Piezo1 and Its Function in Different Blood Cell Lineages
Abstract
:1. Overview of Piezo1 Properties
1.1. Discovery of Piezo Proteins and Structure of Piezo1
1.2. Piezo1 Expression and Cellular Distribution
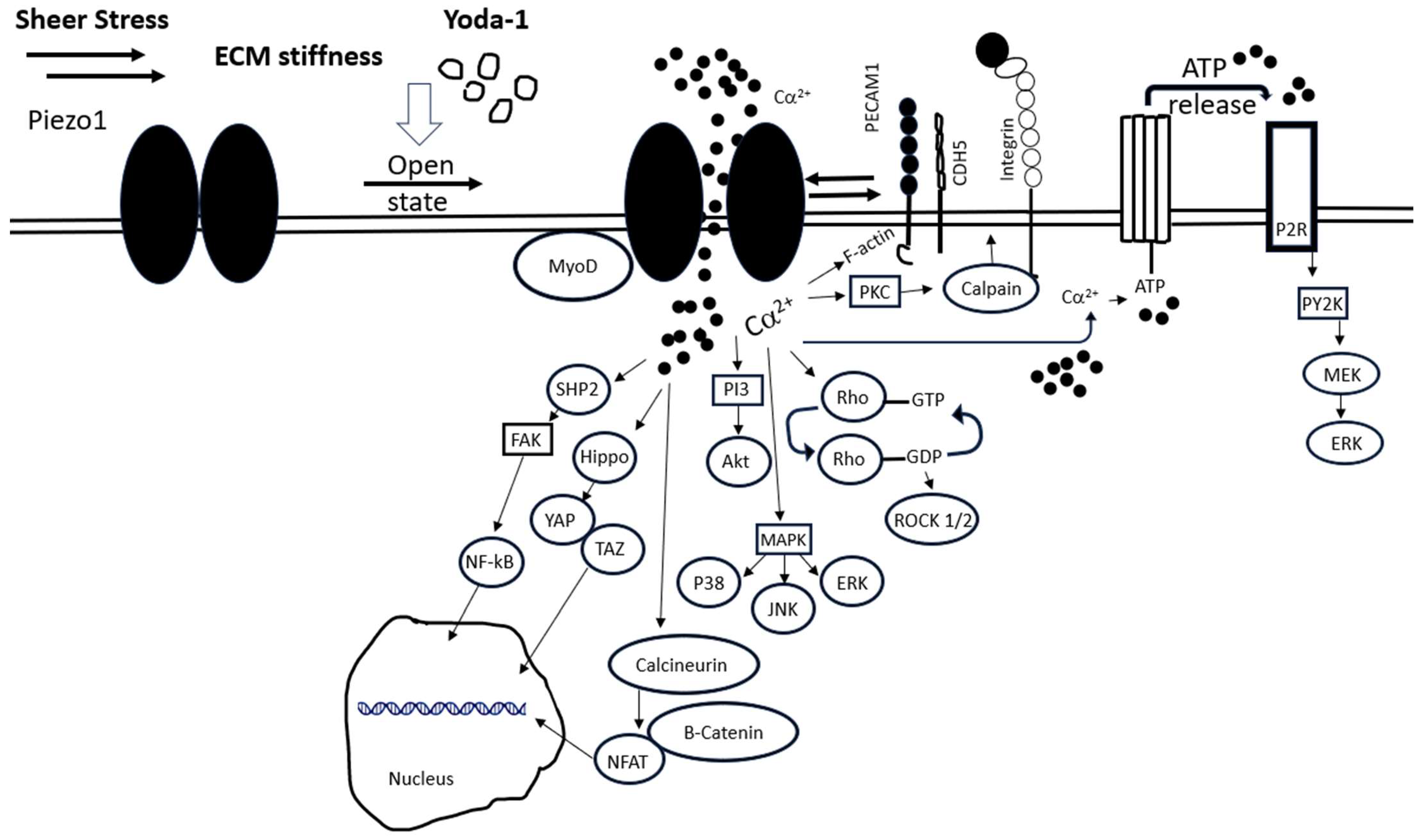
1.3. Mechanogating of Piezo1 and Lipid Interactome
1.4. Piezo1 Intracellular Signaling and Associated Proteins
1.5. Pharmacological Activators and Inhibitors of Piezo1
2. Piezo1 Function in Blood Cell Lineages
2.1. Piezo1 in Megakaryocytes and Platelets
2.2. Piezo1 and Red Blood Cells
2.3. Piezo1 and Myeloid Cells
2.4. Piezo1 and Immune Cells
3. Discussion and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iggo, A.; Andres, K.H. Morphology of cutaneous receptors. Annu. Rev. Neurosci. 1982, 5, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Neher, E.; Sakmann, B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 1976, 260, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Guharay, F.; Sachs, F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J. Physiol. 1984, 352, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Sakmann, B.; Bormann, J.; Hamill, O.P. Ion transport by single receptor channels. Cold Spring Harb. Symp. Quant. Biol. 1983, 48 Pt 1, 247–257. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Viswanath, V.; Patapoutian, A. Trp ion channels and temperature sensation. Annu. Rev. Neurosci. 2006, 29, 135–161. [Google Scholar] [CrossRef]
- Drew, L.J.; Wood, J.N.; Cesare, P. Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J. Neurosci. 2002, 22, RC228. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.; Hao, J.; Rodat-Despoix, L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat. Rev. Neurosci. 2011, 12, 139–153. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef]
- Bagriantsev, S.N.; Gracheva, E.O.; Gallagher, P.G. Piezo proteins: Regulators of mechanosensation and other cellular processes. J. Biol. Chem. 2014, 289, 31673–31681. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.H.; Grandl, J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. eLife 2015, 4, e12088. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.E.; Dubin, A.E.; Patapoutian, A. Piezos thrive under pressure: Mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.Z.; Marshall, K.L.; Min, S.; Daou, I.; Chapleau, M.W.; Abboud, F.M.; Liberles, S.D.; Patapoutian, A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 2018, 362, 464–467. [Google Scholar] [CrossRef]
- Xiao, B. Levering Mechanically Activated Piezo Channels for Potential Pharmacological Intervention. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Szczot, M.; Pogorzala, L.A.; Solinski, H.J.; Young, L.; Yee, P.; Le Pichon, C.E.; Chesler, A.T.; Hoon, M.A. Cell-Type-Specific Splicing of Piezo2 Regulates Mechanotransduction. Cell Rep. 2017, 21, 2760–2771. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Murthy, S.E.; Mathur, J.; Schmidt, M.; Mechioukhi, Y.; Delmas, P.; Patapoutian, A. Piezo1 ion channel pore properties are dictated by C-terminal region. Nat. Commun. 2015, 6, 7223. [Google Scholar] [CrossRef]
- Ge, J.; Li, W.; Zhao, Q.; Li, N.; Chen, M.; Zhi, P.; Li, R.; Gao, N.; Xiao, B.; Yang, M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 2015, 527, 64–69. [Google Scholar] [CrossRef]
- Saotome, K.; Murthy, S.E.; Kefauver, J.M.; Whitwam, T.; Patapoutian, A.; Ward, A.B. Structure of the mechanically activated ion channel Piezo1. Nature 2018, 554, 481–486. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, H.; Chi, S.; Wang, Y.; Wang, J.; Geng, J.; Wu, K.; Liu, W.; Zhang, T.; Dong, M.Q.; et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature 2018, 554, 487–492. [Google Scholar] [CrossRef]
- Guo, Y.R.; MacKinnon, R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. eLife 2017, 6, e33660. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chi, S.; Guo, H.; Li, G.; Wang, L.; Zhao, Q.; Rao, Y.; Zu, L.; He, W.; Xiao, B. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat. Commun. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, H.; Li, X.; Xiao, B. The mechanosensitive Piezo1 channel: A three-bladed propeller-like structure and a lever-like mechanogating mechanism. FEBS J. 2019, 286, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wanggou, S.; Bodalia, A.; Zhu, M.; Dong, W.; Fan, J.J.; Yin, W.C.; Min, H.K.; Hu, M.; Draghici, D.; et al. A Feedforward Mechanism Mediated by Mechanosensitive Ion Channel PIEZO1 and Tissue Mechanics Promotes Glioma Aggression. Neuron 2018, 100, 799–815.e7. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.R.; Zeng, W.Z.; Evans, E.L.; Woo, S.H.; Ma, S.; Abuwarda, H.; Loud, M.; Patapoutian, A.; Pathak, M.M. Spatiotemporal dynamics of PIEZO1 localization controls keratinocyte migration during wound healing. eLife 2021, 10, e65415. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Garcia, J.; Herrera-Fernandez, V.; Serra, S.A.; Rubio-Moscardo, F.; Vogel-Gonzalez, M.; Donate-Macian, P.; Hevia, C.F.; Pujades, C.; Valverde, M.A. The mechanosensitive Piezo1 channel controls endosome trafficking for an efficient cytokinetic abscission. Sci. Adv. 2021, 7, eabi7785. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yin, K.; Wu, K.; Zhang, M.; Wang, S.; Cheng, H.; Zhou, Z.; Xiao, B. The mechanosensitive Piezo1 channel mediates heart mechano-chemo transduction. Nat. Commun. 2021, 12, 869. [Google Scholar] [CrossRef]
- Vaisey, G.; Banerjee, P.; North, A.J.; Haselwandter, C.A.; Mackinnon, R. Piezo1 as a force-through-membrane sensor in red blood cells. eLife 2022, 11, e82621. [Google Scholar] [CrossRef]
- Yang, S.; Miao, X.; Arnold, S.; Li, B.; Ly, A.T.; Wang, H.; Wang, M.; Guo, X.; Pathak, M.M.; Zhao, W.; et al. Membrane curvature governs the distribution of Piezo1 in live cells. Nat. Commun. 2022, 13, 7467. [Google Scholar] [CrossRef]
- Lin, Y.C.; Guo, Y.R.; Miyagi, A.; Levring, J.; MacKinnon, R.; Scheuring, S. Force-induced conformational changes in PIEZO1. Nature 2019, 573, 230–234. [Google Scholar] [CrossRef]
- Yang, X.; Lin, C.; Chen, X.; Li, S.; Li, X.; Xiao, B. Structure deformation and curvature sensing of PIEZO1 in lipid membranes. Nature 2022, 604, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, A.C.; Stommen, A.; Koehler, M.; Cloos, A.S.; Yang, J.; Leclercqz, A.; Tyteca, D.; Alsteens, D. Probing PIEZO1 Localization upon Activation Using High-Resolution Atomic Force and Confocal Microscopy. Nano Lett. 2021, 21, 4950–4958. [Google Scholar] [CrossRef]
- David, L.; Martinez, L.; Xi, Q.; Kooshesh, K.A.; Zhang, Y.; Shah, J.V.; Maas, R.L.; Wu, H. Piezo mechanosensory channels regulate centrosome integrity and mitotic entry. Proc. Natl. Acad. Sci. USA 2023, 120, e2213846120. [Google Scholar] [CrossRef]
- Chen, J.; Miao, J.; Zhou, D.; Liao, J.; Wang, Z.; Lin, Z.; Zhang, C.; Luo, X.; Li, Y.; Li, X.; et al. Upregulation of mechanosensitive channel Piezo1 involved in high shear stress-induced pulmonary hypertension. Thromb. Res. 2022, 218, 52–63. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Babicheva, A.; Jain, P.P.; Rodriguez, M.; Ayon, R.J.; Ravellette, K.S.; Wu, L.; Balistrieri, F.; Tang, H.; et al. Endothelial upregulation of mechanosensitive channel Piezo1 in pulmonary hypertension. Am. J. Physiol. Cell Physiol. 2021, 321, C1010–C1027. [Google Scholar] [CrossRef] [PubMed]
- Iring, A.; Jin, Y.J.; Albarran-Juarez, J.; Siragusa, M.; Wang, S.; Dancs, P.T.; Nakayama, A.; Tonack, S.; Chen, M.; Kunne, C.; et al. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Investig. 2019, 129, 2775–2791. [Google Scholar] [CrossRef] [PubMed]
- Ilkan, Z.; Wright, J.R.; Goodall, A.H.; Gibbins, J.M.; Jones, C.I.; Mahaut-Smith, M.P.; Colbran, R.J. Evidence for shear-mediated Ca2+ entry through mechanosensitive cation channels in human platelets and a megakaryocytic cell line. J. Biol. Chem. 2017, 292, 9204–9217. [Google Scholar] [CrossRef]
- Abbonante, V.; Karkempetzaki, A.I.; Leon, C.; Krishnan, A.; Huang, N.; Di Buduo, C.A.; Cattaneo, D.; Ward, C.M.; Matsuura, S.; Guinard, I.; et al. Newly identified roles for PIEZO1 mechanosensor in controlling normal megakaryocyte development and in primary myelofibrosis. Am. J. Hematol. 2024, 99, 336–349. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, Y.; Liang, B.; Xu, J.; Lin, Y.; Zhou, N.; Li, J.; Jiang, B.; Cheng, J.; Li, C.; et al. Mechanosensitive Piezo1 channels mediate renal fibrosis. JCI Insight 2022, 7, e152330. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Q.; Zhu, H.; Hou, L.; Liu, W.; Yang, Q.; Shen, H.; Chai, G.; Zhang, B.; Chen, S.; et al. Microglial Piezo1 senses Abeta fibril stiffness to restrict Alzheimer’s disease. Neuron 2023, 111, 15–29.e8. [Google Scholar] [CrossRef]
- Velasco-Estevez, M.; Rolle, S.O.; Mampay, M.; Dev, K.K.; Sheridan, G.K. Piezo1 regulates calcium oscillations and cytokine release from astrocytes. Glia 2020, 68, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.; Blaquiere, M.; Janvier, A.; Cresto, N.; Lana, C.; Genin, A.; Hirbec, H.; Audinat, E.; Faucherre, A.; Barbier, E.L.; et al. PIEZO1 expression at the glio-vascular unit adjusts to neuroinflammation in seizure conditions. Neurobiol. Dis. 2023, 187, 106297. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Duan, Y.W.; Zhou, Y.H.; Chen, S.X.; Li, Y.Y.; Zang, Y. NLRP3-Mediated Piezo1 Upregulation in ACC Inhibitory Parvalbumin-Expressing Interneurons Is Involved in Pain Processing after Peripheral Nerve Injury. Int. J. Mol. Sci. 2022, 23, 13035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hou, L.; Li, F.; Zhang, W.; Wu, C.; Xiang, L.; Li, J.; Zhou, L.; Wang, X.; Xiang, Y.; et al. Piezo1-mediated mechanosensation in bone marrow macrophages promotes vascular niche regeneration after irradiation injury. Theranostics 2022, 12, 1621–1638. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Ren, X.; Zhang, Y.; Jiang, F.; Zhou, P. Trimethylamine-N-oxide sensitizes chondrocytes to mechanical loading through the upregulation of Piezo1. Food Chem. Toxicol. 2023, 175, 113726. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.O.; Massey, A.E.; Mata-Daboin, A.D.; Sierra-Valdez, F.J.; Chauhan, S.C.; Cordero-Morales, J.F.; Vasquez, V. Dietary fatty acids fine-tune Piezo1 mechanical response. Nat. Commun. 2019, 10, 1200. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.D.; Bae, C.; Ziegler, L.; Hartley, S.; Nikolova-Krstevski, V.; Rohde, P.R.; Ng, C.A.; Sachs, F.; Gottlieb, P.A.; Martinac, B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat. Commun. 2016, 7, 10366. [Google Scholar] [CrossRef]
- Syeda, R.; Florendo, M.N.; Cox, C.D.; Kefauver, J.M.; Santos, J.S.; Martinac, B.; Patapoutian, A. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016, 17, 1739–1746. [Google Scholar] [CrossRef]
- Corradi, V.; Mendez-Villuendas, E.; Ingólfsson, H.I.; Gu, R.-X.; Siuda, I.; Melo, M.N.; Moussatova, A.; DeGagné, L.J.; Sejdiu, B.I.; Singh, G.; et al. Lipid–Protein Interactions Are Unique Fingerprints for Membrane Proteins. ACS Cent. Sci. 2018, 4, 709–717. [Google Scholar] [CrossRef]
- Lin, Y.; Buyan, A.; Corry, B. Computational studies of Piezo1 yield insights into key lipid–protein interactions, channel activation, and agonist binding. Biophys. Rev. 2022, 14, 209–219. [Google Scholar] [CrossRef]
- Borbiro, I.; Badheka, D.; Rohacs, T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci. Signal. 2015, 8, ra15. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Morales, J.F.; Vasquez, V. How lipids contribute to ion channel function, a fat perspective on direct and indirect interactions. Curr. Opin. Struct. Biol. 2018, 51, 92–98. [Google Scholar] [CrossRef]
- Weinrich, M.; Worcester, D.L.; Bezrukov, S.M. Lipid nanodomains change ion channel function. Nanoscale 2017, 9, 13291–13297. [Google Scholar] [CrossRef] [PubMed]
- Buyan, A.; Cox, C.D.; Barnoud, J.; Li, J.; Chan, H.S.M.; Martinac, B.; Marrink, S.J.; Corry, B. Piezo1 Forms Specific, Functionally Important Interactions with Phosphoinositides and Cholesterol. Biophys. J. 2020, 119, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; De Vecchis, D.; Hyman, A.J.; Povstyan, O.V.; Ludlow, M.J.; Shi, J.; Beech, D.J.; Kalli, A.C. Modeling of full-length Piezo1 suggests importance of the proximal N-terminus for dome structure. Biophys. J. 2021, 120, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Chidlow, J.H., Jr.; Sessa, W.C. Caveolae, caveolins, and cavins: Complex control of cellular signalling and inflammation. Cardiovasc. Res. 2010, 86, 219–225. [Google Scholar] [CrossRef]
- Peter, B.J.; Kent, H.M.; Mills, I.G.; Vallis, Y.; Butler, P.J.; Evans, P.R.; McMahon, H.T. BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science 2004, 303, 495–499. [Google Scholar] [CrossRef]
- Kung, C. A possible unifying principle for mechanosensation. Nature 2005, 436, 647–654. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, L.E.; Kittelmann, M.; Li, J.; Petkovic, M.; Cheng, T.; Jin, P.; Guo, Z.; Gopfert, M.C.; Jan, L.Y.; et al. Ankyrin Repeats Convey Force to Gate the NOMPC Mechanotransduction Channel. Cell 2015, 162, 1391–1403. [Google Scholar] [CrossRef]
- Lewis, A.H.; Grandl, J. Piezo1 ion channels inherently function as independent mechanotransducers. eLife 2021, 10, e70988. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Shi, L.Z.; Yoon, C.W.; Preece, D.; Gomez-Godinez, V.; Lu, S.; Carmona, C.; Woo, S.H.; Chien, S.; Berns, M.W.; et al. Mechanosensor Piezo1 mediates bimodal patterns of intracellular calcium and FAK signaling. EMBO J. 2022, 41, e111799. [Google Scholar] [CrossRef] [PubMed]
- Albarran-Juarez, J.; Iring, A.; Wang, S.; Joseph, S.; Grimm, M.; Strilic, B.; Wettschureck, N.; Althoff, T.F.; Offermanns, S. Piezo1 and G(q)/G(11) promote endothelial inflammation depending on flow pattern and integrin activation. J. Exp. Med. 2018, 215, 2655–2672. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Song, J.; Xu, Y.; Liu, C.; Qian, W.; Bai, T.; Hou, X. Piezo1 regulates intestinal epithelial function by affecting the tight junction protein claudin-1 via the ROCK pathway. Life Sci. 2021, 275, 119254. [Google Scholar] [CrossRef] [PubMed]
- Blythe, N.M.; Muraki, K.; Ludlow, M.J.; Stylianidis, V.; Gilbert, H.T.J.; Evans, E.L.; Cuthbertson, K.; Foster, R.; Swift, J.; Li, J.; et al. Mechanically activated Piezo1 channels of cardiac fibroblasts stimulate p38 mitogen-activated protein kinase activity and interleukin-6 secretion. J. Biol. Chem. 2019, 294, 17395–17408. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-Y.; Gong, H.; Kesteven, S.; Guo, Y.; Wu, J.; Li, J.V.; Cheng, D.; Zhou, Z.; Iismaa, S.E.; Kaidonis, X.; et al. Piezo1 is the cardiac mechanosensor that initiates the cardiomyocyte hypertrophic response to pressure overload in adult mice. Nat. Cardiovasc. Res. 2022, 1, 577–591. [Google Scholar] [CrossRef]
- Mousawi, F.; Peng, H.; Li, J.; Ponnambalam, S.; Roger, S.; Zhao, H.; Yang, X.; Jiang, L.H. Chemical activation of the Piezo1 channel drives mesenchymal stem cell migration via inducing ATP release and activation of P2 receptor purinergic signaling. Stem Cells 2020, 38, 410–421. [Google Scholar] [CrossRef]
- Pathak, M.M.; Nourse, J.L.; Tran, T.; Hwe, J.; Arulmoli, J.; Le, D.T.; Bernardis, E.; Flanagan, L.A.; Tombola, F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 16148–16153. [Google Scholar] [CrossRef]
- Hasegawa, K.; Fujii, S.; Matsumoto, S.; Tajiri, Y.; Kikuchi, A.; Kiyoshima, T. YAP signaling induces PIEZO1 to promote oral squamous cell carcinoma cell proliferation. J. Pathol. 2021, 253, 80–93. [Google Scholar] [CrossRef]
- Liu, S.; Xu, X.; Fang, Z.; Ning, Y.; Deng, B.; Pan, X.; He, Y.; Yang, Z.; Huang, K.; Li, J. Piezo1 impairs hepatocellular tumor growth via deregulation of the MAPK-mediated YAP signaling pathway. Cell Calcium 2021, 95, 102367. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, B.; Fan, Y.; Liu, Y.; Feng, S.; Cong, Q.; Zhang, X.; Zhou, Y.; Yadav, P.S.; Lin, J.; et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ss-catenin. eLife 2020, 9, e52779. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zou, Y.; Teng, P.; Chen, Z.; Wu, Y.; Dai, X.; Li, X.; Hu, Z.; Wu, S.; Xu, Y.; et al. Mechanosensitive Channel PIEZO1 Senses Shear Force to Induce KLF2/4 Expression via CaMKII/MEKK3/ERK5 Axis in Endothelial Cells. Cells 2022, 11, 2191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ma, X.; Lin, Y.; Cheng, D.; Bavi, N.; Secker, G.A.; Li, J.V.; Janbandhu, V.; Sutton, D.L.; Scott, H.S.; et al. MyoD-family inhibitor proteins act as auxiliary subunits of Piezo channels. Science 2023, 381, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Aglialoro, F.; Hofsink, N.; Hofman, M.; Brandhorst, N.; van den Akker, E. Inside Out Integrin Activation Mediated by PIEZO1 Signaling in Erythroblasts. Front. Physiol. 2020, 11, 958. [Google Scholar] [CrossRef] [PubMed]
- Chuntharpursat-Bon, E.; Povstyan, O.V.; Ludlow, M.J.; Carrier, D.J.; Debant, M.; Shi, J.; Gaunt, H.J.; Bauer, C.C.; Curd, A.; Simon Futers, T.; et al. PIEZO1 and PECAM1 interact at cell-cell junctions and partner in endothelial force sensing. Commun. Biol. 2023, 6, 358. [Google Scholar] [CrossRef]
- McHugh, B.J.; Buttery, R.; Lad, Y.; Banks, S.; Haslett, C.; Sethi, T. Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J. Cell Sci. 2010, 123, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Tijore, A.; Cheng, D.; Li, J.V.; Hariharan, A.; Martinac, B.; Tran Van Nhieu, G.; Cox, C.D.; Sheetz, M. Force- and cell state-dependent recruitment of Piezo1 drives focal adhesion dynamics and calcium entry. Sci. Adv. 2022, 8, eabo1461. [Google Scholar] [CrossRef]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical activation of the mechanotransduction channel Piezo1. eLife 2015, 4, e07369. [Google Scholar] [CrossRef]
- Parsonage, G.; Cuthbertson, K.; Endesh, N.; Murciano, N.; Hyman, A.J.; Revill, C.H.; Povstyan, O.V.; Chuntharpursat-Bon, E.; Debant, M.; Ludlow, M.J.; et al. Improved PIEZO1 agonism through 4-benzoic acid modification of Yoda1. Br. J. Pharmacol. 2023, 180, 2039–2063. [Google Scholar] [CrossRef]
- Evans, E.L.; Cuthbertson, K.; Endesh, N.; Rode, B.; Blythe, N.M.; Hyman, A.J.; Hall, S.J.; Gaunt, H.J.; Ludlow, M.J.; Foster, R.; et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br. J. Pharmacol. 2018, 175, 1744–1759. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Ghatak, C.; Yasmann, A.; Nishizawa, K.; Sachs, F.; Ladokhin, A.S.; Sukharev, S.I.; Suchyna, T.M. GsMTx4: Mechanism of Inhibiting Mechanosensitive Ion Channels. Biophys. J. 2017, 112, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wei, Z.; Xin, G.; Li, Y.; Yuan, J.; Ming, Y.; Ji, C.; Sun, Q.; Li, S.; Chen, X.; et al. Piezo1 initiates platelet hyperreactivity and accelerates thrombosis in hypertension. J. Thromb. Haemost. 2021, 19, 3113–3125. [Google Scholar] [CrossRef] [PubMed]
- Zarychanski, R.; Schulz, V.P.; Houston, B.L.; Maksimova, Y.; Houston, D.S.; Smith, B.; Rinehart, J.; Gallagher, P.G. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood 2012, 120, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Cahalan, S.M.; Lukacs, V.; Ranade, S.S.; Chien, S.; Bandell, M.; Patapoutian, A. Piezo1 links mechanical forces to red blood cell volume. eLife 2015, 4, e07370. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Zhang, Y.; Wan, Y.C.S.; Ma, S.; Dong, P.; Lowry, A.J.; Francis, S.J.; Khandelwal, S.; Delahunty, M.; Telen, M.J.; et al. Deciphering and disrupting PIEZO1-TMEM16F interplay in hereditary xerocytosis. Blood 2024, 143, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, C. Sickle cell dehydration: Pathophysiology and therapeutic applications. Clin. Hemorheol. Microcirc. 2018, 68, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Dubin, A.E.; Zhang, Y.; Mousavi, S.A.R.; Wang, Y.; Coombs, A.M.; Loud, M.; Andolfo, I.; Patapoutian, A. A role of PIEZO1 in iron metabolism in mice and humans. Cell 2021, 184, 969–982.e13. [Google Scholar] [CrossRef]
- Cinar, E.; Zhou, S.; DeCourcey, J.; Wang, Y.; Waugh, R.E.; Wan, J. Piezo1 regulates mechanotransductive release of ATP from human RBCs. Proc. Natl. Acad. Sci. USA 2015, 112, 11783–11788. [Google Scholar] [CrossRef]
- Rogers, S.; Lew, V.L. Up-down biphasic volume response of human red blood cells to PIEZO1 activation during capillary transits. PLoS Comput. Biol. 2021, 17, e1008706. [Google Scholar] [CrossRef]
- Rogers, S.; Lew, V.L. PIEZO1 and the mechanism of the long circulatory longevity of human red blood cells. PLoS Comput. Biol. 2021, 17, e1008496. [Google Scholar] [CrossRef]
- Karamatic Crew, V.; Tilley, L.A.; Satchwell, T.J.; AlSubhi, S.A.; Jones, B.; Spring, F.A.; Walser, P.J.; Martins Freire, C.; Murciano, N.; Rotordam, M.G.; et al. Missense mutations in PIEZO1, which encodes the Piezo1 mechanosensor protein, define Er red blood cell antigens. Blood 2023, 141, 135–146. [Google Scholar] [CrossRef]
- Kawamoto, H.; Minato, N. Myeloid cells. Int. J. Biochem. Cell Biol. 2004, 36, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Zhang, X.; Wang, S.; Qin, J.; Liu, Q.; Liu, A.; Sheng, Z.; Feng, Q.; Hu, X.; Peng, J. Ion channel Piezo1 activation promotes aerobic glycolysis in macrophages. Front. Immunol. 2022, 13, 976482. [Google Scholar] [CrossRef] [PubMed]
- Atcha, H.; Jairaman, A.; Holt, J.R.; Meli, V.S.; Nagalla, R.R.; Veerasubramanian, P.K.; Brumm, K.T.; Lim, H.E.; Othy, S.; Cahalan, M.D.; et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 2021, 12, 3256. [Google Scholar] [CrossRef]
- Atcha, H.; Meli, V.S.; Davis, C.T.; Brumm, K.T.; Anis, S.; Chin, J.; Jiang, K.; Pathak, M.M.; Liu, W.F. Crosstalk Between CD11b and Piezo1 Mediates Macrophage Responses to Mechanical Cues. Front. Immunol. 2021, 12, 689397. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Shi, Y.; Zhang, J.; Yang, B.; Wang, P.; Yuan, W.; Zhao, H.; Li, J.; Qin, F.; Hong, L.; et al. TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection. Nat. Commun. 2021, 12, 3519. [Google Scholar] [CrossRef]
- Solis, A.G.; Bielecki, P.; Steach, H.R.; Sharma, L.; Harman, C.C.D.; Yun, S.; de Zoete, M.R.; Warnock, J.N.; To, S.D.F.; York, A.G.; et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 2019, 573, 69–74. [Google Scholar] [CrossRef]
- Aykut, B.A.-O.; Chen, R.A.-O.; Kim, J.I.; Wu, D.; Shadaloey, S.A.A.; Abengozar, R.; Preiss, P.; Saxena, A.A.-O.; Pushalkar, S.A.-O.; Leinwand, J.; et al. Targeting Piezo1 unleashes innate immunity against cancer and infectious disease. Sci. Immunol. 2020, 5, eabb5168. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Jia, A.; Wang, Y.; Yang, Q.; Dong, Y.; Hou, Y.; Cao, Y.; Dong, L.; Bi, Y.; et al. Dendritic cell Piezo1 directs the differentiation of T(H)1 and T(reg) cells in cancer. eLife 2022, 11, e79957. [Google Scholar] [CrossRef]
- Chakraborty, M.; Chu, K.; Shrestha, A.; Revelo, X.S.; Zhang, X.; Gold, M.J.; Khan, S.; Lee, M.; Huang, C.; Akbari, M.; et al. Mechanical Stiffness Controls Dendritic Cell Metabolism and Function. Cell Rep. 2021, 34, 108609. [Google Scholar] [CrossRef]
- Liu, C.S.C.; Raychaudhuri, D.; Paul, B.; Chakrabarty, Y.; Ghosh, A.R.; Rahaman, O.; Talukdar, A.; Ganguly, D. Cutting Edge: Piezo1 Mechanosensors Optimize Human T Cell Activation. J. Immunol. 2018, 200, 1255–1260. [Google Scholar] [CrossRef]
- Tabdanov, E.; Gondarenko, S.; Kumari, S.; Liapis, A.; Dustin, M.L.; Sheetz, M.P.; Kam, L.C.; Iskratsch, T. Micropatterning of TCR and LFA-1 ligands reveals complementary effects on cytoskeleton mechanics in T cells. Integr. Biol. 2015, 7, 1272–1284. [Google Scholar] [CrossRef]
- Pan, Y.; Yoon, S.; Sun, J.; Huang, Z.; Lee, C.; Allen, M.; Wu, Y.; Chang, Y.J.; Sadelain, M.; Shung, K.K.; et al. Mechanogenetics for the remote and noninvasive control of cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2018, 115, 992–997. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.A.-O.; Quinn, T.A.-O.; Scandiuzzi, L.; Basu, I.A.-O.; Partanen, A.; Tomé, W.A.; Macian, F.; Guha, C. Low-Intensity Focused Ultrasound Induces Reversal of Tumor-Induced T Cell Tolerance and Prevents Immune Escape. J. Immunol. 2016, 196, 1964–1976. [Google Scholar] [CrossRef]
- Jairaman, A.; Othy, S.; Dynes, J.L.; Yeromin, A.V.; Zavala, A.; Greenberg, M.L.; Nourse, J.L.; Holt, J.R.; Cahalan, S.M.; Marangoni, F.; et al. Piezo1 channels restrain regulatory T cells but are dispensable for effector CD4(+) T cell responses. Sci. Adv. 2021, 7, eabg5859. [Google Scholar] [CrossRef] [PubMed]
| Cell Type | Piezo1 Function | References |
|---|---|---|
| Megakaryocytes (MKs) | MK development | [38] |
| MK shear stress responses | [37] | |
| Platelets | Platelet biogenesis | [38] |
| Platelet activation | [37,82] | |
| Platelet shear stress responses | [37] | |
| Red blood cells (RBCs) | RBC volume regulation | [83,84] |
| Iron metabolism | [87] | |
| Erythropoiesis | [74] | |
| Er antigen expression | [91] | |
| RBC ATP release | [88] | |
| RBC circulatory aging homeostasis | [89,90] | |
| Macrophages | Metabolic switch to anaerobic glycolysis/inflammatory responses | [93] |
| Macrophage activation/phagocytosis (TLR-4 co-receptor) | [95,96] | |
| Dendritic cells (DCs) | DC-mediated differentiation of TH1 and Treg cells | [99] |
| DC function (Ag presentation) and metabolism control | [100] | |
| T cells | Stabilization of the immunological synapse (TCR-APC cell) | [101] |
| T-cell activation in cancer and autoimmunity | [104,105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karkempetzaki, A.I.; Ravid, K. Piezo1 and Its Function in Different Blood Cell Lineages. Cells 2024, 13, 482. https://doi.org/10.3390/cells13060482
Karkempetzaki AI, Ravid K. Piezo1 and Its Function in Different Blood Cell Lineages. Cells. 2024; 13(6):482. https://doi.org/10.3390/cells13060482
Chicago/Turabian StyleKarkempetzaki, Anastasia Iris, and Katya Ravid. 2024. "Piezo1 and Its Function in Different Blood Cell Lineages" Cells 13, no. 6: 482. https://doi.org/10.3390/cells13060482




