The Use of Immune Regulation in Treating Head and Neck Squamous Cell Carcinoma (HNSCC)
Abstract
1. Introduction
2. Role of the Immune Response in the Development and Progression of HNSCC
3. Overview of Immune Checkpoint Proteins
3.1. Programmed Cell Death Protein-1/Programmed Death Ligand-1 (PD-1/PD-L1)
3.2. Cytotoxic T Lymphocyte Antigen 4 (CTLA-4)
3.3. T-Cell Immunoglobulin Mucin-3 (TIM-3)
3.4. Lymphocyte Activation Gene 3 (LAG-3)
3.5. Glucocorticoid-Induced TNFR Family-Related Protein (GITR)
3.6. V-Domain Ig Suppressor of T-Cell Activation (VISTA)
4. Immune Checkpoint Inhibitor-Based Therapeutics in HNSCC
4.1. Targeting PD-1/PD-L1
4.2. Targeting CTLA-4
4.3. Targeting GITR
5. Combined Immune Checkpoint Inhibitor Therapy in Oral Cancer
5.1. PD-1/CTLA-4 Combination
5.2. PD-1/GITR Combination
6. Immune Checkpoint Inhibitors in Combination with Other Therapies
6.1. Radiotherapy
6.2. Chemotherapy
7. Discussion and Future Perspectives on Oral Cancer Immune Therapy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD-1 | programmed cell death protein 1 |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| ICIs | immune checkpoint inhibitors |
| TME | tumor microenvironment |
| RT | radiotherapy |
| TAAs | tumor-associated antigens |
| MRI | magnetic resonance imaging |
| ERK | extracellular signal-regulated kinase |
| MAPK | mitogen-activated protein kinase |
| NF-κB | nuclear factor kappa B |
| HNSCC | head and neck squamous cell carcinoma |
| R/M | relapsed/metastatic |
| ACT | adaptive cell therapy |
| HPV | human papillomavirus |
| FDA | US Food and Drug Administration |
| ORR | overall response rate |
| BOR | best overall response |
| Tregs | regulatory T cells |
| HNCs | head and neck cancers |
| MDSCs | myeloid-derived suppressor cells |
| tTregs | thymus-derived Tregs |
| pTregs | periphery-derived Tregs |
| FOXP3 | Forkhead Box P3 |
| OS | overall survival |
| EFS | event-free survival |
| CI | confidence interval |
| TILs | tumor-infiltrating lymphocytes |
| HR | hazard ratio |
| BM | bone marrow |
| DCs | dendritic cells |
| CTLs | cytotoxic T lymphocytes |
| NK | natural killer |
| NKT | natural killer T cells |
| TAMs | tumor-associated macrophages |
| pMN | premetastatic niche |
| MMP9 | matrix metalloprotease 9 |
| MET | mesenchymalepithelial transition |
| VEGF | vascular endothelial growth factor |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
| TNF-α | tumor necrosis factor α |
| TCRs | T-cell receptors |
| TIM-3 | T-cell immunoglobulin mucin-3 |
| LAG-3 | lymphocyte activation gene 3 |
| VISTA | V domain Ig suppressor of T-cell activation |
| TIGIT | T-cell immunoglobulin and ITIM domain |
| PD-L1 | programmed death ligand-1 |
| PFS | progression-free survival |
| mAb | monoclonal antibody |
| MTD | maximum tolerated dose |
| CT | chemotherapy |
| IFN-γ | interferon-gamma IFN-γ |
| IMRT | intensity-modulated radiotherapy |
References
- El-Bayoumy, K.; Chen, K.M.; Zhang, S.M.; Sun, Y.W.; Amin, S.; Stoner, G.; Guttenplan, J.B. Carcinogenesis of the Oral Cavity: Environmental Causes and Potential Prevention by Black Raspberry. Chem. Res. Toxicol. 2017, 30, 126–144. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Sloan, P.; Gale, N.; Hunter, K.; Lingen, M.; Nylander, K.; Reibel, J. Tumours of the oral cavity and mobile tongue: Malignant surface epithelial tumors: Squamous cell carcinoma. WHO Classif. Head Neck Tumours 2017, 9, 108–109. [Google Scholar]
- Mignogna, M.D.; Fedele, S.; Lo Russo, L. The world cancer report and the burden of oral cancer. Eur. J. Cancer Prev. 2004, 13, 139–142. [Google Scholar] [CrossRef]
- De Virgilio, A.; Costantino, A.; Mercante, G.; Petruzzi, G.; Sebastiani, D.; Franzese, C.; Scorsetti, M.; Pellini, R.; Malvezzi, L.; Spriano, G. Present and Future of De-intensification Strategies in the Treatment of Oropharyngeal Carcinoma. Curr. Oncol. Rep. 2020, 22, 91. [Google Scholar] [CrossRef]
- Tsou, H.H.; Tsai, H.C.; Chu, C.T.; Cheng, H.W.; Liu, C.J.; Lee, C.H.; Liu, T.Y.; Wang, H.T. Cigarette Smoke Containing Acrolein Upregulates EGFR Signaling Contributing to Oral Tumorigenesis In Vitro and In Vivo. Cancers 2021, 13, 3544. [Google Scholar] [CrossRef]
- Okami, K. Clinical features and treatment strategy for HPV-related oropharyngeal cancer. Int. J. Clin. Oncol. 2016, 21, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Salazar, C.R.; Anayannis, N.; Smith, R.V.; Wang, Y.; Haigentz, M., Jr.; Garg, M.; Schiff, B.A.; Kawachi, N.; Elman, J.; Belbin, T.J.; et al. Combined P16 and human papillomavirus testing predicts head and neck cancer survival. Int. J. Cancer 2014, 135, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Agne, G.R.; Kohler, H.F.; Chulam, T.C.; Pinto, C.A.L.; Vartanian, J.G.; Kowalski, L.P. Oncologic outcomes of microscopic tumor cut-through in locally advanced oral squamous cell carcinoma. Arch. Head Neck Surg. 2022, 51, e20220013. [Google Scholar] [CrossRef]
- Huang, S.H.; O’Sullivan, B. Oral cancer: Current role of radiotherapy and chemotherapy. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e233–e240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, T.; Gan, C.; Wang, K.; Sun, B.; Wang, M.; Zhu, F. Temporal and spatial patterns of recurrence in oral squamous cell carcinoma, a single-center retrospective cohort study in China. BMC Oral Health 2023, 23, 679. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and neck cancer: Changing epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 2008, 83, 489–501. [Google Scholar] [CrossRef]
- Hashim, D.; Genden, E.; Posner, M.; Hashibe, M.; Boffetta, P. Head and neck cancer prevention: From primary prevention to impact of clinicians on reducing burden. Ann. Oncol. 2019, 30, 744–756. [Google Scholar] [CrossRef]
- Kitamura, N.; Sento, S.; Yoshizawa, Y.; Sasabe, E.; Kudo, Y.; Yamamoto, T. Current Trends and Future Prospects of Molecular Targeted Therapy in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 22, 240. [Google Scholar] [CrossRef]
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef]
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jie, H.B.; Lei, Y.; Gildener-Leapman, N.; Trivedi, S.; Green, T.; Kane, L.P.; Ferris, R.L. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer Res. 2015, 75, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J.; Ferris, R.L.; Blumenschein, G., Jr.; Colevas, A.D.; Fayette, J.; Licitra, L.; Kasper, S.; Even, C.; Vokes, E.E.; Worden, F.; et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1104–1115. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Duray, A.; Demoulin, S.; Hubert, P.; Delvenne, P.; Saussez, S. Immune suppression in head and neck cancers: A review. Clin. Dev. Immunol. 2010, 2010, 701657. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Krishnakumar, H.N.; Saladi, S.V. Current and Future Biomarkers for Immune Checkpoint Inhibitors in Head and Neck Squamous Cell Carcinoma. Curr. Oncol. 2022, 29, 4185–4198. [Google Scholar] [CrossRef]
- Seminerio, I.; Descamps, G.; Dupont, S.; de Marrez, L.; Laigle, J.A.; Lechien, J.R.; Kindt, N.; Journe, F.; Saussez, S. Infiltration of FoxP3+ Regulatory T Cells is a Strong and Independent Prognostic Factor in Head and Neck Squamous Cell Carcinoma. Cancers 2019, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.W.; Jiang, J.; Pang, X.; Huang, M.C.; Tang, Y.J.; Liang, X.H.; Tang, Y.L. The Evolving Landscape of PD-1/PD-L1 Pathway in Head and Neck Cancer. Front. Immunol. 2020, 11, 1721. [Google Scholar] [CrossRef] [PubMed]
- Lyford-Pike, S.; Peng, S.; Young, G.D.; Taube, J.M.; Westra, W.H.; Akpeng, B.; Bruno, T.C.; Richmon, J.D.; Wang, H.; Bishop, J.A.; et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Patini, R.; Cordaro, M.; Marchesini, D.; Scilla, F.; Gioco, G.; Rupe, C.; D’Agostino, M.A.; Lajolo, C. Is Systemic Immunosuppression a Risk Factor for Oral Cancer? A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3077. [Google Scholar] [CrossRef]
- Perri, F.; Ionna, F.; Longo, F.; Della Vittoria Scarpati, G.; De Angelis, C.; Ottaiano, A.; Botti, G.; Caponigro, F. Immune Response Against Head and Neck Cancer: Biological Mechanisms and Implication on Therapy. Transl. Oncol. 2020, 13, 262–274. [Google Scholar] [CrossRef]
- Alves, A.; Diel, L.; Ramos, G.; Pinto, A.; Bernardi, L.; Yates, J., 3rd; Lamers, M. Tumor microenvironment and Oral Squamous Cell Carcinoma: A crosstalk between the inflammatory state and tumor cell migration. Oral Oncol. 2021, 112, 105038. [Google Scholar] [CrossRef]
- de Sousa Lopes, M.L.D.; Liu, Y.; Liu, K.Y.; da Silveira, E.J.D.; Poh, C.F. Tumor-associated immune aggregates in oral cancer: Their cellular composition and potential prognostic significance. Med. Hypotheses 2017, 108, 17–23. [Google Scholar] [CrossRef]
- Fehervari, Z.; Sakaguchi, S. CD4+ Tregs and immune control. J. Clin. Investig. 2004, 114, 1209–1217. [Google Scholar] [CrossRef]
- Liu, S.; Liu, D.; Li, J.; Zhang, D.; Chen, Q. Regulatory T cells in oral squamous cell carcinoma. J. Oral Pathol. Med. 2016, 45, 635–639. [Google Scholar] [CrossRef]
- Park, J.Y.; Chung, H.; DiPalma, D.T.; Tai, X.; Park, J.H. Immune quiescence in the oral mucosa is maintained by a uniquely large population of highly activated Foxp3+ regulatory T cells. Mucosal Immunol. 2018, 11, 1092–1102. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef]
- Jiang, X.; Shapiro, D.J. The immune system and inflammation in breast cancer. Mol. Cell. Endocrinol. 2014, 382, 673–682. [Google Scholar] [CrossRef]
- Ma, M.W.; Medicherla, R.C.; Qian, M.; Vega-Saenz de Miera, E.; Friedman, E.B.; Berman, R.S.; Shapiro, R.L.; Pavlick, A.C.; Ott, P.A.; Bhardwaj, N.; et al. Immune response in melanoma: An in-depth analysis of the primary tumor and corresponding sentinel lymph node. Mod. Pathol. 2012, 25, 1000–1010. [Google Scholar] [CrossRef]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef]
- Liu, C.; Wang, M.; Zhang, H.; Li, C.; Zhang, T.; Liu, H.; Zhu, S.; Chen, J. Tumor microenvironment and immunotherapy of oral cancer. Eur. J. Med. Res. 2022, 27, 198. [Google Scholar] [CrossRef] [PubMed]
- Jie, H.B.; Gildener-Leapman, N.; Li, J.; Srivastava, R.M.; Gibson, S.P.; Whiteside, T.L.; Ferris, R.L. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br. J. Cancer 2013, 109, 2629–2635. [Google Scholar] [CrossRef]
- Shang, B.; Liu, Y.; Jiang, S.J.; Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef]
- Echarti, A.; Hecht, M.; Buttner-Herold, M.; Haderlein, M.; Hartmann, A.; Fietkau, R.; Distel, L. CD8+ and Regulatory T cells Differentiate Tumor Immune Phenotypes and Predict Survival in Locally Advanced Head and Neck Cancer. Cancers 2019, 11, 1398. [Google Scholar] [CrossRef]
- de Ruiter, E.J.; Ooft, M.L.; Devriese, L.A.; Willems, S.M. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology 2017, 6, e1356148. [Google Scholar] [CrossRef]
- Borsetto, D.; Tomasoni, M.; Payne, K.; Polesel, J.; Deganello, A.; Bossi, P.; Tysome, J.R.; Masterson, L.; Tirelli, G.; Tofanelli, M.; et al. Prognostic Significance of CD4+ and CD8+ Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Cancers 2021, 13, 781. [Google Scholar] [CrossRef]
- Thyagarajan, A.; Alshehri, M.S.A.; Miller, K.L.R.; Sherwin, C.M.; Travers, J.B.; Sahu, R.P. Myeloid-Derived Suppressor Cells and Pancreatic Cancer: Implications in Novel Therapeutic Approaches. Cancers 2019, 11, 1627. [Google Scholar] [CrossRef]
- Pawelec, G.; Verschoor, C.P.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells: Not Only in Tumor Immunity. Front. Immunol. 2019, 10, 1099. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S. Myeloid derived-suppressor cells: Their role in cancer and obesity. Curr. Opin. Immunol. 2018, 51, 68–75. [Google Scholar] [CrossRef]
- Chan, C.Y.; Yuen, V.W.; Wong, C.C. Hypoxia and the Metastatic Niche. Adv. Exp. Med. Biol. 2019, 1136, 97–112. [Google Scholar] [CrossRef]
- Poschke, I.; Kiessling, R. On the armament and appearances of human myeloid-derived suppressor cells. Clin. Immunol. 2012, 144, 250–268. [Google Scholar] [CrossRef]
- Principi, E.; Raffaghello, L. The role of the P2X7 receptor in myeloid-derived suppressor cells and immunosuppression. Curr. Opin. Pharmacol. 2019, 47, 82–89. [Google Scholar] [CrossRef]
- Pang, X.; Fan, H.Y.; Tang, Y.L.; Wang, S.S.; Cao, M.X.; Wang, H.F.; Dai, L.L.; Wang, K.; Yu, X.H.; Wu, J.B.; et al. Myeloid derived suppressor cells contribute to the malignant progression of oral squamous cell carcinoma. PLoS ONE 2020, 15, e0229089. [Google Scholar] [CrossRef]
- Najafi, M.; Hashemi Goradel, N.; Farhood, B.; Salehi, E.; Nashtaei, M.S.; Khanlarkhani, N.; Khezri, Z.; Majidpoor, J.; Abouzaripour, M.; Habibi, M.; et al. Macrophage polarity in cancer: A review. J. Cell. Biochem. 2019, 120, 2756–2765. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef]
- Evrard, D.; Szturz, P.; Tijeras-Raballand, A.; Astorgues-Xerri, L.; Abitbol, C.; Paradis, V.; Raymond, E.; Albert, S.; Barry, B.; Faivre, S. Macrophages in the microenvironment of head and neck cancer: Potential targets for cancer therapy. Oral Oncol. 2019, 88, 29–38. [Google Scholar] [CrossRef]
- Kumar, A.T.; Knops, A.; Swendseid, B.; Martinez-Outschoom, U.; Harshyne, L.; Philp, N.; Rodeck, U.; Luginbuhl, A.; Cognetti, D.; Johnson, J.; et al. Prognostic Significance of Tumor-Associated Macrophage Content in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Front. Oncol. 2019, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Fu, E.; Liu, T.; Yu, S.; Chen, X.; Song, L.; Lou, H.; Ma, F.; Zhang, S.; Hussain, S.; Guo, J.; et al. M2 macrophages reduce the radiosensitivity of head and neck cancer by releasing HB-EGF. Oncol. Rep. 2020, 44, 698–710. [Google Scholar] [CrossRef]
- Kubota, K.; Moriyama, M.; Furukawa, S.; Rafiul, H.; Maruse, Y.; Jinno, T.; Tanaka, A.; Ohta, M.; Ishiguro, N.; Yamauchi, M.; et al. CD163+CD204+ tumor-associated macrophages contribute to T cell regulation via interleukin-10 and PD-L1 production in oral squamous cell carcinoma. Sci. Rep. 2017, 7, 1755. [Google Scholar] [CrossRef]
- Haque, A.; Moriyama, M.; Kubota, K.; Ishiguro, N.; Sakamoto, M.; Chinju, A.; Mochizuki, K.; Sakamoto, T.; Kaneko, N.; Munemura, R.; et al. CD206+ tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci. Rep. 2019, 9, 14611. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, F.Y.; Kirkwood, K.L. The p38/MKP-1 signaling axis in oral cancer: Impact of tumor-associated macrophages. Oral Oncol. 2020, 103, 104591. [Google Scholar] [CrossRef]
- He, K.F.; Zhang, L.; Huang, C.F.; Ma, S.R.; Wang, Y.F.; Wang, W.M.; Zhao, Z.L.; Liu, B.; Zhao, Y.F.; Zhang, W.F.; et al. CD163+ tumor-associated macrophages correlated with poor prognosis and cancer stem cells in oral squamous cell carcinoma. Biomed. Res. Int. 2014, 2014, 838632. [Google Scholar] [CrossRef]
- Sperk, M.; Domselaar, R.V.; Neogi, U. Immune Checkpoints as the Immune System Regulators and Potential Biomarkers in HIV-1 Infection. Int. J. Mol. Sci. 2018, 19, 2000. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, L.; Chen, J.; Hao, K.; Zhang, S.; Guo, X.; Guo, Z.; Tian, H.; Chen, X. Highly Enhanced Antitumor Immunity by a Three-Barreled Strategy of the l-Arginine-Promoted Nanovaccine and Gene-Mediated PD-L1 Blockade. ACS Appl. Mater. Interfaces 2020, 12, 41127–41137. [Google Scholar] [CrossRef]
- Wang, K.; Chen, J.; Lin, L.; Yan, N.; Yang, W.H.; Cai, K.Y.; Tian, H.Y.; Chen, X.S. Anion receptor-mediated multicomponent synergistic self-assembly of porphyrin for efficient phototherapy to elicit tumor immunotherapy. Nano Today 2022, 46, 101579. [Google Scholar] [CrossRef]
- Toor, S.M.; Sasidharan Nair, V.; Decock, J.; Elkord, E. Immune checkpoints in the tumor microenvironment. Semin. Cancer Biol. 2020, 65, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wu, J.; Chen, J.; Lin, L.; Zhang, S.; Yang, Z.; Sun, P.; Li, Y.; Tian, H.; Chen, X. Targeting dual gene delivery nanoparticles overcomes immune checkpoint blockade induced adaptive resistance and regulates tumor microenvironment for improved tumor immunotherapy. Nano Today 2021, 38, 101194. [Google Scholar] [CrossRef]
- Li, B.; Jin, J.; Guo, D.; Tao, Z.; Hu, X. Immune Checkpoint Inhibitors Combined with Targeted Therapy: The Recent Advances and Future Potentials. Cancers 2023, 15, 2858. [Google Scholar] [CrossRef]
- de Vicente, J.C.; Rodriguez-Santamarta, T.; Rodrigo, J.P.; Blanco-Lorenzo, V.; Allonca, E.; Garcia-Pedrero, J.M. PD-L1 Expression in Tumor Cells Is an Independent Unfavorable Prognostic Factor in Oral Squamous Cell Carcinoma. Cancer Epidemiol. Biomark. Prev. 2019, 28, 546–554. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Mei, Z.; Huang, J.; Qiao, B.; Lam, A.K. Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma. Int. J. Oral Sci. 2020, 12, 16. [Google Scholar] [CrossRef]
- Zandberg, D.P.; Strome, S.E. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014, 50, 627–632. [Google Scholar] [CrossRef]
- Wang, W.; Lau, R.; Yu, D.; Zhu, W.; Korman, A.; Weber, J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int. Immunol. 2009, 21, 1065–1077. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Wang, X.B.; Fan, Z.Z.; Anton, D.; Vollenhoven, A.V.; Ni, Z.H.; Chen, X.F.; Lefvert, A.K. CTLA4 is expressed on mature dendritic cells derived from human monocytes and influences their maturation and antigen presentation. BMC Immunol. 2011, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Jie, H.B.; Schuler, P.J.; Lee, S.C.; Srivastava, R.M.; Argiris, A.; Ferrone, S.; Whiteside, T.L.; Ferris, R.L. CTLA-4+ Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015, 75, 2200–2210. [Google Scholar] [CrossRef]
- Sullivan, T.J.; Letterio, J.J.; van Elsas, A.; Mamura, M.; van Amelsfort, J.; Sharpe, S.; Metzler, B.; Chambers, C.A.; Allison, J.P. Lack of a role for transforming growth factor-beta in cytotoxic T lymphocyte antigen-4-mediated inhibition of T cell activation. Proc. Natl. Acad. Sci. USA 2001, 98, 2587–2592. [Google Scholar] [CrossRef] [PubMed]
- Wing, J.B.; Ise, W.; Kurosaki, T.; Sakaguchi, S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity 2014, 41, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef]
- Anderson, A.C.; Anderson, D.E.; Bregoli, L.; Hastings, W.D.; Kassam, N.; Lei, C.; Chandwaskar, R.; Karman, J.; Su, E.W.; Hirashima, M.; et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 2007, 318, 1141–1143. [Google Scholar] [CrossRef]
- Zhou, Q.; Munger, M.E.; Veenstra, R.G.; Weigel, B.J.; Hirashima, M.; Munn, D.H.; Murphy, W.J.; Azuma, M.; Anderson, A.C.; Kuchroo, V.K.; et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 2011, 117, 4501–4510. [Google Scholar] [CrossRef]
- Golden-Mason, L.; Rosen, H.R. Galectin-9: Diverse roles in hepatic immune homeostasis and inflammation. Hepatology 2017, 66, 271–279. [Google Scholar] [CrossRef]
- Ngiow, S.F.; von Scheidt, B.; Akiba, H.; Yagita, H.; Teng, M.W.; Smyth, M.J. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011, 71, 3540–3551. [Google Scholar] [CrossRef]
- Finn, O.J. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Ann. Oncol. 2012, 23 (Suppl. S8), viii6–viii9. [Google Scholar] [CrossRef]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Huard, B.; Tournier, M.; Hercend, T.; Triebel, F.; Faure, F. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur. J. Immunol. 1994, 24, 3216–3221. [Google Scholar] [CrossRef]
- Workman, C.J.; Vignali, D.A.A. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur. J. Immunol. 2003, 33, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G.; Hipkiss, E.L.; Ravi, S.; Kowalski, J.; Levitsky, H.I.; et al. Role of LAG-3 in regulatory T cells. Immunity 2004, 21, 503–513. [Google Scholar] [CrossRef]
- Woo, S.R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef]
- Botticelli, A.; Zizzari, I.G.; Scagnoli, S.; Pomati, G.; Strigari, L.; Cirillo, A.; Cerbelli, B.; Di Filippo, A.; Napoletano, C.; Scirocchi, F.; et al. The Role of Soluble LAG3 and Soluble Immune Checkpoints Profile in Advanced Head and Neck Cancer: A Pilot Study. J. Pers. Med. 2021, 11, 651. [Google Scholar] [CrossRef]
- Deng, W.W.; Mao, L.; Yu, G.T.; Bu, L.L.; Ma, S.R.; Liu, B.; Gutkind, J.S.; Kulkarni, A.B.; Zhang, W.F.; Sun, Z.J. LAG-3 confers poor prognosis and its blockade reshapes antitumor response in head and neck squamous cell carcinoma. Oncoimmunology 2016, 5, e1239005. [Google Scholar] [CrossRef]
- Chocarro, L.; Bocanegra, A.; Blanco, E.; Fernandez-Rubio, L.; Arasanz, H.; Echaide, M.; Garnica, M.; Ramos, P.; Pineiro-Hermida, S.; Vera, R.; et al. Cutting-Edge: Preclinical and Clinical Development of the First Approved Lag-3 Inhibitor. Cells 2022, 11, 2351. [Google Scholar] [CrossRef]
- Zheng, Y.; Song, A.; Zhou, Y.; Zhong, Y.; Zhang, W.; Wang, C.; Ding, X.; Du, Y.; Zhang, W.; Li, G.; et al. Identification of extracellular vesicles-transported miRNAs in Erlotinib-resistant head and neck squamous cell carcinoma. J. Cell Commun. Signal. 2020, 14, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Stephens, G.L.; McHugh, R.S.; Whitters, M.J.; Young, D.A.; Luxenberg, D.; Carreno, B.M.; Collins, M.; Shevach, E.M. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J. Immunol. 2004, 173, 5008–5020. [Google Scholar] [CrossRef] [PubMed]
- Schaer, D.A.; Budhu, S.; Liu, C.; Bryson, C.; Malandro, N.; Cohen, A.; Zhong, H.; Yang, X.; Houghton, A.N.; Merghoub, T.; et al. GITR pathway activation abrogates tumor immune suppression through loss of regulatory T cell lineage stability. Cancer Immunol. Res. 2013, 1, 320–331. [Google Scholar] [CrossRef]
- Esparza, E.M.; Arch, R.H. Glucocorticoid-induced TNF receptor functions as a costimulatory receptor that promotes survival in early phases of T cell activation. J. Immunol. 2005, 174, 7869–7874. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.; Yamazaki, S.; Takahashi, T.; Ishida, Y.; Sakaguchi, S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 2002, 3, 135–142. [Google Scholar] [CrossRef]
- Le Mercier, I.; Chen, W.; Lines, J.L.; Day, M.; Li, J.; Sergent, P.; Noelle, R.J.; Wang, L. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014, 74, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rubinstein, R.; Lines, J.L.; Wasiuk, A.; Ahonen, C.; Guo, Y.; Lu, L.F.; Gondek, D.; Wang, Y.; Fava, R.A.; et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011, 208, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, Y.; Chen, W.; Putra, J.; Suriawinata, A.A.; Schenk, A.D.; Miller, H.E.; Guleria, I.; Barth, R.J.; Huang, Y.H.; et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc. Natl. Acad. Sci. USA 2015, 112, 6682–6687. [Google Scholar] [CrossRef]
- Wang, J.; Wu, G.; Manick, B.; Hernandez, V.; Renelt, M.; Erickson, C.; Guan, J.; Singh, R.; Rollins, S.; Solorz, A.; et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology 2019, 156, 74–85. [Google Scholar] [CrossRef]
- Harada, H.; Suzu, S.; Hayashi, Y.; Okada, S. BT-IgSF, a novel immunoglobulin superfamily protein, functions as a cell adhesion molecule. J. Cell Physiol. 2005, 204, 919–926. [Google Scholar] [CrossRef]
- Sunshine, J.; Taube, J.M. PD-1/PD-L1 inhibitors. Curr. Opin. Pharmacol. 2015, 23, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Polverini, P.J.; D’Silva, N.J.; Lei, Y.L. Precision Therapy of Head and Neck Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 614–621. [Google Scholar] [CrossRef]
- Falchook, G.S.; Leidner, R.; Stankevich, E.; Piening, B.; Bifulco, C.; Lowy, I.; Fury, M.G. Responses of metastatic basal cell and cutaneous squamous cell carcinomas to anti-PD1 monoclonal antibody REGN2810. J. Immunother. Cancer 2016, 4, 70. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Mehra, R.; Seiwert, T.Y.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; Kang, H.; et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. Br. J. Cancer 2018, 119, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef]
- Yearley, J.H.; Gibson, C.; Yu, N.; Moon, C.; Murphy, E.; Juco, J.; Lunceford, J.; Cheng, J.; Chow, L.Q.M.; Seiwert, T.Y.; et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin. Cancer Res. 2017, 23, 3158–3167. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Irving, B.A.; Hodi, F.S. Molecular pathways: Next-generation immunotherapy-inhibiting programmed death-ligand 1 and programmed death-1. Clin. Cancer Res. 2012, 18, 6580–6587. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Colevas, A.D.; Bahleda, R.; Braiteh, F.; Balmanoukian, A.; Brana, I.; Chau, N.G.; Sarkar, I.; Molinero, L.; Grossman, W.; Kabbinavar, F.; et al. Safety and clinical activity of atezolizumab in head and neck cancer: Results from a phase I trial. Ann. Oncol. 2018, 29, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Rischin, D.; Migden, M.R.; Lim, A.M.; Schmults, C.D.; Khushalani, N.I.; Hughes, B.G.M.; Schadendorf, D.; Dunn, L.A.; Hernandez-Aya, L.; Chang, A.L.S.; et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: Primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J. Immunother. Cancer 2020, 8, e000775. [Google Scholar] [CrossRef] [PubMed]
- Zandberg, D.P.; Algazi, A.P.; Jimeno, A.; Good, J.S.; Fayette, J.; Bouganim, N.; Ready, N.E.; Clement, P.M.; Even, C.; Jang, R.W.; et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur. J. Cancer 2019, 107, 142–152. [Google Scholar] [CrossRef]
- Siu, L.L.; Even, C.; Mesia, R.; Remenar, E.; Daste, A.; Delord, J.P.; Krauss, J.; Saba, N.F.; Nabell, L.; Ready, N.E.; et al. Safety and Efficacy of Durvalumab With or Without Tremelimumab in Patients With PD-L1-Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol. 2019, 5, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Harrington, K.; Tahara, M.; Ferris, R.L.; Gillison, M.; Fayette, J.; Daste, A.; Koralewski, P.; Zurawski, B.; Taberna, M.; et al. Nivolumab Plus Ipilimumab Versus EXTREME Regimen as First-Line Treatment for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: The Final Results of CheckMate 651. J. Clin. Oncol. 2023, 41, 2166–2180. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Carvajal, R.D.; Marabelle, A.; Patel, S.P.; LoRusso, P.M.; Rasmussen, E.; Juan, G.; Upreti, V.V.; Beers, C.; Ngarmchamnanrith, G.; et al. Dose escalation results from a first-in-human, phase 1 study of glucocorticoid-induced TNF receptor-related protein agonist AMG 228 in patients with advanced solid tumors. J. Immunother. Cancer 2018, 6, 93. [Google Scholar] [CrossRef]
- Schwab, K.S.; Kristiansen, G.; Schild, H.H.; Held, S.E.A.; Heine, A.; Brossart, P. Successful Treatment of Refractory Squamous Cell Cancer of the Head and Neck with Nivolumab and Ipilimumab. Case Rep. Oncol. 2018, 11, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M.; Stephens, G.L. The GITR-GITRL interaction: Co-stimulation or contrasuppression of regulatory activity? Nat. Rev. Immunol. 2006, 6, 613–618. [Google Scholar] [CrossRef]
- Siu, L.L.; Steeghs, N.; Meniawy, T.; Joerger, M.; Spratlin, J.L.; Rottey, S.; Nagrial, A.; Cooper, A.; Meier, R.; Guan, X.; et al. Preliminary results of a phase I/IIa study of BMS-986156 (glucocorticoid-induced tumor necrosis factor receptor–related gene [GITR] agonist), alone and in combination with nivolumab in pts with advanced solid tumors. J. Clin. Oncol. 2017, 35, 104. [Google Scholar] [CrossRef]
- Trommer, M.; Yeo, S.Y.; Persigehl, T.; Bunck, A.; Grull, H.; Schlaak, M.; Theurich, S.; von Bergwelt-Baildon, M.; Morgenthaler, J.; Herter, J.M.; et al. Abscopal Effects in Radio-Immunotherapy-Response Analysis of Metastatic Cancer Patients with Progressive Disease Under Anti-PD-1 Immune Checkpoint Inhibition. Front. Pharmacol. 2019, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.C.; Formenti, S.C. Radiation therapy to enhance tumor immunotherapy: A novel application for an established modality. Int. J. Radiat. Biol. 2019, 95, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yao, Y.; Tang, Y.; Xin, Z.; Wu, D.; Ni, C.; Huang, J.; Wei, Q.; Zhang, T. Radiation-induced tumor immune microenvironments and potential targets for combination therapy. Signal Transduct. Target. Ther. 2023, 8, 205. [Google Scholar] [CrossRef]
- Zappasodi, R.; Merghoub, T.; Wolchok, J.D. Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies. Cancer Cell 2018, 34, 690. [Google Scholar] [CrossRef] [PubMed]
- Cruz, E.; Kayser, V. Monoclonal antibody therapy of solid tumors: Clinical limitations and novel strategies to enhance treatment efficacy. Biologics 2019, 13, 33–51. [Google Scholar] [CrossRef]
- Qiao, M.; Jiang, T.; Ren, S.; Zhou, C. Combination Strategies on the Basis of Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer: Where Do We Stand? Clin. Lung Cancer 2018, 19, 1–11. [Google Scholar] [CrossRef]
- Garassino, M.C.; Torri, V.; Colombo, M.P.; Sica, A. Choosing the Best Chemotherapy Agent to Boost Immune Checkpoint Inhibition Activity. Cancer Res. 2018, 78, 5729–5730. [Google Scholar] [CrossRef]
- Lynch, T.J.; Bondarenko, I.; Luft, A.; Serwatowski, P.; Barlesi, F.; Chacko, R.; Sebastian, M.; Neal, J.; Lu, H.; Cuillerot, J.M.; et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: Results from a randomized, double-blind, multicenter phase II study. J. Clin. Oncol. 2012, 30, 2046–2054. [Google Scholar] [CrossRef]
- Arasanz, H.; Zuazo, M.; Bocanegra, A.; Chocarro, L.; Blanco, E.; Martinez, M.; Morilla, I.; Fernandez, G.; Teijeira, L.; Morente, P.; et al. Hyperprogressive Disease: Main Features and Key Controversies. Int. J. Mol. Sci. 2021, 22, 3736. [Google Scholar] [CrossRef]
- Miserocchi, G.; Spadazzi, C.; Calpona, S.; De Rosa, F.; Usai, A.; De Vita, A.; Liverani, C.; Cocchi, C.; Vanni, S.; Calabrese, C.; et al. Precision Medicine in Head and Neck Cancers: Genomic and Preclinical Approaches. J. Pers. Med. 2022, 12, 854. [Google Scholar] [CrossRef]
- Lee, R.H.; Johnson, D.E.; Grandis, J.R. To Tip or Not to Tip: A New Combination for Precision Medicine in Head and Neck Cancer. Cancer Res. 2023, 83, 3162–3164. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Levyn, H.; Shah, J. Recent advances in head and neck surgical oncology. J. Surg. Oncol. 2023, 129, 32–39. [Google Scholar] [CrossRef]
- Lu, L.; Zhan, M.; Li, X.Y.; Zhang, H.; Dauphars, D.J.; Jiang, J.; Yin, H.; Li, S.Y.; Luo, S.; Li, Y.; et al. Clinically approved combination immunotherapy: Current status, limitations, and future perspective. Curr. Res. Immunol. 2022, 3, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C.X. Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 156. [Google Scholar] [CrossRef]
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef]
- Vadevoo, S.M.P.; Gurung, S.; Lee, H.-S.; Gunassekaran, G.R.; Lee, S.-M.; Yoon, J.-W.; Lee, Y.-K.; Lee, B. Peptides as multifunctional players in cancer therapy. Exp. Mol. Med. 2023, 55, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, Y.; Lindstrom, A.R.; Lin, T.Y.; Lam, K.S.; Li, Y. Peptide-based materials for cancer immunotherapy. Theranostics 2019, 9, 7807–7825. [Google Scholar] [CrossRef]
- Hoffmann, F.; Franzen, A.; de Vos, L.; Wuest, L.; Kulcsar, Z.; Fietz, S.; Maas, A.P.; Hollick, S.; Diop, M.Y.; Gabrielpillai, J.; et al. CTLA4 DNA methylation is associated with CTLA-4 expression and predicts response to immunotherapy in head and neck squamous cell carcinoma. Clin. Epigenetics 2023, 15, 112. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Noji, R.; Tohyama, K.; Kugimoto, T.; Kuroshima, T.; Hirai, H.; Tomioka, H.; Michi, Y.; Tasaki, A.; Ohno, K.; Ariizumi, Y.; et al. Comprehensive Genomic Profiling Reveals Clinical Associations in Response to Immune Therapy in Head and Neck Cancer. Cancers 2022, 14, 3476. [Google Scholar] [CrossRef]
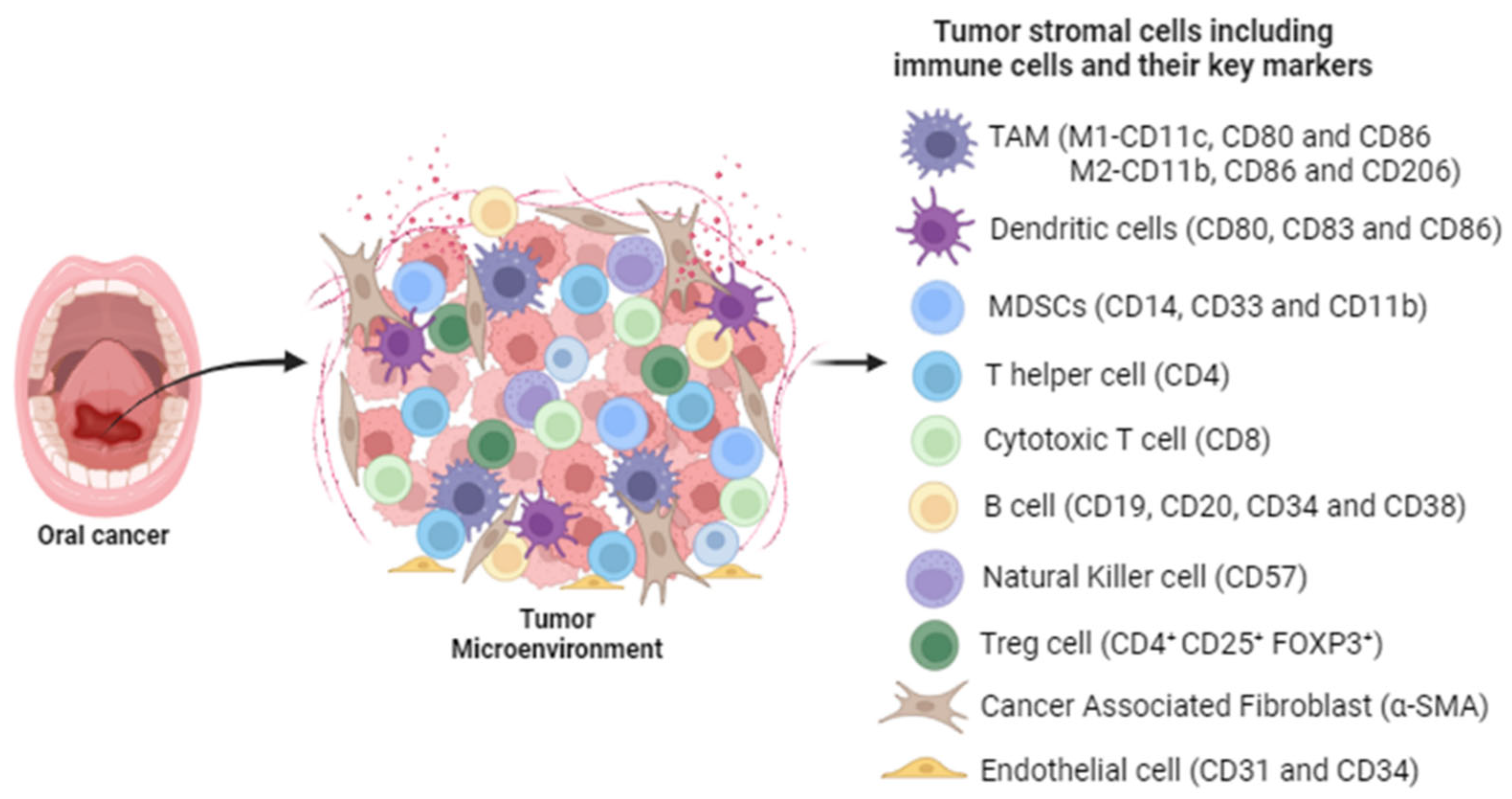
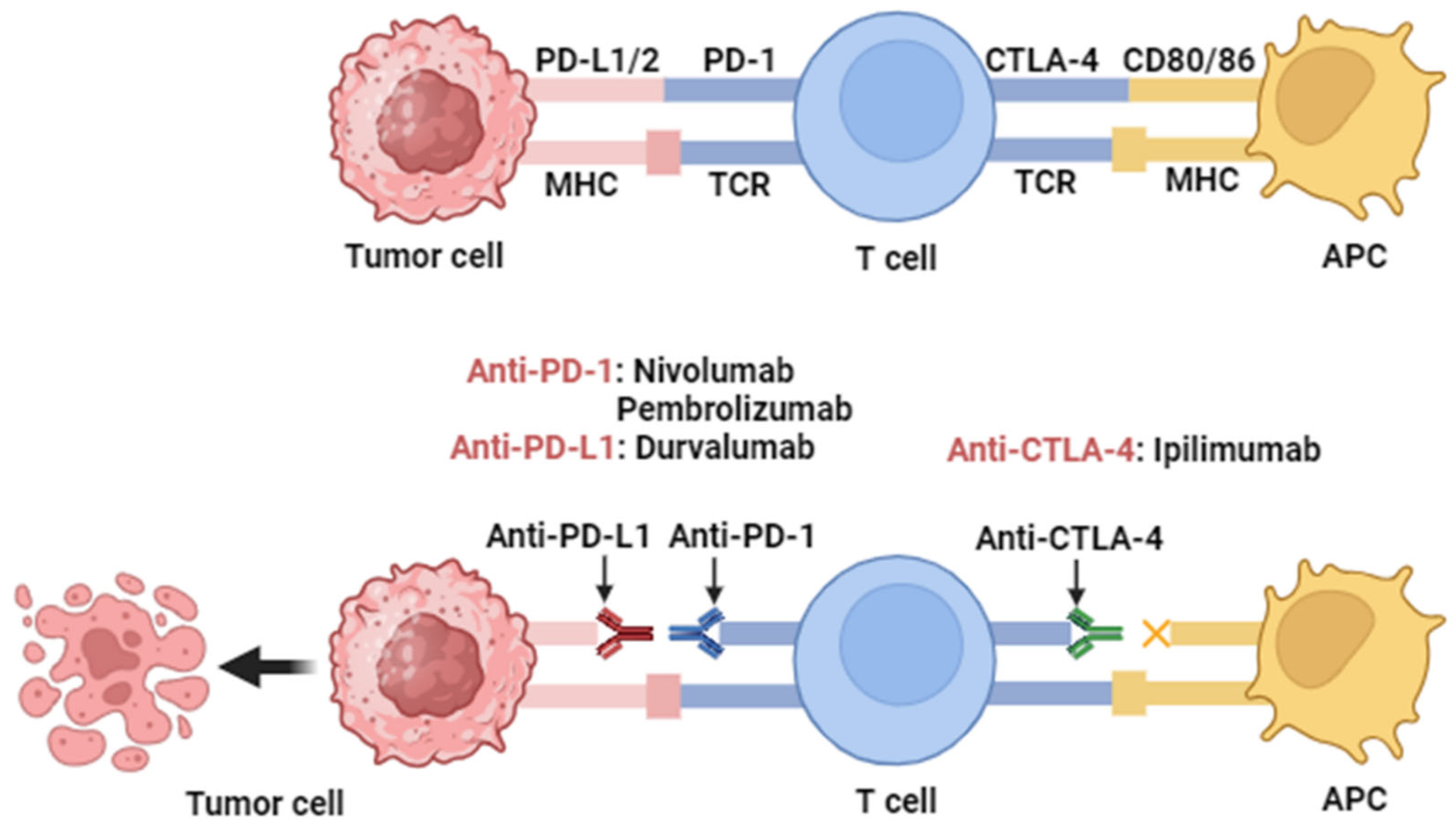
| Serial | ICIs | Target | Phase | Arm/Group | No. of Patients | Combination | Clinical Trial Number | Primary Endpoint | Study Start/Date |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Durvalumab | PD-L1 | I | Recurrent and/or metastatic head and neck squamous cell carcinoma | 71 | Tremelimumab | NCT02262741 | Safety, tolerability, antitumor activity, PK, pharmacodynamics, and immunogenicity | 15/10/2014 |
| 2 | Pembrolizumab | PD-1 | II | Recurrent and/or metastatic head and neck squamous cell carcinoma | 172 | After platinum-based and cetuximab therapy | NCT02255097 | Objective response rate (ORR) | 24/10/2014 |
| 3 | Durvalumab | PD-L1 | I/II | Head and neck cancer solid tumors | 176 | Epacadostat | NCT02318277 | Safety, tolerability, pharmacokinetics, immunogenicity, and preliminary efficacy | 05/01/2015 |
| 4 | Pembrolizumab | PD-1 | III | First-line treatment of recurrent and/or metastatic head and neck squamous cell carcinoma | 882 | Chemotherapy | NCT02358031 | Progression-free survival (PFS) | 19/03/2015 |
| 5 | Pembrolizumab | PD-1 | II | Advanced head and neck squamous cell carcinoma | 78 | Acalabrutinib | NCT02454179 | Overall response rate (ORR) | 01/05/2015 |
| 6 | Nivolumab | PD-1 | II | Metastatic head and neck squamous cell carcinoma (HNSCC) | 65 | Stereotactic body radiotherapy (SBRT) | NCT02684253 | Best overall response (BOR) | 11/02/2016 |
| 7 | Pembrolizumab | PD-1 | I/II | Locally advanced laryngeal squamous cell carcinoma (Grade 3/4) | 9 | Chemotherapy | NCT02759575 | Adverse effects | 01/04/2016 |
| 8 | Pembrolizumab | PD-1 | I | Recurrent and/or metastatic head and neck squamous cell carcinoma | 36 | Talimogene laherparepvec | NCT02626000 | Safety and toxicity | 06/04/2016 |
| 9 | Nivolumab | PD-1 | I | Intermediate and high-risk local regionally advanced head and neck cancer | 40 | Chemotherapy | NCT02764593 | Safety | 01/06/2016 |
| 10 | Nivolumab | PD-1 | II | Recurrent or metastatic squamous cell carcinoma | 425 | Ipilimumab | NCT02823574 | Overall response rate (ORR) | 08/11/2016 |
| 11 | Nivolumab | PD-1 | I/II | Neoadjuvant to surgery in advanced stage head and neck squamous cell carcinoma (HNSCC) | 33 | Ipilimumab | NCT03003637 | Feasibility and safety | 28/02/2017 |
| 12 | Nivolumab | PD-1 | II | Recurrent or metastatic salivary gland carcinoma | 98 | - | NCT03132038 | Progression-free survival (PFS): after 6 months of treatment | 24/03/2017 |
| 13 | Nivolumab | PD-1 | III | Recurrent and/or metastatic head and neck squamous cell carcinoma | 124 | - | NCT05802290 | Adverse effects | 27/11/2017 |
| 14 | Nivolumab | PD-1 | III | Locally advanced squamous cell carcinoma | 74 | Cisplatin in combination with radiotherapy | NCT03349710 | Event-free survival (EFS) | 15/12/2017 |
| 15 | Pembrolizumab | PD-1 | II | Recurrent and/or metastatic head and neck squamous cell carcinoma | 29 | Afatinib | NCT03695510 | Toxicities and efficacies | 24/01/2019 |
| 16 | Durvalumab | PD-L1 | I/II | Head and neck squamous cell carcinoma | 33 | - | NCT03829007 | Treatment regimen | 15/04/2019 |
| 17 | Pembrolizumab | PD-1 | II | First-line treatment of metastatic or unresectable, recurrent head and neck squamous cell carcinoma | 18 | Ulevostinag | NCT04220866 | Safety and efficacy | 04/03/2020 |
| 18 | Ipilimumab | CTLA-4 | I | Head and neck cancer (Stage III-IVB) | 19 | Cetuximab with intensity-modulated radiation therapy | NCT01935921 | Side effects and dosage regimen | 09/04/2013 |
| 19 | Ipilimumab | CTLA-4 | I | Head and neck squamous cell carcinoma | 80 | Pembrolizumab | NCT01986426 | Safety, tolerability, PK, and efficacy | 01/11/2013 |
| 20 | Ipilimumab | CTLA-4 | I/II | Advancedstage head and neck squamous cell carcinoma | 33 | Nivolumab (neoadjuvant to surgery) | NCT03003637 | Feasibility and safety | 28/02/2017 |
| 21 | Ipilimumab | CTLA-4 | I | Head and neck cancer (Stage IVA-B) | 24 | Nivolumab with radiotherapy | NCT03162731 | Side effects | 11/05/2017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-W.; Biswas, P.K.; Islam, A.; Chen, M.-K.; Chueh, P.J. The Use of Immune Regulation in Treating Head and Neck Squamous Cell Carcinoma (HNSCC). Cells 2024, 13, 413. https://doi.org/10.3390/cells13050413
Wang C-W, Biswas PK, Islam A, Chen M-K, Chueh PJ. The Use of Immune Regulation in Treating Head and Neck Squamous Cell Carcinoma (HNSCC). Cells. 2024; 13(5):413. https://doi.org/10.3390/cells13050413
Chicago/Turabian StyleWang, Che-Wei, Pulak Kumar Biswas, Atikul Islam, Mu-Kuan Chen, and Pin Ju Chueh. 2024. "The Use of Immune Regulation in Treating Head and Neck Squamous Cell Carcinoma (HNSCC)" Cells 13, no. 5: 413. https://doi.org/10.3390/cells13050413
APA StyleWang, C.-W., Biswas, P. K., Islam, A., Chen, M.-K., & Chueh, P. J. (2024). The Use of Immune Regulation in Treating Head and Neck Squamous Cell Carcinoma (HNSCC). Cells, 13(5), 413. https://doi.org/10.3390/cells13050413








