Microfluidics-Based Technologies for the Assessment of Castration-Resistant Prostate Cancer
Abstract
:1. Introduction
1.1. Prostate Cancer
1.2. Castration-Resistant Prostate Cancer
1.3. Limitations of Current In Vitro and In Vivo Techniques
1.4. Microfluidic Models to Study Castration Resistance
2. Microfluidic Models
2.1. Prostate Cancer Cell Lines Used in Current Microfluidics Systems
2.1.1. Lymph Node Carcinoma of the Prostate (LNCaP)
2.1.2. DU-145
2.1.3. PC3
2.2. Microfluidic Studies Examining Physical Property of Castration-Resistant Prostate Cancer Cells
2.3. Microfluidic Studies Examining Circulating Castration-Resistant Prostate Cancer Cells
2.3.1. Separation Based on Size or Deformability
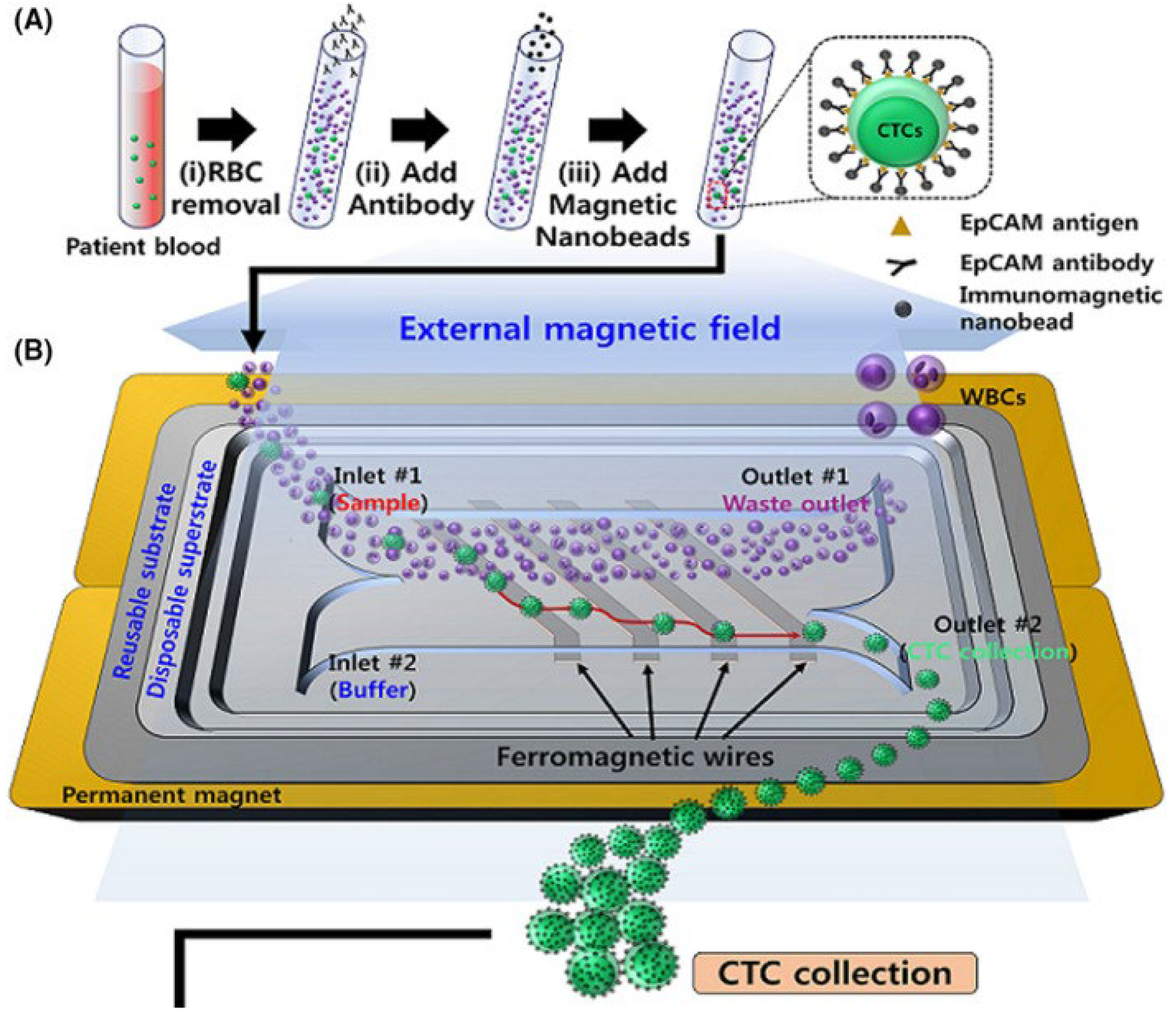
2.3.2. Separation Based on Epithelial Markers
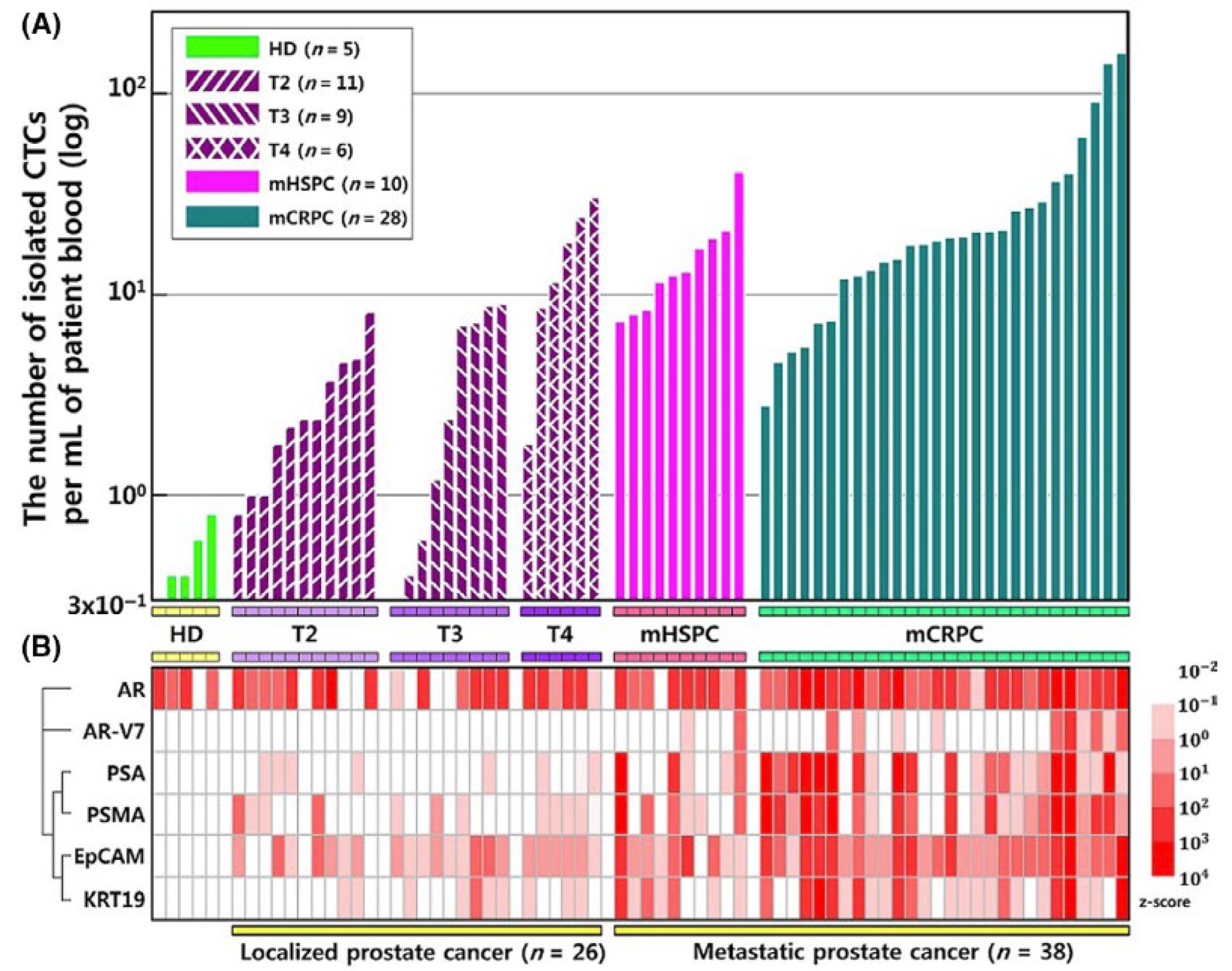
2.3.3. Prostate-Specific Markers
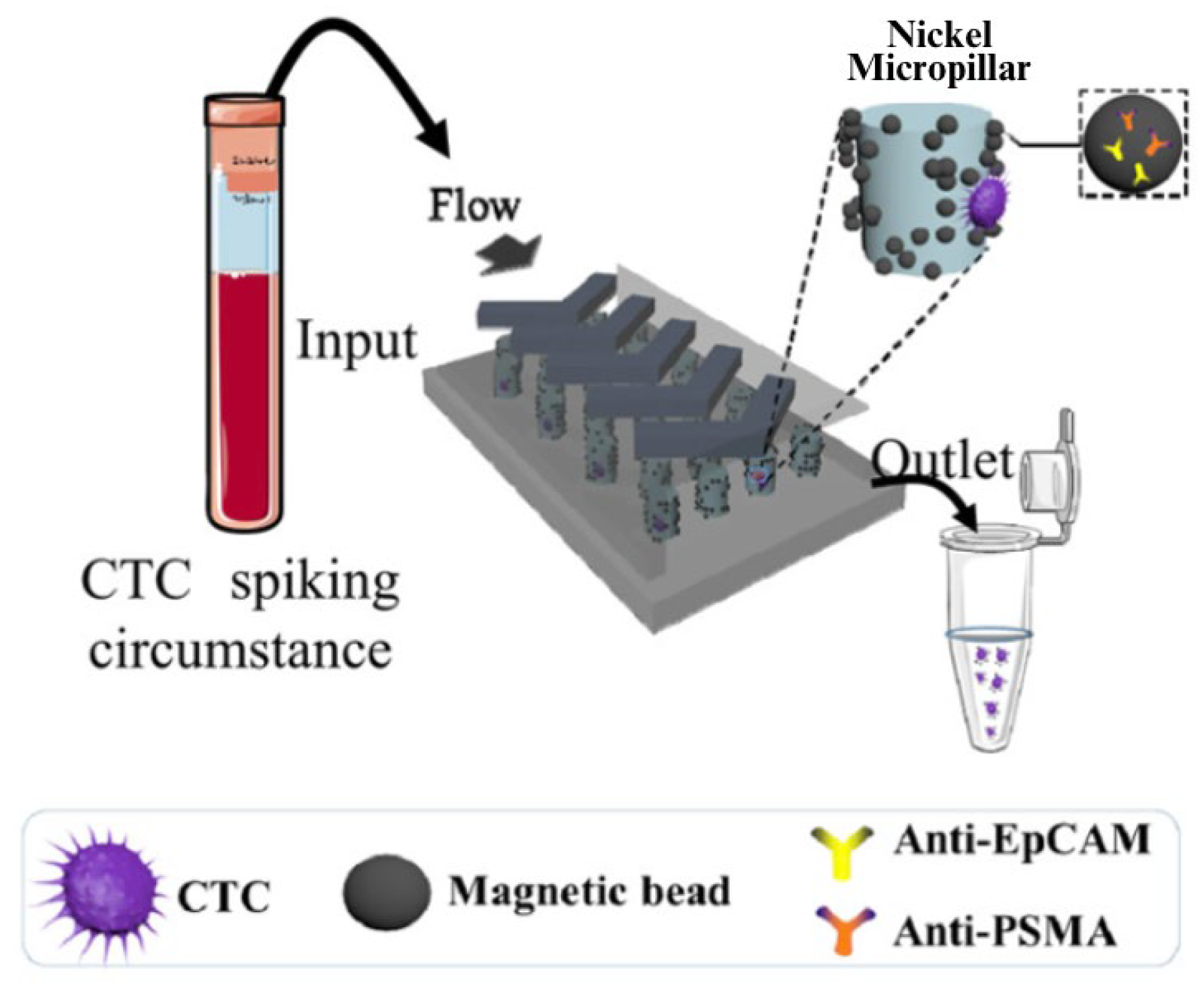
2.3.4. Separation Based on Multiple Characteristics
2.4. Tumor-on-a-Chip Models
2.5. 3D-Printed Microfluidic Devices
3. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| PCa | Prostate cancer |
| PSA | Prostate-specific antigen |
| ADT | Androgen deprivation therapy |
| AR | Androgen receptor |
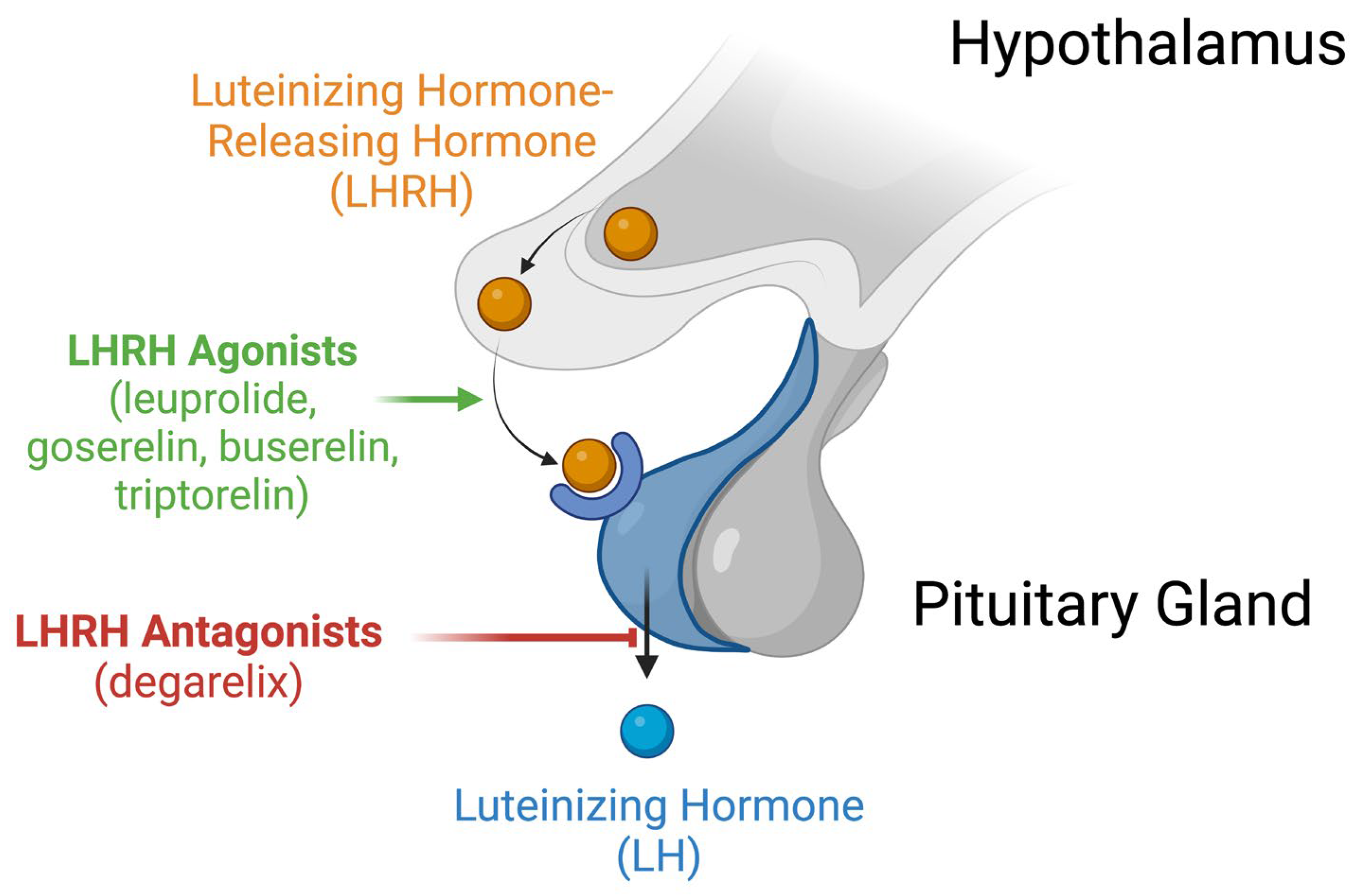
| LHRH | Luteinizing hormone-releasing hormone |
| GnRH | Gonadotropin-releasing hormone |
| LH | Luteinizing hormone |
| LNCaP | Lymph node carcinoma of the prostate |
| PCR | Polymerase chain reaction |
| hK2 | Human kallikrein 2 |
| CTC | Circulating tumor cell |
| EMT | Epithelial-to-mesenchymal transition |
| EpCAM | Epithelial cellular adhesion molecule |
| PSMA | Prostate-specific membrane antigen |
| AR-V7 | Androgen receptor variant 7 |
| KRT19 | Keratin 19 |
| GEDI | Geometrically enhanced differential immunocapture |
| ERG | ETS-related gene |
| SChLAP1 | Second chromosome locus associated with prostate-1 |
References
- Prostate Cancer Statistics. Centers for Disease Control and Prevention. 29 November 2022. Available online: https://www.cdc.gov/cancer/prostate/statistics/index.htm (accessed on 24 July 2023).
- Key Statistics for Prostate Cancer | Prostate Cancer Facts. Available online: https://www.cancer.org/cancer/types/prostate-cancer/about/key-statistics.html (accessed on 24 July 2023).
- Thobe, M.N.; Clark, R.J.; Bainer, R.O.; Prasad, S.M.; Rinker-Schaeffer, C.W. From Prostate to Bone: Key Players in Prostate Cancer Bone Metastasis. Cancers 2011, 3, 478–493. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU Guidelines on Prostate Cancer. Part II: Treatment of Advanced, Relapsing, and Castration-Resistant Prostate Cancer. Eur. Urol. 2014, 65, 467–479. [Google Scholar] [CrossRef]
- Costello, A.J. Considering the role of radical prostatectomy in 21st century prostate cancer care. Nat. Rev. Urol. 2020, 17, 177–188. [Google Scholar] [CrossRef]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J. Urol. 2002, 168, 9–12. [Google Scholar] [CrossRef]
- Denis, L.J.; Griffiths, K. Endocrine treatment in prostate cancer. Semin. Surg. Oncol. 2000, 18, 52–74. [Google Scholar] [CrossRef]
- van Royen, M.E.; van Cappellen, W.A.; de Vos, C.; Houtsmuller, A.B.; Trapman, J. Stepwise androgen receptor dimerization. J. Cell Sci. 2012, 125 Pt 8, 1970–1979. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, S.; Lu, J.; Fu, S.; Li, L.; Zhang, J.; Wang, Z.; Hong, M. FOXO3a-ROS pathway is involved in androgen-induced proliferation of prostate cancer cell. BMC Urol. 2022, 22, 70. [Google Scholar] [CrossRef]
- Schally, A.V.; Block, N.L.; Rick, F.G. Discovery of LHRH and development of LHRH analogs for prostate cancer treatment. Prostate 2017, 77, 1036–1054. [Google Scholar] [CrossRef]
- Crawford, E.D.; Hou, A.H. The role of LHRH antagonists in the treatment of prostate cancer. Oncology (Williston Park) 2009, 23, 626–630. [Google Scholar]
- Memarzadeh, S.; Xin, L.; Mulholland, D.J.; Mansukhani, A.; Wu, H.; Teitell, M.A.; Witte, O.N. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell 2007, 12, 572–585. [Google Scholar] [CrossRef]
- Lu, X.; Horner, J.W.; Paul, E.; Shang, X.; Troncoso, P.; Deng, P.; Jiang, S.; Chang, Q.; Spring, D.J.; Sharma, P.; et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 2017, 543, 728–732. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Crowley, F.; Sterpi, M.; Buckley, C.; Margetich, L.; Handa, S.; Dovey, Z. A Review of the Pathophysiological Mechanisms Underlying Castration-resistant Prostate Cancer. Res. Rep. Urol. 2021, 13, 457–472. [Google Scholar] [CrossRef]
- Mansoorifar, A.; Gordon, R.; Bergan, R.C.; Bertassoni, L.E. Bone-on-a-Chip: Microfluidic Technologies and Microphysiologic Models of Bone Tissue. Adv. Funct. Mater. 2021, 31, 2006796. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, S.J.; McCauley, L.K.; Gallick, G.E. Preclinical Mouse Models of Human Prostate Cancer and Their Utility in Drug Discovery. Curr. Protoc. Pharmacol. 2010, 51, 14.15.1–14.15.27. [Google Scholar] [CrossRef]
- Bischel, L.L.; Casavant, B.P.; Young, P.A.; Eliceiri, K.W.; Basu, H.S.; Beebe, D.J. A microfluidic coculture and multiphoton FAD analysis assay provides insight into the influence of the bone microenvironment on prostate cancer cells. Integr. Biol. (Camb.) 2014, 6, 627–635. [Google Scholar] [CrossRef]
- Sobel, R.E.; Sadar, M.D. Cell lines used in prostate cancer research: A compendium of old and new lines—Part 1. J. Urol. 2005, 173, 342–359. [Google Scholar] [CrossRef]
- Sobel, R.E.; Sadar, M.D. Cell lines used in prostate cancer research: A compendium of old and new lines—Part 2. J. Urol. 2005, 173, 360–372. [Google Scholar] [CrossRef]
- Herbig, M.; Kräter, M.; Plak, K.; Müller, P.; Guck, J.; Otto, O. Real-Time Deformability Cytometry: Label-Free Functional Characterization of Cells. Methods Mol. Biol. 2018, 1678, 347–369. [Google Scholar] [CrossRef]
- Tse, H.T.K.; Gossett, D.R.; Moon, Y.S.; Masaeli, M.; Sohsman, M.; Ying, Y.; Mislick, K.; Adams, R.P.; Rao, J.; Di Carlo, D. Quantitative diagnosis of malignant pleural effusions by single-cell mechanophenotyping. Sci. Transl. Med. 2013, 5, 212ra163. [Google Scholar] [CrossRef]
- Byun, S.; Son, S.; Amodei, D.; Cermak, N.; Shaw, J.; Kang, J.H.; Hecht, V.C.; Winslow, M.M.; Jacks, T.; Mallick, P.; et al. Characterizing deformability and surface friction of cancer cells. Proc. Natl. Acad. Sci. USA 2013, 110, 7580–7585. [Google Scholar] [CrossRef]
- Liu, N.; Du, P.; Xiao, X.; Liu, Y.; Peng, Y.; Yang, C.; Yue, T. Microfluidic-Based Mechanical Phenotyping of Androgen-Sensitive and Non-sensitive Prostate Cancer Cells Lines. Micromachines 2019, 10, 602. [Google Scholar] [CrossRef]
- Luo, D.; Liu, N.; Chen, Y.; Peng, Y.; Yue, T.; Cao, S.; Liu, Y. Microfluidic Assessment of Drug Effects on Physical Properties of Androgen Sensitive and Non-Sensitive Prostate Cancer Cells. Micromachines 2021, 12, 532. [Google Scholar] [CrossRef]
- Otto, O.; Rosendahl, P.; Mietke, A.; Golfier, S.; Herold, C.; Klaue, D.; Girardo, S.; Pagliara, S.; Ekpenyong, A.; Jacobi, A.; et al. Real-time deformability cytometry: On-the-fly cell mechanical phenotyping. Nat. Methods 2015, 12, 199–202. [Google Scholar] [CrossRef]
- Wei, S.C.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.J.; et al. Matrix stiffness drives epithelial–mesenchymal transition and tumour metastasis through a TWIST1–G3BP2 mechanotransduction pathway. Nat. Cell Biol. 2015, 17, 678–688. [Google Scholar] [CrossRef]
- Wei, S.C.; Yang, J. Forcing through Tumor Metastasis: The Interplay between Tissue Rigidity and Epithelial-Mesenchymal Transition. Trends Cell Biol. 2016, 26, 111–120. [Google Scholar] [CrossRef]
- Katsogiannou, M.; Ziouziou, H.; Karaki, S.; Andrieu, C.; Henry de Villeneuve, M.; Rocchi, P. The hallmarks of castration-resistant prostate cancers. Cancer Treat. Rev. 2015, 41, 588–597. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Aaltonen, K.E.; Novosadová, V.; Bendahl, P.O.; Graffman, C.; Larsson, A.M.; Rydén, L. Molecular characterization of circulating tumor cells from patients with metastatic breast cancer reflects evolutionary changes in gene expression under the pressure of systemic therapy. Oncotarget 2017, 8, 45544–45565. [Google Scholar] [CrossRef]
- Babayan, A.; Pantel, K. Advances in liquid biopsy approaches for early detection and monitoring of cancer. Genome Med. 2018, 10, 21. [Google Scholar] [CrossRef]
- Riethdorf, S.; O’Flaherty, L.; Hille, C.; Pantel, K. Clinical applications of the CellSearch platform in cancer patients. Adv. Drug Deliv. Rev. 2018, 125, 102–121. [Google Scholar] [CrossRef]
- Andree, K.C.; van Dalum, G.; Terstappen, L.W.M.M. Challenges in circulating tumor cell detection by the CellSearch system. Mol. Oncol. 2016, 10, 395–407. [Google Scholar] [CrossRef]
- Kitz, J.; Goodale, D.; Postenka, C.; Lowes, L.E.; Allan, A.L. EMT-independent detection of circulating tumor cells in human blood samples and pre-clinical mouse models of metastasis. Clin. Exp. Metastasis 2021, 38, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Renier, C.; Pao, E.; Che, J.; Liu, H.E.; Lemaire, C.A.; Matsumoto, M.; Triboulet, M.; Srivinas, S.; Jeffrey, S.S.; Rettig, M.; et al. Label-free isolation of prostate circulating tumor cells using Vortex microfluidic technology. npj Precis. Oncol. 2017, 1, 15. [Google Scholar] [CrossRef]
- Park, E.S.; Jin, C.; Guo, Q.; Ang, R.R.; Duffy, S.P.; Matthews, K.; Azad, A.; Abdi, H.; Todenhöfer, T.; Bazov, J.; et al. Continuous Flow Deformability-Based Separation of Circulating Tumor Cells Using Microfluidic Ratchets. Small 2016, 12, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Chung, J.I.; Kim, J.; Lee, C.H.; Morgan, T.M.; Byun, S.; Chung, J.; Han, K. Multigene model for predicting metastatic prostate cancer using circulating tumor cells by microfluidic magnetophoresis. Cancer Sci. 2021, 112, 859–870. [Google Scholar] [CrossRef]
- Green, B.J.; Nguyen, V.; Atenafu, E.; Weeber, P.; Duong, B.T.V.; Thiagalingam, P.; Labib, M.; Mohamadi, R.M.; Hansen, A.R.; Joshua, A.M.; et al. Phenotypic Profiling of Circulating Tumor Cells in Metastatic Prostate Cancer Patients Using Nanoparticle-Mediated Ranking. Anal. Chem. 2019, 91, 9348–9355. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.T.; Lee, R.J.; Stott, S.L.; Ting, D.T.; Wittner, B.S.; Ulman, M.; Smas, M.E.; Lord, J.B.; Brannigan, B.W.; Trautwein, J.; et al. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov. 2012, 2, 995–1003. [Google Scholar] [CrossRef]
- Miyamoto, D.T.; Zheng, Y.; Wittner, B.S.; Lee, R.J.; Zhu, H.; Broderick, K.T.; Desai, R.; Fox, D.B.; Brannigan, B.W.; Trautwein, J.; et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science 2015, 349, 1351–1356. [Google Scholar] [CrossRef]
- Gleghorn, J.P.; Pratt, E.D.; Denning, D.; Liu, H.; Bander, N.H.; Tagawa, S.T.; Nanus, D.M.; Giannakakou, P.A.; Kirby, B.J. Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically-enhanced differential immunocapture (GEDI) and a prostate specific antibody. Lab. Chip. 2010, 10, 27–29. [Google Scholar] [CrossRef]
- Galletti, G.; Matov, A.; Beltran, H.; Fontugne, J.; Mosquera, J.M.; Cheung, C.; MacDonald, T.Y.; Sung, M.; O’toole, S.; Kench, J.G.; et al. ERG induces taxane resistance in castration-resistant prostate cancer. Nat. Commun. 2014, 5, 5548. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Wang, Y.; Ji, J.; Cai, B.; Chen, H.; Yang, Z.; Wang, K.; Luo, C.; Zhang, W.; Yuan, C.; et al. Molecular Profiling of Pooled Circulating Tumor Cells from Prostate Cancer Patients Using a Dual-Antibody-Functionalized Microfluidic Device. Anal. Chem. 2018, 90, 3744–3751. [Google Scholar] [CrossRef] [PubMed]
- Gires, O.; Klein, C.A.; Baeuerle, P.A. On the abundance of EpCAM on cancer stem cells. Nat. Rev. Cancer 2009, 9, 143. [Google Scholar] [CrossRef]
- Spizzo, G.; Obrist, P.; Ensinger, C.; Theurl, I.; Dünser, M.; Ramoni, A.; Gunsilius, E.; Eibl, G.; Mikuz, G.; Gastl, G. Prognostic significance of Ep-CAM AND Her-2/neu overexpression in invasive breast cancer. Int. J. Cancer 2002, 98, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Breuhahn, K.; Baeuerle, P.A.; Peters, M.; Prang, N.; Töx, U.; Kohnevolland, R.; Dries, V.; Schirmacher, P.; Leo, E. Expression of epithelial cellular adhesion molecule (Ep-CAM) in chronic (necro-)inflammatory liver diseases and hepatocellular carcinoma. Hepatol. Res. 2006, 34, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gastl, G.; Spizzo, G.; Obrist, P.; Dünser, M.; Mikuz, G. Ep-CAM overexpression in breast cancer as a predictor of survival. Lancet. 2000, 356, 1981–1982. [Google Scholar] [CrossRef] [PubMed]
- van der Gun, B.T.F.; Melchers, L.J.; Ruiters, M.H.J.; de Leij, L.F.M.H.; McLaughlin, P.M.J.; Rots, M.G. EpCAM in carcinogenesis: The good, the bad or the ugly. Carcinogenesis 2010, 31, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Gires, O. EpCAM (CD326) finding its role in cancer. Br. J. Cancer 2007, 96, 417–423. [Google Scholar] [CrossRef]
- Sankpal, N.; Willman, M.; Fleming, T.; Mayfield, J.; Gillanders, W. Transcriptional repression of epithelial cell adhesion molecule (EpCAM) contributes to p53 control of breast cancer invasion. Cancer Res. 2009, 69, 753–757. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, J.K.; Phillips, J.W.; Huang, P.; Cheng, D.; Huang, J.; Witte, O.N. Prostate epithelial cell of origin determines cancer differentiation state in an organoid transformation assay. Proc. Natl. Acad. Sci. USA 2016, 113, 4482–4487. [Google Scholar] [CrossRef] [PubMed]
- Cheaito, K.A.; Bahmad, H.F.; Hadadeh, O.; Saleh, E.; Dagher, C.; Hammoud, M.S.; Shahait, M.; Mrad, Z.A.; Nassif, S.; Tawil, A.; et al. EMT Markers in Locally-Advanced Prostate Cancer: Predicting Recurrence? Front. Oncol. 2019, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Gulhati, P.; Bowen, K.A.; Liu, J.; Stevens, P.D.; Rychahou, P.G.; Chen, M.; Lee, E.Y.; Weiss, H.L.; O’Connor, K.L.; Gao, T.; et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011, 71, 3246–3256. [Google Scholar] [CrossRef] [PubMed]
- Grille, S.J.; Bellacosa, A.; Upson, J.; Klein-Szanto, A.J.; Van Roy, F.; Lee-Kwon, W.; Donowitz, M.; Tsichlis, P.N.; LaRue, L. The Protein Kinase Akt Induces Epithelial Mesenchymal Transition and Promotes Enhanced Motility and Invasiveness of Squamous Cell Carcinoma Lines1. Cancer Res. 2003, 63, 2172–2178. [Google Scholar] [PubMed]
- Pattabiraman, D.R.; Weinberg, R.A. Targeting the Epithelial-to-Mesenchymal Transition: The Case for Differentiation-Based Therapy. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Balk, S.P.; Ko, Y.J.; Bubley, G.J. Biology of Prostate-Specific Antigen. JCO 2003, 21, 383–391. [Google Scholar] [CrossRef]
- Lee, S. Diagnosis of Prostate Cancer. Canadian Cancer Society. February 2021. Available online: https://cancer.ca/en/cancer-information/cancer-types/prostate/diagnosis (accessed on 24 July 2023).
- Neal, D.E.; Clejan, S.; Sarma, D.; Moon, T.D. Prostate specific antigen and prostatitis. I. Effect of prostatitis on serum PSA in the human and nonhuman primate. Prostate 1992, 20, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Leidinger, P.; Hart, M.; Backes, C.; Rheinheimer, S.; Keck, B.; Wullich, B.; Keller, A.; Meese, E. Differential blood-based diagnosis between benign prostatic hyperplasia and prostate cancer: miRNA as source for biomarkers independent of PSA level, Gleason score, or TNM status. Tumour Biol. 2016, 37, 10177–10185. [Google Scholar] [CrossRef] [PubMed]
- Fenton, J.J.; Weyrich, M.S.; Durbin, S.; Liu, Y.; Bang, H.; Melnikow, J. Prostate-Specific Antigen–Based Screening for Prostate Cancer: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 319, 1914. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Kelly, W.K.; Schwartz, L.H.; Heller, G. Prostate cancer clinical trial end points: “RECIST”ing a step backwards. Clin. Cancer Res. 2005, 11, 5223–5232. [Google Scholar] [CrossRef]
- Berthold, D.R.; Pond, G.R.; Roessner, M.; de Wit, R.; Eisenberger, M.; Tannock, I.F.; TAX-327 investigators. Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: Relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin. Cancer Res. 2008, 14, 2763–2767. [Google Scholar] [CrossRef]
- Fleming, M.T.; Morris, M.J.; Heller, G.; Scher, H.I. Post-therapy changes in PSA as an outcome measure in prostate cancer clinical trials. Nat. Clin. Pract. Oncol. 2006, 3, 658–667. [Google Scholar] [CrossRef]
- Ross, J.S.; Sheehan, C.E.; Fisher, H.A.G.; Kaufman, R.P.; Kaur, P.; Gray, K.; Webb, I.; Gray, G.S.; Mosher, R.; Kallakury, B.V.S. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin. Cancer Res. 2003, 9, 6357–6362. [Google Scholar]
- Sweat, S.D.; Pacelli, A.; Murphy, G.P.; Bostwick, D.G. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998, 52, 637–640. [Google Scholar] [CrossRef]
- Stott, S.L.; Lee, R.J.; Nagrath, S.; Yu, M.; Miyamoto, D.T.; Ulkus, L.; Inserra, E.J.; Ulman, M.; Springer, S.; Nakamura, Z.; et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci. Transl. Med. 2010, 2, 25ra23. [Google Scholar] [CrossRef]
- D’Abronzo, L.S.; Lombard, A.P.; Ning, S.; Armstong, C.M.; Leslie, A.R.; Sharifi, M.; Schaaf, Z.A.; Lou, W.; Gao, A.C. Wntless expression promotes lineage plasticity and is associated with neuroendocrine prostate cancer. Am. J. Clin. Exp. Urol. 2022, 10, 299–310. [Google Scholar]
- Kirby, B.J.; Jodari, M.; Loftus, M.S.; Gakhar, G.; Pratt, E.D.; Chanel-Vos, C.; Gleghorn, J.P.; Santana, S.M.; Liu, H.; Smith, J.P.; et al. Functional characterization of circulating tumor cells with a prostate-cancer-specific microfluidic device. PLoS ONE 2012, 7, e35976. [Google Scholar] [CrossRef]
- Yu, J.; Berthier, E.; Craig, A.; de Groot, T.E.; Sparks, S.; Ingram, P.N.; Jarrard, D.F.; Huang, W.; Beebe, D.J.; Theberge, A.B. Reconfigurable open microfluidics for studying the spatiotemporal dynamics of paracrine signalling. Nat. Biomed. Eng. 2019, 3, 830–841. [Google Scholar] [CrossRef]
- Pandya, H.J.; Dhingra, K.; Prabhakar, D.; Chandrasekar, V.; Natarajan, S.K.; Vasan, A.S.; Kulkarni, A.; Shafiee, H. A microfluidic platform for drug screening in a 3D cancer microenvironment. Biosens. Bioelectron. 2017, 94, 632–642. [Google Scholar] [CrossRef]
- Lin, K.C.; Torga, G.; Sun, Y.; Axelrod, R.; Pienta, K.J.; Sturm, J.C.; Austin, R.H. The role of heterogeneous environment and docetaxel gradient in the emergence of polyploid, mesenchymal and resistant prostate cancer cells. Clin. Exp. Metastasis 2019, 36, 97–108. [Google Scholar] [CrossRef]
- Steinberg, E.; Friedman, R.; Goldstein, Y.; Friedman, N.; Beharier, O.; Demma, J.A.; Zamir, G.; Hubert, A.; Benny, O. A fully 3D-printed versatile tumor-on-a-chip allows multi-drug screening and correlation with clinical outcomes for personalized medicine. Commun. Biol. 2023, 6, 1157. [Google Scholar] [CrossRef]
- Neufeld, L.; Yeini, E.; Pozzi, S.; Satchi-Fainaro, R. 3D bioprinted cancer models: From basic biology to drug development. Nat. Rev. Cancer 2022, 22, 679–692. [Google Scholar] [CrossRef]
- Xu, K.; Huang, Y.; Wu, M.; Yin, J.; Wei, P. 3D bioprinting of multi-cellular tumor microenvironment for prostate cancer metastasis. Biofabrication 2023, 15, 035020. [Google Scholar] [CrossRef]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed Microfluidic Devices: Fabrication, Advantages and Limitations—A Mini Review. Anal. Methods. 2016, 8, 6005–6012. [Google Scholar] [CrossRef]
- Gross, B.C.; Anderson, K.B.; Meisel, J.E.; McNitt, M.I.; Spence, D.M. Polymer Coatings in 3D-Printed Fluidic Device Channels for Improved Cellular Adherence Prior to Electrical Lysis. Anal. Chem. 2015, 87, 6335–6341. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef]
- Kang, H.; Lee, D.; Yang, Y.; Oh, D.K.; Seong, J.; Kim, J.; Jeon, N.; Kang, D.; Rho, J. Emerging low-cost, large-scale photonic platforms with soft lithography and self-assembly. Photonics Insights 2023, 2, R04. [Google Scholar] [CrossRef]
- Descamps, L.; Le Roy, D.; Deman, A.L. Microfluidic-Based Technologies for CTC Isolation: A Review of 10 Years of Intense Efforts towards Liquid Biopsy. Int. J. Mol. Sci. 2022, 23, 1981. [Google Scholar] [CrossRef]
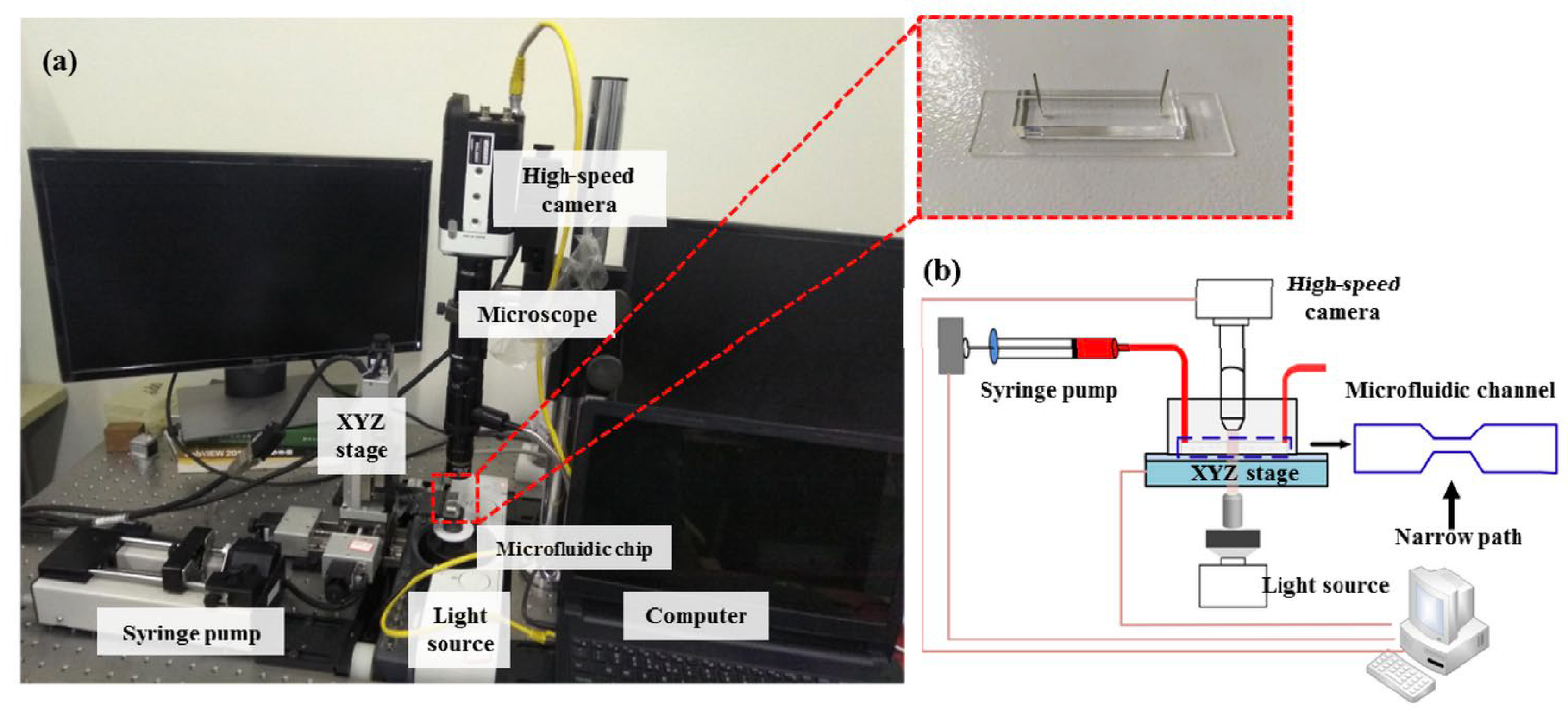

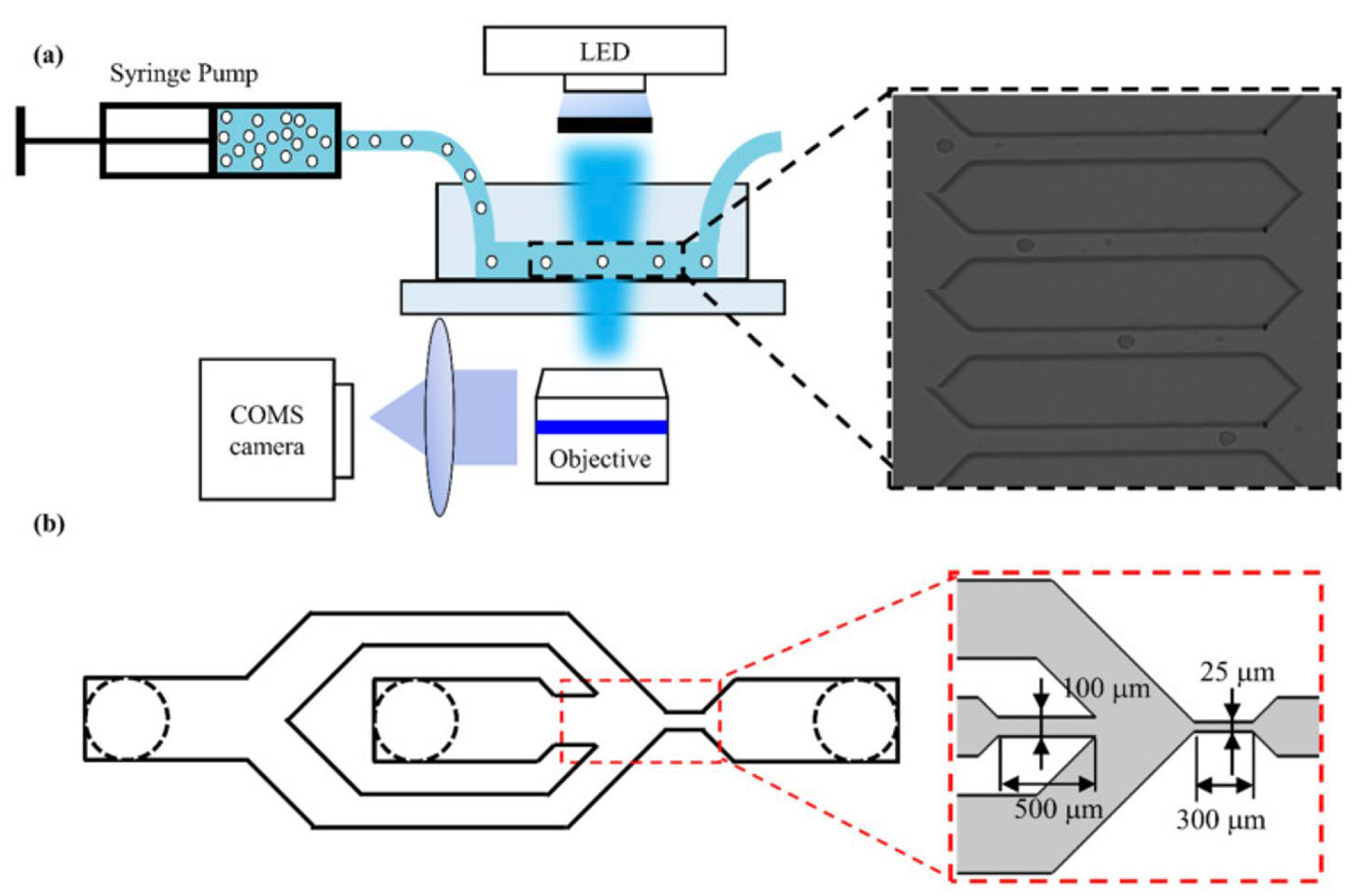

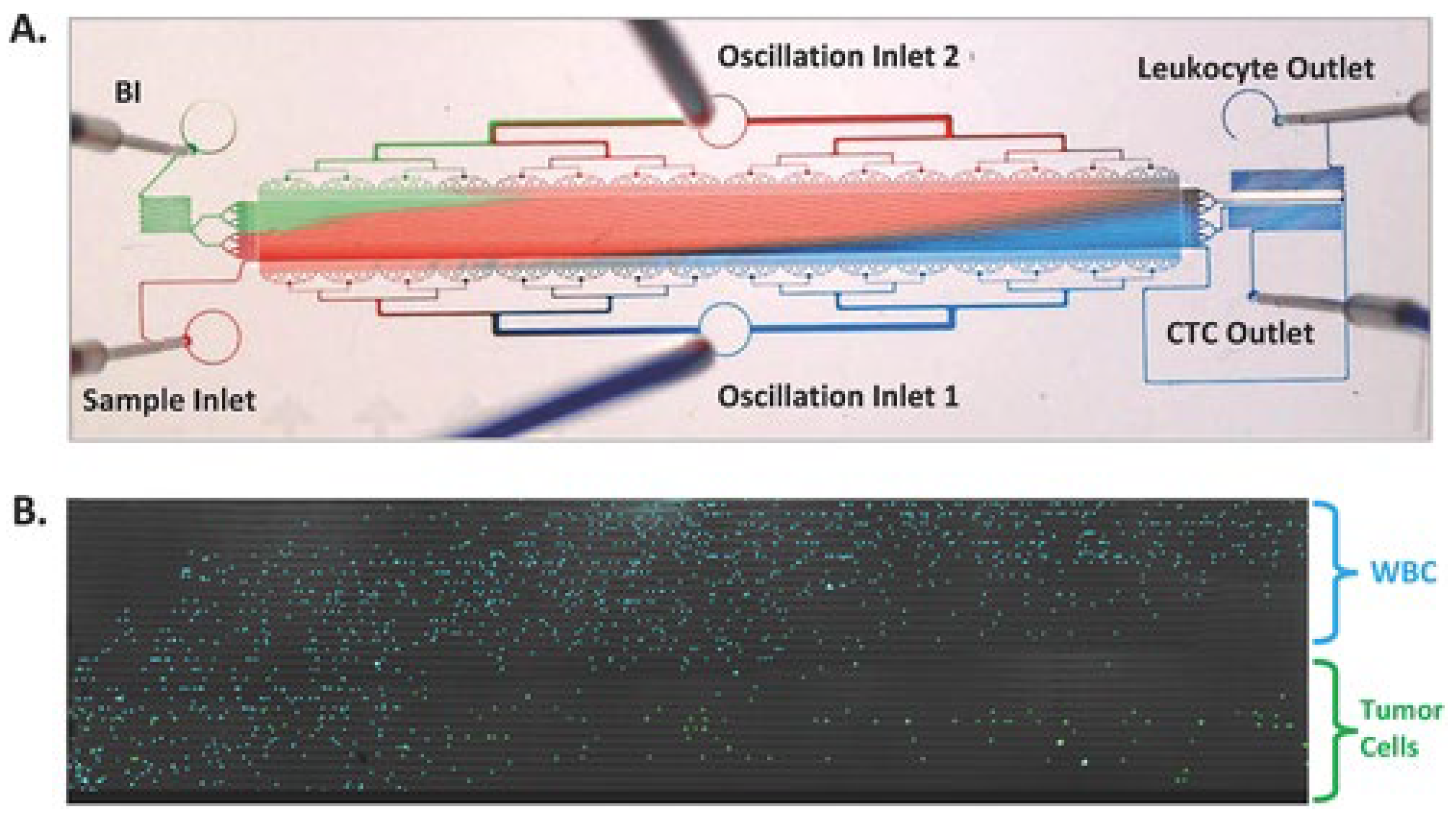


| Reference | Cell Type | Objective | Major Findings |
|---|---|---|---|
| Liu et al. 2019 [25] | LNCaP, DU-145, PC3 | Mechanically phenotype androgen-sensitive and androgen-insensitive human prostate cancer cell lines using a morphological rheological microfluidic method. |
|
| Luo et al. 2021 [26] | LNCaP, PC3 | Evaluate the physical properties of androgen-sensitive and androgen-insensitive prostate cancer cell lines exposed to docetaxel and enzalutamide. |
|
| Reference | Isolation Method | Biomarkers/ Properties | Capture Efficiency | Purity | Summary |
|---|---|---|---|---|---|
| Renier et al. 2017 [37] | Vortex microfluidic technology | Size | 1.88–93.75 CTCs/7.5 mL | 1.74–37.59% |
|
| Park et al. 2016 [38] | Microfluidic ratchet system | Deformability | Median of 178 CTCs/7.5 mL | - |
|
| Cho et al. 2021 [39] | Immunomagnetic nanobeads bound to anti-EpCAM antibodies | Epithelial Markers | Average of 16.7 CTCs/mL | 6.7% |
|
| Green et al. 2019 [40] | Magnetic particles conjugated to EpCAM antibodies | Epithelial Markers | - | - |
|
| Miyamoto et al. [41,42] | Magnetic particles conjugated to EpCAM antibodies | Prostate-Specific Marker Staining | - | - |
|
| Gleghorn et al. 2010 [43] | Geometrically enhanced differential immunocapture and immunocapture using PMSA antibodies | Multiple Characteristics (Size + Prostate-Specific Markers) | Average of 27 CTCs/7.5 mL | 68% |
|
| Galletti et al. 2014 [44] | Geometrically enhanced differential immunocapture and immunocapture using PMSA antibodies | Multiple Characteristics (Size + Prostate-Specific Markers) | - | - |
|
| Yin et al. 2018 [45] | Dual-antibody-functionalized microfluidic device (EpCAM and PMSA) | Multiple Characteristics (Epithelial Markers + Prostate-Specific Markers) | 6.6 CTCs/2 mL | - |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sassi, A.; You, L. Microfluidics-Based Technologies for the Assessment of Castration-Resistant Prostate Cancer. Cells 2024, 13, 575. https://doi.org/10.3390/cells13070575
Sassi A, You L. Microfluidics-Based Technologies for the Assessment of Castration-Resistant Prostate Cancer. Cells. 2024; 13(7):575. https://doi.org/10.3390/cells13070575
Chicago/Turabian StyleSassi, Amel, and Lidan You. 2024. "Microfluidics-Based Technologies for the Assessment of Castration-Resistant Prostate Cancer" Cells 13, no. 7: 575. https://doi.org/10.3390/cells13070575






