HDAC6 Enhances Endoglin Expression through Deacetylation of Transcription Factor SP1, Potentiating BMP9-Induced Angiogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Plasmid Construction
2.3. Lentivirus-Mediated Gene Knockdown and Overexpression
2.4. CRISPR-Cas9-Based HDAC6 Knockout in 293T Cells
2.5. Wound Healing Assay
2.6. Tube Formation Assay
2.7. The Transwell Assay
2.8. Immunoprecipitation
2.9. The Dual-Luciferase Reporter Assay
2.10. Immunofluorescence Staining
2.11. SDS-PAGE and Western Blot
2.12. High-Throughput Transcriptomic Analysis
2.13. Reverse Transcription and Real-Time PCR
2.14. Tandem Mass Tags (TMT) Labeling Proteomic Analysis
2.15. Data Visualization and Statistical Analysis
3. Results
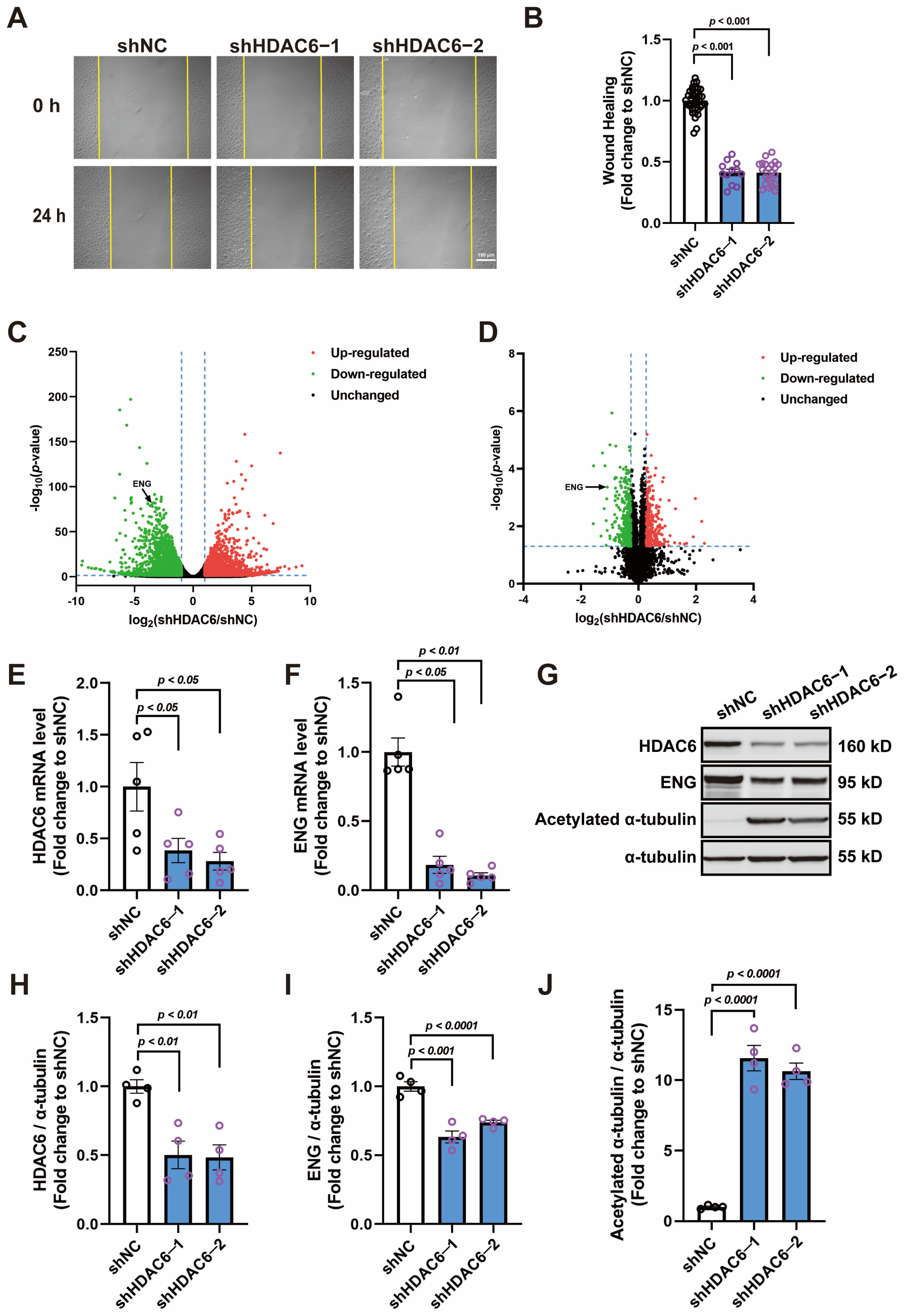
3.1. Knocking down HDAC6 Inhibits the Migration of HUVECs and Reduces ENG Expression
3.2. BMP9 Promotes HUVECs Wound Healing and SMAD1/5/9 Phosphorylation in a Dose-Dependent Manner
3.3. HDAC6 Mediates BMP9 Effects in Promoting Migration and Tube Formation of HUVECs
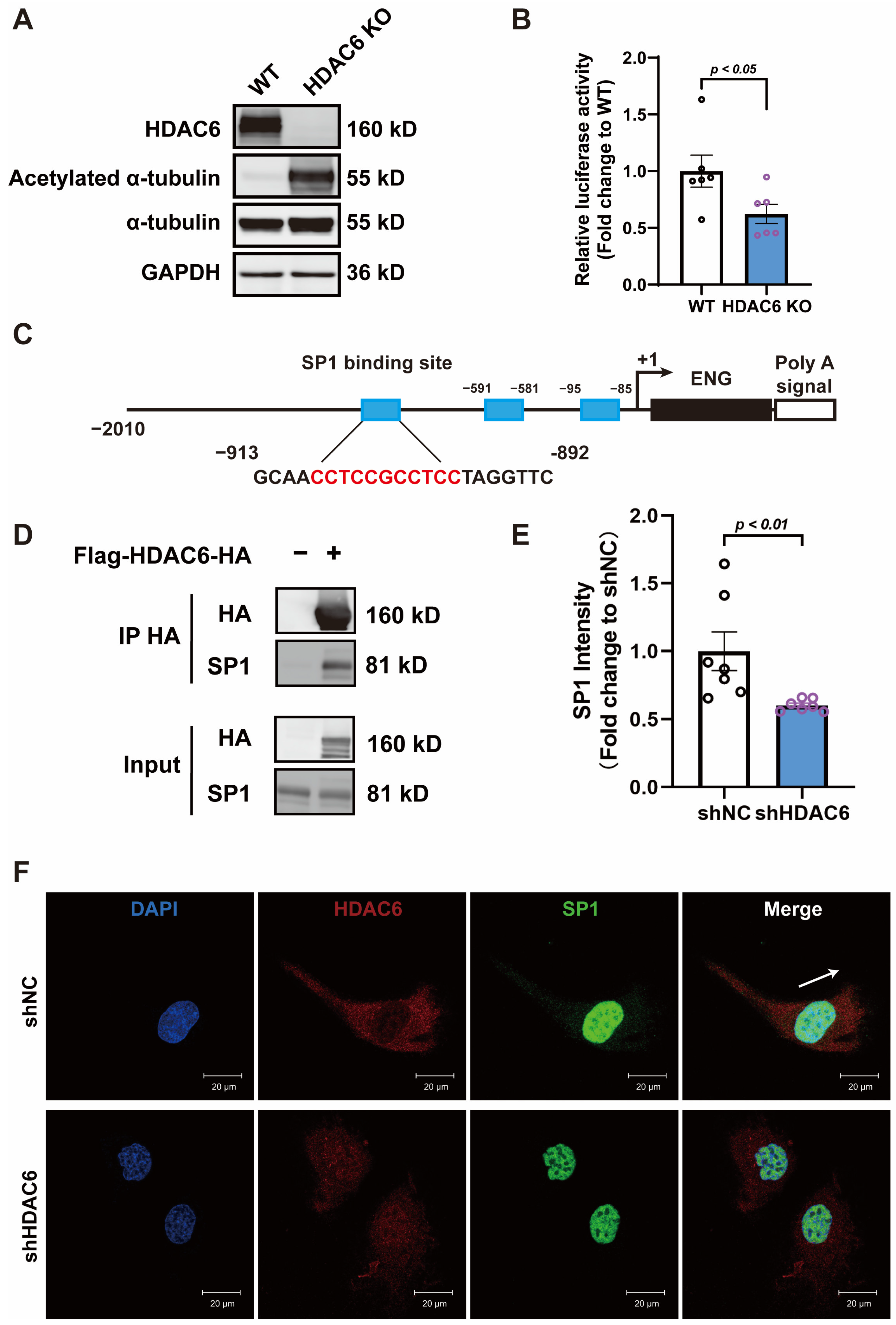
3.4. HDAC6 Regulates ENG Promoter Activity by Interacting with Transcription Factor SP1
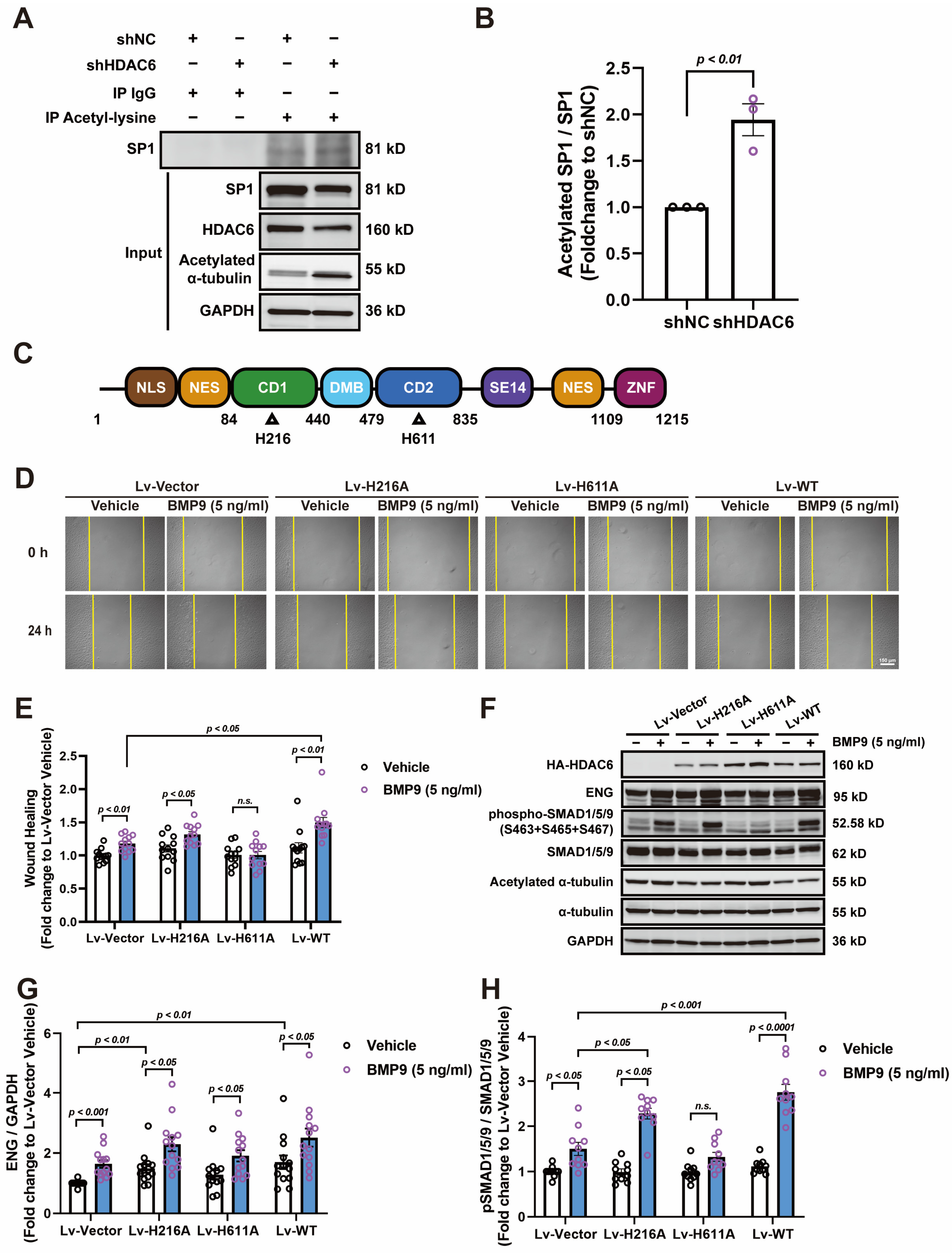
3.5. HDAC6 CD2 Deacetylates SP1 and Regulates ENG-Mediated Angiogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shvedunova, M.; Akhtar, A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2022, 23, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, P.; Tang, F.; Lian, H.; Chen, X.; Zhang, Y.; He, X.; Liu, W.; Xie, C. HDAC6 promotes cell proliferation and confers resistance to temozolomide in glioblastoma. Cancer Lett. 2016, 379, 134–142. [Google Scholar] [CrossRef]
- Valenzuela-Fernandez, A.; Cabrero, J.R.; Serrador, J.M.; Sanchez-Madrid, F. HDAC6: A key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 2008, 18, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, D.; Kroll, J.; Gesierich, S.; Yao, T.P.; Boon, R.A.; Hergenreider, E.; Tjwa, M.; Rossig, L.; Seto, E.; Augustin, H.G.; et al. Class IIb HDAC6 regulates endothelial cell migration and angiogenesis by deacetylation of cortactin. EMBO J. 2011, 30, 4142–4156. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, C.; Yu, L.; Kong, D.; Ma, W.; Lv, B.; Wang, Y.; Wu, W.; Zhou, M.; Cui, G. Upregulation of MDH1 acetylation by HDAC6 inhibition protects against oxidative stress-derived neuronal apoptosis following intracerebral hemorrhage. Cell Mol. Life Sci. 2022, 79, 356. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Zhang, Y.; Matthias, P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Gene Dev. 2007, 21, 3381–3394. [Google Scholar] [CrossRef]
- Gao, Y.S.; Hubbert, C.C.; Lu, J.; Lee, Y.S.; Lee, J.Y.; Yao, T.P. Histone deacetylase 6 regulates growth factor-induced actin remodeling and endocytosis. Mol. Cell Biol. 2007, 27, 8637–8647. [Google Scholar] [CrossRef]
- Deribe, Y.L.; Wild, P.; Chandrashaker, A.; Curak, J.; Schmidt, M.; Kalaidzidis, Y.; Milutinovic, N.; Kratchmarova, I.; Buerkle, L.; Fetchko, M.J.; et al. Regulation of epidermal growth factor receptor trafficking by lysine deacetylase HDAC6. Sci. Signal. 2009, 2, ra84. [Google Scholar] [CrossRef]
- Hai, Y.; Christianson, D.W. Histone deacetylase 6 structure and molecular basis of catalysis and inhibition. Nat. Chem. Biol. 2016, 12, 741–747. [Google Scholar] [CrossRef]
- Miyake, Y.; Keusch, J.J.; Wang, L.; Saito, M.; Hess, D.; Wang, X.; Melancon, B.J.; Helquist, P.; Gut, H.; Matthias, P. Structural insights into HDAC6 tubulin deacetylation and its selective inhibition. Nat. Chem. Biol. 2016, 12, 748–754. [Google Scholar] [CrossRef]
- Saito, M.; Hess, D.; Eglinger, J.; Fritsch, A.W.; Kreysing, M.; Weinert, B.T.; Choudhary, C.; Matthias, P. Acetylation of intrinsically disordered regions regulates phase separation. Nat. Chem. Biol. 2019, 15, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.J. Cortactin signalling and dynamic actin networks. Biochem. J. 2004, 382, 13–25. [Google Scholar] [CrossRef]
- Boyault, C.; Zhang, Y.; Fritah, S.; Caron, C.; Gilquin, B.; Kwon, S.H.; Garrido, C.; Yao, T.P.; Vourc’H, C.; Matthias, P.; et al. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Gene Dev. 2007, 21, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Moreira, E.A.; Kempf, G.; Miyake, Y.; Oliveira, E.B.; Fahmi, A.; Schaefer, J.V.; Dreier, B.; Yamauchi, Y.; Alves, M.P.; et al. Disrupting the HDAC6-ubiquitin interaction impairs infection by influenza and Zika virus and cellular stress pathways. Cell Rep. 2022, 39, 110736. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Feng, M.; Wang, B.; Zheng, Y.; Jiang, D.; Zhao, L.; Mamun, M.; Kang, H.; Nie, H.; Zhang, X.; et al. New insights into the non-enzymatic function of HDAC6. Biomed. Pharmacother. 2023, 161, 114438. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Li, H.; Hu, H.; Li, Y.; Wang, T. The role of HDAC6 in autophagy and NLRP3 inflammasome. Front. Immunol. 2021, 12, 763831. [Google Scholar] [CrossRef]
- Li, D.; Xie, S.; Ren, Y.; Huo, L.; Gao, J.; Cui, D.; Liu, M.; Zhou, J. Microtubule-associated deacetylase HDAC6 promotes angiogenesis by regulating cell migration in an EB1-dependent manner. Protein Cell 2011, 2, 150–160. [Google Scholar] [CrossRef]
- Crouch, E.E.; Bhaduri, A.; Andrews, M.G.; Cebrian-Silla, A.; Diafos, L.N.; Birrueta, J.O.; Wedderburn-Pugh, K.; Valenzuela, E.J.; Bennett, N.K.; Eze, U.C.; et al. Ensembles of endothelial and mural cells promote angiogenesis in prenatal human brain. Cell 2022, 185, 3753–3769. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Arroyo, A.G.; Iruela-Arispe, M.L. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc. Res. 2010, 86, 226–235. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gutierrez, L.; Ferrara, N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat. Rev. Mol. Cell Biol. 2023, 24, 816–834. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Menden, H.L.; Mabry, S.M.; Sampath, V. HDAC6 and ERK/ADAM17 regulate VEGF-induced NOTCH signaling in lung endothelial cells. Cells 2023, 12, 2231. [Google Scholar] [CrossRef] [PubMed]
- Jerkic, M.; Rodriguez-Barbero, A.; Prieto, M.; Toporsian, M.; Pericacho, M.; Rivas-Elena, J.V.; Obreo, J.; Wang, A.; Perez-Barriocanal, F.; Arevalo, M.; et al. Reduced angiogenic responses in adult endoglin heterozygous mice. Cardiovasc. Res. 2006, 69, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Mythreye, K.; Golzio, C.; Katsanis, N.; Blobe, G.C. Endoglin mediates fibronectin/alpha5beta1 integrin and TGF-beta pathway crosstalk in endothelial cells. EMBO J. 2012, 31, 3885–3900. [Google Scholar] [CrossRef]
- David, L.; Mallet, C.; Mazerbourg, S.; Feige, J.J.; Bailly, S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 2007, 109, 1953–1961. [Google Scholar] [CrossRef]
- Lawera, A.; Tong, Z.; Thorikay, M.; Redgrave, R.E.; Cai, J.; van Dinther, M.; Morrell, N.W.; Afink, G.B.; Charnock-Jones, D.S.; Arthur, H.M.; et al. Role of soluble endoglin in BMP9 signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 17800–17808. [Google Scholar] [CrossRef]
- Bourdeau, A.; Dumont, D.J.; Letarte, M. A murine model of hereditary hemorrhagic telangiectasia. J. Clin. Investig. 1999, 104, 1343–1351. [Google Scholar] [CrossRef]
- Snellings, D.A.; Gallione, C.J.; Clark, D.S.; Vozoris, N.T.; Faughnan, M.E.; Marchuk, D.A. Somatic mutations in vascular malformations of hereditary hemorrhagic telangiectasia result in bi-allelic loss of ENG or ACVRL1. Am. J. Hum. Genet. 2019, 105, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Tiscornia, G.; Singer, O.; Verma, I.M. Production and purification of lentiviral vectors. Nat. Protoc. 2006, 1, 241–245. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhu, R.; Wang, F.; Cheng, Y.; Liu, Y. Three differential expression analysis methods for RNA sequencing: Limma, EdgeR, DESeq2. J. Vis. Exp. 2021, 175, 62528. [Google Scholar] [CrossRef]
- Plumitallo, S.; Ruiz-Llorente, L.; Langa, C.; Morini, J.; Babini, G.; Cappelletti, D.; Scelsi, L.; Greco, A.; Danesino, C.; Bernabeu, C.; et al. Functional analysis of a novel ENG variant in a patient with hereditary hemorrhagic telangiectasia (HHT) identifies a new Sp1 binding-site. Gene 2018, 647, 85–92. [Google Scholar] [CrossRef]
- Botella, L.M.; Sanchez-Elsner, T.; Rius, C.; Corbi, A.; Bernabeu, C. Identification of a critical Sp1 site within the endoglin promoter and its involvement in the transforming growth factor-beta stimulation. J. Biol. Chem. 2001, 276, 34486–34494. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.J.; Wang, Y.T.; Chang, W.C. Sp1 deacetylation induced by phorbol ester recruits p300 to activate 12(S)-lipoxygenase gene transcription. Mol. Cell Biol. 2006, 26, 1770–1785. [Google Scholar] [CrossRef]
- Chang, W.C.; Hung, J.J. Functional role of post-translational modifications of Sp1 in tumorigenesis. J. Biomed. Sci. 2012, 19, 94. [Google Scholar] [CrossRef]
- Waby, J.S.; Chirakkal, H.; Yu, C.; Griffiths, G.J.; Benson, R.S.; Bingle, C.D.; Corfe, B.M. Sp1 acetylation is associated with loss of DNA binding at promoters associated with cell cycle arrest and cell death in a colon cell line. Mol. Cancer 2010, 9, 275. [Google Scholar] [CrossRef]
- Simoes-Pires, C.; Zwick, V.; Nurisso, A.; Schenker, E.; Carrupt, P.A.; Cuendet, M. HDAC6 as a target for neurodegenerative diseases: What makes it different from the other HDACs? Mol. Neurodegener. 2013, 8, 7. [Google Scholar] [CrossRef]
- Calogero, A.M.; Basellini, M.J.; Isilgan, H.B.; Longhena, F.; Bellucci, A.; Mazzetti, S.; Rolando, C.; Pezzoli, G.; Cappelletti, G. Acetylated alpha-tubulin and alpha-synuclein: Physiological interplay and contribution to alpha-synuclein oligomerization. Int. J. Mol. Sci. 2023, 24, 12287. [Google Scholar] [CrossRef]
- Wattanathamsan, O.; Chantaravisoot, N.; Wongkongkathep, P.; Kungsukool, S.; Chetprayoon, P.; Chanvorachote, P.; Vinayanuwattikun, C.; Pongrakhananon, V. Inhibition of histone deacetylase 6 destabilizes ERK phosphorylation and suppresses cancer proliferation via modulation of the tubulin acetylation-GRP78 interaction. J. Biomed. Sci. 2023, 30, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, C.; Hassan, S.; Liu, X.; Song, F.; Chen, K.; Zhang, W.; Yang, J. Histone deacetylase 6 in cancer. J. Hematol. Oncol. 2018, 11, 111. [Google Scholar] [CrossRef]
- Jin, G.; Wang, K.; Zhao, Y.; Yuan, S.; He, Z.; Zhang, J. Targeting histone deacetylases for heart diseases. Bioorg. Chem. 2023, 138, 106601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hu, X.; Henning, R.H.; Brundel, B.J. Keeping up the balance: Role of HDACs in cardiac proteostasis and therapeutic implications for atrial fibrillation. Cardiovasc. Res. 2016, 109, 519–526. [Google Scholar] [CrossRef]
- Karnam, K.; Sedmaki, K.; Sharma, P.; Routholla, G.; Goli, S.; Ghosh, B.; Venuganti, V.; Kulkarni, O.P. HDAC6 inhibitor accelerates wound healing by inhibiting tubulin mediated IL-1beta secretion in diabetic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165903. [Google Scholar] [CrossRef] [PubMed]
- Dunaway, L.S.; Pollock, J.S. HDAC1: An environmental sensor regulating endothelial function. Cardiovasc. Res. 2022, 118, 1885–1903. [Google Scholar] [CrossRef]
- Naderinezhad, S.; Zhang, G.; Wang, Z.; Zheng, D.; Hulsurkar, M.; Bakhoum, M.; Su, N.; Yang, H.; Shen, T.; Li, W. A novel GRK3-HDAC2 regulatory pathway is a key direct link between neuroendocrine differentiation and angiogenesis in prostate cancer progression. Cancer Lett. 2023, 571, 216333. [Google Scholar] [CrossRef]
- Wang, D.; Hong, H.; Li, X.X.; Li, J.; Zhang, Z.Q. Involvement of HDAC3-mediated inhibition of microRNA cluster 17-92 in bronchopulmonary dysplasia development. Mol. Med. 2020, 26, 99. [Google Scholar] [CrossRef]
- Seidel, C.; Schnekenburger, M.; Dicato, M.; Diederich, M. Histone deacetylase 6 in health and disease. Epigenomics 2015, 7, 103–118. [Google Scholar] [CrossRef]
- Ten, D.P.; Goumans, M.J.; Pardali, E. Endoglin in angiogenesis and vascular diseases. Angiogenesis 2008, 11, 79–89. [Google Scholar] [CrossRef]
- Saito, T.; Bokhove, M.; Croci, R.; Zamora-Caballero, S.; Han, L.; Letarte, M.; de Sanctis, D.; Jovine, L. Structural basis of the human endoglin-BMP9 interaction: Insights into BMP signaling and HHT1. Cell Rep. 2017, 19, 1917–1928. [Google Scholar] [CrossRef]
- Choi, H.; Kim, B.G.; Kim, Y.H.; Lee, S.J.; Lee, Y.J.; Oh, S.P. BMP10 functions independently from BMP9 for the development of a proper arteriovenous network. Angiogenesis 2023, 26, 167–186. [Google Scholar] [CrossRef]
- Rossi, E.; Bernabeu, C. Novel vascular roles of human endoglin in pathophysiology. J. Thromb. Haemost. 2023, 21, 2327–2338. [Google Scholar] [CrossRef]
- David, L.; Feige, J.J.; Bailly, S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009, 20, 203–212. [Google Scholar] [CrossRef]
- Medina-Jover, F.; Riera-Mestre, A.; Vinals, F. Rethinking growth factors: The case of BMP9 during vessel maturation. Vasc. Biol. 2022, 4, R1–R14. [Google Scholar] [CrossRef] [PubMed]
- Jonker, L. TGF-beta & BMP receptors endoglin and ALK1: Overview of their functional role and status as antiangiogenic targets. Microcirculation 2014, 21, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Alexdottir, M.S.; Magnus, S.H.; Richter, T.R.; Morikawa, M.; Zwijsen, A.; Valdimarsdottir, G. EGFL7 mediates BMP9-induced sprouting angiogenesis of endothelial cells derived from human embryonic stem cells. Stem Cell Rep. 2019, 12, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ohga, N.; Morishita, Y.; Hida, K.; Miyazono, K.; Watabe, T. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J. Cell Sci. 2010, 123, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Mallet, C.; Keramidas, M.; Lamande, N.; Gasc, J.M.; Dupuis-Girod, S.; Plauchu, H.; Feige, J.J.; Bailly, S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ. Res. 2008, 102, 914–922. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Xie, K.; Yang, L.; Cai, S.; Wang, M.; Zhu, Y.; Tao, B.; Zhu, Y. HDAC6 Enhances Endoglin Expression through Deacetylation of Transcription Factor SP1, Potentiating BMP9-Induced Angiogenesis. Cells 2024, 13, 490. https://doi.org/10.3390/cells13060490
Sun C, Xie K, Yang L, Cai S, Wang M, Zhu Y, Tao B, Zhu Y. HDAC6 Enhances Endoglin Expression through Deacetylation of Transcription Factor SP1, Potentiating BMP9-Induced Angiogenesis. Cells. 2024; 13(6):490. https://doi.org/10.3390/cells13060490
Chicago/Turabian StyleSun, Chen, Kuifang Xie, Lejie Yang, Shengyang Cai, Mingjie Wang, Yizhun Zhu, Beibei Tao, and Yichun Zhu. 2024. "HDAC6 Enhances Endoglin Expression through Deacetylation of Transcription Factor SP1, Potentiating BMP9-Induced Angiogenesis" Cells 13, no. 6: 490. https://doi.org/10.3390/cells13060490
APA StyleSun, C., Xie, K., Yang, L., Cai, S., Wang, M., Zhu, Y., Tao, B., & Zhu, Y. (2024). HDAC6 Enhances Endoglin Expression through Deacetylation of Transcription Factor SP1, Potentiating BMP9-Induced Angiogenesis. Cells, 13(6), 490. https://doi.org/10.3390/cells13060490





