Intrauterine Growth Restriction: Need to Improve Diagnostic Accuracy and Evidence for a Key Role of Oxidative Stress in Neonatal and Long-Term Sequelae
Abstract
1. Introduction
2. Inconsistent Diagnostic Criteria of Intrauterine Growth Restriction
3. Approaches to Increase Diagnostic Accuracy and Identify Neonates Being “at Risk”
3.1. Imaging Studies
3.2. Placental Work-Up
3.3. Maternal and Neonatal Serum Biomarkers
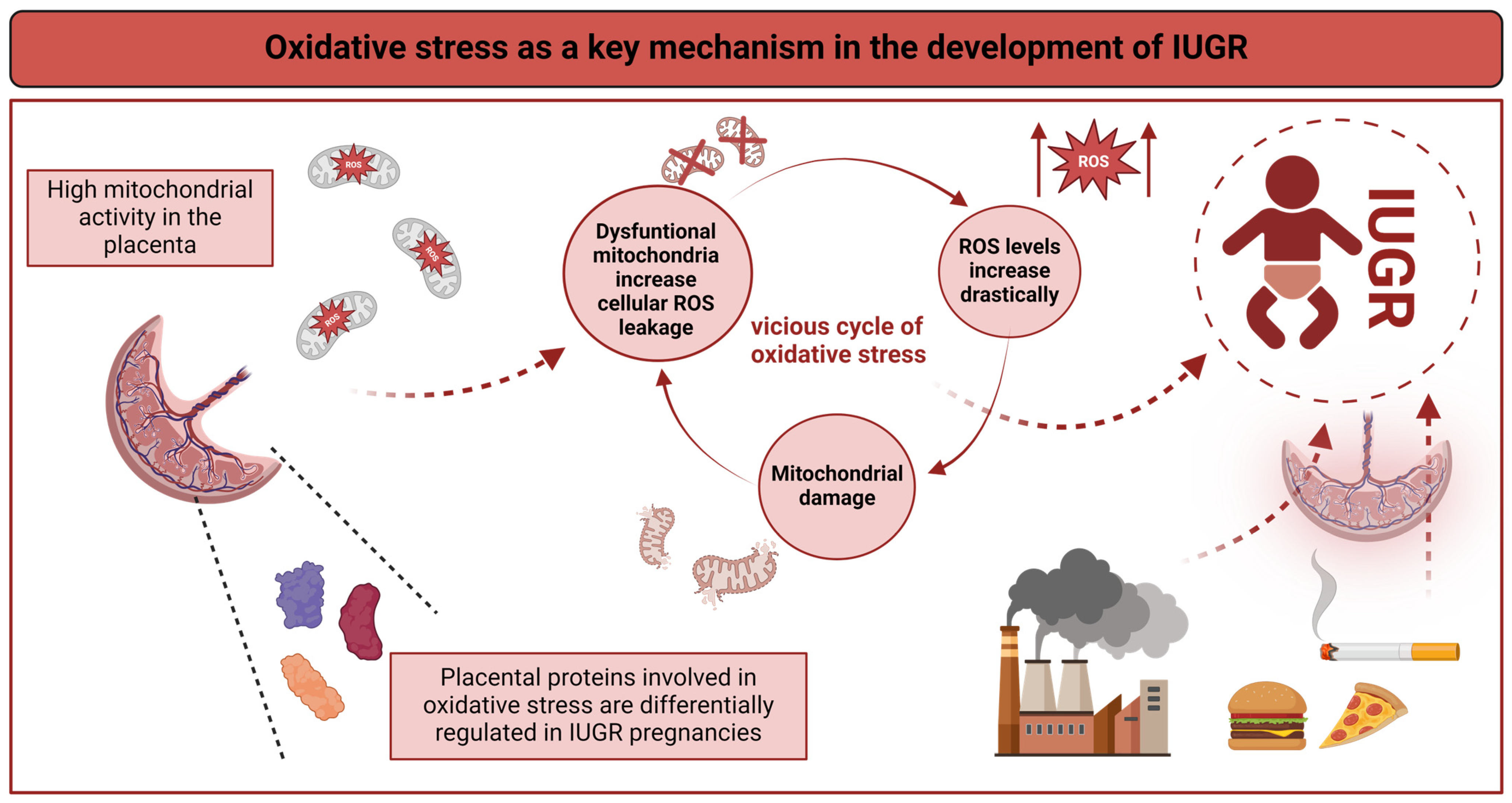
4. Comprehensive Molecular Analyses Identify Oxidative Stress as a Key Mechanism in IUGR
4.1. Oxidative Stress in IUGR
4.2. Oxidative Stress and Cellular Homeostasis
4.3. Oxidative Stress and Inflammation: A Vicious Cycle
5. Follow-Up Care of Children “at Risk” after IUGR Using the Example of Neuropediatric Sequelae
6. Strategies to Prevent or Mitigate IUGR
6.1. The Clinical View—Current Clinical Practice
6.2. The Research View—Future Perspectives
7. Summary and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Barker, D. Infant Mortality, Childhood Nutrition, and Ischaemic Heart Disease in England and Wales. Lancet 1986, 327, 1077–1081. [Google Scholar] [CrossRef]
- Nüsken, K.D.; Schneider, H.; Plank, C.; Trollmann, R.; Nüsken, E.; Rascher, W.; Dötsch, J. Fetal programming of gene expression in growth-restricted rats depends on the cause of low birth weight. Endocrinology 2011, 152, 1327–1335. [Google Scholar] [CrossRef]
- Tzschoppe, A.; Riedel, C.; von Kries, R.; Struwe, E.; Rascher, W.; Dörr, H.G.; Beckmann, M.W.; Schild, R.L.; Goecke, T.W.; Flyvbjerg, A.; et al. Differential effects of low birthweight and intrauterine growth restriction on umbilical cord blood insulin-like growth factor concentrations. Clin. Endocrinol. 2015, 83, 739–745. [Google Scholar] [CrossRef]
- Olga, L.; Sovio, U.; Wong, H.; Smith, G.; Aiken, C. Association between antenatal diagnosis of late fetal growth restriction and educational outcomes in mid-childhood: A UK prospective cohort study with long-term data linkage study. PLoS Med. 2023, 20, e1004225. [Google Scholar] [CrossRef]
- Broere-Brown, Z.A.; Schalekamp-Timmermans, S.; Jaddoe, V.W.V.; Steegers, E.A.P. Deceleration of fetal growth rate as alternative predictor for childhood outcomes: A birth cohort study. BMC Pregnancy Childbirth 2019, 19, 216. [Google Scholar] [CrossRef]
- Musco, H.; Beecher, K.; Chand, K.K.; Colditz, P.B.; Wixey, J.A. Blood biomarkers in the fetally growth restricted and small for gestational age neonate: Associations with brain injury. Dev. Neurosci. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Lees, C.; Stampalija, T.; Hecher, K. Diagnosis and management of fetal growth restriction: The ISUOG guideline and comparison with the SMFM guideline. Ultrasound Obstet. Gynecol. 2021, 57, 884–887. [Google Scholar] [CrossRef]
- Fantasia, I.; Zamagni, G.; Lees, C.; Mylrea-Foley, B.; Monasta, L.; Mullins, E.; Prefumo, F.; Stampalija, T. Current practice in the diagnosis and management of fetal growth restriction: An international survey. Acta Obstet. Gynecol. Scand. 2022, 101, 1431–1439. [Google Scholar] [CrossRef]
- Bardien, N.; Whitehead, C.L.; Tong, S.; Ugoni, A.; McDonald, S.; Walker, S.P. Placental Insufficiency in Fetuses That Slow in Growth but Are Born Appropriate for Gestational Age: A Prospective Longitudinal Study. PLoS ONE 2016, 11, e0142788. [Google Scholar] [CrossRef]
- Lees, C.C.; Stampalija, T.; Baschat, A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Martins, J.G.; Biggio, J.R.; Abuhamad, A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and management of fetal growth restriction: (Replaces Clinical Guideline Number 3, April 2012). Am. J. Obstet. Gynecol. 2020, 223, B2–B17. [Google Scholar] [CrossRef]
- Sovio, U.; White, I.R.; Dacey, A.; Pasupathy, D.; Smith, G.C.S. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: A prospective cohort study. Lancet 2015, 386, 2089–2097. [Google Scholar] [CrossRef]
- Gardosi, J.; Hugh, O. Outcome-based comparative analysis of five fetal growth velocity models to define slow growth. Ultrasound Obstet. Gynecol. 2023, 62, 805–812. [Google Scholar] [CrossRef]
- Lees, C.C.; Romero, R.; Stampalija, T.; Dall’Asta, A.; DeVore, G.A.; Prefumo, F.; Frusca, T.; Visser, G.H.A.; Hobbins, J.C.; Baschat, A.A.; et al. Clinical Opinion: The diagnosis and management of suspected fetal growth restriction: An evidence-based approach. Am. J. Obstet. Gynecol. 2022, 226, 366–378. [Google Scholar] [CrossRef]
- Baschat, A.A. Planning management and delivery of the growth-restricted fetus. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018, 49, 53–65. [Google Scholar] [CrossRef]
- Salavati, N.; Smies, M.; Ganzevoort, W.; Charles, A.K.; Erwich, J.J.; Plösch, T.; Gordijn, S.J. The Possible Role of Placental Morphometry in the Detection of Fetal Growth Restriction. Front. Physiol. 2018, 9, 1884. [Google Scholar] [CrossRef]
- MacDonald, T.M.; Hui, L.; Robinson, A.J.; Dane, K.M.; Middleton, A.L.; Tong, S.; Walker, S.P. Cerebral-placental-uterine ratio as novel predictor of late fetal growth restriction: Prospective cohort study. Ultrasound Obstet. Gynecol. 2019, 54, 367–375. [Google Scholar] [CrossRef]
- La Verde, M.; Savoia, F.; Riemma, G.; Schiattarella, A.; Conte, A.; Hidar, S.; Torella, M.; Colacurci, N.; De Franciscis, P.; Morlando, M. Fetal aortic isthmus Doppler assessment to predict the adverse perinatal outcomes associated with fetal growth restriction: Systematic review and meta-analysis. Arch. Gynecol. Obs. 2023, 309, 79–92. [Google Scholar] [CrossRef]
- Shmueli, A.; Mor, L.; Blickstein, O.; Sela, R.; Weiner, E.; Gonen, N.; Schreiber, L.; Levy, M. Placental pathology in pregnancies with late fetal growth restriction and abnormal cerebroplacental ratio. Placenta 2023, 138, 83–87. [Google Scholar] [CrossRef]
- Hamidi, O.P.; Driver, C.; Steller, J.G.; Peek, E.E.; Monasta, L.; Stampalija, T.; Gumina, D.L.; DeVore, G.R.; Hobbins, J.C.; Galan, H.L. Umbilical Venous Volume Flow in Late-Onset Fetal Growth Restriction. J. Ultrasound Med. 2023, 42, 173–183. [Google Scholar] [CrossRef]
- DeVore, G.R.; Epstein, A. Computing Z-Score Equations for Clinical Use to Measure Fetal Umbilical Vein Size and Flow Using Six Independent Variables of Age and Size. J. Ultrasound Med. 2022, 41, 1949–1960. [Google Scholar] [CrossRef]
- Ferrazzi, E.; Rigano, S.; Bozzo, M.; Bellotti, M.; Giovannini, N.; Galan, H.; Battaglia, F.C. Umbilical vein blood flow in growth-restricted fetuses. Ultrasound Obstet. Gynecol. 2000, 16, 432–438. [Google Scholar] [CrossRef]
- Rizzo, G.; Mappa, I.; Bitsadze, V.; Słodki, M.; Khizroeva, J.; Makatsariya, A.; D’Antonio, F. Role of Doppler ultrasound at time of diagnosis of late-onset fetal growth restriction in predicting adverse perinatal outcome: Prospective cohort study. Ultrasound Obstet. Gynecol. 2020, 55, 793–798. [Google Scholar] [CrossRef]
- Parra-Saavedra, M.; Crovetto, F.; Triunfo, S.; Savchev, S.; Parra, G.; Sanz, M.; Gratacos, E.; Figueras, F. Added value of umbilical vein flow as a predictor of perinatal outcome in term small-for-gestational-age fetuses. Ultrasound Obstet. Gynecol. 2013, 42, 189–195. [Google Scholar] [CrossRef]
- Farsetti, D.; Pometti, F.; Tiralongo, G.M.; Lo Presti, D.; Pisani, I.; Gagliardi, G.; Vasapollo, B.; Novelli, G.P.; Valensise, H. Distinction between SGA and FGR by means of fetal umbilical vein flow and maternal hemodynamics. J. Matern. -Fetal Neonatal Med. 2022, 35, 6593–6599. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, C.; Abdel-Azim, S.; Galeva, S.; Georgiopoulos, G.; Nicolaides, K.H.; Charakida, M. Placental function and fetal weight are associated with maternal hemodynamic indices in uncomplicated pregnancies at 35–37 weeks of gestation. Am. J. Obstet. Gynecol. 2020, 222, 604.e1–604.e10. [Google Scholar] [CrossRef]
- Perrone, S.; Santacroce, A.; de Bernardo, G.; Alagna, M.G.; Carbone, S.F.; Paternò, I.; Buonocore, G. Magnetic Resonance Imaging in Pregnancy with Intrauterine Growth Restriction: A Pilot Study. Dis. Markers 2019, 2019, 4373490. [Google Scholar] [CrossRef]
- Liu, X.L.; Feng, J.; Huang, C.T.; Mei, Y.J.; Xu, Y.K. Use of intravoxel incoherent motion MRI to assess placental perfusion in normal and Fetal Growth Restricted pregnancies on their third trimester. Placenta 2022, 118, 10–15. [Google Scholar] [CrossRef]
- Andescavage, N.; You, W.; Jacobs, M.; Kapse, K.; Quistorff, J.; Bulas, D.; Ahmadzia, H.; Gimovsky, A.; Baschat, A.; Limperopoulos, C. Exploring in vivo placental microstructure in healthy and growth-restricted pregnancies through diffusion-weighted magnetic resonance imaging. Placenta 2020, 93, 113–118. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Milligan, N.; Keating, S.; Windrim, R.; Keunen, J.; Thakur, V.; Ohman, A.; Portnoy, S.; Sled, J.G.; Kelly, E.; et al. The hemodynamics of late-onset intrauterine growth restriction by MRI. Am. J. Obstet. Gynecol. 2016, 214, 367.e1–367.e17. [Google Scholar] [CrossRef]
- Parra-Saavedra, M.; Simeone, S.; Triunfo, S.; Crovetto, F.; Botet, F.; Nadal, A.; Gratacos, E.; Figueras, F. Correlation between histological signs of placental underperfusion and perinatal morbidity in late-onset small-for-gestational-age fetuses. Ultrasound Obstet. Gynecol. 2015, 45, 149–155. [Google Scholar] [CrossRef]
- Almasry, S.M.; Elfayomy, A.K. Morphometric analysis of terminal villi and gross morphological changes in the placentae of term idiopathic intrauterine growth restriction. Tissue Cell 2012, 44, 214–219. [Google Scholar] [CrossRef]
- Egbor, M.; Ansari, T.; Morris, N.; Green, C.J.; Sibbons, P.D. Pre-eclampsia and fetal growth restriction: How morphometrically different is the placenta? Placenta 2006, 27, 727–734. [Google Scholar] [CrossRef]
- Mayhew, T.M.; Manwani, R.; Ohadike, C.; Wijesekara, J.; Baker, P.N. The placenta in pre-eclampsia and intrauterine growth restriction: Studies on exchange surface areas, diffusion distances and villous membrane diffusive conductances. Placenta 2007, 28, 233–238. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Turkoglu, O.; Yilmaz, A.; Kumar, P.; Zeb, A.; Konda, S.; Sherman, E.; Kirma, J.; Allos, M.; Odibo, A.; et al. Metabolomic identification of placental alterations in fetal growth restriction. J. Matern. -Fetal Neonatal Med. 2022, 35, 447–456. [Google Scholar] [CrossRef]
- Sovio, U.; Goulding, N.; McBride, N.; Cook, E.; Gaccioli, F.; Charnock-Jones, D.S.; Lawlor, D.A.; Smith, G.C.S. A maternal serum metabolite ratio predicts fetal growth restriction at term. Nat. Med. 2020, 26, 348–353. [Google Scholar] [CrossRef]
- Paules, C.; Youssef, L.; Miranda, J.; Crovetto, F.; Estanyol, J.M.; Fernandez, G.; Crispi, F.; Gratacós, E. Maternal proteomic profiling reveals alterations in lipid metabolism in late-onset fetal growth restriction. Sci. Rep. 2020, 10, 21033. [Google Scholar] [CrossRef]
- Paules, C.; Dantas, A.P.; Miranda, J.; Crovetto, F.; Eixarch, E.; Rodriguez-Sureda, V.; Dominguez, C.; Casu, G.; Rovira, C.; Nadal, A.; et al. Premature placental aging in term small-for-gestational-age and growth-restricted fetuses. Ultrasound Obstet. Gynecol. 2019, 53, 615–622. [Google Scholar] [CrossRef]
- Grohmann, R.M.; Marçal, V.M.G.; Corazza, I.C.; Peixoto, A.B.; Júnior, E.A.; Nardozza, L.M.M. Maternal Blood Fatty Acid Levels in Fetal Growth Restriction. Rev. Bras. Ginecol. Obs. 2023, 45, 127–133. [Google Scholar] [CrossRef]
- Hannan, N.J.; Stock, O.; Spencer, R.; Whitehead, C.; David, A.L.; Groom, K.; Petersen, S.; Henry, A.; Said, J.M.; Seeho, S.; et al. Circulating mRNAs are differentially expressed in pregnancies with severe placental insufficiency and at high risk of stillbirth. BMC Med. 2020, 18, 145. [Google Scholar] [CrossRef]
- De Alwis, N.; Beard, S.; Binder, N.K.; Pritchard, N.; Kaitu’u-Lino, T.J.; Walker, S.P.; Stock, O.; Groom, K.M.; Petersen, S.; Henry, A.; et al. NR4A2 expression is not altered in placentas from cases of growth restriction or preeclampsia, but is reduced in hypoxic cytotrophoblast. Sci. Rep. 2021, 11, 20670. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Krofta, L. First-Trimester Screening for Fetal Growth Restriction and Small-for-Gestational-Age Pregnancies without Preeclampsia Using Cardiovascular Disease-Associated MicroRNA Biomarkers. Biomedicines 2022, 10, 718. [Google Scholar] [CrossRef]
- Oluklu, D.; Beser, D.M.; Hendem, D.U.; Kara, O.; Yazihan, N.; Sahin, D. Maternal serum midkine level in fetal growth restriction: A case-control study. J. Perinat. Med. 2023, 51, 396–402. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Dymara-Konopka, W.; Laskowska, M.; Grywalska, E.; Hymos, A.; Błażewicz, A.; Leszczyńska-Gorzelak, B. Similar Pro- and Antiangiogenic Profiles Close to Delivery in Different Clinical Presentations of Two Pregnancy Syndromes: Preeclampsia and Fetal Growth Restriction. Int. J. Mol. Sci. 2023, 24, 972. [Google Scholar] [CrossRef]
- Garcia-Manau, P.; Mendoza, M.; Bonacina, E.; Garrido-Gimenez, C.; Fernandez-Oliva, A.; Zanini, J.; Catalan, M.; Tur, H.; Serrano, B.; Carreras, E. Soluble fms-like tyrosine kinase to placental growth factor ratio in different stages of early-onset fetal growth restriction and small for gestational age. Acta Obstet. Gynecol. Scand. 2021, 100, 119–128. [Google Scholar] [CrossRef]
- Verlohren, S.; Brennecke, S.P.; Galindo, A.; Karumanchi, S.A.; Mirkovic, L.B.; Schlembach, D.; Stepan, H.; Vatish, M.; Zeisler, H.; Rana, S. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens. 2022, 27, 42–50. [Google Scholar] [CrossRef]
- Lopes Perdigao, J.; Chinthala, S.; Mueller, A.; Minhas, R.; Ramadan, H.; Nasim, R.; Naseem, H.; Young, D.; Shahul, S.; Chan, S.L.; et al. Angiogenic Factor Estimation as a Warning Sign of Preeclampsia-Related Peripartum Morbidity among Hospitalized Patients. Hypertension 2019, 73, 868–877. [Google Scholar] [CrossRef]
- Dröge, L.A.; Perschel, F.H.; Stütz, N.; Gafron, A.; Frank, L.; Busjahn, A.; Henrich, W.; Verlohren, S. Prediction of Preeclampsia-Related Adverse Outcomes with the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor)-Ratio in the Clinical Routine. Hypertension 2021, 77, 461–471. [Google Scholar] [CrossRef]
- Mylrea-Foley, B.; Bhide, A.; Mullins, E.; Thornton, J.; Marlow, N.; Stampalija, T.; Napolitano, R.; Lees, C.C. Building consensus: Thresholds for delivery in TRUFFLE-2 randomized intervention study. Ultrasound Obstet. Gynecol. 2020, 56, 285–287. [Google Scholar] [CrossRef]
- Kırıcı, P.; Çağıran, F.T.; Kalı, Z.; Tanrıverdi, E.F.; Mavral, N.; Ecin, S.M. Determination of maternal serum pro-inflammatory cytokine changes in intrauterine growth restriction. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1996–2001. [Google Scholar] [CrossRef]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, O.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obs. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef]
- Guvendag Guven, E.S.; Karcaaltincaba, D.; Kandemir, O.; Kiykac, S.; Mentese, A. Cord blood oxidative stress markers correlate with umbilical artery pulsatility in fetal growth restriction. J. Matern. -Fetal Neonatal Med. 2013, 26, 576–580. [Google Scholar] [CrossRef]
- Karowicz-Bilińska, A.; Suzin, J.; Sieroszewski, P. Evaluation of oxidative stress indices during treatment in pregnant women with intrauterine growth retardation. Med. Sci. Monit. 2002, 8, Cr211–Cr216. [Google Scholar]
- Saker, M.; Soulimane Mokhtari, N.; Merzouk, S.A.; Merzouk, H.; Belarbi, B.; Narce, M. Oxidant and antioxidant status in mothers and their newborns according to birthweight. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 141, 95–99. [Google Scholar] [CrossRef]
- Andersson, A.; Lindgren, A.; Arnadottir, M.; Prytz, H.; Hultberg, B. Thiols as a measure of plasma redox status in healthy subjects and in patients with renal or liver failure. Clin. Chem. 1999, 45, 1084–1086. [Google Scholar] [CrossRef]
- Ozler, S.; Oztas, E.; Guler, B.G.; Erel, O.; Caglar, A.T.; Ergin, M.; Danisman, N. Dynamic Thiol/Disulfide Homeostasis in Predicting Adverse Neonatal Outcomes in Fetal Growth Restriction. Fetal Pediatr. Pathol. 2020, 39, 132–144. [Google Scholar] [CrossRef]
- Schoots, M.H.; Bourgonje, M.F.; Bourgonje, A.R.; Prins, J.R.; van Hoorn, E.G.M.; Abdulle, A.E.; Muller Kobold, A.C.; van der Heide, M.; Hillebrands, J.L.; van Goor, H.; et al. Oxidative stress biomarkers in fetal growth restriction with and without preeclampsia. Placenta 2021, 115, 87–96. [Google Scholar] [CrossRef]
- Eroglu, H.; Turgal, M.; Senat, A.; Karakoc, G.; Neselioglu, S.; Yucel, A. Maternal and fetal thiol/disulfide homeostasis in fetal growth restriction. J. Matern. -Fetal Neonatal Med. 2021, 34, 1658–1665. [Google Scholar] [CrossRef]
- Seshadri Reddy, V.; Duggina, P.; Vedhantam, M.; Manne, M.; Varma, N.; Nagaram, S. Maternal serum and fetal cord-blood ischemia-modified albumin concentrations in normal pregnancy and preeclampsia: A systematic review and meta-analysis. J. Matern. -Fetal Neonatal Med. 2018, 31, 3255–3266. [Google Scholar] [CrossRef]
- Lippi, G.; Montagnana, M.; Guidi, G.C. Albumin cobalt binding and ischemia modified albumin generation: An endogenous response to ischemia? Int. J. Cardiol. 2006, 108, 410–411. [Google Scholar] [CrossRef]
- Sinha, M.K.; Roy, D.; Gaze, D.C.; Collinson, P.O.; Kaski, J.C. Role of “Ischemia modified albumin”, a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg. Med. J. 2004, 21, 29–34. [Google Scholar] [CrossRef]
- Gölbaşı, C.; Gölbaşı, H.; Kocahakimoğlu Gültekin, C.; Gülseren, V.; Zeytinli Akşit, M.; Bayraktar, B.; Çolak, A.; Taner, C.E. Ischemia modified albumin levels in intrauterine growth restriction: Levels are increased in fetal cord blood but not in maternal blood. Ginekol. Pol. 2022, 93, 993–998. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Du, X.; Huang, Z.; Quan, N. Sestrin 2, a potential star of antioxidant stress in cardiovascular diseases. Free Radic. Biol. Med. 2021, 163, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Agaoglu, M.O.; Agaoglu, Z.; Yucel, K.Y.; Ozturk, F.H.; Caglar, T. Evaluation of maternal serum sestrin-2 levels in intrauterine growth restriction. Ir. J. Med. Sci. 2023, 192, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- De Alwis, N.; Beard, S.; Binder, N.K.; Pritchard, N.; Kaitu’u-Lino, T.J.; Walker, S.P.; Stock, O.; Groom, K.; Petersen, S.; Henry, A.; et al. DAAM2 is elevated in the circulation and placenta in pregnancies complicated by fetal growth restriction and is regulated by hypoxia. Sci. Rep. 2021, 11, 5540. [Google Scholar] [CrossRef] [PubMed]
- Gęca, T.; Stupak, A.; Nawrot, R.; Goździcka-Józefiak, A.; Kwaśniewska, A.; Kwaśniewski, W. Placental proteome in late-onset of fetal growth restriction. Mol. Med. Rep. 2022, 26, 356. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.L.; Yuan, P.B.; Wang, X.J.; Hang, J.; Shi, X.M.; Zhao, Y.Y.; Wei, Y. The Proteome Landscape of Human Placentas for Monochorionic Twins with Selective Intrauterine Growth Restriction. Genom. Proteom. Bioinform. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Alfian, I.; Chakraborty, A.; Yong, H.E.J.; Saini, S.; Lau, R.W.K.; Kalionis, B.; Dimitriadis, E.; Alfaidy, N.; Ricardo, S.D.; Samuel, C.S.; et al. The Placental NLRP3 Inflammasome and Its Downstream Targets, Caspase-1 and Interleukin-6, Are Increased in Human Fetal Growth Restriction: Implications for Aberrant Inflammation-Induced Trophoblast Dysfunction. Cells 2022, 11, 1413. [Google Scholar] [CrossRef] [PubMed]
- Moros, G.; Boutsikou, T.; Fotakis, C.; Iliodromiti, Z.; Sokou, R.; Katsila, T.; Xanthos, T.; Iacovidou, N.; Zoumpoulakis, P. Insights into intrauterine growth restriction based on maternal and umbilical cord blood metabolomics. Sci. Rep. 2021, 11, 7824. [Google Scholar] [CrossRef]
- Youssef, L.; Simões, R.V.; Miranda, J.; García-Martín, M.L.; Paules, C.; Crovetto, F.; Amigó, N.; Cañellas, N.; Gratacos, E.; Crispi, F. Paired maternal and fetal metabolomics reveal a differential fingerprint in preeclampsia versus fetal growth restriction. Sci. Rep. 2021, 11, 14422. [Google Scholar] [CrossRef]
- Tao, Z.; Chen, Y.; He, F.; Tang, J.; Zhan, L.; Hu, H.; Ding, Z.; Ruan, S.; Chen, Y.; Chen, B.; et al. Alterations in the Gut Microbiome and Metabolisms in Pregnancies with Fetal Growth Restriction. Microbiol. Spectr. 2023, 11, e0007623. [Google Scholar] [CrossRef]
- Dede, H.; Takmaz, O.; Ozbasli, E.; Dede, S.; Gungor, M. Higher Level of Oxidative Stress Markers in Small for Gestational Age Newborns Delivered by Cesarean Section at Term. Fetal Pediatr. Pathol. 2017, 36, 232–239. [Google Scholar] [CrossRef]
- Gupta, P.; Narang, M.; Banerjee, B.D.; Basu, S. Oxidative stress in term small for gestational age neonates born to undernourished mothers: A case control study. BMC Pediatr. 2004, 4, 14. [Google Scholar] [CrossRef]
- Potdar, N.; Singh, R.; Mistry, V.; Evans, M.D.; Farmer, P.B.; Konje, J.C.; Cooke, M.S. First-trimester increase in oxidative stress and risk of small-for-gestational-age fetus. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Kido, T.; Kyono, Y.; Yoshida, A.; Suga, S.; Nakasone, R.; Abe, S.; Tanimura, K.; Nozu, K.; Fujioka, K. Correlation between Severity of Fetal Growth Restriction and Oxidative Stress in Severe Small-for-Gestational-Age Infants. Int. J. Environ. Res. Public Health 2021, 18, 10726. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.F.; Myatt, L. Placental mitochondrial dysfunction with metabolic diseases: Therapeutic approaches. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 165967. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 2010, 31, S66–S69. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Nowaczyk, J.; Poniedziałek, B.; Rzymski, P.; Sikora, D.; Ropacka-Lesiak, M. Platelets in Fetal Growth Restriction: Role of Reactive Oxygen Species, Oxygen Metabolism, and Aggregation. Cells 2022, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Mundal, S.B.; Rakner, J.J.; Silva, G.B.; Gierman, L.M.; Austdal, M.; Basnet, P.; Elschot, M.; Bakke, S.S.; Ostrop, J.; Thomsen, L.C.V.; et al. Divergent Regulation of Decidual Oxidative-Stress Response by NRF2 and KEAP1 in Preeclampsia with and without Fetal Growth Restriction. Int. J. Mol. Sci. 2022, 23, 1966. [Google Scholar] [CrossRef]
- Hoch, D.; Majali-Martinez, A.; Bankoglu, E.E.; Stopper, H.; Glasner, A.; Desoye, G.; Gauster, M.; Hiden, U. Maternal Smoking in the First Trimester and its Consequence on the Early Placenta. Lab. Investig. 2023, 103, 100059. [Google Scholar] [CrossRef]
- Juan-Reyes, S.S.; Gómez-Oliván, L.M.; Juan-Reyes, N.S.; Islas-Flores, H.; Dublán-García, O.; Orozco-Hernández, J.M.; Pérez-Álvarez, I.; Mejía-García, A. Women with preeclampsia exposed to air pollution during pregnancy: Relationship between oxidative stress and neonatal disease—Pilot study. Sci. Total Environ. 2023, 871, 161858. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Niu, Z.; Li, K.; Xie, C.; Wen, X. Adverse Birth Outcomes and Birth Telomere Length: A Systematic Review and Meta-Analysis. J. Pediatr. 2019, 215, 64–74.e6. [Google Scholar] [CrossRef] [PubMed]
- Formanowicz, D.; Malińska, A.; Nowicki, M.; Kowalska, K.; Gruca-Stryjak, K.; Bręborowicz, G.; Korybalska, K. Preeclampsia with Intrauterine Growth Restriction Generates Morphological Changes in Endothelial Cells Associated with Mitochondrial Swelling-An In Vitro Study. J. Clin. Med. 2019, 8, 1994. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, A.L.; Wesolowski, S.R.; Regnault, T.R.H.; Lynch, R.M.; Limesand, S.W. Dimming the Powerhouse: Mitochondrial Dysfunction in the Liver and Skeletal Muscle of Intrauterine Growth Restricted Fetuses. Front. Endocrinol. 2021, 12, 612888. [Google Scholar] [CrossRef] [PubMed]
- Tasta, O.; Swiader, A.; Grazide, M.H.; Rouahi, M.; Parant, O.; Vayssière, C.; Bujold, E.; Salvayre, R.; Guerby, P.; Negre-Salvayre, A. A role for 4-hydroxy-2-nonenal in premature placental senescence in preeclampsia and intrauterine growth restriction. Free Radic. Biol. Med. 2021, 164, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Kajdy, A.; Modzelewski, J.; Cymbaluk-Płoska, A.; Kwiatkowska, E.; Bednarek-Jędrzejek, M.; Borowski, D.; Stefańska, K.; Rabijewski, M.; Torbé, A.; Kwiatkowski, S. Molecular Pathways of Cellular Senescence and Placental Aging in Late Fetal Growth Restriction and Stillbirth. Int. J. Mol. Sci. 2021, 22, 4186. [Google Scholar] [CrossRef]
- Baker, B.C.; Heazell, A.E.P.; Sibley, C.; Wright, R.; Bischof, H.; Beards, F.; Guevara, T.; Girard, S.; Jones, R.L. Hypoxia and oxidative stress induce sterile placental inflammation in vitro. Sci. Rep. 2021, 11, 7281. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.B.; Gierman, L.M.; Rakner, J.J.; Stødle, G.S.; Mundal, S.B.; Thaning, A.J.; Sporsheim, B.; Elschot, M.; Collett, K.; Bjørge, L.; et al. Cholesterol Crystals and NLRP3 Mediated Inflammation in the Uterine Wall Decidua in Normal and Preeclamptic Pregnancies. Front. Immunol. 2020, 11, 564712. [Google Scholar] [CrossRef]
- Gaillard, R.; Steegers, E.A.; Tiemeier, H.; Hofman, A.; Jaddoe, V.W. Placental vascular dysfunction, fetal and childhood growth, and cardiovascular development: The generation R study. Circulation 2013, 128, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Skilton, M.R.; Crispi, F. Human fetal growth restriction: A cardiovascular journey through to adolescence. J. Dev. Orig. Health Dis. 2016, 7, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Chen, Y.; Ye, J.; Ouyang, F.; Jiang, F.; Zhang, J. The optimal postnatal growth trajectory for term small for gestational age babies: A prospective cohort study. J. Pediatr. 2015, 166, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, C.; Marino, C.; Nosarti, C.; Vieno, A.; Visentin, S.; Simonelli, A. Association of Intrauterine Growth Restriction and Small for Gestational Age Status with Childhood Cognitive Outcomes: A Systematic Review and Meta-analysis. JAMA Pediatr. 2020, 174, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Benítez Marín, M.J.; Blanco Elena, J.A.; Marín Clavijo, J.; Jiménez López, J.; Lubián López, D.M.; González Mesa, E. Neurodevelopment Outcome in Children with Fetal Growth Restriction at Six Years of Age: A Retrospective Cohort Study. Int. J. Env. Res. Public Health 2022, 19, 11043. [Google Scholar] [CrossRef] [PubMed]
- Monteith, C.; Flood, K.; Pinnamaneni, R.; Levine, T.A.; Alderdice, F.A.; Unterscheider, J.; McAuliffe, F.M.; Dicker, P.; Tully, E.C.; Malone, F.D.; et al. An abnormal cerebroplacental ratio (CPR) is predictive of early childhood delayed neurodevelopment in the setting of fetal growth restriction. Am. J. Obstet. Gynecol. 2019, 221, 273.e1–273.e9. [Google Scholar] [CrossRef] [PubMed]
- Aisa, M.C.; Barbati, A.; Gerli, S.; Clerici, G.; Nikolova, N.; Giardina, I.; Babucci, G.; De Rosa, F.; Cappuccini, B. Brain 3D-echographic early predictors of neuro-behavioral disorders in infants: A prospective observational study. J. Matern. -Fetal Neonatal Med. 2022, 35, 642–650. [Google Scholar] [CrossRef]
- Dudink, I.; Hüppi, P.S.; Sizonenko, S.V.; Castillo-Melendez, M.; Sutherland, A.E.; Allison, B.J.; Miller, S.L. Altered trajectory of neurodevelopment associated with fetal growth restriction. Exp. Neurol. 2022, 347, 113885. [Google Scholar] [CrossRef]
- Korkalainen, N.; Ilvesmäki, T.; Parkkola, R.; Perhomaa, M.; Mäkikallio, K. Brain volumes and white matter microstructure in 8- to 10-year-old children born with fetal growth restriction. Pediatr. Radiol. 2022, 52, 2388–2400. [Google Scholar] [CrossRef]
- Ananth, C.V.; Peltier, M.R.; Chavez, M.R.; Kirby, R.S.; Getahun, D.; Vintzileos, A.M. Recurrence of ischemic placental disease. Obstet. Gynecol. 2007, 110, 128–133. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Mayer-Pickel, K. The Antiphospholipid Syndrome—The Obstetric Point of View. Z. Gefäßmedizin 2018, 15, 9–13. [Google Scholar]
- Menichini, D.; Feliciello, L.; Neri, I.; Facchinetti, F. L-Arginine supplementation in pregnancy: A systematic review of maternal and fetal outcomes. J. Matern. -Fetal Neonatal Med. 2023, 36, 2217465. [Google Scholar] [CrossRef]
- Schleussner, E.; Lehmann, T.; Kähler, C.; Schneider, U.; Schlembach, D.; Groten, T. Impact of the nitric oxide-donor pentaerythrityl-tetranitrate on perinatal outcome in risk pregnancies: A prospective, randomized, double-blinded trial. J. Perinat. Med. 2014, 42, 507–514. [Google Scholar] [CrossRef]
- Bowkalow, S.; Schleussner, E.; Kähler, C.; Schneider, U.; Lehmann, T.; Groten, T. Pentaerythrityltetranitrate (PETN) improves utero- and feto-placental Doppler parameters in pregnancies with impaired utero-placental perfusion in mid-gestation—A secondary analysis of the PETN-pilot trial. J. Perinat. Med. 2018, 46, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Mottola, M.F. Physical activity and maternal obesity: Cardiovascular adaptations, exercise recommendations, and pregnancy outcomes. Nutr. Rev. 2013, 71 (Suppl. S1), S31–S36. [Google Scholar] [CrossRef] [PubMed]
- Tanner, L.D.; Brock, C.; Chauhan, S.P. Severity of fetal growth restriction stratified according to maternal obesity. J. Matern. -Fetal Neonatal Med. 2022, 35, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Cremer, M.; Flothkötter, M.; Graf, C.; Hauner, H.; Hellmers, C.; Kersting, M.; Krawinkel, M.; Przyrembel, H.; Röbl-Mathieu, M.; et al. Diet and Lifestyle Before and During Pregnancy—Practical Recommendations of the Germany-wide Healthy Start—Young Family Network. Geburtshilfe Frauenheilkd. 2018, 78, 1262–1282. [Google Scholar] [CrossRef] [PubMed]
- Rakhanova, Y.; Almawi, W.Y.; Aimagambetova, G.; Riethmacher, D. The effects of sildenafil citrate on intrauterine growth restriction: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2023, 23, 409. [Google Scholar] [CrossRef] [PubMed]
- Pels, A.; Derks, J.; Elvan-Taspinar, A.; van Drongelen, J.; de Boer, M.; Duvekot, H.; van Laar, J.; van Eyck, J.; Al-Nasiry, S.; Sueters, M.; et al. Maternal Sildenafil vs Placebo in Pregnant Women with Severe Early-Onset Fetal Growth Restriction: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e205323. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.; Fragoso, M.B.T.; Tenório, M.C.S.; Bueno, N.B.; Goulart, M.O.F.; Oliveira, A.C.M. The role played by oral antioxidant therapies in preventing and treating preeclampsia: An updated meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Mesdaghinia, E.; Naderi, F.; Bahmani, F.; Chamani, M.; Ghaderi, A.; Asemi, Z. The effects of zinc supplementation on clinical response and metabolic profiles in pregnant women at risk for intrauterine growth restriction: A randomized, double-blind, placebo-controlled trial. J. Matern. -Fetal Neonatal Med. 2021, 34, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Bo, Q.; Xie, Y.; Lin, Q.; Fu, L.; Hu, C.; Zhang, Z.; Meng, Q.; Xu, F.; Wang, G.; Miao, Z.; et al. Docosahexaenoic acid protects against lipopolysaccharide-induced fetal growth restriction via inducing the ubiquitination and degradation of NF-κB p65 in placental trophoblasts. J. Nutr. Biochem. 2023, 118, 109359. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Xiao, B.; Cui, D.; Lin, Y.; Zeng, J.; Li, J.; Cao, M.J.; Liu, J. Antioxidant Activity of Docosahexaenoic Acid (DHA) and Its Regulatory Roles in Mitochondria. J. Agric. Food Chem. 2021, 69, 1647–1655. [Google Scholar] [CrossRef]
- Surico, D.; Bordino, V.; Cantaluppi, V.; Mary, D.; Gentilli, S.; Oldani, A.; Farruggio, S.; Melluzza, C.; Raina, G.; Grossini, E. Preeclampsia and intrauterine growth restriction: Role of human umbilical cord mesenchymal stem cells-trophoblast cross-talk. PLoS ONE 2019, 14, e0218437. [Google Scholar] [CrossRef]
- Zhang, D.; Fu, L.; Wang, L.; Lin, L.; Yu, L.; Zhang, L.; Shang, T. Therapeutic benefit of mesenchymal stem cells in pregnant rats with angiotensin receptor agonistic autoantibody-induced hypertension: Implications for immunomodulation and cytoprotection. Hypertens. Pregnancy 2017, 36, 247–258. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nüsken, E.; Appel, S.; Saschin, L.; Kuiper-Makris, C.; Oberholz, L.; Schömig, C.; Tauscher, A.; Dötsch, J.; Kribs, A.; Alejandre Alcazar, M.A.; et al. Intrauterine Growth Restriction: Need to Improve Diagnostic Accuracy and Evidence for a Key Role of Oxidative Stress in Neonatal and Long-Term Sequelae. Cells 2024, 13, 501. https://doi.org/10.3390/cells13060501
Nüsken E, Appel S, Saschin L, Kuiper-Makris C, Oberholz L, Schömig C, Tauscher A, Dötsch J, Kribs A, Alejandre Alcazar MA, et al. Intrauterine Growth Restriction: Need to Improve Diagnostic Accuracy and Evidence for a Key Role of Oxidative Stress in Neonatal and Long-Term Sequelae. Cells. 2024; 13(6):501. https://doi.org/10.3390/cells13060501
Chicago/Turabian StyleNüsken, Eva, Sarah Appel, Leon Saschin, Celien Kuiper-Makris, Laura Oberholz, Charlotte Schömig, Anne Tauscher, Jörg Dötsch, Angela Kribs, Miguel A. Alejandre Alcazar, and et al. 2024. "Intrauterine Growth Restriction: Need to Improve Diagnostic Accuracy and Evidence for a Key Role of Oxidative Stress in Neonatal and Long-Term Sequelae" Cells 13, no. 6: 501. https://doi.org/10.3390/cells13060501
APA StyleNüsken, E., Appel, S., Saschin, L., Kuiper-Makris, C., Oberholz, L., Schömig, C., Tauscher, A., Dötsch, J., Kribs, A., Alejandre Alcazar, M. A., & Nüsken, K.-D. (2024). Intrauterine Growth Restriction: Need to Improve Diagnostic Accuracy and Evidence for a Key Role of Oxidative Stress in Neonatal and Long-Term Sequelae. Cells, 13(6), 501. https://doi.org/10.3390/cells13060501





