Comparison of Minced Cartilage Implantation with Autologous Chondrocyte Transplantation in an In Vitro Inflammation Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Isolation and Culture of Minced Cartilage Samples
2.3. Isolation and Culture of Articular Chondrocytes
2.4. Chondrocyte Inflammation Model
2.5. Biochemical Analysis
2.6. RNA Extraction, Reverse Transcription and Gene Expression Analysis
2.7. Histology and Immunohistochemistry
2.8. Statistics
3. Results
3.1. Chondrogenic Differentiation Potential Is Not Affected in MCI Culture
3.2. Collagen 1 Synthesis Is Not Influenced by TNFα in MCI Samples
3.3. MMPs Show Different TNFα-Induced Patterns in ACT and MCI Transplants
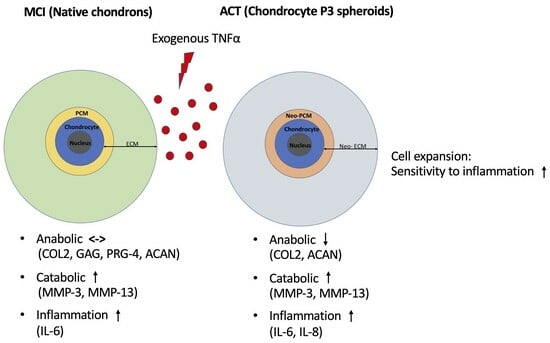
3.4. ACT Constructs Are More Sensitive to TNFα-Induced Inflammation Compared to MCI Grafts
4. Discussion
4.1. Key Findings
4.2. MCI Counteracts Anti-Catabolic and Catabolic Effects of TNFα
4.3. Pericellular Matrix as Key of Chondrogenic Potential
4.4. Mincing Procedure as Factor of Chondrocyte Activation
4.5. Cell Expansion Process in ACT Increases the Sensitivity to Inflammatory Stimuli
4.6. Limitations and Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flanigan, D.C.; Harris, J.D.; Trinh, T.Q.; Siston, R.A.; Brophy, R.H. Prevalence of chondral defects in athletes’ knees: A systematic review. Med. Sci. Sports Exerc. 2010, 42, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Haqqi, T.M. Immunopathogenesis of osteoarthritis. Clin. Immunol. 2013, 146, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.T.; Aman, Z.S.; DePhillipo, N.N.; Dickens, J.F.; Anz, A.W.; LaPrade, R.F. Chondral Lesions of the Knee: An Evidence-Based Approach. J. Bone Jt. Surg. Am. 2021, 103, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Albrecht, D.; Andereya, S.; Angele, P.; Ateschrang, A.; Aurich, M.; Baumann, M.; Bosch, U.; Erggelet, C.; Fickert, S.; et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: A guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee 2016, 23, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Laute, V.; Zinser, W.; John, T.; Becher, C.; Diehl, P.; Kolombe, T.; Fay, J.; Siebold, R.; Fickert, S. Safety and efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology is independent of spheroid dose after 4 years. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1130–1143. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Carey, J.L.; Reardon, P.J.; Peterson, L.; Stuart, M.J.; Krych, A.J. Long-Term Outcomes after Autologous Chondrocyte Implantation: A Systematic Review at Mean Follow-Up of 11.4 Years. Cartilage 2016, 7, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Riboh, J.C.; Cvetanovich, G.L.; Cole, B.J.; Yanke, A.B. Comparative efficacy of cartilage repair procedures in the knee: A network meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3786–3799. [Google Scholar] [CrossRef]
- Barié, A.; Kruck, P.; Sorbi, R.; Rehnitz, C.; Oberle, D.; Walker, T.; Zeifang, F.; Moradi, B. Prospective Long-term Follow-up of Autologous Chondrocyte Implantation With Periosteum Versus Matrix-Associated Autologous Chondrocyte Implantation: A Randomized Clinical Trial. Am. J. Sports Med. 2020, 48, 2230–2241. [Google Scholar] [CrossRef]
- Salzmann, G.M.; Ossendorff, R.; Gilat, R.; Cole, B.J. Autologous Minced Cartilage Implantation for Treatment of Chondral and Osteochondral Lesions in the Knee Joint: An Overview. Cartilage 2020, 13, 1124S–1136S. [Google Scholar] [CrossRef]
- Vonk, L.A.; Windt TS de Kragten, A.H.; Beekhuizen, M.; Mastbergen, S.C.; Dhert, W.J.; Lafeber, F.P.J.G.; Creemers, L.B.; Saris, D.B.F. Enhanced cell-induced articular cartilage regeneration by chondrons; the influence of joint damage and harvest site. Osteoarthr. Cartil. 2014, 22, 1910–1917. [Google Scholar] [CrossRef]
- Vonk, L.A.; Doulabi, B.Z.; Huang, C.; Helder, M.N.; Everts, V.; Bank, R.A. Preservation of the chondrocyte’s pericellular matrix improves cell-induced cartilage formation. J. Cell Biochem. 2010, 110, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Frodl, A.; Siegel, M.; Fuchs, A.; Wagner, F.C.; Schmal, H.; Izadpanah, K.; Yilmaz, T. Minced Cartilage Is a One-Step Cartilage Repair Procedure for Small Defects in the Knee-A Systematic-Review and Meta-Analysis. J. Pers. Med. 2022, 12, 1923. [Google Scholar] [CrossRef] [PubMed]
- Massen, F.K.; Inauen, C.R.; Harder, L.P.; Runer, A.; Preiss, S.; Salzmann, G.M. One-Step Autologous Minced Cartilage Procedure for the Treatment of Knee Joint Chondral and Osteochondral Lesions: A Series of 27 Patients With 2-Year Follow-up. Orthop. J. Sports Med. 2019, 7, 2325967119853773. [Google Scholar] [CrossRef] [PubMed]
- Wodzig, M.H.; Peters, M.J.; Emanuel, K.S.; van Hugten, P.P.; Wijnen, W.; Jutten, L.M.; Boymans, T.A.; Loeffen, D.V.; Emans, P.J. Minced Autologous Chondral Fragments with Fibrin Glue as a Simple Promising One-Step Cartilage Repair Procedure: A Clinical and MRI Study at 12-Month Follow-Up. Cartilage 2022, 13, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P. Cartilage repair surgery for full-thickness defects of the knee in Germany: Indications and epidemiological data from the German Cartilage Registry (KnorpelRegister DGOU). Arch. Orthop. Trauma Surg. 2016, 136, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Angele, P.; Fritz, J.; Albrecht, D.; Koh, J.; Zellner, J. Defect type, localization and marker gene expression determines early adverse events of matrix-associated autologous chondrocyte implantation. Injury 2015, 46 (Suppl. 4), S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Kunisch, E.; Kinne, R.W.; Alsalameh, R.J.; Alsalameh, S. Pro-inflammatory IL-1beta and/or TNF-alpha up-regulate matrix metalloproteases-1 and -3 mRNA in chondrocyte subpopulations potentially pathogenic in osteoarthritis: In situ hybridization studies on a single cell level. Int. J. Rheum. Dis. 2016, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, J.; Rajappa, R.; Deng, L.; Fredberg, J.; Yang, L. Interleukin-1β and tumor necrosis factor-α increase stiffness and impair contractile function of articular chondrocytes. Acta Biochim. Biophys. Sin. 2015, 47, 121–129. [Google Scholar] [CrossRef]
- Salzmann, G.M.; Calek, A.-K.; Preiss, S. Second-Generation Autologous Minced Cartilage Repair Technique. Arthrosc. Tech. 2017, 6, e127–e131. [Google Scholar] [CrossRef]
- Ossendorff, R.; Grad, S.; Stoddart, M.J.; Alini, M.; Schmal, H.; Südkamp, N.; Salzmann, G.M. Autologous Chondrocyte Implantation in Osteoarthritic Surroundings: TNFα and Its Inhibition by Adalimumab in a Knee-Specific Bioreactor. Am. J. Sports Med. 2018, 46, 431–440. [Google Scholar] [CrossRef]
- Labarca, C.; Paigen, K. A simple, rapid, and sensitive DNA assay procedure. Anal. Biochem. 1980, 102, 344–352. [Google Scholar] [CrossRef]
- Farndale, R.W.; Sayers, C.A.; Barrett, A.J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect. Tissue Res. 1982, 9, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Grad, S.; Gogolewski, S.; Alini, M.; Wimmer, M.A. Effects of simple and complex motion patterns on gene expression of chondrocytes seeded in 3D scaffolds. Tissue Eng. 2006, 12, 3171–3179. [Google Scholar] [CrossRef]
- Du, J.; Pfannkuche, J.-J.; Lang, G.; Häckel, S.; Creemers, L.B.; Alini, M.; Grad, S.; Li, Z. Proinflammatory intervertebral disc cell and organ culture models induced by tumor necrosis factor alpha. JOR Spine 2020, 3, e1104. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Albrecht, D.; Aurich, M.; Becher, C.; Behrens, P.; Bichmann, P.; Bode, G.; Brucker, P.; Erggelet, C.; Ezechieli, M.; et al. Empfehlungen der AG Klinische Geweberegeneration zur Behandlung von Knorpelschäden am Kniegelenk. Z. Orthop. Unfall 2022, 161, 57–64. [Google Scholar] [CrossRef]
- Saklatvala, J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature 1986, 322, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.P.; Caron, J.P.; Evans, C.; Robbins, P.D.; Georgescu, H.I.; Jovanovic, D.; Fernandes, J.C.; Martel-Pelletier, J. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997, 40, 1012–1019. [Google Scholar] [CrossRef]
- Pelttari, K.; Lorenz, H.; Boeuf, S.; Templin, M.F.; Bischel, O.; Goetzke, K.; Hsu, H.-Y.; Steck, E.; Richter, W. Secretion of matrix metalloproteinase 3 by expanded articular chondrocytes as a predictor of ectopic cartilage formation capacity in vivo. Arthritis Rheum. 2008, 58, 467–474. [Google Scholar] [CrossRef]
- Shah, S.S.; Mithoefer, K. Scientific Developments and Clinical Applications Utilizing Chondrons and Chondrocytes with Matrix for Cartilage Repair. Cartilage 2020, 13, 1195S–1205S. [Google Scholar] [CrossRef]
- Hing, W.A.; Sherwin, A.F.; Poole, C.A. The influence of the pericellular microenvironment on the chondrocyte response to osmotic challenge. Osteoarthr. Cartil. 2002, 10, 297–307. [Google Scholar] [CrossRef]
- Albrecht, F.; Roessner, A.; Zimmermann, E. Closure of osteochondral lesions using chondral fragments and fibrin adhesive. Arch. Orthop. Trauma. Surg. 1983, 101, 213–217. [Google Scholar] [CrossRef]
- Levinson, C.; Cavalli, E.; Sindi, D.M.; Kessel, B.; Zenobi-Wong, M.; Preiss, S.; Salzmann, G.; Neidenbach, P. Chondrocytes From Device-Minced Articular Cartilage Show Potent Outgrowth Into Fibrin and Collagen Hydrogels. Orthop. J. Sports Med. 2019, 7, 2325967119867618. [Google Scholar] [CrossRef]
- Evuarherhe, A.; Condron, N.B.; Knapik, D.M.; Haunschild, E.D.; Gilat, R.; Huddleston, H.P.; Kaiser, J.T.; Parvaresh, K.C.; Wagner, K.R.; Chubinskaya, S.; et al. Effect of Mechanical Mincing on Minimally Manipulated Articular Cartilage for Surgical Transplantation. Am. J. Sports Med. 2022, 50, 2515–2525. [Google Scholar] [CrossRef]
- Bonasia, D.E.; Marmotti, A.; Mattia, S.; Cosentino, A.; Spolaore, S.; Governale, G.; Castoldi, F.; Rossi, R. The Degree of Chondral Fragmentation Affects Extracellular Matrix Production in Cartilage Autograft Implantation: An In Vitro Study. Arthroscopy 2015, 31, 2335–2341. [Google Scholar] [CrossRef]
- Schneider, S.; Ossendorff, R.; Holz, J.; Salzmann, G.M. Arthroscopic Minced Cartilage Implantation (MCI): A Technical Note. Arthrosc. Tech. 2021, 10, e97–e101. [Google Scholar] [CrossRef]
- Darling, E.M.; Athanasiou, K.A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 2005, 23, 425–432. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossendorff, R.; Grede, L.; Scheidt, S.; Strauss, A.C.; Burger, C.; Wirtz, D.C.; Salzmann, G.M.; Schildberg, F.A. Comparison of Minced Cartilage Implantation with Autologous Chondrocyte Transplantation in an In Vitro Inflammation Model. Cells 2024, 13, 546. https://doi.org/10.3390/cells13060546
Ossendorff R, Grede L, Scheidt S, Strauss AC, Burger C, Wirtz DC, Salzmann GM, Schildberg FA. Comparison of Minced Cartilage Implantation with Autologous Chondrocyte Transplantation in an In Vitro Inflammation Model. Cells. 2024; 13(6):546. https://doi.org/10.3390/cells13060546
Chicago/Turabian StyleOssendorff, Robert, Lisa Grede, Sebastian Scheidt, Andreas C. Strauss, Christof Burger, Dieter C. Wirtz, Gian M. Salzmann, and Frank A. Schildberg. 2024. "Comparison of Minced Cartilage Implantation with Autologous Chondrocyte Transplantation in an In Vitro Inflammation Model" Cells 13, no. 6: 546. https://doi.org/10.3390/cells13060546
APA StyleOssendorff, R., Grede, L., Scheidt, S., Strauss, A. C., Burger, C., Wirtz, D. C., Salzmann, G. M., & Schildberg, F. A. (2024). Comparison of Minced Cartilage Implantation with Autologous Chondrocyte Transplantation in an In Vitro Inflammation Model. Cells, 13(6), 546. https://doi.org/10.3390/cells13060546