Souza, G.H.B.; Santos, O.D.H.; Cardoso, L.M. Salt-dependent hypertension and inflammation: Targeting the gut-brain axis and the immune system with Brazilian green propolis. Inflammopharmacology 2020, 28, 1163–1182. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xu, J.; Li, G.; Liu, T.; Guo, X.; Wang, H.; Luo, L. Ethanol extract of propolis prevents high-fat diet-induced insulin resistance and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2020, 130, 108939. [Google Scholar] [CrossRef]
- Garzarella, E.U.; Navajas-Porras, B.; Pérez-Burillo, S.; Ullah, H.; Esposito, C.; Santarcangelo, C.; Hinojosa-Nogueira, D.; Pastoriza, S.; Zaccaria, V.; Xiao, J.; et al. Evaluating the effects of a standardized polyphenol mixture extracted from poplar-type propolis on healthy and diseased human gut microbiota. Biomed. Pharmacother. 2022, 148, 112759. [Google Scholar] [CrossRef]
- Nakajima, M.; Arimatsu, K.; Minagawa, T.; Matsuda, Y.; Sato, K.; Takahashi, N.; Nakajima, T.; Yamazaki, K. Brazilian propolis mitigates impaired glucose and lipid metabolism in experimental periodontitis in mice. BMC Complement. Altern. Med. 2016, 16, 329. [Google Scholar] [CrossRef]
- Mujica, V.; Orrego, R.; Pérez, J.; Romero, P.; Ovalle, P.; Zúñiga-Hernández, J.; Arredondo, M.; Leiva, E. The Role of Propolis in Oxidative Stress and Lipid Metabolism: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2017, 2017, 4272940. [Google Scholar] [CrossRef]
- Huang, X.; Wu, X.; Yan, S.; Lan, T. Lipid-lowering effect of propolis in mice with Triton-WR1339-induced hyperlipidemia and its mechanism for regulating lipid metabolism. J. South. Med. 2018, 38, 1020–1024. [Google Scholar]
- Zakerkish, M.; Jenabi, M.; Zaeemzadeh, N.; Hemmati, A.A.; Neisi, N. The Effect of Iranian Propolis on Glucose Metabolism, Lipid Profile, Insulin Resistance, Renal Function and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 7289. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Feng, Z.; Dong, J.; Zhang, H. Phenolic Compounds of Propolis Alleviate Lipid Metabolism Disorder. Evid.-Based Complement. Altern. Med. 2021, 2021, 7615830. [Google Scholar] [CrossRef]
- Zhao, L.; Yao, L.; Chen, R.; He, T.; Qiu, S.; Chen, G.; Chen, H.; Qiu, S.-X. Pinostrobin from plants and propolis against human coronavirus HCoV-OC43 by modulating host AHR/CYP1A1 pathway and lipid metabolism. Antiviral Res. 2023, 212, 105570. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Wilkinson, F.L.; Sandhu, M.A.; Lightfoot, A.P. The Interplay of Oxidative Stress and Inflammation: Mechanistic Insights and Therapeutic Potential of Antioxidants. Oxidative Med. Cell. Longev. 2021, 2021, 9851914. [Google Scholar] [CrossRef]
- Baierle, M.; Nascimento, S.N.; Moro, A.M.; Brucker, N.; Freitas, F.; Gauer, B.; Durgante, J.; Bordignon, S.; Zibetti, M.; Trentini, C.M.; et al. Relationship between inflammation and oxidative stress and cognitive decline in the institutionalized elderly. Oxidative Med. Cell. Longev. 2015, 2015, 804198. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Rowiński, R.; Kornatowski, M.; Dąbrowski, A.; Kędziora-Kornatowska, K.; Strachecka, A. Relation of Moderate Physical Activity to Blood Markers of Oxidative Stress and Antioxidant Defense in the Elderly. Oxidative Med. Cell. Longev. 2019, 2019, 5123628. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, Z.; Naseri, M.; Vatandoost, F. Propolis: Chemical Composition and Its Applications in Endodontics. Iran. Endod. J. 2018, 13, 285–292. [Google Scholar] [PubMed]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- Pahlavani, N.; Malekahmadi, M.; Firouzi, S.; Rostami, D.; Sedaghat, A.; Moghaddam, A.B.; Ferns, G.A.; Navashenaq, J.G.; Reazvani, R.; Safarian, M.; et al. Molecular and Cellular Mechanisms of the Effects of Propolis in Inflammation, Oxidative Stress, and Glycemic Control in Chronic Diseases. Nutr. Metab. 2020, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Chaudhari, A.Z.; Teli, D.; Balar, P.; Vora, L. Propolis and Their Active Constituents for Chronic Diseases. Biomedicines 2023, 11, 259. [Google Scholar] [CrossRef]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a025130. [Google Scholar] [CrossRef]
- Laffon, B.; Bonassi, S.; Costa, S.; Valdiglesias, V. Genomic instability as a main driving factor of unsuccessful ageing: Potential for translating the use of micronuclei into clinical practice. Mutat. Res. Rev. 2021, 787, 108359. [Google Scholar] [CrossRef]
- Rattan, S.I.S. Molecular Basis of Nutrition and Aging: Molecular and Cellular Basis of Aging; Academic Press: New York, NY, USA, 2016; pp. 3–9. [Google Scholar]
- López-Gil, L.; Pascual-Ahuir, A.; Proft, M. Genomic Instability and Epigenetic Changes during Aging. Int. J. Mol. Sci. 2023, 24, 14279. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Chen, H.; Collins, A.R.; Connell, M.; Damia, G.; Dasgupta, S.; Malhotra, M.; Meeker, A.K.; Amedei, A.; Amin, A.; et al. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin. Cancer Biol. 2015, 35, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Petr, M.A.; Tulika, T.; Carmona-Marin, L.M.; Scheibye-Knudsen, M. Protecting the Aging Genome. Trends Cell Biol. 2020, 30, 117–132. [Google Scholar] [CrossRef]
- Das, S.; Das, J.; Paul, A.; Samadder, A.; Khuda-Bukhsh, A.R. Apigenin, a bioactive flavonoid from Lycopodium clavatum, stimulates nucleotide excision repair genes to protect skin keratinocytes from ultraviolet B-induced reactive oxygen species and DNA damage. J. Acupunct. Meridian Stud. 2013, 6, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Karapetsas, A.; Voulgaridou, G.P.; Konialis, M.; Tsochantaridis, I.; Kynigopoulos, S.; Lambropoulou, M.; Stavropoulou, M.I.; Stathopoulou, K.; Aligiannis, N.; Bozidis, P.; et al. Propolis Extracts Inhibit UV-Induced Photodamage in Human Experimental In Vitro Skin Models. Antioxidants 2019, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Widelski, J.; Gaweł-Bęben, K.; Czech, K.; Paluch, E.; Bortkiewicz, O.; Kozachok, S.; Mroczek, T.; Okińczyc, P. Extracts from European Propolises as Potent Tyrosinase Inhibitors. Molecules 2022, 28, 55. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, M.I.; Stathopoulou, K.; Cheilari, A.; Benaki, D.; Gardikis, K.; Chinou, I.; Aligiannis, N. NMR metabolic profiling of Greek propolis samples: Comparative evaluation of their phytochemical compositions and investigation of their anti-ageing and antioxidant properties. J. Pharm. Biomed. Anal. 2021, 194, 113814. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Jazvinšćak Jembrek, M. Molecular and Cellular Mechanisms of Propolis and Its Polyphenolic Compounds against Cancer. Int. J. Mol. Sci. 2022, 23, 10479. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Cho, H.A.; Jun, C.H.; Kim, H.J.; Cho, S.B.; Choi, S.K. A Review of Hepatocellular Carcinoma in Elderly Patients Focused on Management and Outcomes. In Vivo 2019, 33, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Harjumäki, R.; Pridgeon, C.S.; Ingelman-Sundberg, M. CYP2E1 in Alcoholic and Non-Alcoholic Liver Injury. Roles of ROS, Reactive Intermediates and Lipid Overload. Int. J. Mol. Sci. 2021, 22, 8221. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, E.A.; Chen, C.Y.; Wu, H.N.; Chien, C.C.; Chen, S.C. Propolis alleviates 4-aminobiphenyl-induced oxidative DNA damage by inhibition of CYP2E1 expression in human liver cells. Environ. Toxicol. 2021, 36, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, C.; Xi, S.; Qian, F.; Peng, X.; Huang, J.; Tang, F. Radioprotective Effect of Flavonoids on Ionizing Radiation-Induced Brain Damage. Molecules 2020, 25, 5719. [Google Scholar] [CrossRef]
- Ni, J.; Wu, Z.; Meng, J.; Zhu, A.; Zhong, X.; Wu, S.; Nakanishi, H. The Neuroprotective Effects of Brazilian Green Propolis on Neurodegenerative Damage in Human Neuronal SH-SY5Y Cells. Oxidative Med. Cell. Longev. 2017, 2017, 7984327. [Google Scholar] [CrossRef]
- Kumari, S.; Nayak, G.; Lukose, S.T.; Kalthur, S.G.; Bhat, N.; Hegde, A.R.; Mutalik, S.; Kalthur, G.; Adiga, S.K. Indian propolis ameliorates the mitomycin C-induced testicular toxicity by reducing DNA damage and elevating the antioxidant activity. Biomed. Pharmacother. 2017, 95, 252–263. [Google Scholar] [CrossRef]
- Santos, G.S.; Tsutsumi, S.; Vieira, D.P.; Bartolini, P.; Okazaki, K. Effect of Brazilian propolis (AF-08) on genotoxicity, cytotoxicity and clonogenic death of Chinese hamster ovary (CHO-K1) cells irradiated with (60)Co gamma-radiation. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2014, 762, 17–23. [Google Scholar] [CrossRef]
- Sharma, H.; Kanwal, R.; Bhaskaran, N.; Gupta, S. Plant flavone apigenin binds to nucleic acid bases and reduces oxidative DNA damage in prostate epithelial cells. PLoS ONE 2014, 9, e91588. [Google Scholar] [CrossRef] [PubMed]
- Mangan, D. Iron: An underrated factor in aging. Aging 2021, 13, 23407–23415. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Mao, L.; Tang, M.; Yan, Z.-Y.; Shao, J.; Huang, C.-H.; Sheng, Z.-G.; Zhu, B.-Z. Caffeic Acid Phenyl Ester (CAPE) Protects against Iron-Mediated Cellular DNA Damage through Its Strong Iron-Binding Ability and High Lipophilicity. Antioxidants 2021, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Barroso, L.; Puškár, M.; Ševčovičová, A.; Almeida-Aguiar, C.; Oliveira, R. Analysis of the effects of propolis extracts on DNA damage. In Študentská Vedecká Konferencia; Univerzita Komenského v Bratislave: Bratislava, Slovakia, 2016; pp. 20–25. [Google Scholar]
- Oršolić, N.; Sirovina, D.; Gajski, G.; Garaj-Vrhovac, V.; Jembrek, M.J.; Kosalec, I. Assessment of DNA damage and lipid peroxidation in diabetic mice: Effects of propolis and epigallocatechin gallate (EGCG). Mutat. Res. 2013, 757, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Jurk, D.; Passos, J.F.; Fagagna, F.A. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Pellegrini, M.V. Biochemistry, Telomere and Telomerase; StatPearls: Treasure Island, FL, USA, 2022; p. 35015454. [Google Scholar]
- Shay, J.W.; Wright, W.E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef]
- Galiè, S.; Canudas, S.; Muralidharan, J.; García-Gavilán, J.; Bulló, M.; Salas-Salvadó, J. Impact of Nutrition on Telomere Health: Systematic Review of Observational Cohort Studies and Randomized Clinical Trials. Adv. Nutr. 2020, 11, 576–601. [Google Scholar] [CrossRef]
- Nasir, N.F.; Kannan, T.P.; Sulaiman, S.A.; Shamsuddin, S.; Azlina, A.; Stangaciu, S. The relationship between telomere length and beekeeping among Malaysians. Age 2015, 37, 9797. [Google Scholar] [CrossRef]
- Sarkar, J.; Liu, Y. The origin of oxidized guanine resolves the puzzle of oxidation-induced telomere-length alterations. Nat. Struct. Mol. Biol. 2016, 23, 1070–1071. [Google Scholar] [CrossRef][Green Version]
- Legault, J.; Perron, T.; Mshvildadze, V.; Girard-Lalancette, K.; Perron, S.; Laprise, C.; Sirois, P.; Pichette, A. Antioxidant and anti-inflammatory activities of quercetin 7-O-β-D-glucopyranoside from the leaves of Brasenia schreberi. J. Med. Food 2011, 14, 1127–1134. [Google Scholar] [CrossRef]
- Tawani, A.; Kumar, A. Structural Insight into the interaction of Flavonoids with Human Telomeric Sequence. Sci. Rep. 2015, 5, 17574. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int. J. Mol. Sci. 2017, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Parekh, N.; Garg, A.; Choudhary, R.; Gupta, M.; Kaur, G.; Ramniwas, S.; Shahwan, M.; Tuli, H.S.; Sethi, G. The Role of Natural Flavonoids as Telomerase Inhibitors in Suppressing Cancer Growth. Pharmaceuticals 2023, 16, 605. [Google Scholar] [CrossRef]
- Bhatiya, M.; Pathak, S.; Jothimani, G.; Duttaroy, A.K.; Banerjee, A.A. Comprehensive Study on the Anti-cancer Effects of Quercetin and Its Epigenetic Modifications in Arresting Progression of Colon Cancer Cell Proliferation. Arch. Immunol. Ther. Exp. 2023, 71, 6. [Google Scholar] [CrossRef]
- Robinson, N.J.; Schiemann, W.P. Telomerase in Cancer: Function, Regulation, and Clinical Translation. Cancers 2022, 14, 808. [Google Scholar] [CrossRef] [PubMed]
- Avci, C.B.; Sahin, F.; Gunduz, C.; Selvi, N.; Aydin, H.H.; Oktem, G.; Topcuoglu, N.; Saydam, G. Protein phosphatase 2A (PP2A) has a potential role in CAPE-induced apoptosis of CCRF-CEM cells via affecting human telomerase reverse transcriptase activity. Hematology 2007, 12, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, C.; Biray, C.; Kosova, B.; Yilmaz, B.; Eroglu, Z.; Sahin, F.; Omay, S.B.; Cogulu, O. Evaluation of Manisa propolis effect on leukemia cell line by telomerase activity. Leuk. Res. 2005, 29, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Cogulu, O.; Biray, C.; Gunduz, C.; Karaca, E.; Aksoylar, S.; Sorkun, K.; Salih, B.; Ozkinay, F. Effects of Manisa propolis on telomerase activity in leukemia cells obtained from the bone marrow of leukemia patients. Int. J. Food Sci. Nutr. 2009, 60, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, U.C.; Bagca, B.G.; Karaca, E.; Durmaz, A.; Durmaz, B.; Aykut, A.; Kayalar, H.; Avci, C.B.; Susluer, S.Y.; Pariltay, E.; et al. Propolis Extract Regulates microRNA Expression in Glioblastoma and Brain Cancer Stem Cells. Anticancer Agents Med. Chem. 2022, 22, 378–389. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar]
- Zheng, Y.; Wu, Y.; Tao, L.; Chen, X.; Jones, T.J.; Wang, K.; Hu, F. Chinese Propolis Prevents Obesity and Metabolism Syndromes Induced by a High Fat Diet and Accompanied by an Altered Gut Microbiota Structure in Mice. Nutrients 2020, 12, 959. [Google Scholar] [CrossRef]
- Malard, F.; Dore, J.; Gaugler, B.; Mohty, M. Introduction to host microbiome symbiosis in health and disease. Mucosal Immunol. 2020, 14, 547–554. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Chen, Z.-D. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Salazar, A.M.; Aparicio, R.; Clark, R.I.; Rera, M.; Walker, D.W. Intestinal barrier dysfunction: An evolutionarily conserved hallmark of aging. Dis. Models Mech. 2023, 16, dmm049969. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.; Manary, M.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef]
- Ratiner, K.; Abdeen, S.K.; Goldenberg, K.; Elinav, E. Utilization of Host and Microbiome Features in Determination of Biological Aging. Microorganisms 2022, 10, 668. [Google Scholar] [CrossRef]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108, 4586–4591. [Google Scholar] [CrossRef]
- Fransen, F.; van Beek, A.A.; Borghuis, T.; Aidy, S.E.; Hugenholtz, F.; van der Gaast-de Jongh, C.; Savelkoul, H.F.J.; De Jonge, M.I.; Boekschoten, M.V.; Smidt, H.; et al. Aged Gut Microbiota Contributes to Systemical Inflammaging after Transfer to Germ-Free Mice. Front. Immunol. 2017, 8, 1385. [Google Scholar] [CrossRef]
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef]
- Si, J.; Vázquez-Castellanos, J.F.; Gregory, A.C.; Decommer, L.; Rymenans, L.; Proost, S.; Centelles Lodeiro, J.; Weger, M.; Notdurfter, M.; Leitner, C.; et al. Long-term life history predicts current gut microbiome in a population-based cohort study. Nat. Aging 2022, 2, 885–895. [Google Scholar] [CrossRef]
- Tamura, M.; Hoshi, C.; Kobori, M.; Takahashi, S.; Tomita, J.; Nishimura, M.; Nishihira, J. Quercetin metabolism by fecal microbiota from healthy elderly human subjects. PLoS ONE 2017, 12, e0188271. [Google Scholar] [CrossRef]
- Lan, H.; Hong, W.; Qian, D.; Peng, F.; Li, H.; Liang, C.; Du, M.; Gu, J.; Mai, J.; Bai, B.; et al. Quercetin modulates the gut microbiota as well as the metabolome in a rat model of osteoarthritis. Bioengineered 2021, 12, 6240–6250. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Roquetto, A.R.; Monteiro, N.E.S.; Moura, C.S.; Toreti, V.C.; de Pace, F.; Santos, A.D.; Park, Y.K.; Amaya-Farfan, J. Green propolis modulates gut microbiota, reduces endotoxemia and expression of TLR4 pathway in mice fed a high-fat diet. Food Res. Int. 2015, 76, 796–803. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Salamone, D.; Rivellese, A.A.; Vetrani, C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetol. 2021, 58, 1131–1138. [Google Scholar] [CrossRef]
- Williams, G.R.; Rier, H.N.; McDonald, A.; Shachar, S.S. Sarcopenia. aging in cancer. J. Geriatr. Oncol. 2019, 10, 374–377. [Google Scholar] [CrossRef]
- Dunne, R.F.; Loh, K.P.; Williams, G.R.; Jatoi, A.; Mustian, K.M.; Mohile, S.G. Cachexia and Sarcopenia in Older Adults with Cancer: A Comprehensive Review. Cancers 2019, 11, 1861. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Apitherapy for Age-Related Skeletal Muscle Dysfunction (Sarcopenia): A Review on the Effects of Royal Jelly, Propolis, and Bee Pollen. Foods 2020, 9, 1362. [Google Scholar] [CrossRef]
- Berthelot, J.M.; Sellam, J.; Maugars, Y.; Berenbaum, F. Cartilage-gut-microbiome axis: A new paradigm for novel therapeutic opportunities in osteoarthritis. RMD Open 2019, 5, e001037. [Google Scholar] [CrossRef]
- Braakhuis, A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Olcoz, J.L.; Jover, R.; Jorquera, F.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Functional Interactions between Gut Microbiota Transplantation, Quercetin, and High-Fat Diet Determine Non-Alcoholic Fatty Liver Disease Development in Germ-Free Mice. Mol. Nutr. Food Res. 2019, 63, e1800930. [Google Scholar] [CrossRef]
- Soleimani, D.; Miryan, M.; Tutunchi, H.; Navashenaq, J.G.; Sadeghi, E.; Ghayour-Mobarhan, M.; Ferns, G.A.; Ostadrahimi, A. A systematic review of preclinical studies on the efficacy of propolis for the treatment of inflammatory bowel disease. Phytother. Res. 2021, 35, 701–710. [Google Scholar] [CrossRef]
- Aabed, K.; Bhat, R.S.; Al-Dbass, A.; Moubayed, N.; Algahtani, N.; Merghani, N.M.; Alanazi, A.; Zayed, N.; El-Ansary, A. Bee pollen and propolis improve neuroinflammation and dysbiosis induced by propionic acid, a short chain fatty acid in a rodent model of autism. Lipids Health Dis. 2019, 18, 200. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, J.; Yang, H.; Li, G.; Li, H.; Deng, Z.; Zhang, B. Propolis polyphenols: A review on the composition and anti-obesity mechanism of different types of propolis polyphenols. Front. Nutr. 2023, 10, 1066789. [Google Scholar] [CrossRef]
- Guan, R.; Ma, N.; Liu, G.; Wu, Q.; Su, S.; Wang, J.; Geng, Y. Ethanol extract of propolis regulates type 2 diabetes in mice via metabolism and gut microbiota. J. Ethnopharmacol. 2023, 310, 116385. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, H.; Xu, K.; Du, Y.; Liu, J.; Wang, J.; Jiang, Y. Fecal metabolomics reveals the positive effect of ethanol extract of propolis on T2DM mice. Food Sci. Hum. Wellness 2023, 12, 161–172. [Google Scholar] [CrossRef]
- Zhong, X.-C.; Liu, Y.-M.; Gao, X.-X.; Krausz, K.W.; Niu, B.; Gonzalez, F.J.; Xie, C. Caffeic acid phenethyl ester suppresses intestinal FXR signaling and ameliorates nonalcoholic fatty liver disease by inhibiting bacterial bile salt hydrolase activity. Acta Pharmacol. Sin. 2023, 44, 145–156. [Google Scholar] [CrossRef]
- Chien, Y.H.; Yu, Y.H.; Chen, Y.W. Taiwanese green propolis ameliorates metabolic syndrome via remodeling of white adipose tissue and modulation of gut microbiota in diet-induced obese mice. Biomed. Pharmacother. 2023, 160, 114386. [Google Scholar] [CrossRef]
- Elbatreek, M.H.; Mahdi, I.; Ouchari, W.; Mahmoud, M.F.; Sobeh, M. Current advances on the therapeutic potential of pinocembrin: An updated review. Biomed. Pharmacother. 2023, 157, 114032. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Khandia, R.; Dadar, M.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Iqbal, H.M.N.; Singh, K.P.; Joshi, S.K.; et al. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells 2019, 8, 674. [Google Scholar] [CrossRef]
- Leidal, A.M.; Levine, B.; Debnath, J. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 2018, 20, 1338–1348. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Li, L.H.; Rao, Y.K.; Ju, T.C.; Nai, Y.S.; Chen, Y.W.; Hua, K.F. Mechanistic insight into the attenuation of gouty inflammation by Taiwanese green propolis via inhibition of the NLRP3 inflammasome. J. Cell. Physiol. 2019, 234, 4081–4094. [Google Scholar] [CrossRef]
- Hori, J.I.; Zamboni, D.S.; Carrão, D.B.; Goldman, G.H.; Berretta, A.A. The Inhibition of Inflammasome by Brazilian Propolis (EPP-AF). Evid.-Based Complement. Altern. Med. 2013, 2013, 418508. [Google Scholar] [CrossRef]
- Almeida-da-Silva, C.L.C.; Savio, L.E.B.; Coutinho-Silva, R.; Ojcius, D.M. The role of NOD-like receptors in innate immunity. Front. Immunol. 2023, 14, 1122586. [Google Scholar] [CrossRef]
- Biasizzo, M.; Kopitar-Jerala, N. Interplay Between NLRP3 Inflammasome and Autophagy. Front. Immunol. 2020, 11, 591803. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Arias, C.; Saavedra, N.; Saavedra, K.; Alvear, M.; Cuevas, A.; Maria-Engler, S.S.; Abdalla, D.S.P.; Salazar, L.A. Propolis Reduces the Expression of Autophagy-Related Proteins in Chondrocytes under Interleukin-1β Stimulus. Int. J. Mol. Sci. 2019, 20, 3768. [Google Scholar] [CrossRef]
- Karim, M.R.; Fisher, C.R.; Kapphahn, R.J.; Polanco, J.R.; Ferrington, D.A. Investigating AKT activation and autophagy in immunoproteasome-deficient retinal cells. PLoS ONE 2020, 15, e0231212. [Google Scholar] [CrossRef]
- Arias, C.; Salazar, L.A. Ethanolic Extract of Propolis Modulates Autophagy-Related microRNAs in Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2023, 24, 14767. [Google Scholar] [CrossRef]
- Arias, C.; Vásquez, B.; Salazar, L.A. Propolis as a Potential Therapeutic Agent to Counteract Age-Related Changes in Cartilage: An In Vivo Study. Int. J. Mol. Sci. 2023, 24, 14272. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Yang, H.; Jing, X.; Wang, W.; Liu, X.; Zhang, B.; Liu, X.; Shao, Y.; Cui, X. Activation of the Nrf-2 pathway by pinocembrin safeguards vertebral endplate chondrocytes against apoptosis and degeneration caused by oxidative stress. Life Sci. 2023, 333, 122162. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, J.H. Recent Advances in Understanding Nrf2 Agonism and Its Potential Clinical Application to Metabolic and Inflammatory Diseases. Int. J. Mol. Sci. 2022, 23, 2846. [Google Scholar] [CrossRef]
- Yuan, W.; Chang, H.; Liu, X.; Wang, S.; Liu, H.; Xuan, H. Brazilian Green Propolis Inhibits Ox-LDL-Stimulated Oxidative Stress in Human Umbilical Vein Endothelial Cells Partly through PI3K/Akt/mTOR-Mediated Nrf2/HO-1 Pathway. Evid.-Based Complement. Altern. Med. 2019, 2019, 5789574. [Google Scholar] [CrossRef]
- Xuan, H.; Yuan, W.; Chang, H.; Liu, M.; Hu, F. Anti-inflammatory effects of Chinese propolis in lipopolysaccharide-stimulated human umbilical vein endothelial cells by suppressing autophagy and MAPK/NF-κB signaling pathway. Inflammopharmacology 2019, 27, 561–571. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, L.; Zhao, Q.; Zhao, Z.; Zhi, F.; Qin, Y.; Cui, J. SKP2 attenuates NF-κB signaling by mediating IKKβ degradation through autophagy. J. Mol. Cell Biol. 2018, 10, 205–215. [Google Scholar] [CrossRef]
- Elumalai, P.; Muninathan, N.; Megalatha, S.T.; Suresh, A.; Kumar, K.S.; Jhansi, N.; Kalaivani, K.; Krishnamoorthy, G. An Insight into Anticancer Effect of Propolis and Its Constituents: A Review of Molecular Mechanisms. Evid.-Based Complement. Altern. Med. 2022, 2022, 5901191. [Google Scholar] [CrossRef]
- Chang, H.; Wang, Y.; Yin, X.; Liu, X.; Xuan, H. Ethanol extract of propolis and its constituent caffeic acid phenethyl ester inhibit breast cancer cells proliferation in inflammatory microenvironment by inhibiting TLR4 signal pathway and inducing apoptosis and autophagy. BMC Complement. Altern. Med. 2017, 17, 471. [Google Scholar] [CrossRef]
- Ghavami, S.; Cunnington, R.; Gupta, S.; Yeganeh, B.; Filomeno, K.L.; Freed, D.H.; Chen, S.; Klonisch, T.; Halayko, A.J.; Ambrose, E.; et al. Autophagy is a regulator of TGF-β1-induced fibrogenesis in primary human atrial myofibroblasts. Cell Death Dis. 2015, 6, e1696. [Google Scholar] [CrossRef]
- Yang, N.; Dang, S.; Shi, J.; Wu, F.; Li, M.; Zhang, X.; Li, Y.; Jia, X.; Zhai, S. Caffeic acid phenethyl ester attenuates liver fibrosis via inhibition of TGF-β1/Smad3 pathway and induction of autophagy pathway. Biochem. Biophys. Res. Commun. 2017, 486, 22–28. [Google Scholar] [CrossRef]
- Kubiliene, L.; Jekabsone, A.; Zilius, M.; Trumbeckaite, S.; Simanaviciute, D.; Gerbutaviciene, R.; Majiene, D. Comparison of aqueous, polyethylene glycol-aqueous, and ethanolic propolis extracts: Antioxidant and mitochondria modulating properties. BMC Complement. Altern. Med. 2018, 18, 165. [Google Scholar] [CrossRef]
- Tan, W.; Pasinelli, P.; Trotti, D. Role of mitochondria in mutant SOD1 linked amyotrophic lateral sclerosis. Biochim. Biophys. Acta 2014, 1842, 1295–1301. [Google Scholar] [CrossRef]
- Tafuri, F.; Ronchi, D.; Magri, F.; Comi, G.P.; Corti, S. SOD1 misplacing and mitochondrial dysfunction in amyotrophic lateral sclerosis pathogenesis. Front. Cell. Neurosci. 2015, 9, 336. [Google Scholar] [CrossRef]
- Ueda, T.; Inden, M.; Shirai, K.; Sekine, S.-I.; Masaki, Y.; Kurita, H.; Ichihara, K.; Inuzuka, T.; Hozumi, I. The effects of Brazilian green propolis that contains flavonols against mutant copper-zinc superoxide dismutase-mediated toxicity. Sci. Rep. 2017, 7, 2882. [Google Scholar] [CrossRef]
- Liu, R.; Wu, C.X.; Zhou, D.; Yang, F.; Tian, S.; Zhang, L.; Zhang, T.T.; Du, G.H. Pinocembrin protects against β-amyloid-induced toxicity in neurons through inhibiting receptor for advanced glycation end products (RAGE)-independent signaling pathways and regulating mitochondrion-mediated apoptosis. BMC Med. 2012, 10, 105. [Google Scholar] [CrossRef]
- Prasuhn, J.; Davis, R.L.; Kumar, K.R. Targeting Mitochondrial Impairment in Parkinson’s Disease: Challenges and Opportunities. Front. Cell Dev. Biol. 2021, 8, 615461. [Google Scholar] [CrossRef]
- Zambrano, K.; Barba, D.; Castillo, K.; Noboa, L.; Argueta-Zamora, D.; Robayo, P.; Arizaga, E.; Caicedo, A.; Gavilanes, A.W.D. Fighting Parkinson’s disease: The return of the mitochondria. Mitochondrion 2022, 64, 34–44. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, S.; Wang, J.; Shen, Y.; Zhang, J.; Wu, Q. Nrf2/HO-1 mediates the neuroprotective effect of mangiferin on early brain injury after subarachnoid hemorrhage by attenuating mitochondria-related apoptosis and neuroinflammation. Sci. Rep. 2017, 7, 11883. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Miao, Y.; Cui, Q.; Zhao, W.; Zhang, J.; Wang, H. Pinocembrin protects SH-SY5Y cells against MPP+-induced neurotoxicity through the mitochondrial apoptotic pathway. J. Mol. Neurosci. 2014, 53, 537–545. [Google Scholar] [CrossRef]
- Fontanilla, C.V.; Ma, Z.; Wei, X.; Klotsche, J.; Zhao, L.; Wisniowski, P.; Dodel, R.C.; Farlow, M.R.; Oertel, W.H.; Du, Y. Caffeic acid phenethyl ester prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration. Neuroscience 2011, 188, 135–141. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Petcu, E.B.; Capitanescu, B.; Hermann, D.M.; Radu, E.; Gresita, A. Ageing as a risk factor for cerebral ischemia: Underlying mechanisms and therapy in animal models and in the clinic. Mech. Ageing Dev. 2020, 190, 111312. [Google Scholar] [CrossRef]
- Balion, Z.; Ramanauskienė, K.; Jekabsone, A.; Majienė, D. The Role of Mitochondria in Brain Cell Protection from Ischaemia by Differently Prepared Propolis Extracts. Antioxidants 2020, 9, 1262. [Google Scholar] [CrossRef]
- Guang, H.M.; Du, G.H. Protections of pinocembrin on brain mitochondria contribute to cognitive improvement in chronic cerebral hypoperfused rats. Eur. J. Pharmacol. 2006, 542, 77–83. [Google Scholar] [CrossRef]
- Boutab, K.; Kebsa, W.; Alyane, M.; Lahouel, M. Polyphenolic fraction of Algerian propolis protects rat kidney against acute oxidative stress induced by doxorubicin. Indian J. Nephrol. 2011, 21, 101–106. [Google Scholar]
- El-Aziz, E.A.E.A.; Elgayar, S.F.; Mady, F.M.; Abourehab, M.A.S.; Hasan, O.A.; Reda, L.M.; Alaaeldin, E. The Potential of Optimized Liposomes in Enhancement of Cytotoxicity and Apoptosis of Encapsulated Egyptian Propolis on Hep-2 Cell Line. Pharmaceutics 2021, 13, 2184. [Google Scholar] [CrossRef]
- Oliveira, R.D.; Celeiro, S.P.; Barbosa-Matos, C.; Freitas, A.S.; Cardoso, S.M.; Viana-Pereira, M.; Almeida-Aguiar, C.; Baltazar, F. Portuguese Propolis Antitumoral Activity in Melanoma Involves ROS Production and Induction of Apoptosis. Molecules 2022, 27, 3533. [Google Scholar] [CrossRef]
- Salman, B.N.; Kalantari, M.; Mohebbi Rad, M.; Saburi, E. Comparison of cytotoxic and apoptosis-inducing effects of MTA, propolis, and propolis-MTA on immature dental pulp stem cells. Eur. Arch. Paediatr. Dent. 2023, 24, 797–802. [Google Scholar] [CrossRef]
- Campos, J.F.; Dos Santos, H.F.; Bonamigo, T.; de Campos Domingues, N.L.; de Picoli Souza, K.; Dos Santos, E.L. Stingless Bee Propolis: New Insights for Anticancer Drugs. Oxidative Med. Cell. Longev. 2021, 2021, 2169017. [Google Scholar] [CrossRef]
- Frión-Herrera, Y.; Díaz-García, A.; Ruiz-Fuentes, J.; Rodríguez-Sánchez, H.; Sforcin, J.M. Brazilian green propolis induced apoptosis in human lung cancer A549 cells through mitochondrial-mediated pathway. J. Pharm. Pharmacol. 2015, 67, 1448–1456. [Google Scholar] [CrossRef]
- Kuo, Y.Y.; Chen, W.T.; Lin, G.B.; Lu, C.H.; Chao, C.Y. Study on the effect of a triple cancer treatment of propolis, thermal cycling-hyperthermia, and low-intensity ultrasound on PANC-1 cells. Aging 2023, 15, 7496–7512. [Google Scholar] [CrossRef]
- Fu, Y.K.; Wang, B.J.; Tseng, J.C.; Huang, S.H.; Lin, C.Y.; Kuo, Y.Y.; Hour, T.C.; Chuu, C.P. Combination treatment of docetaxel with caffeic acid phenethyl ester suppresses the survival and the proliferation of docetaxel-resistant prostate cancer cells via induction of apoptosis and metabolism interference. J. Biomed. Sci. 2022, 29, 16. [Google Scholar] [CrossRef]
- Guimarães, N.S.; Mello, J.C.; Paiva, J.S.; Bueno, P.C.; Berretta, A.A.; Torquato, R.J.; Nantes, I.L.; Rodrigues, T. Baccharis dracunculifolia, the main source of green propolis, exhibits potent antioxidant activity and prevents oxidative mitochondrial damage. Food Chem. Toxicol. 2012, 50, 1091–1097. [Google Scholar] [CrossRef]
- Sung, Y.; Yu, Y.C.; Han, J.M. Nutrient sensors and their crosstalk. Exp. Mol. Med. 2023, 55, 1076–1089. [Google Scholar] [CrossRef]
- Micó, V.; Berninches, L.; Tapia, J.; Daimiel, L. NutrimiRAging: Micromanaging Nutrient Sensing Pathways through Nutrition to Promote Healthy Aging. Int. J. Mol. Sci. 2017, 18, 915. [Google Scholar] [CrossRef]
- Dakic, T.; Jevdjovic, T.; Vujovic, P.; Mladenovic, A. The Less We Eat, the Longer We Live: Can Caloric Restriction Help Us Become Centenarians? Int. J. Mol. Sci. 2022, 23, 6546. [Google Scholar] [CrossRef]
- Lucia, C.; Murphy, T.; Steves, C.J.; Dobson, R.J.B.; Proitsi, P.; Thuret, S. Lifestyle mediates the role of nutrient-sensing pathways in cognitive aging: Cellular and epidemiological evidence. Commun. Biol. 2020, 3, 157. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C. Nutrient Sensing, Signaling and Ageing: The Role of IGF-1 and mTOR in Ageing and Age-Related Disease. Subcell. Biochem. 2018, 90, 49–97. [Google Scholar] [PubMed]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, J.Z.; Sun, Y.; Le, Z.; Zhang, P.; Yu, D.; Liu, Y. Insulin-like growth factor 1 receptor-mediated cell survival in hypoxia depends on the promotion of autophagy via suppression of the PI3K/Akt/mTOR signaling pathway. Mol. Med. Rep. 2017, 15, 2136–2142. [Google Scholar] [CrossRef]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Fernandes, S.A.; Demetriades, C. The Multifaceted Role of Nutrient Sensing and mTORC1 Signaling in Physiology and Aging. Front. Aging 2021, 2, 707372. [Google Scholar] [CrossRef]
- Kim, J.; Guan, K.L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 2019, 21, 63–71. [Google Scholar] [CrossRef]
- Chrienova, Z.; Nepovimova, E.; Kuca, K. The role of mTOR in age-related diseases. J. Enzyme Inhib. Med. Chem. 2021, 36, 1679–1693. [Google Scholar] [CrossRef]
- Ham, D.J.; Börsch, A.; Chojnowska, K.; Lin, S.; Leuchtmann, A.B.; Ham, A.S.; Thürkauf, M.; Delezie, J.; Furrer, R.; Burri, D.; et al. Distinct and additive effects of calorie restriction and rapamycin in aging skeletal muscle. Nat. Commun. 2022, 13, 2025. [Google Scholar] [CrossRef]
- Wang, H.F.; Li, H.; Yuan, M.; Fan, H.; Cai, Z. Role of AMPK in autophagy. Front. Physiol. 2022, 13, 2479. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, G.H.; Qu, J. Mitochondrial sirtuins, metabolism, and aging. J. Genet. Genom. 2022, 49, 287–298. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef]
- Ye, X.; Li, M.; Hou, T.; Gao, T.; Zhu, W.; Yang, Y. Sirtuins in glucose and lipid metabolism. Oncotarget 2017, 8, 1845–1859. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, H.; Xu, N.; Zhou, S.; Peng, Y.; Zhu, J.; Liu, S.; Chang, Y. Chrysin improves diabetic nephropathy by regulating the AMPK-mediated lipid metabolism in HFD/STZ-induced DN mice. J. Food Biochem. 2022, 46, e14379. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Abedi, F.; Samarghandian, S. Chrysin attenuates inflammatory and metabolic disorder indices in aged male rat. Biomed. Pharmacother. 2019, 109, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Oriquat, G.; Masoud, I.M.; Kamel, M.A.; Aboudeya, H.M.; Bakir, M.B.; Shaker, S.A. The Anti-Obesity and Anti-Steatotic Effects of Chrysin in a Rat Model of Obesity Mediated through Modulating the Hepatic AMPK/mTOR/lipogenesis Pathways. Molecules 2023, 28, 1734. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Chitturi, S.; Farrell, G.C. Mechanisms and implications of age-related changes in the liver: Nonalcoholic Fatty Liver Disease in the elderly. Curr. Gerontol. Geriatr. Res. 2011, 2011, 831536. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Fan, S.; Li, X.; Yang, S.; Ji, C.; Ji, F.; Zhou, B. Four flavonoids from propolis ameliorate free fatty acids-induced non-alcoholic steatohepatitis in HepG2 cells: Involvement of enhanced AMPK activation, mTOR-NF-κBp65 interaction, and PTEN expression. J. Funct. Foods 2023, 102, 105460. [Google Scholar] [CrossRef]
- Cardinault, N.; Tourniaire, F.; Astier, J.; Couturier, C.; Bonnet, L.; Seipelt, E.; Karkeni, E.; Letullier, C.; Dlalah, N.; Georgé, S.; et al. Botanic Origin of Propolis Extract Powder Drives Contrasted Impact on Diabesity in High-Fat-Fed Mice. Antioxidants 2021, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Cardinault, N.; Tourniaire, F.; Astier, J.; Couturier, C.; Perrin, E.; Dalifard, J.; Seipelt, E.; Mounien, L.; Letullier, C.; Bonnet, L.; et al. Poplar Propolis Ethanolic Extract Reduces Body Weight Gain and Glucose Metabolism Disruption in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2020, 64, e2000275. [Google Scholar] [CrossRef]
- Ichi, I.; Hori, H.; Takashima, Y.; Adachi, N.; Kataoka, R.; Okihara, K.; Hashimoto, K.; Kojo, S. The beneficial effect of propolis on fat accumulation and lipid metabolism in rats fed a high-fat diet. J. Food Sci. 2009, 74, H127–H131. [Google Scholar] [CrossRef] [PubMed]
- Sani, L.; Cardinault, N.; Astier, J.; Darmon, P.; Landrier, J.F. Poplar Propolis Improves Insulin Homeostasis in Non-Diabetic Insulin-Resistant Volunteers with Obesity: A Crossover Randomized Controlled Trial. Antioxidants 2023, 12, 1481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, X.; Zhang, G.; Kong, L.; Peng, W.; Zhang, H. Galangin and Pinocembrin from Propolis Ameliorate Insulin Resistance in HepG2 Cells via Regulating Akt/mTOR Signaling. Evid.-Based Complement. Altern. Med. 2018, 2018, 7971842. [Google Scholar] [CrossRef]
- Ueda, M.; Hayashibara, K.; Ashida, H. Propolis extract promotes translocation of glucose transporter 4 and glucose uptake through both PI3K- and AMPK-dependent pathways in skeletal muscle. BioFactors 2013, 39, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Xu, N.; Zhang, X.C.; Zhu, Y.Y.; Liu, S.W.; Chang, Y.N. Chrysin Improves Glucose and Lipid Metabolism Disorders by Regulating the AMPK/PI3K/AKT Signaling Pathway in Insulin-Resistant HepG2 Cells and HFD/STZ-Induced C57BL/6J Mice. J. Agric. Food Chem. 2021, 69, 5618–5627. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Yuan, W.; Wu, H.; Yin, X.; Xuan, H. Bioactive components and mechanisms of Chinese poplar propolis alleviates oxidized low-density lipoprotein-induced endothelial cells injury. BMC Complement. Altern. Med. 2018, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Feng, J.; Zhang, J.; Kang, X.; Qian, D. Quercetin modulates AMPK/SIRT1/NF-κB signaling to inhibit inflammatory/oxidative stress responses in diabetic high-fat diet-induced atherosclerosis in the rat carotid artery. Exp. Ther. Med. 2020, 20, 280. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Lu, L.; Liu, Y.; Ma, J.; Yang, L.; Li, L.; Guo, H.; Yu, S.; Ren, J.; Bai, H.; et al. Quercetin improves ischemia/reperfusion-induced cardiomyocyte apoptosis: An in vitro and in vivo study via SIRT1/PGC-1α signaling. J. Cell. Biochem. 2019, 120, 9747–9757. [Google Scholar] [CrossRef]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018, 103, 1585–1591. [Google Scholar] [CrossRef]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Heger, V.; Tyni, J.; Hunyadi, A.; Horáková, L.; Lahtela-Kakkonen, M.; Rahnasto-Rilla, M. Quercetin-based derivatives as sirtuin inhibitors. Biomed. Pharmacother. 2019, 111, 1326–1333. [Google Scholar] [CrossRef]
- Akter, R.; Afrose, A.; Rahman, M.R.; Chowdhury, R.; Nirzhor, S.S.R.; Khan, R.I.; Kabir, M.T. A Comprehensive Analysis into the Therapeutic Application of Natural Products as SIRT6 Modulators in Alzheimer’s Disease, Aging, Cancer, Inflammation, and Diabetes. Int. J. Mol. Sci. 2021, 22, 4180. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef]
- Tseng, J.C.; Lin, C.Y.; Su, L.C.; Fu, H.H.; Yang, S.D.; Chuu, C.P. CAPE suppresses migration and invasion of prostate cancer cells via activation of non-canonical Wnt signaling. Oncotarget 2016, 7, 38010–38024. [Google Scholar] [CrossRef]
- Havermann, S.; Chovolou, Y.; Humpf, H.U.; Wätjen, W. Caffeic acid phenethylester increases stress resistance and enhances lifespan in Caenorhabditis elegans by modulation of the insulin-like DAF-16 signalling pathway. PLoS ONE 2014, 9, e100256. [Google Scholar] [CrossRef] [PubMed]
- Davy, P.M.C.; Allsopp, R.C.; Donlon, T.A.; Morris, B.J.; Willcox, D.C.; Willcox, B.J. FOXO3 and Exceptional Longevity: Insights From Hydra to Humans. Curr. Top. Dev. Biol. 2018, 127, 193–212. [Google Scholar] [PubMed]
- Fafián-Labora, J.A.; O’Loghlen, A. Classical and Nonclassical Intercellular Communication in Senescence and Ageing. Trends Cell Biol. 2020, 30, 628–639. [Google Scholar] [CrossRef]
- Saavedra, D.; Añé-Kourí, A.L.; Barzilai, N.; Caruso, C.; Cho, K.H.; Fontana, L.; Franceschi, C.; Frasca, D.; Ledón, N.; Niedernhofer, L.J.; et al. Aging and chronic inflammation: Highlights from a multidisciplinary workshop. Immun. Ageing 2023, 20, 25. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Gao, W.; Wu, J.; Wei, J.; Pu, L.; Guo, C.; Yang, J.; Yang, M.; Luo, H. Brazilian green propolis improves immune function in aged mice. J. Clin. Biochem. Nutr. 2014, 55, 7–10. [Google Scholar] [CrossRef]
- Zhu, A.; Wu, Z.; Zhong, X.; Ni, J.; Li, Y.; Meng, J.; Du, C.; Zhao, X.; Nakanishi, H.; Wu, S. Brazilian Green Propolis Prevents Cognitive Decline into Mild Cognitive Impairment in Elderly People Living at High Altitude. J. Alzheimer’s Dis. 2018, 63, 551–560. [Google Scholar] [CrossRef]
- Ali, A.A.; Kamal, M.M.; Khalil, M.G.; Ali, S.A.; Elariny, H.A.; Bekhit, A.; Wahid, A. Behavioral, Biochemical and Histopathological effects of Standardised Pomegranate extract with Vinpocetine, Propolis or Cocoa in a rat model of Parkinson’s disease. Exp. Aging Res. 2022, 48, 191–210. [Google Scholar] [CrossRef]
- Islam, M.T.; Tuday, E.; Allen, S.; Kim, J.; Trott, D.W.; Holland, W.L.; Donato, A.J.; Lesniewski, L.A. Senolytic Drugs, Dasatinib and Quercetin, Attenuate Adipose Tissue Inflammation, and Ameliorate Metabolic Function in Old Age. Aging Cell 2023, 22, e13767. [Google Scholar] [CrossRef]
- Conklin, C.M.; Bechberger, J.F.; MacFabe, D.; Guthrie, N.; Kurowska, E.M.; Naus, C.C. Genistein and quercetin increase connexin43 and suppress growth of breast cancer cells. Carcinogenesis 2007, 28, 93–100. [Google Scholar] [CrossRef]
- Klaips, C.L.; Jayaraj, G.G.; Hartl, F.U. Pathways of cellular proteostasis in aging and disease. J. Cell Biol. 2018, 217, 51–63. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Bhargava, P.; Kumari, A.; Putri, J.F.; Ishida, Y.; Terao, K.; Kaul, S.C.; Sundar, D.; Wadhwa, R. Caffeic acid phenethyl ester (CAPE) possesses pro-hypoxia and anti-stress activities: Bioinformatics and experimental evidences. Cell Stress Chaperones 2018, 23, 1055–1068. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation, and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A.; Baghdady, A.M.; Ali, S.A.; Ahmed, M.I. GRP78 targeting: Hitting two birds with a stone. Life Sci. 2020, 260, 118317. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Motoyama, M.; Kimura, S.; Takashima, M.; Ikawa, T.; Oh-Hashi, K.; Kamatari, Y.O. Artepillin C, a major component of Brazilian green propolis, inhibits endoplasmic reticulum stress and protein aggregation. Eur. J. Pharmacol. 2021, 912, 174572. [Google Scholar] [CrossRef]
- Liu, M.Q.; Chen, Z.; Chen, L.X. Endoplasmic reticulum stress: A novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol. Sin. 2016, 37, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Estébanez, B.; de Paz, J.A.; Cuevas, M.J.; González-Gallego, J. Endoplasmic Reticulum Unfolded Protein Response, Aging and Exercise: An Update. Front. Physiol. 2018, 9, 1744. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Sun, H.W.; Zhang, J.J.; Zhang, X.-W.; Zhao, L.; Guo, S.-D.; Li, Y.-Y.; Jiao, P.; Wang, H.; Qin, S.-T.; et al. Ethanol extract of propolis protects macrophages from oxidized low-density lipoprotein-induced apoptosis by inhibiting CD36 expression and endoplasmic reticulum stress-C/EBP homologous protein pathway. BMC Complement. Altern. Med. 2015, 15, 230. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, R.; Takakura, K.; Takatou, S.; Le, T.M.; Nishiuchi, T.; Nakamura, Y.; Konishi, T.; Matsugo, S.; Hori, O. 3,4-dihydroxybenzalacetone and caffeic acid phenethyl ester induce preconditioning ER stress and autophagy in SH-SY5Y cells. J. Cell Physiol. 2018, 233, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Omoruyi, S.I.; Hiss, D.C.; Ekpo, O.E. GRP78/BIP/HSPA5 as a Therapeutic Target in Models of Parkinson’s Disease: A Mini Review. Adv. Pharmacol. Sci. 2019, 2019, 2706783. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, Y.; Salmani, B.; Mirzaei-Behbahani, B.; Taleb, M.; Meratan, A.A.; Ramezani, M.; Nikfarjam, N.; Becker, S.; Rezaei-Ghaleh, N. Polyphenols-Based Nanosheets of Propolis Modulate Cytotoxic Amyloid Fibril Assembly of α-Synuclein. ACS Chem. Neurosci. 2022, 13, 3168–3179. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lim, W.; Bazer, F.W.; Song, G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J. Cell Physiol. 2017, 232, 3786–3797. [Google Scholar] [CrossRef]
- Wadhwa, R.; Nigam, N.; Bhargava, P.; Dhanjal, J.K.; Goyal, S.; Grover, A.; Sundar, D.; Ishida, Y.; Terao, K.; Kaul, S.C. Molecular Characterization and Enhancement of Anticancer Activity of Caffeic Acid Phenethyl Ester by γ Cyclodextrin. J. Cancer 2016, 7, 1755–1771. [Google Scholar] [CrossRef]
- Wang, J.; Bhargava, P.; Yu, Y.; Sari, A.N.; Zhang, H.; Ishii, N.; Yan, K.; Zhang, Z.; Ishida, Y.; Terao, K.; et al. Novel Caffeic Acid Phenethyl Ester-Mortalin Antibody Nanoparticles Offer Enhanced Selective Cytotoxicity to Cancer Cells. Cancers 2020, 12, 2370. [Google Scholar] [CrossRef]
- Bhargava, P.; Grover, A.; Nigam, N.; Kaul, A.; Doi, M.; Ishida, Y.; Kakuta, H.; Kaul, S.C.; Terao, K.; Wadhwa, R. Anticancer activity of the supercritical extract of Brazilian green propolis and its active component, artepillin C: Bioinformatics and experimental analyses of its mechanisms of action. Int. J. Oncol. 2018, 52, 925–932. [Google Scholar]
- Li, W.; Yang, C.; Shi, Z.; Long, Q.; Cheng, Z.; He, S.; Dong, J.; Liu, T.; Wang, C. Caffeic Acid Phenethyl Ester Inhibits Ubiquitination and Degradation of p53 and Blocks Cervical Cancer Cell Growth. Curr. Mol. Med. 2023, 23, 960–970. [Google Scholar] [CrossRef]
- Ganesan, A. Epigenetics: The first 25 centuries. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170067. [Google Scholar] [CrossRef]
- Lacal, I.; Ventura, R. Epigenetic Inheritance: Concepts, Mechanisms and Perspectives. Front. Mol. Neurosci. 2018, 11, 292. [Google Scholar] [CrossRef]
- Tammen, S.A.; Friso, S.; Choi, S.W. Epigenetics: The link between nature and nurture. Mol. Asp. Med. 2013, 34, 753–764. [Google Scholar] [CrossRef]
- Saul, D.; Kosinsky, R.L. Epigenetics of Aging and Aging-Associated Diseases. Int. J. Mol. Sci. 2021, 22, 401. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shukla, S.; Sinha, S.; Meeran, S.M. Epigenetic targets in cancer and aging: Dietary and therapeutic interventions. Expert Opin. Ther. Targets 2016, 20, 689–703. [Google Scholar] [CrossRef]
- Gambhir, S.; Vyas, D.; Hollis, M.; Aekka, A.; Vyas, A. Nuclear factor kappa B role in inflammation-associated gastrointestinal malignancies. World J. Gastroenterol. 2015, 21, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.L.; Michishita, E.; Adler, A.S.; Damian, M.; Berber, E.; Lin, M.; McCord, R.A.; Ongaigui, K.C.; Boxer, L.D.; Chang, H.Y.; et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 2009, 136, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Verzella, D.; Pescatore, A.; Capece, D.; Vecchiotti, D.; Ursini, M.V.; Franzoso, G.; Alesse, E.; Zazzeroni, F. Life, death, and autophagy in cancer: NF-κB turns up everywhere. Cell Death Dis. 2020, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.G.; Wang, J.L.; Yang, S.L.; Wu, J.L. Aberrant epigenetic alteration in Eca9706 cells modulated by nanoliposomal quercetin combined with butyrate mediated via epigenetic-NF-κB signaling. Asian Pac. J. Cancer Prev. 2014, 15, 4539–4543. [Google Scholar] [CrossRef][Green Version]
- Wu, K.K.; Liou, J.Y.; Cieslik, K. Transcriptional Control of COX-2 via C/EBPbeta. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Shi, D.; Liu, L.; Wang, J.; Xie, X.; Kang, T.; Deng, W. Quercetin Suppresses Cyclooxygenase-2 Expression and Angiogenesis through Inactivation of P300 Signaling. PLoS ONE 2011, 6, e22934. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Hussain, A.; Haque, S.; Raina, R.; Afroze, N. Quercetin modifies 5′CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J. Cell Biochem. 2019, 120, 18357–18369. [Google Scholar] [CrossRef]
- Zaccaria, V.; Curti, V.; Di Lorenzo, A.; Baldi, A.; Maccario, C.; Sommatis, S.; Mocchi, R.; Daglia, M. Effect of Green and Brown Propolis Extracts on the Expression Levels of microRNAs, mRNAs and Proteins, Related to Oxidative Stress and Inflammation. Nutrients 2017, 9, 1090. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Ijaz, B.; Ahmad, Z.; Farooq, N.; Sarwar, M.B.; Husnain, T. Modification of miRNA Expression through Plant Extracts and Compounds against Breast Cancer: Mechanism and Translational Significance. Phytomedicine 2020, 68, 153168. [Google Scholar] [CrossRef]
- Benameur, T.; Soleti, R.; Porro, C. The Potential Neuroprotective Role of Free and Encapsulated Quercetin Mediated by miRNA against Neurological Diseases. Nutrients 2021, 13, 1318. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, Q.; Kang, M.; Jiang, S.; Yang, F.; Gong, J.; Ou, G.; Wang, S. Regulation of Main ncRNAs by Polyphenols: A Novel Anticancer Therapeutic Approach. Phytomedicine 2023, 120, 155072. [Google Scholar] [CrossRef]
- Shaker, S.A.; Alshufta, S.M.; Gowayed, M.A.; El-Salamouni, N.S.; Bassam, S.M.; Megahed, M.A.; El-Tahan, R.A. Propolis-Loaded Nanostructured Lipid Carriers Halt Breast Cancer Progression through miRNA-223 Related Pathways: An In-Vitro/In-Vivo Experiment. Sci. Rep. 2023, 13, 15752. [Google Scholar] [CrossRef]
- Wang, H.; Jo, Y.; Oh, J.; Kim, N. Quercetin delays postovulatory aging of mouse oocytes by regulating SIRT expression and MPF activity. Oncotarget 2017, 8, 38631–38641. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.A.D.; Malta-Santos, H.; Rebouças-Silva, J.; Teles, F.; Galvão, E.B.S.; Souza, S.P.; Dutra, F.R.D.; Gomes, M.M.D.; Teixeira, M.B.; Conceição, L.F.M.R.; et al. Effects of Standardized Brazilian Green Propolis Extract (EPP-AF®) on Inflammation in Haemodialysis Patients: A Clinical Trial. Int. J. Nephrol. 2022, 2022, 1035475. [Google Scholar]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated Methods for the Quantification of Biologically Active Constituents of Poplar-Type Propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef]
- Bankova, V. Chemical Diversity of Propolis and the Problem of Standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Behl, T.; Gupta, A.; Sehgal, A.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Najmi, A.; Bhatia, S.; Bungau, S. Exploring Protein Tyrosine Phosphatases (PTP) and PTP-1B Inhibitors in Management of Diabetes Mellitus. Biomed. Pharmacother. 2022, 153, 113405. [Google Scholar] [CrossRef]
- Takahashi, H.; Nguyen, B.C.Q.; Uto, Y.; Shahinozzaman, M.; Tawata, S.; Maruta, H. 1,2,3-Triazolyl esterization of PAK1-blocking propolis ingredients, artepillin C (ARC), and caffeic acid (CA), for boosting their anti-cancer/anti-PAK1 activities along with cell-permeability. Drug Discov. Ther. 2017, 11, 104–109. [Google Scholar] [CrossRef]
- Vasileva, B.; Staneva, D.; Grozdanova, T.; Petkov, H.; Trusheva, B.; Alipieva, K.; Popova, M.; Miloshev, G.; Bankova, V.; Georgieva, M. Natural Deep Eutectic Extracts of Propolis, Sideritis scardica, and Plantago major Reveal Potential Antiageing Activity during Yeast Chronological Lifespan. Oxidative Med. Cell. Longev. 2022, 2022, 8368717. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mahajan, P.; Kaur, R.; Gautam, S. Nanotechnology and its challenges in the food sector: A review. Mater. Today Chem. 2020, 17, 100332. [Google Scholar] [CrossRef] [PubMed]
- Côté, M.; Lamarche, B. Artificial Intelligence in Nutrition Research: Perspectives on Current and Future Applications. App. Physiol. Nutr. Metab. 2021, 47, 1–8. [Google Scholar] [CrossRef]
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A Critical Review of the Use of Surfactant-Coated Nanoparticles in Nanomedicine and Food Nanotechnology. Int. J. Nanomed. 2021, 16, 3937–3999. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Voulgaridou, G.; Moschos, P.; Levidi, D.; Anastasiou, T.; Dedes, V.; Diplari, E.-M.; Fourfouri, N.; Giaginis, C.; Panoutsopoulos, G.I.; et al. Artificial Intelligence, Nutrition, and Ethical Issues: A Mini-Review. Clin. Nutr. Open Sci. 2023, 50, 46–56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).