The Role of Oxidative Stress in Tumorigenesis and Progression
Abstract
:1. Introduction
2. Oxidative Stress and Tumorigenesis
3. Oxidative Stress and Tumor Metabolism
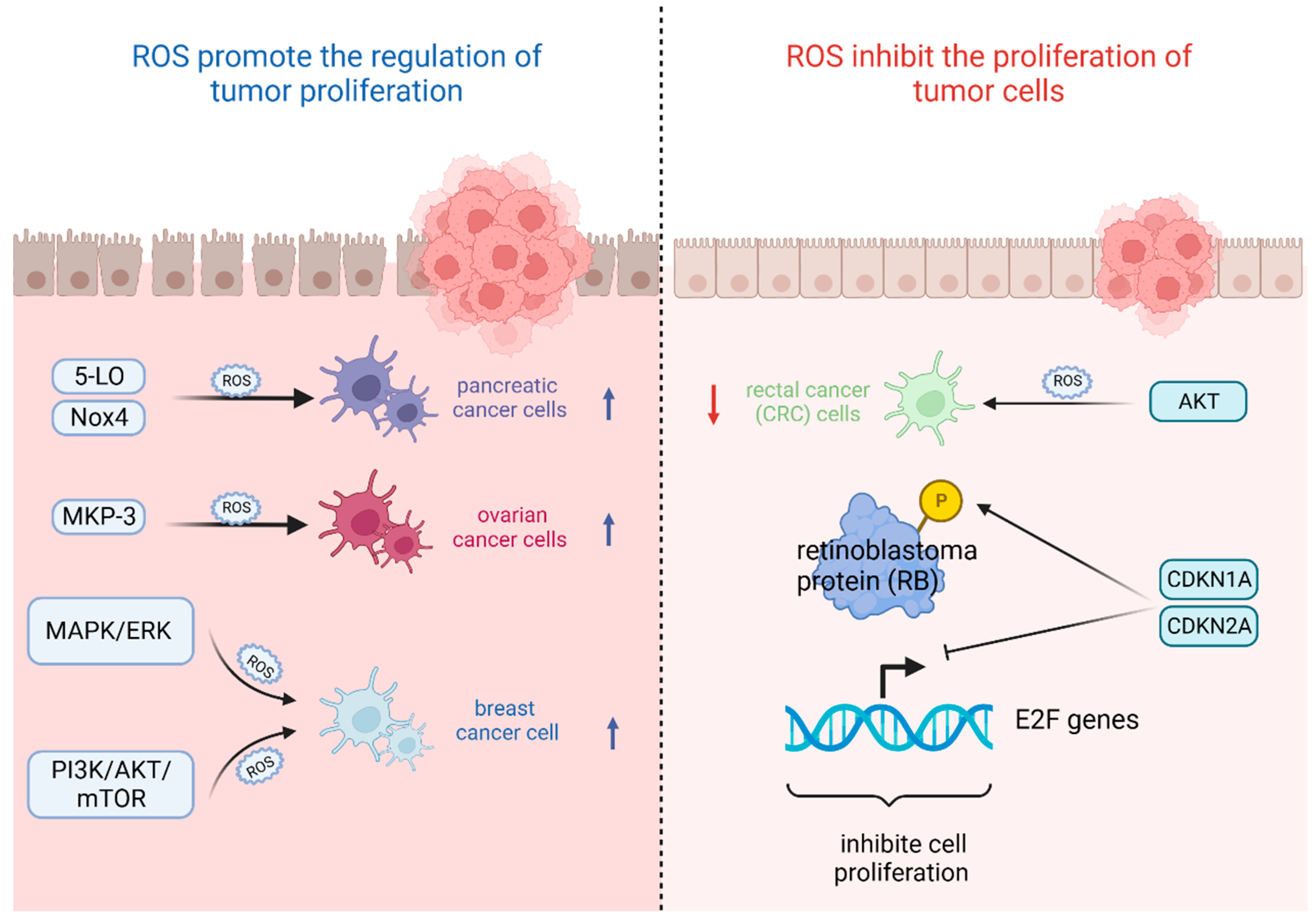
4. Oxidative Stress and Tumor Cell Proliferation
5. Oxidative Stress and Tumor Immunity
6. Oxidative Stress and Tumor Metastasis
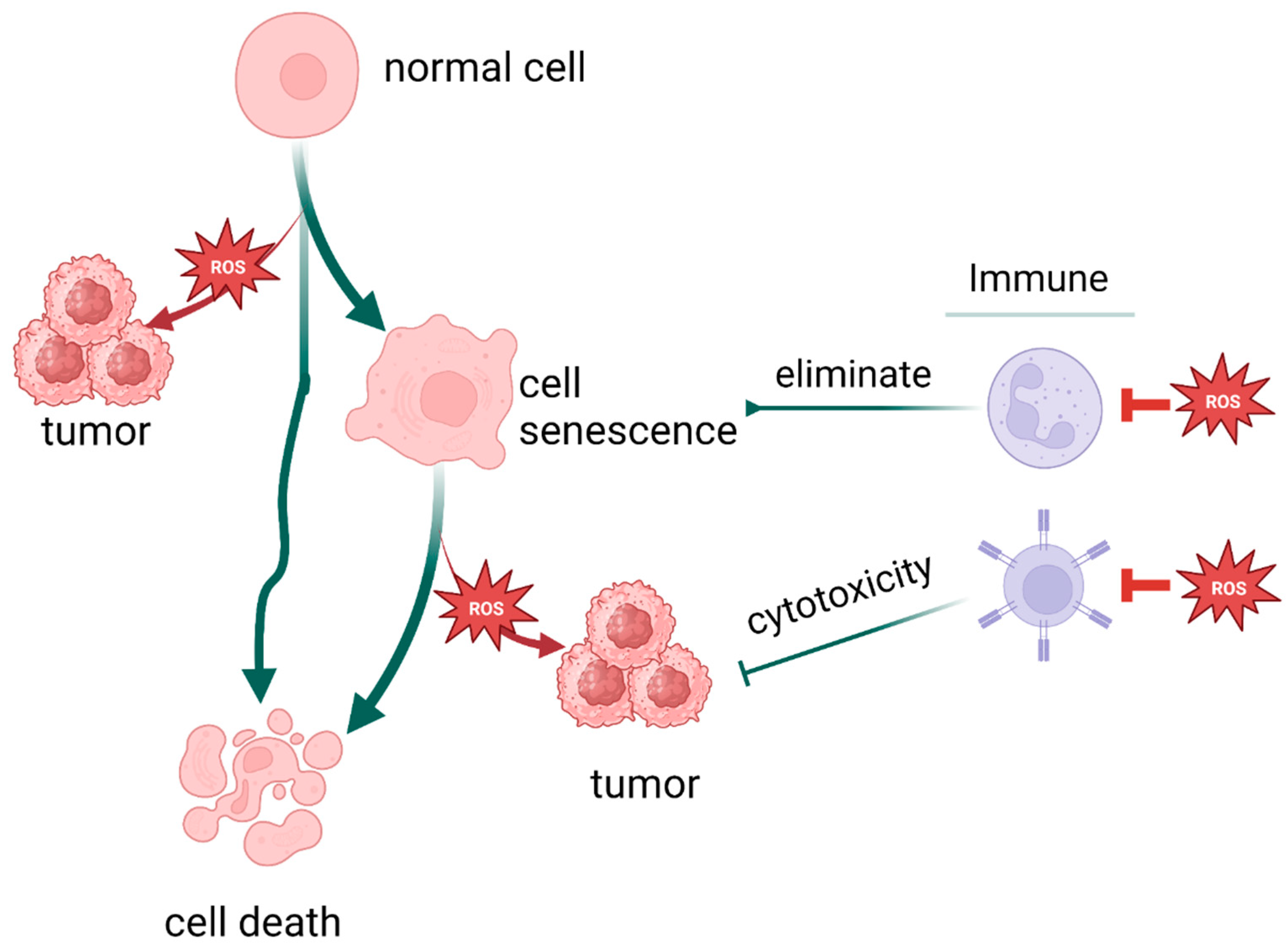
7. Oxidative Stress and the Relationship Between Aging and Tumors
8. Oxidative Stress and the Relationship Between Death and Tumors
9. Oxidative Stress and Tumor Treatment
| Drug Name | Type | Clinical Phase | Specific Mechanism | Function | References |
|---|---|---|---|---|---|
| Arsenic trioxide | Chemically synthesized drug | In p53-mutated pediatric cancer, phase 2 | Including autophagy, apoptosis, necroptosis, and ferroptosis | Promote oxidative stress in tumor cells | [187] |
| Gemcitabine | Chemically synthesized drug | In biliary tract cancer, phase 3 trial | Inhibiting nuclear replication, promoting p-STAT3 binding to the promoters of Bmi1, Nanog, and Sox2 genes. | Promote oxidative stress in tumor cells | [156,188,189,190] |
| Elesclomol | Chemically synthesized drug | In ovarian, fallopian tube or primary peritoneal cancer, phase 2 | Promoting cupproposis and killing cancer cells | Promote oxidative stress in tumor cells | [191] |
| Rotenone | Natural active substance | In colon cancer | Inhibiting the PI3K/AKT/mTOR signaling pathway | Promote oxidative stress in tumor cells | [192] |
| Fucoidan | Natural active substance | In hepatocellular carcinoma, phase 2 | Boosting ROS and mitochondrial superoxide generation and draining ATP | Promote oxidative stress in tumor cells | [193] |
| 2-ME | Chemically synthesized drug | In patients with solid tumors, phase 1 | Inhibiting angiogenesisin, increasing CD3+ cell number and promoting tumour necrosis. | Promote oxidative stress in tumor cells | [194] |
| Naringenin | Natural active substance | In human tongue carcinoma CAL-27 cells | Inducing cell death via modulation of the Bid and Bcl-xl signaling pathways | Promote oxidative stress in tumor cells | [195] |
| BT-Br | Chemically synthesized drug | In castration-resistant prostate cancer DU145 cells | Binding to NADPH and inducing ferroptosis | Promote oxidative stress in tumor cells | [196] |
| Atovaquone | Chemically synthesized drug | In non-small cell lung carcinoma, early phase 1 | Inducing tumor cell apoptosis by elevating ROS levels | Promote oxidative stress in tumor cells | [197] |
| Metformin | Chemically synthesized drug | In advanced breast cancer, phase 2 | Increasing FOXO3a, p-FOXO3a, AMPK, p-AMPK, and MnSOD levels | Inhibit oxidative stress in tumor cells | [198] |
| Rapamycin | Chemically synthesized drug | In angiofibromas, phase 2 | Targeting mTOR, inhibits tumor proliferation | Inhibit oxidative stress in pre-cancerous cells | [199] |
| Pirfenidone | Chemically synthesized drug | In neurofibromatosis type 1 and progressive plexiform neurofibromas, phase 2 | Suppressing CAF activation | Inhibit oxidative stress in CAF cells | [200] |
| ME-143 | Chemically synthesized drug | In refractory solid tumors, phase 1 | Targeting NADPH oxidase, blocking ROS production | Inhibit oxidative stress in tumor cells | [201] |
| Carboplatin | Chemically synthesized drug | In locally advanced triple negative breast cancer, phase 2 | Facilitating early and durable CAR T cell infiltration | Promote oxidative stress in TME | [202] |
| Apatinib | Chemically synthesized drug | In metastatic colorectal cancer, phase 2 | Alleviating hypoxia, increasing infiltration of CD8+ T cells, reducing recruitment of TAMs | Promote oxidative stress in TME | [153,203] |
| Propofol | Chemically synthesized drug | In pediatric tumor, phase 4 | Inducing oxidative stress and apoptosis | Promote oxidative stress in tumor cells | [204] |
| Doxorubicin | Chemically synthesized drug | In advanced solid tumors, phase 1 | Perturbing mitochondrial structure and function in tumor cells | Promote oxidative stress in tumor cells | [205] |
| Sunitinib | Chemically synthesized drug | In advanced solid tumors, phase 1 | Alleviating the tumor hypoxia, improving pericyte coverage on endothelial cells | Promote oxidative stress in TME | [206] |
| Salidroside | Natural active substance | In human gastric cancer cell line | Downregulating Src-associated signaling pathway and HSP70 expression | Inhibit oxidative stress in tumor cells | [207] |
| Lipoxin A4 | Natural active substance | In pancreatic cancer cells | Suppressing the ROS/ERK/MMPs pathway | Inhibit oxidative stress in tumor cells | [208] |
| Lobaplatin | Chemically synthesized drug | In human gastric carcinoma cell line BGC-823 | Decreasing mitochondrial membrane potential | Promote oxidative stress in tumor cells | [209] |
| Quercetin | Natural active substance | In metastatic breast cancer, phase 1 | Inhibiting signaling pathways, including MAPK/ERK1/2, JAK/STAT, AMPKα1/ASK1/p38, etc. and inducing cell cycle arrest | Inhibit oxidative stress in tumor cells | [159] |
| Curcumin | Natural active substance | In advanced pancreatic cancer, phase 2 | Promoting apoptosis through inhibiting NF-κB | Inhibit oxidative stress in tumor cells | [160] |
| α-T-K | Chemically synthesized drug | In clinical immunotherapy of sensitized anti-PD-1 | Reprogramming M2 macrophages, elevating the curative effect of PD-1 antibody | Inhibit oxidative stress in TME | [85] |
| Artesunate | Natural active substance | In hepatocellular carcinoma, phase 1 | Promoting the accumulation of intracellular lipid peroxides to induce cancer cell ferroptosis | Promote oxidative stress in tumor cells | [113] |
| MRTX1133 | Chemically synthesized drug | In advanced non-small cell lung cancer with KRAS G12D mutation, phase 3 | Inhibiting KRAS G12D mutation, eliminating ROS, and alleviating intratumoral immunosuppression | Promote oxidative stress in tumor cells | [173,210] |
| Lapatinib | Chemically synthesized drug | In advanced or metastatic breast cancer, phase 1 | Inhibiting EGFR and apoptotic pathways | Promote oxidative stress in tumor cells | [174,211] |
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Full Name | Abbreviations | Full Name |
| ROS | reactive oxygen species | PAC1 | phosphatase of activated cells 1 |
| NF-κB | nuclear factor -κB | EMT | epithelial-mesenchymal transition |
| Nrf2 | NF-E2-related factor 2 | HSF1 | heat shock transcription factor 1 |
| PRAK | p38 regulated/activated protein kinase | E-cadherin | epithelial calmodulin |
| ERK | extracellular regulated kinase | CPT2 | carnitine palmitoyltransferase 2 |
| MAPK | mitogen-activated protein kinase | MMP | matrix metalloproteinases |
| PI3K | phosphatidylinositol 3-kinase | ccRCC | clear cell renal cell carcinoma |
| AKT | protein kinase B | NAC | N-acetylcysteine |
| mTOR | mammalian target of rapamycin | OSCC | oral squamous cell carcinoma |
| NOX | NADPH oxidase | AURKA | Aurora kinase A |
| mtROS | mitochondrial ROS | ASK | apoptosis stimulating kinase |
| NAC | N-acetylcysteine | SAPK | stress activated protein kinase |
| TSG | tumor suppressor gene | JNK | c-JunNH2 terminal kinase |
| DNMT | DNA methyltransferase | D-gal | D-galactose |
| CDX1 | caudal type homeobox-1 | AR | aldose reductase |
| LINE-1 | long-interspersed nuclear element-1 | IR | ionizing radiation |
| RUNX3 | runt-related transcription factor 3 | ATM | ataxia telangiectasia mutated |
| CRC | rectal cancer | SASP | senescent-associatedsecretory phenotype |
| RB | retinoblastoma | TSG | tumor suppressor gene |
| HCC | hepatocellular carcinoma | TERT | telomerase reverse transcriptase |
| LKB1 | Live kinase B1 | Trx | thioredoxin |
| MKP | mitogen-activated protein kinase phosphatase | Cytc | cytochrome c |
| PTEN | phosphatase and tensin homolog deleted on chromosome 10 | ER | endoplasmic reticulum |
| PML | promyelocytic leukemia | FINs | ferroptosis inducers |
| SIRT1 | sirtuin 1 | ICIs | immune checkpoint inhibitor |
| IDH2 | isocitrate dehydrogenase 2 | mtHSP70 | mitochondrial HSP70 |
| SOD2 | superoxide dismutase 2 | MFF1 | mitochondrial fission factor 1 |
| FOXO3a | Forkhead box O3 | VDAC1 | voltage-dependent anion channel 1 |
| PGC | peroxisome proliferators-activated receptor γ coactivator | ALDH | aldehyde dehydrogenase |
| TFAM | mitochondrial transcription factor A | STAT3 | Signal Transducer and Activator of Transcription 3 |
| Drp | dynamin-related protein | JAK | Janus kinase |
| PINK | PTEN induced putative kinase | NSCLC | non-small cell lung cancer cells |
| KHK-A | protein kinase activity of fructokinase A | RIOS | oxidative stress damage |
| MDA | malondialdehyde | POLRMT | mitochondrial RNA polymerase |
| TME | tumor microenvironment | AMPK | AMP-activated protein kinase |
| MDSCs | myeloid-derived suppressor cells | MDM2 | Murine double minute 2 |
| Tregs | regulatory T cells | CTL | cytotoxic T lymphocyte |
| TAMs | tumor-associated macrophages | TSTR | T cells in a cellular stress response |
| IL | interleukin |
References
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Lutsenko, S.V.; Terentiev, A.A. Reactive Oxygen and Nitrogen Species-Induced Protein Modifications: Implication in Carcinogenesis and Anticancer Therapy. Cancer Res. 2018, 78, 6040–6047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ren, J.; Lu, J.; Li, P.; Zhang, W.; Wang, H.; Tang, B. Elucidating the Relationship between ROS and Protein Phosphorylation through In Situ Fluorescence Imaging in the Pneumonia Mice. Anal. Chem. 2021, 93, 10907–10915. [Google Scholar] [CrossRef] [PubMed]
- Block, K.; Gorin, Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat. Rev. Cancer 2012, 12, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Garg, M.; Braunstein, G.; Koeffler, H.P. LAMC2 as a therapeutic target for cancers. Expert. Opin. Ther. Targets 2014, 18, 979–982. [Google Scholar] [CrossRef]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef]
- Moore, J.M.; Correa, R.; Rosenberg, S.M.; Hastings, P.J. Persistent damaged bases in DNA allow mutagenic break repair in Escherichia coli. PLoS Genet. 2017, 13, e1006733. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Crisafulli, G.; Sogari, A.; Reilly, N.M.; Arena, S.; Lamba, S.; Bartolini, A.; Amodio, V.; Magrì, A.; Novara, L.; et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science 2019, 366, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Messina, S.; De Simone, G.; Ascenzi, P. Cysteine-based regulation of redox-sensitive Ras small GTPases. Redox Biol. 2019, 26, 101282. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.; Chandel, N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Döppler, H.; DelGiorno, K.E.; Zhang, L.; Leitges, M.; Crawford, H.C.; Murphy, M.P.; Storz, P. Mutant KRas-Induced Mitochondrial Oxidative Stress in Acinar Cells Upregulates EGFR Signaling to Drive Formation of Pancreatic Precancerous Lesions. Cell Rep. 2016, 14, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Sawalha, A.H. The Role of Oxidative Stress in Epigenetic Changes Underlying Autoimmunity. Antioxid. Redox Signal. 2022, 36, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kang, K.A.; Kim, K.C.; Na, S.Y.; Chang, W.Y.; Kim, G.Y.; Kim, H.S.; Hyun, J.W. Oxidative stress causes epigenetic alteration of CDX1 expression in colorectal cancer cells. Gene 2013, 524, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ni, X. ROS-mediated DNA methylation pattern alterations in carcinogenesis. Curr. Drug Targets 2015, 16, 13–19. [Google Scholar] [CrossRef]
- Wongpaiboonwattana, W.; Tosukhowong, P.; Dissayabutra, T.; Mutirangura, A.; Boonla, C. Oxidative stress induces hypomethylation of LINE-1 and hypermethylation of the RUNX3 promoter in a bladder cancer cell line. Asian Pac. J. Cancer Prev. 2013, 14, 3773–3778. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Z.; Zou, Z.; Xiao, G.; Luo, G.; Yang, H. Clinicopathological significance of RUNX3 gene hypermethylation in hepatocellular carcinoma. Tumour Biol. 2014, 35, 10333–10340. [Google Scholar] [CrossRef]
- Poungpairoj, P.; Whongsiri, P.; Suwannasin, S.; Khlaiphuengsin, A.; Tangkijvanich, P.; Boonla, C. Increased Oxidative Stress and RUNX3 Hypermethylation in Patients with Hepatitis B Virus-Associated Hepatocellular Carcinoma (HCC) and Induction of RUNX3 Hypermethylation by Reactive Oxygen Species in HCC Cells. Asian Pac. J. Cancer Prev. 2015, 16, 5343–5348. [Google Scholar] [CrossRef]
- Huang, R.; Chen, H.; Liang, J.; Li, Y.; Yang, J.; Luo, C.; Tang, Y.; Ding, Y.; Liu, X.; Yuan, Q.; et al. Dual Role of Reactive Oxygen Species and their Application in Cancer Therapy. J. Cancer 2021, 12, 5543–5561. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.; Bonanni, B. Relationship Between Metabolic Reprogramming and Mitochondrial Activity in Cancer Cells. Understanding the Anticancer Effect of Metformin and Its Clinical Implications. Anticancer Res. 2015, 35, 5789–5796. [Google Scholar] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Ruan, Y.; Che, X.; Feng, W. Dual role of PRDX1 in redox-regulation and tumorigenesis: Past and future. Free. Radic. Biol. Med. 2024, 210, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Zhao, M.; Liu, B.; Han, X.; Li, Y.; Wang, W.; Zhang, Q.; Lv, P.; Xing, L.; Shen, H.; et al. TNF-α-mediated upregulation of SOD-2 contributes to cell proliferation and cisplatin resistance in esophageal squamous cell carcinoma. Oncol. Rep. 2019, 42, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Huang, Y.; Wu, J.; Wang, Y.; Chen, A.; Guo, Q.; Zhang, Y.; Zhang, S.; Wang, L.; et al. An oncolytic system produces oxygen selectively in pancreatic tumor cells to alleviate hypoxia and improve immune activation. Pharmacol. Res. 2024, 199, 107053. [Google Scholar] [CrossRef] [PubMed]
- Zakic, T.; Pekovic-Vaughan, V.; Cvoro, A.; Korac, A.; Jankovic, A.; Korac, B. Redox and metabolic reprogramming in breast cancer and cancer-associated adipose tissue. FEBS Lett. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Ju, H.Q.; Lin, J.F.; Tian, T.; Xie, D.; Xu, R.H. NADPH homeostasis in cancer: Functions, mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 2020, 5, 231. [Google Scholar] [CrossRef] [PubMed]
- Niwa-Kawakita, M.; Ferhi, O.; Soilihi, H.; Le Bras, M.; Lallemand-Breitenbach, V.; de Thé, H. PML is a ROS sensor activating p53 upon oxidative stress. J. Exp. Med. 2017, 214, 3197–3206. [Google Scholar] [CrossRef]
- Niwa-Kawakita, M.; Wu, H.C.; Thé, H.; Lallemand-Breitenbach, V. PML nuclear bodies, membrane-less domains acting as ROS sensors? Semin. Cell Dev. Biol. 2018, 80, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, S.; Li, H.; Duan, Q.; Zhang, Z.; Shen, Q.; Wang, C.; Yin, T. ROS/KRAS/AMPK Signaling Contributes to Gemcitabine-Induced Stem-Like Cell Properties in Pancreatic Cancer. Mol. Ther. Oncolytics 2019, 14, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; de Souza, C.T. Multi-regulatory network of ROS: The interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. J. Physiol. Biochem. 2017, 73, 487–494. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Shen, L. Mitochondrial proteins in heart failure: The role of deacetylation by SIRT3. Pharmacol. Res. 2021, 172, 105802. [Google Scholar] [CrossRef]
- Kitamura, H.; Takeda, H.; Motohashi, H. Genetic, metabolic and immunological features of cancers with NRF2 addiction. FEBS Lett. 2022, 596, 1981–1993. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, X.; Shao, F.; Lv, G.; Lv, H.; Lee, J.H.; Qian, X.; Wang, Z.; Xia, Y.; Du, L.; et al. The protein kinase activity of fructokinase A specifies the antioxidant responses of tumor cells by phosphorylating p62. Sci. Adv. 2019, 5, eaav4570. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Zhou, W.; Wang, J.; Huang, X.; Zuo, Y.; Wang, T.S.; Gao, X.; Xu, Y.Y.; Zou, S.W.; Liu, Y.B.; et al. Arginine Methylation of MDH1 by CARM1 Inhibits Glutamine Metabolism and Suppresses Pancreatic Cancer. Mol. Cell 2016, 64, 673–687. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Du, W.; Jiang, P.; Mancuso, A.; Stonestrom, A.; Brewer, M.D.; Minn, A.J.; Mak, T.W.; Wu, M.; Yang, X. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nat. Cell Biol. 2013, 15, 991–1000. [Google Scholar] [CrossRef]
- Edderkaoui, M.; Hong, P.; Vaquero, E.C.; Lee, J.K.; Fischer, L.; Friess, H.; Buchler, M.W.; Lerch, M.M.; Pandol, S.J.; Gukovskaya, A.S. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G1137–G1147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chi, Y.; Gao, K.; Zhang, X.; Yao, J. p53 protein-mediated up-regulation of MAP kinase phosphatase 3 (MKP-3) contributes to the establishment of the cellular senescent phenotype through dephosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2). J. Biol. Chem. 2015, 290, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Shen, Z.; Yang, Q.; Sui, F.; Pu, J.; Ma, J.; Ma, S.; Yao, D.; Ji, M.; Hou, P. Vitamin C kills thyroid cancer cells through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways via distinct mechanisms. Theranostics 2019, 9, 4461–4473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lin, T.; Nie, G.; Hu, R.; Pi, S.; Wei, Z.; Wang, C.; Xing, C.; Hu, G. Cadmium and molybdenum co-induce pyroptosis via ROS/PTEN/PI3K/AKT axis in duck renal tubular epithelial cells. Environ. Pollut. 2021, 272, 116403. [Google Scholar] [CrossRef] [PubMed]
- Pani, G.; Galeotti, T.; Chiarugi, P. Metastasis: Cancer cell’s escape from oxidative stress. Cancer Metastasis Rev. 2010, 29, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Hara-Chikuma, M. Aquaporin-3 Controls Breast Cancer Cell Migration by Regulating Hydrogen Peroxide Transport and Its Downstream Cell Signaling. Mol. Cell Biol. 2016, 36, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Hu, J.; Lv, Y.; Bai, B.; Shan, L.; Chen, K.; Dai, S.; Zhu, H. Pyrvinium pamoate inhibits cell proliferation through ROS-mediated AKT-dependent signaling pathway in colorectal cancer. Med. Oncol. 2021, 38, 21. [Google Scholar] [CrossRef]
- Toyoshima, H.; Hunter, T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Coats, S.; Flanagan, W.M.; Nourse, J.; Roberts, J.M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 1996, 272, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Nevins, J.R. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998, 9, 585–593. [Google Scholar] [PubMed]
- Brown, K.; Xie, S.; Qiu, X.; Mohrin, M.; Shin, J.; Liu, Y.; Zhang, D.; Scadden, D.T.; Chen, D. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013, 3, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Shin, S.; Baek, M.C.; Yea, K. Modification of immune cell-derived exosomes for enhanced cancer immunotherapy: Current advances and therapeutic applications. Exp. Mol. Med. 2024, 56, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Y.; Gao, Y.; Ju, Y.; Zhao, Y.; Wu, Z.; Gao, S.; Zhang, B.; Pang, X.; Zhang, Y.; et al. The PRAK-NRF2 axis promotes the differentiation of Th17 cells by mediating the redox homeostasis and glycolysis. Proc. Natl. Acad. Sci. USA 2023, 120, e2212613120. [Google Scholar] [CrossRef] [PubMed]
- Paardekooper, L.M.; Vos, W.; van den Bogaart, G. Oxygen in the tumor microenvironment: Effects on dendritic cell function. Oncotarget 2019, 10, 883–896. [Google Scholar] [CrossRef]
- Sena, L.A.; Li, S.; Jairaman, A.; Prakriya, M.; Ezponda, T.; Hildeman, D.A.; Wang, C.R.; Schumacker, P.T.; Licht, J.D.; Perlman, H.; et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013, 38, 225–236. [Google Scholar] [CrossRef]
- Bao, Y.; Li, G.; Li, S.; Zhang, H.; Wu, X.; Yan, R.; Wang, Z.; Guo, C.; Jin, Y. Multifunctional Tumor-Targeting Carbon Dots for Tumor Microenvironment Activated Ferroptosis and Immunotherapy in Cancer Treatment. ACS Appl. Mater. Interfaces 2023, 15, 56834–56845. [Google Scholar] [CrossRef]
- Hu, X.; Ding, S.; Lu, G.; Lin, Z.; Liao, L.; Xiao, W.; Ding, Y.; Zhang, Y.; Wang, Z.; Gong, W.; et al. Apolipoprotein C-III itself stimulates the Syk/cPLA2-induced inflammasome activation of macrophage to boost anti-tumor activity of CD8+ T cell. Cancer Immunol. Immunother. CII 2023, 72, 4123–4144. [Google Scholar] [CrossRef]
- Hu, Z.; Teng, X.L.; Zhang, T.; Yu, X.; Ding, R.; Yi, J.; Deng, L.; Wang, Z.; Zou, Q. SENP3 senses oxidative stress to facilitate STING-dependent dendritic cell antitumor function. Mol. Cell 2021, 81, 940–952.e945. [Google Scholar] [CrossRef] [PubMed]
- Akbal, A.; Dernst, A.; Lovotti, M.; Mangan, M.S.J.; McManus, R.M.; Latz, E. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell. Mol. Immunol. 2022, 19, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Liu, L.; Fan, Z. Mechanisms and effects of NLRP3 in digestive cancers. Cell Death Discov. 2024, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Arrè, V.; Scialpi, R.; Centonze, M.; Giannelli, G.; Scavo, M.P.; Negro, R. The ‘speck’-tacular oversight of the NLRP3-pyroptosis pathway on gastrointestinal inflammatory diseases and tumorigenesis. J. Biomed. Sci. 2023, 30, 90. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.S.; Costantini, S.; de Lima, V.C.C.; de Andrade, V.P.; Rialland, M.; Cedric, R.; Budillon, A.; Magalhães, K.G. NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer. J. Biomed. Sci. 2021, 28, 26. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Kanneganti, T.D. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer 2019, 19, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Vatner, R.E.; Janssen, E.M. STING, DCs and the link between innate and adaptive tumor immunity. Mol. Immunol. 2019, 110, 13–23. [Google Scholar] [CrossRef]
- Cao, L.; Tian, H.; Fang, M.; Xu, Z.; Tang, D.; Chen, J.; Yin, J.; Xiao, H.; Shang, K.; Han, H.; et al. Activating cGAS-STING pathway with ROS-responsive nanoparticles delivering a hybrid prodrug for enhanced chemo-immunotherapy. Biomaterials 2022, 290, 121856. [Google Scholar] [CrossRef]
- Li, W.; Lu, L.; Lu, J.; Wang, X.; Yang, C.; Jin, J.; Wu, L.; Hong, X.; Li, F.; Cao, D.; et al. cGAS-STING-mediated DNA sensing maintains CD8+ T cell stemness and promotes antitumor T cell therapy. Sci. Transl. Med. 2020, 12, eaay9013. [Google Scholar] [CrossRef]
- Gan, Y.; Li, X.; Han, S.; Liang, Q.; Ma, X.; Rong, P.; Wang, W.; Li, W. The cGAS/STING Pathway: A Novel Target for Cancer Therapy. Front. Immunol. 2021, 12, 795401. [Google Scholar] [CrossRef]
- Krueger, P.D.; Osum, K.C.; Jenkins, M.K. CD4+ Memory T-Cell Formation during Type 1 Immune Responses. Cold Spring Harb. Perspect. Biol. 2021, 13, a038141. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D. The metabolic spectrum of memory T cells. Immunol. Cell Biol. 2019, 97, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Aboelella, N.S.; Brandle, C.; Kim, T.; Ding, Z.C.; Zhou, G. Oxidative Stress in the Tumor Microenvironment and Its Relevance to Cancer Immunotherapy. Cancers 2021, 13, 986. [Google Scholar] [CrossRef]
- OuYang, L.Y.; Wu, X.J.; Ye, S.B.; Zhang, R.X.; Li, Z.L.; Liao, W.; Pan, Z.Z.; Zheng, L.M.; Zhang, X.S.; Wang, Z.; et al. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J. Transl. Med. 2015, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Kraaij, M.D.; Savage, N.D.; van der Kooij, S.W.; Koekkoek, K.; Wang, J.; van den Berg, J.M.; Ottenhoff, T.H.; Kuijpers, T.W.; Holmdahl, R.; van Kooten, C.; et al. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2010, 107, 17686–17691. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zheng, W.; Liu, J.; Zhang, Y.; Qin, H.; Wu, H.; Xue, B.; Lu, Y.; Shen, P. Oxidative stress in malignant melanoma enhances tumor necrosis factor-α secretion of tumor-associated macrophages that promote cancer cell invasion. Antioxid. Redox Signal. 2013, 19, 1337–1355. [Google Scholar] [CrossRef]
- Yu, X.; Lao, Y.; Teng, X.L.; Li, S.; Zhou, Y.; Wang, F.; Guo, X.; Deng, S.; Chang, Y.; Wu, X.; et al. SENP3 maintains the stability and function of regulatory T cells via BACH2 deSUMOylation. Nat. Commun. 2018, 9, 3157. [Google Scholar] [CrossRef]
- Balwit, J.M.; Kalinski, P.; Sondak, V.K.; Coulie, P.G.; Jaffee, E.M.; Gajewski, T.F.; Marincola, F.M. Review of the 25th annual scientific meeting of the International Society for Biological Therapy of Cancer. J. Transl. Med. 2011, 9, 60. [Google Scholar] [CrossRef]
- Notarangelo, G.; Spinelli, J.B.; Perez, E.M.; Baker, G.J.; Kurmi, K.; Elia, I.; Stopka, S.A.; Baquer, G.; Lin, J.R.; Golby, A.J.; et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8+ T cell function. Science 2022, 377, 1519–1529. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ibrahim, K.E.; Kundu, S.; Attia, S.M.; Alanazi, W.A.; AlSharari, S.D. Inhibition of spleen tyrosine kinase signaling protects against acute lung injury through blockade of NADPH oxidase and IL-17A in neutrophils and γδ T cells respectively in mice. Int. Immunopharmacol. 2019, 68, 39–47. [Google Scholar] [CrossRef]
- Wu, J.J.; Fan, H.J.; Fan, Q.X.; Zhang, M.Z. Roles and mechanisms of E2F-1 and PAC1 in signaling oxidative stress-induced apoptosis of Saos-2 cells. Zhonghua Yi Xue Za Zhi 2012, 92, 1219–1221. [Google Scholar]
- Dan, L.; Liu, L.; Sun, Y.; Song, J.; Yin, Q.; Zhang, G.; Qi, F.; Hu, Z.; Yang, Z.; Zhou, Z.; et al. The phosphatase PAC1 acts as a T cell suppressor and attenuates host antitumor immunity. Nat. Immunol. 2020, 21, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, X.; Zhang, J.; Lu, Y.; Shi, Y.; Zhu, C.; Liu, Y.; Qin, B.; Luo, Z.; Du, Y.; et al. Dual Inhibition of Endoplasmic Reticulum Stress and Oxidation Stress Manipulates the Polarization of Macrophages under Hypoxia to Sensitize Immunotherapy. ACS Nano 2021, 15, 14522–14534. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Dai, E.; Li, Y.; Han, G.; Pei, G.; Ingram, D.R.; Thakkar, K.; Qin, J.J.; Dang, M.; Le, X.; et al. Pan-cancer T cell atlas links a cellular stress response state to immunotherapy resistance. Nat. Med. 2023, 29, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Muthuramalingam, K.; Cho, M. Redox Regulation of NOX Isoforms on FAK((Y397))/SRC((Y416)) Phosphorylation Driven Epithelial-to-Mesenchymal Transition in Malignant Cervical Epithelial Cells. Cells 2020, 9, 1555. [Google Scholar] [CrossRef]
- Hjelmeland, A.B.; Patel, R.P. SOD2 acetylation and deacetylation: Another tale of Jekyll and Hyde in cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 23376–23378. [Google Scholar] [CrossRef]
- He, C.; Danes, J.M.; Hart, P.C.; Zhu, Y.; Huang, Y.; de Abreu, A.L.; O’Brien, J.; Mathison, A.J.; Tang, B.; Frasor, J.M.; et al. SOD2 acetylation on lysine 68 promotes stem cell reprogramming in breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 23534–23541. [Google Scholar] [CrossRef]
- Xi, C.; Hu, Y.; Buckhaults, P.; Moskophidis, D.; Mivechi, N.F. Heat shock factor Hsf1 cooperates with ErbB2 (Her2/Neu) protein to promote mammary tumorigenesis and metastasis. J. Biol. Chem. 2012, 287, 35646–35657. [Google Scholar] [CrossRef]
- Li, W.; Cao, L.; Han, L.; Xu, Q.; Ma, Q. Superoxide dismutase promotes the epithelial-mesenchymal transition of pancreatic cancer cells via activation of the H2O2/ERK/NF-κB axis. Int. J. Oncol. 2015, 46, 2613–2620. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, Q.; Yang, Z.; Ni, Y.; Agbana, Y.L.; Bai, H.; Yi, Z.; Yi, X.; Kuang, Y.; Zhu, Y. G6PD facilitates clear cell renal cell carcinoma invasion by enhancing MMP2 expression through ROS-MAPK axis pathway. Int. J. Oncol. 2020, 57, 197–212. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, K.; Chen, Y.; Chen, H.; Nice, E.C.; Huang, C. Redox regulation in tumor cell epithelial-mesenchymal transition: Molecular basis and therapeutic strategy. Signal Transduct. Target. Ther. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Vlachostergios, P.J. Loss of tumor suppressive properties of lipid metabolism enzyme CPT2 in ovarian carcinoma: Comment on “CPT2 down-regulation promotes tumor growth and metastasis through inducing ROS/NFκB pathway in ovarian cancer” by Zhang et al. Transl. Oncol. 2021, 14, 101067. [Google Scholar] [CrossRef] [PubMed]
- Nikitovic, D.; Corsini, E.; Kouretas, D.; Tsatsakis, A.; Tzanakakis, G. ROS-major mediators of extracellular matrix remodeling during tumor progression. Food Chem. Toxicol. 2013, 61, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, S.W.; Kim, J.R. Reactive oxygen species regulate urokinase plasminogen activator expression and cell invasion via mitogen-activated protein kinase pathways after treatment with hepatocyte growth factor in stomach cancer cells. J. Exp. Clin. Cancer Res. 2009, 28, 73. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Im, M.; Yim, N.H.; Jung, Y.P.; Ma, J.Y. Aqueous extract of Bambusae Caulis in Taeniam inhibits PMA-induced tumor cell invasion and pulmonary metastasis: Suppression of NF-κB activation through ROS signaling. PLoS ONE 2013, 8, e78061. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.H.; Hu, J.; Shi, M.; Zhang, L.; Chen, H. Combined treatment with N-acetylcysteine and gefitinib overcomes drug resistance to gefitinib in NSCLC cell line. Cancer Med. 2020, 9, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Dong, H.; Wang, Q. AURKA contributes to the progression of oral squamous cell carcinoma (OSCC) through modulating epithelial-to-mesenchymal transition (EMT) and apoptosis via the regulation of ROS. Biochem. Biophys. Res. Commun. 2018, 507, 83–90. [Google Scholar]
- Mohamad Kamal, N.S.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell Biol. 2020, 99, 151108. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Aging, cellular senescence, and cancer. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free. Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Li, Z.; Xiong, J.; Geng, Z.; Wei, W.; Zhang, Y.; Wu, G.; Zhuang, T.; Tian, X.; Liu, Z.; et al. LARP7 ameliorates cellular senescence and aging by allosterically enhancing SIRT1 deacetylase activity. Cell Rep. 2021, 37, 110038. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Si, F.; Bagley, D.; Ma, F.; Zhang, Y.; Tao, Y.; Shaw, E.; Peng, G. Blockades of effector T cell senescence and exhaustion synergistically enhance antitumor immunity and immunotherapy. J. Immunother. Cancer 2022, 10, e005020. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, T.; Kriesen, S.; Neels, O.; Hildebrandt, G.; Manda, K. Investigation of epothilone B-induced cell death mechanisms in human epithelial cancer cells -in consideration of combined treatment with ionizing radiation. Cancer Investig. 2015, 33, 213–224. [Google Scholar] [CrossRef]
- Vaddavalli, P.L.; Schumacher, B. The p53 network: Cellular and systemic DNA damage responses in cancer and aging. Trends Genet. 2022, 38, 598–612. [Google Scholar] [CrossRef]
- Ichijo, H.; Nishida, E.; Irie, K.; ten Dijke, P.; Saitoh, M.; Moriguchi, T.; Takagi, M.; Matsumoto, K.; Miyazono, K.; Gotoh, Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997, 275, 90–94. [Google Scholar] [CrossRef]
- Huang, Z.; Gan, S.; Zhuang, X.; Chen, Y.; Lu, L.; Wang, Y.; Qi, X.; Feng, Q.; Huang, Q.; Du, B.; et al. Artesunate Inhibits the Cell Growth in Colorectal Cancer by Promoting ROS-Dependent Cell Senescence and Autophagy. Cells 2022, 11, 2472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, X.; Ren, M.; Wang, Z. Ferroptosis: A New Research Direction of Artemisinin and Its Derivatives in Anti-Cancer Treatment. Am. J. Chin. Med. 2024, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Mausset-Bonnefont, A.L.; Jorgensen, C.; Louis-Plence, P.; Brondello, J.M. Cellular senescence impact on immune cell fate and function. Aging Cell 2016, 15, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Prieur, A.; Peeper, D.S. Cellular senescence in vivo: A barrier to tumorigenesis. Curr. Opin. Cell Biol. 2008, 20, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, M.; Zanoni, M.; Pirini, F.; Tumedei, M.M.; Ravaioli, S.; Rapposelli, I.G.; Frassineti, G.L.; Bravaccini, S. Pancreatic Cancer and Cellular Senescence: Tumor Microenvironment under the Spotlight. Int. J. Mol. Sci. 2021, 23, 254. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Jedrychowski, M.P.; Schweppe, D.K.; Huttlin, E.L.; Yu, Q.; Heppner, D.E.; Li, J.; Long, J.; Mills, E.L.; Szpyt, J.; et al. A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging. Cell 2020, 180, 968–983. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.; Verma, A.; Marderstein, A.R.; White, J.; Bhinder, B.; Garcia Medina, J.S.; Elemento, O. Pan-cancer analysis reveals molecular patterns associated with age. Cell Rep. 2021, 37, 110100. [Google Scholar] [CrossRef]
- Papaconstantinou, J. The Role of Signaling Pathways of Inflammation and Oxidative Stress in Development of Senescence and Aging Phenotypes in Cardiovascular Disease. Cells 2019, 8, 1383. [Google Scholar] [CrossRef]
- Macip, S.; Igarashi, M.; Fang, L.; Chen, A.; Pan, Z.Q.; Lee, S.W.; Aaronson, S.A. Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. Embo J. 2002, 21, 2180–2188. [Google Scholar] [CrossRef]
- Lv, F.; Li, N.; Kong, M.; Wu, J.; Fan, Z.; Miao, D.; Xu, Y.; Ye, Q.; Wang, Y. CDKN2a/p16 Antagonizes Hepatic Stellate Cell Activation and Liver Fibrosis by Modulating ROS Levels. Front. Cell Dev. Biol. 2020, 8, 176. [Google Scholar] [CrossRef]
- Aubert, G.; Lansdorp, P.M. Telomeres and aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef]
- Gasek, N.S.; Kuchel, G.A.; Kirkland, J.L.; Xu, M. Strategies for Targeting Senescent Cells in Human Disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef]
- Antonangeli, F.; Zingoni, A.; Soriani, A.; Santoni, A. Senescent cells: Living or dying is a matter of NK cells. J. Leukoc. Biol. 2019, 105, 1275–1283. [Google Scholar] [CrossRef]
- Schank, M.; Zhao, J.; Wang, L.; Li, Z.; Cao, D.; Nguyen, L.N.; Dang, X.; Khanal, S.; Nguyen, L.N.T.; Thakuri, B.K.C.; et al. Telomeric injury by KML001 in human T cells induces mitochondrial dysfunction through the p53-PGC-1α pathway. Cell Death Dis. 2020, 11, 1030. [Google Scholar] [CrossRef]
- Ullah, H.; Di Minno, A.; Santarcangelo, C.; Khan, H.; Daglia, M. Improvement of Oxidative Stress and Mitochondrial Dysfunction by β-Caryophyllene: A Focus on the Nervous System. Antioxidants 2021, 10, 546. [Google Scholar] [CrossRef]
- AlBasher, G.; AlKahtane, A.A.; Alarifi, S.; Ali, D.; Alessia, M.S.; Almeer, R.S.; Abdel-Daim, M.M.; Al-Sultan, N.K.; Al-Qahtani, A.A.; Ali, H.; et al. Methotrexate-induced apoptosis in human ovarian adenocarcinoma SKOV-3 cells via ROS-mediated bax/bcl-2-cyt-c release cascading. Onco Targets Ther. 2019, 12, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cui, L.; Ye, J.; Yang, G.; Lu, G.; Fang, X.; Zeng, Z.; Zhou, J. Dioscin facilitates ROS-induced apoptosis via the p38-MAPK/HSP27-mediated pathways in lung squamous cell carcinoma. Int. J. Biol. Sci. 2020, 16, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, J. Cytochrome c in cancer therapy and prognosis. Biosci. Rep. 2022, 42, BSR20222171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jing, S.; Liu, S.; Shen, X.; Cai, L.; Zhu, C.; Zhao, Y.; Pang, M. Double-activation of mitochondrial permeability transition pore opening via calcium overload and reactive oxygen species for cancer therapy. J. Nanobiotechnol. 2022, 20, 188. [Google Scholar] [CrossRef]
- Liu, X.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H.; Li, Y. Mitochondrial-Endoplasmic Reticulum Communication-Mediated Oxidative Stress and Autophagy. Biomed. Res. Int. 2022, 2022, 6459585. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, K.Y.; Yu, S.N.; Park, S.G.; Yu, H.S.; Seo, Y.K.; Ahn, S.C. Monensin Induces PC-3 Prostate Cancer Cell Apoptosis via ROS Production and Ca2+ Homeostasis Disruption. Anticancer Res. 2016, 36, 5835–5843. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.M.; Seo, S.U.; Min, K.J.; Im, S.S.; Nam, J.O.; Chang, J.S.; Kim, S.; Park, J.W.; Kwon, T.K. Corosolic Acid Induces Non-Apoptotic Cell Death through Generation of Lipid Reactive Oxygen Species Production in Human Renal Carcinoma Caki Cells. Int. J. Mol. Sci. 2018, 19, 1309. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.W.; Sviderskiy, V.O.; Terzi, E.M.; Papagiannakopoulos, T.; Moreira, A.L.; Adams, S.; Sabatini, D.M.; Birsoy, K.; Possemato, R. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 2017, 551, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Lin, B.; Zhou, M.; Wu, L.; Zheng, T. Role of ferroptosis in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2018, 144, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tavana, O.; Chu, B.; Erber, L.; Chen, Y.; Baer, R.; Gu, W. NRF2 Is a Major Target of ARF in p53-Independent Tumor Suppression. Mol. Cell 2017, 68, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Punganuru, S.R.; Madala, H.R.; Arutla, V.; Srivenugopal, K.S. Selective killing of human breast cancer cells by the styryl lactone (R)-goniothalamin is mediated by glutathione conjugation, induction of oxidative stress and marked reactivation of the R175H mutant p53 protein. Carcinogenesis 2018, 39, 1399–1410. [Google Scholar] [CrossRef]
- Kang, R.; Kroemer, G.; Tang, D. The tumor suppressor protein p53 and the ferroptosis network. Free. Radic. Biol. Med. 2019, 133, 162–168. [Google Scholar] [CrossRef]
- Kaul, S.C.; Aida, S.; Yaguchi, T.; Kaur, K.; Wadhwa, R. Activation of wild type p53 function by its mortalin-binding, cytoplasmically localizing carboxyl terminus peptides. J. Biol. Chem. 2005, 280, 39373–39379. [Google Scholar] [CrossRef]
- Trinh, D.L.; Elwi, A.N.; Kim, S.W. Direct interaction between p53 and Tid1 proteins affects p53 mitochondrial localization and apoptosis. Oncotarget 2010, 1, 396–404. [Google Scholar] [CrossRef]
- Arena, G.; Cissé, M.Y.; Pyrdziak, S.; Chatre, L.; Riscal, R.; Fuentes, M.; Arnold, J.J.; Kastner, M.; Gayte, L.; Bertrand-Gaday, C.; et al. Mitochondrial MDM2 Regulates Respiratory Complex I Activity Independently of p53. Mol. Cell 2018, 69, 594–609. [Google Scholar] [CrossRef]
- Fasano, C.; Disciglio, V.; Bertora, S.; Lepore Signorile, M.; Simone, C. FOXO3a from the Nucleus to the Mitochondria: A Round Trip in Cellular Stress Response. Cells 2019, 8, 1110. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ni, Z.; Li, B.S.; Yong, X.; Yang, X.; Zhang, J.W.; Zhang, D.; Qin, Y.; Jie, M.M.; Dong, H.; et al. hTERT promotes the invasion of gastric cancer cells by enhancing FOXO3a ubiquitination and subsequent ITGB1 upregulation. Gut 2017, 66, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Tackmann, N.R.; Zhang, Y. Mouse modelling of the MDM2/MDMX-p53 signalling axis. J. Mol. Cell Biol. 2017, 9, 34–44. [Google Scholar] [CrossRef]
- Huang, W.; Xie, W.; Zhong, H.; Cai, S.; Huang, Q.; Liu, Y.; Zeng, Z.; Liu, Y. Cytosolic p53 Inhibits Parkin-Mediated Mitophagy and Promotes Acute Liver Injury Induced by Heat Stroke. Front. Immunol. 2022, 13, 859231. [Google Scholar] [CrossRef] [PubMed]
- Chibaya, L.; Karim, B.; Zhang, H.; Jones, S.N. Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2003193118. [Google Scholar] [CrossRef]
- Chen, H.; Lin, X.; Yi, X.; Liu, X.; Yu, R.; Fan, W.; Ling, Y.; Liu, Y.; Xie, W. SIRT1-mediated p53 deacetylation inhibits ferroptosis and alleviates heat stress-induced lung epithelial cells injury. Int. J. Hyperth. 2022, 39, 977–986. [Google Scholar] [CrossRef]
- Jin, Y.; Gu, W.; Chen, W. Sirt3 is critical for p53-mediated ferroptosis upon ROS-induced stress. J. Mol. Cell Biol. 2021, 13, 151–154. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhang, D.; Yang, Y.T.; Li, X.Y.; Li, H.N.; Zhang, X.P.; Long, J.Y.; Lu, Y.Q.; Liu, L.; Yang, G.; et al. Suppression of microRNA-222-3p ameliorates ulcerative colitis and colitis-associated colorectal cancer to protect against oxidative stress via targeting BRG1 to activate Nrf2/HO-1 signaling pathway. Front. Immunol. 2023, 14, 1089809. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, J.; Xin, T.; Xu, S.; Liu, X.; Gao, Y.; Huang, H. Eleutheroside E functions as anti-cervical cancer drug by inhibiting the phosphatidylinositol 3-kinase pathway and reprogramming the metabolic responses. J. Pharm. Pharmacol. 2022, 74, 1251–1260. [Google Scholar] [CrossRef]
- Chen, B.; Song, Y.; Zhan, Y.; Zhou, S.; Ke, J.; Ao, W.; Zhang, Y.; Liang, Q.; He, M.; Li, S.; et al. Fangchinoline inhibits non-small cell lung cancer metastasis by reversing epithelial-mesenchymal transition and suppressing the cytosolic ROS-related Akt-mTOR signaling pathway. Cancer Lett. 2022, 543, 215783. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Feng, H.; Wu, H.; Jin, Z.; Shen, X.; Kuang, J.; Huo, Z.; Chen, X.; Gao, H.; Ye, F.; et al. Targeting autophagy enhances apatinib-induced apoptosis via endoplasmic reticulum stress for human colorectal cancer. Cancer Lett. 2018, 431, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.S.; Tan, M.J.; Sng, M.K.; Teo, Z.; Phua, T.; Choo, C.C.; Li, L.; Zhu, P.; Tan, N.S. Cancer-associated fibroblasts enact field cancerization by promoting extratumoral oxidative stress. Cell Death Dis. 2017, 8, e2562. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shen, X.; Zhi, F.; Wen, Z.; Gao, Y.; Xu, J.; Yang, B.; Bai, Y. An overview of arsenic trioxide-involved combined treatment algorithms for leukemia: Basic concepts and clinical implications. Cell Death Discov. 2023, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Pandit, B.; Royzen, M. Recent Development of Prodrugs of Gemcitabine. Genes 2022, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Skwarski, M.; McGowan, D.R.; Belcher, E.; Di Chiara, F.; Stavroulias, D.; McCole, M.; Derham, J.L.; Chu, K.-Y.; Teoh, E.; Chauhan, J.; et al. Mitochondrial Inhibitor Atovaquone Increases Tumor Oxygenation and Inhibits Hypoxic Gene Expression in Patients with Non–Small Cell Lung Cancer. Clin. Cancer Res. 2021, 27, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.T.; Huang, H.C.; Lin, J.K. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol. Carcinog. 2010, 49, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Dey, D.; Biswas, P.K.; Rahaman, T.I.; Saha, S.; Parvez, A.; Khan, D.A.; Lily, N.J.; Saha, K.; Sohel, M.; et al. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. Int. J. Mol. Sci. 2022, 23, 11746. [Google Scholar] [CrossRef]
- Pricci, M.; Girardi, B.; Giorgio, F.; Losurdo, G.; Ierardi, E.; Di Leo, A. Curcumin and Colorectal Cancer: From Basic to Clinical Evidences. Int. J. Mol. Sci. 2020, 21, 2364. [Google Scholar] [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell. Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef]
- Edupuganti, V.; Tyndall, J.; Gamble, A.B. Self-immolative Linkers in Prodrugs and Antibody Drug Conjugates in Cancer Treatment. Recent Pat. Anticancer Drug Discov. 2021, 16, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody–Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Walker, I.; Newell, H. Do molecularly targeted agents in oncology have reduced attrition rates? Nat. Rev. Drug Discov. 2009, 8, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zuo, J.; Li, B.; Chen, R.; Luo, K.; Xiang, X.; Lu, S.; Huang, C.; Liu, L.; Tang, J.; et al. Drug-induced oxidative stress in cancer treatments: Angel or devil? Redox Biol. 2023, 63, 102754. [Google Scholar] [CrossRef] [PubMed]
- Camarillo, J.M.; Rose, K.L.; Galligan, J.J.; Xu, S.; Marnett, L.J. Covalent Modification of CDK2 by 4-Hydroxynonenal as a Mechanism of Inhibition of Cell Cycle Progression. Chem. Res. Toxicol. 2016, 29, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Schexnayder, C.; Broussard, K.; Onuaguluchi, D.; Poché, A.; Ismail, M.; McAtee, L.; Llopis, S.; Keizerweerd, A.; McFerrin, H.; Williams, C. Metformin Inhibits Migration and Invasion by Suppressing ROS Production and COX2 Expression in MDA-MB-231 Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3692. [Google Scholar] [CrossRef]
- Weng, M.S.; Chang, J.H.; Hung, W.Y.; Yang, Y.C.; Chien, M.H. The interplay of reactive oxygen species and the epidermal growth factor receptor in tumor progression and drug resistance. J. Exp. Clin. Cancer Res. 2018, 37, 61. [Google Scholar] [CrossRef]
- Briere, D.M.; Li, S.; Calinisan, A.; Sudhakar, N.; Aranda, R.; Hargis, L.; Peng, D.H.; Deng, J.; Engstrom, L.D.; Hallin, J.; et al. The KRAS(G12C) Inhibitor MRTX849 Reconditions the Tumor Immune Microenvironment and Sensitizes Tumors to Checkpoint Inhibitor Therapy. Mol. Cancer Ther. 2021, 20, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.B.; Cheng, N.; Markosyan, N.; Sor, R.; Kim, I.K.; Hallin, J.; Shoush, J.; Quinones, L.; Brown, N.V.; Bassett, J.B.; et al. Efficacy of a Small-Molecule Inhibitor of KrasG12D in Immunocompetent Models of Pancreatic Cancer. Cancer Discov. 2023, 13, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Alqahtani, A.M.; Youssif, B.G.M.; Gouda, A.M. Globally Approved EGFR Inhibitors: Insights into Their Syntheses, Target Kinases, Biological Activities, Receptor Interactions, and Metabolism. Molecules 2021, 26, 6677. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Y.; Zhong, P.; Wang, J.; Weng, Q.; Qian, Y.; Han, J.; Zou, C.; Liang, G. EGFR inhibition attenuates diabetic nephropathy through decreasing ROS and endoplasmic reticulum stress. Oncotarget 2017, 8, 32655–32667. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, K.; Wang, X.; Jia, Z.; Yang, Y.; Duan, Y.; Huang, L.; Wu, Z.X.; Zhang, J.Y.; Ding, X. Cholesterol promotes EGFR-TKIs resistance in NSCLC by inducing EGFR/Src/Erk/SP1 signaling-mediated ERRα re-expression. Mol. Cancer 2022, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Cho, P.; Selfors, L.M.; Kuiken, H.J.; Kaul, R.; Fujiwara, T.; Harris, I.S.; Zhang, T.; Gygi, S.P.; Brugge, J.S. 3D Culture Models with CRISPR Screens Reveal Hyperactive NRF2 as a Prerequisite for Spheroid Formation via Regulation of Proliferation and Ferroptosis. Mol. Cell 2020, 80, 828–844. [Google Scholar] [CrossRef]
- Hiebert, P.; Wietecha, M.S.; Cangkrama, M.; Haertel, E.; Mavrogonatou, E.; Stumpe, M.; Steenbock, H.; Grossi, S.; Beer, H.D.; Angel, P.; et al. Nrf2-Mediated Fibroblast Reprogramming Drives Cellular Senescence by Targeting the Matrisome. Dev. Cell 2018, 46, 145–161. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). J. Am. Med. Assoc. 2009, 301, 39–51. [Google Scholar] [CrossRef]
- The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). J. Am. Med. Assoc. 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Dentro, S.C.; Leshchiner, I.; Haase, K.; Tarabichi, M.; Wintersinger, J.; Deshwar, A.G.; Yu, K.; Rubanova, Y.; Macintyre, G.; Demeulemeester, J.; et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell 2021, 184, 2239–2254. [Google Scholar] [CrossRef] [PubMed]
- Dando, I.; Cordani, M.; Dalla Pozza, E.; Biondani, G.; Donadelli, M.; Palmieri, M. Antioxidant Mechanisms and ROS-Related MicroRNAs in Cancer Stem Cells. Oxid. Med. Cell Longev. 2015, 2015, 425708. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Wang, J.Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R., Jr.; Yang, D.H.; Chen, Z.S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updates 2018, 41, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Tuveson, D.A. The promise and perils of antioxidants for cancer patients. N. Engl. J. Med. 2014, 371, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.C.; Llovet, J.M. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015, 149, 1226–1239. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jin, Z.; Zhang, S.; Zhang, X.; Li, P.; Yang, H.; Ma, Y. Arsenic trioxide elicits prophylactic and therapeutic immune responses against solid tumors by inducing necroptosis and ferroptosis. Cell. Mol. Immunol. 2023, 20, 51–64. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef]
- Coleman, J.A.; Yip, W.; Wong, N.C.; Sjoberg, D.D.; Bochner, B.H.; Dalbagni, G.; Donat, S.M.; Herr, H.W.; Cha, E.K.; Donahue, T.F.; et al. Multicenter Phase II Clinical Trial of Gemcitabine and Cisplatin as Neoadjuvant Chemotherapy for Patients with High-Grade Upper Tract Urothelial Carcinoma. J. Clin. Oncol. 2023, 41, 1618–1625. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, Q.; Zhao, H.; Liu, T.; Wu, H.; Shen, Q.; Wang, C.; Yin, T. Gemcitabine treatment promotes pancreatic cancer stemness through the Nox/ROS/NF-κB/STAT3 signaling cascade. Cancer Lett. 2016, 382, 53–63. [Google Scholar] [CrossRef]
- Guo, B.; Yang, F.; Zhang, L.; Zhao, Q.; Wang, W.; Yin, L.; Chen, D.; Wang, M.; Han, S.; Xiao, H.; et al. Cuproptosis Induced by ROS Responsive Nanoparticles with Elesclomol and Copper Combined with αPD-L1 for Enhanced Cancer Immunotherapy. Adv. Mater. 2023, 35, e2212267. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, Y.; Dai, M.; Li, Y.; Peng, R.; Yu, S.; Liu, H. Rotenone restrains colon cancer cell viability, motility and epithelial-mesenchymal transition and tumorigenesis in nude mice via the PI3K/AKT pathway. Int. J. Mol. Med. 2020, 46, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Pawar, J.S.; Ghosh, I. Fucoidan induces ROS-dependent epigenetic modulation in cervical cancer HeLa cell. Int. J. Biol. Macromol. 2021, 181, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Peta, K.T.; Durandt, C.; van Heerden, M.B.; Joubert, A.M.; Pepper, M.S.; Ambele, M.A. Effect of 2-methoxyestradiol treatment on early- and late-stage breast cancer progression in a mouse model. Cell Biochem. Funct. 2023, 41, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lai, J.; Su, J.; Li, J.; Li, C.; Zhu, B.; Li, Y. Naringenin-induced Oral Cancer Cell Apoptosis Via ROS-mediated Bid and Bcl-xl Signaling Pathway. Curr. Cancer Drug Targets 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Y.; Chen, Y.Y.; Wang, M.S.; Tong, J.J.; Xu, M.; Zhao, C.; Lin, H.Y.; Mei, L.C.; Dong, J.; Zhang, W.L.; et al. A catalase inhibitor: Targeting the NADPH-binding site for castration-resistant prostate cancer therapy. Redox Biol. 2023, 63, 102751. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, Y. Atovaquone exerts its anticancer effect by inhibiting Na(+)/K(+)-ATPase ion transport in canine cancer cells. Vet. World 2023, 16, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.G.; Shende, M.; Inci, G.; Park, S.H.; Jung, J.S.; Kim, S.B.; Kim, J.H.; Mo, Y.W.; Seo, J.H.; Feng, J.H.; et al. Combination of metformin/efavirenz/fluoxetine exhibits profound anticancer activity via a cancer cell-specific ROS amplification. Cancer Biol. Ther. 2023, 24, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Cancer prevention with rapamycin. Oncotarget 2023, 14, 342–350. [Google Scholar] [CrossRef]
- Antar, S.A.; Saleh, M.A.; Al-Karmalawy, A.A. Investigating the possible mechanisms of pirfenidone to be targeted as a promising anti-inflammatory, anti-fibrotic, anti-oxidant, anti-apoptotic, anti-tumor, and/or anti-SARS-CoV-2. Life Sci. 2022, 309, 121048. [Google Scholar] [CrossRef]
- Pant, S.; Burris, H.A., 3rd; Moore, K.; Bendell, J.C.; Kurkjian, C.; Jones, S.F.; Moreno, O.; Kuhn, J.G.; McMeekin, S.; Infante, J.R. A first-in-human dose-escalation study of ME-143, a second generation NADH oxidase inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2014, 32, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.H.; Zhu, J.J.; Lister, N.L.; Harrison, S.G.; Keerthikumar, S.; Goode, D.L.; Urban, R.Q.; Byrne, D.J.; Azad, A.; Vela, I.; et al. Low-dose carboplatin modifies the tumor microenvironment to augment CAR T cell efficacy in human prostate cancer models. Nat. Commun. 2023, 14, 5346. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Ren, S.; Jiang, T.; Zhu, B.; Li, X.; Zhao, C.; Jia, Y.; Shi, J.; Zhang, L.; Liu, X.; et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol. Res. 2019, 7, 630–643. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Liu, P.; Sun, C.; Pei, L.J.; Huang, Y.G. Propofol Augments Paclitaxel-Induced Cervical Cancer Cell Ferroptosis In Vitro. Front. Pharmacol. 2022, 13, 816432. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.; Dass, C.R. Doxorubicin Action on Mitochondria: Relevance to Osteosarcoma Therapy? Curr. Drug Targets 2018, 19, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, Y.; Okazaki, T.; Ebihara, S.; Komatsu, R.; Nihei, M.; Kobayashi, M.; Hirano, T.; Sugiura, H.; Tamada, T.; Tanaka, N.; et al. Beneficial effects of sunitinib on tumor microenvironment and immunotherapy targeting death receptor5. Oncoimmunology 2019, 8, e1543526. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Tang, T.; Sheng, L.; Ma, Y.; Liu, Y.; Yan, L.; Qi, S.; Ling, L.; Zhang, Y. Salidroside inhibits the proliferation and migration of gastric cancer cells via suppression of Src-associated signaling pathway activation and heat shock protein 70 expression. Mol. Med. Rep. 2018, 18, 147–156. [Google Scholar] [CrossRef]
- Zong, L.; Li, J.; Chen, X.; Chen, K.; Li, W.; Li, X.; Zhang, L.; Duan, W.; Lei, J.; Xu, Q.; et al. Lipoxin A4 Attenuates Cell Invasion by Inhibiting ROS/ERK/MMP Pathway in Pancreatic Cancer. Oxid. Med. Cell Longev. 2016, 2016, 6815727. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Yang, F.; Yu, Y.; Zeng, A.; Ye, T.; Yin, W.; Xie, Y.; Fu, Z.; Zhao, C. Lobaplatin induces BGC-823 human gastric carcinoma cell apoptosis via ROS- mitochondrial apoptotic pathway and impairs cell migration and invasion. Biomed. Pharmacother. 2016, 83, 1239–1246. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, S.; Ni, X.; Shen, S.; Wang, J.; Sun, W.; Suo, T.; Liu, H.; Ni, X.; Liu, H. KRAS G12D mutation eliminates reactive oxygen species through the Nrf2/CSE/H (2)S axis and contributes to pancreatic cancer growth. Acta Biochim. Biophys. Sin. 2022, 54, 1731–1739. [Google Scholar] [CrossRef]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef]
- Min, S.; Kim, J.Y.; Cho, H.M.; Park, S.; Hwang, J.M.; You, H.; Chan Chae, Y.; Lee, W.J.; Sun, W.; Kang, D.; et al. Heat shock protein 60 couples an oxidative stress-responsive p38/MK2 signaling and NF-κB survival machinery in cancer cells. Redox Biol. 2022, 51, 102293. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Rong, J.; Lai, Y.; Tao, L.; Yuan, X.; Shu, X. The Degree of Helicobacter pylori Infection Affects the State of Macrophage Polarization through Crosstalk between ROS and HIF-1α. Oxid. Med. Cell Longev. 2020, 2020, 5281795. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Liu, P.; Xie, X. AMPK and Cancer. Exp. Suppl. 2016, 107, 203–226. [Google Scholar] [PubMed]
- Khanna-Chopra, R.; Jajoo, A.; Semwal, V.K. Chloroplasts and mitochondria have multiple heat tolerant isozymes of SOD and APX in leaf and inflorescence in Chenopodium album. Biochem. Biophys. Res. Commun. 2011, 412, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Yu, J.; Wang, H.; Yu, A.; Xiao, L.; Li, Z.; Zhang, H.; Zhang, Y.; Wu, Y. Rational design of a reversible fluorescent probe for sensing GSH in mitochondria. Anal. Chim. Acta 2022, 1220, 340081. [Google Scholar] [CrossRef]
- Mäntymaa, P.; Siitonen, T.; Guttorm, T.; Säily, M.; Kinnula, V.; Savolainen, E.R.; Koistinen, P. Induction of mitochondrial manganese superoxide dismutase confers resistance to apoptosis in acute myeloblastic leukaemia cells exposed to etoposide. Br. J. Haematol. 2000, 108, 574–581. [Google Scholar] [CrossRef]
- Ale-Agha, N.; Jakobs, P.; Goy, C.; Zurek, M.; Rosen, J.; Dyballa-Rukes, N.; Metzger, S.; Greulich, J.; von Ameln, F.; Eckermann, O.; et al. Mitochondrial Telomerase Reverse Transcriptase Protects from Myocardial Ischemia/Reperfusion Injury by Improving Complex I Composition and Function. Circulation 2021, 144, 1876–1890. [Google Scholar] [CrossRef]
- Missiroli, S.; Perrone, M.; Gafà, R.; Nicoli, F.; Bonora, M.; Morciano, G.; Boncompagni, C.; Marchi, S.; Lebiedzinska-Arciszewska, M.; Vezzani, B.; et al. PML at mitochondria-associated membranes governs a trimeric complex with NLRP3 and P2X7R that modulates the tumor immune microenvironment. Cell Death Differ. 2023, 30, 429–441. [Google Scholar] [CrossRef]
- Vaseva, A.V.; Moll, U.M. Identification of p53 in mitochondria. Methods Mol. Biol. 2013, 962, 75–84. [Google Scholar] [PubMed]
- Savu, D.I.; Moisoi, N. Mitochondria–Nucleus communication in neurodegenerative disease. Who talks first, who talks louder? Biochim. Biophys. Acta Bioenerg. 2022, 1863, 148588. [Google Scholar] [CrossRef] [PubMed]
- Correia-Melo, C.; Marques, F.D.; Anderson, R.; Hewitt, G.; Hewitt, R.; Cole, J.; Carroll, B.M.; Miwa, S.; Birch, J.; Merz, A.; et al. Mitochondria are required for pro-ageing features of the senescent phenotype. Embo J. 2016, 35, 724–742. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Breda, C.N.S.; Davanzo, G.G.; Basso, P.J.; Saraiva Câmara, N.O.; Moraes-Vieira, P.M.M. Mitochondria as central hub of the immune system. Redox Biol. 2019, 26, 101255. [Google Scholar] [CrossRef]
- Corpet, A.; Kleijwegt, C.; Roubille, S.; Juillard, F.; Jacquet, K.; Texier, P.; Lomonte, P. PML nuclear bodies and chromatin dynamics: Catch me if you can! Nucleic Acids Res. 2020, 48, 11890–11912. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Deng, Z.; Lei, C.; Ding, X.; Li, J.; Wang, C. The Role of Oxidative Stress in Tumorigenesis and Progression. Cells 2024, 13, 441. https://doi.org/10.3390/cells13050441
Li K, Deng Z, Lei C, Ding X, Li J, Wang C. The Role of Oxidative Stress in Tumorigenesis and Progression. Cells. 2024; 13(5):441. https://doi.org/10.3390/cells13050441
Chicago/Turabian StyleLi, Kexin, Zhangyuzi Deng, Chunran Lei, Xiaoqing Ding, Jing Li, and Changshan Wang. 2024. "The Role of Oxidative Stress in Tumorigenesis and Progression" Cells 13, no. 5: 441. https://doi.org/10.3390/cells13050441
APA StyleLi, K., Deng, Z., Lei, C., Ding, X., Li, J., & Wang, C. (2024). The Role of Oxidative Stress in Tumorigenesis and Progression. Cells, 13(5), 441. https://doi.org/10.3390/cells13050441







